Abstract
How to cite this article: Khilnani GC, Tiwari P, Zirpe KG, Chaudhary D, Govil D, Dixit S, et al. Guidelines for the Use of Procalcitonin for Rational Use of Antibiotics. Indian J Crit Care Med 2022;26(S2):S77–S94.
Keywords: Antibiotics, Guidelines, Procalcitonin, Sepsis, Stewardship
Executive Summary
Procalcitonin (PCT) In Sepsis
Should Baseline PCT Levels be done in Patients with Sepsis?
Evidence statement
Procalcitonin is detectable in the serum within 4–6 hours after induction, which is most often a bacterial infection. It reaches its peak within 24 hours and then starts its decline in the case of adequate treatment, with levels reducing by roughly 50% daily according to its half-life. A baseline PCT is required for guiding antibiotic therapy to look at subsequent increases or decreases in levels. Most randomized controlled trials (RCTs) have done a baseline value in suspected sepsis patients and utilized it in PCT-based algorithms to limit the duration of antibiotic therapy.
Recommendations
Baseline serum PCT levels may be done in patients with sepsis (1B).
What is the Role of PCT in the Diagnosis of Sepsis?
Evidence Statement
Procalcitonin can diagnose sepsis only with moderate accuracy. The cut-off values for the diagnosis of sepsis are not well defined and need further clarification.
Recommendations
Procalcitonin alone should not be used for the diagnosis of sepsis (1A).
We suggest that PCT be used to differentiate culture-negative sepsis from non-sepsis (2B).
Should PCT be done for Discontinuation of Antibiotics in Sepsis?
Evidence Statement
Procalcitonin-guided treatment reduces the duration of antibiotic therapy and duration of hospital stay in adult critically ill patients with sepsis without any increase in mortality. Procalcitonin-based protocols are associated with significant overall hospitalization cost savings.
Recommendations
Procalcitonin levels can be used to support the discontinuation or de-escalation of antibiotics in patients with sepsis (2A).
How often should PCT Level be repeated after the Baseline Value?
Evidence Statement
There is no evidence to suggest the optimal frequency of PCT testing. In various studies, serum PCT has been tested every 24–72 hours.
Recommendations
Procalcitonin levels may be repeated every 72 hours or earlier if clinically indicated (3B).
What Level of PCT should be used for Discontinuing Antibiotics in Sepsis?
Evidence Statement
There is significant heterogeneity among studies regarding the cut-off value of PCT for discontinuation of antibiotics. In most studies, the reduction of PCT levels below 0.25–0.5 ng/mL, or an 80–90% decline from baseline led to antibiotic de-escalation.
Recommendations
Procalcitonin levels of less than 0.5 ng/mL or a drop by more than 80% from baseline should be used along with clinical judgment for decision-making regarding antibiotic de-escalation (3A).
What is the Role of PCT Value in deciding the Severity and Prognosis of Infections?
Evidence Statement
Elevated baseline PCT and non-clearance of PCT were associated with increased mortality in patients with sepsis. Procalcitonin can differentiate between gram-negative and gram-positive sepsis.
Recommendations
Procalcitonin may be used to define the severity of infection in a patient with sepsis (2B).
What are the Infections in which PCT is not an Effective Biomarker?
Evidence Statement
Procalcitonin may not be an effective marker in some infections. It may be inconsistently raised in infections with Legionella, Mycoplasma, and Chlamydia, whereas localized bacterial infections such as tonsillitis, sinusitis, cystitis, and uncomplicated soft tissue infections abscesses, or empyema can cause it to be falsely negative. Procalcitonin may be raised in non-bacterial infections like scrub typhus and malaria. Among fungal infections, it may or may not be raised in candidal infections and may be negative in aspergillosis and mucormycosis.
Recommendations
Physicians should be aware of infections where PCT is not a reliable marker [useful practice point (UPP)].
PCT in Lower Respiratory Tract Infections (LRTIs)
What is the Utility and Optimal Timing of Measurement of Serum PCT for Antibiotic Administration in Patients with Community-acquired Pneumonia (CAP) and other Community-acquired LRTIs?
Evidence Statement
In patients with CAP, baseline PCT alone has limited utility in clinical decision-making regarding the initiation of antibiotics. In clinical trials, PCT measurements have been performed at randomization or emergency or hospital admission.
Recommendations
Baseline PCT should not be routinely measured for initiation of antibiotics in all patients with CAP (1A).
Baseline PCT levels should be obtained in patients with severe CAP for subsequent de-escalation of antibiotics (2A).
If indicated, baseline serum PCT should be preferably measured at admission (3A).
Should Baseline PCT Values in Community-acquired LRTIs be used to decide Antibiotic Initiation?
Evidence Statement
Baseline PCT levels have not been consistently shown to reduce antibiotic exposure in community-acquired LRTIs. Most guidelines recommend against withholding antibiotics in LRTIs or CAP based on baseline PCT.
Recommendations
Baseline PCT levels alone should not be used to withhold empiric antibiotic therapy in patients with LRTIs, including CAP (1A).
Based on clinical judgment, prompt initiation of optimal empiric antibiotic therapy is recommended in patients with LRTIs, including CAP (1A).
Can PCT Levels be used to differentiate between Viral and Bacterial Etiology in CAP?
Evidence Statement
Higher PCT levels are strongly associated with typical bacterial infections. Baseline PCT levels have varied sensitivity in differentiating bacterial and viral CAP. There are currently no established threshold values of PCT to discriminate bacterial from viral pneumonia.
Recommendations
Procalcitonin levels alone should not be used to differentiate between bacterial and viral etiology in patients with CAP (1A).
Should Serum PCT be used to determine Duration of Antibiotic Therapy in Patients with CAP?
Evidence Statement
In patients with CAP, PCT based antibiotic de-escalation is associated with decreased antibiotic exposure and significant cost benefits without worsening short-term outcomes.
Recommendations
Procalcitonin should be used, along with clinical judgement, to take decisions regarding de-escalation in patients with CAP regarding antibiotics beyond 5–7 days (1A).
What Cut-off of PCT should be used to De-escalate Antibiotic Therapy in CAP?
Evidence Statement
For PCT, various studies have used absolute cut-offs of 0.25 ng/mL for mild-to-moderate disease and 0.5 ng/mL for severe disease. Also, a decline of 80–90% from baseline has also been used for antibiotic de-escalation. In patients with renal failure, a cut-off of 0.5 ng/mL has been proposed.
Recommendations
Procalcitonin levels of 0.5 ng/mL or a decline of 80% from baseline should be used for the de-escalation of antibiotics in severe CAP or patients requiring intensive care (3A).
Should PCT Levels be used to decide Antibiotic Initiation in Hospital Acquired Pneumonia (HAP) and VAP?
Evidence Statement
Procalcitonin alone has limited utility for diagnosing or predicting outcome of hospital-acquired or ventilator-associated pneumonia (VAP). Baseline PCT values may be used to aid clinical parameters for decision-making.
Recommendations
Baseline PCT levels alone should not be used to make the clinical decision regarding antibiotic initiation in patients with VAP and HAP (1A).
How often should PCT Level be repeated after the baseline value in VAP and HAP?
Evidence Statement
Serial PCT levels have been used in antibiotic de-escalation and antibiotic stewardship studies. Most studies have measured PCT daily in critically ill and every 48–72 hours in stable patients. Most studies on VAP have done daily PCT.
Recommendations
Serial serum PCT level measurement, preferably every 48 hours, should be used in antibiotic de-escalation (UPP).
Should PCT be used for the De-escalation of Antibiotics in Patients with VAP?
Evidence Statement
Procalcitonin-based antibiotic de-escalation has been associated with decreased antibiotic exposure and better outcomes without any significant increase in treatment failure.
Recommendations
Procalcitonin should be used as a part of an antibiotic stewardship program for antibiotic de-escalation in patients with VAP on antibiotics beyond 7 days (1A).
What cut-off value of PCT should be used to consider de-escalation of antibiotic therapy in VAP?
Evidence Statement
For PCT, various studies have used absolute cut-offs of 0.5 ng/mL for severe disease. Also, a decline of 80–90% from baseline has been used for antibiotic de-escalation. In patients with renal failure, a cut-off of 0.5 ng/mL has been proposed.
Recommendations
Procalcitonin levels of 0.5 ng/mL or a decline of 80% from baseline along with clinical criteria should be used for de-escalation in VAP and HAP (3A).
A decline of 80% or more from baseline and clinical criteria can be used for de-escalation if baseline values are available (UPP).
PCT in Other Infections
Can PCT be used to guide Antibiotic Therapy in Urinary Tract Infections?
Evidence Statement
Procalcitonin has been used to differentiate urosepsis and bacteremia among patients with urinary tract infections (UTIs), however, the sensitivity and specificity remain variable. Procalcitonin has been used for the decision to stop antibiotics among patients with UTI. The PCT-guided algorithms led to lesser use of antibiotics without significant adverse effects. Different studies have used different cut-off values.
Recommendations
Procalcitonin may be used to diagnose bacteremia in UTI patients (2B).
Procalcitonin–pyuria-based algorithm can be used to guide antibiotic therapy in UTI patients (1B).
We suggest against the use of PCT as marker of treatment outcome in patients with febrile UTI (2B).
What is the Utility of Procalcitonin in Infections among Patients with Cirrhosis and End-stage Liver Disease?
Evidence Statement
Procalcitonin has been used for the diagnosis of infections among patients with cirrhosis/end-stage liver disease. The sensitivity and specificity (modest) are variable. Different studies have used different cut-off values.
Recommendations
Procalcitonin alone should not be used for diagnosis of infection or spontaneous bacterial peritonitis (SBP) among patients with cirrhosis and end-stage liver disease (2A).
Procalcitonin may be considered for the diagnosis of infection or SBP in combination with clinical and other laboratory parameters (2B).
What is the Utility of PCT in Infections among Patients with Acute Pancreatitis?
Evidence Statement
Procalcitonin has been used for the prediction of the severity of acute pancreatitis. However, the performance of PCT is not better than existing clinical scores. A PCT-guided antibiotic therapy led to a reduction in antibiotic use and hospital stay without any adverse effects.
Recommendations
We recommend against the use of PCT for the diagnosis of acute severe pancreatitis (2A).
We suggest using PCT for guiding antibiotic therapy among patients with severe acute pancreatitis (1B).
What is the Utility of PCT in Infections among Patients with Spontaneous and Secondary Bacterial Peritonitis?
Evidence Statement
Procalcitonin has been used to diagnose SBP with variable sensitivity and specificity. There is heterogeneity in the methods and cut-off for PCT used by different studies for the diagnosis of SBP. Procalcitonin has been used for prediction of risk of septic shock and mortality among patients with secondary peritonitis. The PCT-guided antibiotics therapy leads to a reduction in antibiotic use and hospital stay without any adverse effect on secondary peritonitis.
Recommendations
Procalcitonin alone should not be used for the diagnosis of SBP (2A).
Procalcitonin may be considered for diagnosis of SBP in combination with clinical features (1B).
Procalcitonin can be used for risk stratification among patients with secondary peritonitis (2A).
We recommend use of PCT for guiding antibiotic therapy among patients with severe secondary peritonitis (2A).
Should Baseline PCT Levels be used to initiate Antibiotic Therapy in Immunocompromised Patients with Suspected Sepsis?
Evidence Statement
Immunocompromised patients have an increased incidence of severe and life-threatening infections. There may be a need for prolonged antibiotic therapy in these patients. Serum PCT has been shown to be raised in immunocompromised patients with bacterial infections in observational studies. There are no RCTs that have examined the performance of serum PCT in guiding antibiotic therapy in immunocompromised patients. Patients with immunocompromise due to HIV infection or malignancy have elevated baseline serum PCT levels and there is heterogeneity in the cut-off levels as well as the diagnostic accuracy among different studies.
Recommendations
Serum PCT alone should not be used for the diagnosis of infection in immunocompromised patients (2A).
Serum PCT should not be used to guide antibiotic therapy in immunocompromised patients (2B).
Introduction
The rapid emergence of antibiotic resistance is a big problem that endangers the progress of humankind in encountering infections and saving lives. In the early 20th century, it was thought that antibiotics would be the panacea to disease and make death due to infection rare.1 However, natural selection and adaptation of microbes, aging, immunosuppression (e.g., obesity, diabetes mellitus, and others), prolonged hospitalization, overuse, and misuse of antimicrobial agents have increased infections with multidrug-resistant organisms. Infections with multidrug-resistant organisms, traditionally thought to be nosocomial, have become prevalent in community-acquired infections, thereby compounding the problem.2 Two main approaches have been suggested to encounter this problem. The first is to invest in new antibiotic discoveries. However, drug development and clinical trials are complex, expensive, and time-consuming, and ultimately, only a few new antibiotics enter the real world. The second option is the optimal use of available antibiotics.3 This approach has been refined into the concept of antibiotic stewardship. “Antibiotic stewardship” encompasses multiple coordinated interventions to improve appropriate antimicrobial use by promoting optimal drug regimen selection, and dosing, and minimizing the duration and adverse effects of antibiotics without compromising clinical outcomes. Antibiotic stewardship also improves the susceptibility of microbes to existing drugs.4 It is crucial while practicing antibiotic stewardship to ensure that no deserving patient is denied timely antimicrobial therapy. At the same time, prolonged use of antimicrobials adds to the cost of care and leads to the selective propagation of drug-resistant organisms. Both initiation and de-escalation require clinical judgment and some objective tests to minimize the overuse of antibiotics. Various biomarkers have been studied to aid optimal antibiotic usage and stewardship. Serum PCT is the most commonly utilized for this purpose worldwide.
