Abstract
The paradigm of surface-expressed programmed death ligand 1 (PDL1) signalling to immune cell programmed death 1 (PD1) to inhibit antitumour immunity has helped to develop effective and revolutionary immunotherapies using antibodies blocking these cell-extrinsic interactions. The recent discovery of cancer cell-intrinsic PDL1 signals has broadened understanding of pathologic tumour PDL1 signal consequences that now includes control of tumour growth and survival pathways, stemness, immune effects, DNA damage responses and gene expression regulation. Many such effects are PD1-independent. These insights demonstrate that the prevailing cell-extrinsic PDL1 signalling paradigm is useful, but incomplete in important respects. This Perspective discusses historical and recent advances in understanding cancer cell-intrinsic PDL1 signals, mechanisms for signal controls and important immunopathologic consequences including resistance to cytotoxic agents, targeted small molecules and immunotherapies. Cancer cell-intrinsic PDL1 signals present novel drug discovery targets and also have potential as reliable treatment response biomarkers. Cancer cell-intrinsic PD1 signals and cell-intrinsic PDL1 signals in non-cancer cells are discussed briefly, as are PDL1 signals from soluble and vesicle-bound PDL1 and PDL1 isoforms. We conclude with suggestions for addressing the most pressing challenges and opportunities in this rapidly developing field.
Programmed death ligand 1 (PDL1; also known as CD274 and B7-H1) is an immune checkpoint molecule that was discovered to be expressed by heart, placenta, lung and skeletal muscle tissues and to regulate T cell proliferation and IL-10 secretion1 in 1999. Soon after, researchers identified PDL1 expression on cancer cells and that PDL1 interaction with the receptor programmed death 1 (PD1) on T cells led to inhibition of T cell activation2 including through induction of T cell apoptosis3, thereby inhibiting antitumour immunity. Antibodies blocking PDL1 or PD1 improve antitumour immunity and prolonged survival in mouse cancer models and humans3-9. Immune checkpoint blockade (ICB) agents including these antibodies, as well as antibodies blocking the CTLA4 immune checkpoint, are now US Food and Drug Administration (FDA)-approved cancer immunotherapies. The research leading to their identification was recognized with a Nobel Prize in Medicine in 2018 shared by Jim Allison and Tasuku Honjo. The principal anticancer mechanism of anti-PDL1 or anti-PD1 antibodies is thought to be preventing the cell surface PDL1-mediated inhibition of PD1+ antitumour T cells3,10-12, thereby promoting antitumour immunity13 (reviewed in REFS6,14,15). Although some patients with cancer experience durable and complete treatment responses from ICB, most fail to respond6,16 and reliable anti-PDL1 or anti-PD1 treatment response biomarkers are lacking8,9,14, suggesting the existence of alternative and/or additional PDL1-related immunopathogenesis and treatment resistance mechanisms.
The well-described cell-extrinsic immunopathogenic PDL1–PD1 pathway in cancer is represented by cancer or non-cancer cell surface-expressed PDL1 that extrinsically engages PD1 expressed on the surface of immune cells, leading to signalling downstream of PD1 to inhibit antitumour immunity. PDL1 reverse signalling refers to PD1 engaging cell surface PDL1 and induction of intracellular PDL1 signalling. Canonical PD1–PDL1 signals is defined here (and generally assumed in most literature) as PD1 signalling induced in immune cells by immune or tumour cell surface PDL1 to immune cell PD1 (REFS6,14) (BOX 1). Cancer cell-intrinsic PDL1 signals, that is, cellular functions induced by surface, cytosolic or nuclear PDL1, can be immunopathogenic, but are much less studied or understood, and have not previously been precisely defined.
Box 1∣. Cell-extrinsic versus cell-intrinsic programmed death ligand 1 signalling mechanisms.
Current immune checkpoint blockade (ICB) dogma states that tumour programmed death ligand 1 (PDL1) is expressed on the surface of cancer or immune cells and is pathogenic primarily by inhibiting programmed death 1 (PD1)-expressing CD8+ antitumour T cells3,6,10-15. However, the multiverse of tumour PDL1 signalling extends beyond cell-extrinsic PDL1 signalling to immune cells to include important cell-intrinsic effects. We define ‘cell-extrinsic’ PDL1 signalling as any signal mediated by surface PDL1 outside the PDL1-expressing cell to alter signalling in adjacent tumour or non-tumour cells. Specifically, such signals include canonical cell-extrinsic PDL1 engagement with PD1-expressing immune cells and downstream signalling in immune cells. Adjacent cancer cells can also express PD1, or as yet little studied tumour surface PDL1 receptors (for example, CD80 ( REF.73) and integrins76), resulting in cell-extrinsic PDL1 surface signalling-driven cancer cell-intrinsic consequences. By contrast, we define cancer ‘cell-intrinsic’ PDL1 signalling as any PDL1-driven signal altering biology within, or inside, that PDL1-expressing cancer cell. For example, PD1 engagement with cancer cell surface PDL1 could elicit PDL1 reverse signalling as now seen in immune cells68,77,134,135. Thus, PDL1 and PD1 could be both ligands and receptors for each other in a non-mutually exclusive manner. Although CD80-mediated surface PDL1 reverse signalling might be possible, it has not been described in the literature. Cancer cell surface PDL1 can also transduce cell-intrinsic signals independent of PD1 ligation27 but mechanistic underpinnings are incompletely defined. Surface PDL1 could also signal intrinsically by acting as a co-receptor for other cancer cell surface receptors as suggested by reports of integrin33 and (weak) extracellular growth factor receptor (EGFR)137 interactions in human bladder and lung cancer cells, respectively, but more work is required to understand the functional significance and generalizability of data. Remarkably, cell-intrinsic PDL1 signals can also be transmitted by fully intracellular PDL1, independent of any PD1 engagement, through macromolecular interactions. Intracellular PDL1 (for example, nuclear or cytosolic) participates in specific protein–protein37-38 and protein–nucleic acid34-36 interactions to regulate cell-intrinsic tumour pathogenic signals (FIG. 1b; TABLE 1). The US Food and Drug Administration (FDA) approved anti-PD1 or anti-PDL1 ICB antibodies were designed to disrupt surface PDL1–PD1 interactions specifically. Based on mechanistic work on tumour PDL1 signalling so far, many pathogenic cell-intrinsic PDL1 effects are refractory to inhibition by currently available ICB agents, thus warranting the need for additional tumour PDL1 targeting strategies or use of cell-intrinsic PDL1 effects as treatment response biomarkers.
Cell-intrinsic PDL1 signals can originate from PDL1 in specific subcellular locations (for example, cytosol and nucleus), often directed there by post-translational modifications (described later in the section Cancer cell-intrinsic signalling via intracellular PDL1), and might be elicited by surface PDL1–PD1 engagement, but can be PD1-independent. PDL1 signalling can originate from secreted, vesicular or exosomal PDL1 (REFS17,18) or non-stromal cells or platelets19-22, and anti-surface PDL1 antibody can affect tumour-intrinsic PDL1 signals23-28 through as yet incompletely defined mechanisms. This Perspective focuses on how cancer cell-intrinsic PDL1 signals control signalling biology within and among cancer cells (TABLE 1).
Table 1∣.
Programmed death ligand 1 signal outcomes and mechanisms
| Cancer cell PDL1 signal |
Subcellular PDL1 location |
Functional consequence | Mechanisms | Tumour types | Experimental models and context |
|---|---|---|---|---|---|
| Intrinsic | Surface | Increase myeloid-derived suppressor cell recruitment and anti-PD1 resistance | CD8+T cell IFNγ induces cancer cell PDL1 signalling to promote STAT3-dependent, cell-intrinsic NLRP3 inflammasome activation | Melanoma | In vivo mouse models28 |
| Intrinsic | Unknown, but localized to carboxy-terminal tail (RMLDVEKC motif) | Inhibits STAT3 activation | Inhibits IFNα, 1FNβ and IFNγ signals and sensitivity in mouse cells | Melanoma | In vitro studies of mouse cell lines27 |
| Intrinsic | Surface | Anti-PDL1 antibodies sensitized mouse B16 melanoma cells directly to IFNβ-mediated cytotoxicity in vitro | Unknown | Melanoma, colon, breast | In vitro studies of mouse cell lines27 |
| Intrinsic | Surface | PDL1 suppresses FAS-mediated apoptosis | Unknown but involves PD1-induced, tumour surface PDL1 back-signalling | P815 mastocytoma | In vitro studies of mouse cell lines29 |
| Intrinsic | Surface | Promote SNAIL protein stability and immune-independent metastasis | Antagonize PTP1B, promote MAPK signalling | Breast | In vitro and in vivo human79 and mouse79 models |
| Intrinsic | Intracellular/perinuclear | Increase tumour resistance to chemotherapy | Enhance DNA-PK-mediated RAS–MAPK activation | Breast | In vitro studies of human cell lines81 |
| Intrinsic | Surface | Increase tumour susceptibility to chemotherapy | Promote expression of pro-apoptotic proteins BIM and BIK | Colon (BRAFV600E) | In vitro and in vivo human25 and mouse25 |
| Intrinsic | Nuclear | Anti-PD1 resistance | Potentially by mediating transcription of immune checkpoint ligands (e.g. VISTA, galectin 9) | Heterotopic colon cancer | In vivo mouse model36 |
| Intrinsic | Nuclear | Possibly regulate tumour immunogenicity through hypoxia-mediated pyroptosis | Activate gasdermin C to induce tumour necrosis and tumour growth promoting inflammation | Breast | In vitro studies with human35 cell lines and in vivo mouse models35 |
| Intrinsic | Nuclear | Increase cell proliferation by enhancing GAS6–MERTK signalling | Nuclear PDL1 binds to SP1 transcription factor to increase Gas6 gene transcription | Non-small-cell lung cancer | In vitro and in vivo studies of human cell lines80 |
| Intrinsic | Unknown | Renders tumour immune cytolytic-resistant | Unknown, related to anti-apoptosis? | Melanoma | In vitro experiments of a syngeneic mouse cell line28 |
| Intrinsic | Unknown | Regulates MHC class I expression possibly affecting antigen presentation | Controls transcription of immune response genes | Breast | In vitro studies of human cell lines36,91 |
| Intrinsic | Cytosol | Cancer cell-intrinsic PDL1 regulates expression of genes involved in the DNA damage response | Cytoplasmic PDL1 binds to specific mRNAs with a GAAGAA/U motif to outcompete the RNA exosome | Breast, colon | In vitro studies of human cell lines34 |
| Intrinsic | Unknown | Could alter chemokines/TIL trafficking | Controls tumour NF-κB-mediated transcription (e.g. binds to RelA) | Breast, colon | In vitro studies of mouse91 and human36 cell lines |
| Intrinsic | Unknown | Alter autophagic flux and response to autophagy inhibitors | Could affect antigen processing | Melanoma, bladder | In vitro studies of mouse23,24 and human23,24 cell lines |
| Intrinsic | Unknown | Promote tumour stemness | mTORC1-driven Oct4 gene expression | Melanoma, ovarian | In vitro and in vivo studies of mouse cell lines133 |
| Intrinsic | Unknown | Increase tumour glucose metabolism | Possibly allow tumours to outcompete T cells for glucose, causing T cell dysfunction by driving mTORC1 activation | Sarcoma, B16 melanoma, L cells, MC38 colon | In vitro studies of a mouse cell line95 |
| Intrinsic | Unknown | Increase tumour metastases | Reduce immunotherapy efficacy due to metastases | B16 melanoma, breast | In vivo studies of mouse24 and human85 models |
| Intrinsic | Nuclear | Promote genomic stability | Facilitate cohesin complex formation by binding cohesin subunit SA1 | Rectal | In vitro studies of human38 cell lines |
| Intrinsic | Nuclear | Promote sister chromatid cohesion through amino-terminal YSR-like motif | Facilitate cohesin complex retention on chromatin | TNBC | In vitro studies of human cell lines37 |
| Intrinsic, isoform | Unknown | Epithelial–mesenchymal transition signature | Activating PI3K–AKT signals; GSK3β-mediated phosphorylation, ubiquitination and degradation of SNAIL; RAS–ERK activation | Colorectal, glioblastoma, non-small-cell lung cancer, nasopharyngeal, oesophageal, TNBC | In vitro studies of human31,84,85,89 and rat84 models |
| Extrinsic | Surface | Reduce cell growth rate and possibly drive anti-PD1/PDL1-associated tumour growth | Tumour surface PDL1 engages tumour surface PD1 to suppress p-AKT and/or p-ERK activation | Non-small-cell lung cancer | In vitro and in vivo study of human72 cell lines/xenografts |
| Extrinsic | Surface | Increase cell growth rate and possibly explain anti-PD1/ PDL1 treatment response | Tumour surface PDL1 engages tumour surface PD1 to increase via p-S6 signals | Melanoma | In vitro and in vivo studies of mouse71 and human71 models; IHC from patient samples |
| Extrinsic | Surface | Inhibit PD1-expressing antitumour immune cells, especially T cells | Direct immune cell engagement with PDL1 back-signalling | Lung, ovary, colon, melanoma, mastocytoma, myeloma, non-small-cell lung cancer, renal cell, pancreatic, gastric, breast and non-tumour models | In vitro and in vivo human and mouse models3-12,14,15 |
| Extrinsic | Surface | Prevent T cell co-stimulation | Interacting with CD80 in cis or in trans | Non-tumour models | In vivo mouse models51,52 |
| Extrinsic | Surface | Induce T cell anergy, exhaustion and/or death | Direct immune cell engagement with back-signalling | Non-tumour models | In vivo human and mouse studies68,134,135 |
| Extrinsic | Surface | Inhibit T cell memory | Reduce effector memory, T cell stem cell and resident T cell memory through unclear mechanisms | Melanoma, non-small-cell lung cancer | In vivo human136 and mouse136 studies |
BIK, BCL-2-interacting killer; BIM, BCL-2-like protein 11; DNA-PK, DNA-dependent protein kinase; ERK, extracellular-signal-related kinase; IFNγ, interferon-γ; GSK3β, glutathione synthase kinase 3β; IHC, immunohistochemistry; MAPK, mitogen-activated protein kinase; MERTK, tyrosine-protein kinase MER; MHC, major histocompatibility complex; mTORC1, mammalian target of rapamycin complex 1; NF-κB, nuclear factor-κB; NLRP3, NOD-, LRR- and pyrin domain-containing 3; PD1, programmed death 1; PDL1, programmed death ligand 1; p-S6, phospho-S6 ribosomal protein; PTP1B, protein-tyrosine phosphatase 1B; RelA, REL-associated protein; SA1, cohesion subunit SA1; SP1, specificity protein 1; STAT3, signal transducer and activator of transcription 3; TIL, tumour infiltrating lymphocyte; TNBC, triple-negative breast cancer; VISTA, V-type immunoglobulin domain-containing suppressor of T cell activation.
