Abstract
Bid is an abundant proapoptotic protein of the Bcl-2 family that is crucial for the induction of death receptor-mediated apoptosis in primary tissues such as liver. Bid action has been proposed to involve the relocation of its truncated form, tBid, to mitochondria to facilitate the release of apoptogenic cytochrome c. The mechanism of Bid relocation to mitochondria was unclear. We report here novel biochemical evidence indicating that Bid has lipid transfer activity between mitochondria and other intracellular membranes, thereby explaining its dynamic relocation to mitochondria. First, physiological concentrations of phospholipids such as phosphatidic acid and phosphatidylgycerol induced an accumulation of full-length Bid in mitochondria when incubated with light membranes enriched in endoplasmic reticulum. Secondly, native and recombinant Bid, as well as tBid, displayed lipid transfer activity under the same conditions and at the same nanomolar concentrations leading to mitochondrial relocation and release of cytochrome c. Thus, Bid is likely to be involved in the transport and recycling of mitochondrial phospholipids. We discuss how this new role of Bid may relate to its proapoptotic action.
Apoptosis is a fundamental process in tissue development and homeostasis. It is increasingly appreciated that proteins of the Bcl-2 family are crucially involved in the control of apoptotic pathways (6, 34). Members of this family such as Bax and Bid act as promoters of apoptosis and translocate to mitochondria to facilitate the release of cytochrome c into the cytosol, where it binds to a complex of proteins, the apoptosome (4, 6, 9, 14, 16, 22, 24, 34, 38, 39). The apoptosome then promotes the self-cleavage of procaspase enzymes, which leads to the activation of the caspase cascade of cell execution (14). Emerging evidence indicates that Bid is involved in various pathways of apoptosis that interplay the activation of caspases with mitochondrial dysfunction (14, 16, 23, 24, 28, 36–39). Death receptors such as Fas/CD95 activate apical caspases that cleave full-length Bid (16, 24, 36–39). The C-terminal part of cleaved Bid, tBid, is believed to subsequently migrate to mitochondria (16, 22–26, 28, 36–39) where it promotes the release of cytochrome c and other proteins resident in the intermembrane space (9, 16, 22, 24).
Two hypotheses are currently considered to explain why Bid specifically migrates to mitochondria during apoptosis. In the first hypothesis, Bid acts as a ligand for other proteins of the Bcl-2 family (35), including Bak (36) and Bax (9), which are tethered to the mitochondrial outer membrane (OM). The second hypothesis postulates that tBid has a propensity for binding to cardiolipin (CL) (25), a membrane lipid unique to mitochondria (18). More recently, it has also been suggested that posttranslational myristoylation of tBid enhances its targeting to mitochondria (38).
The aim of this work was to clarify the mechanism of Bid relocation to mitochondria, especially in primary tissues such as liver and kidney, where Bid is expressed at relatively high levels (10, 35) and is involved in physiological pathways of apoptosis (11, 16, 28, 30, 37, 39). We found that certain phospholipids promote a redistribution of Bid from light membranes to mitochondria and report, for the first time, that Bid displays lipid transfer activity. These novel data suggest that Bid relocation to mitochondria depends upon its underlying involvement in the transport and recycling of phospholipids between intracellular organelles.
MATERIALS AND METHODS
Antibodies and other reagents.
Primary antibodies were obtained from various commercial sources: anti-Bak monoclonal and polyclonal antibodies were from Oncogene-CN Biosciences, BD-PharMingen, and Upstate Biotechnology; anti-Bcl-xL polyclonal antibodies were from Transduction Laboratories; Bax RP was from BD-PharMingen; Bax N20 was from Santa Cruz; anti-Bad was from R&D Systems; and anti-cytochrome c was from BD-PharMingen. For characterization of subcellular fractions we used antibodies against endoplasmic reticulum (ER) P450 reductase, OM porin/VDAC (both from Santa Cruz), aldolase (provided by P. Savory), subunit IV of mitochondrial cytochrome oxidase (from Molecular Probes), and mitochondrial cytochrome c1, which was kindly provided by D. Gonzalez-Halphen (University of Mexico, Mexico City, Mexico). To detect multiple forms of native Bid, we systematically used different antibodies, including the following commercial products: R&D Systems no. AF860 (raised against the whole recombinant mouse protein), C-20 from Santa Cruz (raised against the C terminus of Bid), N-19 and D-19 from Santa Cruz (raised against the N terminus of human and mouse Bid, respectively), and BD-PharMingen no. 68836E (raised against a peptide comprising residues 129 to 146 of mouse Bid). To verify the specificity of Bid immunoblotting, we carried out competition experiments with 10 to 20 μg of the antigenic peptides per ml and comparison with the reactivity of mouse recombinant Bid.
Among other reagents, phosphate-buffered saline (PBS) was obtained from Oxoid, cell culture media was from Gibco, fluorescent probes were from Molecular Probes, caspase substrates and inhibitors were from BD-PharMingen and Alexis, and electrophoresis reagents were from Bio-Rad. Other chemicals were purchased from Sigma and were of high analytical grade.
Cell culture.
Human cell lines (T-cell lymphoma lines CEM C7A and Jurkat and colon cancer cell lines KM12 and HT29) were grown in RPMI 1640 medium supplemented with fetal bovine serum (10% [vol/vol]) and l-glutamine (2 mM) (15). Murine IC.DP cells were cultured and deprived of interleukin 3 to induce apoptosis, as described previously (21).