Several randomized trials and meta-analyses have addressed the use of PCT for antibiotic stewardship in community-acquired and hospital-acquired infections. This guideline frames evidence-based recommendations for serum PCT in antibiotic stewardship for various infections.
Scope of Guidelines
These guidelines address the current evidence-based use of PCT in antibiotic stewardship for infections including sepsis, respiratory infections, infections involving other organ systems, and immunocompromised patients. The guidelines also include recommendations on using PCT in patients with coronavirus disease-2019 (COVID-19). These guidelines review the current status of the utility of PCT in the diagnosis of infections, initial prescription of antibiotics, de-escalation of antibiotics, and evidence-based recommendations for use in clinical practice. These guidelines should be used along with clinical judgment and local scenarios, including the feasibility and availability of PCT. Also, as the evaluation of the utility of PCT in pediatric patients was not in purview, these guidelines should be used only for adults.
Methodology
These guidelines were framed by an expert panel commissioned under the aegis of the Indian Society for Critical Care Medicine. The panel comprised members (list enclosed) with expertise in handling various severe infections and practical and research experience on the utility of biomarkers in infections. The experts were divided into three groups. A thorough literature review was performed by searching various electronic databases (PubMed, Embase, and Cochrane) and cross-references. Various international and national guidelines were also reviewed. Relevant literature and manuscripts were shared among all experts. All members discussed and arrived at a consensus regarding the relevant questions that needed to be answered during the framing of these guidelines. In subsequent meetings, the relevant literature on each topic was presented, and evidence statements and recommendations were formulated. A modified grade system was utilized to classify the quality of evidence and the strength of recommendations (Table 1). Subsequently, all the experts reviewed the executive summary and suggested modifications based on their literature review and experience. Thus, the draft guidelines were formulated using consensus. The draft document was circulated to all members, and their comments and suggestions were incorporated to prepare the final report.
Table 1.
Modified grade system for classification of quality of evidence and strength of recommendations for this guideline
| Quality of evidence | Level |
|---|---|
| Evidence from more than or equal to 1 good quality and well-conducted RCTs or meta-analysis of RCTs | 1 |
| Evidence from at least one RCT of moderate quality, well-designed clinical trial without randomization; or cohort or case-controlled studies | 2 |
| Evidence from descriptive studies, reports of expert committees, or opinions of respected authorities based on clinical experience | 3 |
| Not backed by sufficient evidence; however, a consensus reached by the working group, based on clinical experience and expertise | UPP |
PCT: A Brief Overview
Procalcitonin, the precursor for calcitonin, is secreted in low physiologic quantities by the thyroid gland and pulmonary-endocrine cells and has a half-life of 20–24 hours.5 Physiologically, serum PCT levels are usually below 0.05 ng/mL. However, PCT production can be induced by systemic inflammation, including sepsis and systemic inflammatory response syndrome (SIRS). This induction occurs in most extra thyroid organs, including the liver, pancreas, kidney, lung, intestine, and white blood cells.6 Induction of PCT occurs via various proinflammatory cytokines, including tissue necrosis factor (TNF)-alpha and interleukin 1β (IL-1β). However, PCT levels may be falsely increased in non-infectious conditions like trauma, burns, medullary C cell carcinoma, neuroendocrine cell tumors (small cell lung carcinoma and carcinoids), immunomodulatory therapy, which causes proinflammatory cytokine increase, cardiogenic shock, early neonatal life, chronic renal disease, dialysis, and advanced cirrhosis.
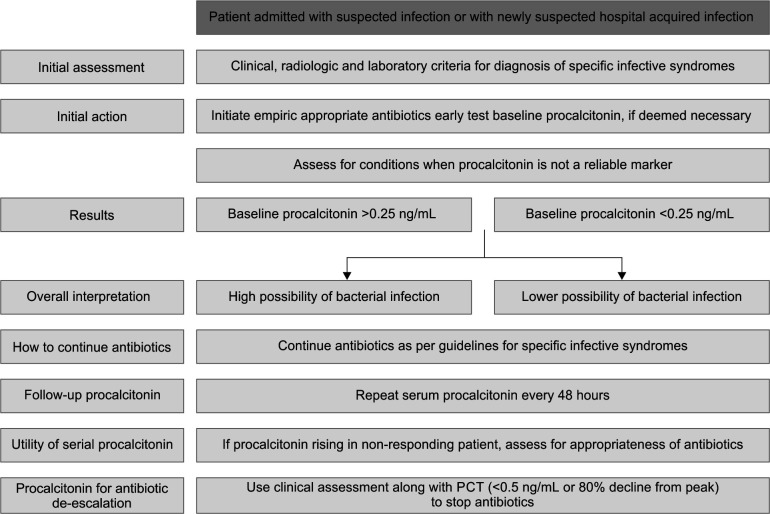
Most chemical assays for PCT have a functional sensitivity of 0.06 ng/mL, require only 20–50 μL of plasma or serum sample, and have a turnaround time of less than an hour. If induced, serum PCT levels increase 6–12 hours after infection; PCT levels decline over 24 hours with optimal therapy. Cut-offs for PCT are often used in conjunction with clinical algorithms. A cut-off of 0.1–0.5 ng/mL or a reduction of more than 80% from baseline is commonly utilized for decision-making on antibiotics.7 Flowchart 1 provides a consensus overview of the utility of serum PCT for the rational use of antibiotics in admitted patients with infections.
Flowchart 1.
Overview of the utility of PCT for rational use of antibiotics in admitted patients with infections
PCT in Sepsis
Should Baseline PCT Levels be done in Patients with Sepsis?
Sepsis, a dysregulated host response to infection and causing life-threatening organ dysfunction, is a global healthcare problem. Biomarkers can have a supportive role in identifying specific physiopathological pathways in sepsis. In this regard, serum PCT has emerged as a promising candidate.
Dandona et al. demonstrated a rapid increase in serum PCT in healthy volunteers four hours after endotoxin injection.8 Assicot et al. demonstrated a rapid increase in serum PCT levels with the onset of infections, correlation with severity, and rapid reduction with antibiotic therapy.9 Though baseline serum PCT is a sensitive marker for sepsis diagnosis, inherent limitations preclude recommendations for its use in initial diagnosis and decision-making regarding antibiotic initiation.10–13 Majority of RCTs evaluating PCT-based algorithms to decide the duration of antibiotic treatment in critically ill sepsis patients have used both absolute values and 80% reduction from baseline.7,13 Though not recommended for antibiotic initiation, baseline PCT levels shall be required for antibiotic cessation if criteria utilizing percentage decline from baseline value are utilized.
Evidence Statement
Procalcitonin is detectable in the serum within four to six hours after induction, which is most often in a bacterial infection. It reaches its peak within 24 hours and then starts its decline in the case of adequate treatment, with levels reducing by roughly 50% daily according to its half-life. A baseline PCT is required for guiding antibiotic therapy to look at subsequent increases or decreases in levels. Most RCTs have done a baseline value in suspected sepsis patients and utilized it in PCT-based algorithms to limit the duration of antibiotic therapy.
Recommendations
Baseline serum PCT levels may be done in patients with sepsis (1B).
What is the Role of PCT in the Diagnosis of Sepsis?
Up to 30–40% of sepsis patients remain culture negative.14,15 These patients pose a challenge in decision-making regarding antibiotic administration. There is an urgent need for accurate biomarkers to diagnose or exclude suspected sepsis from non-infective causes. In a prospective observational study (n = 208), PCT had a sensitivity of 92.2% and a negative predictive value (NPV) of 82.5% for the diagnosis of sepsis; the optimal cut-off point for PCT was 1.43 ng/mL. The receiver operating characteristic (ROC) curve [area under the curves (AUCs)] for PCT was 0.892 [confidence interval (CI), 0.836–0.947; p = 0.028].16 Tan et al.11 performed a meta-analysis of 11 studies comprising 1,368 patients to evaluate the accuracy of PCT in sepsis diagnosis. Serum PCT had a pooled sensitivity of 0.80 (95% CI: 0.69–0.87) and specificity of 0.77 (95% CI: 0.60–0.88) for the diagnosis of sepsis; the overall area under the SROC curve was 0.85 (95% CI: 0.82–0.88). However, overall accuracy was below 90%, with low positive and negative likelihood ratios. Also, individual studies had taken different cut-offs for diagnosis ranging from 0.57 to 6.03 ng/mL.
Evidence Statement
Procalcitonin can diagnose sepsis only with moderate accuracy. The cut-off values for the diagnosis of sepsis are not well defined and need further clarification.
Recommendations
Procalcitonin alone should not be used for the diagnosis of sepsis (1A).
We suggest that PCT be used to differentiate culture-negative sepsis from non-sepsis (2B).
Should PCT be done for Discontinuation of Antibiotics in Sepsis?
The optimal duration of antibiotic administration in patients with sepsis is variable. Guidelines suggest using PCT for shortening the duration of antimicrobial therapy in sepsis.12 In a meta-analysis of 15 studies evaluating PCT-guided antimicrobial administration in sepsis, the PCT-guided approach led to a significant reduction in duration of antibiotic therapy (p <0.001) and hospital stay (p = 0.049) without any difference in 28-day mortality (p = 0.626).17 Andriolo et al. also reported significantly reduced antibiotic exposure without any mortality benefit in their meta-analysis of 12 RCTs of sepsis patients.18 Pepper et al. performed a systematic review and meta-analysis of 16 RCTs involving 5,158 critically ill adult patients to evaluate the effect of PCT-guided antibiotic discontinuation on mortality. In this meta-analysis, PCT-guided antibiotic discontinuation was associated with decreased mortality [risk ratio (RR), 0.89; 95% CI: 0.83–0.97] and decreased antibiotic duration (mean difference, 1.31 days; 95% CI: from –2.27 to –0.35); survival benefit was not significant in critically ill patients with sepsis (10 RCTs; RR 0.94; 95% CI: 0.85–1.03). Also, the meta-analysis noted significant methodologic limitations in the studies included and qualified the imputed results as being low certainty.19 In a subsequent patient-level meta-analysis of critically ill sepsis patients, the PCT arm had significantly lower mortality [odds ratio (OR) 0.89, 95% CI: 0.8–0.99)] and shorter duration of treatment (adjusted coefficient −1.19 days, 95% CI: from −1.73 to −0.66; p <0.001).20 Similar findings were reported in other meta-analyses.13,21 Vishalashi et al. found antibiotic duration significantly lower in the PCT group in their single-center open RCT of 90 sepsis patients (4.98 ± 2.56 vs 7.73 ± 3.06 days, p <0.001). Also, patients in the PCT group had lesser ICU stay (5.98 ± 2.73 days vs 8.80 ± 3.35 days, p <0.001) and significantly lower secondary infection rate (4.4% vs 26.7%, p = 0.014), with comparable readmission and mortality.22 Significant reduction in antibiotic duration (11.3 vs 8.6 days; 95% CI: from −3.59 to −1.40) and mortality [adjusted OR, 0.88 (95% CI: 0.78−0.98)] was reported in 5002 critically ill patients with infections and chronic kidney disease, using PCT-based algorithms in a patient-level meta-analysis.23 In patients with catheter-related bloodstream infections (CRBSI), PCT-based algorithms led to a non-significant reduction in antibiotic duration (weighted mean difference: 1.218, 95% CI: from −0.155 to 2.591) and hospital stay 5.69 (95% CI: from −5.81 to 17.20) without any adverse effects on mortality.24 According to the analysis of real-world data of United States hospitals by Voermans et al., utilization of PCT-based algorithms in critically ill sepsis and LRTIs patients was associated with significant cost savings without any increase in mortality.25 In another cohort before-after study, adoption of PCT based protocols resulted in 14% overall cost savings.26 In the Procalcitonin-guided treatment on duration of antibiotic therapy and cost in septic patients (PRODA) trial, PCT-based algorithms led to a 4-day reduction in antibiotic duration (p <0.01) with a cost saving of US$30 in sepsis patients.27
Evidence Statement
The PCT-guided treatment reduces the duration of antibiotic therapy and duration of hospital stay in adult critically ill patients with sepsis without any increase in mortality. Procalcitonin-based protocols are associated with significant overall hospitalization cost savings.
Recommendations
Procalcitonin levels can be used to support discontinuation or de-escalation of antibiotics in patients with sepsis (2A).
How often should PCT Level be repeated after the Baseline Value?
There is limited literature on the optimal frequency of repetition of PCT. As the half-life is 20–24 hours, most trials assessing its utility in sepsis have tested PCT daily or every 48 hours. In three RCTs, PCT was done daily until day 7; antibiotic stoppage, or the patient was transferred out of ICU.28–30 Hochreiter et al., Schroeder et al., and Liu et al. also did daily PCT in their trials.31–34 Annane et al. repeated PCT on day 3 and day 5 post-randomization.35 Deliberato et al. repeated serum PCT levels on the fifth and seventh days.36 However, in this trial, if blood cultures were positive, PCT was repeated every 48 hours until death, discharge, or stoppage of treatment. In another trial, Oliveira et al. repeated36 PCT on the fourth or fifth day and then daily until the seventh day or stoppage of antibiotics.37 Bloos et al.38 repeated PCT on day one after randomization and then every 3 days till day 14, whereas Jeon et al. measured PCT at baseline and every other day till day 14, or stoppage of antibiotics.27 Procalcitonin-based strategies effectively reduced antibiotic exposure and hospital stay duration without affecting mortality in all these RCTs.
Evidence Statement
There is no evidence to suggest the optimal frequency of PCT testing. In various studies, serum PCT has been tested every 24–72 hours.
Recommendations
Procalcitonin levels may be repeated every 72 hours or earlier if clinically indicated (3B).
What Level of PCT should be used for discontinuing Antibiotics in Sepsis?
