Abstract
Background
Respiratory syncytial virus (RSV) is a leading cause of acute respiratory infections worldwide. While historically RSV research has been focused on children, data on RSV infection in adults are limited. The goal of this study was to establish the prevalence of RSV in community-dwelling Italian adults and analyze its genetic variability during the 2021/22 winter season.
Methods
In this cross-sectional study, a random sample of naso-/oropharyngeal specimens from symptomatic adults seeking for SARS-CoV-2 molecular testing between December 2021 and March 2022 were tested for RSV and other respiratory pathogens by means of reverse-transcription polymerase chain reaction. RSV-positive samples were further molecularly characterized by sequence analysis.
Results
Of 1,213 samples tested, 1.6% (95% CI: 0.9–2.4%) were positive for RSV and subgroups A (44.4%) and B (55.6%) were identified in similar proportions. The epidemic peak occurred in December 2021, when the RSV prevalence was as high as 4.6% (95% CI: 2.2–8.3%). The prevalence of RSV detection was similar (p = 0.64) to that of influenza virus (1.9%). All RSV A and B strains belonged to the ON1 and BA genotypes, respectively. Most (72.2%) RSV-positive samples were also positive for other pathogens being SARS-CoV-2, Streptococcus pneumoniae and rhinovirus the most frequent. RSV load was significantly higher among mono-detections than co-detections.
Conclusion
During the 2021/22 winter season, characterized by the predominant circulation of SARS-CoV-2 and some non-pharmaceutical containment measures still in place, a substantial proportion of Italian adults tested positive for genetically diversified strains of both RSV subtypes. In view of the upcoming registration of vaccines, establishment of the National RSV surveillance system is urgently needed.
Keywords: Respiratory syncytial virus, RSV, Adult population, RSV epidemiology, RSV molecular characteristics
Background
Human respiratory syncytial virus (RSV) is a leading cause of acute respiratory infections (ARIs) worldwide and belongs to the Paramyxoviridae family, genus Orthopneumovirus [1]. Of 11 proteins encoded by the viral RNA, glycoproteins G and F play the prominent role in the virus–host interaction: the latter functions as fusion agent, while the former is responsible for cell attachment [2]. The F protein is highly conserved and immunogenic, which makes it particularly attractive for vaccine development. By contrast, the G protein is subjected to frequent mutations and, on the basis of this latter, RSV is classified into two antigenically distinct subgroups A (RSV-A) and B (RSV-B). In a typical season, both subgroups co-circulate, although one usually predominates over the other [3–5].
Traditionally, research on the epidemiology and burden of RSV infections has been focused on young children. Indeed, RSV was first isolated in a child with bronchopneumonia [6], and RSV is responsible for a large burden of disease in infants [7–9], typically exceeding that of seasonal influenza [10]. However, the burden of disease in adults is considerable as well. For instance, it has been estimated that in 2015 in industrialized countries a total of 1,500,000 (95% CI: 300,000–6,900,000) episodes of RSV-associated ARIs occurred among older adults and 14.5% of these patients required hospitalization [11].
Since the early 2020, non-pharmaceutical interventions adopted to limit the spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have modified seasonal circulation pattern of other respiratory viruses, including RSV. Most available surveys [12–14] have reported a dramatic decrease in RSV detections during the different waves of COVID-19 pandemic. On the other hand, in Shenzhen (China) contrary to seasonal influenza, the detection rate of RSV in children < 14 years increased from 6.6% in 2019 to 25.6% in 2020 [15]. RSV and SARS-CoV-2 co-occurrence seems to be infrequent: a systematic review and meta-analysis by Musuuza et al. [16] has estimated the prevalence of SARS-CoV-2 and RSV co-infection to be 3.8%, and RSV superinfection in SARS-CoV-2-positive individuals to be 0.4%. A recent large study [17] conducted among hospitalized SARS-CoV-2-positive adults in the United Kingdom has revealed a prevalence of RSV of 3.16%.
To date, there is no centralized surveillance system of RSV in Italy and previous research has been mostly focused on the pediatric population [18]. Few available epidemiological surveys have demonstrated a substantial RSV burden in the Italian adults. For instance, in four consecutive winter seasons (from 2014/15 to 2017/18) a total of 3.3–12.2% and 8.1–14.2% of influenza-like illness (ILI) cases in working-age and older adults, respectively, were caused by RSV [19]. During the 2019/20 season, 22.6% of hospitalized Italian adults with respiratory symptoms were diagnosed with RSV [20]. With regard to RSV viral population, a gradual increase in detections of the RSV-A genotype ON1 has been reported, while RSV-B is currently represented by BA strains [19, 21, 22]. Similarly to other countries, during the first COVID-19 pandemic waves, circulation of RSV in Italy decreased significantly [23–25]. However, a sharp increase in RSV detections among young children was observed in late 2021 [26], suggesting resurgence of RSV in Italy [27]. To our knowledge, no studies on the epidemiology of RSV infection in adults have been conducted in Italy since the start of COVID-19 pandemic. The objective of this study was to quantify the prevalence of RSV (co)-detections and analyze its genetic variability among symptomatic community-dwelling adults seeking for SARS-CoV-2 molecular diagnosis during the 2021/22 winter season.
Methods
Study population
In this cross-sectional study, nasopharyngeal and/or oropharyngeal swab specimens from symptomatic community-dwelling adult individuals seeking for SARS-CoV-2 molecular diagnostics were tested for RSV and other respiratory pathogens. In particular, eligible specimens came from subjects self-presenting at a community point for SARS-CoV-2 swab with any respiratory symptom(s). These samples were collected between December 1, 2021 and March 31, 2022 and processed at the regional reference laboratory for COVID-19 diagnostics located at San Martino Polyclinic Hospital (Genoa, Italy). The study period was characterized by the highest SARS-CoV-2 incidence [28], which was driven by the Omicron variant of concern [29]. Individuals of both sexes, aged ≥ 18 years and residing in the Metropolitan City of Genoa were eligible.
The minimum sample size was determined a priori as follows: by assuming a true RSV prevalence of 3% when α is 95% and precision is 0.01, at least 1,118 samples were required. By assuming that 15% of specimens would have an insufficient volume, a total of 1,286 samples eluted in the universal transport medium (UTM) (Copan Italia S.p.A.; Brescia, Italy) were randomly extracted from the available set of specimens stored at -80 °C.