Fractionation and Fas activation of primary tissues.
Fas treatment was performed ex vivo with whole organs. Livers were removed from either male or female mice and immediately incubated at room temperature (usually for 1 h) with 5 to 20 μg of the Fas antibody agonist Jo-2 (BD-PharMingen) per ml, similarly to previous studies (16, 37, 39). In most experiments, 10 μM lactacystin was added during Jo-2 treatment to block proteasome cleavage of Bid. Subcellular fractionation of mammalian tissues (from mouse, pig, bovine, and cultured cells) was carried out as follows. After being soaked with ice-cold PBS, organs were cleaned of connective tissue, cut with scissors and suspended in isolation medium (10 mM K-HEPES, 0.25 M mannitol, 1 mM EGTA, 0.2% bovine serum albumin [BSA] [pH 7.4]), generally containing a cocktail of protease inhibitors (0.1% [vol/vol] of P3840 [Sigma]). The cleaned tissues were homogenized with either a Polytron (for kidney or heart) or a Teflon pestle homogenizer (for liver) and then centrifuged at 600 × g for 5 min. The supernatants were filtered to remove fats and centrifuged at 10,000 × g for 10 to 15 min at 4°C. The supernatants containing cytosol (S10) were used in cell-free assays or taken aside for further fractionation (normally at approximately 20,000 × g for 45 min to obtain light membranes enriched in ER; fraction P20), while the pellets containing crude mitochondria were resuspended in assay buffer (20 mM K-HEPES, 0.12 M mannitol, 0.08 M KCl, 1 mM EDTA [pH 7.4]), gently homogenized, and then recentrifuged at 10,000 × g for 15 min. This wash was repeated, and the final pellets of mitochondria were homogenized in a minimal volume of assay medium containing protease inhibitors. The protein content of the various fractions was determined by using the Bio-Rad Bradford miniassay in the presence of the nonionic detergent Triton X-100 to solubilize membrane proteins; BSA was used as a standard.
Isolated proteins.
Recombinant Bak and Bcl-xL, both with C-terminus truncation, were expressed in and purified from Escherichia coli and kindly provided by B. Corfe of this laboratory. Recombinant mouse and human Bid was obtained in purified and active form from R&D Systems. In addition, a sample of human recombinant Bid was kindly provided by D. Green (La Jolla Institute for Allergy and Immunology, San Diego, Calif.). Recombinant human caspases were purchased from BD-PharMingen. Caspase 8-cleaved Bid was obtained after incubation of the full-length recombinant protein with recombinant caspase 8 (22), which was subsequently removed by ion-exchange chromatography. Native Bid was isolated from cytosolic extracts of mouse kidney (or pig kidney cortex) by a procedure modified from that described earlier by Luo et al. (24). Briefly, frozen cytosolic extracts were thawed and clarified by extensive centrifugation at 12,000 × g and then diluted with assay medium containing 2 mM dithiothreitol and protease inhibitors.
Subsequently, the extracts were heated at 70°C for 15 min, followed by centrifugation at 4°C for 40 min at 12,000 × g. The supernatant contained most of native Bid—which is strongly thermostable (24, 26)—and was filtered through 100-kDa filters (Sartorius) by extensive centrifugation. The filtrates were further fractionated and then concentrated by ultrafiltration with 30- and 5-kDa filters (Sartorius or Amicon). The crude Bid preparations thus obtained were generally used without further manipulation, since they did not show significant contamination by other proteins of the Bcl-2 family.
Immunoblotting.
Cell samples were washed with PBS and suspended in lysis buffer (10 mM K-HEPES, 0.15 M NaCl, 2 mM EDTA, 0.1% NP-40 [pH 7.5], supplemented with protease inhibitors) (15). Tissue fractions were diluted with assay buffer containing protease inhibitors and adjusted to a final protein concentration of 1 mg/ml with concentrated sodium dodecyl sulfate (SDS)-sample buffer. Protein samples were separated by SDS-polyacrylamide gel electrophoresis, routinely with 15% acrylamide gels, and transferred to polyvinylidene difluoride membranes (NEN) (15, 21). Membranes were blocked with 5% defatted dried milk in PBS containing 0.05% Tween-20 (PBST) and probed with primary antibodies in PBST either at room temperature for 1 h or at 4°C overnight. After PBST washes, the membranes were treated with secondary antibodies (Dako) in PBST containing 5% dried milk for 45 to 60 min. Blots were visualized by chemiluminescence (NEN Life Sciences) and analyzed using a GS 700 scanning densitometer with Molecular Analyst software (Bio-Rad). To evaluate the possible interference of lipids on the immunodetection of Bid, blotted polyvinylidene difluoride membranes were dried and delipidized in 10 ml of 2:1 (vol/vol) of chloroform:methanol for 5 min. They were subsequently blocked with a mixture of defatted dry milk and fatty acid-free BSA and probed with antibodies as described above.
Assays of cytochrome c release and OM permeability.
Equal amounts of proteins from both cytosolic extracts and mitochondria were suspended at a final concentration of 1 mg/ml in assay buffer. In some experiments, the reaction mixtures were supplemented with nanomolar concentrations of recombinant Bid or light fractions enriched in ER (P20). The complete mixtures were incubated at 30°C for 30 min, and mitochondria were separated by centrifugation for 10 min at 12,000 rpm in a Eppendorf minicentrifuge refrigerated at 4°C. The supernatants were removed, while the pellets were carefully washed with assay buffer before resuspension with sample buffer.