Cancer cell-intrinsic PDL1 effects were first demonstrated in 2008 as anti-apoptotic, through a PD1-dependent mechanism that required the PDL1 cytoplasmic tail, although the specific subcellular location was not defined29. This was followed by our 2016 demonstrations that cancer cell-intrinsic PDL1 exerts control of proliferation, immune-independent metastasis, mammalian target of rapamycin complex 1 (mTORC1) signalling, autophagy, small-molecule drug resistance and stemness in mouse and human ovarian cancer and melanoma cells24,30. Although specific subcellular PDL1 locations were not defined, we showed PD1 dependence for immune-independent, anti-PDL1-driven cancer cell proliferation suppression in vitro. Reports swiftly emerged that cancer cell-intrinsic PDL1 from unspecified subcellular locations in cancer cells promotes expression of distinct genes24,31 and resistance to cytotoxic chemotherapy, radiation and other treatments23,24,32, including anti-PD1 resistance28, and suppresses signal transducer and activator of transcription 3 (STAT3) and interferon-β (IFNβ) signalling27, specific immune cell recruitment28, IFNγ resistance33 and DNA damage32. Cytosolic PDL1 promotes expression of genes involved in the DNA damage response34. Nuclear PDL1 increases tumour pyroptosis35, gene expression35,36, anti-PD1 resistance35, sister chromatid cohesion37 and genomic stability38. None of these reports tested PD1 dependency in detail. Inhibiting cancer cell-intrinsic PDL1 signals with small molecules or antibodies for therapeutic benefit has been demonstrated20,28,36,39.
Exploiting therapeutic vulnerabilities from cancer cell-intrinsic PDL1 signals or when soluble, vesicular, exosomal or aberrantly expressed, or its isoforms17,40-45 (BOX 2, represents largely untapped opportunities warranting further investigation. Given the rapidly improving understanding of cell-intrinsic PDL1 signals, in this Perspective we discuss cancer cell-intrinsic PDL1 signals and suggest novel treatment strategies and response biomarkers. We cover mechanisms for cancer cell-intrinsic PDL1 signalling and major consequences. Finally, we address the targeting of tumour cell-intrinsic PDL1 signals in drug discovery.
Box 2 ∣. PDL1 from extracellular PDL1 and non-cancer cells, and related molecules.
Non-cancer cells and exosomes
Aside from tumour cells, programmed death ligand 1 (PDL1) signals on non-tumour cells and structures can mediate homeostatic and/or pathologic outcomes. For instance, endothelial cell PDL1 generates regulatory T cells21 that can inhibit antitumour or autoimmune responses. PDL1-deficient versus control conventional dendritic cells intrinsically produce more inflammatory cytokines in tumours70. In the non-tumour context, cell-intrinsic PDL1 signals in dermal dendritic cells can promote their migration to draining lymph nodes and subsequent activation of T cells by enhancing signalling downstream of chemokine receptors (for example, ERKs and F-actin polymerization)138. T cell-intrinsic PDL1 inhibits CD4+ T cell activation by activating signal transducer and activator of transcription 3 (STAT3)68. Natural killer cell PDL1 can improve the cells’ antitumour cytotoxicity through a PD1-independent, p38 mitogen-activated protein kinase (MAPK)-dependent pathway67. PDL1 promotes pyroptosis in human pulmonary arterial smooth muscle cells under hypoxia that augments pulmonary vascular fibrosis69. PDL1 presented by platelets can suppress antitumour immunity in mouse heterotopic MC38 colon cancer and various human PDL1-negative cancer lines in vitro19. Both haematopoietic (including immune cell) and non-haematopoietic PDL1 contribute to virus defence22. We anticipate that cell-intrinsic PDL1 signals from immune and non-immune cells can contribute to anticancer immunity, an area deserving additional studies. X-irradiated Cd274-knockout mice survive a significantly shorter time versus X-irradiated wild-type mice, an effect the authors attributed to defective DNA damage repair34 but which could be multifactorial, and is nonetheless consistent with cell-intrinsic PDL1 effects in normal cells. Some non-cancer cell PDL1 signals appear to overlap functionally with cell-intrinsic PDL1 signals in cancer cells (TABLE 1). The role of cell-intrinsic PDL1 signals in normal cells under homeostatic conditions or during cellular transformation requires much further investigation. We reported that adipocyte PDL1 suppresses response to immune checkpoint blockade (ICB) and the peroxisome proliferator-activated receptor-γ (PPARγ) antagonist GW9662 can selectively reduce adipocyte PDL1 to improve ICB in murine models of breast cancer20, demonstrating a proof of concept for pharmacologic cell-type selective, cell-intrinsic PDL1 targeting. Details of exosome biology and exosomal PDL1 (REF.17) and effects of soluble PDL1 on cancer immunotherapy18 were recently comprehensively reviewed.
Other immunoglobulin superfamily members
PDL1 is an immunoglobulin superfamily member. Interestingly, other immunoglobulin superfamily members were recently reported to mediate cancer cell-intrinsic signals, some similar to PDL1 signals in various mouse and human cancers including in vivo effects on distinct treatments. These immunoglobulin superfamily members include programmed death 1 (PD1) (REFS23,24,71,72,139), PDL2 (the other PD1 ligand)73,140,141, CD80 (the other PDL1 receptor)73, B7-H3 (REFS142-145), CD90 (REFS146,147), AXL148 and IgA itself126. Fat mass and obesity-associated protein promotes PD1 expression associated with increased mTOR in human MDA-MB-231 and BT549 cells, and mouse 4T1 TNBC cells, but a mechanistic relation to PDL1–PD1 signals was not demonstrated149. PDL1-independent mTOR promotion from PD1 on colon cancer and human hepatoma cells in vitro was reported150. Myeloid cell PD1 inhibits their phagocytosis151 and T cell-intrinsic V-type immunoglobulin domain-containing suppressor of T cell activation (VISTA) promotes T cell quiescence (R. Noelle, personal communication).
PDL1 structure and immune cell PD1 engagement
PDL1 is a 290 amino acid, type 1 transmembrane protein in the immunoglobulin superfamily resembling the immunoglobulin light chain (FIG. 1a). It engages its major receptor PD1, in trans as well as in cis46,47, to inhibit antitumour PD1+ T cell functions48, by antagonizing T cell receptor and CD28 co-stimulatory signals49, authoritatively reviewed elsewhere50. PDL1 also engages its other known receptor, the immune co-signalling molecule CD80, in trans51 as well as in cis52 each to prevent T cell co-stimulation.
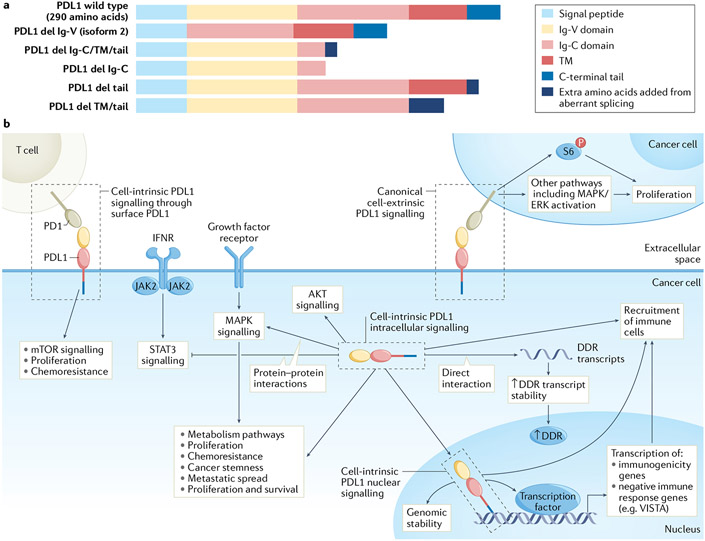
Fig. 1 ∣. Programmed death ligand 1 signalling in cancer cells.
a ∣ Programmed death ligand 1 (PDL1) comprises several domains, the order and presence of which differ between wild-type PDL1 and its isoforms. PDL1 isoforms can be secreted by loss of transmembrane anchoring through splicing or retained in subcellular compartments where they could control cell-intrinsic signals, all requiring further investigation. PDL1 isoforms aside from full-length (isoform 1) PDL1 or its enzymatic cleavage product in vesicles, exosomes or soluble form are not described, b ∣ Surface PDL1 exhibits immunoglobulin-like topology with amino-terminal extracellular structure (yellow and light red rectangles) attached to a transmembrane (TM) domain (dark red rectangle) and a short carboxy-terminal tail (blue rectangle). The immunoglobulin-like extracellular domain interacts with the immunoglobulin-like domain of extracellular programmed death 1 (PD1). Cell-intrinsic full-length intracellular PDL1 (for example, cytoplasmic or nuclear) has same sequence as PDL1 expressed on the cell surface, but its N-terminal domain is likely unfolded and, thus, could interact with proteins distinctly from surface-expressed PDL1, and likely cannot engage PD1 using extracellular topology. Surface PDL1 appears capable of reverse signalling through its cell-intrinsic C-terminal tail (cell-intrinsic PDL1 surface signalling, or reverse PDL1 signalling), requiring additional mechanistic studies to confirm, or could signal to adjacent cancer cells expressing PD1 (cell-extrinsic PDL1 signalling), CD80 or other receptors such as specific integrins. PDL1 in cytosol can directly interact with resident molecules, for example, mRNA and others described in text, or in nucleus to act as a co-transcription factor, or mediate other effects described in text. Tumour surface PDL1 signalling to PD1 on distinct cancer cells (cell-extrinsic PDL1 surface signalling) can promote cancer cell proliferation, for example by increasing phosphorylation of ribosomal S6 protein (p-S6) in PD1-expressing cancer cells among other effects. Cytosolic PDL1 can control mRNA stability to regulate expression of genes involved in the DNA damage response (DDR) or could directly promote major signalling pathways such as mitogen-activated protein kinase (MAPK)/extracellular-signal-related kinase (ERK) or signal transducer and activator of transcription 3 (STAT3) through protein–protein interactions. Nuclear PDL1 exerts transcriptional control of specific target genes involved in tumour immunogenicity or can have non-transcriptional effects on chromosome stability through sister chromatid. These cell-intrinsic PDL1 signals affect antitumour immune responses distinctly from surface PDL1. del, deletion; IFNR, interferon receptor; VISTA, V-type immunoglobulin domain-containing suppressor of T cell activation.
PDL1 domains.
PDL1 protein has five major domains (FIG. 1a). The domains lack canonical signalling motifs as observed in classical receptor tyrosine kinases but cell-intrinsic PDL1 signals can similarly be altered by post-translational modifications35,36,53. For example, PDL1 glycosylation enhances its protein stability54 and binding to known interacting partners such as PD1 (REF.50), whereas PDL1 acetylation inhibits its nuclear translocation that suppresses anti-PD1 efficacy36. Cell-intrinsic PDL1 signals can come from full-length 290 amino acid PDL1 found at the cell surface as well as the cytosol and nucleus35,36. As surface-expressed PDL1 still contains a short 30 amino acid cytoplasmic tail27,55, studies distinguishing cell-intrinsic signalling effects derived from surface versus fully cytosolic PDL1 must account for this mechanistic possibility if subcellular effects are considered. Mouse and human PDL1 share high protein sequence and structural similarity, although differences in sequence can affect anti-PDL1 binding and PD1 engagement56. Anti-PDL1 can increase CD80 signals to activate antitumour T cells57 but specific consequences are little understood as the PDL1–CD80 interaction is much less studied compared with the PD1–PDL1 interaction.
Human PDL1 isoform 1 is the full-length protein generally referred to as ‘PDL1’, a convention used here. When fully intracellular, its Ig-V and Ig-C domains (which are extracellular when PDL1 is on the cell surface) are intracellular and can participate directly in cell-intrinsic PDL1 signalling. At least five other PDL1 isoforms are described, all in human but not mouse cells, some having signal functions and/or prognostic significance41-43,58 (FIG. 1a). A secreted carboxy-terminal tail-deficient isoform was detected in relapsed non-small-cell lung cancer that generated ICB resistance by competing for anti-PDL1 (REF.59). Additional functions of these PDL1 isoforms, including cell-intrinsic consequences and mechanisms, merit more investigation.