Molecular detection of RSV by real-time RT-PCR
Each specimen was first screened for RSV by means of the Resp-4-Plex kit (Abbott Molecular Inc., Des Plaines, IL, USA) used with the fully automated Alinity m System (Abbott Molecular Inc., Des Plaines, IL, USA) and according to the manufacturer’s instructions. This kit is a multiplex real-time reverse transcription polymerase chain reaction (RT-PCR) for the qualitative detection and differentiation of RNA from SARS-CoV-2, RSV, influenza A and B viruses. According to the manufacturer, the limit of detection is 0.300 and 0.100 median tissue culture infectious dose (TCID50)/ml for RSV-A and RSV-B, respectively [30].
To discern RSV subgroup and detect the presence of other respiratory pathogens, samples positive for RSV were further tested by means of the Allplex Respiratory Panel (RP) assays (Seegene Inc.; Seoul, Republic of Korea) according to the manufacturer’s instructions. Briefly, nucleic acids were first extracted using the STARMag Universal Cartridge Kit (Seegene Inc.; Seoul, Republic of Korea) on the automated Nimbus IVD (Seegene Inc.; Seoul, Republic of Korea) platform. For this purpose, 200 µl of each specimen was extracted and eluted with 100 µl of elution buffer and set up for RT-PCR. RT-PCR was then performed on a CFX96 instrument (Bio-Rad Laboratories, Inc; Hercules, CA, USA) with the Allplex RPs 1–4 kits. These four panels are able to detect the most common respiratory pathogens - both viruses [RP 1: RSV-A, RSV-B, influenza viruses A, A(H1N1), A(H1N1)pdm09, A(H3N2) and B; RP 2: adenovirus (AdV), enterovirus (EV), metapneumovirus (MPV), parainfluenza (PIV) viruses 1–4; RP 3: bocaviruses (BoV) 1–4, coronaviruses (CoV) 229E, NL63, OC43, rhinovirus (RV)] and bacteria [RP 4: Streptococcus pneumoniae (SP), Bordetella parapertussis (BPP), Bordetella pertussis (BP), Chlamydophila pneumoniae (CP), Haemophilus influenzae (HI), Legionella pneumophila (LP), Mycoplasma pneumoniae (MP)]. For each RT-PCR, 8 µl of the extracted nucleic acid in a final volume of 25 µl was used. The diagnostic accuracy of this assay in detecting RSV-A and RSV-B is 100% [31].
Samples showing cycle threshold (Ct) values < 40 in at least one assay were deemed positive. Ct values were used as a proxy measure of viral load: lower Ct values indicate higher viral load.
Sequencing and molecular characterization of RSV A and B
All samples tested positive in either commercial RT-PCR assay were sequenced. Viral RNA was first extracted from 140 µl of sample using the QIAamp Viral RNA Mini Kit (QIAGEN; Hilden, Germany) and eluted in 50 µl of the supplied elution buffer. RT-PCR on the extracted RNA was then performed using the SuperScript IV One-Step RT-PCR System (Invitrogen; Carlsbad, CA, USA) with the following temperature profile: 1 cycle at 45 °C for 30 min and 98 °C for 2 min, 40 cycles at 98 °C for 30 s, 55 °C for 30 s, 72 °C for 2 min and a final extension of 72 °C for 5 min.
The first amplification of gene G (irrespective of the RSV subgroup) was performed by using specific primers [32]. Subsequently, a nested PCR using 5 µl of the amplified cDNA as template was performed. The amplification was carried out using Platinum II Taq Hot-Start DNA Polymerase (Invitrogen; Carlsbad, CA, USA) with the following conditions: 1 cycle at 94 °C for 2 min, 40 cycles at 94 °C for 30 s, 55 °C for 30 s, 68 °C for 2 min and a final extension at 68 °C for 5 min.
Full G gene amplicons of 969 bp and 954 bp of RSV-A and RSV-B, respectively, were visualized on 1.5% agarose gel with Midori Green Direct (NIPPON Genetics EUROPE; Düren, Germany) as intercalating dye. PCR products were then purified with ExoSAP-IT PCR Product Cleanup Reagent (Applied Biosystems; Waltham, MA, USA) as per manufacturer’s instructions. Finally, the sequencing reactions were performed on SeqStudio Genetic Analyzer System (Applied Biosystems; Waltham, MA, USA) for Sanger sequencing. The obtained sequences were assembled with SeqScape Software v4.0 (Applied Biosystems; Waltham, MA, USA).
Phylogenetic analysis of RSV-A and RSV-B
Overlapping sequences of the G gene were edited and assembled by using the ClustalW program implemented in the BioEdit software v. 7.2 [33]. All nucleotide sequences obtained were deposited in GenBank database (https://www.ncbi.nlm.nih.gov/genbank/) under the accession numbers (ANs) ON707106–ON707123. Multiple sequence alignments, including study and reference sequences, were carried out by using the ClustalW program, implemented in the BioEdit software. To identify RSV-A and RSV-B genotypes, nucleotide sequence alignments were phylogenetically analyzed by MEGA software, version 6.0 [34].
Nucleotide sequence identities were established by generating sequence identity matrices implemented in BioEdit and expressed as mean percentage with ranges. Cluster analysis of the study sequences was conducted in MEGA. Phylogenetic trees were generated by using the maximum likelihood method based on the Hasegawa-Kishino-Yano and the Tamura-Nei model with a gamma-shaped distribution model for RSV-A and RSV-B, respectively. The best amino acid substitution models were ascertained by analyzing sequence datasets within the “Models tool” implemented in MEGA. Confidence estimates were assessed by conducting a bootstrap analysis with 1,000 replicates and estimates ≥ 70% were considered significant. The phylogenetic relationship among study strains was assessed by calculating pairwise p-distances expressed as averages crude rates ± standard deviations (SDs).
Statistical analysis
Prevalence was expressed as percentage with Clopper-Pearson exact 95% confidence intervals (95% CIs). The McNemar’s test was used to compare the overall prevalence of RSV and influenza virus detections. Prevalence estimates were compared by means of the Fisher’s exact test. Unpaired t test was used to compare Ct values between RSV mono-detections and co-detections and the corresponding effect size was expressed as Cohen’s d. The Firth’s penalized logistic regression was applied to estimate the adjusted (for age, sex and month of swab) odds ratio (aOR) of testing positive for RSV according to the SARS-CoV-2 positivity status.
Statistical analysis was performed using R stat packages v. 4.1.0 (R Core Team; Vienna, Austria) [35].