Various trials of sepsis patients have taken different cut-offs of PCT to decide on antibiotic cessation. Nobre et al. divided their algorithm based on baseline PCT levels. If baseline PCT levels were more than one mg/L, a drop in PCT more than 90% from baseline or an absolute value below 0.25mg/L was used for antibiotic discontinuation.28 If baseline PCT levels were <1 mg/L, day 3 PCT <0.1 mg/L along with clinical evaluation was used to decide antibiotic discontinuation.28 Two other studies discontinued antibiotic therapy if there was clinical resolution and PCT <1 ng/mL, or PCT >1 ng/mL with a 25 to 35% decline from baseline over 3 days. 31,32 Other studies have used absolute cut-offs of less than 0.1 ng/mL or 0.25 ng/mL.33,35 Deliberato et al. discontinued antibiotics if PCT dropped more than 90% of peak level or below 0.5 ng/mL.36 Three trials combined clinical judgment and PCT decline of 90% from baseline or absolute value below 0.25 ng/mL as criteria for antibiotic discontinuation.29,30,33 Oliveira et al. stopped antibiotics when PCT levels dropped by 90% if baseline levels were more than >1 ng/mL; if baseline levels were less than one ng/mL, antibiotics were discontinued when PCT values became less than 0.1 ng/mL.37 Bloos et al. used a cut-off of below 1 ng/mL or a 50% decline from the previous value for antibiotic cessation.38 Also, de Jong et al. and Jeon et al. stopped antibiotics if PCT declined by at least 80% from peak value or below 0.5 ng/mL.27,34 Vishalashi et al. discontinued antibiotics at a serum PCT value of below 0.01 ng/mL or a decline of above 80% from baseline.22
Evidence Statement
There is significant heterogeneity among studies regarding the cut-off value of PCT for discontinuation of antibiotics. In most studies, the reduction of PCT levels below 0.25–0.5 ng/mL, or an 80–90% decline from baseline, led to antibiotic de-escalation.
Recommendations
Procalcitonin levels of less than 0.5 ng/mL or a drop by above 80% from baseline along with clinical judgment should be used for decision-making regarding antibiotic de-escalation (3A).
What is the Role of PCT Value in deciding the Severity and Prognosis of Infections?
Serum PCT has been evaluated as a predictor of the severity and prognosis of infections. Baseline serum PCT was an independent predictor of 30-day mortality in a multicentric prospective study of 6,970 patients with varied diagnoses presenting in the emergency department.39 A meta-analysis of PCT in sepsis included 23 observational studies with 3,944 patients. Studies had different PCT cut-offs, but the elevated PCT and non-clearance of PCT were associated with increased mortality in septic patients with pooled relative risks of 2.60 (95% CI: 2.05–3.30) and 3.05 (95% CI: 2.35–3.95), respectively. In a large multicentric observational study (n = 858), the inability to decrease PCT by more than 80% by day 4 was an independent predictor of 28-day all-cause mortality with a hazard ratio of 1.97 (95% CI: 1.18–3.30; p <0.009).40 In a prospective evaluation by Jekarl et al., an elevated baseline serum PCT was an independent predictor of mortality among 248 patients with suspected sepsis admitted to the emergency department (OR 2.004 95% CI:1.24–3.24).41
Evidence Statement
Elevated baseline PCT and non-clearance of PCT were associated with increased mortality in patients with sepsis. Procalcitonin can differentiate between gram-negative and gram-positive sepsis.
Recommendations
Procalcitonin may be used to define the severity of infection in a patient with sepsis (2B).
What are the Infections in Which PCT is not an Effective Biomarker?
Procalcitonin is more specific for bacterial infections than other biomarkers, such as white blood cell count, erythrocyte sedimentation rate, and C-reactive protein.42 However, false positives can still occur. Procalcitonin is elevated in typical respiratory bacterial infections. The degree to which PCT rises varies among pathogens, with higher levels observed in patients with LRTIs caused by typical bacteria compared with atypical bacteria or other pathogens. In one multicenter study evaluating 1,735 hospitalized patients with CAP, median PCT levels were higher in patients with pneumonia caused by typical bacteria (2.5 ng/mL) compared with atypical bacteria (0.20 ng/mL) and viruses (0.09 ng/mL).43 Among atypical bacteria, Legionella species cause modest elevations in PCT while Mycoplasma and Chlamydia species may not result in detectable elevations43 Other infections in which PCT also rises are scrub typhus, Plasmodium spp. (malaria), candidiasis and Clostridium difficile-associated disease.44–49 In Mycobacterial disease, both rise and lack of PCT have been reported.50,51 Procalcitonin may not rise in localized infections such as tonsillitis, sinusitis, cystitis, uncomplicated skin/soft tissue infections, abscesses, or empyema.52,53 Also, PCT may be falsely normal in viral infections, borreliosis, chlamydia pneumoniae, mycoplasma pneumonia, aspergillosis, coccidioidomycosis, and mucormycosis.43,54,55 (Table 2).
Table 2.
Infections for which PCT may not be an effective marker
| May be raised but inconsistent results: Atypical bacteria – Legionella species, Mycoplasma and Chlamydia species |
| Non-bacterial infections associated with positive PCT: Scrub typhus, Plasmodium spp., Candidiasis, Clostridium difficile-associated disease |
| Procalcitonin negative in bacterial infection – localized infections such as tonsillitis, sinusitis, cystitis, uncomplicated skin/soft tissue infections, abscesses, or empyema |
| Procalcitonin negative in infections – Viral infections, Borrelia spp., Chlamydia pneumoniae, Mycoplasma pneumoniae, Aspergillosis, Coccidioidomycosis, and Mucormycosis |
Evidence Statement
Procalcitonin may not be an effective marker in some infections. It may be inconsistently raised in infections with Legionella, Mycoplasma, and Chlamydia, whereas localized bacterial infections like tonsillitis, sinusitis, cystitis, uncomplicated soft tissue infections abscesses, or empyema can cause it to be falsely negative. Procalcitonin may be raised in non-bacterial infections such as scrub typhus and malaria. Among fungal infections, it may or may not be raised in candidal infections and may be negative in aspergillosis and mucormycosis.
Recommendations
Physicians should be aware of infections where PCT is not a reliable marker (UPP).
PCT in LRTIs
What are the Utility and Optimal Timing of Measurement of Serum PCT for Antibiotic Administration in Patients with CAP and Other Community-acquired LRTIs?
Procalcitonin is a promising biomarker to limit antimicrobial usage in patients with suspected LRTIs. In the Procalcitonin Antibiotic Consensus Trial (ProACT) evaluating the PCT-based strategy applied on antibiotic exposure, PCT levels were tested at emergency admission.56 The median time from sample collection to report availability was 77 minutes. In other studies, baseline PCT has been measured at randomization.34
Evidence Statement
In patients with CAP, baseline PCT alone has limited utility in clinical decision-making regarding the initiation of antibiotics. In clinical trials, PCT measurements have been performed at randomization or emergency or hospital admission.
Recommendations
Baseline PCT should not be routinely measured for initiation of antibiotics in all patients with CAP (1A).
Baseline PCT levels should be obtained in patients with severe CAP for subsequent de-escalation of antibiotics (2A).
If indicated, baseline serum PCT should be preferably measured at admission (3A).
Should Baseline PCT Values in Community-acquired LRTIs be used to decide Antibiotic Initiation?
Schuetz et al.,57 in their meta-analysis (26 RCTs, n = 6,706), showed a significant reduction in antibiotic exposure in patients with acute LRTIs. Notably, the reduction in antibiotic exposure was predominantly attributable to the early cessation of antibiotics in patients with pneumonia and intensive care settings and not to lesser initiation of antibiotics. Multiple limitations of the studies included in the meta-analysis have been highlighted.58 These include the inclusion of a wide variety of acute respiratory tract infections and conditions, including exacerbations of COPD, asthma, and influenza; pneumonia was not required as an inclusion criterion in some studies and was an exclusion criterion in others. Out of the five studies including CAP for PCT-based treatment, four excluded seriously ill patients. Also, among studies including critically ill patients, up to 50% of patients had extra-pulmonary infections.34,38 Schuetz et al. performed a subsequent systematic review and gave consensus recommendations on PCT use in CAP, in which they advised immediate antibiotic initiation in all CAP patients who were clinically unstable or deemed to be at high risk of CAP.7 The ProACT recruited 1,656 patients from 14 hospitals in the United States to evaluate the PCT-based strategy applied during emergency admission on antibiotic exposure.56 The results included FDA recommendations with antibiotics recommended if PCT levels were more than 0.25 ng/mL. However, the trial failed to demonstrate any significant difference in antibiotic days (difference, −0.05 day; 95% CI: from −0.6 to 0.5; p = 0.87) or adverse outcomes (difference, −1.5% points; 95% CI: from −4.6 to 1.7; p <0.001 for noninferiority) within 30 days. There was no significant difference in short and long-term mortality in the two groups overall and subgroup analysis by type of LRTIs.59 Retrospective studies in real-world settings have shown that PCT-based algorithms lead to a non-significant reduction in antibiotic exposure.60
In a large cohort study of 1,705 randomly sampled COVID-19 patients admitted in 38 United States of America hospitals, 56.6% patients received empiric antibiotics, whereas only 3.5% had a community-acquired infection. Baseline PCT levels were available for 910 patients, and though levels less than 0.1 ng/mL had 98.9% NPV for bacterial co-infection, 33% patients received antibiotics irrespective of PCT levels.61
Elevated baseline PCT (>0.25 ng/mL) correlated with Acute Physiology and Chronic Health Evaluation II scores (9 vs 8; p = 0.04) and C-reactive proteins levels (111 μg/mL vs 79 μg/mL; p = 0.007), but had no relation with mortality (OR = 1.00; 95% Cl: 0.97 to 1.02; p = 0.713), length of ICU stay (p = 0.9) or length of hospital stay (p = 0.14) in a single center retrospective study of 223 hospitalized COVID-19 patients.62 Vazzana et al. did a meta-analysis of early studies of COVID-19. They analyzed data from 14 studies comprising 3,492 patients. Procalcitonin was increased in 22.8% of patients with the severe course, and 30.6% with adverse outcomes, implying low sensitivity for severity and outcomes. Elevated PCT, however, did have higher odds of severe disease (OR: 5.92; 95% CI: 3.20–10.94), and adverse outcomes (OR: 13.1; 95% CI: 7.37–23.1).63 In a retrospective cohort study of 332 COVID-19 patients admitted to an emergency, PCT showed an adjusted OR of 2.11 (95% CI: 1.36–3.61) for the development of severe disease.64 Moreover, PCT >0.5 ng/mL corresponds to an almost 5 times higher risk of severe infection (OR, 4.76; 95% CI: 2.74–8.29) compared to patients with lower PCT. Another meta-analysis also correlated PCT >0.5 ng/mL with higher odds of severe COVID-19 infection (OR, 4.76; 95% CI: 2.74–8.29).65 In a time series analysis of admitted COVID-19 patients from 105 hospitals in England, introduction of PCT testing in emergency and acute admission units resulted in significant initial reduction in total antibiotic usage (−1.08 days; 95% CI: from −1.81 to −0.36 days), but this effect was not sustained, and was lost at a rate of 0.05 days (95% CI: 0.02–0.08) per week. Also, PCT use did not result in any reduction in antibiotic usage in ICU setting.66 Guidelines discourage the use of PCT at baseline to decide on antibiotic initiation.67–69
Evidence Statement
Baseline PCT levels have not been consistently shown to reduce antibiotic exposure in community-acquired LRTIs. Most guidelines recommend against withholding antibiotics in LRTIs or CAP based on baseline PCT.
Recommendations
Baseline PCT levels alone should not be used to withhold empiric antibiotic therapy in patients with LRTIs, including CAP (1A).
Based on clinical judgment, prompt initiation of optimal empiric antibiotic therapy is recommended in patients with LRTIs, including CAP (1A).
Can PCT Levels be used to differentiate between Viral and Bacterial Etiology in CAP?
Recent trials suggest that PCT-based guidelines can reduce antibiotic use for respiratory infections. Self et al. evaluated the accuracy of PCT in discriminating bacterial from viral etiology of CAP. Among the 1,735 patients included, median PCT concentration was significantly lower in viral [0.09 ng/mL; interquartile range (IQR) <0.05–0.54 ng/mL] as compared to atypical bacterial (0.20 ng/mL; IQR, <0.05–0.87 ng/mL; p = 0.05), and typical bacterial pathogens (2.5 ng/mL; IQR, 0.29–12.2 ng/mL; p <0.01). Procalcitonin discriminated bacterial pathogens from viral pathogens with an area under the ROC curve of 0.73 (95% CI: 0.69–0.77).43
In a recent meta-analysis, Kamat et al.70 performed a meta-analysis of 12 studies in 2,408 patients with CAP to evaluate the utility of serum PCT in differentiating etiology. In this evaluation, serum PCT was found to have a low sensitivity [0.55 (95% CI: 0.37–0.71; I2 = 95.5%) and specificity (0.76, 95% CI: 0.62–0.86)] in differentiating bacterial from viral etiologies. In a multicentric retrospective study of 962 hospitalized COVID-19 patients, baseline PCT levels did not add to diagnostic accuracy over clinical criteria for the detection of CAP.71 Various guidelines have also cautioned against using PCT to differentiate etiologies in pneumonia.67–69
Evidence Statement
Higher PCT levels are strongly associated with typical bacterial infections. Baseline PCT levels have varied sensitivity in differentiating bacterial and viral CAP. There are currently no established threshold values of PCT to discriminate bacterial from viral pneumonia.
Recommendations
Procalcitonin levels alone should not be used to differentiate between bacterial and viral etiology in patients with CAP (1A).
