Key Points
Question
Is treatment to a goal low-density lipoprotein cholesterol (LDL-C) level between 50 and 70 mg/dL noninferior to a strategy using high-intensity statin therapy among patients with coronary artery disease?
Findings
In this randomized noninferiority trial that included 4400 patients with coronary artery disease, the rate of the 3-year composite of all-cause death, myocardial infarction, stroke, or any coronary revascularization was 8.1% in the treat-to-target strategy group compared with 8.7% in the high-intensity statin therapy group, a difference that met the prespecified noninferiority margin of 3.0 percentage points.
Meaning
Among patients with coronary artery disease, the treat-to-target LDL-C strategy was noninferior to the high-intensity statin strategy for major clinical outcomes.
Abstract
Importance
In patients with coronary artery disease, some guidelines recommend initial statin treatment with high-intensity statins to achieve at least a 50% reduction in low-density lipoprotein cholesterol (LDL-C). An alternative approach is to begin with moderate-intensity statins and titrate to a specific LDL-C goal. These alternatives have not been compared head-to-head in a clinical trial involving patients with known coronary artery disease.
Objective
To assess whether a treat-to-target strategy is noninferior to a strategy of high-intensity statins for long-term clinical outcomes in patients with coronary artery disease.
Design, Setting, and Participants
A randomized, multicenter, noninferiority trial in patients with a coronary disease diagnosis treated at 12 centers in South Korea (enrollment: September 9, 2016, through November 27, 2019; final follow-up: October 26, 2022).
Interventions
Patients were randomly assigned to receive either the LDL-C target strategy, with an LDL-C level between 50 and 70 mg/dL as the target, or high-intensity statin treatment, which consisted of rosuvastatin, 20 mg, or atorvastatin, 40 mg.
Main Outcomes and Measures
Primary end point was a 3-year composite of death, myocardial infarction, stroke, or coronary revascularization with a noninferiority margin of 3.0 percentage points.
Results
Among 4400 patients, 4341 patients (98.7%) completed the trial (mean [SD] age, 65.1 [9.9] years; 1228 females [27.9%]). In the treat-to-target group (n = 2200), which had 6449 person-years of follow-up, moderate-intensity and high-intensity dosing were used in 43% and 54%, respectively. The mean (SD) LDL-C level for 3 years was 69.1 (17.8) mg/dL in the treat-to-target group and 68.4 (20.1) mg/dL in the high-intensity statin group (n = 2200) (P = .21, compared with the treat-to-target group). The primary end point occurred in 177 patients (8.1%) in the treat-to-target group and 190 patients (8.7%) in the high-intensity statin group (absolute difference, –0.6 percentage points [upper boundary of the 1-sided 97.5% CI, 1.1 percentage points]; P < .001 for noninferiority).
Conclusions and Relevance
Among patients with coronary artery disease, a treat-to-target LDL-C strategy of 50 to 70 mg/dL as the goal was noninferior to a high-intensity statin therapy for the 3-year composite of death, myocardial infarction, stroke, or coronary revascularization. These findings provide additional evidence supporting the suitability of a treat-to-target strategy that may allow a tailored approach with consideration for individual variability in drug response to statin therapy.
Trial Registration
ClinicalTrials.gov Identifier: NCT02579499
This randomized clinical trial compares the efficacy of a treat-to-target low-density lipoprotein cholesterol (LDL-C) strategy of 50 to 70 mg/dL as the goal vs high-intensity statin therapy for the 3-year composite of death, myocardial infarction, stroke, or coronary revascularization in patients with coronary artery disease.
Introduction
Patients with coronary artery disease (CAD) are considered to be at high risk or very high risk for future adverse cardiovascular events.1,2 For this patient population, intensive reduction of low-density lipoprotein cholesterol (LDL-C) levels via 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitor (statin) therapy is recommended by meta-analyses, which have shown an association between absolute LDL-C level reduction with statins and a proportional reduction in major vascular events.1,2,3,4 For the management of LDL-C, some guidelines recommend initial statin treatment with high-intensity statins to achieve at least a 50% reduction in LDL-C levels.1,5 High-intensity or maximally tolerated intensity can be maintained without a target goal.1,5 The use of high-intensity statin might be simple because it reduces the need to adjust statin intensity according to follow-up of LDL-C levels, but it raises concerns about individual variability in drug response and the adverse effects of long-term use of high-intensity statins.6 An alternative approach is to begin with moderate-intensity statins and titrate to a specific LDL-C goal.2,7,8,9 This treat-to-target strategy could allow a tailored approach and facilitate patient-physician communication, which can enhance adherence to therapy. However, that strategy has not been well evaluated in randomized clinical trials and thus lacks sufficient evidence.2,7,8,9 Furthermore, these alternatives have not been compared head-to-head in a clinical trial involving patients with known CAD.
In the LODESTAR (Low-Density Lipoprotein Cholesterol-Targeting Statin Therapy Versus Intensity-Based Statin Therapy in Patients With Coronary Artery Disease) Trial, it was hypothesized that high-intensity statin therapy would be less needed in a treat-to-target strategy compared with a high-intensity statin strategy. Consequently, it could be advantageous in regard to the safety concerns related to the long-term use of high-intensity statin therapy if equally effective. Therefore, the noninferiority of the treat-to-target strategy, with an LDL-C level between 50 and 70 mg/dL as the target, compared with a high-intensity statin strategy on 3-year clinical outcomes in patients with CAD was evaluated.
Methods
Study Design
This trial was an investigator-initiated, multicenter, randomized, open-label, noninferiority trial conducted at 12 centers in South Korea. The trial protocol was approved by the institutional review board at each participating center. The study was performed according to the principles of the Declaration of Helsinki. The study protocol, statistical analysis plan, and summary of their changes are available in Supplement 1. There were no preplanned trial discontinuation rules. The data and safety monitoring board (DSMB) responsible for ensuring participant safety acted in an advisory capacity to monitor patient safety, evaluate study progress, and review the study process. For safety monitoring, adverse events were centrally collected, the DSMB reviewed the blinded safety data, and the DSMB statistician provided unblinded summary tables. The DSMB discussed outcomes and safety data and determined whether early stopping was needed for benefit or harm. Study coordination, data management, and site management services were performed at the Cardiovascular Research Center (Seoul, South Korea). Designated trial monitors reviewed the investigational data at appropriate intervals to ensure their accuracy, completeness, and adherence to the protocol.
Study Population
Patients with clinically diagnosed CAD, including stable ischemic heart disease or acute coronary syndrome (unstable angina, acute myocardial infarction), were enrolled (Figure 1). Details about the inclusion and exclusion criteria are provided in eTable 1 in Supplement 2. All participants provided written informed consent.
Figure 1. Recruitment, Randomization, and Follow-up in a Trial of Treat-to-Target Strategy or High-Intensity Statin Therapy for Coronary Artery Disease.