Results
Prevalence of RSV
A total of 1,213 samples were tested by the Resp-4-Plex assay and the main characteristics of subjects are reported in Table 1.
Table 1.
Characteristics of adults with respiratory symptoms included in this study and results of SARS-CoV-2 RNA detection in their naso- or oropharyngeal samples (n = 1,213)
| Variable | Level | % (n) | 95% CI |
|---|---|---|---|
| Sex | Female | 58.3 (707) | 55.5–61.1 |
| Male | 41.7 (506) | 38.9–44.5 | |
| Age, years | 18–64 | 53.1 (644) | 50.2–55.9 |
| ≥ 65 | 46.9 (569) | 44.1–49.8 | |
| Median | 61 | 43–80a | |
| Month of sample | Dec 2021 | 18.0 (218) | 15.8–20.3 |
| Jan 2022 | 29.3 (355) | 26.7–31.9 | |
| Feb 2022 | 31.7 (384) | 29.0–34.4 | |
| Mar 2022 | 21.1 (256) | 18.8–23.5 | |
| SARS-CoV-2 RNA detection | Yes | 78.4 (951) | 76.0–80.7 |
| No | 21.6 (262) | 19.3–24.0 |
a Interquartile range
Briefly, their mean age was 60.5 ± 21.5 years and females slightly prevailed (58.3%). As expected, most (78.6%) samples were positive for SARS-CoV-2.
Nineteen out of 1,213 (1.6%) samples resulted positive for RSV, giving an overall prevalence rate of 1.6% (95% CI: 0.9–2.4%). The overall detection rates among working-age (18–64 years) [1.7% (95% CI: 0.9–3.0%)] and older (≥ 65 years) [1.4% (95% CI: 0.6–2.8%)] adults were similar (p = 0.83). Most positive cases were detected in December 2021 when the prevalence was as high as 4.6% (95% CI: 2.2–8.3%). In the subsequent three months of 2022 [January: 2.0% (95% CI: 0.8–4.0%); February: 0.5% (95% CI: 0.1–1.9%); March: 0.0% (95% CI: 0.0–1.4%)], the prevalence of RSV gradually decreased.
Twenty-three samples [1.9% (95% CI: 1.2–2.8%)] were positive for influenza viruses and this prevalence was similar to that of RSV (p = 0.64). Of influenza positive samples, 91.3% (21/23) and 8.7% (2/23) belonged to A and B types, respectively. Contrary to RSV detections that mostly occurred in December 2021, most (82.6%; 19/23) influenza virus detections were reported in March 2022.
Of specimens tested positive for RSV by the Resp-4-Plex assay, 18 samples proved also positive by the Allplex RP 1. RSV-A (44.4%; 8/18) and RSV-B (55.6%; 10/18) were detected in similar proportions. As shown in Table 2, most (72.2%; 13/18) RSV-positive subjects tested positive for at least one other respiratory pathogen. SARS-CoV-2 (n = 7), SP (n = 3) and Rhinovirus (n = 2) were the most frequent co-detections. Seasonal coronavirus 229E, enterovirus and HI were detected once each. No RSV/ influenza virus co-detections were found.
Table 2.
Characteristics of RSV-positive subjects
| # | Sex | Age, years | Sample date | RSV | Co-detections (Ct) | |
|---|---|---|---|---|---|---|
| Resp-4-Plex (Ct) | Allplex Respiratory Panel 1 (Ct) | |||||
| 1 | F | 42 | 13/12/2021 | POS (22.17) | RSV-B (24.79) | SP (27.42) |
| 2 | F | 86 | 16/12/2021 | POS (22.43) | RSV-A (28.25) | – |
| 3 | F | 71 | 16/12/2021 | POS (21.65) | RSV-B (23.68) | – |
| 4 | M | 70 | 19/12/2021 | POS (19.35) | RSV-A (22.56) | – |
| 5 | F | 29 | 20/12/2021 | POS (18.91) | RSV-A (25.55) | RV (39.09) |
| 6 | M | 47 | 20/12/2021 | POS (20.47) | RSV-A (23.38) | SP (27.96) |
| 7 | F | 52 | 20/12/2021 | POS (21.09) | RSV-A (24.94) | SARS-CoV-2 (26.86); HI (38.33) |
| 8 | F | 30 | 26/12/2021 | POS (18.18) | RSV-A (22.66) | SARS-CoV-2 (23.81); RV (39.33) |
| 9 | M | 53 | 26/12/2021 | POS (37.31) | RSV-B (41.79) | SARS-CoV-2 (31.90) |
| 10 | F | 79 | 28/12/2021 | POS (21.39) | RSV-B (25.62) | – |
| 11 | F | 21 | 02/01/2022 | POS (25.88) | RSV-B (25.09) | CoV-229E (25.42); SP (32.94) |
| 12 | F | 21 | 02/01/2022 | POS (31.77) | RSV-B (33.39) | EV (33.75) |
| 13 | F | 50 | 02/01/2022 | POS (23.55) | RSV-B (26.16) | – |
| 14 | F | 72 | 03/01/2022 | POS (30.57) | RSV-B (33.94) | SARS-CoV-2 (26.47) |
| 15 | F | 49 | 03/01/2022 | POS (21.65) | RSV-A (29.30) | SP (35.11) |
| 16 | F | 39 | 09/01/2022 | POS (31.94) | RSV-B (35.31) | SARS-CoV-2 (32.29) |
| 17 | M | 83 | 23/01/2022 | POS (20.38) | RSV-B (23.13) | – |
| 18 | M | 91 | 23/02/2022 | POS (34.72) | RSV-A (38.17) | SARS-CoV-2 (30.51) |
| 19 | F | 91 | 23/02/2022 | POS (36.42) | NEG | SARS-CoV-2 (20.66) |
CoV-229E, coronavirus 229E; Ct, cycle threshold; EV, enterovirus; HI, Hemophilus influenzae; NA, not available; RV, rhinovirus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SP, Streptococcus pneumoniae
Phylogenetic analysis
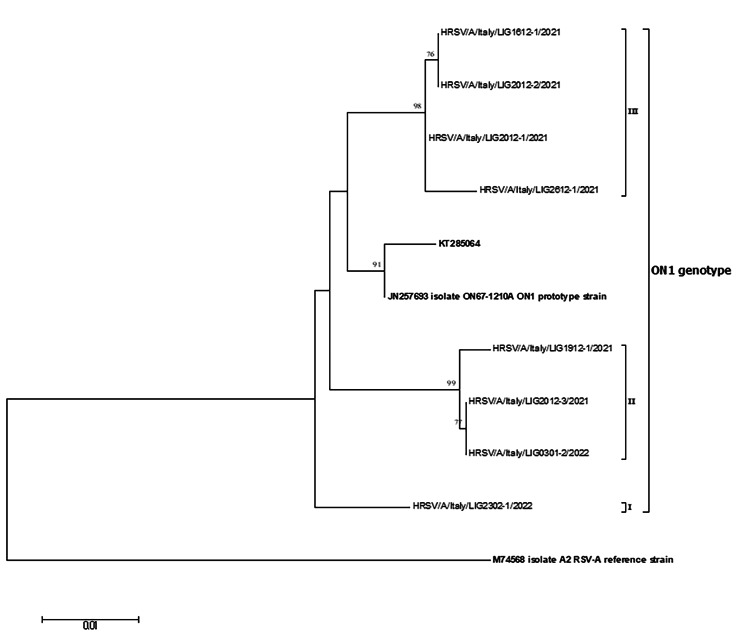
Sequencing was successful for 18 samples. As shown in Fig. 1, all RSV-A strains belonged to the ON1 genotype.
