Abstract
Assess the incidence, risk factors, clinical and microbiological features, and outcome of both probable invasive and invasive group A Streptococcus (GAS) infections in children and adults in the BrusselsCapital Region between 2005 and 2020. A retrospective, multicentric study was performed in three university hospitals in Brussels. Patients were identified through the centralized laboratory information system. Epidemiological and clinical data were collected from patients’ hospital records. A total of 467 cases were identified. Incidence has increased from 2.1 to 10.9/100,000 inhabitants between 2009 and 2019 in non-homeless adults while it was above 100/100,000 on homeless in years with available denominators. Most of GAS were isolated from blood (43.6%), and the most common clinical presentation was skin and soft tissue infections (42.8%). A third of all the patients needed surgery, a quarter was admitted to the intensive care unit, and 10% of the adult patients died. Wounds and chickenpox disease were the main risk factors for children. Tobacco, alcohol abuse, wounds or chronic skin lesion, being homeless, and diabetes were identified as major predisposing factors for adults. The most common emm clusters were D4, E4, and AC3; 64% of the isolates were theoretically covered by the 30-valent M-protein vaccine. The burden of invasive and probable invasive GAS infections is on the rise in the studied adult population. We identified potential interventions that could contribute to decrease this burden: appropriate care of wounds, specifically among homeless and patients with risk factors such as diabetes and systematic chickenpox vaccination for children.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10096-023-04568-y.
Keywords: Group A Streptococcus, Streptococcus pyogenes, Invasive infection, Incidence, Emm type, Epidemiology, Chickenpox
Introduction
Streptococcus pyogenes, or group A Streptococcus (GAS), is a Gram-positive bacterium accountable for a wide range of diseases in children and adults. These include common, benign infections like pharyngitis or superficial skin infections, but also invasive infections like bacteremia, meningitis, pneumonia, and necrotizing fasciitis (NF). Invasive GAS (iGAS) diseases can be complicated by streptococcal toxic shock syndrome (STSS), which has a case fatality rate (CFR) of 38% [1]. Although rare in Europe, chronic rheumatic heart disease (RHD), which is a complication of GAS disease, remains a major public health issue in developing countries with an estimated prevalence of 33 million people affected worldwide in 2015 and at least 233,000 deaths each year [2, 3].
Recent epidemiological studies have shown that iGAS disease represents a high global burden with a sustained, high incidence and is associated with high morbidity and mortality with 163,000 deaths per year worldwide [2]. The most vulnerable populations to iGAS infections are young children less than 1 year of age and adults over 65 years of age [1]. Other risk factors (RFs) for iGAS disease are poverty, homelessness [4, 5], overcrowded housing conditions [6], diabetes, immunosuppression, intravenous (IV) drug use, chickenpox disease, and recent surgery [7].
The M protein located at the GAS surface is both a virulence factor and a vaccine antigen. The N-terminal end of its encoding gene (emm) is to date used as a reference locus for epidemiological typing. More than 220 different variants (emm types) of this protein have already been identified, and an increasing number of subtypes are still being identified. This variability can be a potential barrier for M protein–based vaccine development. The most advanced vaccines are currently the 30-valent serotype-specific vaccine [8] and the conserved M protein vaccine (minimal epitope J8 vaccine) [9]. In 2014, a new functional classification based on 48 emm clusters [10] containing closely related M proteins was proposed to facilitate the design of studies concerning M protein function, GAS vaccine development, virulence, and surveillance.
Unlike other Streptococci, GAS has until today remained universally susceptible to penicillin and vancomycin. The most frequent antibiotic resistances are to tetracycline followed by clarithromycin and clindamycin. There are variations between countries. Resistance rate to tetracycline has decreased in Germany [11] between 2003 and 2007. However, it has increased in Spain between 1996 and 2006 [12]. Clindamycin resistance is reported in 6–7% of the tested isolates across different settings over the past 15 years [13, 14].
Although it is important to gain data about the local RF, the outcome of the disease, and the emm type distribution to guide the implementation of a future vaccine, limited recent research has been done on the epidemiology of iGAS infections in the Brussels-Capital Region. This study aims to fill that gap. The main goals of this study are to estimate the burden of probable iGAS (piGAS) and iGAS infections among children and adults, to characterize the clinical presentation, to identify predisposing factors and outcomes of these infections, to describe the molecular characteristics and antibiotic susceptibility of GAS isolates responsible to these infections, and to assess the percentage piGAS and iGAS infections in the Brussels-Capital Region theoretically covered by the 30-valent vaccine candidate.
Materials and methods
Case definition
Invasive disease was defined by the isolation of GAS from a normally sterile site (blood, bone, pleural fluid, synovial fluid, peritoneal fluid, or cerebrospinal fluid (CSF)).
Probable invasive disease was defined as an unwell patient with GAS isolated from a non-sterile site (deep skin or retropharyngeal aspiration) who required one or more of the following: hospitalization for intravenous antibiotics, surgery, or admission to the intensive care unit (ICU).
Clinical syndromes of piGAS and iGAS disease were categorized as isolated bacteremia, pneumonia/empyema, skin and soft tissue infection (SSTI) with or without bacteremia, NF, STSS, septic arthritis, osteitis, bursitis, abdominal/peritoneal infection, meningitis, pharyngeal abscess with and without bacteremia, and endometritis (pregnancy or not pregnancy related).
Clinical syndromes are presented in Supplementary Information 1. In case of concurrent diagnosis in the same patient, the most severe diagnosis was retained.
IGAS infections were considered healthcare related if they occurred at least 48 h after the time of admission or if the patient underwent surgery within the 7 days preceding the onset of iGAS. IGAS or piGAS infections were considered associated to chickenpox if it occurred within a maximum time span of 7 days from the onset of the varicella infection. The pediatric population was limited to children between 0 and 16 years. An adult was defined as a patient at least 17 years old.
The 2010 case definition [15] based on two major criteria (hypotension and the involvement of at least two organs/systems) was applied for the diagnosis of STSS.
Study design
This is a retrospective study covering data from January 1, 2005 to December 31, 2020 in three university hospitals in the Brussels-Capital Region: CUB Erasme (858 beds), CHU Brugmann (853 beds), and CHU Saint-Pierre (582 beds). Among the 3 hospitals, only CHU Saint-Pierre and Erasme Hospital have pediatric beds. In 2019, the catchment population of the 3 hospitals was estimated at 411,231 inhabitants representing 34% of the total population of the Brussels-Capital Region. The laboratory information system (LIS) of their consolidated laboratory (Laboratoire Hospitalier Universitaire de Bruxelles-Universitair Laboratorium Brussel (LHUB-ULB)) was used to identify all the patients that presented at least one GAS isolate in a clinical sample of the following sites: blood, CSF, bone biopsy, pleural or peritoneal fluid, and deep skin or retropharyngeal aspiration during the study period. Data for each patient was then obtained by reviewing their medical files. An additional group of 21 patients was included from a previous study performed between 2016 and 2018 at CHU Saint-Pierre [4].
Patients with non-invasive infections or those who declared that they did not wish their data to be used were excluded. In case of two iGAS infections with two different hospitalizations, we included only the first infection in the study. Polymicrobial infections have also been included.