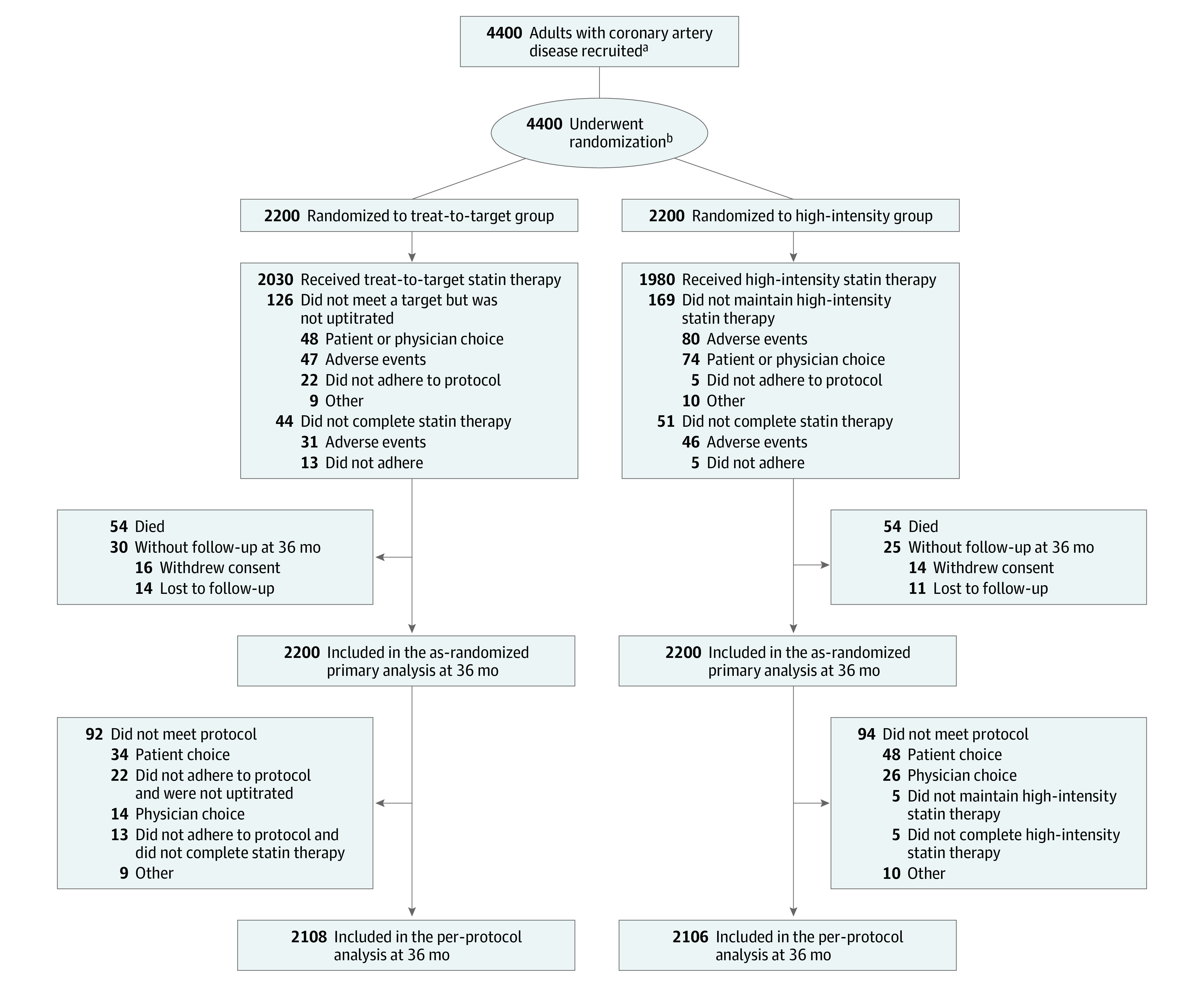
aData regarding screening were not collected.
bRandomization was stratified by baseline low-density lipoprotein cholesterol levels of 100 mg/dL or greater, acute coronary syndrome, and the presence of diabetes.
Randomization and Study Procedures
Eligible patients were randomized in a 1:1 manner to receive a statin using either the targeted strategy of titrated-intensity statin therapy (treat-to-target) or the strategy of high-intensity statin therapy. Web-response permuted-block randomization (mixed blocks of 4 or 6) was used at each participating site to allocate the patients, who were stratified by baseline LDL-C levels of 100 mg/dL or greater, acute coronary syndrome, and the presence of diabetes. Furthermore, each group of patients was secondarily randomized in a 1:1 manner to receive 1 of 2 statins, rosuvastatin or atorvastatin (once daily). The allocation sequence was computer-generated by an external programmer not involved in the trial, and physicians or research coordinators accessed the web-response system. To convert cholesterol to mmol/L, multiply by 0.0259.
The intensity of statin treatment was divided into high or moderate intensity according to the 2013 American College of Cardiology/American Heart Association guideline for the treatment of blood cholesterol.5 Patients were treated with rosuvastatin, 10 mg, or atorvastatin, 20 mg, for the moderate-intensity statin therapy and rosuvastatin, 20 mg, or atorvastatin, 40 mg, for the high-intensity statin therapy. In the treat-to-target group, the target LDL-C level chosen was the lowest recommended LDL-C level for our population in the latest guidelines at the time of trial design (August 2015),7,8,10 which was below 70 mg/dL, and the statin intensity was titrated as follows. For statin-naive patients, moderate-intensity statin therapy was initiated. For those who were already taking a statin, an equivalent intensity was maintained when the LDL-C level at randomization was below 70 mg/dL, and the intensity was uptitrated when the LDL-C level was 70 mg/dL or greater. During follow-up, in the treat-to-target group, uptitration for those with an LDL-C level of 70 mg/dL or greater, maintenance of the same intensity for those with an LDL-C level of 50 mg/dL or greater to less than 70 mg/dL, and downtitration for those with an LDL-C level less than 50 mg/dL was performed. In the high-intensity statin group, the maintenance of high-intensity statin therapy was recommended without adjustment regardless of follow-up LDL-C levels during the study period. However, given the adherence, tolerance, and clinical situations of individual patients, up- or downtitration of statin intensity not following the study protocol may have been exceptionally allowed in both groups at the physician’s discretion, but those changes required a detailed report of reasons. Nonstatin add-on therapy, such as ezetimibe, was not recommended strongly in the treat-to-target group, even when the target was not achieved with high-intensity statin therapy, to focus on the strategy for choosing statin intensity and avoid confounding by any imbalance in their use. For other medical treatments, guideline-directed medical therapy was strongly recommended, and it was also allowed to be changed by a personal physician, except for the change in the statin intensity. Risk factor modification, including blood pressure or glucose control, dietary changes, weight reduction, exercise, and smoking cessation, was encouraged.