Fig. 1.
Phylogenetic tree based on the partial G gene sequences of RSV-A strains
Mean nucleotide sequence identity between study RSV-A sequences and the ON1 prototype strain (strain ON67-1210 A, AN: JN257693) was 98.3% (range: 97.9–98.8%). The mean intra-genotypic p-distance of the study ON1 strains was 0.018 ± 0.010. The study sequences were further divided into three branches. In particular, while the HRSV/A/Italy/LIG2302-1/2022 strain was stand-alone (branch I), strains HRSV/A/Italy/LIG1912-1/2021, HRSV/A/Italy/LIG2012-3/2021 and HRSV/A/Italy/LIG0301-2/2022 sequences clustered together (branch II, bootstrap 99%) sharing a mean intra-group p-distance of 0.003 ± 0.002. The remaining four ON1 sequences clustered into the third branch (bootstrap 98%) sharing a mean intra-group p-distance of 0.003 ± 0.003.
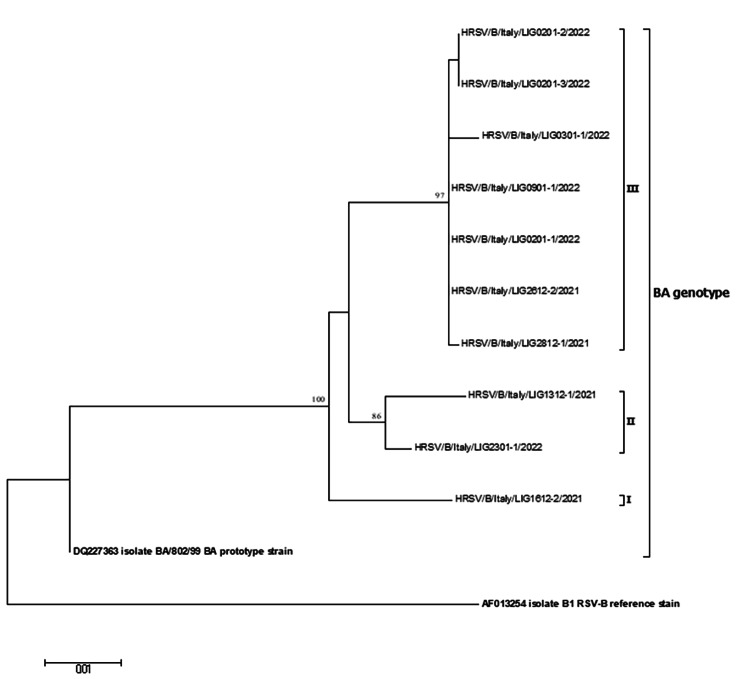
For what concerns RSV-B, all study sequences belonged to the BA group and the average nucleotide identity to the BA prototype strain (BA/802/99, AN: DQ227363) was 95.4% (range: 94.9–95.7%). At nucleotide level, the mean intra-genotypic p-distance of the study sequences was 0.014 ± 0.012. The phylogenetic analysis (Fig. 2) showed that the study sequences were further segregated into three branches: strain HRSV/B/Italy/LIG1612-2/2021 was stand-alone (branch I), strains HRSV/B/Italy/LIG1312-1/2021 and HRSV/B/Italy/LIG2301-1/2022 clustered together (branch II, bootstrap 86%) with a mean intra-group p-distance of 0.016, while the remaining seven BA sequences defined branch III (bootstrap 97%) sharing a mean intra-group p-distance of 0.002 ± 0.002.
Fig. 2.
Phylogenetic tree based on the partial G gene sequences of RSV-B strains
Factors associated with RSV infection
In the multivariable analysis, RSV positivity was similar between males and females and between working-age and older adults. On the other hand, the prevalence of RSV among SARS-CoV-2-negative adults was 4.6%, while only 0.7% of SARS-CoV-2-positive subjects were also positive for RSV with an aOR of 5.26 (95% CI: 2.13–14.29).
For what concerns RSV load, subjects with RSV mono-detection (n = 6) showed significantly (p = 0.016) lower Ct values (mean 21.5 ± 1.5) than those with co-detections (n = 13; mean 24.9 ± 2.2) with a |d| of 0.93 (95% CI: 0.16–2.02). A similar picture was observed by Allplex RP1 assay (27.0 ± 7.0 vs. 29.9 ± 6.4; p = 0.029). This difference was driven by viral co-detections: the Resp-4-Plex Ct values in samples positive for both RSV and other viruses (n = 8) were generally lower (mean: 30.0; range: 18.9–37.3) compared with those positive (n = 3) for both RSV and bacteria (mean: 21.4; range: 20.5–22.2). For what concerns SARS-CoV-2, subjects negative for RSV (n = 944) had on average higher (p = 0.095) SARS-CoV-2 load than those with a concomitant (n = 7) RSV detection (Ct values of 24.2 ± 8.0 vs. 27.5 ± 7.5).
Discussion
In the present study, the overall prevalence of RSV in a representative sample of Italian community-dwelling working-age and older adults seeking for SARS-CoV-2 diagnosis was established. Our main findings may be summarized as follows: (i) during the 2021/22 winter season, up to 4.6% of samples tested positive for RSV and RSV detection rate was similar to that of influenza virus; (ii) RSV-A and RSV-B circulated in approximately equal proportions and a substantial level of phylogenetic diversity was observed within each subgroup; (iii) among RSV-positive subjects, the detection of other respiratory viruses and bacteria was frequent; (iv) as shown by significantly lower Ct values, RSV viral load was higher among mono-detections than co-detections.