To evaluate OM permeability, we measured the latency in the rate of cytochrome c oxidase activity (22). Mitochondria were equilibrated at room temperature in assay buffer at a final protein concentration of 0.02 mg/ml, and the reaction of cytochrome oxidase was started by the injection of 15 to 20 μM of reduced cytochrome c (obtained by dithionite reduction) and followed spectrophotometrically at 550 nm (7). The content of mitochondrial cytochromes was determined from the reduced minus oxidized optical spectra, as previously described (7).
Lipid transfer assays.
Assays of lipid transfer between donor and acceptor liposomes were carried out using protocols similar to those routinely used with plant lipid transfer proteins (LTP) (12, 27). The highly fluorescent lipid probes, 2-(4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene-3-pentanoyl)-1-hexadecanoyl-sn-glycero-3-phosphocholine (BODIPY FL C5-HPC) (2) and 2-(4,4- difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene-3-pentanoyl)-1-hexadecanoyl-sn-glycero-3-phosphate (BODIPY FL C5-HPA) were used at final concentrations of 20 to 100 nM. All lipid suspensions were in assay buffer. Donor liposomes were prepared by either ethanol injection (27) or sonication (2, 25) of mixtures of lipid probes and other phospholipids. Acceptor liposomes were prepared by rapid ethanol injection (27) or extrusion through 0.2-μm-pore-size filters to produce large unilamellar vesicles (LUV) (38). Calculations of the rate constants for the spontaneous transfer of lipids among liposomes were carried out as described earlier (2, 29).
In a typical experiment, donor vesicles containing a final concentration of 20 to 40 nM BODIPY FL C5-HPA were equilibrated at room temperature (22 to 23°C) in assay buffer, and the baseline level of the self-quenched fluorescence of the probe was recorded for a few minutes. Subsequently, an excess of acceptor vesicles was added to a final concentration of 1 to 4 μg/ml, and the spontaneous rate of diffusion of the lipid probe into the acceptor membrane was monitored by the increased fluorescence emission. This spontaneous lipid transfer from donor to acceptor membranes usually reached near equilibrium within 15 min (cf. reference 2) and could be accelerated by the presence of Bid preparations. Total fluorescence of the probe was determined after solubilization of the donor-acceptor mixture with 0.2% Triton X-100. Fluorescence was measured with a Perkin-Elmer LS50B luminescence spectrometer with excitation at 485 or 490 nm (5-nm bandwidth). Emission intensity was recorded with a 10-nm bandwidth in either spectral or time drive mode. In some experiments, Bid and other proteins were added after the spontaneous transfer among donor and acceptor liposomes had reached equilibrium. In other experiments, mitochondria from mouse liver or pig heart were used as membrane acceptors and separated by centrifugation to validate the transfer of lipid from the donor liposomes.
RESULTS
Bid distribution in mitochondria is affected by phospholipids.
Studies with recombinant preparations of Bid have indicated that its proapoptotic association with mitochondria may involve interactions with membrane lipids (22, 23, 25). In particular, it has been reported that recombinant human tBid may specifically interact with CL (25), a negatively charged lipid characteristic of mitochondria (18). Because it was not known whether these lipid interactions were essential to the action of native Bid, we investigated in depth the relationships between phospholipids and native Bid in primary tissues such as liver.
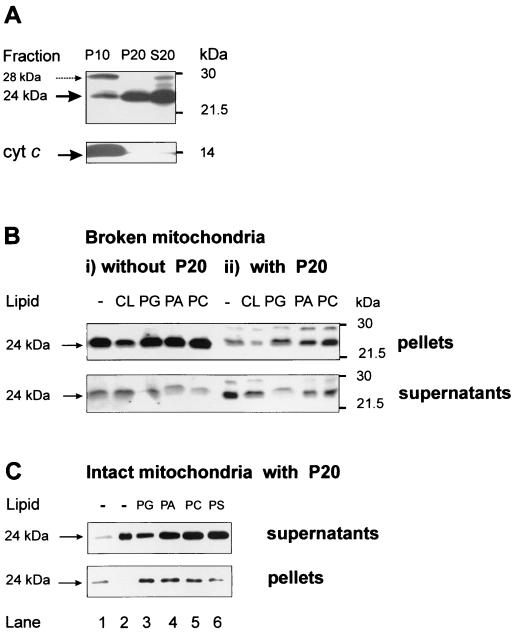
To study native Bid we used a panel of several Bid-specific antibodies, which commonly detected the full-length protein as a reactive band migrating with an apparent size of 24 kDa. Following subfractionation of liver and other primary tissues such as kidney, the 24-kDa band was found to be dominant in Bid immunoblots of cytosolic extracts and also of light membranes enriched in ER, especially those sedimenting at 20,000 × g (the P20 fraction) (Fig. 1A). Conversely, Bid immunoblots of isolated mitochondria consistently showed an additional band with an apparent size of around 28 kDa that appeared to be loosely associated with the outer mitochondrial membrane (Fig. 1A and results not shown). Given that the molecular identification of this 28-kDa band proved to be elusive and that recombinant and isolated native Bid showed a single band corresponding to the 24-kDa band seen in primary tissues, we focused here on the 24-kDa band of native Bid and its interaction with lipids.
FIG. 1.