Collection of clinical data
Study data was collected through the REDCap [16] application and included for each patient clinical diagnosis; socio-demographic data like age, sex, body mass index (BMI), ethnicity, and housing status (homeless or institutionalized); possible addictions; comorbidities; healthcare-related infection or not; ICU admission; treatment (e.g., surgery including amputation, immunoglobulins, or clindamycin); length of hospital stay; mortality; emm type; and antibiotic resistance of the GAS. The Charlson Comorbidity Index (CCI) [17] was calculated for each patient, allowing us to obtain their expected survival rate at 10 years. Death was considered related to GAS infection if the patient passed away during the hospitalization period for piGAS or iGAS infection.
The infection was considered related to pregnancy if it occurred during the pregnancy or within 30 days postpartum. For the patients that presented arterial hypotension, we reviewed their diagnosis to determine if they would meet the criteria for STSS.
Microbiological methods
GAS culture, identification, and antibiotic susceptibility testing
At LHUB-ULB, GAS was isolated from clinical samples of the following: blood, CSF, bone biopsy, pleural or peritoneal fluid, and deep skin or retropharyngeal aspiration by culture into sheep blood Columbia agars. Before 2010, GAS was identified through microscopic and macroscopic examinations (Gram staining, beta-hemolysis) and using the Latex Agglutination System (Prolex; Pro-Lab Diagnostics, Richmond Hill, Ontario, Canada). Since 2010, the identification is performed by MALDI-TOF MS (Bruker Daltonics, Bremen, Germany).
Systematic antibiotic susceptibility testing (AST) was conducted using the Kirby-Bauer disk diffusion method on Mueller–Hinton agar to the following antimicrobial agents: penicillin, erythromycin, vancomycin, clindamycin, tetracycline, and fluoroquinolones. A D-zone test was performed systematically to determine inducible clindamycin resistance. Classification as “susceptible,” “intermediate,” or “resistant” to the tested antibiotic was done according to the Clinical and Laboratory Standards Institute (CLSI) guidelines [18] before 2010 and according to the updated guidelines of the European Committee on Antimicrobial Susceptibility Testing (EUCAST) [19] breakpoints from 2010 onwards. Since 2013, most iGAS isolates were transferred to the Belgian National Reference Centre (NRC) in Antwerp for the following analyses: emm type and minimal inhibitory concentration (MIC) test.
Since 2015, isolates from iGAS disease were systematically stored at − 80 °C. If these frozen isolates had not yet been emm typed, they were sent to the NRC for emm typing in February 2021.
Statistical analysis
Simple descriptive statistics were used to summarize patients’ characteristics: median and interquartile ranges (IQRs) for quantitative data, frequencies, and percentages for qualitative data. Analyses comparing groups of patients were performed with the Mann–Whitney U test, when the data was quantitative, and Fischer’s exact test, when the data was qualitative. For comparison of more than two groups, the Kruskal–Wallis test has been used. Incidence rate was calculated using the catchment population per hospital based on the market share of hospital admissions per year, as reported by the Federal Public Service Public Health, and the number of inhabitants per municipality for the respective year available from 2009 onwards. As previous study has shown high incidence of GAS infection in the homeless population of Brussels [4] and considering that homeless are not included in the official population registry, incidence calculations were performed differently for non-homeless and homeless adults. Data of the number of the homeless of the Brussels-Capital Region were extracted from the Bruss'Help’s reports (https://www.brusshelp.org/index.php/fr/missions/analyse/les-chiffres). Children were not included as the majority of children’s cases were from one hospital. The evolution of the incidence density between the years 2009 and 2019 was estimated by linear regression. All analyses and graphs were produced using SAS statistical software (version 9.4; SAS Institute, Cary, NC, USA). All reported p values are two-sided.
Results
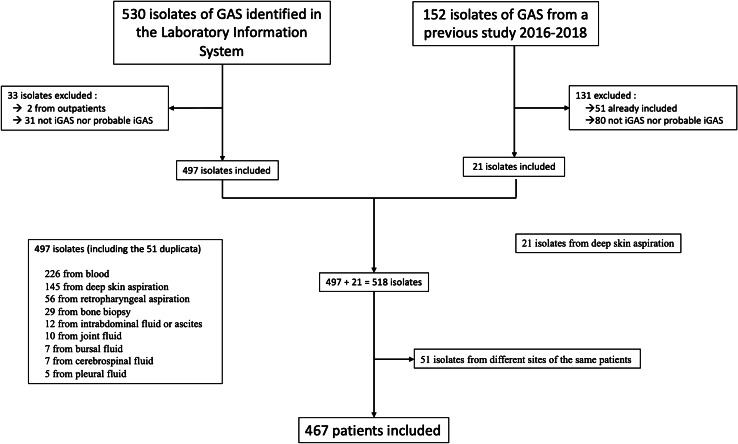
A total of 467 patients (adults [n = 407] and children [n = 60]) with invasive (n = 270) and probable invasive (n = 197) GAS infections were retained for the study period in the three following hospitals: CHU Saint-Pierre (n = 260 (51.2%)), CHU Brugmann (n = 129 (31.8%)), and CUB Erasme (n = 78 (17.0%)), for a total of 518 GAS-positive samples.
As illustrated in Fig. 1, 226 (43.6%) isolates were cultured from blood, 166 (32.0%) from deep skin aspirations (145 identified through the laboratory information system and 21 collected during a previous study), 56 (10.8%) from retropharyngeal aspirations, 29 (5.6%) from bone biopsies, 12 (2.3%) from intra-abdominal fluid or ascites, 10 (1.9%) from joint fluid, 7 (1.5%) from bursal fluid, 7 (1.5%) from CSF, and 5 (1.0%) from pleural fluid. For 30 patients, GAS was isolated from two sites. Distribution of the cases according to the years of diagnosis, housing condition, age, and iGAS and piGAS status is described in Supplementary Table 1.
Fig. 1.
Flow chart summarizing the patients included based on the identification of GAS isolate samples
Description of the study population
The median age was 3 years (IQR 2–6 years) among children and 45 years (IQR 34–62 years) among adults. The male/female ratio was different among adults (1.9) and children (0.9) (p < 0.009). There was also a significant difference between the ethnicity of children and that of adults: the majority of the children belonged to North African or Sub-Saharan ethnicities, while the majority of the adults were Caucasians (Table 1) (p < 0.0001). Homeless patients and IVD users represented 89/407 (21.9%) and 36/407 (8.8%) of the adult cases, respectively.
Table 1.