Few available studies on the confirmed cases of RSV among Italian adults were conducted on a limited number of individuals sourced from different settings. For instance, the cumulative four-season (from 2014/15 to 2017/18) prevalence of RSV among community-dwelling adults with ILI was 4.8%, 9.3% and 12.0% for subjects aged 16–45, 46–65 and > 65 years, respectively [19]. By contrast, in the 2019/20 winter season only 3.0% of adults with ILI tested positive for RSV [36], which approaches our estimate. A high variability of RSV prevalence has been also reported in hospital setting. While Calderaro et al. [20] reported a positivity rate of 22.6% among adult inpatients with respiratory symptoms, the estimates provided by Leli et al. [37] were 2.0% and 7.4% for working-age and older adults, respectively. The heterogeneity in estimates is likely driven by a number of factors, including winter season (i.e., some seasons are characterized by higher RSV activity [38]), study period (e.g., inclusion of summer months decreases the overall prevalence), inclusion criteria and other features of the study population (e.g., different set of symptoms may have different prediction scores). Finally, our study was conducted during the period in which non-pharmaceutical interventions (e.g., mask wearing) were in place that may have had an impact on RSV circulation as well.
We found that RSV-A and RSV-B circulated in similar proportions. An analogous trend was observed during the 2016/17 Italian winter season [19, 39]. Phylogenetically, all RSV-A and RSV-B detections belonged to the ON1 and BA genotypes, respectively; these latter are currently the predominant strains in Italy [19] and Europe [40, 41]. Within both subtypes, the overall p-distance was 1.4–1.8%, which is coherent with previous Italian studies [19, 21]. Indeed, it is known that multiple RSV genotypes may co-circulate during the same season [19, 21, 22], which may denote that local epidemics are caused by a mix of strains seeded from abroad and persistence of local viruses [42]. Continuous monitoring of the molecular dynamics of the local RSV population is warranted in view of the near future prophylactic and therapeutic opportunities.
In our study, the rate of RSV co-detections, most of which were due to SARS-CoV-2, was relatively high. In our opinion, this finding is mostly driven by the fact that the study period (form December 1, 2021 to March 31, 2022) was characterized by the highest SARS-CoV-2 incidence [28]. Analogously, the study population was composed of communing-dwelling adults seeking for SARS-CoV-2 molecular testing. Other than SARS-CoV-2 viral co-detections were significantly less frequent, which is consistent with previous research [20]. With regard to bacteria co-detections, it is likely that these were ascribable to the transient colonization. Indeed, the nasopharyngeal carriage of both SP and HI is common among Italian adults [43, 44]. We then observed that compared with RSV mono-detections, samples tested positive for both RSV and another respiratory pathogen (especially if viral) showed significantly lower viral loads. These findings are in line with a pediatric study conducted during the 2015/16 season: Ct values in RSV single detections were on median 1.5 points lower (25.5 vs. 27.0; p = 0.05) than in RSV co-detections [45]. This observation may be explained by a negative viral inference that has been described for RSV co-infections with influenza A virus, rhinovirus and metapneumovirus [46]. A prospective cohort study on the RSV transmission dynamics [47] has brought to light that prior infection with a respiratory virus may lead to either an up-regulation of innate immunity or non-specific cross-reactivity that reduces shedding of a subsequent infection, while presence of RSV co-infections may indicate a low immunity associated with poor viral clearance. Furthermore, RSV detections are less frequent during the periods characterized by high influenza activity and the incidence of RSV may peak 1–2 months earlier [46]. This is consistent with our results when RSV and influenza virus type A circulated in two distinct periods. Analogously, RSV–MPV and RSV-RV co-infections seems to be relatively rare [46]. In the present study no RSV–MPV co-detections were found, while two subjects also positive for RV had viral loads just above the limit of detection (Ct = 39). Less is instead known about a possible inference between RSV and SARS-CoV-2. Bai et al. [48] have demonstrated that RSV had no effect on SARS-CoV-2 infectivity. Although the systematic evidence suggests that co-detections of both viruses are infrequent [16], most available studies were conducted during the period in which several non-pharmaceutical interventions to contain pandemic were in place, which reduced circulation of other common viruses.
This study has some limitations, which may interfere with the interpretation of our findings. The main study drawback was the lack of itemized clinical record data. The study population was composed of adults with respiratory symptoms – either ARI or ILI – and, based on the previous Italian studies [19, 20, 36–38], it is likely that RSV prevalence differs between these two clinical syndromes. Second, the overall RSV prevalence may be underestimated, since most detections occurred in December 2021, while no samples collected earlier were available. In other words, it appears likely that RSV circulated in earlier months of 2021 and therefore we cannot rule out an earlier incidence peak. In this regard, Movva et al. [9] have established that the COVID-19 pandemic may have changed the RSV epidemiology and the number of RSV detections is likely underestimated. Third, the phylogenetic analysis was performed on the G gene only and therefore some important evolutionary patterns in other genome regions were not assessed. Indeed, the lack of whole RSV genome data was identified as a main knowledge gap [49]. Finally, considering the cross-sectional nature of the study, for samples positive for RSV and other respiratory pathogens we were unable to rule out which infection occurred first.
In conclusion, during the 2021/22 winter season, which was characterized by the predominant circulation of SARS-CoV-2 variants of concern Delta and Omicron, a significant proportion of Italian adults were positive for genetically diversified strains of both RSV subgroups. In view of the near future availability of RSV vaccines, the establishment of a national surveillance system should be seen as a public health priority. Future large-scale longitudinal studies should investigate the natural history of an RSV episode (e.g., incidence, complications, prescriptions, outcomes) in adults since these data are essential for policy-oriented health technology assessment of the upcoming vaccines.
Acknowledgements
The authors thank Dr Carlo Simone Trombetta, Dr Allegra Ferrari for their support of the study.