Most commercial anti-PDL1 antibodies recognize the Ig-V domain which engages PD1 (REF.46), and thereby block PD1 interaction, but do not recognize PDL1 isoforms lacking the Ig-V (PD1-binding) domain such as isoform 2 (which lacks the amino-terminal Ig-V moiety)58. Some human anti-PDL1 detection antibodies that recognize the C-terminal tail (for example, clones 405.9A11, E1L3N) are commercially available60, do not interfere with PD1 engagement and should theoretically bind to PDL1 isoform 2, but that has not been reported. Similar limitations can apply to detecting other PDL1 isoforms.
Cancer cell-extrinsic PDL1-related treatment outcomes.
Cancer cell-extrinsic PDL1-related treatment outcomes have been reviewed extensively14,61-63. In brief, the principal anticancer mechanism of anti-PDL1 or anti-PD1 is thought to be preventing PD1+ antitumour T cells from inhibition by cell surface-expressed PDL1 (REFS3,10-12), resulting in T cell reinvigoration13, reduced T cell exhaustion or death and increased T cell memory and intratumoural antitumour immune cell infiltrates6,14,15, including effects in chronic infection64 that parallel cancer data.
Signalling mechanisms
Although PDL1 is generally considered to be surface-expressed, it is found in many subcellular compartments (FIG. 1b. Cytoplasmic PDL1 can be recycled from endosomes to the cell surface65,66. Functional nuclear PDL1 has been reported35-37, but its cellular source remains unresolved. Although there are profound differences in cell-extrinsic versus cell-intrinsic PDL1 signals, the same PDL1 molecule could mediate either signal depending on subcellular location (BOX 1; FIG. 1b. The conformation of PDL1 on the cell surface (as well as in soluble form45 or in exosomes17,18) is immunoglobulin-like and three-dimensional. This conformation is likely lost in fully intracellular PDL1 (although linear Ig-V and Ig-C domains could remain largely unchanged), which we predict affects interactions with potential intracellular binding partners. For example, PDL1–PD1 engagement is through their immunoglobulin-like regions46 that require complex tertiary conformations which would be lost in intracellular compartments. Thus, known PD1-dependent cell-intrinsic PDL1 effects24,29 likely can only occur through surface-expressed PDL1 (FIG. 1b. In addition, post-translational modifications directing PDL1 to distinct subcellular compartments or arising in such compartments can affect PDL1 signalling, such as acetylation, reducing nuclear PDL1 to improve anti-PD1 efficacy in a heterotopic colon cancer model36 (FIG. 2. Cell-intrinsic PDL1 signals observed in cancer cells have also been detected in non-cancer cells and in the non-cancer, healthy context including natural killer cells67, T cells68, adipocytes20, arterial smooth muscle cells69 and dendritic cells70, implicating PDL1 as a homeostatic molecule. We anticipate that PDL1 signals in non-cancerous cells could differ from malignant counterparts and from other tumour genetic backgrounds and histologies just as PDL1 signal effects in cancer cells can differ among tumour types (BOX 1; TABLE 1. Much work in these contexts is merited to understand pathologic versus homeostatic outcomes. Additional PDL1 moiety signalling features are described in BOX 3.
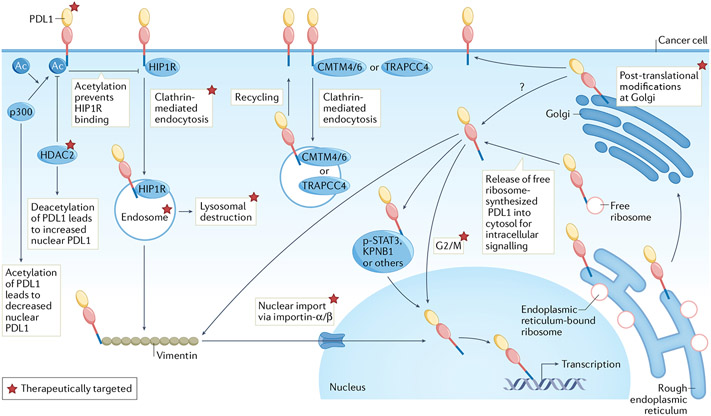
Fig. 2 ∣. Regulation of intracellular PDL1 and potential targeting.
Programmed death ligand 1 (PDL1) is synthesized in endoplasmic reticulum followed by Golgi processing and surface presentation. Huntingtin-interacting protein 1-related protein (HIP1R) targets PDL1 for lysosomal destruction whereas CKLF-like MARVEL transmembrane domain-containing protein 4/6 (CMTM4/6) or trafficking protein particle complex subunit 4 (TRAPPC4) recycles endocytic PDL1 from cytoplasm to surface. HIP1R-dependent clathrin-mediated endocytosis of surface, non-acetylated PDL1 (controlled by histone deacetylase 2 (HDAC2) and acetyltransferase p300) facilitates PDL1 (from free ribosomes) interaction with vimentin followed by nuclear import via importin-α/β. We show p300 and HDAC2 as acting on cell surface PDL1, but those interactions could also occur in cytosol or nucleus. PDL1 can interact with p-STAT3 in triple-negative breast cancer (TNBC) during hypoxia or associate with karyopherin B1 (KPNB1) in lung cancer, either pathway increasing nuclear PDL1. Free ribosomes (that is, not endoplasmic reticulum-bound) can theoretically synthesize PDL1 for direct release into the cytosol without modification in the endoplasmic reticulum and Golgi. Thus, the cellular source of nuclear PDL1 could be from the surface or cytosol, or directly from other organelles following PDL1 processing such as endoplasmic reticulum/Golgi. PDL1 can participate in macromolecular interactions, for example, the endoplasmic reticulum-associated protein degradation pathway, but whether endoplasmic reticulum-bound PDL1 mediates cell-intrinsic signalling is unknown. Apart from acetylation, phosphorylation, glycosylation, palmitoylation and polyubiquitination, other post-translational PDL1 modifications regulate its subcellular distribution, but mechanistic details are not comprehensively characterized. Exogenous ligands and cellular stressors can upregulate PDL1 expression or redistribute surface PDL1 to other locations including the cytosol and nucleus or into organelles such as lysosomes or autophagosomes. Examples include tumour necrosis factor (TNF), interferons, DNA damage (from exogenous sources such as cytotoxic chemotherapy) or environmental cues such as hypoxia or nutrient (for example, glucose) availability. PDL1 cell-intrinsic trafficking is significantly controlled by the cell cycle, decreasing in the G1 phase and increasing in S/G2/M cycles. Regulation of PDL1 cell-intrinsic signalling directly from mitochondria, endoplasmic reticulum, autophagosomes or other non-nuclear organelles remains to be elucidated. Altering cell-intrinsic PDL1 content or signalling apart from surface PD1–PDL1 signal disruption using US Food and Drug Administration (FDA)-approved antibodies elicits distinct therapeutic vulnerabilities. Total tumour PDL1 can be reduced using approaches including agents targeting PDL1 post-translational modifications such as palmitoylation and glycosylation (for example, peptide mimics, glutathione synthase kinase 3β (GSK3β) inhibitors), antibodies disrupting PDL1–CMTM4 or CMTM6 interactions (for example, H1A), repurposed FDA-approved drugs (for example, the photosensitizer verteporfin) and natural products (for example, curcumin). Subcellular PDL1 redistribution can be altered using agents disrupting clathrin-mediated endocytosis (for example, pitstop 2), macromolecular interactions (for example, cytoskeleton or signal transducer and activator of transcription 3 (STAT3) inhibitors) and importin-α/β molecules for nuclear import (for example, FDA-approved ivermectin). Therapies sequestering PDL1 on cell surface or in other compartments such as endosomes or autophagosomes include FDA-approved cell-cycle inhibitors (for example, palbociclib, ribociclib), endocytic inhibitors (for example, Dyngo-4a) and autophagy inhibitors (for example, hydroxychloroquine).
Box 3 ∣. Effects from the programmed death ligand 1 C-terminal tail.
Programmed death ligand 1 C-terminal tail signals
The short (30 amino acid) human programmed death ligand 1 (PDL1) carboxy-terminal tail lacks canonical signalling motifs and is the least structured PDL1 domain. Yet, it binds nucleic acids to promote mRNA stability34; binds specific DNA promoter regions to enhance gene expression36; has a hypoxia-mediated p-STAT3 binding site that promotes PDL1 nuclear translocation and gasdermin C-driven pryoptosis35; has an acetylation site, acetylation of which inhibits nuclear PDL1 translocation36; has a lysosome-targeting motif to promote its degradation79; contains palmitoylation115 and phosphorylation112 sites that inhibit its ubiquitination; promotes genomic stability38; and promotes immune checkpoint blockade (ICB) resistance through other distinct mechanisms27,28,36. Most of these properties have not yet been reported for mouse PDL1 but are likely given structural similarities to human PDL1 (REF.56).
The mouse C-terminal tail has defined motifs that overall inhibit interferon-β (IFNβ)-mediated cell cytotoxicity, and also inhibit signal transducer and activator of transcription (STAT3), which could contribute to cell-intrinsic resistance to type I and II interferons27. In this report, genetic PDL1 silencing increased interferon-mediated tumour cytotoxicity through enhanced caspase 7 activity, although caspases 3 and 9 were also inhibited by PDL1. Additionally, this report did not distinguish cell-intrinsic effects on IFNβ signalling mediated by intracellular (cytosolic or nuclear) or surface PDL1. Specific mechanisms for mouse PDL1 C-terminal tail regulation of these outcomes (for example, caspase expression) and PDL1-driven STAT3-mediated interferon sensitivity in human cells is possible but as yet unreported.
Cell-intrinsic PDL1 effects not mediated by the C-terminal tail
PDL1 signals other than from its C-terminal tail are, thus far, only reported in human cells. A tail-deleted PDL1 secreted isoform can mediate immune suppression59, discussed above.PDL1 isoform C lacking the C-terminal tail and transmembrane domain promoted colorectal cancer cell proliferation and epithelial–mesenchymal transition43. A report of non-C-terminal tail PDL1 signals provided some evidence that protein kinase B (PKB; also known as AKT) and F-actin accumulation are regulated by PDL1 amino acids 128–237 (REF83), essentially spanning the Ig-C domain, far amino-terminal to the C-terminal tail. However, functional tests of this PDL1 moiety were not demonstrated. By contrast, functional studies of a YSR-like motif in the PDL1 N terminus at the Ig-V/Ig-C domain boundaries abrogated nuclear PDL1 stabilization of the cohesin complex, but not its PD1 binding ability, producing defective sister chromatid cohesion37, suggesting properties of cell-intrinsic PDL1 independent of its C-terminal tail. Additional signal functions of non-tail PDL1 moieties remain to be elucidated in detail, including in mouse cells.
Cancer cell-intrinsic signalling via surface PDL1.
Surface PDL1 signalling includes engagement of immune cell PD1 (REF.2) or CD80 (REF.51), but reports on functional CD80 engagement with PDL1 are limited. Although some cancer cells express PD1 (REFS24,71,72) or CD80 (REF.73), cis interactions with PDL1 have not yet been reported in cancer cells. In melanoma, PD1 on cancer cells can interact with cancer cell surface PDL1 leading to mTORC1 activation, cell proliferation and in vivo tumour growth71 (FIG. 1b; TABLE 1). Whereas triple-negative breast cancer (TNBC) cells can also express both PD1 and PDL1, similar interactions leading to intrinsic PDL1 signalling have not been reported, although PDL1 contributed to proliferation and growth in vitro and in vivo37 which could be, in part, because of cell type-specific differences in the levels of PD1 N-glycosylation that affect PDL1 engagement (as shown in T cells)74 or other post-translational modifications of PDL1 that affect PD1 engagement. For example, a study of human MDA-MB-231 TNBC cells observed markedly reduced glycosylation of surface PDL1 relative to nuclear PDL1 (REF.36) implicating PDL1 glycosylation, or potentially other post-translational modifications, as a determinant of cell-intrinsic PDL1 signalling from the cell surface versus other subcellular compartments.
Therapeutically, cancer cell-intrinsic signalling in trans via the PD1-PDL1 axis on cancer cells may partially explain immune-independent anti-PDL1-mediated inhibition of proliferation in vitro in melanoma24,71 and ovarian cancer24 and in vivo in melanoma in NSG mice24,71. The transduction of cell-intrinsic signals by surface PDL1 occurs through cancer cell-expressed PD1 immunoreceptor tyrosine-based inhibitory motif and immunoreceptor tyrosine-based switch motif sites, and can drive cell growth via phospho-S6 ribosomal protein (p-S6) in melanoma cells71 (FIG. 1b), although the activity of this axis requires validation in other tumour types. By contrast, cancer cell-intrinsic PD1–PDL1 signalling, possibly in trans, in non-small-cell lung cancer cells in vitro and in vivo in NSG mice instead led to growth suppression by antagonizing p38 mitogen-activated protein kinase (MAPK)-extracellular-signal-regulated kinase (ERK) signals72. Although in vitro culture conditions appear similar in these distinct reports, more work is needed to clarify these discordant data which likely include effects from specific genetic backgrounds, tumour biology or cell-specific post-translational PDL1 or PD1 modifications35,36,53,75.