Clinical and laboratory findings were assessed at baseline, and all patients were scheduled for follow-up visits at 6 weeks and 3, 6, 12, 24, and 36 months. General health status, use of drugs, and the occurrence of clinical end points or adverse events were assessed at baseline and each follow-up visit. Serial follow-up of lipid profiles, including total cholesterol, LDL-C, high-density lipoprotein cholesterol, and triglyceride levels, was performed at 6 weeks and 12, 24, and 36 months. When the dose or type of study medication was changed during follow-up, patients were recommended to present for a laboratory test within 4 to 6 weeks in both groups. To monitor adverse effects related to the statin therapy, plasma glucose, aspartate aminotransferase, alanine aminotransferase, creatinine, and creatine kinase levels were assessed at 6 weeks and 12, 24, and 36 months. Hemoglobin A1c was assessed at 12, 24, and 36 months.
Study End Point
The primary end point was major adverse cardiac and cerebrovascular events, defined as a composite of all-cause death, myocardial infarction (MI), stroke, and any coronary revascularization at 3 years. Death was classified as cardiovascular death and noncardiovascular death. Cardiovascular death was defined as death due to MI, sudden cardiac death, heart failure, stroke, cardiovascular procedures, cardiovascular hemorrhage, and any death in which a cardiovascular cause could not be excluded, as adjudicated by the clinical end points committee.11 MI was defined based on clinical symptoms, electrocardiographic changes, or abnormal findings during imaging studies, combined with an increase in the creatine kinase myocardial band fraction above the upper normal limit or an increase in the troponin-T or troponin-I level greater than the 99th percentile of the upper normal limit.12 Stroke was defined as an acute cerebrovascular event resulting in a neurologic deficit for longer than 24 hours or the presence of an acute infarction in imaging studies.13 Any coronary revascularization included percutaneous coronary intervention or coronary artery bypass graft surgery.11 Clinically indicated revascularization was defined as an invasive angiographic percent diameter stenosis of 50% or greater with ischemic symptoms or signs or a percent diameter stenosis of 70% or greater even in the absence of symptoms or signs.11 Staged coronary revascularizations planned at randomization were not considered as adverse events.
Secondary end points were the occurrence of (1) new-onset diabetes, (2) hospitalization due to heart failure, (3) deep vein thrombosis or pulmonary thromboembolism, (4) endovascular revascularization for peripheral artery disease, (5) aortic intervention or surgery, (6) end-stage kidney disease, (7) discontinuation of study drugs due to intolerance, (8) cataract operation, and (9) composite of laboratory abnormalities. Each secondary end point is defined in the eAppendix in Supplement 2. An independent clinical end point committee blinded to the therapy assignment and the primary results of the trial before the locking of the database was responsible for categorizing each clinical event.
Sample Size Calculation
According to the latest guideline at the time of trial design and several randomized trials demonstrating the superiority of fixed-dose high-intensity statin therapy to fixed-dose moderate-intensity statin therapy in patients with CAD,5,14,15,16 the high-intensity statin approach was regarded as the standard therapy. The treat-to-target strategy, which had not been evaluated in randomized trials for our population, was considered to be the experimental therapy. Our aim was to determine whether a treat-to-target strategy was noninferior to a high-intensity statin strategy in terms of the 3-year occurrence of the primary end point in all participants as randomized. Based on previous studies that compared different statin intensities in patients with CAD, the expected event rate of the primary end point was 4% per year in the high-intensity statin group.14,17 Assuming that the 2 strategies had equivalent efficacy, the expected event rate of the primary end point at 3 years was estimated to be 12% in each group. A noninferiority margin of 3.0 percentage points was primarily chosen with a consideration that this was not clinically different between the 2 groups. A total of 3686 patients was required, with a 2.5% 1-sided α error rate and 80% power. Considering a 15% loss to follow-up and balancing the 2 types of statins (rosuvastatin and atorvastatin), a total of 4400 patients (2200 patients in each group) was required.
Statistical Analyses
Categorical data are presented as numbers (percentages). Continuous data are presented as means (SDs) and medians (IQRs) for normal and skewed distributions, respectively. The cumulative incidence of the primary end point at 3 years was estimated using Kaplan-Meier curves for a time-to-event analysis from the time of randomization to the occurrence of the first event of interest during follow-up. The test of noninferiority was performed for the primary end point using the Com-Nougue approach to estimating the z statistic for the Kaplan-Meier failure rates with the Greenwood formula for estimating the standard error. It was predetermined that noninferiority would be declared if the upper boundary of the 1-sided 97.5% CI for the event rate difference was less than 3.0 percentage points.
The primary analysis was performed with all participants randomly assigned to a treatment group and after excluding participants who did not receive the allocated therapy (participants who discontinued statin therapy, those who did not undergo uptitration despite the nonachievement of the goal in the treat-to-target group, or who did not maintain high-intensity statin treatment or added nonstatin drug to moderate- or low-intensity statin treatment in the high-intensity statin group). Prespecified subgroup analyses were performed for clinically relevant factors: age, sex, body mass index, hypertension, diabetes, chronic kidney disease, clinical presentation at randomization, and baseline LDL-C levels. Data regarding drug use were collected by the record of the physicians’ prescription. Study drug adherence was measured by self-reported pill count.
Data were collected and analyzed according to the predefined statistical analysis plan. No imputation was used to infer missing values. Those with missing data for primary or secondary end points were censored at the time of withdrawal of consent or loss to follow-up. All analyses were conducted using SAS version 9.2 (SAS Institute). All tests were 2-sided except for the noninferiority test. A P value <.05 was considered statistically significant.
Results
Between September 9, 2016, and November 27, 2019, 4400 participants with CAD were randomly assigned to receive either the treat-to-target strategy (n = 2200) or the high-intensity statin therapy (n = 2200) (Figure 1). The baseline characteristics of the participants did not differ between the groups (Table 1). At randomization, 74% of participants were more than 1 year beyond their initial diagnosis or coronary revascularization. Before randomization, 25% and 57% were taking a high-intensity statin and a moderate-intensity statin, respectively. Among the 4400 participants, 4341 participants (98.7%) completed the 3-year clinical follow-up. The total person-years of follow-up was 6449 in the treat-to-target group and 6461 in the high-intensity statin therapy group. For the overall study period, in the treat-to-target group, statin intensity was uptitrated in 378 participants (17%), downtitrated in 208 patients (9%), and maintained without changes in 1614 participants (73%) (eTable 2 in Supplement 2). In the treat-to-target group, 53% were taking the high-intensity statin at 1 year, 55% at 2 years, and 56% at 3 years; the corresponding rates in the high-intensity statin therapy group were 93%, 91%, and 89%, respectively (Figure 2A; eTables 3 and 4 in Supplement 2). For the overall study period in the treat-to-target group, 43% and 54% received moderate-intensity statin and high-intensity statin, respectively (eTable 3 in Supplement 2). Ezetimibe was used more in the treat-to-target group than in the high-intensity statin therapy group from 6 months, mostly as a combination therapy with high-intensity statin therapy (Figure 2B; eTable 4 in Supplement 2). Other cardiovascular medications did not differ statistically between the groups during the study period (eTable 5 in Supplement 2).