List of abbreviations
- AdV
Adenovirus
- AN
Accession number
- aOR
adjusted odds ratio
- ARI
Acute respiratory infection
- BoV
Bocavirus
- BP
Bordetella pertussis
- BPP
Bordetella parapertussis
- CoV
Coronavirus
- CP
Chlamydophila pneumoniae
- Ct
Cycle threshold
- EV
Enterovirus
- HI
Haemophilus influenzae
- ILI
Influenza-like illness
- LP
Legionella pneumophila
- MP
Mycoplasma pneumoniae
- MPV
Metapneumovirus
- PIV
Parainfluenza virus
- RP
Respiratory panel
- RSV
Human respiratory syncytial virus
- RSV-A
Human respiratory syncytial virus subgroup A
- RSV-B
Human respiratory syncytial virus subgroup B
- RT-PCR
Real-time reverse transcription polymerase chain reaction
- RV
Rhinovirus
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- SD
Standard deviation
- SP
Streptococcus pneumoniae
- TCID
Tissue culture infectious dose
- UTM
Universal transport medium
Author contributions
GI, AD, AO, and DP designed the study. BB, AD, CG, FS, EP performed laboratory analyses. PLL and MO were responsible for data collection. AD carried out statistical analysis. AD and DP were responsible for writing and editing of the manuscript. AO, EP and GI were responsible for the critical review of the manuscript. GI was responsible for supervision of the study. All authors have read and approved the final manuscript.
Funding
The study was funded by Janssen Cilag.
Data availability
The datasets generated and/or analysed during the current study are not publicly available due to the data nature but are available from the corresponding author on reasonable request.
Declarations
Ethical approval and consent to participate
The study was approved by the Ethics Committee of the Liguria Region (Genoa, Italy) (n° 230/2022). The study was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical and Laboratory Practices guidelines. All subjects gave their informed consent to the enrolling physicians.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Battles MB, McLellan JS. Respiratory syncytial virus entry and how to block it. Nat Rev Microbiol. 2019;17(4):233–45. doi: 10.1038/s41579-019-0149-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borchers AT, Chang C, Gershwin ME, Gershwin LJ. Respiratory syncytial virus–a comprehensive review. Clin Rev Allergy Immunol. 2013;45(3):331–79. doi: 10.1007/s12016-013-8368-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ciarlitto C, Vittucci AC, Antilici L, Concato C, Di Camillo C, Zangari P, Villani A. Respiratory syncytial virus A and B: three bronchiolitis seasons in a third level hospital in Italy. Ital J Pediatr. 2019;45(1):115. doi: 10.1186/s13052-019-0704-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peret TC, Hall CB, Hammond GW, Piedra PA, Storch GA, Sullender WM, Tsou C, Anderson LJ. Circulation patterns of group A and B human respiratory syncytial virus genotypes in 5 communities in North America. J Infect Dis. 2000;181(6):1891–6. doi: 10.1086/315508. [DOI] [PubMed] [Google Scholar]
- 5.Mazur NI, Higgins D, Nunes MC, Melero JA, Langedijk AC, Horsley N, Buchholz UJ, Openshaw PJ, McLellan JS, Englund JA, Mejias A, Karron RA, Simões EA, Knezevic I, Ramilo O, Piedra PA, Chu HY, Falsey AR, Nair H, Kragten-Tabatabaie L, Greenough A, Baraldi E, Papadopoulos NG, Vekemans J, Polack FP, Powell M, Satav A, Walsh EE, Stein RT, Graham BS, Bont LJ. Respiratory Syncytial Virus Network (ReSViNET) Foundation. The respiratory syncytial virus vaccine landscape: lessons from the graveyard and promising candidates. Lancet Infect Dis. 2018;18(10):e295–e311. doi: 10.1016/S1473-3099(18)30292-5. [DOI] [PubMed] [Google Scholar]
- 6.Chanock R, Finberg L. Recovery from infants with respiratory illness of a virus related to chimpanzee coryza agent (Cca) Am J Epidemiol. 1957;66:281–90. doi: 10.1093/oxfordjournals.aje.a119901. [DOI] [PubMed] [Google Scholar]
- 7.Haddadin Z, Rankin DA, Lipworth L, Suh M, McHenry R, Blozinski A, George SS, Fernandez KN, Varjabedian R, Spieker AJ, Shepard DS, Halasa NB. Respiratory virus surveillance in infants across different clinical settings. J Pediatr. 2021;234:164–171e2. doi: 10.1016/j.jpeds.2021.03.036. [DOI] [PubMed] [Google Scholar]
- 8.Suh M, Movva N, Jiang X, Bylsma LC, Reichert H, Fryzek JP, Nelson CB. Respiratory syncytial virus is the leading cause of United States infant hospitalizations, 2009–2019: a study of the national (nationwide) inpatient sample. J Infect Dis. 2022;226(Suppl 2):154–S163. doi: 10.1093/infdis/jiac120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Movva N, Suh M, Reichert H, Hintze B, Sendak MP, Wolf Z, Carr S, Kaminski T, White M, Fisher K, Wood CT, Fryzek JP, Nelson CB, Malcolm WF. Respiratory syncytial virus during the COVID-19 pandemic compared to historic levels: a retrospective cohort study of a health system. J Infect Dis. 2022;226(Suppl 2):175–S183. doi: 10.1093/infdis/jiac220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansen CL, Chaves SS, Demont C, Viboud C. Mortality associated with influenza and respiratory syncytial virus in the US, 1999–2018. JAMA Netw Open. 2022;5(2):e220527. doi: 10.1001/jamanetworkopen.2022.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi T, Denouel A, Tietjen AK, Campbell I, Moran E, Li X, Campbell H, Demont C, Nyawanda BO, Chu HY, Stoszek SK, Krishnan A, Openshaw P, Falsey AR, Nair H. RESCEU Investigators. Global disease burden estimates of respiratory syncytial virus-associated acute respiratory infection in older adults in 2015: a systematic review and meta-analysis. J Infect Dis. 2020;222(Suppl 7):577–S583. doi: 10.1093/infdis/jiz059. [DOI] [PubMed] [Google Scholar]