Demographic characteristics of patients with iGAS and piGAS infections by age group
| Total children, N = 60 |
< 1 year, N = 2 |
1–5 years, N = 42 |
6–16 years, N = 16 |
Total adults, N = 407 |
17–39 years, N = 163 |
40–59 years, N = 136 | 60–79 years, N = 79 |
> 80 years, N = 29 |
All, N = 467 |
p value1 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | |||||||||||
| Male | 29 (48.3) | 1 (50) | 23 (54.8) | 5 (31.3) | 267 (65.6) | 101 (62.0) | 103 (75.7) | 45 (57.0) | 18 (62.1) | 296 (63.4) | < 0.01 |
| Female | 31 (51.7) | 1 (50) | 19 (45.2) | 11 (68.8) | 140 (34.4) | 62 (38.0) |
33 (24.3) |
34 (43.0) | 11 (37.9) | 171 (36.6) | |
| Ethnicity | |||||||||||
| Europe | 16/59 (27.1) | 1 (50) | 10 (23.8) | 5/15 (33.3) | 268/404 (66.3) | 81/162 (50) | 98/135 (72.6) | 65 (82.3) | 24 (82.8) | 284/464 (61.2) | < 0.01 |
| North Africa | 25/59 (42.4) | 1 (50) | 16 (38.1) | 8/15 (53.3) | 79/404 (19.6) | 47/162 (29.0) | 19/135 (14.0) | 9 (11.4) | 4 (13.8) | 104/464 (22.4) | |
| Sub-Saharan Africa | 13/59 (22.0) | 0 | 11 (26.2) | 2/15 (13.3) | 42/404 (10.4) | 25/162 (15.4) | 13/135 (9.6) | 3 (3.8) | 1 (3.5) | 55/464 (11.8) | |
| Other | 5/59 (8.5) | 0 | 5 (12.0) | 0 | 16/404 (39.0) | 9/162 (5.6) | 5/135 (3.7) | 2 (2.5) | 0 | 21/464 (5.2) | |
Data are expressed in numbers and percentages. Denominators are smaller for sex and ethnic group due to missing data in the medical records
1For the comparison of results among the different age groups. p < 0.05 was considered statistically significant
Incidence of iGAS and piGAS infection
The incidence of piGAS and iGAS infection for non-homeless adults in the three hospitals significantly increased per year from 2.1 cases/1,000,000/year to 10.9 cases/100,000 people/year in 2019 (p = 0.01). Denominators for the homeless population in the Brussels-Capital Region were only available for a selected number of years. Overall, incidence among homeless was above 100 cases/100,000/year (Table 2).
Table 2.
Incidence of GAS infection among non-homeless and homeless adults in three hospitals of the Brussels-Capital Region, 2009–2010
| Year/incidence (/100,000 patient-years) | Non-homeless adults | Homeless | ||||
|---|---|---|---|---|---|---|
| piGAS | iGAS | Total | iGAS | piGAS | Total | |
| 2009 | 1.8 | 0.3 | 2.1 | |||
| 2010 | 2.1 | 1.8 | 3.9 | 140.6 | 0 | 140.6 |
| 2011 | 4.6 | 3.4 | 8.0 | |||
| 2012 | 4.2 | 1.1 | 5.4 | |||
| 2013 | 3 | 1.7 | 4.7 | |||
| 2014 | 4.6 | 3.3 | 7.9 | 95.3 | 63.6 | 158.9 |
| 2015 | 3.9 | 2.1 | 6.1 | |||
| 2016 | 3.5 | 2 | 5.6 | 84.4 | 225.0 | 309.3 |
| 2017 | 3.6 | 3.8 | 7.4 | |||
| 2018 | 4.3 | 1.9 | 6.3 | 72.1 | 48.1 | 120.2 |
| 2019 | 5.6 | 5.3 | 10.9 | |||
| 2020 | 94.1 | 150.6 | 244.7 | |||
Predisposing factors and comorbidities
As Table 3 shows, the most predisposing risk factors for presenting piGAS or iGAS infection among adults were tobacco consumption (202/399 (50.6%)); skin lesions, wounds, or chronic skin disease (145/407 (35.6%)); alcohol abuse (120/401 (30.0%)); obesity (67/293 (22.9%)); homelessness (89/407 (21.9%)); and diabetes (74/407 (18.2%)). One-fifth (84/407) of the adults had a CCI of > 5, translating into a life expectancy at 10 years of 2%. Homeless people had significantly more wounds and chronic skin disease than non-homeless people (p < 0.05) and also higher rates of smoking, alcohol, and IV drug abuse (p < 0.05) (Supplementary Table 2).
Table 3.
Underlying conditions of patients with iGAS and piGAS infections by age group
| Total children, N = 60 |
< 1 year, N = 2 |
1–5 years, N = 42 |
6–16 years, N = 16 |
Total adults, N = 407 |
17–39 years, N = 163 |
40–59 years, N = 136 | 60–79 years, N = 79 | > 80 years, N = 29 |
All, N = 467 |
p value1 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Skin lesion | 2 (3.3) | 0 | 1 (2.4) | 1 (6.3) | 79 (19.4) | 33 (20.2) | 28 (20.6) | 15 (19.0) | 3 (10.3) | 81 (17.3) | < 0.2 |
| Chronic skin disease | 1 (1.7) | 0 | 1 (2.4) | 0 | 66 (16.2) | 16 (9.8) | 28 (20.6) | 14 (17.7) | 8 (27.6) | 67 (14.3) | < 0.005 |
| Chickenpox2 | 11 (18.3) | 0 | 11 (26.2) | 0 | 1 (0.2) | 1 (0.6) | 0 | 0 | 0 | 12 (2.6) | < 0.0001 |
| Diabetes | 0 | 0 | 0 | 0 | 74 (18.2) | 7 (4.3) | 22 (16.1) | 38 (48.1) | 8 (27.6) | N/A | |
| Obesity | N/A | N/A | N/A | N/A | 67/293 (22.9) | 18/100 (18.0) | 19/102 (18.6) | 27/68 (39.7) | 3/23 (13.0) | N/A | |
| Cancer3 | 0 | 0 | 0 | 0 | 44 (10.8) | 2 (1.2) | 11 (8.1) | 22 (27.8) | 9 (31.0) | N/A | |
| COPD | 0 | 0 | 0 | 0 | 48 (11.8) | 3 (1.8) | 17 (12.5) | 20 (25.3) | 8 (27.6) | N/A | |
| Liver disease | 0 | 0 | 0 | 0 | 55 (13.5) | 13 (8) | 28 (20.6) | 12 (15.2) | 2 (6.9) | N/A | |
| CCI > 5 | N/A | N/A | N/A | N/A | 84 (20.6) | 0 | 15 (11.0) | 47 (59.5) | 22 (75.9) | N/A | |
| Institutionalized | N/A | N/A | N/A | N/A | 18 (4.4) | 1 (0.6) | 2 (1.5) | 6 (7.6) | 9 (31) | N/A | < 0.0001 |
| Homeless | N/A | N/A | N/A | N/A | 89 (21.9) | 42 (24.8) | 42 (25.8) | 5 (6.3) | 0 | N/A | < 0.0001 |
| Smoker | N/A | N/A | N/A | N/A | 202/399 (50.6) | 78/159 (49.0) | 85/134 (63.4) | 32/77 (41.6) | 7 (24.1) | N/A | < 0.007 |
| IVDU | N/A | N/A | N/A | N/A | 36 (8.8) | 23 (14.1) | 13 (9.6) | 0 | 0 | N/A | < 0.02 |
| Alcohol abuse | N/A | N/A | N/A | N/A | 120/401 (30.0) | 41/160 (25.6) | 54/134 (40.3) | 22/78 (28.2) | 3 (10.3) | N/A | < 0.07 |
Data are expressed in numbers and percentages. Denominators are smaller for smoker and alcohol abuse because of lack of data in the medical records
N/A not applicable, COPD chronic obstructive pulmonary disease, CCI Charlson Comorbidity Index, IVDU intravenous drug use
1For the comparison of results among the different age groups. p < 0.05 was considered statistically significant
2Within a maximum time span of 7 days before the piGAS or iGAS infection
3Cancer includes solid tumor, leukemia, and lymphoma
Pregnant women represented 20.7% of the adult female patients between 16 and 49 years. Of note, 26.2% (11/42) of the children aged 1 to 5 years were suffering from chickenpox disease in the 7 days before the GAS infection. Finally, 25.3% of the patients did not have any identifiable risk factor.