Table 1. Baseline Characteristics in the Study Population of the LODESTAR Trial.
| No. (%) | ||
|---|---|---|
| Treat-to-target group (n = 2200) | High-intensity statin group (n = 2200) | |
| Age, mean (SD), y | 65 (10) | 65 (10) |
| Sex | ||
| Female | 626 (29) | 602 (27) |
| Male | 1574 (72) | 1598 (73) |
| Weight, mean (SD), kg | 67 (11) | 67 (11) |
| Height, mean (SD), cm | 164 (8) | 165 (8) |
| Body mass index, mean (SD)a | 24.7 (2.9) | 24.7 (2.9) |
| Medical historyb | ||
| Hypertension | 1473 (67) | 1464 (67) |
| Diabetes | 735 (33) | 733 (33) |
| Diabetes with insulin treatment | 81 (4) | 81 (4) |
| Chronic kidney disease | 153 (7) | 166 (8) |
| End-stage kidney disease on dialysis | 13 (1) | 16 (1) |
| Previous PCI | 1243 (57) | 1214 (55) |
| Previous CABG | 154 (7) | 180 (8) |
| Previous stroke | 135 (6) | 128 (6) |
| Current smoking | 303 (14) | 300 (14) |
| Estimated glomerular filtration rate, mean (SD), mL/min/1.73 m2 | 88 (17) | 88 (18) |
| Lipid levels, mean (SD), mg/dLc | ||
| Low-density lipoprotein cholesterol | 86 (33) | 87 (31) |
| High-density lipoprotein cholesterol | 47 (12) | 47 (12) |
| Total cholesterol | 156 (38) | 157 (37) |
| Triglyceride | 138 (83) | 137 (83) |
| Clinical presentation at randomization | ||
| Acute myocardial infarction within 1 y | 159 (7) | 179 (8) |
| >1 y after myocardial infarction | 338 (15) | 337 (15) |
| Unstable angina or revascularization within 1 y | 381 (17) | 407 (19) |
| >1 y after unstable angina or revascularization | 910 (41) | 874 (40) |
| Detection of CAD at screening without symptoms | 412 (19) | 403 (18) |
| Lipid-lowering therapy before randomization | ||
| Statind | ||
| High intensity | 529 (24) | 576 (26) |
| Moderate intensity | 1284 (58) | 1240 (56) |
| Low intensity | 53 (2) | 40 (2) |
| None | 334 (16) | 334 (16) |
| Ezetimibe | 253 (12) | 226 (10) |
Abbreviations: CABG, coronary artery bypass grafting; CAD, coronary artery disease; LODESTAR, Low-Density Lipoprotein Cholesterol-Targeting Statin Therapy Versus Intensity-Based Statin Therapy in Patients With Coronary Artery Disease; PCI, percutaneous coronary intervention.
Calculated as weight in kilograms divided by height in meters squared.
History was collected by self-report except chronic kidney disease. Chronic kidney disease was defined as estimated glomerular filtration of less than 60 mL/min/1.73 m2 of body surface area.
Reference values may vary based on laboratory and location. To convert cholesterol to mmol/L, multiply by 0.0259; and triglyceride to mmol/L, multiply by 0.0113.
The intensity of statin treatment was divided according to the 2013 American College of Cardiology/American Heart Association guideline for the treatment of blood cholesterol.
Figure 2. Lipid-Lowering Therapy During the Study Period.
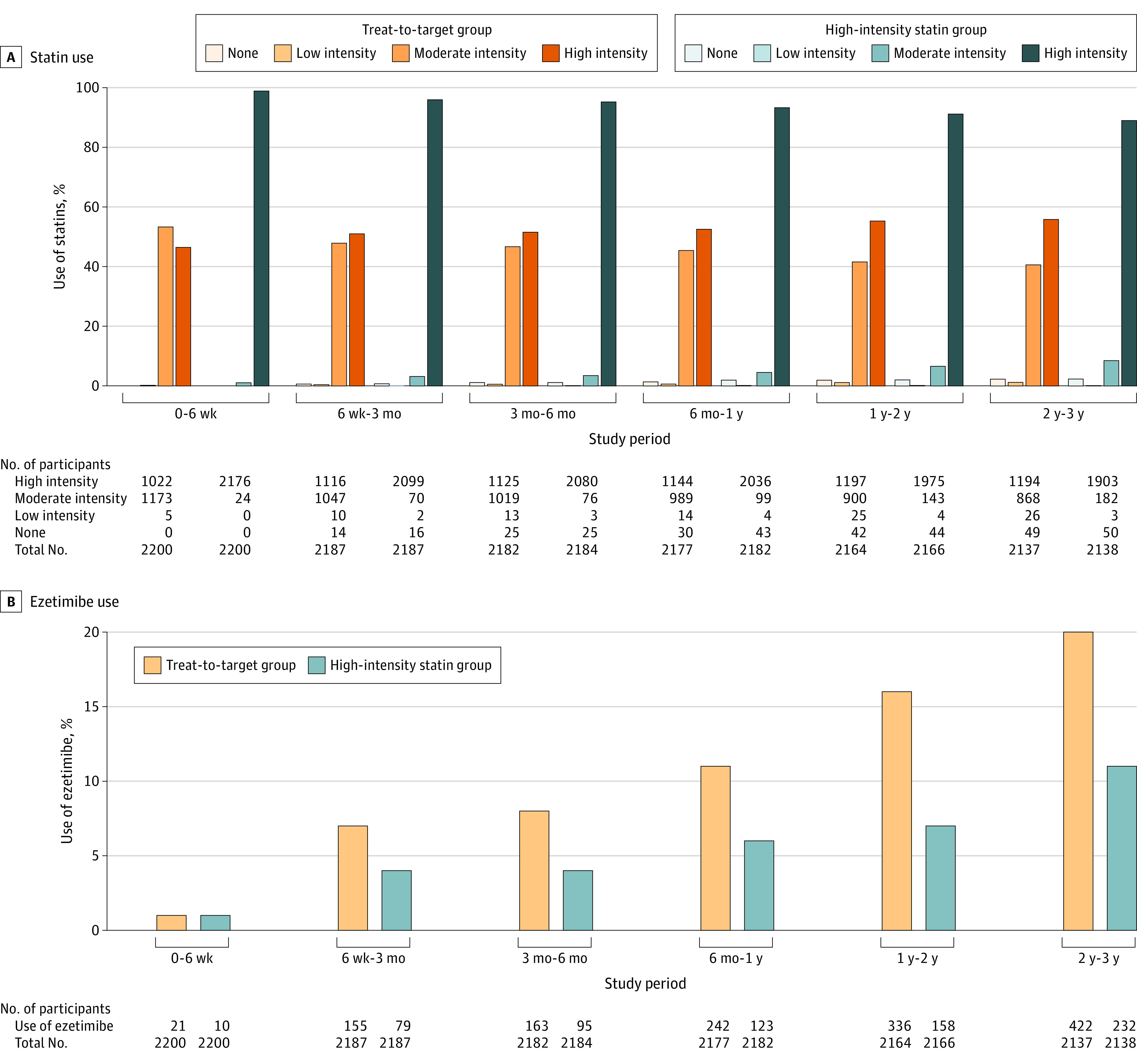
The changes in LDL-C level during study period are presented in Figure 3A and eTable 6 in Supplement 2. At 6 weeks, the mean (SD) LDL-C level was significantly higher in the treat-to-target group than the high-intensity statin therapy group (69.6 [21.2] mg/dL vs 66.8 [21.8] mg/dL; difference, 2.8 mg/dL [95% CI, 1.3 to 4.3]; P < .001). After 6 weeks, the LDL-C levels did not differ between the groups. During the overall study period, the mean (SD) LDL-C level was 69.1 (17.8) mg/dL in the treat-to-target group and 68.4 (20.1) mg/dL in the high-intensity statin therapy group, which was not a significant difference (P = .21). The proportion of participants with an LDL-C level below 70 mg/dL, which was the goal for the treat-to-target group, was 55.7% at 6 weeks, 59.2% at 3 months, 57.7% at 6 months, 55.7% at 1 year, 60.8% at 2 years, and 58.2% at 3 years (eTable 7 in Supplement 2). This proportion was significantly lower in the treat-to-target group than the high-intensity statin therapy group at 6 weeks and 3 months (eTable 7 in Supplement 2). The changes in the other lipid profiles are also presented in eFigure 1 and eTable 6 in Supplement 2.