- 12.van Summeren J, Meijer A, Aspelund G, Casalegno JS, Erna G, Hoang U, Lina B, VRS study group in Lyon. de Lusignan S, Teirlinck AC, Thors V, Paget J. Low levels of respiratory syncytial virus activity in Europe during the 2020/21 season: what can we expect in the coming summer and autumn/winter? Euro Surveill. 2021;26(29):2100639. doi: 10.2807/1560-7917.ES.2021.26.29.2100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Letafati A, Aghamirmohammadali FS, Rahimi-Foroushani A, Hasani SA, Mokhtari-Azad T, Yavarian J. No human respiratory syncytial virus but SARS-CoV-2 found in children under 5 years old referred to Children Medical Center in 2021, Tehran, Iran. J Med Virol. 2022 doi: 10.1002/jmv.27685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engels G, Sack J, Weissbrich B, Hartmann K, Knies K, Härtel C, Streng A, Dölken L, Liese JG, CoPraKid Study Group Very low incidence of SARS-CoV-2, influenza and RSV but high incidence of rhino-, adeno- and endemic coronaviruses in children with acute respiratory infection in primary care pediatric practices during the second and third wave of the SARS-CoV-2 pandemic. Pediatr Infect Dis J. 2022;41(4):e146–8. doi: 10.1097/INF.0000000000003460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li L, Wang H, Liu A, Wang R, Zhi T, Zheng Y, Bao Y, Chen Y, Wang W. Comparison of 11 respiratory pathogens among hospitalized children before and during the COVID-19 epidemic in Shenzhen, China. Virol J. 2021;18(1):202. doi: 10.1186/s12985-021-01669-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Musuuza JS, Watson L, Parmasad V, Putman-Buehler N, Christensen L, Safdar N. Prevalence and outcomes of co-infection and superinfection with SARS-CoV-2 and other pathogens: a systematic review and meta-analysis. PLoS ONE. 2021;16(5):e0251170. doi: 10.1371/journal.pone.0251170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swets MC, Russell CD, Harrison EM, Docherty AB, Lone N, Girvan M, Hardwick HE, Investigators ISARIC4C, Visser LG, Openshaw PJM, Groeneveld GH, Semple MG, Baillie JK. SARS-CoV-2 co-infection with influenza viruses, respiratory syncytial virus, or adenoviruses. Lancet. 2022;399(10334):1463–4. doi: 10.1016/S0140-6736(22)00383-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Azzari C, Baraldi E, Bonanni P, Bozzola E, Coscia A, Lanari M, Manzoni P, Mazzone T, Sandri F, Checcucci Lisi G, Parisi S, Piacentini G, Mosca F. Epidemiology and prevention of respiratory syncytial virus infections in children in Italy. Ital J Pediatr. 2021;47(1):198. doi: 10.1186/s13052-021-01148-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pellegrinelli L, Galli C, Bubba L, Cereda D, Anselmi G, Binda S, Gramegna M, Pariani E. Respiratory syncytial virus in influenza-like illness cases: Epidemiology and molecular analyses of four consecutive winter seasons (2014–2015/2017–2018) in Lombardy (Northern Italy) J Med Virol. 2020;92(12):2999–3006. doi: 10.1002/jmv.25917. [DOI] [PubMed] [Google Scholar]
- 20.Calderaro A, De Conto F, Buttrini M, Piccolo G, Montecchini S, Maccari C, Martinelli M, Di Maio A, Ferraglia F, Pinardi F, Montagna P, Arcangeletti MC, Chezzi C. Human respiratory viruses, including SARS-CoV-2, circulating in the winter season 2019–2020 in Parma, Northern Italy. Int J Infect Dis. 2021;102:79–84. doi: 10.1016/j.ijid.2020.09.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pierangeli A, Trotta D, Scagnolari C, Ferreri ML, Nicolai A, Midulla F, Marinelli K, Antonelli G, Bagnarelli P. Rapid spread of the novel respiratory syncytial virus a ON1 genotype, central Italy, 2011 to 2013. Euro Surveill. 2014;19(26):20843. doi: 10.2807/1560-7917.es2014.19.26.20843. [DOI] [PubMed] [Google Scholar]
- 22.Esposito S, Piralla A, Zampiero A, Bianchini S, Di Pietro G, Scala A, Pinzani R, Fossali E, Baldanti F, Principi N. Characteristics and their clinical relevance of respiratory syncytial virus types and genotypes circulating in Northern Italy in five consecutive winter seasons. PLoS ONE. 2015;10(6):e0129369. doi: 10.1371/journal.pone.0129369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nenna R, Matera L, Pierangeli A, Oliveto G, Viscido A, Petrarca L, La Regina DP, Mancino E, Di Mattia G, Villani A, Midulla F. First COVID-19 lockdown resulted in most respiratory viruses disappearing among hospitalised children, with the exception of rhinoviruses. Acta Paediatr. 2022 Mar;10. 10.1111/apa.16326. [DOI] [PMC free article] [PubMed]
- 24.Ippolito G, La Vecchia A, Umbrello G, Di Pietro G, Bono P, Scalia S, Pinzani R, Tagliabue C, Bosis S, Agostoni C, Marchisio PG. Disappearance of seasonal respiratory viruses in children under two years old during COVID-19 pandemic: a monocentric retrospective study in Milan, Italy. Front Pediatr. 2021;9:721005. doi: 10.3389/fped.2021.721005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vittucci AC, Piccioni L, Coltella L, Ciarlitto C, Antilici L, Bozzola E, Midulla F, Palma P, Perno CF, Villani A. The disappearance of respiratory viruses in children during the COVID-19 pandemic. Int J Environ Res Public Health. 2021;18(18):9550. doi: 10.3390/ijerph18189550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Indolfi G, Resti M, Zanobini A, Associazione Ospedali Pediatrici Italiani Research Group on Bronchiolitis. ;. Outbreak of respiratory syncytial virus bronchiolitis in Italy.Clin Infect Dis. 2022 Feb10:ciac120. doi: 10.1093/cid/ciac120. [DOI] [PubMed]
- 27.Bozzola E. Respiratory syncytial virus resurgence in Italy: the need to protect all neonates and young infants. Int J Environ Res Public Health. 2021;19(1):380. doi: 10.3390/ijerph19010380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Italian National Institute of Health. Data on integrated COVID-19 surveillance in Italy. Available online: https://www.epicentro.iss.it/coronavirus/sars-cov-2-dashboard (accessed on 31 January 2023).
- 29.Piazza MF, Amicizia D, Marchini F, Astengo M, Grammatico F, Battaglini A, Sticchi C, Paganino C, Lavieri R, Andreoli GB, Orsi A, Icardi G, Ansaldi F. Who is at higher risk of SARS-CoV-2 reinfection? Results from a Northern Region of Italy. Vaccines (Basel) 2022;10(11):1885. doi: 10.3390/vaccines10111885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Food and Drug Administration (FDA). Alinity m Resp-4-Plex AMP kit. Available online: https://www.fda.gov/media/146491/download (accessed on 3 June 2022).