Phospholipids change the mitochondrial distribution of Bid. (A) Different forms of Bid-reactive bands were detected in three subcellular fractions obtained with healthy mouse liver. P10 was the pellet of crude mitochondria obtained by the first centrifugation at 10,000 × g, while P20 was the pellet containing mainly ER-associated light membranes that was obtained by centrifugation of the S10 supernatant at 20,000 × g. S20 was the cytosolic extract remaining in the supernatants after the latter centrifugation and showed a pattern of Bid immunoblots similar to that of fraction S10. Exactly equal protein concentrations (24 μg) of each fraction were immunoblotted with a mixture of the C-20 and the R&D antibodies to detect all forms of Bid. Blots for cytochrome c (bottom) and subunit IV of cytochrome oxidase (not shown) were used as mitochondrial markers. The presence of a 28-kDa band reacting with Bid antibodies is noted by the dashed arrow. (B) Exogenous phospholipids do not affect Bid distribution in purified mitochondria alone (panels i) but change Bid distribution when the same mitochondria are coincubated with the ER-rich fraction P20 (panels ii). Mitochondria and light membranes were initially coprecipitated by centrifugation of a postnuclear supernatant of mouse liver at 22,000 × g. After the frozen pellets were thawed, mitochondria were separated from fraction P20 by centrifugation (see Materials and Methods) and further purified by multiple washes at 9,000 × g. Purified mitochondria were resuspended in the caspase assay buffer (20 mM K-HEPES, 0.25 M sucrose, 2 mM dithiothreitol, 1 mM EDTA [pH 7.4], supplemented with 0.1% [vol/vol] of protease inhibitors) at 2 mg/ml and then incubated at 30°C for 30 min with 0.2 mg of each phospholipid (all synthetic dioleyl analogues dissolved in chloroform except for cardiolipin) per ml in either the absence (panels i) or presence (panels ii) of 1 mg of fraction P20 per ml. Control (−) samples contained equivalent amounts of solvent (less than 1% of final volume). At the end of the incubation, the samples were centrifuged at 10,000 × g under cold conditions, and equal volumes of pellets and supernatants were analyzed by Western blotting with the R&D antibody. The two panels are taken from duplicate blots with slightly different exposures. (C) Intact mouse liver mitochondria were diluted to 1 mg/ml in assay buffer (20 mM K-HEPES, 0.12 M mannitol, 0.08 M KCl, 1 mM EDTA [pH 7.4], supplemented with protease inhibitors) and incubated at 30°C for 30 min with preformed liposomes (0.1 mg/ml, obtained after drying the chloroform solutions of different phospholipids). A total of 0.9 mg of fraction P20 per ml obtained separately was also added to the incubation mixture, and the mitochondria were subsequently separated by centrifugation as described in the legend to panel B. Equivalent amounts of the supernatants (top) and pellets (bottom) were probed with C-20 together with the R&D antibody as described in the legend to panel A. For reference, lanes 1 and 2 in contained mitochondria without lipids and P20 alone, respectively. Note that exogenous lipids, and especially PG, increased the proportion of the 24-kDa band of Bid that associated with the mitochondrial pellets.
To verify potential interactions with lipids, we investigated first whether exogenous phospholipids could modify Bid immunodetection, since binding to negatively charged lipids is known to produce aberrant electrophoretic migrations of lipid-interacting proteins in the presence of SDS (20, 33). Lipid extraction and reconstitution of mitochondria indicated that CL could partly affect the immunodetection of full-length Bid (not shown). In addition, incubation of mitochondria with exogenous CL induced an apparent decrease in the intensity of the 24-kDa band associated with mitochondria (Fig. 1Bi; left). This was due at least in part to tight binding of Bid to CL (cf. references 13 and 25) that masked the epitopes for antibody binding, since lipid extraction of the blotting membrane removed most of the immunodetection interferences induced by CL (results not shown).
Phospholipids other than CL had little effect on the detection and distribution of Bid forms in purified mitochondria, as shown in Fig. 1Bi. However, exogenous phospholipids dramatically changed the distribution of native Bid after incubation with mixtures of mitochondria and ER-rich fractions such as P20 (Fig. 1Bii and C). This lipid-dependent redistribution of Bid forms was most evident with freeze-thawed mitochondria coincubated with the light membranes of fraction P20 and subsequently separated from these light membranes by centrifugation at 10,000 × g. In the absence of lipids, most of the 24-kDa band that was normally associated with the light membranes (Fig. 1A) remained in the supernatants (Fig. 1Bii, lane 1, and C, lane 2, top), but in the presence of certain lipids this band migrated from the supernatants to the mitochondrial pellets (Fig. 1B and C). The redistribution of Bid was induced by phosphatidylglycerol (PG), phosphatidic acid (PA), and also phosphatidylcoline (PC) but not by other lipids, including the negatively charged phosphatidylserine (PS) (Fig. 1C).
Interestingly, physiological concentrations (i.e., those equal to the CL content of mitochondria) of PG and, to a lesser extent, PA and PC shifted the distribution of the 24-kDa band of Bid from the supernatants containing light membranes to the mitochondrial pellets when added either in chloroform solutions (Fig. 1Bii) or as preformed liposomes (Fig. 1C). Thus, certain phospholipids such as PG can specifically promote a redistribution of native Bid from light membranes to mitochondria in the absence of apoptotic stimuli.
Bid displays lipid transfer activity.
Given that mitochondria and ER compartments are the major sites of phospholipid metabolism (5, 18), we reasoned that the lipid-induced distribution changes of Bid between mitochondria and ER-rich membranes (Fig. 1) may reflect an underlying connection with the cellular traffic of phospholipids. The transport and recycling of mitochondrial phospholipids is poorly understood (18), but a few proteins, notably plant LTP, are known to transport phospholipids and other lipid metabolites to mitochondria (12, 27). We investigated whether isolated Bid displayed lipid transfer activity when assayed with the methods used for plant and other established LTP (12, 27, 33).