Potential contributions of CD80 on cancer cells to cell-intrinsic PDL1 signals or functional outputs have not been reported. However, cancer cell surface PDL1 seems to induce cancer cell-intrinsic signals in some contexts through non-PD1 receptors such as β4 integrin on various human cervical cancer cells in vitro76. In various human bladder cancer cells, surface PDL1 interacted with surface β6 integrin to drive cell proliferation via FAK signalling and resistance to cisplatin-induced apoptosis in vitro33. PD1-induced cancer cell surface PDL1 reverse signalling, likely through the PDL1 cytoplasmic tail, has been reported in tumours including Hodgkin lymphoma, driving cell growth and death resistance in this and other human lines in vitro29,77. Reverse signals likely also include as yet unreported cell-specific and/or context-specific differences. Anti-PDL1 antibody effects on tumour-intrinsic PDL1 signals23-28,78 are discussed later in Interrupting cell-intrinsic PDL1 signals.
Cancer cell-intrinsic signalling via intracellular PDL1.
Direct protein–protein interactions are now a major known mechanism for cell-intrinsic PDL1 signal control in cancer cells. Interactions of PDL1 with glutathione synthase kinase 3β (GSK3β) and β-transducin repeats-containing protein (β-TRCP)54, CKLF-like MARVEL transmembrane domain-containing protein 6 (CMTM6)65,66 or CMTM4 (REF.66) can regulate total intracellular PDL1 content by reducing PDL1 protein degradation globally through proteasomal or lysosomal pathways. Other interactions, including with histone deacetylase 2 (HDAC2)36 and Huntingtin-interacting protein 1-related protein (HIP1R)79, can facilitate surface PDL1 relocation to intracellular compartments such as the cytosol, nucleus or lysosome through clathrin-mediated endocytosis36. When intracellular, other interactions with vimentin, p-STAT3 (Y703) and karyopherin B1 (KPNB1) can additionally promote, whereas p300 inhibits PDL1 nuclear translocation35,36,80. Once nuclear, PDL1 can promote transcription of pro-tumour genes such as Gas6 (REF.80) (FIG. 1b; TABLE 1). Nuclear PDL1 can alter gene expression through specific DNA promoter region binding (mapped by PDL1 chromatin immunoprecipitation and deep sequencing)36, possibly by acting as a co-factor to known transcription factors35,36,80 such as specificity protein 1 (SP1).
Remarkably, cytosolic PDL1 has also been shown to bind to and stabilize mRNAs, thereby increasing expression of specific DNA damage repair proteins containing GAAGAA/U motifs in MDA-MB-231 cells34. Perinuclear or nuclear PDL1 associated with DNA-dependent protein kinase (DNA-PK) to activate MAPK or ERKs and promote cell survival signals in a cancer-specific manner also in MDA-MD-231 TNBC cells81. These latter two mechanisms appear to promote resistance to selected cytotoxic chemotherapies in vitro independent of PD1 or CD80. However, additional mechanistic studies are required to determine formally whether these competing cell-intrinsic PDL1 signalling consequences indeed mediate resistance to DNA damaging agents and to assess generalizability of such findings. As DNA damage can induce nuclear PDL1 accumulation82 and PDL1 can also bind DNA directly36, we predict that some control of DNA damage repair and, possibly chemoresistance occurs through direct participation of nuclear PDL1 in DNA repair pathways (for example, homologous recombination), although no mechanistic details have been reported.
Other protein interactions with intracellular PDL1 have been reported. However, the consequences on cancer cell functions of such interactions are incompletely understood and some studies are largely correlative. Such PDL1 interactions include with adaptin β2, keratins, importin α1, REL-associated protein (RelA; also known as p65) and interferon-regulated factors (IRFs) (all in REF.36 with some additional interactions discovered using PDL1 co-immunoprecipitation mass spectrometry), protein kinase B (PKB; also known as AKT)83, HRAS84, actin36,83, protein-tyrosine phosphatase 1B (PTP1B)85 and the Nijmegen breakage syndrome protein 1 (NBS1) involved in DNA repair86. Molecular, biochemical or functional studies, which should involve specific binding defective PDL1 mutants, are needed to understand the full phenotypic extent and formally prove the functional significance of these observed protein–protein interaction involving PDL1.
Intracellular PDL1 can also interact with large macromolecular structures, including with the nuclear cohesin complex to facilitate sister chromatid cohesion37 and with cohesin subunit SA1 (SA1) to suppress genomic instability and/or aneuploidy38. The former study provided strong molecular evidence for intracellular PDL1 promotion of genomic stability by utilizing a PDS5 cohesin-associated factor B binding-defective PDL1 point mutant that failed to restore sister chromatid cohesion fully in CD274-knockout MDA-MB-231 cells. By contrast, PDL1 can associate with lysosomes79, autophagosomes24 and seven distinct oligomeric Golgi proteins87 but intrinsic cellular and phenotypic effects of these interactions remain unclear. Potential effects include PDL1-mediated suppression (melanoma, ovarian cancer) or promotion (bladder cancer) of autophagy23,24. PDL1 association with autophagosomes suggests a potential mechanistic autophagy control effect requiring investigation. This exceptional diversity of potential interactions and signal outcomes makes a simple, unifying view of PDL1-regulated gene product expression difficult, especially as most outcomes do not appear to involve canonical signal motifs. Much work is needed to understand why and how PDL1 binds to and regulates so many diverse molecules and to understand its manifold roles in homeostatic versus dysfunctional processes. We speculate that PDL1 participation in phase separation88 could help to provide a basis for this large array of macromolecular interaction effects. BOX 3 describes specific PDL1 domains mediating cancer cell-intrinsic signalling from intracellular PDL1.
Cell-intrinsic PDL1 from undefined subcellular locations.
Cancer cell-intrinsic PD1 engages PDL1 for mTORC1 activation in melanoma cells71 and can also promote cancer cell proliferation23,24. Of note, we showed a role of cancer cell-intrinsic, PDL1-driven, immune-independent promotion of B16 melanoma metastasis24, which could be from mTORC1-mediated cell proliferation or due to reported activation of epithelial to mesenchymal transition in TNBC, glioblastoma multiforme and carcinomas of the nasopharynx, lung and colon31,43,84,85,89,90 that could affect invasion and motility. Cancer cell-intrinsic PDL1 control of pro-growth mTORC1 signalling was discussed above, demonstrating that PDL1-driven mTORC1 activation and autophagy suppression are not necessarily related and that PDL1 effects can differ by tumour type. PDL1-mediated mTOR control mechanisms have not been reported in detail, including relative effects on and consequences of downstream mTORC1 targets, areas for investigation.
In certain tumours, cancer stem cells have higher PDL1 expression levels than non-stem cells30. Cancer cell-intrinsic PDL1 promotes tumour stemness-like properties by increasing expression of canonical stemness genes (for example, Oct4 and Nanog), self-renewal and in vivo tumorigenesis in mouse and human melanoma and ovarian cancer cells30. However, more specific mechanisms for PDL1-mediated control of stemness properties and consequences for tumour pathogenesis or treatment resistance have not been reported in detail.
Cancer cell-intrinsic PDL1 immune effects
Immune outcomes from cancer cell surface PDL1 engaging immune cell surface PD1 are well described and are a basis for FDA-approved ICB antibodies8,9. By contrast it is little appreciated that cancer cell-intrinsic PDL1 signalling can also alter anticancer immunity. For example, as mentioned in the previous section, cell-intrinsic PDL1 can inhibit STAT3 activation (FIG. 1b) to inhibit sensitivity to IFNα, IFNβ and IFNγ and their signals in mouse melanoma cells in vitro27, promote tumour immunogenic cell death through pyroptosis in vitro in human and in vivo in mouse TNBC35, and suppress FAS–FASL-mediated apoptosis in a mouse mastocytoma model in vitro29. Interestingly, cancer cell-intrinsic nuclear PDL1 can promote transcription of major histocompatibility complex (MHC) class 1 expression in various human TNBC cell lines in vitro36,91 that theoretically could inhibit natural killer cells or improve antigen-specific immune recognition (TABLE 1). Cancer cell-intrinsic PDL1 could also control antigen processing or presentation based on its known autophagic control23,24 contributing to antigen processing92.
Although nuclear PDL1 can upregulate MHC class 1 expression, which could enhance antitumour immune responses, it can simultaneously increase transcription of inhibitory immune checkpoint molecules (for example, V-type immunoglobulin domain-containing suppressor of T cell activation (VISTA), B7-H3 and galectin-9) in various human TNBC cells in vitro36 that could counteract the effects of the former and reduce ICB efficacy. These findings suggest opposing consequences of cell-intrinsic PDL1 on antitumour immune responses, the net effect of which could differ in distinct tumours, environments or treatments. In vivo, nuclear (de-acetylated) PDL1 was shown to mediate anti-PD1 resistance through as yet undefined mechanisms which result in reduced activated tumour-infiltrating T cells in heterotopic MC38 colon cancer36 and, possibly, by increasing hypoxia-dependent, tumour-associated inflammation in 4T1 murine TNBC35.
Cancer cell-intrinsic PDL1 signals can also regulate the DNA damage response in human breast and colon cancer cells34 that could supress accumulation of mutations14 and/or sensing of damaged nucleic acid in the cytoplasm (for example, by cyclic GMP–AMP synthase (cGAS)/stimulator of interferon genes (STING)), both potentially affecting tumour immunogenicity93, or could promote anti-PD1 resistance through NOD-, LRR- and pyrin domain-containing 3 (NLRP3) inflammasome-dependent recruitment of immunosuppressive cell subsets including macrophages as shown in a murine BRAFV600E/PTEN melanoma model28. Cancer cell-intrinsic PDL1 controls tumour nuclear factor-κB (NF-κB) signalling36, and we speculate downstream immune cell trafficking effects through differential chemokine expression as noted in RNA-sequencing data sets of PDL1 knockdown versus control MC38 tumours91. Thus, targeting cell-intrinsic PDL1 signals could further enhance the known immune potentiating of selected agents, for example poly(ADP-ribose) polymerase (PARP) inhibitors94, a possibility that remains largely untested.
Therapeutic anti-PDL1 antibodies designed to disrupt cell surface PDL1/PD1 ligation can also alter some cancer cell-intrinsic PDL1 signals. We reported that anti-PDL1 directly suppresses melanoma, ovarian and bladder cancer cell proliferation in vitro, including potential tumour PD1 participation independent of immunity23,24. Anti-PDL1 antibodies could improve melanoma sensitivity to type I interferons independent of PD1 (REF.27), and activate STAT3-mediated suppression of pro-tumour NLRP3 inflammasome in mouse melanoma cells in vitro27. Because melanoma PDL1 promotes NLRP3 activation to augment anti-PD1 resistance in vivo28, cell-intrinsic PDL1 targeting with anti-PDL1 antibodies to reverse this mechanism of resistance to anti-PD1 seems possible but has not been explored, which could include combining anti-PD1 with anti-PDL1. An RNA-sequencing study tested atezolizumab (human anti-PDL1) effects on gene expression in human MDA-MB-231 TNBC in vitro to show that it reduced epithelial–mesenchymal transition and cell-growth and hypoxia pathways78, suggesting unappreciated cell-intrinsic effects of clinically utilized anti-PDL1 agents that require study.
An anti-PDL1 antibody increased tumour cell apoptosis and sensitivity to cytotoxic chemotherapies in vitro81, but as this antibody was later shown to deplete total tumour cell PDL1 by suppressing its interaction from CMTM6 to increase lysosomal PDL1 degradation34, the mechanism for apoptosis induced by anti-PDL1 here needs clarification. Specifically, the effect of anti-PDL1 blocking antibodies altering cell-intrinsic PDL1 signals could be due to disruption of non-canonical signals generated from surface PDL1 or may, consequently, result from altered subcellular redistribution of surface PDL1 to the cytoplasm or nucleus. Tumour PD1 or CD80 contributions and other mechanistic details for these novel anti-PDL1 effects are largely unreported.
Metabolic effects of cancer cell-intrinsic PDL1 can theoretically increase sarcoma capacity to outcompete T cells for glucose, likely by promoting mTORC1 activation in sarcoma cells, leading to reduction of T cell antitumour efficacy95. Some metabolic effects were induced by anti-PDL1 antibody How these cell-intrinsic PDL1 signals affect antitumour immunity or ICB efficacy in vivo requires further investigation.
Of note, some cancer cell-intrinsic PDL1 signals, especially from intracellular PDL1, are clearly refractory to inhibition by anti-PDL1 antibodies (for example, chromosome maintenance by sister chromatid cohesion)37. Interestingly, in contrast to a study in melanoma cells, anti-PDL1 (and anti-PD1) augmented human non-small-cell lung cancer proliferation in vitro, possibly by activating AKT–ERK1/2 signals72, and could also explain some tumour type and context-specific anti-PDL1 treatment failures. Thus, new ways to target pathologic cell-intrinsic PDL1 signals in cancer cells which could drive immunotherapy resistance and rethinking of efficacy mechanisms for anti-PDL1 (and anti-PD1) ICB and the meaning of tumour PDL1 status as an ICB biomarker are needed. As immune cells also express PDL1 that generates cell-intrinsic signals67,68,70 (BOX 2), additional work investigating such effects on antitumour immunity and ICB response is warranted.
Cellular regulation of PDL1 content
Cell-autonomous regulation of cell-intrinsic and subcellular PDL1 content.