Should Serum PCT be used to determine Duration of Antibiotic Therapy in Patients with CAP?
In a patient-level meta-analysis of 6,706 acute respiratory tract infection patients from 26 RCTs, PCT-guided algorithms led to significantly lower 30-day mortality (9% in PCT vs 10% in the control arm; adjusted OR 0.83; 95% CI: 0.70–0.99; p = 0.037). Procalcitonin guidance also led to a reduction in antibiotic exposure by 2.4 days (95% CI: from –2.71 to –2.15, p <0.0001) and a reduction in antibiotic-related side effects (16% vs 22%, adjusted OR 0.68; 95% CI: 0.57–0.82, p <0.0001). In pneumonia and ICU patients, reduction in antibiotic exposure was predominantly due to early cessation.57 Ito et al. evaluated the impact of PCT-based therapy on antibiotic duration and costs in 352 hospitalized CAP patients. Significant cost benefits (approximately 15%, p = 0.005) and reduction in antibiotic duration (8.6 days vs 12.6 days; p <0.001) was observed, without any increase in recurrence rates or mortality.72 In a pragmatic multicenter RCT of 285 CAP patients with Pneumonia Severity Index class IV or class V, the PCT-based strategy did not significantly reduce the duration of antibiotics.73
Evidence Statement
In patients with CAP, PCT-based antibiotic de-escalation is associated with decreased antibiotic exposure and significant cost benefits without worsening short-term outcomes.
Recommendations
Procalcitonin should be used, along with clinical judgment, to take decisions regarding de-escalation in patients with CAP on antibiotics beyond 5−7 days (1A).
What Cut-off of PCT should be used to De-escalate Antibiotic Therapy in CAP?
Most studies evaluating PCT in acute respiratory infections including CAP have used a cut-off of 0.5 ng/mL or a drop of 80% from baseline to decide on antibiotic de-escalation.7 Recent guidelines recommend PCT guidance to be used for antibiotic de-escalation when duration of antibiotics is more than 5−7 days.68
Evidence Statement
For PCT, various studies have used absolute cut-offs of 0.25 ng/mL for mild-to-moderate disease and 0.5 ng/mL for severe disease. Also, a decline of 80−90% from baseline has also been used for antibiotic de-escalation. In patients with renal failure, a cut-off of 0.5 ng/mL has been proposed.
Recommendations
Procalcitonin levels of 0.5 ng/mL or a decline of 80% from baseline should be used for de-escalation in severe CAP or patients requiring intensive care (3A).
Should PCT Levels be used to decide Antibiotic Initiation in HAP and VAP?
Serum PCT was significantly higher in patients with VAP than controls in a single-center prospective observational study of 80 post-cardiac valve replacement patients.74 In another small prospective study from China, PCT had a diagnostic sensitivity of 60.0%, specificity of 87.5%, and AUC 0.76 (0.62–0.90) for VAP diagnosis. Sotillo–Díaz et al. performed a meta-analysis of 7 prospective studies (373 patients) to evaluate the role of PCT in VAP diagnosis. In this study, PCT had a pooled sensitivity of 76% (69–82), specificity of 79% (74–84), and positive and negative likelihood ratios of 4.35 (2.48–7.62), 0.26 (0.15–0.46) for VAP diagnosis.75 In a multicentric prospective study of 138 mechanically ventilated patients, PCT kinetics, that is, slope, peak, or difference between lowest and peak PCT (Δmax), could not predict the development of VAP.76 Serum or bronchoalveolar lavage (BAL) PCT failed to improve the diagnostic accuracy of the Clinical Pulmonary Infection Score (CPIS) in diagnosing VAP in a prospective evaluation of 86 suspected cases.77 In a prospective study involving 91 suspected VAP patients, PCT levels were not different in VAP compared to controls (p = 0.45). Also, there was no significant difference in PCT levels between VAP, VAP-shock, and controls (p = 0.17).78 In a single-center study of 85 ICU patients (45 VAP patients and 45 controls), serum PCT had a low sensitivity (51.5%) for diagnosing culture-proven VAP compared to various clinical criteria.79 Combination of PCT >0.25 ng/mL along with positive lung ultrasound had a high sensitivity (81.3%) and specificity (85.5%) for VAP diagnosis in a single-center prospective study.80 However, serum PCT could not accurately differentiate between ventilator-associated tracheobronchitis and VAP in 689 patients with ventilator-associated LRTI from a prospective multinational TAVeM database.81
In a single-center retrospective observational study of 115 VAP patients, high serum PCT level was an independent predictor of 60-day mortality (OR = 2.38, 95% CI: 1.26–4.50, p = 0.008).82 Bloos et al., in their multicentric prospective study of 175 severe pneumonia patients, found baseline and peak serum PCT to be an independent predictor of 30-day mortality, with similar performance characteristics as acute physiology and chronic health examination (APACHE) II.83 Persistently elevated PCT on third-day post-admission was associated with increased mortality in a small prospective single-center study.84 However, PCT kinetics did not independently predict the development of septic shock or mortality in another prospective study.85 Therefore, the diagnostic and prognostic utility of PCT needs more evidence for a recommendation for use in VAP. International guidelines recommend against using PCT for antibiotic initiation in HAP and VAP.86,87
Evidence Statement
Procalcitonin alone has limited utility in diagnosing or predicting the outcome of hospital-acquired or VAP. Baseline PCT values may be used to aid clinical parameters for decision-making.
Recommendations
Baseline PCT levels alone should not be used to make the clinical decision regarding antibiotic initiation in patients with VAP and HAP (1A)
How often should PCT Levels be repeated after the Baseline Value in VAP and HAP?
Stolz et al.,88 in their RCT on VAP patients, did daily PCT levels for the first 10 days. In another single-center RCT, Mazlan et al. did PCT on days 1, 3, 7, and 9.89 The SAPS trial also did daily PCT till ICU discharge or until 3 days after the antibiotic cessation.34 Wongsurakiat et al.,90 used single spot PCT measurement (<0.5 ng/mL) on day 8th in their trial. Beye et al. also measured daily PCT in their cohort study.91
Evidence Statement
Serial PCT levels have been used in antibiotic de-escalation and antibiotic stewardship studies. Most studies have measured PCT daily in critically ill and every 48–72 hours in stable patients. Most studies on VAP have done daily PCT.
Recommendations
Serial serum PCT level measurement, preferably every 48 hours, should be used in antibiotic de-escalation (UPP).
Should PCT be used for the de-escalation of Antibiotics in Patients with VAP?
Serum PCT reduces antibiotic therapy exposure in acute respiratory infections and critically ill sepsis patients.13,57 However, limited studies have evaluated PCT-guided antibiotic discontinuation in VAP. Stolz et al.88 performed a multicentric RCT of 101 VAP patients and compared PCT-based antibiotic discontinuation strategy with guideline-based strategy. In this trial, the PCT-based strategy significantly increased the number of antibiotic-free days alive after VAP onset (median 13 days vs 9.5 days; p <0.05), translating into a reduction of 27% antibiotic exposure (p = 0.038). Even after adjustment for confounders, antibiotic discontinuation on day 28 was significantly higher in the PCT group (HR 1.6, 95% CI: 1.02–2.71). There was no change in the number of days spent on mechanical ventilation, ICU stay, duration of hospital stay, or mortality. Pontet et al. also showed a significant reduction in antibiotic duration (p <0.001) with PCT-based treatment.92 In another single-center RCT, a PCT-based algorithm significantly reduced antibiotic duration (−1.25 days, 95% CI: from −2.48 to 0.01; p = 0.049).89 The SAPS trial evaluated the efficacy and safety of PCT for antibiotic discontinuation in 1,575 critically ill patients. The majority of the patients (65%) had a pulmonary source of infection. In this study, PCT aided strategy led to a lesser median duration of antibiotics (5 days vs 7 days; mean difference 1.22, 0·65–1.78, p <0.0001).34 along with mortality benefit (20% vs 25%, 54%, 95% CI: 1.2–9.5, p = 0·0122).
In a small non-randomized study of 46 VAP patients (70% with non-fermentative GNB etiology), day 8 serum PCT and clinical criteria led to a greater number of antibiotic-free days alive (14.6 ± 5.4 days vs 5.9 ± 5.7 days, p <0.001) without any adverse outcomes.90 In a 5-year cohort study evaluating the impact of PCT in the real world scenario, PCT guidance led to significantly lesser antimicrobial duration (9.5 vs 8.0 days; p = 0.02), without any increase in unfavorable outcomes (46.9% vs 51.3%; p = 0.69) in patients with VAP.91 In a retrospective analysis, infections with VAP due to multidrug-resistant bacteria had a similar rise in PCT compared to sensitive bacterial infections. With appropriate antibiotic therapy, the time taken for PCT decline (by 80% of baseline or below 0.5 ng/mL) was significantly higher with MDR VAP (7.2 ± 2.9 days vs 5 ± 1.8 days; p = 0.03).93 PCT aided strategies led to lesser mortality (OR 0.67, 95% CI: 0.48–0.96), along with reduction in antibiotic exposure (–3.2 days, 95% CI: from −4.45 to −1.95) without any increase in adverse outcomes.86 Most international guidelines recommend antibiotics for up to 7 days for most responding patients with VAP,87,94 thus making PCT more useful in individualizing the duration of antibiotic therapy for patients who require treatment for more than a week. There are a few pertinent scenarios where PCT will likely reduce antibiotic exposure, for example, in patients with MDR infections, on reserved drugs like polymyxins, or immunocompromised status.88
Evidence Statement
Procalcitonin-based antibiotic de-escalation has been associated with decreased antibiotic exposure and better outcomes without any significant increase in treatment failure.
Recommendations
Procalcitonin should be used as a part of an antibiotic stewardship program for antibiotic de-escalation in VAP in patients on antibiotics beyond 7 days (1A).
What Cut-off Value of PCT should be used to Consider de-escalation of Antibiotic Therapy in VAP?
Stolz et al.88 and Pontet et al.92 used a cut-off of 0.5 ng/mL or an 80% drop in PCT values and clinical judgment for de-escalation of antibiotics in VAP. A similar cut-off was also used in the SAPS trial.34 Most studies in a meta-analysis evaluating PCT in acute respiratory infections also had the same cut-off.7 A single-center RCT used a combination of PCT value below 0.5 ng/mL and drop of 80% to decide de-escalation.89 whereas another study used absolute PCT below 0.5 ng/mL on day 8 for antibiotic de-escalation.90
Evidence Statement
For PCT, various studies have used absolute cut-offs of 0.5 ng/mL for severe disease. Also, a decline of 80 to 90% from baseline has been used for antibiotic de-escalation. In patients with renal failure, a cut-off of 0.5 ng/mL has been proposed.
Recommendations
Procalcitonin levels of 0.5 ng/mL or a decline of 80% from baseline along with clinical criteria should be used for de-escalation in VAP and HAP (3A).
A decline of 80% or more from baseline and clinical criteria can be used for de-escalation if baseline values are available (UPP).
PCT in Other Infections
Can PCT be used to guide Antibiotic Therapy in Urinary Tract Infections?
Urinary tract infection is one of the most common bacterial infections. Fever in patients with UTI should be considered to reflect the presence of tissue inflammation. Although febrile UTI is generally regarded as acute pyelonephritis, the exact site of inflammation may be difficult to clinically define, and fever can be an early sign of sepsis. Febrile UTI responds well to treatment and in most cases is not associated with serious complications. However, UTI with bacteremia can be complicated by sepsis or death. Consequently, it is essential to be able to differentiate bacteremic patients with febrile UTI from non-bacteremic patients at the initial presentation.
Also, UTIs are common drivers of antibiotic use and hospitalizations. Any reduction of antibiotic exposure is important to diminish selection pressure for antibiotic resistance, costs, and drug-related side effects.
Procalcitonin is a prohormone of calcitonin that can be used as a candidate marker for the diagnosis of active bacterial infections. The use of biomarkers might improve the management of patients with UTIs in terms of diagnosis of bacteremia as well as the antibiotic duration.
Evidence
Cees van Nieuwkoop et al.94 conducted a prospective observational multicenter cohort study regarding biomarker PCT as an aid in predicting bacteremia. Authors showed that PCT above 0.25 ng/mL had the best diagnostic performance (sensitivity 0.95; 95% CI: 0.89–0.98, specificity 0.50; 95% CI: 0.46–0.55). Using PCT as a single decision tool, would result in 40% fewer blood cultures being taken, while still identifying 94–99% of patients with bacteremia. In an observational study involving adults patients with UTI in emergency department, Julián–Jiménez et al.95 reported a threshold of 1.16 ng/mL of PCT that showed largest area under the ROC at 0.993 with a sensitivity of 100%, a specificity of 97%, a positive predictive value of 84% and a NPV of 100%; hence, the most relevant in guiding medical decision-making. A retrospective analysis by Lee et al.96 reported that a PCT level of 0.5 ng/dL was associated with bacteremia (OR 2.03; 95 % CI: 1.07–3.86). Another retrospective study conducted in emergency department by Levine et al.97 reported an AUC for PCT to predict UTI was 0.717; 95% CI: 0.643–0.791. They found a PCT threshold of 0.25 ng/mL corresponded to the best combination of sensitivity (67%) and specificity (63%), with a positive predictive value and NPV of 26 and 91%, respectively. Xin Luo et al.98 conducted a retrospective study to identify the predictive accuracy of PCT/albumin ratio in discriminating between patients with urosepsis and those with febrile UTIs. Their results showed that patients in the urosepsis group had higher PCT/albumin ratios compared to those in the febrile UTI group [2.254 (0.978, 6.299) vs 0.021 (0.004, 0.095); p <0.001]. Based on the multivariate logistic analysis, the PCT/albumin ratio (OR 1.029, 95% CI: 1.013–1.045, p <0.001) was an independent predictor of urosepsis. The area under the ROC curve (AUC) for the PCT/albumin ratio was 0.937 (95% CI: 0.894–0.980); p <0.001. The sensitivity and specificity of the PCT/albumin ratio cut-off values (>0.44) were 84.62 and 96.00%, respectively. In an open-label, RCT Drozdov et al.99 reported that a PCT-pyuria-based algorithm reduced antibiotic exposure by 30% when compared to current guidelines without apparent negative effects on clinical outcomes. A secondary analysis of a randomized placebo-controlled trial involving adults with a diagnosis of UTI treated with either 7 days or 14 days of antibiotics, Stalenhoef et al.100 reported that PCT concentrations at presentation were positively correlated with bacteremia (τ = 0.33, p <0.001) and presence of shaking chills (τ = 0.25, p <0.001). However, in this PCT did not predict treatment outcomes in patients with community-acquired febrile UTI.