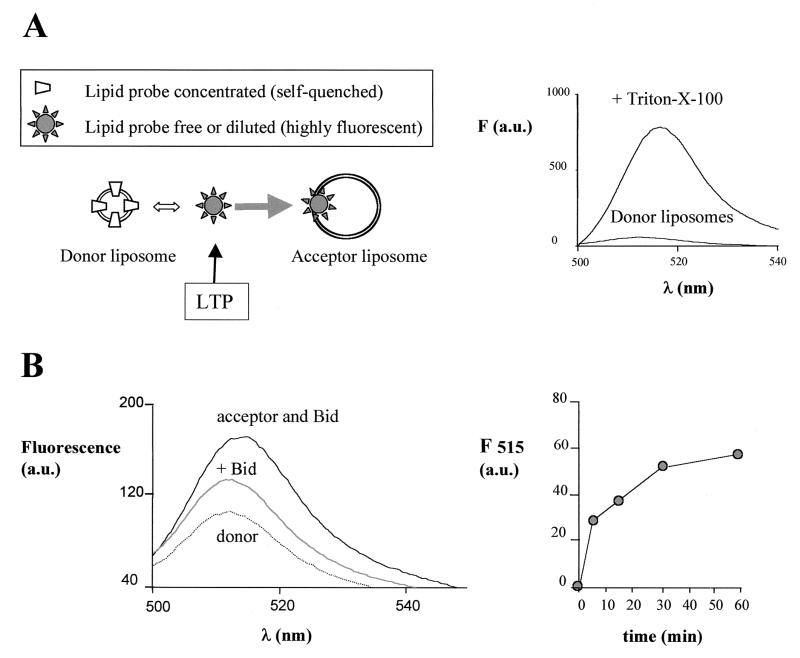
A scheme illustrating the principles of lipid transfer and its measurement with fluorescent probes is presented in Fig. 2A, left panel. Lipid transfer is a complex process involving extraction of the lipid probe from the donor lipid membrane, binding to the protein, transport of the protein-bound probe through the aqueous phase, and its subsequent release from the protein into the acceptor lipid membrane. Transfer of lipids also occurs spontaneously among donor and acceptor liposomes due to the equilibrium dynamics of the lipids between the membrane and aqueous phase (27, 29). Lipid transfer proteins are believed to accelerate the entire process by facilitating the removal of the lipid probe from the donor liposomes and enhancing its water solubility by loose binding (27, 33). To allow direct measurement of the time course of lipid transfer, the activity of Bid was followed by monitoring the dequenching of fluorescent lipid probes leaving donor liposomes, where their high concentration induces strong fluorescence quenching, and their subsequent distribution in acceptor liposomes, where their dilution allows full fluorescence emission (2, 12, 27, 29). Maximal increase in probe fluorescence was obtained after treatment of donor liposomes with Triton-X-100, as shown in Fig. 2A, right panel.
FIG. 2.
Bid displays lipid transfer activity with liposomes. (A) The scheme in the left panel illustrates the principles of the lipid transfer assay between donor and acceptor liposomes using fluorescent lipid probes (cf. references 2 and 27). The arrow indicates the action of an LTP, which fundamentally shifts the equilibrium dynamics of the whole process to the right. The right panel illustrates these principles experimentally. Donor liposomes containing 100 nM BODIPY FL C5-HPA show a quenched fluorescence due to the high molar fraction (see below) of the probe with respect to the other lipids in the membrane, but high fluorescence after solubilization and dilution of the probe with the detergent Triton X-100. (B) Lipid transfer activity was measured with a final concentration of 100 nM BODIPY FL C5-HPA under conditions equivalent to those in OM permeability and cell-free assays (see Materials and Methods). The donor liposomes were prepared by rapid injection of an ethanolic solution containing 1:3 (wt/wt) of the lipid probe with a mixture of purified phospholipids (44% PC, 31% PI, and 25% PS) in assay buffer. The acceptor liposomes were prepared by ethanol injection of a lipid mixture containing (wt/wt) 20% CL, 35% PC, 25% PI, and 18% PS and added at a final concentration of 3.5 μg/ml (i.e., 15-fold in excess of the concentration of donor liposomes). The results were obtained by recording time-resolved emission spectra with excitation at 485 nm. The dotted spectrum represents the fluorescence emission of donor liposomes alone, which was stable with time. Upon addition of native Bid isolated from mouse kidney (equivalent to a final concentration of approximately 10 nM Bid), a rapid increase of fluorescence occurred that stabilized to the middle spectrum shown. The subsequent addition of acceptor liposomes induced a large increase in fluorescence with time: the top spectrum was recorded after 15 min of incubation. The entire time course of the peak emission at 515 nm is shown in the right panel.
We evaluated in detail the capacity of Bid to transfer fluorescent lipids between donor and acceptor liposomes prepared by different methods. Preparations of both isolated native (Fig. 2B) and recombinant (Fig. 3 and 4) Bid accelerated the transfer of BODIPY FL C5-HPA, a fluorescent analog of PA, to acceptor liposomes with lipid composition similar to that of mitochondrial membranes. Although native and recombinant mouse Bid showed a comparable concentration dependence of their LTP-like activity, results were more reproducible with fresh preparations of the recombinant proteins. For instance, recombinant mouse Bid was able to accelerate substantially the spontaneous transfer of the PA-derived lipid probe between sonicated donor liposomes and LUV added in excess of the donor liposomes (Fig. 3A). Of note, Bid also demonstrated lipid transfer activity with other probes like BODIPY FL C5-HPC, albeit with different qualitative and quantitative properties (data not shown).