Factors and mechanisms controlling cancer cell or immune cell PDL1 expression have been comprehensively reviewed53,96,97, and major endogenous PDL1 regulation controls are shown in FIG. 2. Although not well appreciated, total cancer cell PDL1 content is dependent on the cell cycle, with relatively increased PDL1 levels during the G2/M phase in human MDA-MB-231 TNBC cells37 and decreased levels during the G1 phase in human MDA-MB-231 and HCC1954 TNBC and human HeLa cervical cancer lines98, all in vitro. Nuclear PDL1 likely accumulates in the G2/M phase and is consistent with cell-intrinsic, nuclear PDL1 control of sister chromatid cohesion/chromosomal stability in MDA-MB-231 cells37 but has yet to be assessed. Similarly, clinically observed PDL1 3′ untranslated region truncation leads to high cancer cell total PDL1 expression largely seen in certain leukaemias, lymphomas and gastric adenocarcinomas, associated with higher progression, but not affecting anti-PDL1 responsiveness44. Although subcellular-specific PDL1 expression has also not been explored in these studies, we predict that such augmented PDL1 expression could promote observed tumour progression by increasing cell-intrinsic PDL1 signals.
PDL1 distribution between the cell surface and cytosol is regulated homeostatically by CMTM4/CMTM6/TRAPPC4 (REFS65,66,99) and HIP1R79 that promote or inhibit (respectively) endosomal to surface PDL1 recycling (FIG. 2). Thus, tumours that express high levels of endosomal recycling scaffolds such as CMTM6, as shown in MEL-53 human melanoma cells in vitro65, can suppress antitumour immunity. Genetic silencing of Trappc4 reduced PDL1 expression in mouse colon epithelial cells to reduce carcinogen-induced colon cancer, and in a heterotopic mouse MC38 colon cancer model to improve anti-PDL1 efficacy in vivo and to improve T cell cytotoxicity against human RKO colon cancer cells in vitro99. In both cases, silenced genes reduced tumour surface PDL1 by promoting its lysosomal degradation. By contrast, tumours with high HIP1R expression could have increased cytoplasmic or nuclear, relative to surface, PDL1. Although CMTM6, TRAPPC4 or HIP1R could regulate surface PDL1 content homeostatically, we note here that certain exogenous cellular stressors such as cytotoxic doxorubicin82 or other chemotherapies35 could also facilitate the redistribution of surface PDL1 to the cytosol or nucleus, originally observed in MDA-MB-231 TNBC82, but the mechanistic underpinning of such effects remains to be elucidated.
It had been previously suggested that nuclear PDL1 was an artefact of immunofluorescence microscopy100, but the existence of functional nuclear PDL1 has since been reported in convincing studies using nuclear localization-defective PDL1 mutants35,36. For example, post-translational HDAC2-dependent de-acetylation of the PDL1 C-terminal tail at K263 or the PDL1 K263 point mutant reduced nuclear PDL1 levels, whereas p300-dependent acetylation opposes this effect36. The observation that vimentin+ circulating cancer cells in patients with metastatic colon cancer showed nuclear PDL1 expression was of unclear functional importance but suggested that factors controlling epithelial–mesenchymal transition can facilitate nuclear PDL1 accumulation. In support, the same study describing acetylated PDL1 showed that incubation of human HCC1937 TNBC cells with transforming growth factor-β (TGFβ) increased nuclear PDL1 expression35, presumably by promoting vimentin expression. Vimentin contributes to promoting nuclear PDL1 translocation through a mechanism that may involve acetylation of surface-derived PDL1, but it likely facilitates general nuclear-cytoplasmic shuttling of PDL1 derived from other non-surface subcellular locations (for example, cytosol) independent of acetylation36, which requires additional investigation.
Additional mechanisms of nuclear PDL1 entry have also been rigorously demonstrated. Hypoxia-induced binding of p-STAT3 to the human PDL1 C-terminal tail increases PDL1 nuclear localization, which may be augmented by many environmental and anticancer agents in breast cancer in vitro35. This study identified putative PDL1 nuclear localization and retention sequences35. Whether PDL1 nuclear entry always requires a binding partner warrants more experimentation. Although PDL1 C-terminal tail post-translational modifications augment PDL1 nuclear localization35,36, factors mediating PDL1 nuclear import versus those regulating PDL1 nuclear retention or protein stability as seen in other nuclear protein regulation (for example, breast cancer type 1 susceptibility protein (BRCA1)101 and androgen receptor102) have not been reported to date. PDL1 nuclear translocation in lung cancer was facilitated through KPNB1 binding to promote GAS6–tyrosine-protein kinase MER (MERTK signalling)80 but whether this mechanism is dependent on acetylation or the PDL1 C-terminal tail is not known. We anticipate that moieties aside from the PDL1 C-terminal tail could increase PDL1 nuclear localization and mediate related signals, likely depending on specific tumour type and genetic backgrounds, which are areas warranting additional investigation.
The cellular source for nuclear PDL1 remains unresolved. Nuclear PDL1 could result from post-translationally modified PDL1 in the endoplasmic reticulum and/or Golgi followed by clathrin-mediated endocytosis from the surface, as with other surface proteins such as extracellular growth factor receptor (EGFR)103, or directly from cytosolic PDL1 synthesized by free/unbound ribosomes (FIG. 2). One study reported hyper-N-glycosylated (~150 kDa) nuclear PDL1 compared with cytosolic or membrane-bound PDL1 (~55 kDa) in MDA-MB-231 human TNBC cells37, suggesting that hyper-glycosylation might increase nuclear PDL1 translocation and/or retention and implicates a unique endoplasmic reticulum/Golgi-dependent, cell surface-independent pathway for nuclear PDL1 accumulation (FIG. 2). Discordantly, however, another report also using MDA-MB-231 cells did not observe a 150 kDa species of PDL1 in the nucleus and found that PDL1 glycosylation was dispensable for nuclear localization, rigorously demonstrated using a glycosylation-defective (4NQ) mutant PDL1 and subcellular fractionations36, further underscoring the need for additional studies of PDL1 subcellular localization control. The specific mechanisms for nuclear PDL1 accumulation and retention appear context specific and can be further altered by certain cellular stress factors. The source or site of post-translational modifications affecting nuclear PDL1 content remains incompletely understood.
Potential therapeutic strategies
General considerations.
Although cytokines, notably IFNγ and tumour necrosis factor (TNF), are well known to augment PDL1 expression, studies generally do not distinguish effects on surface versus other subcellular locations. One study reported that IFNγ is less effective in inducing tumour nuclear PDL1 than TNF35. Additional work is needed to understand whether these cytokines can drive specific cell-intrinsic PDL1 signalling outcomes such as chemoresistance, including studying effects in distinct tumour types and immune cells. Indeed, in vivo tumour PDL1 induced by IFNγ (primarily derived from T cells) compared with induction by TNF (mainly derived from myeloid cells) elicited distinct tumour signals and tumour biological behaviour104. We speculate that differential outcomes could be, in part, the result of differential subcellular PDL1 distribution elicited by distinct cytokines. For example, TGFβ can increase nuclear PDL1 in some human breast cancer cell lines in vitro, which could be vimentin-dependent in TNBC36, whereas IFNγ appears to promote cell-intrinsic surface PDL1-driven NLRP3 inflammasome activation28. In addition, we reported sexually dimorphic PDL1 effects on anti-PDL1 immunotherapy and cytokine production in B16 melanoma in vitro and in vivo70, suggesting potential PDL1–sex hormone interactions which require investigation. IFNγ induces PDL1 largely through transcription whereas TNF induces PDL1 largely through protein stabilization by inhibiting its ubiquitination105. As cytokines such as TNF and IFNγ have pleiotropic effects, the ability to target cancer cell-intrinsic PDL1 signals elicited by specific cytokine microenvironmental factors will depend on better mechanistic understanding of their net effects and influence on PDL1 post-translational modifications.
Optimal targeting of cancer cell-intrinsic PDL1 signals requires a thorough understanding of these signals and their consequences, of signals in specific tumour types, in individual tumours from a given host owing to mutational and microenvironmental differences, and of off-target or secondary treatment effects. Considerations here are based on best available data at the time of writing that will surely change as new information becomes available. As it is unlikely that single treatment approaches will cure most cancers, combination strategies merit consideration based on understanding specific cell-intrinsic versus cell-extrinsic PDL1 signals in given settings.
Cancer cell-intrinsic PDL1 signals can be exploited clinically in three areas: as therapies alone or combined with other agents, as treatment response biomarkers or as prognosis biomarkers. No strategy specifically depleting tumour PDL1 has yet been tested clinically to our knowledge.
Strategies for global cellular PDL1 protein reduction.
Studies of cancer cell PDL1 expression reduction effects on treatment have largely used genetic tumour PDL1 depletion in established cell lines, for example leading to reduced resistance to small-molecule drugs such as mTOR inhibitors24 or cytotoxic chemotherapy23. Aside from genetic PDL1 depletion itself, genetic reduction of gene products promoting PDL1 expression can reduce tumour PDL1 content for therapeutic utility65,99, and could selectively reduce cytosolic or nuclear PDL1 based on their mechanisms, as discussed above in Cell-autonomous regulation of cell-intrinsic and subcellular PDL1 content. Genetic approaches are useful for proof of concept but are not practical current clinical strategies. Potential cell-intrinsic PDL1 signal mechanisms that can be or have been therapeutically targeted are shown in FIG. 2.
Several groups have now demonstrated small molecules depleting tumour PDL1 as cancer treatment. Verteporfin, a benzoporphyrin FDA-approved for retinal diseases, reduced cancer cell PDL1 content in distinct human cancer lines and immune and stromal cells through autophagy and inhibition of STAT1 and IRF1-dependent CD274 (PDL1 gene) transcription. It improved PARP inhibitor efficacy in mouse ovarian tumours in vivo39. The natural product curcumin inhibited COP9 signalosome complex 5 protein to deplete cancer cell PDL1 content by promoting its ubiquitination and improved anti-CTLA4 immunotherapy efficacy in mouse 4T1 TNBC105. The natural quinolizidine alkaloid product oxymatrine epigenetically reduced PDL1 expression in cultured human SW620 and HCT116 colorectal cell lines and was cytotoxic to them106. Aspirin (acetylsalicylic acid) transcriptionally suppressed PDL1 protein and inhibited proliferation of human A549 and H1299 non-small-cell lung cancer cells in vitro107.
Small-molecule PDL1 and PD1 inhibitors are described108,109, most of which inhibit surface PDL-PD1 interactions. The subset that reduce cellular PDL1 content could also reduce cancer cell-intrinsic PDL1 signals. Those designed to inhibit PDL1–PD1 surface interactions could also reduce cell-intrinsic PDL1 signals originating from the cell surface. Therapeutically, increasing PDL1 with the cyclin-dependent kinase 4/6 (CDK4/6) inhibitors palbociclib or ribociclib98, or decreasing it with the EGFR inhibitor gefitinib54, improved anti-PD1 immunotherapy in mouse models in vivo (for example, 4T1, B16F10, CT26), demonstrating alternative means to control tumour PDL1 for therapeutic benefit. The EGFR inhibitors erlotininb110 and osemertinib111 inhibit tumour PDL1 expression, notably in EGFR-mutated human small cell lung cancer lines. Glucose deprivation in vitro or a ketogenic diet in vivo in CT26 tumour-bearing mice reduced tumour PDL1 expression via adenosine monophosphate-activated protein kinase (AMPK)-dependent PDL1 C-terminal tail phosphorylation that reduced PDL1–CMTM4/6 interactions, thereby increasing PDL1 lysosomal targeting for degradation112. Metformin (FDA-approved diabetes drug) AMPK dependently phosphorylates PDL1 at S195, leading to subsequent endoplasmic reticulum accumulation and depletion of tumour PDL1, thereby improving anti-CTLA4 immunotherapy efficacy in mouse 4T1 TNBC113. In hepatocellular carcinoma, the JAK inhibitor ruxolitinib depleted tumour PDL1 in vitro by preventing IL-6-driven JAK1 phosphorylation at Y112 to stabilize PDL1 (REF.114).
The anti-PDL1 antibody H1A prevented PDL1–CMTM6 interaction, thereby redirecting PDL1 to lysosomes for degradation in MDA-MB-231 TNBC cells34 to prevent cell-intrinsic PDL1-driven radioresistance. In a similar process, a synthetic peptide diverted PDL1 to lysosomes for degradation through an HIP1R-dependent mechanism, in human colorectal cells79, differing from HIP1R-mediated surface to nuclear PDL1 translocation through PDL1 deacetylation36. A peptide inhibitor of PDL1 palmitoylation in the C-terminal cytoplasmic tail or the small molecule 2-bromopalmitate (general palmitoylation inhibitor) depleted PDL1 by promoting its ubiquitination in vitro and in vivo, respectively, in MC38 mouse colon cancer cells, thereby improving antitumour T cell functions115. Neither PDL1 intrinsic signals from specific cancer cell subcellular locations nor PD1 dependence altered by these PDL1 depletion or antagonist agents were described in detail.
Casimersen, eteplirsen and golodirsen are oligonucleotide drugs that alter gene splicing, FDA-approved for Duchenne muscular dystrophy. Similar agents could potentially inhibit pathogenic PDL1 isoforms arising from splicing events while limiting autoimmune side effects. We mined drug libraries to identify 17 candidate PDL1 depletion drugs that depleted tumour PDL1 >2.6-fold in vitro, including in specific subcellular locations that phenocopied genetic PDL1 depletion effects and improved small-molecule DNA damage response inhibitor efficacy in vivo (patent application PCT/US2021/022244). The latter approach is likely the most practical for unbiased identification and rapid clinical translation of PDL1-depleting drugs in humans.