Figure 3. Changes in LDL-C Levels and Kaplan-Meier Curves for the Primary End Pointa.
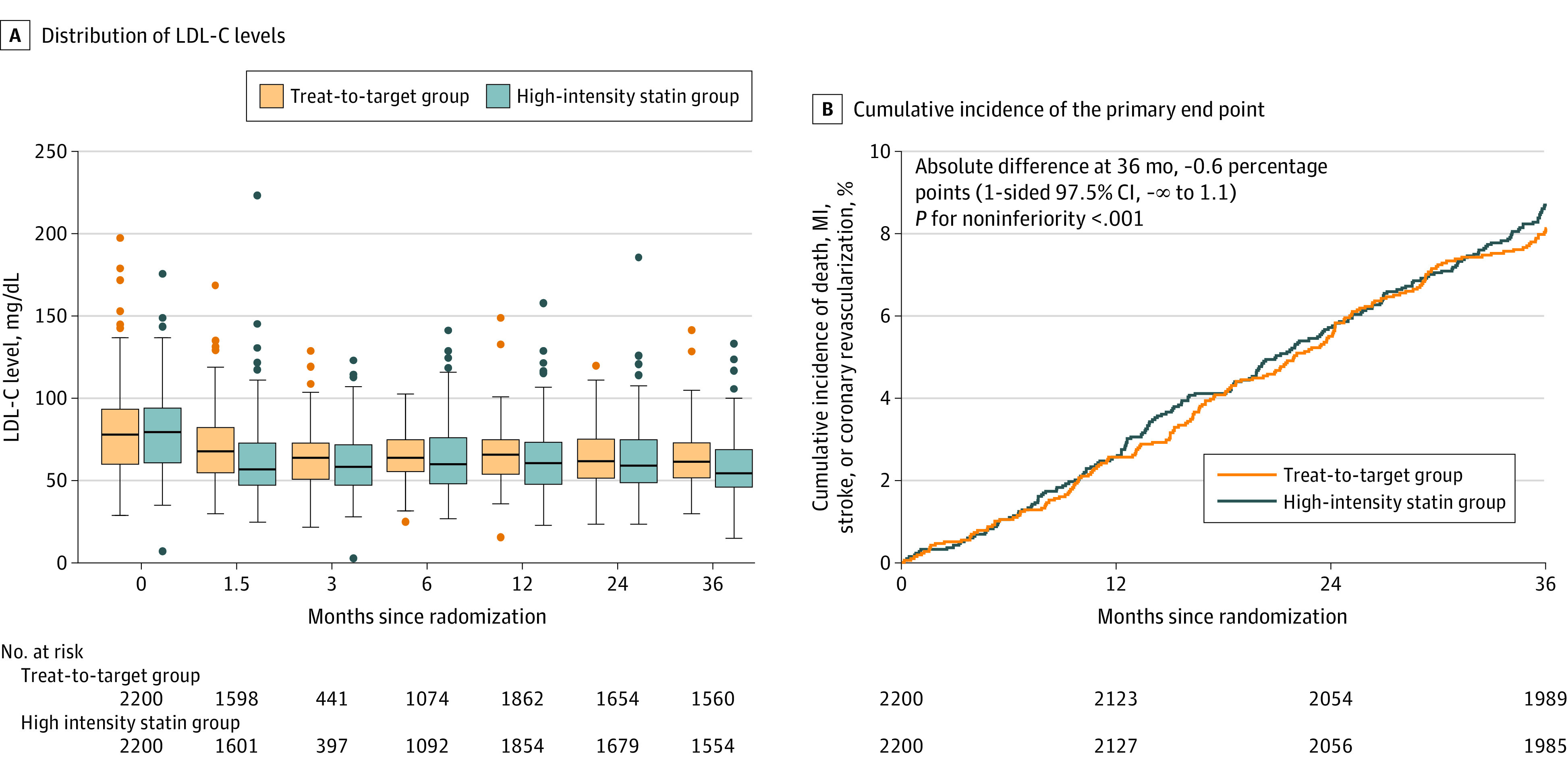
In panel A, the middle lines in the box plots represent the median values, boxes represent the IQRs, whiskers extend to the most extreme observed values with 1.5 × the IQR of the nearer quartile, and dots represent observed values outside that range. Reference values may vary based on laboratory and location. LDL-C indicates low-density lipoprotein cholesterol; MI, myocardial infarction.
aChanges in total cholesterol, triglyceride, and high-density lipoprotein cholesterol levels over time are also presented in eFigure 1 in Supplement 2.
The primary end point occurred in 177 participants (8.1%) in the treat-to-target group and 190 participants (8.7%) in the high-intensity statin therapy group (absolute difference, −0.6 percentage points [upper boundary of the 1-sided 97.5% CI, 1.1 percentage points]; P < .001 for noninferiority) (Table 2, Figure 3B, and eFigure 2 in Supplement 2). All-cause death occurred in 54 participants (2.5%) in the treat-to-target group and 54 (2.5%) in the high-intensity statin therapy group (absolute difference, <0.1% [95% CI, −0.9% to 0.9%]; P = .99). MIs were observed in 34 participants (1.6%) and 26 participants (1.2%), respectively (absolute difference, 0.4% [95% CI, −0.3% to 1.1%]; P = .23). The occurrence of stroke did not differ statistically between the groups either (0.8% vs 1.3%; absolute difference, −0.5% [95% CI, −1.1% to 0.1%]; P = .13) (Table 2). This finding was consistent in the per-protocol population (eTables 8 and 9 in Supplement 2). The primary end point occurred in 8.3% of the treat-to-target group and 8.5% of the high-intensity statin therapy group (absolute difference, −0.2 percentage points [upper boundary of the 1-sided 97.5% CI, 1.5 percentage points]; P < .001 for noninferiority) (eFigure 2 and eTable 9 in Supplement 2).