Clinical manifestations
As Table 4 shows, the most common clinical presentation for adults (175/407 (43.0%)) and children (25/60 (41.7%)) was SSTI. The second and third most frequent clinical presentations among adults were pharyngeal abscess without bacteremia (52/407 (12.8%)) and STSS (46/407 (11.3%)). Among the children, it was pharyngeal abscess without bacteremia (6/60 (10.0%)) and ear, nose, and throat infections and/or mastoiditis with bacteremia (5/60 (8.3%)), while no child presented septic shock nor NF, 4/407 (1%) of the adults developed septic shock, and 6/407 (1.5%) were diagnosed with NF. Among pregnant women, the most predominant disease was postpartum endometritis (6/17 (35.3%)), followed by SSTI (5/17 (29.4%)) and isolated bacteremia (5/17 (29.4%)). Almost half (8/17; 47.1%) of these pregnancy-associated infections was healthcare related while less than 10% (44/467; 9.4%) of the whole study population was suffering from healthcare-related infection. There was a significant difference in the clinical manifestations between homeless and non-homeless patients: 69.7% (62/89) of the homeless suffered from SSTI with (15/89) or without (47/89) bacteremia as compared to 35.5% of the non-homeless adults (Supplementary Table 2).
Table 4.
Clinical manifestations of patients with iGAS and piGAS infections by age group
| Clinical manifestation | Total children, N = 60 |
< 1 year, N = 2 |
1–5 years, N = 42 |
6–16 years, N = 16 |
Total adults, N = 407 |
17–39 years, N = 163 |
40–59 years, N = 136 | 60–79 years, N = 79 | > 80 years, N = 29 |
All, N = 467 |
|---|---|---|---|---|---|---|---|---|---|---|
| SSTI without bacteremia | 12 (20) | 0 | 4 (9.5) | 8 (50.0) | 116 (28.5) | 58 (35.6) | 50 (36.8) | 7 (8.9) | 1 (3.4) | 128 (27.4) |
| SSTI with bacteremia | 13 (21.7) | 0 | 13 (31) | 0 | 59 (14.5) | 11 (8) | 20 (14.7) | 18 (22.8) | 10 (34.5) | 72 (15.4) |
| Pharyngeal abscess without bacteremia | 6 (10) | 0 | 1 (2.4) | 5 (31.3) | 52 (12.8) | 38 (23.3) | 12 (8.8) | 1 (1.3) | 1 (3.4) | 58 (12.4) |
| Pharyngeal abscess with bacteremia | 0 | 0 | 0 | 0 | 2 (0.5) | 2 (1.2) | 0 | 0 | 0 | 2 (0.4) |
| Isolated bacteremia | 1 (1.7) | 0 | 1 (2.4) | 0 | 29 (7.1) | 8 (4.9) | 6 (4.4) | 9 (11.4) | 6 (20.7) | 30 (6.4) |
| Pneumonia1 | 4 (6.7) | 1 (50) | 3 (7.1) | 0 | 16 (3.9) | 7 (4.2) | 2 (1.5) | 6 (7.6) | 1 (3.4) | 20 (4.3) |
| Osteomyelitis | 2 (3.3) | 0 | 2 (4.8) | 0 | 24 (5.9) | 4 (2.5) | 8 (5.9) | 9 (11.4) | 3 (10.3) | 26 (5.6) |
| Arthritis | 3 (5) | 0 | 1 (2.4) | 2 (12.5) | 11 (2.7) | 5 (3.1) | 5 (3.7) | 1 (1.3) | 0 | 14 (3) |
| ENT/mastoiditis with bacteremia | 5 (8.3) | 1 (50) | 4 (9.5) | 0 | 5 (1.2) | 1 (0.6) | 1 (0.7) | 3 (3.8) | 0 | 10 (2.1) |
| ENT/mastoiditis without bacteremia | 4 (6.7) | 0 | 4 (9.5) | 0 | 2 (0.5) | 1 (0.6) | 1 (0.7) | 0 | 0 | 6 (1.3) |
| Necrotizing fasciitis | 0 | 0 | 0 | 0 | 6 (1.5) | 2 (1.2) | 2 (1.5) | 2 (2.5) | 0 | 6 (1.3) |
| Intra-abdominal infection | 2 (3.3) | 0 | 2 (4.8) | 0 | 8 (2) | 1 (0.6) | 6 (4.4) | 1 (1.3) | 0 | 10 (2.1) |
| Meningitis | 3 (5) | 0 | 2 (4.8) | 1 (6.3) | 4 (1) | 1 (0.6) | 1 (0.7) | 2 (2.5) | 0 | 7 (1.5) |
| UTI with bacteremia | 2 (3.3) | 0 | 2 (4.8) | 0 | 5 (1.2) | 1 (0.6) | 1 (0.7) | 3 (3.8) | 0 | 7 (1.5) |
| Not pregnancy-related endometritis | 0 | 0 | 0 | 0 | 6 (1.5) | 4 (2.5) | 2 (1.5) | 0 | 0 | 6 (1.3) |
| Pregnancy-related endometritis | 0 | 0 | 0 | 0 | 6 (1.5) | 5 (3.1) | 1 (0.7) | 0 | 0 | 6 (1.3) |
| Bursitis | 0 | 0 | 0 | 0 | 6 (1.5) | 4 (2.5) | 1 (0.7) | 1 (1.3) | 0 | 6 (1.3) |
| Toxic shock syndrome | 3 (5.0) | 0 | 3 (7.1) | 0 | 46 (11.3) | 9 | 16 (11.8) | 14 (17.7) | 7 (24.1) | 49 (10.5) |
| Catheter-related blood stream infection | 0 | 0 | 0 | 0 | 1 (0.2) | 1 (0.6) | 0 | 0 | 0 | 1 (0.2) |
| Endocarditis/pericarditis | 0 | 0 | 0 | 0 | 3 (0.7) | 0 | 1 (0.7) | 2 (2.5) | 0 | 3 (0.6) |
| Septic shock associated | 0 | 0 | 0 | 0 | 4 (1.0) | 0 | 2 (1.5) | 0 | 2 (6.9) | 4 (0.8) |
Data are expressed in numbers and percentages
SSTI skin and soft tissue infection; ENT ear, nose, and throat, UTI urinary tract infection
1Included empyema
Outcome and mortality
Outcome of patients is presented in Table 5. Overall, 31.9% (149/467) of the patients required surgery with general anesthesia as a direct consequence of GAS infection (procedures included washout, incision and drainage of abscesses, exploratory surgery, debridement, or amputation), and of these, 22/149 (14.8%) required amputation of a phalanx or a limb. ICU admission was required for 26.8% of the cases. Mortality rate was 10.1% among adult patients while no child died during the study period. Of note, 31.7% (13/41) of the patients who died were under 50 years old and 24.4% (10/41) was over 80 years old. Of those who died, 3/41 had received IV immunoglobulin (IVIG) treatment and 13/41 had received clindamycin. Clindamycin was significantly more frequently administered to patients with iGAS than those with piGAS disease. The outcome was significantly different between piGAS and iGAS disease in terms of ICU admission (p < 0.0001), number of amputations (p < 0.005), and deaths (p < 0.0001).