The BET bromodomain inhibitor JQ1 suppressed tumour PDL1 in a mouse model for ovarian cancer and promoted survival through improved antitumour cytotoxic T cell effects116. The small molecule tomivosertib inhibits eIF4E phosphorylation and reduced PDL1 expression in a mouse model for hepatocellular carcinoma to reduce tumour growth117. None of the foregoing studies of FDA-approved or investigational agents reported effects on specific subcellular PDL1 expression. Much additional work is needed to understand optimal use of these promising strategies. Mechanisms regulating tumour PDL1 expression are extensively reviewed96,97, whose deep understanding will help to optimize such approaches and identify additional agents to reduce cell-intrinsic PDL1 signalling.
Naturally PDL1-deficient tumours could be more susceptible to IFNα, IFNβ or IFNγ27, or DNA-damaging cytotoxic agents23. Although several studies report that cell-intrinsic tumour PDL1 mediates chemoresistance, there are important exceptions such as tumour PDL1 expression enhancing chemosensitivity in BrafV600E mutated colon cancers cells in vitro25. We reported that expression of tumour PDL1 positively correlates with autophagy inhibitor susceptibility in melanoma and ovarian cancer24. Thus, as depleting PDL1 could increase efficacy of some therapies, it could equally increase resistance to others in distinct cancers. Understanding these cell-intrinsic PDL1 signalling effects will help to inform current treatment regimens involving PDL1 targeting agents and could explain emergence of resistance and/or relapse in specific cancers.
Altering subcellular PDL1 content.
As PDL1 in distinct subcellular locations has differing signal and therapeutic effects, depleting PDL1 in specific subcellular locations could be a better strategy than global PDL1 depletion. Expression of nuclear (but not total) PDL1 in circulating cancer cells in patients with metastatic colorectal or prostate cancer predicted shorter survival versus patients whose circulating cancer cells lacked nuclear PDL1 (REF.118). B16 lung metastases exhibited higher nuclear PDL1 versus the primary subcutaneous tumours that expressed predominantly surface membrane PDL1 (REF.36). Thus, if nuclear PDL1 contributes to ICB resistance of lung metastases as we predict, a nuclear PDL1 reduction agent could improve efficacy. For example, small-molecule HDAC2 inhibitors (for example, Santacruzamate A) reduced tumour PDL1 nuclear accumulation and improved anti-PD1 immunotherapy in heterotopic MC38 colon cancer36. Additionally, a clathrin-dependent endocytosis inhibitor (pitstop 2) and an inhibitor of importin-α/β-dependent nuclear transport (FDA-approved anti-parasitic agent ivermectin)36 each reduced PDL1 nuclear translocation in human TNBC MDA-MB-231 cells in vitro36, although functional consequences were not reported.
Metformin reduces PDL1 protein levels through inducing endoplasmic reticulum accumulation of PDL1 that promoted its degradation113 and could potentially block specific cell-intrinsic PDL1 signalling that requires processing by the endoplasmic reticulum, but may not affect endoplasmic reticulum-independent pathologic consequences. Endosomal inhibitors could selectively reduce cytoplasmic PDL1 by inhibiting endosomal surface-cytoplasm PDL1 recycling. Identifying other agents trapping PDL1 on the cell surface is warranted. Interestingly, a novel compound (‘drug A’) designed to disrupt PD1–PDL1 surface interaction by inducing PDL1 dimerization and cytosolic uptake reduced growth of heterotopic MC38 colon cancer in vivo119. Although this strategy could improve antitumour T cell responses similar to anti-PDL1–PD1 blockade, an unwanted consequence could be paradoxically increased cell-intrinsic PDL1 signalling by intracellular PDL1, driving resistance to certain anticancer agents such as cytotoxic chemotherapy or ICB by non-canonical mechanisms. Thus, a more complete understanding of cell-intrinsic PDL1 signalling from subcellular locations will better inform the design of treatment combinations using novel agents that alter PDL1 cellular distribution. Of note, a report focusing on tumour glucose metabolism using mouse sarcoma, B16 melanoma, L cells and MC38 colon cancer cells incidentally showed that anti-PDL1 antibodies in vitro increased PDL1 in tumour cytosol95 associated with altered tumour glucose metabolism which could potentially alter cell-intrinsic PDL1 signals, but underlying mechanisms or similar effects using in human cells were not reported.
As discussed above, tumour PDL1 induced by myeloid cell TNF–IL-1β (through NF-κB) versus T cell IFNγ (through STAT1) generated distinct tumour biological phenotypes104 through unknown mechanisms. We hypothesize that these differences could be from a known differential subcellular PDL1 distribution elicited by the distinct cytokines35. Alterations of cytokine-producing cells or agents to affect tumour subcellular PDL1 content could affect cell-intrinsic PDL1 signals through the mechanisms described above and shown in FIG. 2. Specific cellular stressors such as hypoxia increase nuclear PDL1 (REF35), implicating selected hypoxia-modulating agents such as bevacizumab120 as therapeutic cancer cell-intrinsic PDL1 targeting strategies.
Interrupting cell-intrinsic PDL1 signals.
Anti-PDL1 ICB antibodies sensitized mouse B16 melanoma cells directly to IFNβ-mediated cytotoxicity via inhibition of STAT3 in vitro27. This report also described clinically occurring somatic CD274 mutations governing interferon-mediated cytotoxicity sensitivity in human cancers, suggesting clinically relevant cell-intrinsic PDL1 effects that merit investigation. Other reports23,24,26,78,95, discussed above in the section Cancer cell-intrinsic PDL1 immune effects, suggest that anti-PDL1 ICB antibodies can also alter some cell-intrinsic PDL1 signals, the scope of which remains incompletely defined. Understanding the additional signalling effects of existing anti-PDL1 (and antibodies inhibiting other immune checkpoints) could lead to new insights into effective treatment combinations.
PDL1 post-translational modifications53 could be specifically targeted to alter outcomes of these modifications, which was demonstrated using EGFR inhibitors to reduce PDL1 glycosylation, thereby reducing T cell suppression in vitro54, a strategy that could be used to prevent PDL1 nuclear translocation as glycosylation could promote nuclear PDL1 accumulation37. Remarkably, as intracellular PDL1 can bind DNA and globally influence expression of many genes36, it could regulate gene expression at the epigenetic level and could be explored using techniques such as ATAC-seq of PDL1 replete versus knockout cells. If so, PDL1 transcriptional regulation of gene expression programmes could be altered by available and FDA-approved small-molecule epigenetic inhibitors such as 5-azacytidine.
Finally, work is required to understand how the above cancer cell-intrinsic PDL1 targeting strategies influence antitumour T cell-mediated immunity. As subcellular compartment-specific cancer cell PDL1 mediates such varied immune outcomes, it is likely that some tumour PDL1-depleting strategies will paradoxically improve anti-PDL1 treatments, for example by altering local chemokines or tumour immunogenicity (FIG. 1b; TABLE 1). Some approaches, for example, the HDAC2 inhibitor Santacruzamate A36, will mediate effects that could alter antitumour immunity. The net clinical effect of cell-intrinsic PDL1 targeting agents will likely require tumour, context and adjunct treatment-specific studies (for example, engineered cytokines and anti-CTLA4).
Conclusions and future considerations
The paradigm of a surface PDL1–PD1 signalling axis is correct but too simplistic and incomplete based on current understanding of the multiplicity of intracellular interactions between PDL1 and many molecules and macromolecular structures governing diverse pathologic processes. This cell-intrinsic PDL1 signalling differs from surface PDL1 signals to PD1 on distinct cells and requires re-thinking of this important paradigm. Likewise, the multiplicity of unexpected cell-intrinsic signal effects of anti-PDL1 antibodies (and likely other ICB antibodies) requires careful reconsideration of their mechanisms of action that can influence patient and combination treatment selection.
Cancer cell-intrinsic PDL1 generates signals affecting growth, survival, metastatic, metabolic, differentiation, stemness and treatment resistance pathways. While mediating biologic mayhem, these pathways also afford novel, actionable treatment targets for potentially more effective interventions or to improve efficacy of existing treatments. We speculate that understanding these cancer cell-intrinsic PDL1 signals could help to explain the incomplete ICB response predicted by expression of tumour PDL1 (REF.14), or hyper-progression after ICB121. The existence of tumour cell-intrinsic PDL1 signalling thus warrants a more nuanced appreciation of tumour PDL1 status clinically. The remarkable discovery that pathologic cell-intrinsic PDL1 signals can originate from intracellular sources such as the cytoplasm or nucleus implicates PDL1 expression in these locations as useful treatment or prognostic biomarkers requiring much further investigation in humans.
Cancer cell-intrinsic PDL1 signal effects can differ by tumour type, likely owing to mutational landscapes in distinct tumours, variations in individual tumours in patients, tumour microenvironmental factors and differential post-translational PDL1 modifications now recognized to contribute to immune checkpoint efficacy75 and cell-intrinsic PDL1 signalling35,36, among other considerations. Additional PDL1 signals arise from disparate sources including exosomes or vesicles bearing PDL1, soluble PDL1 that arises through distinct mechanisms45, platelets19, non-hematopoietic cells22 (BOX 2) and isoforms arising through various mechanisms (FIG. 1a). Assessing cell-intrinsic PDL1 or consequent signalling effects in human clinical trials warrants much additional investigation. A better understanding of the mechanisms directing PDL1 to, or retaining it in, specific subcellular compartments is needed.
Several approaches have already demonstrated the utility of specifically targeting cell-intrinsic PDL1 signals using novel molecules34,79 or existing research reagents115. Repurposing FDA-approved agents is another rapidly translatable approach that has been validated preclinically24,27,36,39,107, as is using natural products generally regarded as safe, such as curcumin105 or oxymatrine106. Investigational agents also deplete specific subcellular34,39 and non-surface20 PDL1 signals. Virtually all of the agents we discuss could suppress cell-intrinsic PDL1 signals in tumour or non-cancer cells. Approaches specifically targeting PDL1 in cancer cells (as opposed to immune or stromal cells) merit further study as off-tumour drug effects might be undesirable in some instances, especially when used in combination with other antitumour agents. Some approaches can be clinically translated rapidly.
Work is needed to place pathologic effects of exosomal, vesicular and soluble PDL1 (REFS17,18), including its isoforms17,40-45 (BOX 2; FIG. 1a), in context relative to effects of cancer cell-intrinsic and cell-extrinsic PDL1 signals, which also requires study in distinct tumours. For example, PDL1 regulation of local chemokines will affect tumour-infiltrating lymphocytes known to alter ICB. PDL1 control of the DNA damage response34 or DNA damage32 could suppress tumour immunogenicity and PDL1 reduction could increase tumour immunogenicity from increased cytosolic nucleic acid activation of cGAS-STING122. PDL1 control of pyroptosis35 or apoptosis29 could likewise be actionable.
In some instances, cancer cell-intrinsic PDL1 signals are beneficial to treatment. For example, genetic PDL1 depletion suppresses tumour sensitivity to small-molecule autophagy inhibitors24 or selected cytotoxic agents25. These effects are tumour-specific, which likely relates to tumour-specific mutations, tissues or origin, the local microenvironment and other factors. Differentiating such detrimental versus beneficial effects of targeting cell-intrinsic PDL1 is an important goal.
Some PDL1 biology merits additional study for translational potential. For example, the cell cycle dependence of PDL1 expression suggests that combining cytotoxic agents with anti-PDL1 could be improved using cytotoxic agents specifically active in the cell cycle phase when PDL1 expression peaks, such as gemcitabine123. Agents active across the entire cell cycle might be less effectively combined, such as taxanes124 and platinum drugs125. These distinctions are important in that taxanes and platinum agents are in FDA-approved anti-PDL1 (and anti-PD1) combinations. Immune cell-intrinsic PDL1 signals could alter tumour immune surveillance or treatment responses but are little studied. There are no reported effects of altering cancer cell-intrinsic PDL1 signals in clinical trials, or of testing specific subcellular PDL1 expression levels as a treatment response biomarker to our knowledge.
PDL1 is not unique in its potential for pathologic cancer cell-intrinsic signals. It is an immunoglobulin superfamily member whose other members thus far studied share signalling similarities with PDL1, although this aspect is as yet little studied. A recent report that tumour microenvironmental IgA mediates cell-intrinsic tumour signals126 suggests that immunoglobulins themselves mediate important cell-intrinsic signals, but no known immune checkpoint molecule has been shown to mediate cell-intrinsic signalling from its immunoglobulin structure per se. Cell-intrinsic signal effects of other immunoglobulin superfamily members (BOX 2) is an area warranting additional investigation.
Essentially, all in vivo tumour PDL1 biology studies have been done in established tumours. Whether PDL1 specifically affects carcinogenesis and whether cell-intrinsic PDL1 signal effects differ in tumours arising from PDL1 null versus PDL1-expressing cells of origin have yet to be reported but are important to understand. Such studies would require the development of cancer cell of origin (for example, melanocyte) specific Cd274-knockout mouse models.
PDL1 depletion could combine well with small-molecule DNA damage response inhibitors such as PARP inhibitors known to augment cGAS–STING-driven immunity127,128 but also with agents inhibiting other DNA damage response pathways. As PDL1 can also directly bind to DNA36 or RNA34, cell-intrinsic PDL1 could theoretically regulate immunogenicity of nucleic acids themselves and their recognition by innate immune sensing pathways not limited to cGAS–STING (for example, Toll-like receptors-retinoic acid-inducible gene 1–mitochondrial antiviral signalling protein)129 or transcription and/or stabilization of mRNAs encoding immune stimulatory effectors. As PDL1 depletion in cancer cells alters immune cell trafficking chemokines91 and could engender tumour immunogenicity35, we expect PDL1 depletion could improve efficacy of immunotherapies, including FDA-approved anti-PDL1 or anti-PD1 ICB antibodies, despite reduced tumour PDL1 expression, and other modalities known to benefit from antitumour immunity such as cytotoxic chemotherapy130. Of note, specific DNA damage such as double-strand DNA breaks induce PDL1 expression in an ataxia telangiectasia and Rad3-related ATR-Checkpoint kinase 1-dependent mechanism131,132. Use of selective small-molecule inhibitors of the ATR–ataxia telangiectasia mutated (ATM) pathway could prevent DNA damage-induced PDL1 expression resulting in simultaneous suppression of intrinsic and extrinsic PDL1 signals.