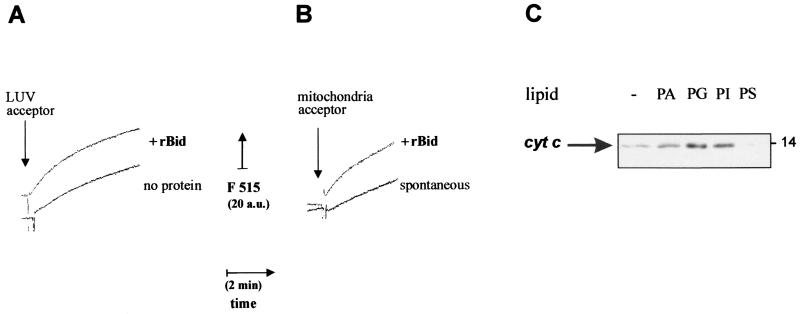
FIG. 3.
Correlation between lipid transfer and release of cytochrome c by recombinant Bid. (A) Lipid transfer was measured as time courses with sonicated donor liposomes prepared with a 1:3 (wt/wt) mixture of BODIPY FL C5-HPA with phospholipids (50% PC, 25% PI, and 25% PS) and acceptor liposomes consisting of LUV. These LUV were prepared by filter extrusion (38) with a phospholipid mixture closely resembling that of the OM (8, 25), namely, 50% PC, 30% PE, 12.5% PI, 5% PG, and 2.5% CL. Before the assay, both donor and LUV liposomes were purified by gel filtration with a Sigma PD10 column. Donor liposomes were equilibrated in assay buffer at a final concentration of 30 nM, and then 2 μg of LUV acceptors per ml were added in either the absence (bottom trace) or the presence (top, trace) of 250 ng of recombinant mouse Bid (rBid) per ml (i.e., 10 nM). Fluorescence emission at 515 nm was continuously monitored with excitation at 490 nm. (B) Lipid transfer from sonicated donor liposomes was measured as shown in panel A using 2.5 μg of mouse liver mitochondria per ml as acceptor membranes. The spontaneous rate of transfer (bottom trace) was accelerated when 30 nM recombinant mouse Bid (rBid) was coincubated with donor liposomes prior to the addition of mitochondria (top trace) (C) Mouse liver mitochondria were incubated for 15 min with 10 nM recombinant Bid in assay buffer. Except for the control sample (−), mitochondria were also incubated with 0.1 mg/ml liposomes formed by different negatively charged phospholipids and then separated by centrifugation as in the experiment shown in Fig. 1C. Equal volumes of the supernatants were immunoblotted for cytochrome c as in routine cell-free assays (22).
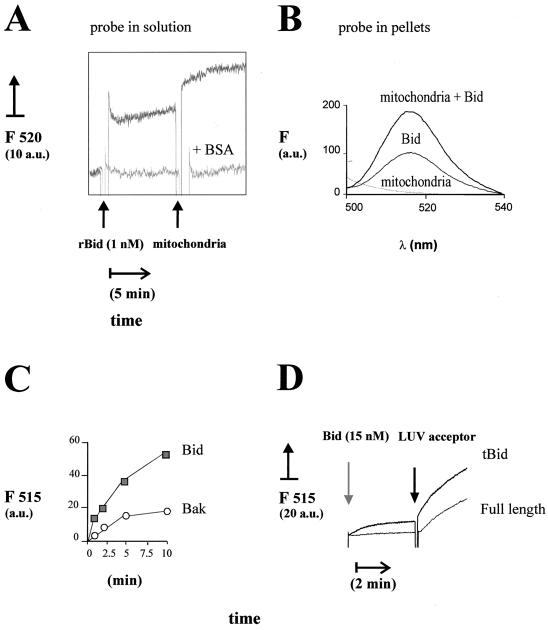
FIG. 4.