Evidence Statement
Procalcitonin has been used to differentiate urosepsis and bacteremia among patients with UTI, however, the sensitivity and specificity remain variable. Procalcitonin has been used for the decision to stop antibiotics among patients with UTI. Procalcitonin-guided algorithms led to lesser use of antibiotics without significant adverse effects. Different studies have used different cut-off values.
Recommendations
Procalcitonin may be used to diagnose bacteremia in UTI patients (2B).
Procalcitonin-pyuria based algorithm can be used to guide antibiotic therapy in UTI patients (1B).
We suggest against the use of PCT as a marker of treatment outcome in patients with febrile urinary tract infection (2B).
What is the Utility of PCT in Infections among Patients with Cirrhosis and End-stage Liver Disease?
Bacterial infections are among the most frequent and serious complications in patients with liver cirrhosis. In these individuals, the presence of iatrogenic factors (diagnostic and therapeutic), and the alteration of different immune mechanisms, favor the development of infections. It is therefore very important to establish an early and firm diagnosis of bacterial infection in cirrhotic individuals. However, the signs and symptoms inherent to the infection are often missing or are difficult to identify in these subjects. The use of biomarker/s for infection, such as PCT, in the diagnostic algorithm therefore may be particularly useful.
Procalcitonin has been used as a diagnostic marker for peritonitis. A retrospective study by Villarreal et al.101 reported a cut-off value of 0.8 ng/mL for PCT with a sensitivity 83%, specificity 75% and AUC of 0.82 (0.702–0.93) for diagnosis of infection among cirrhotic patients. Infection reported included pneumonia (70%), intra-abdominal infection (18%) and bacteremia (5%). Among patients with severe chronic Hepatitis-B, Yuan et al.102 reported a cut-off value for PCT of 0.48 ng/mL for the diagnosis of secondary bacterial peritonitis (SBP). The study showed a significant correlation between PCT concentration, CRP and WBC count. They also showed that PCT concentrations were more accurate than WBC count for the diagnosis of SBP among these patients. A meta-analysis by Yang et al.103 evaluated the overall diagnostic accuracy of PCT levels for identifying SBP due to end-stage liver disease. The meta-analysis revealed a sensitivity of 0.82 (95% CI: 0.79–0.87), specificity of 0.86 (95% CI: 0.82–0.89), positive likelihood ratio of 4.94 (95% CI: 2.28–10.70), negative likelihood ratio of 0.22 (95% CI: 0.10–0.52), and diagnostic OR of 22.55 (95% CI: 7.01–108.30). The AUC was 0.92. Procalcitonin has been used in combination with other markers such as leukocyte counts or morphological characteristics. Wang et al.104 evaluated a new bio-score combined with PCT, mean fluorescence intensity of mature neutrophils, and difference in hemoglobin concentration between newly formed and mature red blood cells in the diagnosis of ascites infection in cirrhotic patients. In ROC analysis, the AUC for PCT was 0.852; whereas the combination bio-score further improved the diagnostic efficacy with an AUC of 0.937 (95% CI: 0.901–0.994, p <0.001).
Evidence Statement
Procalcitonin has been used for the diagnosis of infections among patients with cirrhosis/end-stage liver disease. The sensitivity and specificity (modest) are variable. Different studies have used different cut-off values.
Recommendations
Procalcitonin alone should not be used for diagnosis of infection or SBP among patients with cirrhosis and end-stage liver disease (2A).
Procalcitonin may be considered for the diagnosis of infection or SBP in combination with clinical and other laboratory parameters (2B).
What is the Utility of PCT Infections among Patients with Acute Pancreatitis?
Acute pancreatitis is an acute inflammatory process ranging from mild discomfort with localized inflammation to severe disease with multiple organ failure. Multiple scoring systems, including the Ranson, Glasgow, and APACHE II scores, and several biochemical markers have been developed for the early prediction of severity of acute pancreatitis which facilitates early treatment in an intensive care unit. Serum PCT is an early marker of systemic bacterial infection, sepsis, and multi-organ failure. The major complications of acute pancreatitis are infected pancreatic necrosis, sepsis, and multi-organ failure. Therefore, it is hypothesized is that increased serum levels of PCT could predict severe acute pancreatitis and poor treatment outcome and guide regarding the management of patients with acute severe pancreatitis. A study by Woo et al.105 reported that levels of serum PCT were significantly higher in severe acute pancreatitis (p = 0.001). Authors reported an accuracy of 77% for serum PCT as a predicting marker was similar to the APACHE II score, worse than the Ranson score (93.2%) and better than the Balthazar CT index (65.9%). In this study, the most effective cut-off level of serum PCT was estimated at 1.77 ng/mL (AUC 0.797, 95% CI: 0.658–0.935). An RCT by Qu et al.106 investigated the clinical usefulness of PCT for guiding the duration of antibiotic therapy in patients with severe acute pancreatitis. This study showed that for the PCT-guided group, the duration of antibiotic therapy and hospitalization was significantly shorter than the control group, 10.89 ± 2.85 vs 16.06 ± 2.48 days, p <0.001, and 16.66 ± 4.02 days vs 23.81 ± 7.56 days, p <0.001, respectively. The approach was safe without any negative clinical effects and the cost of hospitalization was significantly lower.
Evidence Statement
Procalcitonin has been used for the prediction of the severity of acute pancreatitis. However, performance of PCT is not better than existing clinical scores. The PCT-guided antibiotics therapy led to a reduction in antibiotic use and hospital stay without any adverse effects.
Recommendations
We recommend against the use of PCT for the diagnosis of acute severe pancreatitis (2A).
We suggest using PCT for guiding antibiotic therapy among patients with severe acute pancreatitis (1B).
What is the Utility of PCT in Infections among Patients with Spontaneous and Secondary Bacterial Peritonitis?
Bacterial peritonitis is an inflammation of the peritoneum by micro-organisms such as gram-negative bacilli. It frequently occurs in children and adults and can endanger life, particularly in patients who have decompensated cirrhosis or in patients receiving continuous ambulatory peritoneal dialysis therapy. Notably, the delayed diagnosis of peritonitis is associated with high mortality. Consequently, diagnosis of bacterial peritonitis continues to be a major clinical challenge, and an accurate biomarker for the early identification of peritonitis would be of great diagnostic value. A prospective study by Abdel–Razik et al.107 demonstrated that at a cut-off value of 0.94 ng/mL, serum PCT had 94.3% sensitivity and 91.8% specificity for detecting bacterial peritonitis. There are other studies also assessed the utility of PCT for the diagnosis of SBP. Yang et al.108 performed a meta-analysis of 18 studies (n = 1,827) to assess the accuracy of PCT for detection of SBP. Their meta-analysis showed that the pooled sensitivity and specificity of serum PCT for the diagnosis of SBP were 0.83 (95% CI: 0.76–0.89) and 0.92 (95% CI: 0.87–0.96), respectively. The positive likelihood ratio was 11.06 (95% CI: 6.31–19.38), the negative likelihood ratio was 0.18 (95% CI: 0.12–0.27) and the diagnostic odds ratio (DOR) was 61.52 (95% CI: 27.58–137.21). The AUC was 0.94. They also reported that PCT performed better than CRP for the diagnosis of SBP. The authors also highlighted the methodological limitations and heterogeneity among the studies; medical decisions should be based on both clinical findings and PCT test results.
Secondary peritonitis, caused by the spread of bacteria into the peritoneal cavity due to perforation or other mechanisms, is an important cause of abdominal sepsis with a high risk of mortality and morbidity in surgical intensive care units. Mortality in SBP is over 60% and is associated with disease severity indices like APACHE II/III and Mannheim Peritonitis Index. The PCT-based algorithms have been used to guide antibiotic therapy in several clinical settings. Procalcitonin has the potential to be used as adjunct to clinical criteria for decision making regarding antibiotic duration. A retrospective study by Pupelis et al.109 involving patients admitted to the surgical ICU with secondary peritonitis reported that higher PCT levels (>15.3 ng/mL) were associated with an increased risk for septic shock and an increased risk of mortality (19.6 ng/mL). Another study by Akcay et al.110 evaluated the role of PCT in determining the severity of secondary peritonitis. They found that PCT was a better predictor of outcome than CRP in secondary peritonitis. Procalcitonin has also been used for guiding antibiotics therapy. A study by Huang et al.111 found that PCT algorithm significantly improves time to antibiotic discontinuation (p = 0.001) with a median duration of antibiotic exposure in the PCT group of 3.4 days (IQR 2.2 days) vs 6.1 days (IQR 3.2 days) in control; p = 0.001). Similar results were reported by Maseda et al.112 and reported that PCT guidance produced 50% reduction in antibiotic duration (p <0.001).
Evidence Statement
Procalcitonin has been used to diagnose SBP with variable sensitivity and specificity. There is heterogeneity in the methods and cut-off for PCT used by different studies for the diagnosis of SBP. Procalcitonin has been used for the prediction of the risk of septic shock and mortality among patients with secondary peritonitis. The PCT-guided antibiotic therapy leads to a reduction in antibiotic use and hospital stay without any adverse effect on secondary peritonitis.
Recommendations
Procalcitonin alone should not be used for the diagnosis of SBP (2A).
Procalcitonin may be considered for diagnosis of SBP in combination clinical features (1B).
Procalcitonin can be used for risk stratification among patients with secondary peritonitis (2A).
We recommend the use of PCT for guiding antibiotic therapy among patients with severe secondary peritonitis (2A).
Should Baseline PCT Levels be used to initiate Antibiotic Therapy in Immunocompromised Patients with Suspected Sepsis?
Immunocompromised patients have an increased incidence of severe and life-threatening infections. Moreover, these patients may have a need for prolonged antibiotic therapy. Hence, these patients have been excluded from most trials of antibiotic guidance using serum PCT.113 Observational studies have examined the role of serum PCT as a diagnostic tool for bacterial sepsis in immunocompromised patients. Bele et al. 114 prospectively studied patients with human immunodeficiency virus (HIV) infection, hematological malignancies, and solid organ cancers and found that serum PCT levels greater than 0.5 ng/mL can detect bacterial infection with a sensitivity and specificity of 100 and 63%, respectively. They concluded that despite its limited specificity, serum PCT may be used as a “rule out” test for bacterial infection in the immunosuppressed. Among hematological and solid organ cancer patients undergoing bronchoscopy for suspected infection, Stolz et al.115 reported that serum PCT had an AUC of 0.746 (p = 0.001) for predicting bacterial infection. Other studies have reported that while serum PCT level correlates with bacterial sepsis in immunocompromised hospitalized patients and organ transplant recipients, it is not accurate enough to be used as a sole criterion116,117 In pediatric oncology patients, the AUC of serum PCT for the diagnosis of bacterial infection has been found to range between 0.636 and 0.729 with poor sensitivity (45–55%) and a good specificity.118,119
Patients with cirrhosis are at increased risk of bacterial infections including SBP. A meta-analysis of 10 diagnostic studies revealed that serum PCT has a sensitivity and specificity of 79 and 89% respectively for the diagnosis of bacterial infections in cirrhotic patients. They found that serum PCT has a positive likelihood ratio of 7.38 and can be used as a “rule-in” test for sepsis. These results were valid for both the diagnosis of SBP and other systemic bacterial infections.120
In hematological malignancies, serum PCT has been found to be significantly higher with infectious fever compared with tumor-related or drug-related fever.121 Furthermore, in studies of patients with hematological malignancies, a combination of a high CRP with a low serum PCT has been found to predict a fungal etiology for sepsis.122,123 In lung transplant recipients, raised serum PCT may help in differentiating bacterial infection from rejection when they may have similar clinical presentations.124
Arora et al. studied the performance of serum PCT in diagnosing bloodstream infections (BSI) in patients with or without immunosuppressant medication use. They found no significant difference in the diagnostic performance of serum PCT between immunosuppressed and non-immunosuppressed patients despite a decreased likelihood of the SIRS in immunosuppressed patients.125 On the contrary, others have argued that cut-off values for serum PCT for diagnosing bacterial infections may be different in immunosuppressed and immunocompetent individuals. Mikuła et al. found that serum PCT levels inversely correlate with CD4 counts in HIV patients with sepsis. This may suggest a higher baseline serum PCT level in patients with low CD4 counts secondary to reactivation of occult infections.126 Similarly, in non-neutropenic patients with cancer, baseline serum PCT level is significantly higher in patients with stage IV or metastatic disease.127 Despite this, in immunosuppressed (leukopenic) patients with sepsis, serum PCT levels were found to be significantly lower than in immunocompetent sepsis patients beyond the third day of illness on serial estimation.128
Evidence Statement
Immunocompromised patients have an increased incidence of severe and life-threatening infections. There may be a need for prolonged antibiotic therapy in these patients. Serum PCT has been shown to be raised in immunocompromised patients with bacterial infections in observational studies. There are no RCTs that have examined the performance of serum PCT in guiding antibiotic therapy in immunocompromised patients. Patients with immunocompromise due to HIV infection or malignancy have elevated baseline serum PCT levels and there is heterogeneity in the cut-off levels as well as the diagnostic accuracy among different studies.