Table 5.
Outcome of children with iGAS and piGAS infection versus adults with iGAS and piGAS infection
| Outcome | Children, N = 60 | Adults, N = 407 | Total, N = 467 | ||
|---|---|---|---|---|---|
| piGAS, N = 22 (%) | iGAS, N = 38 (%) | piGAS, N = 175 (%) | iGAS, N = 232 (%) | ||
| ICU | 1 (4.5) | 10 (26.3) | 13 (7.4) | 101 (43.5) | 125 (26.8) |
| Surgery with GA | 12 (54.5) | 9 (23.7) | 52 (29.7) | 83 (35.8) | 149 (31.9) |
| Amputation | 0 | 0 | 3 (1.7) | 19 (8.2) | 22 (4.7) |
| Death | 0 | 0 | 1 (0.5) | 40 (17.2) | 41 (8.8) |
Data are expressed in numbers and percentages
ICU intensive care unit, GA general anesthesia
Antibiotic susceptibility testing results
No isolate resistant or intermediate to penicillin or vancomycin was identified. Strains were most frequently resistant to tetracycline (12%), followed by erythromycin (5.0%), clindamycin (4.4%), and levofloxacin/ciprofloxacin (2.9%) (Table 6).
Table 6.
Antibiotic susceptibility test results for iGAS and probable invasive isolates between 2005 and 2020
| Antibiotic | Susceptibility, n (%) | ||
|---|---|---|---|
| S | I | R | |
| Penicillin, N = 465 | 465 (100) | 0 | 0 |
| Vancomycin, N = 464 | 464 (100) | 0 | 0 |
| Erythromycin, N = 463 | 426 (92.0) | 14 (3.0) | 23 (5.0) |
| Clindamycin, N = 450 | 423 (94.0) | 7 (1.6) | 20 (4.4) |
| Fluoroquinolone, N = 311 | 294 (94.5) | 8 (2.6) | 9 (2.9) |
| Tetracycline, N = 58 | 49 (84.5) | 2 (3.4) | 7 (12.0) |
S sensitive, I intermediate, R resistant
Emm type distribution
From 2011 to 2020, emm typing was performed on a total of 122 collected isolates. Thirty-seven different emm types from 11 different emm clusters were identified. The six most common emm clusters were D4 (20.5% of isolates), E4 (14.4%), AC3 (13.9%), E2 (9.8%), E6 (9.0%), and E3 (9.0%); they covered 76.7% of all isolates; 63.9% of the isolate emm types are included in the 30-valent vaccine. The most common emm types for piGAS and iGAS in the total study cohort were in decreasing order as follows: emm types 64, 77, 83, 101, and 27 and types 1, 11, 64, and 4, respectively (Table 7).
Table 7.
Emm type distribution of 122 GAS isolates from iGAS and piGAS in adult homeless, adult not homeless, and children cohort
| Emm type | Emm cluster | Non-homeless adults | Homeless adults | Children iGAS (n) | ||
|---|---|---|---|---|---|---|
| piGAS (n) | iGAS (n) | piGAS (n) | iGAS (n) | |||
| 1 | A-C3 | 12 | 5 | |||
| 3 | A-C5 | 4 | ||||
| 4 | E1 | 3 | 2 | |||
| 5 | Single-protein emm-cluster clade Y | 2 | 2 | |||
| 6 | Single-protein emm-cluster clade Y | 2 | 1 | |||
| 11 | E6 | 8 | ||||
| 12 | A-C4 | 1 | 1 | 1 | 1 | |
| 22 | E4 | 1 | ||||
| 25 | E3 | 1 | ||||
| 27 | E2 | 2 | 2 | |||
| 28 | E4 | 2 | ||||
| 29 | Single-protein emm-cluster clade Y | 1 | ||||
| 44 | E3 | 1 | 1 | |||
| 49 | E3 | 2 | 1 | |||
| 50 | E2 | 1 | ||||
| 60 | E1 | 1 | ||||
| 63 | E6 | 1 | ||||
| 64 | D4 | 1 | 3 | 6 | 3 | |
| 66 | E2 | 1 | ||||
| 73 | E4 | 1 | ||||
| 75 | E6 | 1 | ||||
| 77 | E4 | 1 | 4 | 2 | ||
| 81 | E6 | 1 | ||||
| 83 | D4 | 1 | 2 | |||
| 84 | E4 | 1 | 1 | |||
| 87 | E3 | 1 | 3 | |||
| 89 | E4 | 1 | 2 | 2 | ||
| 90 | E2 | 1 | 2 | |||
| 92 | E2 | 1 | ||||
| 94 | E6 | 1 | ||||
| 101 | D4 | 1 | 2 | 2 | ||
| 104 | E2 | 2 | ||||
| 108 | D4 | 2 | 1 | |||
| 162 | 2 | 1 | ||||
| 169 | E4 | 1 | 1 | |||
| 184 | D5 | 1 | ||||
| 209 | E3 | 1 | ||||
| Total | 8 | 69 | 17 | 15 | 13 | |
n number of isolates, iGAS invasive group A Streptococcus, piGAS probable invasive group A Streptococcus
The emm type covered in the proposed 30-valent vaccine (types 1, 2, 3, 4, 5, 6, 11, 12, 14, 18, 19, 22, 24, 28, 29, 44, 49, 58, 73, 75, 77, 78, 81, 82, 83, 87, 89, 92, 114, and 118)
In the homeless population, emm types 64, 27, and 101 were among the three most frequent emm types for piGAS and iGAS. There was a significant difference in the emm cluster distribution between isolates from homeless and those with residence. D4 and E2 represented 75% of the emm clusters among the homeless population, while E4 and AC3, the two most common clusters, represented 38% of the clusters among people with residences (Table 7).
The emm cluster distributions were also different among the subgroups of invasive and probable invasive isolates.
D4 was the most common emm cluster among piGAS infections with 52%, while AC3 represented 18% of the iGAS infections.
Discussion
This is a retrospective, multicentric study evaluating the incidence, clinical and microbiological features, and the outcome of iGAS and piGAS infections in children and adults in Brussels.