Specificity of the LTP-like activity displayed by recombinant Bid. (A) Donor and acceptor liposomes were mixed first for over 10 min under the conditions of the experiment shown in Fig. 2B to reach equilibrium in the spontaneous lipid transfer. A total of 1 nM (25 ng/ml) recombinant human Bid was then added as indicated, and the fluorescence changes were recorded for about 10 min. The top trace was obtained in the absence of other additions, while the bottom trace was obtained in the presence of 25 μg of BSA per ml which strongly inhibits lipid transfer (5, 33). Subsequently, 50 μg of freeze-thawed pig heart mitochondria per ml (equivalent to approximately 20 μg of membrane lipids per ml) was added to further stimulate lipid transfer. The final concentration of the lipid probe was 50 nM. (B) The increase in fluorescence of the PA analog probe was measured at 1 h after the addition of pig heart mitochondria in experiments similar to those shown in panel A. Subsequently, the reaction mixtures were centrifuged at 10,000 × g for 15 min to separate the mitochondria, and the pellets were resuspended in assay buffer containing 0.5% (vol/vol) Triton X-100 and then diluted in the cuvette. The fluorescence recovered in the pellets was measured as described in the legend to Fig. 2. The bottom spectrum was obtained with mitochondria alone (without the lipid probe), the intermediate spectrum was obtained with acceptor and donor liposomes plus Bid, and the top spectrum was obtained with donor and acceptor liposomes plus Bid and mitochondria. (C) Isolated native Bid (10 nM) (filled symbols) or recombinant Bak (10 nM) (open symbols) was incubated with donor liposomes, and lipid transfer was monitored by time-resolved spectra after addition of 3.5 μg of acceptor liposomes per ml as in Fig. 2B. The time course of the intensity in the emission maximum (at 515 nm; F515) is shown. The spontaneous transfer to the acceptor liposomes was essentially equivalent to that measured in the presence of Bak and is not shown, for the sake of clarity. Similar results were obtained using pig heart mitochondria as acceptors (not shown). (D) Caspase 8-cleaved Bid (tBid) and its parent full-length preparation of recombinant mouse Bid were added to donor liposomes (obtained by sonication as shown in Fig. 3) diluted to a final concentration of 30 nM BODIPY FL C5-HPA. After approximately 3 min, 2.5 μg of LUV acceptor liposomes per ml was added, and the initial rate of lipid transfer was recorded as shown in Fig. 3A. Similar differences in the rate of lipid transfer of tBid and full-length Bid were seen in a wide range of protein concentrations up to 200 nM (not shown). a.u., arbitrary units.
Specificity of the lipid transfer activity displayed by Bid.
We also measured the lipid transfer capacity of Bid using isolated mitochondria as acceptor membranes. When liver mitochondria were used, there was a rapid spontaneous transfer of the lipid probe from the donor liposomes (Fig. 3B). In this case, it was impossible to assign LTP activity to endogenous Bid alone, since liver is rich in LTP such as the nonspecific fatty acid transfer protein that may be associated with isolated mitochondria and contribute to the transport of the lipid probe that we observed. Nevertheless, addition of exogenous recombinant mouse Bid accelerated the transfer of the lipid probe to the membranes of mouse liver mitochondria under the same conditions as those of the cell-free assay (Fig. 3B).
To investigate the correlation between lipid transfer and the capacity of Bid to release cytochrome c, cell-free assays were conducted with liver mitochondria and liposomes formed by different negatively charged lipids. Phospholipids such as PG and PA that altered the mitochondrial distribution of endogenous Bid (Fig. 1) also enhanced the release of cytochrome c induced by recombinant Bid (Fig. 3C). On the contrary, lipids such as PS which did not change the distribution of endogenous Bid (Fig. 1C) failed to enhance the release of cytochrome c induced by recombinant Bid (Fig. 3C). To limit the interfering spontaneous transfer of lipid probes that occurred with liver mitochondria, we used other mitochondria such as those of pig heart, which contained very low levels of nonspecific LTP and endogenous Bid. The lipid transfer activity of Bid to pig heart mitochondria was clearly evident with concentrations as low as 1 nM (Fig. 4A) and was validated by the fluorescence recovery of the lipid probe in the mitochondria separated from the assay mixture by centrifugation (Fig. 4B).
Often the addition of isolated Bid induced a rapid increase in fluorescence (Fig. 2B, left, and Fig. 4), which most likely reflected partial release of the lipid probe from the donor liposomes and/or the protein and redistribution in solution (2, 27). This effect, together with the LTP-like activity, was abolished by inactivating isolated Bid with repeated freeze-thaw cycles or boiling, and was strongly inhibited by BSA (Fig. 4A), due to its avid binding to lipids in solution (5). To verify further the specificity in lipid transfer, we compared Bid activity to that of recombinant Bak and Bcl-xL proteins lacking their membrane-anchoring C terminus, to mimic the size and water solubility of Bid. Native or recombinant Bid was much more effective than Bak in lipid transfer activity to liposomes or mitochondria (Fig. 4C). Note that the data showing activity in the presence of Bak (Fig. 4C) were not significantly different from the background data of spontaneous lipid transfer (cf. Fig. 3A and B). Conversely, C-terminus-truncated Bcl-xL was completely inactive in transporting the lipid probe to mitochondria (not shown).
In previous studies it has been shown that caspase cleavage of recombinant Bid produces structural and functional changes in the protein, including an enhanced capacity of cytochrome c release with respect to full-length Bid (4, 16, 23, 24, 36). It was thus important to verify whether caspase cleavage would affect the lipid transfer activity displayed by Bid. As shown in Fig. 4D, recombinant mouse Bid cleaved by caspase 8 (tBid) displayed a higher rate of transfer of BODIPY FL C5-HPA to LUV acceptors than the parent preparation of full-length Bid. Under these conditions, the relative efficiency of lipid transfer was 2.1 ± 0.1-fold higher for tBid than for full-length Bid. The enhanced LTP activity of caspase 8-cleaved Bid was seen in parallel to its stronger capacity of releasing cytochrome c than that of full-length Bid (results not shown). Thus, caspase cleavage increased the LTP-like activity of recombinant Bid, similar to the increased binding of recombinant Bid to mitochondrial lipids reported earlier (25). Studies are under way to clarify whether Bid and tBid have a different specificity for phospholipids.
DISCUSSION
The majority of current information on Bid and other proapoptotic Bcl-2 proteins has been obtained with transformed cells in culture or with recombinant proteins. In all these studies, relocation of the proapoptotic proteins to mitochondria has been considered to be central for their action, but the biochemical reasons for this relocation have not been identified. The aim of our work was to clarify the mechanism of the mitochondrial relocation of Bid. To this end, we focused the present study on adult tissues such as liver and kidney, where Bid is expressed at relatively high levels (Fig. 1 and references 10 and 35) and is crucially involved in the pathway of apoptosis triggered by Fas activation (11, 16, 28, 30, 37, 39).