Recommendations
Serum PCT alone should not be used for the diagnosis of infection in immunocompromised patients (2A).
Serum PCT should not be used to guide antibiotic therapy in immunocompromised patients (2B).
Acknowledgment
The authors acknowledge Thermo Fisher Scientific India Pvt. Ltd. for providing logistic support.
Footnotes
Source of support: Nil
Conflict of interest: None
Orcid
Gopi Chand Khilnani https://orcid.org/0000-0003-0820-0624
Pawan Tiwari https://orcid.org/0000-0002-5136-4221
Kapil Gangadhar Zirpe https://orcid.org/0000-0002-8140-727X
Dhruva Chaudhry https://orcid.org/0000-0001-5138-2908
Deepak Govil https://orcid.org/0000-0002-4624-1614
Subhal Dixit https://orcid.org/0000-0002-1441-0807
Atul Prabhakar Kulkarni https://orcid.org/0000-0002-5172-7619
Subhash Kumar Todi https://orcid.org/0000-0003-2306-6080
Vijay Hadda https://orcid.org/0000-0001-5820-3685
Neetu Jain https://orcid.org/0000-0002-9776-4490
Manjunath B Govindagoudar https://orcid.org/0000-0003-1010-5191
Srinivas Samavedam https://orcid.org/0000-0001-6737-8663
Simant Kumar Jha https://orcid.org/0000-0001-9814-1052
Niraj Tyagi https://orcid.org/0000-0001-5862-9731
Madhusudan R Jaju https://orcid.org/0000-0002-1819-8073
Anita Sharma https://orcid.org/0000-0001-8521-7302
References
- 1.2022. Antimicrobial resistance.https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance Available at: Accessed on: 1st May 2022. [Google Scholar]
- 2.Carlet J, Pulcini C, Piddock LJV. Antibiotic resistance: A geopolitical issue. Clin Microbiol Infect. 2014;20(10):949–953. doi: 10.1111/1469-0691.12767. [DOI] [PubMed] [Google Scholar]
- 3.Laxminarayan R, Duse A, Wattal C, Zaidi AKM, Wertheim HFL, Sumpradit N, et al. Antibiotic resistance: The need for global solutions. Lancet Infect Dis. 2013;13(12):1057–1098. doi: 10.1016/S1473-3099(13)70318-9. [DOI] [PubMed] [Google Scholar]
- 4.Barlam TF, Cosgrove SE, Abbo LM, MacDougall C, Schuetz AN, Septimus EJ, et al. Implementing an antibiotic stewardship program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51–e77. doi: 10.1093/cid/ciw118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Snider RH, Nylen ES, Becker KL. Procalcitonin and its component peptides in systemic inflammation: immunochemical characterization. J Investig Med. 1997;45(9):552–560. 9444882 [PubMed] [Google Scholar]
- 6.Becker KL, Snider R, Nylen ES. Procalcitonin in sepsis and systemic inflammation: A harmful biomarker and a therapeutic target. Br J Pharmacol. 2010;159(2):253–264. doi: 10.1111/j.1476-5381.2009.00433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schuetz P, Bolliger R, Merker M, Christ–Crain M, Stolz D, Tamm M, et al. Procalcitonin-guided antibiotic therapy algorithms for different types of acute respiratory infections based on previous trials. Expert Rev. Anti Infect Therapy. 2018;16(7):555–564. doi: 10.1080/14787210.2018.1496331. [DOI] [PubMed] [Google Scholar]
- 8.Dandona P, Nix D, Wilson MF, Aljada A, Love J, Assicot M, et al. Procalcitonin increase after endotoxin injection in normal subjects. J Clin Endocrinol Metab. 1994;79(6):1605–1608. doi: 10.1210/jcem.79.6.7989463. [DOI] [PubMed] [Google Scholar]
- 9.Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet. 1993;341(8844):515–518. doi: 10.1016/0140-6736(93)90277-n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pontrelli G, De Crescenzo F, Buzzetti R, Jenkner A, Balduzzi S, Calò Carducci F, et al. Accuracy of serum procalcitonin for the diagnosis of sepsis in neonates and children with systemic inflammatory syndrome: A meta-analysis. BMC Infect Dis. 2017;17(1):302. doi: 10.1186/s12879-017-2396-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan M, Lu Y, Jiang H, Zhang L. The diagnostic accuracy of procalcitonin and C-reactive protein for sepsis: A systematic review and meta-analysis. J Cell Biochem. 2019;120(4):5852–5859. doi: 10.1002/jcb.27870. [DOI] [PubMed] [Google Scholar]
- 12.Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021;49(11):e1063–e1143. doi: 10.1097/CCM.0000000000005337. [DOI] [PubMed] [Google Scholar]
- 13.Gutiérrez–Pizarraya A, León–García MDC, De Juan–Idígoras R, Garnacho–Montero J. Clinical impact of procalcitonin-based algorithms for duration of antibiotic treatment in critically ill adult patients with sepsis: A meta-analysis of randomized clinical trials. Expert Rev Anti Infect Therapy. 2022;20(1):103–112. doi: 10.1080/14787210.2021. [DOI] [PubMed] [Google Scholar]
- 14.Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, et al. Sepsis in European intensive care units: Results of the SOAP study. Crit Care Med. 2006;34(2):344–353. doi: 10.1097/01.ccm.0000194725.48928.3a. [DOI] [PubMed] [Google Scholar]
- 15.Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302(21):2323–2329. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 16.Anand D, Das S, Bhargava S, Srivastava LM, Garg A, Tyagi N, et al. Procalcitonin as a rapid diagnostic biomarker to differentiate between culture-negative bacterial sepsis and systemic inflammatory response syndrome: A prospective, observational, cohort study. J Crit Care. 2015;30(1):218.e7–218.e12. doi: 10.1016/j.jcrc.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 17.Zhang T, Wang Y, Yang Q, Dong Y. Procalcitonin-guided antibiotic therapy in critically ill adults: A meta-analysis. BMC Infect Dis. 2017;17(1):514. doi: 10.1186/s12879-017-2622-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andriolo BN, Andriolo RB, Salomão R, Atallah ÁN. Effectiveness and safety of procalcitonin evaluation for reducing mortality in adults with sepsis, severe sepsis or septic shock. Cochrane Database Syst Rev. 2017;1(1):CD010959. doi: 10.1002/14651858.CD010959.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pepper DJ, Sun J, Rhee C, Welsh J, Powers JH, Danner RL, et al. Procalcitonin-guided antibiotic discontinuation and mortality in critically ill adults. Chest. 2019;155(6):1109–1118. doi: 10.1016/j.chest.2018.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wirz Y, Meier MA, Bouadma L, Luyt CE, Wolff M, Chastre J, et al. Effect of procalcitonin-guided antibiotic treatment on clinical outcomes in intensive care unit patients with infection and sepsis patients: A patient-level meta-analysis of randomized trials. Crit Care. 2018;22(1):191. doi: 10.1186/s13054-018-2125-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elnajdy D, El-Dahiyat F. Antibiotics duration guided by biomarkers in hospitalized adult patients: A systematic review and meta-analysis. Infect Dis (Lond) 2022;54(6):387–402. doi: 10.1080/23744235.2022.2037701. [DOI] [PubMed] [Google Scholar]
- 22.Vishalashi SG, Gupta P, Verma PK. Serum procalcitonin as a biomarker to determine the duration of antibiotic therapy in adult patients with sepsis and septic shock in intensive care units: A prospective study. Indian J Crit Care Med. 2021;25(5):507–511. doi: 10.5005/jp-journals-10071-23802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heilmann E, Gregoriano C, Wirz Y, Luyt CE, Wolff M, Chastre J, et al. Association of kidney function with effectiveness of procalcitonin-guided antibiotic treatment: A patient-level meta-analysis from randomized controlled trials. Clin Chem Lab Med (CCLM) 2021;59(2):441–453. doi: 10.1515/cclm-2020-0931. [DOI] [PubMed] [Google Scholar]
- 24.Robati Anaraki M, Nouri–Vaskeh M, Abdoli Oskouie S. Effectiveness of procalcitonin-guided antibiotic therapy to shorten treatment duration in critically-ill patients with bloodstream infections: A systematic review and meta-analysis. Infez Med. 2020;28(1):37–46. 32172259 [PubMed] [Google Scholar]
- 25.Voermans AM, Mewes JC, Broyles MR, Steuten LMG. Cost-effectiveness analysis of a procalcitonin-guided decision algorithm for antibiotic stewardship using real-world U.S. hospital data. OMICS. 2019;23(10):508–515. doi: 10.1089/omi.2019.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chow J, Markossian TW, Albarillo FS, Donahey EE, Bobay KL. Impact of a procalcitonin-based protocol on antibiotic exposure and costs in critically ill patients. Crit Care Explor. 2021;3(11):e0571. doi: 10.1097/CCE.0000000000000571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeon K, Suh JK, Jang EJ, Cho S, Ryu HG, Na S, et al. Procalcitonin-guided treatment on duration of antibiotic therapy and cost in septic patients (PRODA): A multi-center randomized controlled trial. J Korean Med Sci. 2019;34(14):e110. doi: 10.3346/jkms.2019.34.e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nobre V, Harbarth S, Graf JD, Rohner P, Pugin J. Use of procalcitonin to shorten antibiotic treatment duration in septic patients: A randomized trial. Am J Respir Crit Care Med. 2008;177(5):498–505. doi: 10.1164/rccm.200708-1238OC. [DOI] [PubMed] [Google Scholar]
- 29.Xu XL, Yan FD, Yu JQ, Chen QH, Lin H, Zheng RQ. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment of sepsis patients. Zhonghua Yi Xue Za Zhi. 2017;97(5):343–346. doi: 10.3760/cma.j.issn.0376-2491.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Shehabi Y, Sterba M, Garrett PM, Rachakonda KS, Stephens D, Harrigan P, et al. Procalcitonin algorithm in critically ill adults with undifferentiated infection or suspected sepsis. A randomized controlled trial. Am J Respir Crit Care Med. 2014;190(10):1102–10. doi: 10.1164/rccm.201408-1483OC. [DOI] [PubMed] [Google Scholar]
- 31.Hochreiter M, Köhler T, Schweiger AM, Keck FS, Bein B, von Spiegel T, et al. Procalcitonin to guide duration of antibiotic therapy in intensive care patients: a randomized prospective controlled trial. Crit Care. 2009;13(3):R83. doi: 10.1186/cc7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schroeder S, Hochreiter M, Koehler T, Schweiger AM, Bein B, Keck FS, et al. Procalcitonin (PCT)-guided algorithm reduces length of antibiotic treatment in surgical intensive care patients with severe sepsis: Results of a prospective randomized study. Langenbecks Arch Surg. 2009;394(2):221–226. doi: 10.1007/s00423-008-0432-1. [DOI] [PubMed] [Google Scholar]
- 33.Liu Bh, Li Hf, Lei Y, Zhao Sx, Sun Ml. Clinical significance of dynamic monitoring of procalcitonin in guiding the use of antibiotics in patients with sepsis in ICU. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2013;25(11):690–693. doi: 10.3760/cma.j.issn.2095-4352.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 34.de Jong E, van Oers JA, Beishuizen A, Vos P, Vermeijden WJ, Haas LE, et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: A randomised, controlled, open-label trial. Lancet Infect Dis. 2016;16(7):819–827. doi: 10.1016/S1473-3099(16)00053-0. [DOI] [PubMed] [Google Scholar]
- 35.Annane D, Maxime V, Faller JP, Mezher C, Clec’h C, Martel P, et al. Procalcitonin levels to guide antibiotic therapy in adults with non-microbiologically proven apparent severe sepsis: A randomised controlled trial. BMJ Open. 2013;3(2):e002186. doi: 10.1136/bmjopen-2012-002186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deliberato RO, Marra AR, Sanches PR, Martino MDV, Ferreira CE dos S, Pasternak J, et al. Clinical and economic impact of procalcitonin to shorten antimicrobial therapy in septic patients with proven bacterial infection in an intensive care setting. Diagn Microbiol Infect Dis. 2013;76(3):266–271. doi: 10.1016/j.diagmicrobio.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 37.Oliveira CF, Botoni FA, Oliveira CRA, Silva CB, Pereira HA, Serufo JC, et al. Procalcitonin versus C-reactive protein for guiding antibiotic therapy in sepsis: A randomized trial. Crit Care Med. 2013;41(10):2336–2343. doi: 10.1097/CCM.0b013e31828e969f. [DOI] [PubMed] [Google Scholar]
- 38.Bloos F, Trips E, Nierhaus A, Briegel J, Heyland DK, Jaschinski U, et al. Effect of sodium selenite administration and procalcitonin-guided therapy on mortality in patients with severe sepsis or septic shock: A randomized clinical trial. JAMA Intern Med. 2016;176(9):1266–1276. doi: 10.1001/jamainternmed.2016.2514. [DOI] [PubMed] [Google Scholar]
- 39.Sager R, Wirz Y, Amin D, Amin A, Hausfater P, Huber A, et al. Are admission procalcitonin levels universal mortality predictors across different medical emergency patient populations? Results from the multi-national, prospective, observational TRIAGE study. Clin Chem Lab Med. 2017;55(12):1873–1880. doi: 10.1515/cclm-2017-0144. [DOI] [PubMed] [Google Scholar]