These are interesting times for the development of advanced cancer immunotherapies and rational combinatory approaches to improve the outcomes of patients with cancer. We expect that the targeting of cancer cell-intrinsic PDL1 signals will prove useful in this arena.
Acknowledgements
The authors thank H. Bai, C. Clark, Y. Deng, E. Galvan, H. Gupta, Y. Hu, A. Kancharla, S. Kari, R. Li, C. Murray, R. Reyes and D. Zhang for valuable contributions to their cell-intrinsic PDL1 signal studies. They apologize to colleagues whose important works in this area were not cited due to space constraints.
Footnotes
Competing interests
T.J.C. and A.V.R.K. have filed a patent on using intracellular PDL1 to predict anticancer responses and drugs that reduce tumour PDL1 for treatment purposes.
References
- 1.Dong H, Zhu G, Tamada K & Chen L B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat. Med 5, 1365–1369 (1999). [DOI] [PubMed] [Google Scholar]
- 2.Freeman GJ et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med 192, 1027–1034 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dong H et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med 8, 793–800 (2002). [DOI] [PubMed] [Google Scholar]
- 4.Iwai Y et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl Acad. Sci. USA 99, 12293–12297 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baumeister SH, Freeman GJ, Dranoff G & Sharpe AH Coinhibitory pathways in immunotherapy for cancer. Annu. Rev. Immunol 34, 539–573 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Zou W, Wolchok JD & Chen L PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci. Transl Med 8, 328rv324 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curiel TJ et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat. Med 9, 562–567 (2003). [DOI] [PubMed] [Google Scholar]
- 8.Ribas A & Wolchok JD Cancer immunotherapy using checkpoint blockade. Science 359, 1350–1355 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gong J, Chehrazi-Raffle A, Reddi S & Salgia R Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J. immunother. Cancer 6, 8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paterson AM et al. The programmed death-1 ligand 1:B7-1 pathway restrains diabetogenic effector T cells in vivo. J. Immunol 187, 1097–1105 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brahmer JR et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med 366, 2455–2465 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taube JM et al. Colocalization of inflammatory response with B7-H1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci. Transl Med 4, 127ra137 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang AC et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 545, 60–65 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Topalian SL, Taube JM, Anders RA & Pardoll DM Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat. Rev. Cancer 16, 275–287 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pauken KE et al. The PD-1 pathway regulates development and function of memory CD8+ T cells following respiratory viral infection. Cell Rep. 31, 107827 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma P, Hu-Lieskovan S, Wargo JA & Ribas A Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 168, 707–723 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ayala-Mar S, Donoso-Quezada J & Gonzalez-Valdez J Clinical implications of exosomal PD-L1 in cancer immunotherapy. J. Immunol. Res 2021, 8839978 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bocanegra A et al. PD-L1 in systemic immunity: unraveling its contribution to PD-1/PD-l1 blockade immunotherapy. Int. J. Mol. Sci 21, 5918 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zaslavsky AB et al. Platelet PD-L1 suppresses anti-cancer immune cell activity in PD-L1 negative tumors. Sci. Rep 10, 19296 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu B et al. Adipose PD-L1 modulates PD-1/PD-L1 checkpoint blockade immunotherapy efficacy in breast cancer. Oncoimmunology 7, e1500107 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krupnick AS et al. Murine vascular endothelium activates and induces the generation of allogeneic CD4+25+Foxp3+ regulatory T cells. J. Immunol 175, 6265–6270 (2005). [DOI] [PubMed] [Google Scholar]
- 22.Mueller SN et al. PD-L1 has distinct functions in hematopoietic and nonhematopoietic cells in regulating T cell responses during chronic infection in mice. J. Clin. Invest 120, 2508–2515 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang D et al. Bladder cancer cell-intrinsic PD-L1 signals promote mTOR and autophagy activation that can be inhibited to improve cytotoxic chemotherapy. Cancer Med. 10, 2137–2152 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clark CA et al. Tumor-intrinsic PD-L1 signals regulate cell growth, pathogenesis, and autophagy in ovarian cancer and melanoma. Cancer Res. 76, 6964–6974 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng D et al. BRAF(V600E)-induced, tumor intrinsic PD-L1 can regulate chemotherapy-induced apoptosis in human colon cancer cells and in tumor xenografts. Oncogene 38, 6752–6766 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng F et al. PD-L1 promotes self-renewal and tumorigenicity of malignant melanoma initiating cells. Biomed. Res. int 2017, 1293201 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gato-Canas M et al. PDL1 signals through conserved sequence motifs to overcome interferon-mediated cytotoxicity. Cell Rep. 20, 1818–1829 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Theivanthiran B et al. A tumor-intrinsic PD-L1/NLRP3 inflammasome signaling pathway drives resistance to anti-PD-1 immunotherapy. J. Clin. Invest 130, 2570–2586 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Azuma T et al. B7-H1 is a ubiquitous antiapoptotic receptor on cancer cells. Blood 111, 3635–3643 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta HB et al. Tumor cell-intrinsic PD-L1 promotes tumor-initiating cell generation and functions in melanoma and ovarian cancer. Signal Transduct. Target. Ther 1, 16030 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu W et al. PD-L1 promotes tumor growth and progression by activating WIP and β-catenin signaling pathways and predicts poor prognosis in lung cancer. Cell Death Dis. 11, 506 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song N et al. PD-L1 upregulation accompanied with epithelial-mesenchymal transition attenuates sensitivity to ATR inhibition in p53 mutant pancreatic cancer cells. Med. Oncol 37, 47 (2020). [DOI] [PubMed] [Google Scholar]
- 33.Cao D et al. Retinoic acid-related orphan receptor C regulates proliferation, glycolysis, and chemoresistance via the PD-L1/ITGB6/STAT3 signaling axis in bladder cancer. Cancer Res. 79, 2604–2618 (2019). [DOI] [PubMed] [Google Scholar]
- 34.Tu X et al. PD-L1 (B7-H1) competes with the RNA exosome to regulate the DNA damage response and can be targeted to sensitize to radiation or chemotherapy. Mol. Cell 74, 1215–1226.e4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hou J et al. PD-L1-mediated gasdermin C expression switches apoptosis to pyroptosis in cancer cells and facilitates tumour necrosis. Nat. Cell Biol 22, 1264–1275 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao Y et al. Acetylation-dependent regulation of PD-L1 nuclear translocation dictates the efficacy of anti-PD-1 immunotherapy. Nat. Cell Biol 22, 1064–1075 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu J et al. Regulation of sister chromatid cohesion by nuclear PD-L1. Cell Res. 30, 590–601 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang W et al. PD-L1 regulates genomic stability via interaction with cohesin-SA1 in the nucleus. Signal Transduct. Target. Ther 6, 81 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liang J et al. Verteporfin inhibits PD-L1 through autophagy and the STAT1–IRF1–TRIM28 signaling axis, exerting antitumor efficacy. Cancer Immunol. Res 8, 952–965 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Y et al. Development of a sandwich ELISA for evaluating soluble PD-L1 (CD274) in human sera of different ages as well as supernatants of PD-L1+ cell lines. Cytokine 56, 231–238 (2011). [DOI] [PubMed] [Google Scholar]
- 41.Mahoney KM et al. A secreted PD-L1 splice variant that covalently dimerizes and mediates immunosuppression. Cancer Immunol. Immunother 68, 421–432 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hassounah NB et al. Identification and characterization of an alternative cancer-derived PD-L1 splice variant. Cancer Immunol. Immunother. 68, 407–420 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang C et al. Distinct roles of programmed death ligand 1 alternative splicing isoforms in colorectal cancer. Cancer Sci. 112, 178–193 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kataoka K et al. Aberrant PD-L1 expression through 3′-UTR disruption in multiple cancers. Nature 534, 402–406(2016). [DOI] [PubMed] [Google Scholar]
- 45.Romero Y, Wise R & Zolkiewska A Proteolytic processing of PD-L1 by ADAM proteases in breast cancer cells. Cancer Immunol. Immunother 69, 43–55 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin DY et al. The PD-1/PD-L1 complex resembles the antigen-binding Fv domains of antibodies and T cell receptors. Proc. Natl Acad. Sci. USA 105, 3011–3016 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao Y et al. Antigen-presenting cell-intrinsic PD-1 neutralizes PD-L1 in cis to attenuate PD-1 signaling in T cells. Cell Rep. 24, 379–390.e6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fernandes RA et al. Immune receptor inhibition through enforced phosphatase recruitment. Nature 586, 779–784 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hui E et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science 355, 1428–1433 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patsoukis N, Wang Q, Strauss L & Boussiotis VA Revisiting the PD-1 pathway. Sci. Adv 6, eabd2712 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Butte MJ, Keir ME, Phamduy TB, Sharpe AH & Freeman GJ Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity 27, 111–122 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao Y et al. PD-L1:CD80 cis-heterodimer triggers the co-stimulatory receptor CD28 while repressing the inhibitory PD-1 and CTLA-4 pathways. Immunity 51, 1059–1073.e9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Y et al. Regulation of PD-L1: emerging routes for targeting tumor immune evasion. Front. Pharmacol 9, 536 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li CW et al. Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Nat. Commun 7, 12632 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zak KM et al. Structural biology of the immune checkpoint receptor PD-1 and its ligands PD-L1/PD-L2. Structure 25, 1163–1174 (2017). [DOI] [PubMed] [Google Scholar]
- 56.Magiera-Mularz K et al. Human and mouse PD-L1: similar molecular structure, but different druggability profiles. iScience 24, 101960 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mayoux M et al. Dendritic cells dictate responses to PD-L1 blockade cancer immunotherapy. Sci. Transl Med 12, eaav7431 (2020). [DOI] [PubMed] [Google Scholar]
- 58.He XH, Xu LH & Liu Y Identification of a novel splice variant of human PD-L1 mRNA encoding an isoform-lacking Igv-like domain. Acta Pharmacol. Sin 26, 462–468 (2005). [DOI] [PubMed] [Google Scholar]
- 59.Gong B et al. Secreted PD-L1 variants mediate resistance to PD-L1 blockade therapy in non-small cell lung cancer. J. Exp. Med 216, 982–1000 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mahoney KM et al. PD-L1 antibodies to its cytoplasmic domain most clearly delineate cell membranes in immunohistochemical staining of tumor cells. Cancer Immunol. Res 3, 1308–1315 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Page DB, Postow MA, Callahan MK, Allison JP & Wolchok JD Immune modulation in cancer with antibodies. Annu. Rev. Med 65, 185–202 (2014). [DOI] [PubMed] [Google Scholar]
- 62.Chen DS & Mellman I Elements of cancer immunity and the cancer-immune set point. Nature 541, 321–330 (2017). [DOI] [PubMed] [Google Scholar]
- 63.Hegde PS & Chen DS Top 10 challenges in cancer immunotherapy. Immunity 52, 17–35 (2020). [DOI] [PubMed] [Google Scholar]
- 64.Im SJ et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 537, 417–421 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Burr ML et al. CMTM6 maintains the expression of PD-L1 and regulates anti-tumour immunity. Nature 549, 101–105 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mezzadra R et al. Identification of CMTM6 and CMTM4 as PD-L1 protein regulators. Nature 549, 106–110 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dong W et al. The mechanism of anti-PD-L1 antibody efficacy against PD-L1-negative tumors identifies NK cells expressing PD-L1 as a cytolytic effector. Cancer Discov. 9, 1422–1437 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Diskin B et al. PD-L1 engagement on T cells promotes self-tolerance and suppression of neighboring macrophages and effector T cells in cancer. Nat. Immunol 21, 442–454 (2020). [DOI] [PubMed] [Google Scholar]
- 69.Zhang M et al. Programmed death-ligand 1 triggers PASMCs pyroptosis and pulmonary vascular fibrosis in pulmonary hypertension. J. Mol. Cell Cardiol 138, 23–33 (2020). [DOI] [PubMed] [Google Scholar]
- 70.Lin PY et al. B7-H1-dependent sex-related differences in tumor immunity and immunotherapy responses. J. Immunol 185, 2747–2753 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kleffel S et al. Melanoma cell-intrinsic PD-1 receptor functions promote tumor growth. Cell 162, 1242–1256 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang X et al. Tumor cell-intrinsic PD-1 receptor is a tumor suppressor and mediates resistance to PD-1 blockade therapy. Proc. Natl Acad. Sci. USA 117, 6640–6650 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mir MA & Agrewala JN Signaling through CD80: an approach for treating lymphomas. Expert Opin. Ther. Targets 12, 969–979 (2008). [DOI] [PubMed] [Google Scholar]
- 74.Sun L et al. Targeting glycosylated PD-1 induces potent antitumor immunity. Cancer Res. 80, 2298–2310 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang X et al. NEK2 inhibition triggers anti-pancreatic cancer immunity by targeting PD-L1. Nat. Commun 12, 4536 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang S et al. Programmed death ligand 1 promotes lymph node metastasis and glucose metabolism in cervical cancer by activating integrin β4/SNAI1/SIRT3 signaling pathway. Oncogene 37, 4164–4180 (2018). [DOI] [PubMed] [Google Scholar]