- 31.Gimferrer L, Andrés C, Rando A, Piñana M, Codina MG, Martin MDC, Fuentes F, Rubio S, Alcubilla P, Pumarola T, Antón A. Evaluation of Seegene Allplex Respiratory Panel 1 kit for the detection of influenza virus and human respiratory syncytial virus. J Clin Virol. 2018;105:31–4. doi: 10.1016/j.jcv.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pretorius MA, van Niekerk S, Tempia S, Moyes J, Cohen C, Madhi SA, Venter M, SARI Surveillance Group Replacement and positive evolution of subtype A and B respiratory syncytial virus G-protein genotypes from 1997–2012 in South Africa. J Infect Dis. 2013;208(Suppl 3):227–37. doi: 10.1093/infdis/jit477. [DOI] [PubMed] [Google Scholar]
- 33.Hall T. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–8. [Google Scholar]
- 34.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. 2013;30(12):2725–9. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.R Core Team. R: A language and environment for statistical computing. Available online: http://www.R-project.org/ (accessed on 6 June 2022).
- 36.Galli C, Pellegrinelli L, Bubba L, Primache V, Anselmi G, Delbue S, Signorini L, Binda S, Cereda D, Gramegna M, Pariani E, The Ili Sentinel Physicians Group When the COVID-19 pandemic surges during influenza season: Lessons learnt from the sentinel laboratory-based surveillance of influenza-like illness in Lombardy during the 2019–2020 season. Viruses. 2021;13(4):695. doi: 10.3390/v13040695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leli C, Di Matteo L, Gotta F, Vay D, Piceghello A, Cornaglia E, Cavallo V, Busso S, Carrabba L, Mazzeo R, Rocchetti A. Prevalence of respiratory viruses by multiplex PCR: a four-and-a-half year retrospective study in an italian general hospital. Infez Med. 2021;29(1):94–101. [PubMed] [Google Scholar]
- 38.Pellegrinelli L, Galli C, Bubba L, Seiti A, Anselmi G, Primache V, Signorini L, Delbue S, Binda S, Pariani E. Respiratory syncytial virus in pediatric influenza-like illness cases in Lombardy, Northern Italy, during seven consecutive winter seasons (from 2014–2015 to 2020–2021) Influenza Other Respir Viruses. 2022;16(3):481–91. doi: 10.1111/irv.12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ciarlitto C, Vittucci AC, Antilici L, Concato C, Di Camillo C, Zangari P, Villani A. Respiratory Syncityal Virus A and B: three bronchiolitis seasons in a third level hospital in Italy. Ital J Pediatr. 2019;45(1):115. doi: 10.1186/s13052-019-0704-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tabatabai J, Ihling CM, Rehbein RM, Schnee SV, Hoos J, Pfeil J, Grulich-Henn J, Schnitzler P. Molecular epidemiology of respiratory syncytial virus in hospitalised children in Heidelberg, Southern Germany, 2014–2017. Infect Genet Evol. 2022;98:105209. doi: 10.1016/j.meegid.2022.105209. [DOI] [PubMed] [Google Scholar]
- 41.Gaymard A, Bouscambert-Duchamp M, Pichon M, Frobert E, Vallee J, Lina B, Casalegno JS, Morfin F. Genetic characterization of respiratory syncytial virus highlights a new BA genotype and emergence of the ON1 genotype in Lyon, France, between 2010 and 2014. J Clin Virol. 2018;102:12–8. doi: 10.1016/j.jcv.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 42.Vos LM, Oosterheert JJ, Kuil SD, Viveen M, Bont LJ, Hoepelman AIM, Coenjaerts FEJ. High epidemic burden of RSV disease coinciding with genetic alterations causing amino acid substitutions in the RSV G-protein during the 2016/2017 season in The Netherlands.J Clin Virol. 2019Mar;112:20–26. doi: 10.1016/j.jcv.2019.01.007. [DOI] [PubMed]
- 43.Giufrè M, Dorrucci M, Lo Presti A, Farchi F, Cardines R, Camilli R, Pimentel de Araujo F, Mancini F, Ciervo A, Corongiu M, Pantosti A, Cerquetti M, Valdarchi C, FIMMG Group Nasopharyngeal carriage of Haemophilus influenzae among adults with co-morbidities. Vaccine. 2022;40(5):826–32. doi: 10.1016/j.vaccine.2021.12.030. [DOI] [PubMed] [Google Scholar]
- 44.Ansaldi F, de Florentiis D, Canepa P, Ceravolo A, Rappazzo E, Iudici R, Martini M, Botti G, Orsi A, Icardi G, Durando P. Carriage of Streptoccoccus pneumoniae in healthy adults aged 60 years or over in a population with very high and long-lasting pneumococcal conjugate vaccine coverage in children: rationale and perspectives for PCV13 implementation. Hum Vaccin Immunother. 2013;9(3):614–20. doi: 10.4161/hv.23253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beveridge S, Piya B, Stewart L, Lindegren ML, Markus T, Schaffner W, Halasa NB. Comparison of viral loads in patients with co-infections vs. single-virus infections. Open Forum Infect Dis. 2017;4(Suppl 1):310. doi: 10.1093/ofid/ofx163.723. [DOI] [Google Scholar]
- 46.Piret J, Boivin G. Viral interference between respiratory viruses. Emerg Infect Dis. 2022;28(2):273–81. doi: 10.3201/eid2802.211727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Munywoki PK, Koech DC, Agoti CN, Kibirige N, Kipkoech J, Cane PA, Medley GF, Nokes DJ. Influence of age, severity of infection, and co-infection on the duration of respiratory syncytial virus (RSV) shedding. Epidemiol Infect. 2015;143(4):804–12. doi: 10.1017/S0950268814001393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bai L, Zhao Y, Dong J, Liang S, Guo M, Liu X, Wang X, Huang Z, Sun X, Zhang Z, Dong L, Liu Q, Zheng Y, Niu D, Xiang M, Song K, Ye J, Zheng W, Tang Z, Tang M, Zhou Y, Shen C, Dai M, Zhou L, Chen Y, Yan H, Lan K, Xu K. Coinfection with influenza a virus enhances SARS-CoV-2 infectivity. Cell Res. 2021;31(4):395–403. doi: 10.1038/s41422-021-00473-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Langedijk AC, Harding ER, Konya B, Vrancken B, Lebbink RJ, Evers A, Willemsen J, Lemey P, Bont LJ. A systematic review on global RSV genetic data: identification of knowledge gaps. Rev Med Virol. 2022;32(3):e2284. doi: 10.1002/rmv.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available due to the data nature but are available from the corresponding author on reasonable request.