Between 2009 and 2019, the incidence of the disease rose significantly among non-homeless adults. Increasing incidence with similar ranges has been observed in other studies using similar definition, for example in France (2007 to 2022) [20], Iceland (1975 to 2012) [21], and Alaska (1975 to 2012) [22]. Little data about the evolution of the incidence is available from other countries between 2012 and 2020, and the incidences at the end of this study period (2017–2020) are higher than any incidences observed in other countries. Comparison to other countries has to be made carefully because the study was not nationwide but restricted to the most deprived area of the Brussels-Capital Region. Different factors could contribute to the rising incidence of iGAS and piGAS infections in this area. Firstly, the rising prevalence of risk factors such as obesity [23] and diabetes [24] in Belgium could partly explain this increasing incidence. Secondly, as illustrated by doubling of the number of homeless people in the past 10 years [25], poverty is rising among the Brussels population [26]. Both CHU Saint-Pierre and Brugmann are located in the less affluent areas of the Brussels-Capital Region [27], and the vast majority of the samples identified (80%) originated from those two hospitals. Subjects living in proximity to these hospitals have increased risk of diabetes and poverty [26–28].
In this study, the most commonly identified predisposing factors for iGAS and piGAS infections among adults were tobacco consumption, chronic skin diseases or wounds, alcohol abuse, obesity, and being homeless (Table 3). The presence of a wound is a classic RF which has been reported in numerous studies [1, 5, 7] and illustrates the need to properly educate patients with skin lesions or at risk of chronic wounds about hygiene and disinfection of wounds. Wounds and chronic skin disease were more frequently detected among homeless. Other factors such as smoking, alcohol, and IV drug abuse were also more frequently reported among homeless. The latter three risk factors combined with neglected skin care often seen in homeless people retard the process of skin healing and expose this vulnerable population at higher risk of iGAS and piGAS infections compared to the non-homeless population. Almost one-fifth (18%) of the children had a chickenpox infection within the 7 days preceding the GAS infection. There is evidence that iGAS infections in children with chickenpox have a more fatal outcome compared to children without chickenpox co-infections [29]. Chickenpox is also a known risk factor for NF [29, 30]. Varicella vaccination has been shown to be an effective prevention tool for iGAS disease in several developed countries [30–32]. Nevertheless, a quarter of the patients in our study did not have any identified risk factor. The fact that iGAS and piGAS infections also affect people without an underlying risk factor makes it difficult to propose a prevention strategy for those patients.
Overall, the most common clinical presentations of iGAS and piGAS infections were SSTI, pharyngeal abscess, and STSS (Table 4). It remains difficult to compare these results to international surveillances of invasive infections due to lack of coherent methodology across studies. Definitions and clinical interpretations from physicians vary between the regions and countries, which can result in different study results, even when the clinical reality is not so different. Even though most of the studies have shown that SSTI is the most common clinical presentation of iGAS infections, in most surveillance studies of iGAS infections, pharyngeal abscess is not included.
Fifty-four patients in our study had initially been described as suffering from septic shock in their medical file. However, analyzing their files retrospectively has shown that 49 out of those 54 patients fulfilled the diagnosis criteria of STSS, making this the 3rd most common diagnosis for the patients included in this study. This could mean that there is a low application or knowledge of the CDC diagnosis criteria among physicians. Most notably, a retrospective study in the USA [33] showed that cases of STSS in children were classified as a septic shock and thus the diagnosis of toxic shock was not documented even through the patient fulfilled the diagnosis criteria. The consequence is that the administration of clindamycin and IVIG as a treatment option for those cases is underconsidered, and this might have an impact on the outcome for those patients. The case definition of STSS was changed in 2010, and given the different treatment approaches, it is important to correctly distinguish between septic shock and STSS.
The recommended treatment for STSS is IVIG associated with clindamycin. However, 6% of the isolates were not susceptible to clindamycin, similar to rates reported in France (6.0%) [13]. Furthermore, clindamycin was not administered in over two-third (68.3%) of fatal cases in our study, despite recommendations to use them in case of severe infections [34]. Similar observations have been made in the UK [35]. These numbers suggest the need for education of health professionals and the inclusion of clindamycin as a standard treatment for severe iGAS in local guidelines. While, inter alia, the USA, Canada, France, and Ireland have national recommendations to use clindamycin in severe iGAS infections, there is currently no national recommendation to do the same in Belgium.
The iGAS and piGAS disease presentations were serious in this study. A quarter of the children and 43% of the adults with iGAS were admitted to the ICU, and general anesthesia was required in 31.9% of the overall population. No child included in this study died, but 17% of the adults with iGAS did. This is similar to the case fatality rate observed in 11 other European countries. Patients with probable invasive disease have a significantly better outcome than definitive invasive disease (Table 5). Nevertheless, it is important to include these patients in a surveillance study because they also have serious clinical presentations. One-third of all the adult patients and 54.5% of the children with piGAS disease needed surgery with general anesthesia. This result shows that piGAS has also an impact on the healthcare system and that excluding those patients would underestimate the disease burden of GAS infection in the Brussels-Capital Region.
Emm type 1 was the most common emm type among iGAS cases in the present study and has also been identified as most common emm type responsible for iGAS in different epidemiological studies in Europe and North America [11, 20–22, 36], The second and third most frequent emm types 28 and 89 associated with iGAS in other European countries were less represented in our study population. Emm type 64 was found in higher frequency as compared to other European countries, likely reflecting the high proportion of strains identified among homeless [30]. Overall, as compared to the non-homeless population, there were a limited number of emm types circulating in the homeless population in Brussels. Clonal dissemination of emm types among homeless populations was previously reported in different studies in Europe and North America [4, 5, 37, 38].
There was also a difference in the clinical presentation according to the emm types. The majority of the emm types from piGAS infections were from D4 cluster (52%). Among the homeless population, 76% of the emm types from piGAS belong to the D4 cluster. D4 cluster has previously been associated to skin infections [10]. The emm types of D4 cluster have the specificity to be able to bind plasminogen, which is present in wounds. This highlights the need for closer surveillance and education about hygiene and wound care among the homeless due to an increased risk of outbreaks among this specific population [4, 39].
Finally, the most advanced 30-valent vaccine covers 79% of isolates from iGAS infections in the USA [40] and 78% in Europe [41]. In the present study, 63.9% of iGAS and piGAS infections would be covered by this vaccine. Cross-reactions have been demonstrated in vitro [42] and suggest a broader cross-reaction coverage of the 30-valent vaccine in humans. However, in vivo studies are needed to confirm this.
Limitations
There are a number of limitations to this study that need to be taken into account. First, after analyzing all the cases from a study performed between 2016 and 2018, we realized that some of those cases did meet our study inclusion criteria, but were not identified through our inclusion process. This suggests that some cases might have been missed out and that a prospective study design for further surveillance could lead to more complete data collection. Second, only two out of three hospitals participating in the study have pediatric beds. Third, the emm type distribution covered only the 2013–2020 period, but 83.6% of blood isolates had an available emm type. Fourth, including non-invasive isolates fell outside of the scope of this study, even though pharyngeal infections with invasive infections contribute most significantly to the burden of GAS worldwide. Including the piGAS in this study allowed to give a better idea of the burden of the disease. In further research, it would be interesting to expand studies to pharyngeal and cutaneous isolates and have a better surveillance of this pathogen. The microbiological data would be of the utmost interest as the prevention of pharyngeal infection is one of the aims of the 30-valent vaccine.