- 40.Schuetz P, Birkhahn R, Sherwin R, Jones AE, Singer A, Kline JA, et al. Serial procalcitonin predicts mortality in severe sepsis patients: Results from the Multicenter Procalcitonin MOnitoring SEpsis (MOSES) Study. Crit Care Med. 2017;45(5):781–789. doi: 10.1097/CCM.0000000000002321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jekarl DW, Lee S, Kim M, Kim Y, Woo SH, Lee WJ. Procalcitonin as a prognostic marker for sepsis based on SEPSIS-3. J Clin Lab Anal. 2019;33(9):e22996. doi: 10.1002/jcla.22996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stockmann C, Ampofo K, Killpack J, Williams DJ, Edwards KM, Grijalva CG, et al. Procalcitonin accurately identifies hospitalized children with low risk of bacterial community-acquired pneumonia. J Pediatric Infect Dis Soc. 2018;7(1):46–53. doi: 10.1093/jpids/piw091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Self WH, Balk RA, Grijalva CG, Williams DJ, Zhu Y, Anderson EJ, et al. Procalcitonin as a marker of etiology in adults hospitalized with community-acquired pneumonia. Clin Infect Dis. 2017;65(2):183–190. doi: 10.1093/cid/cix317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rao K, Walk ST, Micic D, Chenoweth E, Deng L, Galecki AT, et al. Procalcitonin levels associate with severity of Clostridium difficile infection. PLoS One. 2013;8(3):e58265. doi: 10.1371/journal.pone.0058265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shapiro DS, Friedmann R, Husseini A, Ivgi H, Yinnon AM, Assous MV. Can procalcitonin contribute to the diagnosis of Clostridium difficile colitis? Isr Med Assoc J. 2017;19(5):313–316. 28513121 [PubMed] [Google Scholar]
- 46.Raineri SM, Cortegiani A, Vitale F, Iozzo P, Giarratano A. Procalcitonin for the diagnosis of invasive candidiasis: What is the evidence? J Intensive Car. 2017;5:58. doi: 10.1186/s40560-017-0252-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Charles PE, Dalle F, Aho S, Quenot JP, Doise JM, Aube H, et al. Serum procalcitonin measurement contribution to the early diagnosis of candidemia in critically ill patients. Intensive Care Med. 2006;32(10):1577–1578. doi: 10.1007/s00134-006-0306-3. [DOI] [PubMed] [Google Scholar]
- 48.Uzzan B, Izri A, Durand R, Deniau M, Bouchaud O, Perret GY. Serum procalcitonin in uncomplicated falciparum malaria: A preliminary study. Travel Med Infect Dis. 2006;4(2):77–80. doi: 10.1016/j.tmaid.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 49.Chiwakata CB, Manegold C, Bönicke L, Waase I, Jülch C, Dietrich M. Procalcitonin as a parameter of disease severity and risk of mortality in patients with Plasmodium falciparum malaria. J Infect Dis. 2001;183(7):1161–1164. doi: 10.1086/319283. [DOI] [PubMed] [Google Scholar]
- 50.Ugajin M, Miwa S, Shirai M, Ohba H, Eifuku T, Nakamura H, et al. Usefulness of serum procalcitonin levels in pulmonary tuberculosis. Eur Respir J. 2011;37(2):371–375. doi: 10.1183/09031936.00011910. [DOI] [PubMed] [Google Scholar]
- 51.Rasmussen TA, Søgaard OS, Camara C, Andersen PL, Wejse C. Serum procalcitonin in pulmonary tuberculosis. Int J Tubercul Lung Dis. 2011;15(2):251–256. 21219690 [PubMed] [Google Scholar]
- 52.Christensen AMG, Thomsen MK, Ovesen T, Klug TE. Are procalcitonin or other infection markers useful in the detection of group A streptococcal acute tonsillitis? Scand J Infect Dis. 2014;46(5):376–383. doi: 10.3109/00365548.2014.885656. [DOI] [PubMed] [Google Scholar]
- 53.Pallin DJ, Bry L, Dwyer RC, Lipworth AD, Leung DY, Camargo CA, Jr, et al. Toward an objective diagnostic test for bacterial cellulitis. PLoS One. 2016;11(9):e0162947. doi: 10.1371/journal.pone.0162947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roques M, Chretien ML, Favennec C, Lafon I, Ferrant E, Legouge C, et al. Evolution of procalcitonin, C-reactive protein and fibrinogen levels in neutropenic leukaemia patients with invasive pulmonary aspergillosis or mucormycosis. Mycoses. 2016;59(6):383–390. doi: 10.1111/myc.12487. [DOI] [PubMed] [Google Scholar]
- 55.Sakata KK, Grys TE, Chang YHH, Vikram HR, Blair JE. Serum procalcitonin levels in patients with primary pulmonary coccidioidomycosis. Ann Am Thorac Soc. 2014;11(8):1239–1243. doi: 10.1513/AnnalsATS.201404-180BC. [DOI] [PubMed] [Google Scholar]
- 56.Huang DT, Yealy DM, Filbin MR, Brown AM, Chang CCH, Doi Y, et al. Procalcitonin-guided use of antibiotics for lower respiratory tract infection. New Eng J Med. 2018;379(3):236–249. doi: 10.1056/NEJMoa1802670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schuetz P, Wirz Y, Sager R, Christ–Crain M, Stolz D, Tamm M, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2017;10(1):CD007498. doi: 10.1002/14651858.CD007498.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kamat IS, Ramachandran V, Eswaran H, Abers MS, Musher DM. Low procalcitonin, community acquired pneumonia, and antibiotic therapy. Lancet Infect Dis. 2018;18(5):496–497. doi: 10.1016/S1473-3099(18)30215-9. [DOI] [PubMed] [Google Scholar]
- 59.Huang DT, Yealy DM, Angus DC. Longer-term outcomes of the ProACT trial. N Engl J Med. 2020;382(5):485–486. doi: 10.1056/NEJMc1910508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.DeSear KE, Thompson–Leduc P, Kirson N, Chritton JJ, Ie S, Van Schooneveld TC, et al. ProCommunity: Procalcitonin use in real-world US community hospital settings. Curr Med Res Opin. 2020;36(9):1529–1532. doi: 10.1080/03007995.2020.1793748. [DOI] [PubMed] [Google Scholar]
- 61.Vaughn VM, Gandhi TN, Petty LA, Patel PK, Prescott HC, Malani AN, et al. Empiric antibacterial therapy and community-onset bacterial coinfection in patients hospitalized with coronavirus disease 2019 (COVID-19): A multi-hospital cohort study. Clin Infect Dis. 2021;72(10):e533–e541. doi: 10.1093/cid/ciaa1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jackson I, Jaradeh H, Aurit S, Aldamen A, Narechania S, Destache C, et al. Role of procalcitonin as a predictor of clinical outcomes in hospitalized patients with COVID-19. Int J Infect Dis. 2022;119:47–52. doi: 10.1016/j.ijid.2022.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vazzana N, Dipaola F, Ognibene S. Procalcitonin and secondary bacterial infections in COVID-19: Association with disease severity and outcomes. Acta Clinica Belgica. 2022;77(2):268–272. doi: 10.1080/17843286.2020.1824749. [DOI] [PubMed] [Google Scholar]
- 64.Tong–Minh K, van der Does Y, Engelen S, de Jong E, Ramakers C, Gommers D, et al. High procalcitonin levels associated with increased intensive care unit admission and mortality in patients with a COVID-19 infection in the emergency department. BMC Infect Dis. 2022;22(1):165. doi: 10.1186/s12879-022-07144-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lippi G, Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): A meta-analysis. Clin Chim Acta. 2020;505:190–191. doi: 10.1016/j.cca.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Llewelyn MJ, Grozeva D, Howard P, Euden J, Gerver SM, Hope R, et al. Impact of introducing procalcitonin testing on antibiotic usage in acute NHS hospitals during the first wave of COVID-19 in the UK: A controlled interrupted time series analysis of organization-level data. J Antimicrob Chemother. 2022;77(4):1189–1196. doi: 10.1093/jac/dkac017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gupta D, Agarwal R, Aggarwal AN, Singh N, Mishra N, Khilnani GC, et al. Guidelines for diagnosis and management of community-and hospital-acquired pneumonia in adults: Joint ICS/NCCP(I) recommendations. Lung India. 2012;29(6):27. doi: 10.4103/0970-2113.99248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Khilnani, G, Zirpe, K, Hadda, V, Mehta, Y, Madan, K, Kulkarni, A, et al. Guidelines for antibiotic prescription in intensive care unit. Indian J Crit Care Med. 2019;23(Suppl. 1):S1–S63. doi: 10.5005/jp-journals-10071-23101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Metlay JP, Waterer GW, Long AC, Anzueto A, Brozek J, Crothers K, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45–e67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kamat IS, Ramachandran V, Eswaran H, Guffey D, Musher DM. Procalcitonin to distinguish viral from bacterial pneumonia: A systematic review and meta-analysis. Clin Infect Dis. 2020;70(3):538–542. doi: 10.1093/cid/ciz545. [DOI] [PubMed] [Google Scholar]
- 71.Fabre V, Karaba S, Amoah J, Robinson M, Jones G, Dzintars K, et al. The role of procalcitonin results in antibiotic decision-making in coronavirus disease 2019 (COVID-19). Infect Control Hosp Epidemiol. 2022;43(5):570–575. doi: 10.1017/ice.2021.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ito A, Ishida T, Tokumasu H, Washio Y, Yamazaki A, Ito Y, et al. Impact of procalcitonin-guided therapy for hospitalized community-acquired pneumonia on reducing antibiotic consumption and costs in Japan. J Infect Chemother. 2017;23(3):142–147. doi: 10.1016/j.jiac.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 73.Montassier E, Javaudin F, Moustafa F, Nandjou D, Maignan M, Hardouin JB, et al. Guideline-based clinical assessment versus procalcitonin-guided antibiotic use in pneumonia: A pragmatic randomized trial. Ann Emerg Med. 2019;74(4):580–591. doi: 10.1016/j.annemergmed.2019.02.025. [DOI] [PubMed] [Google Scholar]
- 74.Song YY, Zhang B, Gu JW, Zhang YJ, Wang Y. The predictive value of procalcitonin in ventilator-associated pneumonia after cardiac valve replacement. Scand J Clin Lab Invest. 2020;80(5):423–426. doi: 10.1080/00365513.2020.1762242. [DOI] [PubMed] [Google Scholar]
- 75.Sotillo–Díaz JC, Bermejo–López E, García–Olivares P, Peral–Gutiérrez JA, Sancho–González M, Guerrero–Sanz JE. Role of plasma procalcitonin in the diagnosis of ventilator-associated pneumonia: Systematic review and metaanalysis. Med Intensiva. 2014;38(6):337–346. doi: 10.1016/j.medin.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 76.Póvoa P, Martin–Loeches I, Ramirez P, Bos LD, Esperatti M, Silvestre J, et al. Biomarker kinetics in the prediction of VAP diagnosis: results from the BioVAP study. Ann Intensive Care. 2016;6(1):32. doi: 10.1186/s13613-016-0134-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jung B, Embriaco N, Roux F, Forel JM, Demory D, Allardet–Servent J, et al. Microbiogical data, but not procalcitonin improve the accuracy of the clinical pulmonary infection score. Intensive Care Med. 2010;36(5):790–798. doi: 10.1007/s00134-010-1833-5. [DOI] [PubMed] [Google Scholar]
- 78.Re MFC, Rocchetti NS, Settecase CJ, Bagilet DH. Diagnostic value of procalcitonin in ventilator-associated pneumonia. Med Clin (Barc) 2019;152(6):216–221. doi: 10.1016/j.medcli.2018.06.019. [DOI] [PubMed] [Google Scholar]
- 79.Rahimibashar F, Miller AC, Yaghoobi MH, Vahedian–Azimi A. A comparison of diagnostic algorithms and clinical parameters to diagnose ventilator-associated pneumonia: A prospective observational study. BMC Pulm Med. 2021;21(1):161. doi: 10.1186/s12890-021-01527-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou J, Song J, Gong S, Hu W, Wang M, Xiao A, et al. Lung ultrasound combined with procalcitonin for a diagnosis of ventilator-associated pneumonia. Respir Care. 2019;64(5):519–527. doi: 10.4187/respcare.06377. [DOI] [PubMed] [Google Scholar]
- 81.Coelho L, Rabello L, Salluh J, Martin–Loeches I, Rodriguez A, Nseir S, et al. C-reactive protein and procalcitonin profile in ventilator-associated lower respiratory infections. J Crit Care. 2018;48:385–389. doi: 10.1016/j.jcrc.2018.09.036. [DOI] [PubMed] [Google Scholar]
- 82.Li B, Zhao X, Li S. Serum procalcitonin level and mortality risk in critically ill patients with ventilator-associated pneumonia. CPB. 2015;37(5):1967–1972. doi: 10.1159/000438557. [DOI] [PubMed] [Google Scholar]
- 83.Bloos F, Marshall JC, Dellinger RP, Vincent JL, Gutierrez G, Rivers E, et al. Multinational, observational study of procalcitonin in ICU patients with pneumonia requiring mechanical ventilation: A multicenter observational study. Crit Care. 2011;15(2):R88. doi: 10.1186/cc10087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tanrıverdi H, Tor MM, Kart L, Altın R, Atalay F, SumbSümbüloğlu V. Prognostic value of serum procalcitonin and C-reactive protein levels in critically ill patients who developed ventilator-associated pneumonia. Ann Thorac Med. 2015;10(2):137–142. doi: 10.4103/1817-1737.151442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hillas G, Vassilakopoulos T, Plantza P, Rasidakis A, Bakakos P. C-reactive protein and procalcitonin as predictors of survival and septic shock in ventilator-associated pneumonia. Eur Respir J. 2010;35(4):805–811. doi: 10.1183/09031936.00051309. [DOI] [PubMed] [Google Scholar]