Table 2. Primary and Secondary End Points at 3 Years After Randomizationa.
| Outcome | Patients, No. (%) | Absolute difference, % (95% CI)b | P value | |
|---|---|---|---|---|
| Treat-to-target group (n = 2200) | High-intensity statin group (n = 2200) | |||
| Primary end point | ||||
| Death, myocardial infarction, stroke, or coronary revascularization | 177 (8.1) | 190 (8.7) | −0.6 (−∞ to 1.1)c | <.001d |
| Components of primary end point | ||||
| Death | 54 (2.5) | 54 (2.5) | <0.1 (−0.9 to 0.9) | .99 |
| Cardiac death | 16 | 13 | ||
| Myocardial infarction | 34 (1.6) | 26 (1.2) | 0.4 (−0.3 to 1.1) | .23 |
| Stroke | 17 (0.8) | 27 (1.3) | −0.5 (−1.1 to 0.1) | .13 |
| Ischemic | 12 | 20 | ||
| Hemorrhagic | 5 | 7 | ||
| Coronary revascularizatione | 112 (5.2) | 114 (5.3) | −0.1 (−1.4 to 1.2) | .89 |
| Secondary end points | ||||
| New-onset diabetes | 121 (5.6) | 150 (7.0) | −1.3 (−2.8 to 0.1) | .07 |
| Initiation of antidiabetic medication | 73 | 105 | ||
| Cataract operation | 43 (2.0) | 42 (1.9) | 0.1 (−0.8 to 0.9) | .90 |
| Discontinuation of statin therapy | 31 (1.5) | 46 (2.2) | −0.7 (−1.5 to 0.1) | .09 |
| Composite of laboratory abnormalitiesf | 18 (0.8) | 30 (1.3) | −0.5 (−1.1 to 0.1) | .11 |
| Aminotransferase elevation | 8 | 12 | ||
| Creatine kinase elevation | 3 | 8 | ||
| Creatinine elevation | 7 | 11 | ||
| Peripheral artery revascularization | 12 (0.6) | 17 (0.8) | −0.2 (−0.8 to 0.3) | .35 |
| Hospitalization due to heart failure | 13 (0.6) | 7 (0.3) | 0.3 (−0.1 to 0.7) | .17 |
| End-stage kidney disease | 3 (0.1) | 10 (0.5) | −0.3 (−0.7 to 0.0) | .05 |
| Deep vein thrombosis or pulmonary embolism | 4 (0.2) | 5 (0.2) | <0.1 (−0.3 to 0.2) | .74 |
| Deep vein thrombosis | 2 | 5 | ||
| Pulmonary embolism | 3 | 0 | ||
| Aortic intervention or surgery | 2 (0.1) | 3 (0.1) | NR | |
| Endovascular therapy | 1 | 2 | ||
| Surgical therapy | 1 | 1 | ||
| Composite of new-onset diabetes, aminotransferase or creatine kinase elevation, or end-stage kidney disease (post hoc) | 132 (6.1) | 177 (8.2) | −2.1 (−3.6 to −0.5) | .009 |
Abbreviation: NR, not reported.
Primary and secondary end points were evaluated as randomized 3 years after randomization. The listed percentages were estimated using the Kaplan-Meier method, so the values might not calculate mathematically. Differences in event rates are not reported for aortic intervention because of the low numbers of events.
The between-group difference was measured in the treat-to-target group compared with the high-intensity statin group. The widths of the confidence intervals have not been adjusted for multiplicity and cannot be used to infer treatment effects.
A 1-sided 97.5% CI was calculated for the primary end point.
The P value for noninferiority is for an upper boundary of the 97.5% CI of the between-group difference in the primary end point, which was 1.1 percentage points. Other P values were 2-sided.
All coronary revascularizations were clinically indicated by an invasive angiographic percent diameter stenosis of 50% or greater with ischemic symptoms or signs or 70% or greater even without symptoms or signs.
Aminotransferase elevation was defined as greater than baseline level and more than 3 times the upper limit of reference. Creatine kinase elevation was defined as greater than baseline level and more than 5 times the upper limit of reference. Creatinine level elevation was defined as greater than 50% increase from baseline and greater than the upper limit of reference. Reference values may vary based on laboratory and location.
No prespecified secondary end points differed statistically between the groups (Table 2). However, as a post hoc secondary end point, a composite of new-onset diabetes, aminotransferase or creatine kinase elevation, or end-stage kidney disease was significantly lower in the treat-to-target group vs the high-intensity statin group (6.1% vs 8.2%; absolute difference, −2.1% [95% CI −3.6% to −0.5%]; P = .009). The reasons for discontinuing statin therapy are described in eTable 10 in Supplement 2. Those findings were consistent in the per-protocol population (eTable 9 in Supplement 2). The results of the prespecified subgroup analyses are provided in eFigure 3 in Supplement 2. The effect of the treat-to-target strategy vs the high-intensity statin therapy was consistent for the primary end point across all subgroups.
Discussion
In this multicenter, randomized clinical trial involving patients with CAD, the treat-to-target strategy of 50 to 70 mg/dL as an LDL-C goal was noninferior to the high-intensity statin therapy in terms of the 3-year composite of all-cause death, MI, stroke, or any coronary revascularization. In the treat-to-target group, the mean LDL-C level was higher at 6 weeks compared with the high-intensity statin therapy group; however, it did not differ after 6 weeks. A lower proportion (around 60%) of participants with an LDL-C level below 70 mg/dL in this study might be explained by relatively low implementation of combination therapy, such as ezetimibe to high-intensity statin therapy, possibly due to (1) absence of recommendation of combination therapy in the study protocol because the purpose of this trial was to focus on the management of statin therapy alone; (2) a combination therapy was not frequently used in the initial period of this trial (September 2016), but it has been recommended in recent guidelines with much evidence and has been increasingly used; and (3) patients’ reluctance to add more drugs to their high-intensity statin regimen to manage LDL-C levels.