Conclusion
The increasing high incidence of iGAS and probable iGAS in the Brussels-Capital Region is a potential public health threat. Identified RFs were tobacco consumption, alcohol abuse, homelessness, skin lesion, diabetes, and obesity. However, one-quarter of the patients had no identified RF for infection. Prevention tools such as early detection of infection and appropriate care of wounds, hygienic measures in the hospital to prevent nosocomial infections, systematic varicella vaccines among children, and implementation of the GAS vaccine may reduce iGAS disease in the Brussels-Capital Region. Our study confirms that disease presentation can be very severe, and the outcome poor. To positively impact outcome, early detection of STSS by the physician is crucial as it impacts the treatment option.
Supplementary Information
Additional file 1: Table 1. Number of cases of adults, children, iGAS andpiGAS infections, non-homeless and homeless adults per year between 2005 and2020. Table 2. Comparison of characteristics, risk factors andclinical presentations of homeless versus non-homeless adults with piGAS andiGAS infections.
Author contribution
Conceptualization: L. Z. and N. D.; data collection and analysis: L. Z., D. M., V. Y. M. D., M. H., E. M., M. H., A. B., V. M., H. G., M. H., P. S., and N. D. Statistical analysis: M. D. The first draft of the manuscript was written by L. Z. and N. D., and all authors commented and revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by F.R.S.-FNRS research grants (PDR T.0255.16 and CDR J.0019.17) and the Fund Iris-Research (managed by the King Baudouin Foundation, grant 2019-J1820690-212134). N. D. is a post-doctorate clinical master specialist of the F.R.S.-FNRS.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Informed consent requirement was waived by the three university hospital’s local ethics committees in view of the retrospective nature of the study, and all procedures performed were part of routine care. All 3 ethics committees approved the study and the references are the following: Erasme (P2020/473), CHU Saint-Pierre (O.M.007), and CHU Brugmann (CE2020/156).
Consent for publication
Exemption from patient’s consent because this is a retrospective study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nelson GE, Pondo T, Toews KA, Farley MM, Lindegren ML, Lynfield R, et al. Epidemiology of invasive group A streptococcal infections in the United States, 2005–2012. Clin Infect Dis Off Publ Infect Dis Soc Am. 2016;63(4):478–86. doi: 10.1093/cid/ciw248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005;5(11):685–694. doi: 10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- 3.Watkins DA, Johnson CO, Colquhoun SM, Karthikeyan G, Beaton A, Bukhman G, et al. Global, regional, and national burden of rheumatic heart disease, 1990–2015. N Engl J Med. 2017;377(8):713–722. doi: 10.1056/NEJMoa1603693. [DOI] [PubMed] [Google Scholar]
- 4.Pilon P, Savard N, Aho J, Caron J, Urbanek A, Paré R, et al. Invasive group A streptococcal infection outbreaks of type emm118 in a long-term care facility, and of type emm74 in the homeless population, Montréal. Quebec Can Commun Dis Rep. 2019;45(1):26–31. doi: 10.14745/ccdr.v45i01a03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dauby N, Miendje Deyi VY, Delforge V, Martiny D, Mekkaoui L, Hallin M, et al. Streptococcus pyogenes infections with limited emm-type diversity in the homeless population of Brussels, 2016–2018. Int J Infect Dis. 2019;81:52–56. doi: 10.1016/j.ijid.2019.01.026. [DOI] [PubMed] [Google Scholar]
- 6.Avire NJ, Whiley H, Ross K. A review of Streptococcus pyogenes: public health risk factors, prevention and control. Pathogens. 2021;10(2):248. doi: 10.3390/pathogens10020248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Factor SH, Levine OS, Schwartz B, Harrison LH, Farley MM, McGeer A, et al. Invasive group A streptococcal disease: risk factors for adults. Emerg Infect Dis. 2003;9(8):970–977. doi: 10.3201/eid0908.020745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dale JB, Penfound TA, Chiang EY, Walton WJ. New 30-valent M protein-based vaccine evokes cross-opsonic antibodies against non-vaccine serotypes of group A streptococci. Vaccine. 2011;29(46):8175–8178. doi: 10.1016/j.vaccine.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steer AC, Carapetis JR, Dale JB, Fraser JD, Good MF, Guilherme L, et al. Status of research and development of vaccines for Streptococcus pyogenes. Vaccine. 2016;34(26):2953–2958. doi: 10.1016/j.vaccine.2016.03.073. [DOI] [PubMed] [Google Scholar]
- 10.Sanderson-Smith M, De Oliveira DMP, Guglielmini J, McMillan DJ, Vu T, Holien JK, et al. A systematic and functional classification of Streptococcus pyogenes that serves as a new tool for molecular typing and vaccine development. J Infect Dis. 2014;210(8):1325–1338. doi: 10.1093/infdis/jiu260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imöhl M, Reinert RR, Ocklenburg C, van der Linden M. Epidemiology of invasive Streptococcus pyogenes disease in Germany during 2003–2007. FEMS Immunol Med Microbiol. 2010;58(3):389–396. doi: 10.1111/j.1574-695X.2010.00652.x. [DOI] [PubMed] [Google Scholar]
- 12.Pérez-Trallero E, Martín-Herrero JE, Mazón A, García-Delafuente C, Robles P, Iriarte V, et al. Antimicrobial resistance among respiratory pathogens in Spain: latest data and changes over 11 years (1996–1997 to 2006–2007) Antimicrob Agents Chemother. 2010;54(7):2953–2959. doi: 10.1128/AAC.01548-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tamayo J, Pérez-Trallero E, Gómez-Garcés JL, Alós JI, Spanish Group for the Study of Infection in the Primary Health Care Setting Resistance to macrolides, clindamycin and telithromycin in Streptococcus pyogenes isolated in Spain during 2004. J Antimicrob Chemother. 2005;56(4):780–2. doi: 10.1093/jac/dki286. [DOI] [PubMed] [Google Scholar]
- 14.Lepoutre A, Doloy A, Bidet P, Leblond A, Perrocheau A, Bingen E, et al. Epidemiology of invasive Streptococcus pyogenes infections in France in 2007. J Clin Microbiol. 2011;49(12):4094–4100. doi: 10.1128/JCM.00070-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Streptococcal Toxic Shock Syndrome (STSS) (Streptococcus pyogenes) (2010) Case definition | CDC. Available from: https://ndc.services.cdc.gov/case-definitions/streptococcal-toxic-shock-syndrome-2010/. Accessed 26 Nov 2022
- 16.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap) - a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 18.Wayne PA (2010) Clinical and laboratory standards Institute: performance standards for antimicrobial susceptibility testing: 20th informational supplement. CLSI document M100-S20
- 19.European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. https://www.eucast.org/ast_of_bacteria