- 77.Jalali S et al. Reverse signaling via PD-L1 supports malignant cell growth and survival in classical Hodgkin lymphoma. Blood Cancer J. 9, 22 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Saleh R, Taha RZ, Sasidharan Nair V, Alajez NM & Elkord E PD-L1 blockade by atezolizumab downregulates signaling pathways associated with tumor growth, metastasis, and hypoxia in human triple negative breast cancer. Cancers 11, 1050 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang H et al. HIP1R targets PD-L1 to lysosomal degradation to alter T cell-mediated cytotoxicity. Nat. Chem. Biol 15, 42–50 (2019). [DOI] [PubMed] [Google Scholar]
- 80.Du W et al. KPNB1-mediated nuclear translocation of PD-L1 promotes non-small cell lung cancer cell proliferation via the Gas6/MerTK signaling pathway. Cell Death Differ. 28, 1284–1300 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu X et al. Targeting B7-H1 (PD-L1) sensitizes cancer cells to chemotherapy. Heliyon 4, e01039 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ghebeh H et al. Doxorubicin downregulates cell surface B7-H1 expression and upregulates its nuclear expression in breast cancer cells: role of B7-H1 as an anti-apoptotic molecule. Breast Cancer Res. 12, R48 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen RQ et al. The binding of PD-L1 and Akt facilitates glioma cell invasion upon starvation via Akt/autophagy/F-actin signaling. Front. Oncol 9, 1347 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Qiu XY et al. PD-L1 confers glioblastoma multiforme malignancy via Ras binding and Ras/Erk/EMT activation. Biochim. Biophys. Acta Mol. Basis Dis 1864, 1754–1769 (2018). [DOI] [PubMed] [Google Scholar]
- 85.Chen C et al. PD-L1 tumor-intrinsic signaling and its therapeutic implication in triple-negative breast cancer. JCI Insight 6, e131458 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shen B et al. PD-L1 and MRN synergy in platinum-based chemoresistance of head and neck squamous cell carcinoma. Br. J. Cancer 122, 640–647 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huttlin EL et al. The BioPlex network: a systematic exploration of the human interactome. Cell 162, 425–440 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Alberti S & Hyman AA Biomolecular condensates at the nexus of cellular stress, protein aggregation disease and ageing. Nat. Rev. Mol. Cell Biol 22, 196–213 (2021). [DOI] [PubMed] [Google Scholar]
- 89.Fei Z et al. PD-L1 induces epithelial–mesenchymal transition in nasopharyngeal carcinoma cells through activation of the PI3K/AKT pathway. Oncol. Res 27, 801–807 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang Y et al. The canonical TGF-β/Smad signalling pathway is involved in PD-L1-induced primary resistance to EGFR-TKIs in EGFR-mutant non-small-cellung cancer. Respir. Res 20, 164 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lau J et al. Tumour and host cell PD-L1 is required to mediate suppression of anti-tumour immunity in mice. Nat. Commun 8, 14572 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.You L et al. The crosstalk between autophagic and endo-/exosomal pathways in antigen processing for MHC presentation in anticancer T cell immune responses. J. Hematol. Oncol 10, 165 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Decout A, Katz JD, Venkatraman S & Ablasser A The cGAS–STING pathway as a therapeutic target in inflammatory diseases. Nat. Rev. Immunol 21, 548–569 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sen T et al. Targeting DNA damage response promotes antitumor immunity through STING-mediated T-cell activation in small cell lung cancer. Cancer Discov. 9, 646–661 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chang CH et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell 162, 1229–1241 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen S et al. Mechanisms regulating PD-L1 expression on tumor and immune cells. J. Immunother Cancer 7, 305 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cha JH, Chan LC, Li CW, Hsu JL & Hung MC Mechanisms controlling PD-L1 expression in cancer. Mol. Cell 76, 359–370 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang J et al. Cyclin D-CDK4 kinase destabilizes PD-L1 via cullin 3-SPOP to control cancer immune surveillance. Nature 553, 91–95 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ren Y et al. TRAPPC4 regulates the intracellular trafficking of PD-L1 and antitumor immunity. Nat. Commun 12, 5405 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Polioudaki H et al. Nuclear localization of PD-L1: artifact or reality? Cell Oncol. 42, 237–242 (2019). [DOI] [PubMed] [Google Scholar]
- 101.Tarsounas M & Sung P The antitumorigenic roles of BRCA1–BARD1 in DNA repair and replication. Nat. Rev. Mol. Cell Biol 21, 284–299 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lv S et al. Regulation and targeting of androgen receptor nuclear localization in castration-resistant prostate cancer. J. Clin. Invest 131, e141335 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gao YS, Hubbert CC & Yao TP The microtubule-associated histone deacetylase 6 (HDAC6) regulates epidermal growth factor receptor (EGFR) endocytic trafficking and degradation. J. Biol. Chem 285, 11219–11226 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wei Y et al. The local immune landscape determines tumor PD-L1 heterogeneity and sensitivity to therapy. J. Clin. Invest 129, 3347–3360 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lim SO et al. Deubiquitination and stabilization of PD-L1 by CSN5. Cancer Cell 30, 925–939 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hua S, Gu M, Wang Y, Ban D & Ji H Oxymatrine reduces expression of programmed death-ligand 1 by promoting DNA demethylation in colorectal cancer cells. Clin. Transl. Oncol 23, 750–756 (2021). [DOI] [PubMed] [Google Scholar]
- 107.Zhang Y, Lv C, Dong Y & Yang Q Aspirin-targeted PD-L1 in lung cancer growth inhibition. Thorac. Cance 11, 1587–1593 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wu Q et al. Small molecule inhibitors targeting the PD-1/PD-L1 signaling pathway. Acta Pharmacol. Sin 42, 1–9 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu C, Seeram NP & Ma H Small molecule inhibitors against PD-1/PD-L1 immune checkpoints and current methodologies for their development: a review. Cancer Cell Int. 21, 239 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Azuma K et al. Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann. Oncol 25, 1935–1940 (2014). [DOI] [PubMed] [Google Scholar]
- 111.Jiang XM et al. Osimertinib (AZD9291) decreases programmed death ligand-1 in EGFR-mutated non-small cell lung cancer cells. Acta Pharmacol. Sin 38, 1512–1520 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dai X et al. Energy status dictates PD-L1 protein abundance and anti-tumor immunity to enable checkpoint blockade. Mol. Cell 81, 2317–2331.e6 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cha JH et al. Metformin promotes antitumor immunity via endoplasmic-reticulum-associated degradation of PD-L1. Mol. Cell 71, 606–620.e7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chan LC et al. IL-6/JAK1 pathway drives PD-L1 Y112 phosphorylation to promote cancer immune evasion. J. Clin. Invest 129, 3324–3338 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yao H et al. Inhibiting PD-L1 palmitoylation enhances T-cell immune responses against tumours. Nat. Biomed. Eng 3, 306–317 (2019). [DOI] [PubMed] [Google Scholar]
- 116.Zhu H et al. BET bromodomain inhibition promotes anti-tumor immunity by suppressing PD-L1 expression. Cell Rep. 16, 2829–2837 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xu Y et al. Translation control of the immune checkpoint in cancer and its therapeutic targeting. Nat. Med 25, 301–311 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Satelli A et al. Potential role of nuclear PD-L1 expression in cell-surface vimentin positive circulating tumor cells as a prognostic marker in cancer patients. Sci. Rep 6, 28910 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Park JJ et al. Checkpoint inhibition through small molecule-induced internalization of programmed death-ligand 1. Nat. Commun 12, 1222 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Byers LA & Heymach JV Dual targeting of the vascular endothelial growth factor and epidermal growth factor receptor pathways: rationale and clinical applications for non-small-cell lung cancer. Clin. Lung Cancer 8 (Suppl. 2), S79–S85 (2007). [DOI] [PubMed] [Google Scholar]
- 121.Champiat S et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin. Cancer Res 23, 1920–1928 (2017). [DOI] [PubMed] [Google Scholar]
- 122.Kwon J & Bakhoum SF The cytosolic DNA-sensing cGAS–STING pathway in cancer. Cancer Discov. 10, 26–39 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tolis C, Peters GJ, Ferreira CG, Pinedo HM & Giaccone G Cell cycle disturbances and apoptosis induced by topotecan and gemcitabine on human lung cancer cell lines. Eur. J. Cancer 35, 796–807 (1999). [DOI] [PubMed] [Google Scholar]
- 124.Abal M, Andreu JM & Barasoain I Taxanes: microtubule and centrosome targets, and cell cycle dependent mechanisms of action. Curr. Cancer Drug Targets 3, 193–203 (2003). [DOI] [PubMed] [Google Scholar]
- 125.Donaldson KL, Goolsby GL & Wahl AF Cytotoxicity of the anticancer agents cisplatin and taxol during cell proliferation and the cell cycle. Int. J. Cancer 57, 847–855 (1994). [DOI] [PubMed] [Google Scholar]
- 126.Biswas S et al. IgA transcytosis and antigen recognition govern ovarian cancer immunity. Nature 591, 464–470 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ding L et al. PARP inhibition elicits STING-dependent antitumor immunity in Brca1-deficient ovarian cancer. Cell Rep. 25, 2972–2980.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pantelidou C et al. PARP inhibitor efficacy depends on CD8+ T-cell recruitment via intratumoral STING pathway activation in BRCA-deficient models of triple-negative breast cancer. Cancer Discov. 9, 722–737 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Qiao Y et al. Human cancer cells sense cytosolic nucleic acids through the RIG-I–MAVS pathway and cGAS-STING pathway. Front. Cell Dev. Biol 8, 606001 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang W et al. Effector T cells abrogate stroma-mediated chemoresistance in ovarian cancer. Cell 165, 1092–1105 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sato H et al. DNA double-strand break repair pathway regulates PD-L1 expression in cancer cells. Nat. Commun 8, 1751 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Vendetti FP et al. ATR kinase inhibitor AZD6738 potentiates CD8+ T cell-dependent antitumor activity following radiation. J. Clin. Invest 128, 3926–3940 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gupta HB et al. Tumor-intrinsic PD-L1 regulates tumor initiating cell virulence and stemness genes, and TCF1+ stem-like T cells through Raptor in ovarian cancer, which correlates with survival in high grade serous ovarian cancer. J. Immunol 202 (Suppl. 1), 195.29 (2019). [Google Scholar]
- 134.Lecis D, Sangaletti S, Colombo MP & Chiodoni C Immune checkpoint ligand reverse signaling: looking back to go forward in cancer therapy. Cancers 11, 624 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fanelli G et al. PD-L1 signaling on human memory CD4+ T cells induces a regulatory phenotype. PLoS Biol. 19, e3001199 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Okla K, Farber DL & Zou W Tissue-resident memory T cells in tumor immunity and immunotherapy. J. Exp. Med 218, e2020160 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li T et al. Exploring a tumor-intrinsic PD-L1 signal with proximity-dependent biotin identification in lung cancer cells. Biochemistry 58, 2293–2296 (2019). [DOI] [PubMed] [Google Scholar]
- 138.Erin D et al. PD-L1 reverse signaling in dermal dendritic cells promotes dendritic cell migration required for skin immunity. Cell Rep. 33, 108258 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cao Z et al. An unexpected role for p53 in regulating cancer cell-intrinsic PD-1 by acetylation. Sci. Adv 7, eabf4148 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Latchman Y et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol 2, 261–268 (2001). [DOI] [PubMed] [Google Scholar]
- 141.Ren T et al. Osteosarcoma cell intrinsic PD-L2 signals promote invasion and metastasis via the RhoA–ROCK–LIMK2 and autophagy pathways. Cell Death Dis. 10, 261 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chen YW, Tekle C & Fodstad O The immunoregulatory protein human B7H3 is a tumor-associated antigen that regulates tumor cell migration and invasion. Curr. Cancer Drug Targets 8, 404–413 (2008). [DOI] [PubMed] [Google Scholar]
- 143.Tekle C et al. B7-H3 contributes to the metastatic capacity of melanoma cells by modulation of known metastasis-associated genes. Int. J. Cancer 130, 2282–2290 (2012). [DOI] [PubMed] [Google Scholar]
- 144.Liu H et al. B7-H3 silencing increases paclitaxel sensitivity by abrogating Jak2/Stat3 phosphorylation. Mol. Cancer Ther 10, 960–971 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jiang B, Liu F, Liu Z, Zhang T & Hua D B7-H3 increases thymidylate synthase expression via the PI3k–Akt pathway. Tumour Biol. 37, 9465–9472 (2016). [DOI] [PubMed] [Google Scholar]
- 146.Gao L et al. CD90 affects the biological behavior and energy metabolism level of gastric cancer cells by targeting the PI3K/AKT/HIF-1α signaling pathway. Oncol. Lett 21, 191 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Avril T et al. CD90 expression controls migration and predicts dasatinib response in glioblastoma. Clin. Cancer Res 23, 7360–7374 (2017). [DOI] [PubMed] [Google Scholar]
- 148.Brand TM et al. The receptor tyrosine kinase AXL mediates nuclear translocation of the epidermal growth factor receptor. Sci. Signal 10, eaag1064 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 149.Yang S et al. m6A mRNA demethylase FTO regulates melanoma tumorigenicity and response to anti-PD-1 blockade. Nat. Commun 10, 2782 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yao H et al. A peptidic inhibitor for PD-1 palmitoylation targets its expression and functions. RSC Chem. Biol 2, 192205 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gordon SR et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 545, 495–499 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]