- 86.Torres A, Niederman MS, Chastre J, Ewig S, Fernandez–Vandellos P, Hanberger H, et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: Guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana del Tórax (ALAT). Eur Respir J. 2017;50(3):1700582. doi: 10.1183/13993003.00582-2017. [DOI] [PubMed] [Google Scholar]
- 87.Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stolz D, Smyrnios N, Eggimann P, Pargger H, Thakkar N, Siegemund M, et al. Procalcitonin for reduced antibiotic exposure in ventilator-associated pneumonia: a randomised study. Eur Respir J. 2009;34(6):1364–1375. doi: 10.1183/09031936.00053209. [DOI] [PubMed] [Google Scholar]
- 89.Mazlan MZ, Ismail MAH, Ali S, Salmuna ZN, Shukeri WFWM, Omar M. Efficacy and safety of the point-of-care procalcitonin test for determining the antibiotic treatment duration in patients with ventilator-associated pneumonia in the intensive care unit: A randomised controlled trial. Anaesthesiol Intensive Therapy. 2021;53(3):207–214. doi: 10.5114/ait.2021.104300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wongsurakiat P, Tulatamakit S. Clinical pulmonary infection score and a spot serum procalcitonin level to guide discontinuation of antibiotics in ventilator-associated pneumonia: a study in a single institution with high prevalence of non-fermentative gram-negative bacilli infection. Ther Adv Respir Dis. 2018;12:1753466618760134. doi: 10.1177/1753466618760134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Beye F, Vigneron C, Dargent A, Prin S, Andreu P, Large A, et al. Adhering to the procalcitonin algorithm allows antibiotic therapy to be shortened in patients with ventilator-associated pneumonia. J Crit Care. 2019;53:125–131. doi: 10.1016/j.jcrc.2019.05.022. [DOI] [PubMed] [Google Scholar]
- 92.Pontet J, Bazzano F, Miraballes R, Bentancourt S, Cancela M. Procalcitonin (PCT) guided antibiotic treatment in ventilator associated pneumonia (VAP). Multi–centre, clinical prospective, randomized–controlled study. Index Infectológico. 2008;175:63. [Google Scholar]
- 93.Huespe I, Prado E, Staneloni I, Contrera N, Denaday L, San Roman E, et al. Kinetics of procalcitonin in infections caused by multidrug-resistant bacteria. Medicina (B Aires). 2020;80(6):599–605. 33254103 [PubMed] [Google Scholar]
- 94.van Nieuwkoop C, Bonten TN, van’t Wout JW, Kuijper EJ, Groeneveld GH, Becker MJ, et al. Procalcitonin reflects bacteremia and bacterial load in urosepsis syndrome: a prospective observational study. Crit Care. 2010;14(6):R206. doi: 10.1186/cc9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Julián–Jiménez A, Gutiérrez–Martín P, Lizcano–Lizcano A, López–Guerrero MA, Barroso–Manso Á, Heredero-Gálvez E. Usefulness of procalcitonin and C-reactive protein for predicting bacteremia in urinary tract infections in the emergency department. Actas Urol Esp. 2015:502–510. doi: 10.1016/j.acuro.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 96.Lee H, Lee YS, Jeong R, Kim YJ, Ahn S. Predictive factors of bacteremia in patients with febrile urinary tract infection: An experience at a tertiary care center. Infection. 2014;42(4):669–674. doi: 10.1007/s15010-014-0615-3. [DOI] [PubMed] [Google Scholar]
- 97.Levine AR, Tran M, Shepherd J, Naut E. Utility of initial procalcitonin values to predict urinary tract infection. Am J Emerg Med. 2018;36(11):1993–1997. doi: 10.1016/j.ajem.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 98.Luo X, Yang X, Li J, Zou G, Lin Y, Qing G, et al. The procalcitonin/albumin ratio as an early diagnostic predictor in discriminating urosepsis from patients with febrile urinary tract infection. Medicine (Baltimore) 2018;97(28):e11078. doi: 10.1097/MD.0000000000011078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Drozdov D, Schwarz S, Kutz A, Grolimund E, Rast AC, Steiner D, et al. Procalcitonin and pyuria-based algorithm reduces antibiotic use in urinary tract infections: A randomized controlled trial. BMC Med. 2015;13:104. doi: 10.1186/s12916-015-0347-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stalenhoef JE, van Nieuwkoop C, Wilson DC, van der Starre WE, Delfos NM, Leyten EMS, et al. Biomarker guided triage can reduce hospitalization rate in community acquired febrile urinary tract infection. J Infect. 2018;77(1):18–24. doi: 10.1016/j.jinf.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 101.Villarreal E, Vacacela K, Gordon M, Calabuig C, Alonso R, Ruiz J, et al. Usefulness of procalcitonin for diagnosing infection in critically ill patients with liver cirrhosis. Med Intensiva. 2016;40(2):84–89. doi: 10.1016/j.medin.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 102.Yuan LY, Ke ZQ, Wang M, Li Y. Procalcitonin and C-reactive protein in the diagnosis and prediction of spontaneous bacterial peritonitis associated with chronic severe hepatitis B. Ann Lab Med. 2013;33(6):449–454. doi: 10.3343/alm.2013.33.6.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yang Y, Li L, Qu C, Zeng B, Liang S, Luo Z, et al. Diagnostic accuracy of serum procalcitonin for spontaneous bacterial peritonitis due to end-stage liver disease: A meta-analysis. Medicine (Baltimore) 2015;94(49):e2077. doi: 10.1097/MD.0000000000002077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang H, Li Y, Zhang F, Yang N, Xie N, Mao Y, et al. Combination of PCT, sNFI and dCHC for the diagnosis of ascites infection in cirrhotic patients. BMC Infect Dis. 2018;18(1):389. doi: 10.1186/s12879-018-3308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Woo SM, Noh MH, Kim BG, Hsing CT, Han JS, Ryu SH, et al. Comparison of serum procalcitonin with Ranson, APACHE-II, Glasgow and Balthazar CT severity index scores in predicting severity of acute pancreatitis. Korean J Gastroenterol. 2011;58(1):31–37. doi: 10.4166/kjg.2011.58.1.31. [DOI] [PubMed] [Google Scholar]
- 106.Qu R, Ji Y, Ling Y, Ye CY, Yang SM, Liu YY, et al. Procalcitonin is a good tool to guide duration of antibiotic therapy in patients with severe acute pancreatitis. A randomized prospective single-center controlled trial. Saudi Med J. 2012;33(4):382–387. [PubMed] [Google Scholar]
- 107.Abdel–Razik A, Mousa N, Elhammady D, Elhelaly R, Elzehery R, Elbaz S, et al. Ascitic fluid calprotectin and serum procalcitonin as accurate diagnostic markers for spontaneous bacterial peritonitis. Gut Liver. 2016;10(4):624–631. doi: 10.5009/gnl15120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yang Sk, Xiao L, Zhang H, Xu Xxuan, Song P ai, Liu F you, et al. Significance of serum procalcitonin as biomarker for detection of bacterial peritonitis: A systematic review and meta-analysis. BMC Infect Dis. 2014;14(1):452. doi: 10.1186/1471-2334-14-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pupelis G, Drozdova N, Mukans M, Malbrain MLNG. Serum procalcitonin is a sensitive marker for septic shock and mortality in secondary peritonitis. Anaesthesiol Intensive Ther. 2014;46(4):262–273. doi: 10.5603/AIT.2014.0043. [DOI] [PubMed] [Google Scholar]
- 110.Akcay I, Okoh AK, Yalav O, Eray IC, Rencuzogullari A, Dalci K, et al. The prognostic value of pro-calcitonin, CRP and thyroid hormones in secondary peritonitis: A single-center prospective study. Ulus Travma Acil Cerrahi Derg. 2014;20(5):343–352. doi: 10.5505/tjtes.2014.98354. [DOI] [PubMed] [Google Scholar]
- 111.Huang TS, Huang SS, Shyu YC, Lee CH, Jwo SC, Chen PJ, et al. A procalcitonin-based algorithm to guide antibiotic therapy in secondary peritonitis following emergency surgery: A prospective study with propensity score matching analysis. PLoS One. 2014;9(3):e90539. doi: 10.1371/journal.pone.0090539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Maseda E, Suarez-de-la-Rica A, Anillo V, Tamayo E, García–Bernedo CA, Ramasco F, et al. Procalcitonin-guided therapy may reduce length of antibiotic treatment in intensive care unit patients with secondary peritonitis: A multicenter retrospective study. J Crit Care. 2015;30(3):537–542. doi: 10.1016/j.jcrc.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 113.El Haddad H, Chaftari AM, Hachem R, Chaftari P, Raad II. Biomarkers of sepsis and bloodstream infections: The role of procalcitonin and proadrenomedullin with emphasis in patients with cancer. Clin Infect Dis. 2018;67(6):971–977. doi: 10.1093/cid/ciy331. [DOI] [PubMed] [Google Scholar]
- 114.Bele N, Darmon M, Coquet I, Feugeas JP, Legriel S, Adaoui N, et al. Diagnostic accuracy of procalcitonin in critically ill immunocompromised patients. BMC Infect Dis. 2011;11:224. doi: 10.1186/1471-2334-11-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stolz D, Stulz A, Müller B, Gratwohl A, Tamm M. BAL neutrophils, serum procalcitonin, and C-reactive protein to predict bacterial infection in the immunocompromised host. Chest. 2007;132(2):504–514. doi: 10.1378/chest.07-0175. [DOI] [PubMed] [Google Scholar]
- 116.Savaş Bozbaş Ş, Er Dedekargınoğlu B, Ulubay G, Haberal M. Role of serum procalcitonin levels in solid-organ transplant patients. Exp Clin Transplant. 2016;14(Suppl. 3):116–120. 27805529 [PubMed] [Google Scholar]
- 117.Mauro MV, Cavalcanti P, Perugini D, Noto A, Sperlì D, Giraldi C. Diagnostic utility of LightCycler SeptiFast and procalcitonin assays in the diagnosis of bloodstream infection in immunocompromised patients. Diagn Microbiol Infect Dis. 2012;73(4):308–311. doi: 10.1016/j.diagmicrobio.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 118.Gunasekaran V, Radhakrishnan N, Dinand V, Sachdeva A. Serum procalcitonin for predicting significant infections and mortality in pediatric oncology. Indian Pediatr. 2016;53(12):1075–1078. 27889712 [PubMed] [Google Scholar]
- 119.Vyles D, Gnagi F, Bulloch B, Muenzer J, Hu C. Procalcitonin as a marker of bacteremia in patients with fever and acute lymphoblastic leukemia. Pediatr Emerg Care. 2016;32(9):590–593. doi: 10.1097/PEC.0000000000000660. [DOI] [PubMed] [Google Scholar]
- 120.Lin KH, Wang FL, Wu MS, Jiang BY, Kao WL, Chao HY, et al. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection in patients with liver cirrhosis: a systematic review and meta-analysis. Diagn Microbiol Infect Dis. 2014;80(1):72–78. doi: 10.1016/j.diagmicrobio.2014.03.029. [DOI] [PubMed] [Google Scholar]
- 121.Schüttrumpf S, Binder L, Hagemann T, Berkovic D, Trümper L, Binder C. Procalcitonin: a useful discriminator between febrile conditions of different origin in hemato–oncological patients? Ann Hematol. 2003;82(2):98–103. doi: 10.1007/s00277-002-0584-y. [DOI] [PubMed] [Google Scholar]
- 122.Marková M, Brodská H, Malíčková K, Válková V, Cetkovský P, Kolář M, et al. Substantially elevated C-reactive protein (CRP), together with low levels of procalcitonin (PCT), contributes to diagnosis of fungal infection in immunocompromised patients. Support Care Cancer. 2013;21(10):2733–2742. doi: 10.1007/s00520-013-1844-1. [DOI] [PubMed] [Google Scholar]
- 123.Stoma I, Karpov I, Uss A, Krivenko S, Iskrov I, Milanovich N, et al. Combination of sepsis biomarkers may indicate an invasive fungal infection in haematological patients. Biomarkers. 2019;24(4):401–406. doi: 10.1080/1354750X.2019.1600023. [DOI] [PubMed] [Google Scholar]
- 124.Sammons C, Doligalski CT. Utility of procalcitonin as a biomarker for rejection and differentiation of infectious complications in lung transplant recipients. Ann Pharmacother. 2014;48(1):116–122. doi: 10.1177/1060028013508085. [DOI] [PubMed] [Google Scholar]
- 125.Arora R, Sahni N. Can serum procalcitonin aid in the diagnosis of blood stream infection in patients on immunosuppressive medications? Clin Chim Acta. 2018;483:204–208. doi: 10.1016/j.cca.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 126.Mikuła T, Cianciara J, Wiercińska–Drapało A. Is there any influence of immune deficit on procalcitonin results? Hum Immunol. 2011;72(12):1194–1197. doi: 10.1016/j.humimm.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 127.Shomali W, Hachem R, Chaftari AM, Jiang Y, Bahu R, Jabbour J, et al. Can procalcitonin distinguish infectious fever from tumor-related fever in non-neutropenic cancer patients? Cancer. 2012;118(23):5823–5829. doi: 10.1002/cncr.27602. [DOI] [PubMed] [Google Scholar]
- 128.al-Nawas B, Shah PM. Procalcitonin in patients with and without immunosuppression and sepsis. Infection. 1996;24(6):434–436. doi: 10.1007/BF01713044. [DOI] [PubMed] [Google Scholar]