Clinical guidelines1,2,5,7,8,9 for choosing statin intensity selectively have recommended 2 strategies: (1) a strategy with a target LDL-C level or (2) a strategy that begins with high-intensity statin treatment without a predefined LDL target. Though both strategies are widely accepted and used in current clinical practice, they have not been directly compared for their effectiveness or safety. Furthermore, the treat-to-target strategy has not been well evaluated in randomized trials, particularly in patients with CAD, though the recent TST (Treat Stroke to Target) and EMPATHY (Standard vs Intensive Statin Therapy for Hypercholesterolemic Patients With Diabetic Retinopathy) trials compared target LDL-C levels.18,19 In the TST trial, a target LDL-C level of less than 70 mg/dL was compared with a target of 90 to 110 mg/dL in patients with an ischemic stroke, and a greater reduction in a composite of cardiovascular events was observed in the lower-target group.18 In the EMPATHY trial, a target LDL-C level of less than 70 mg/dL was compared with a target of 100 to 120 mg/dL in patients with hypercholesterolemia, diabetic retinopathy, and no history of CAD. The study found no significant differences in cardiovascular events, possibly because of low between-group differences in LDL-C levels.19 Along with those 2 trials, the current findings add evidence supporting the suitability of the treat-to-target strategy. A lower use of high-intensity statin in those in the treat-to-target group compared with the high-intensity statin therapy group (54% vs 92%) indicated that the treat-to-target strategy was a tailored approach that accounted for individual variability in therapeutic response to statin therapy.20,21 Patients with a good therapeutic response to a statin might not need a high-intensity dose. In addition, the current findings of a numerically lower rate of secondary end points (new-onset diabetes, end-stage kidney disease, or composite of laboratory abnormalities) and a significantly lower rate of composite of secondary end points (number needed to harm = 48 patients) may favor the treat-to-target strategy in regard to the safety issues. There are concerns about discontinuation or nonadherence to statin therapy, especially to high-intensity statin therapy, due to statin-associated muscle symptoms as well as new-onset type 2 diabetes, hepatotoxicity, and kidney toxicity.22 A recent study with 50 928 National Health and Nutrition Examination Survey participants also showed that LDL-C levels have plateaued, and high-intensity statin use has failed to grow significantly most likely due to perceived safety concerns since the 2013 American College of Cardiology/American Heart Association guideline adopted a fixed-dose strategy rather than a treat-to-goal recommendation.23
With regard to the treat-to-target strategy, however, the following concerns need to be considered. First, the validity of the target for those with CAD needs to be confirmed. The goal in the latest European guideline has been lowered to below 55 mg/dL.2 Second, these findings highlight the need for intensive efforts to attain the target LDL-C level. In the treat-to-target group, the proportion who met the target was 56% at 1 year, 61% at 2 years, and 58% at 3 years. Those numbers are attributed to the relatively low use of nonstatin add-on therapy such as ezetimibe (20% in the treat-to-target group and 11% in the high-intensity statin group at 3 years), though recent guidelines strongly recommend its use with a target or threshold.1,2 The EMPATHY trial, which did not show a decrease in cardiovascular events at the lower target, also suggested the importance of target attainment; only 29% in the lower-target group actually met the target.19 Third, the mean LDL-C level was higher in the treat-to-target group early in the study period, most likely due to the time required for titration. In the current trial, although the protocol mandated that moderate-intensity therapy be initiated regardless of baseline LDL-C levels in statin-naive patients, a target LDL-C level below 70 mg/dL might have been achieved earlier if high-intensity therapy had been initiated when the baseline LDL-C level was greater than 100 mg/dL because the lower bound expected reduction in LDL-C level with a moderate-intensity dose is 30%. This trial included relatively low-risk patients with CAD; 74% of them participated in the trial more than 1 year after their initial diagnosis or coronary revascularization, but the initial intensity needs to be selected according to the baseline LDL-C levels, as well as the expected degree of LDL-C level reduction across intensity levels, particularly in patients who need to achieve their target rapidly.24,25 A recent Swedish nationwide cohort study showed that early LDL-C level reduction after MI was associated with reduced cardiovascular outcomes and all-cause mortality during follow-up.24
Limitations
This trial has several limitations. First, this trial was open label. However, an independent committee blinded to therapy assignment adjudicated all clinical events and assessed the clinical end points. Second, lower event rates than anticipated were observed, which might mean that the fixed noninferiority margin of 3.0 percentage points allowed for an overly generous CI for the hazard ratio and that this study was underpowered. Third, the comparison of individual clinical outcomes within the primary end point was difficult because of the small number of events. Fourth, given that this trial recruited exclusively patients with CAD, a strategic comparison of results from other subsets of patients, such as those indicated to statin therapy for primary prevention, might be necessary. Fifth, implementation of the strategy was not complete, with only approximately 60% in the treat-to-target strategy group achieving an LDL-C level below 70 mg/dL. This may imply that nonstatin add-on therapy should be actively considered in a significant proportion of the patients who cannot achieve sufficient reduction in LDL-C levels by using statin monotherapy. Sixth, the follow-up period was 3 years in this trial, which may be relatively short to reflect longer-term effects of 2 strategies.
Conclusions
Among patients with CAD, a treat-to-target LDL-C strategy of 50 to 70 mg/dL as the goal was noninferior to a high-intensity statin therapy for the 3-year composite of death, MI, stroke, or coronary revascularization. These findings provide additional evidence supporting the suitability of a treat-to-target strategy that may allow a tailored approach with consideration for individual variability in drug response to statin therapy.
Trial Protocol
eAppendix. Supplementary Methods for Definition of Secondary Endpoints
Table 1. Inclusion and Exclusion Criteria
eTable 2. Changes in Statin Intensity in the Treat-to-Target Strategy Group
eTable 3. Statin Intensity Between Groups
eTable 4. Lipid-Lowering Therapy
eTable 5. Cardiovascular Medications
eTable 6. Serial Changes in Lipid Profiles
eTable 7. Patients With LDL-C Below 70 mg/dL
eTable 8. Patients Excluded From The Per-Protocol Population
eTable 9. Primary and Secondary Endpoints in the Per-Protocol Population
eTable 10. Reasons for Discontinuing Statin Therapy by Adverse Effect
eFigure1. Lipid Profile During Study Period
eFigure 2. Treatment Difference for the Primary Endpoint
eFigure 3. Subgroup Analyses for the Primary Endpoint
Nonauthor Collaborators. LODESTAR Investigators
Data Sharing Statement
References