- 20.Plainvert C, Loubinoux J, Bidet P, Doloy A, Touak G, Dmytruk N, et al. Épidémiologie des infections invasives à Streptococcus pyogenes (France 2007–2011) Arch Pédiatrie. 2014;21:S62–S68. doi: 10.1016/S0929-693X(14)72262-6. [DOI] [PubMed] [Google Scholar]
- 21.Olafsdottir LB, Erlendsdóttir H, Melo-Cristino J, Weinberger DM, Ramirez M, Kristinsson KG, et al. Invasive infections due to Streptococcus pyogenes: seasonal variation of severity and clinical characteristics, Iceland, 1975 to 2012. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull. 2014;19(17):5–14. [PubMed] [Google Scholar]
- 22.Rudolph K, Bruce MG, Bruden D, Zulz T, Reasonover A, Hurlburt D, et al. Epidemiology of invasive group A streptococcal disease in Alaska, 2001 to 2013. J Clin Microbiol. 2016;54(1):134–141. doi: 10.1128/JCM.02122-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vers une Belgique en bonne santé (2018) Statut pondéral. Available from: https://www.belgiqueenbonnesante.be/fr/etat-de-sante/determinants-de-sante/statut-ponderal. Accessed 4 May 2021
- 24.For a Healthy Belgium (2018) Diabetes. Available from: https://www.healthybelgium.be/en/health-status/non-communicable-diseases/diabetes. Accessed 4 May
- 25.Dénombrement des personnes sans-abri et mal-logées en Région de Bruxelles-Capitale (2021) 6ème edition. Bruss’help. Available from: http://www.brusshelp.org/images/Denombrement2020_vdef.pdf. Accessed 30 Nov 2022
- 26.Observatoire de la Santé et du Social de Bruxelles-Capitale . Baromètre social 2020. Bruxelles: Commission communautaire commune; 2020. [Google Scholar]
- 27.Dossier (2019/2). Tous égaux face à la santé à Bruxelles? Données récentes et cartographie sur les inégalités sociales de santé. Available from: https://www.ccc-ggc.brussels/fr/observatbru/publications/dossier-20192-tous-egaux-face-la-sante-bruxelles-donnees-recentes-et. Accessed 9 May 2021
- 28.Olmo D del (2020) Une étude pour mieux aider les personnes les plus impactées dans certains quartiers. Question Santé. Available from: https://questionsante.org/nos-publications-periodiques/bruxelles-sante/e-mag-bxl-sante/259-par-categorie/prevention/1392-une-etude-pour-mieux-aider-les-personnes-les-plus-impactees-dans-certains-quartiers. Accessed 9 May 2021
- 29.Laupland KB, Davies HD, Low DE, Schwartz B, Green K, McGeer A. Invasive group A streptococcal disease in children and association with varicella-zoster virus infection. Ontario Group A Streptococcal Study Group. Pediatrics. 2000;105(5):60. doi: 10.1542/peds.105.5.e60. [DOI] [PubMed] [Google Scholar]
- 30.Lamagni TL, Darenberg J, Luca-Harari B, Siljander T, Efstratiou A, Henriques-Normark B, et al. Epidemiology of severe Streptococcus pyogenes disease in Europe. J Clin Microbiol. 2008;46(7):2359–2367. doi: 10.1128/JCM.00422-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Imöhl M, van der Linden M, Reinert RR, Ritter K. Invasive group A streptococcal disease and association with varicella in Germany, 1996–2009. FEMS Immunol Med Microbiol. 2011;62(1):101–109. doi: 10.1111/j.1574-695X.2011.00788.x. [DOI] [PubMed] [Google Scholar]
- 32.Frère J, Bidet P, Tapiéro B, Rallu F, Minodier P, Bonacorsi S, et al. Clinical and microbiological characteristics of invasive group A streptococcal infections before and after implementation of a universal varicella vaccine program. Clin Infect Dis Off Publ Infect Dis Soc Am. 2016;62(1):75–77. doi: 10.1093/cid/civ793. [DOI] [PubMed] [Google Scholar]
- 33.Gaensbauer JT, Birkholz M, Smit MA, Garcia R, Todd JK. Epidemiology and clinical relevance of toxic shock syndrome in US children. Pediatr Infect Dis J. 2018;37(12):1223–1226. doi: 10.1097/INF.0000000000002002. [DOI] [PubMed] [Google Scholar]
- 34.Stevens DL, Bryant AE. Severe group A streptococcal infections. In: Ferretti JJ, Stevens DL, Fischetti VA, editors (2016) Streptococcus pyogenes : basic biology to clinical manifestations. Oklahoma City (OK): University of Oklahoma Health Sciences Center. [cited 2021 May 9]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK333425/
- 35.Adalat S, Dawson T, Hackett SJ, Clark JE. In association with the British Paediatric Surveillance Unit. Toxic shock syndrome surveillance in UK children. Arch Dis Child. 2014;99(12):1078–82. doi: 10.1136/archdischild-2013-304741. [DOI] [PubMed] [Google Scholar]
- 36.Villalón P, Sáez-Nieto JA, Rubio-López V, Medina-Pascual MJ, Garrido N, Carrasco G, et al. Invasive Streptococcus pyogenes disease in Spain: a microbiological and epidemiological study covering the period 2007–2019. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2021;40(11):2295–2303. doi: 10.1007/s10096-021-04279-2. [DOI] [PubMed] [Google Scholar]
- 37.Bundle N, Bubba L, Coelho J, Kwiatkowska R, Cloke R, King S, Rajan-Iyer J, Courtney-Pillinger M, Beck CR, Hope V, Lamagni T, Brown CS, Jermacane D, Glass R, Desai M, Gobin M, Balasegaram S, Anderson C (2017). Ongoing outbreak of invasive and non-invasive disease due to group A Streptococcus (GAS) type emm66 among homeless and people who inject drugs in England and Wales, January to December 2016. Euro Surveill 22(3):pii=30446 [DOI] [PMC free article] [PubMed]
- 38.Mosites E, Frick A, Gounder P, Castrodale L, Li Y, Rudolph K, et al. Outbreak of invasive infections from subtype emm26.3 group A Streptococcus among homeless adults—Anchorage, Alaska, 2016–2017. Clin Infect Dis Off Publ Infect Dis Soc Am. 2018;66(7):1068–74. doi: 10.1093/cid/cix921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dohoo C, Stuart R, Finkelstein M, Bradley K, Gournis E. Risk factors associated with group A Streptococcus acquisition in a large, urban homeless shelter outbreak. Can J Public Health Rev Can Sante Publique. 2020;111(1):117–124. doi: 10.17269/s41997-019-00258-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Loughlin RE, Roberson A, Cieslak PR, Lynfield R, Gershman K, Craig A, et al. The epidemiology of invasive group A streptococcal infection and potential vaccine implications: United States, 2000–2004. Clin Infect Dis Off Publ Infect Dis Soc Am. 2007;45(7):853–862. doi: 10.1086/521264. [DOI] [PubMed] [Google Scholar]
- 41.Luca-Harari B, Darenberg J, Neal S, Siljander T, Strakova L, Tanna A, et al. Clinical and microbiological characteristics of severe Streptococcus pyogenes disease in Europe. J Clin Microbiol. 2009;47(4):1155–1165. doi: 10.1128/JCM.02155-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aranha MP, Penfound TA, Salehi S, Botteaux A, Smeesters P, Dale JB, et al. Design of broadly cross-reactive M protein-based group A streptococcal vaccines. J Immunol Baltim Md 1950. 2021;207(4):1138–49. doi: 10.4049/jimmunol.2100286. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table 1. Number of cases of adults, children, iGAS andpiGAS infections, non-homeless and homeless adults per year between 2005 and2020. Table 2. Comparison of characteristics, risk factors andclinical presentations of homeless versus non-homeless adults with piGAS andiGAS infections.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.