- 1.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1082-e1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mach F, Baigent C, Catapano AL, et al. ; ESC Scientific Document Group . 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111-188. doi: 10.1093/eurheartj/ehz455 [DOI] [PubMed] [Google Scholar]
- 3.Silverman MG, Ference BA, Im K, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA. 2016;316(12):1289-1297. doi: 10.1001/jama.2016.13985 [DOI] [PubMed] [Google Scholar]
- 4.Baigent C, Blackwell L, Emberson J, et al. ; Cholesterol Treatment Trialists’ (CTT) Collaboration . Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670-1681. doi: 10.1016/S0140-6736(10)61350-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stone NJ, Robinson JG, Lichtenstein AH, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25)(suppl 2):S1-S45. doi: 10.1161/01.cir.0000437738.63853.7a [DOI] [PubMed] [Google Scholar]
- 6.Smith SC Jr, Grundy SM. 2013 ACC/AHA guideline recommends fixed-dose strategies instead of targeted goals to lower blood cholesterol. J Am Coll Cardiol. 2014;64(6):601-612. doi: 10.1016/j.jacc.2014.06.1159 [DOI] [PubMed] [Google Scholar]
- 7.Jacobson TA, Ito MK, Maki KC, et al. National lipid association recommendations for patient-centered management of dyslipidemia: part 1: full report. J Clin Lipidol. 2015;9(2):129-169. doi: 10.1016/j.jacl.2015.02.003 [DOI] [PubMed] [Google Scholar]
- 8.Expert Dyslipidemia Panel of the International Atherosclerosis Society Panel members . An International Atherosclerosis Society position paper: global recommendations for the management of dyslipidemia: full report. J Clin Lipidol. 2014;8(1):29-60. doi: 10.1016/j.jacl.2013.12.005 [DOI] [PubMed] [Google Scholar]
- 9.Anderson TJ, Grégoire J, Pearson GJ, et al. 2016 Canadian cardiovascular Society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2016;32(11):1263-1282. doi: 10.1016/j.cjca.2016.07.510 [DOI] [PubMed] [Google Scholar]
- 10.Reiner Z, Catapano AL, De Backer G, et al. ; European Association for Cardiovascular Prevention & Rehabilitation; ESC Committee for Practice Guidelines (CPG) 2008-2010 and 2010-2012 Committees . ESC/EAS guidelines for the management of dyslipidaemias: the task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J. 2011;32(14):1769-1818. doi: 10.1093/eurheartj/ehr158 [DOI] [PubMed] [Google Scholar]
- 11.Hicks KA, Tcheng JE, Bozkurt B, et al. ; American College of Cardiology; American Heart Association . 2014 ACC/AHA Key data elements and definitions for cardiovascular endpoint events in clinical trials: a report of the American College of Cardiology/American Heart Association task force on clinical data standards (writing committee to develop cardiovascular endpoints data standards). Circulation. 2015;132(4):302-361. doi: 10.1161/CIR.0000000000000156 [DOI] [PubMed] [Google Scholar]
- 12.Thygesen K, Alpert JS, Jaffe AS, et al. ; Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction . Third universal definition of myocardial infarction. Circulation. 2012;126(16):2020-2035. doi: 10.1161/CIR.0b013e31826e1058 [DOI] [PubMed] [Google Scholar]
- 13.Sacco RL, Kasner SE, Broderick JP, et al. ; American Heart Association Stroke Council, Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention; Council on Peripheral Vascular Disease; Council on Nutrition, Physical Activity and Metabolism . An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(7):2064-2089. doi: 10.1161/STR.0b013e318296aeca [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cannon CP, Braunwald E, McCabe CH, et al. ; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators . Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350(15):1495-1504. doi: 10.1056/NEJMoa040583 [DOI] [PubMed] [Google Scholar]
- 15.LaRosa JC, Grundy SM, Waters DD, et al. ; Treating to New Targets (TNT) Investigators . Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352(14):1425-1435. doi: 10.1056/NEJMoa050461 [DOI] [PubMed] [Google Scholar]
- 16.Pedersen TR, Faergeman O, Kastelein JJ, et al. ; Incremental Decrease in End Points Through Aggressive Lipid Lowering (IDEAL) Study Group . High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA. 2005;294(19):2437-2445. doi: 10.1001/jama.294.19.2437 [DOI] [PubMed] [Google Scholar]
- 17.Gibson CM, Pride YB, Hochberg CP, Sloan S, Sabatine MS, Cannon CP; TIMI Study Group . Effect of intensive statin therapy on clinical outcomes among patients undergoing percutaneous coronary intervention for acute coronary syndrome: PCI-PROVE IT: A PROVE IT-TIMI 22 (Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22) Substudy. J Am Coll Cardiol. 2009;54(24):2290-2295. doi: 10.1016/j.jacc.2009.09.010 [DOI] [PubMed] [Google Scholar]
- 18.Amarenco P, Kim JS, Labreuche J, et al. ; Treat Stroke to Target Investigators . A comparison of two LDL cholesterol targets after ischemic stroke. N Engl J Med. 2020;382(1):9-19. doi: 10.1056/NEJMoa1910355 [DOI] [PubMed] [Google Scholar]
- 19.Itoh H, Komuro I, Takeuchi M, et al. ; EMPATHY Investigators . Intensive treat-to-target statin therapy in high-risk Japanese patients with hypercholesterolemia and diabetic retinopathy: report of a randomized study. Diabetes Care. 2018;41(6):1275-1284. doi: 10.2337/dc17-2224 [DOI] [PubMed] [Google Scholar]
- 20.Boekholdt SM, Hovingh GK, Mora S, et al. Very low levels of atherogenic lipoproteins and the risk for cardiovascular events: a meta-analysis of statin trials. J Am Coll Cardiol. 2014;64(5):485-494. doi: 10.1016/j.jacc.2014.02.615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allahyari A, Jernberg T, Hagström E, Leosdottir M, Lundman P, Ueda P. Application of the 2019 ESC/EAS dyslipidaemia guidelines to nationwide data of patients with a recent myocardial infarction: a simulation study. Eur Heart J. 2020;41(40):3900-3909. doi: 10.1093/eurheartj/ehaa034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ward NC, Watts GF, Eckel RH. Statin toxicity. Circ Res. 2019;124(2):328-350. doi: 10.1161/CIRCRESAHA.118.312782 [DOI] [PubMed] [Google Scholar]
- 23.Gao Y, Shah LM, Ding J, Martin SS. US trends in cholesterol screening, lipid levels, and lipid-lowering medication use in US adults, 1999 to 2018. J Am Heart Assoc. 2023;12(3):e028205. doi: 10.1161/JAHA.122.028205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schubert J, Lindahl B, Melhus H, et al. Low-density lipoprotein cholesterol reduction and statin intensity in myocardial infarction patients and major adverse outcomes: a Swedish nationwide cohort study. Eur Heart J. 2021;42(3):243-252. doi: 10.1093/eurheartj/ehaa1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koskinas KC, Windecker S, Pedrazzini G, et al. Evolocumab for early reduction of LDL cholesterol levels in patients with acute coronary syndromes (EVOPACS). J Am Coll Cardiol. 2019;74(20):2452-2462. doi: 10.1016/j.jacc.2019.08.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eAppendix. Supplementary Methods for Definition of Secondary Endpoints
Table 1. Inclusion and Exclusion Criteria
eTable 2. Changes in Statin Intensity in the Treat-to-Target Strategy Group
eTable 3. Statin Intensity Between Groups
eTable 4. Lipid-Lowering Therapy
eTable 5. Cardiovascular Medications
eTable 6. Serial Changes in Lipid Profiles
eTable 7. Patients With LDL-C Below 70 mg/dL
eTable 8. Patients Excluded From The Per-Protocol Population
eTable 9. Primary and Secondary Endpoints in the Per-Protocol Population
eTable 10. Reasons for Discontinuing Statin Therapy by Adverse Effect
eFigure1. Lipid Profile During Study Period
eFigure 2. Treatment Difference for the Primary Endpoint
eFigure 3. Subgroup Analyses for the Primary Endpoint
Nonauthor Collaborators. LODESTAR Investigators
Data Sharing Statement