The changes in Bid immunoblots that we observed during Fas treatment of liver or kidney indicated an altered distribution of Bid forms in cell fractions. In particular, activation of Fas ex vivo with the Jo-2 antibody transiently induced an increased association of full-length Bid (24-kDa band) to mitochondria concomitant with an increase in the permeability of the OM (our unpublished observations). Intriguingly, this transient increase in the mitochondrial content of full-length Bid mirrors the effects of exogenous phospholipids on Bid distribution (Fig. 1C). Hence, the lipid-dependent redistribution of full-length Bid between light membranes and mitochondria we observed here (Fig. 1) could be relevant to the mechanism of Bid relocation to mitochondria during Fas-induced apoptosis. Of note, an increased mitochondrial content of full-length Bid has been reported also in cells treated with apoptotic stimuli other than Fas ligation (16, 17).
Two mechanisms have been proposed for the action of Bid on mitochondria: (i) interaction with other proteins of the Bcl-2 family such as Bax and Bak, leading to activation that then facilitates the release of cytochrome c (9, 14, 35, 36), and (ii) direct perturbation of the lipid structure of OM to release cytochrome c (22, 23, 25). That nanomolar concentrations of Bid display lipid transfer activity (Fig. 3 and 4) appears to be consistent with a direct interaction of Bid with membrane lipids. However, we cannot exclude the possibility that lipids also enhance the interaction of Bid with native Bak resident in liver mitochondria and are currently addressing this possibility in detail.
Our novel observations suggest that mitochondrial relocation of Bid depends upon its interaction with phospholipids and their transport to mitochondria, since exogenous phospholipids alter the distribution of Bid forms between ER-rich membranes and mitochondria (Fig. 1) and isolated Bid exhibits lipid transfer activity between liposomes and mitochondria (Fig. 3 and 4). Notably, recombinant Bid has an activity of 3 to 4 pmol per min per microgram of protein with a fluorescent analog of PA (Fig. 4A), which compares well with the activity of established LTP from plants (27) or mammals, e.g., phosphatidylinositol (PI) transfer protein (33). Because LTP generally catalyze the transport as well as the exchange of different phospholipids (12, 27, 32, 33), it is likely that Bid is able to bind and transport a variety of lipid molecules. The distribution changes in Bid forms that we observed with chemically different phospholipids (Fig. 1) suggest that Bid may transport and exchange certain negatively charged lipids, as well as PC. Like some plant LTP (32), Bid is able to disrupt the integrity of lipid membranes (cf. references 22 and 23). By further analogy with plant LTP, which bind lysolipids more tightly than diacyllipids (12), it is conceivable that Bid can also bind and transport lysolipids. Accordingly, low concentrations of lysolipids affect the relocation of Bid to mitochondria (M. Degli Esposti, unpublished results).
Negatively charged lipids are synthesized in both ER compartments and mitochondria with lysophosphatidate as a common precursor (5, 18). Lysophosphatidate-metabolizing enzymes have been discovered in association with membrane fission of exocytotic vesicles (31), while LTP specific for mitochondrial PA-based lipids have not been identified. Bid could be one mammalian protein capable of transporting PA metabolites between mitochondria and other cellular compartments, but would this activity be essential for its proapoptotic function?
We envisage that Bid is normally involved in the transport and recycling of mitochondrial phospholipids, processes that can be severely perturbed by the induction of apoptosis. Indeed, the activation of Fas and other proapoptotic pathways induces an imbalance in lipid metabolism and membrane remodeling, with increased activity of phospholipases (1, 3, 17, 19). We propose that the consequent alteration in lipid traffic enhances the capacity of Bid to transport lysolipids, rather than phospholipids, to the OM. Here, the detergent-like effect of these lysolipids may physically overcome the natural capacity to maintain membrane integrity or stimulate the cytochrome c-releasing action of resident proteins such as Bak. In either case, the lysolipids accumulated by Bid on the mitochondrial OM would facilitate the release of cytochrome c in the cytosol, with subsequent activation of the apoptosome.
In conclusion, we present here the first biochemical basis for explaining mitochondrial relocation of Bid, an important proapoptotic protein of the Bcl-2 family. Future studies will reveal the chemical details of the newly identified activity of Bid and will clarify its connections with the action of other proteins of the Bcl-2 family.
ACKNOWLEDGMENTS
M.D.E. was funded by an Alliance Grant from the Institut de Recherches Servier (Paris, France).
We thank P. Masdehors, M. Smith, E. Beaulieu, A. Ghelli, I. Cristea, B. Corfe, D. James, G. Griffiths, J. H. Kim, and R. Kluck for discussion and help in different aspects of this work.
REFERENCES
- 1.Atsumi G, Tajima M, Hadano A, Nakatani Y, Murakami M, Kudo I. Fas-induced arachidonic acid release is mediated by Ca2+-independent phospholipase A2 but not cytosolic phospholipase A2, which undergoes proteolytic inactivation. J Biol Chem. 1998;273:13870–13877. doi: 10.1074/jbc.273.22.13870. [DOI] [PubMed] [Google Scholar]
- 2.Bai J, Pagano R E. Measurement of spontaneous transfer and transbilayer movement of BODIPY-labeled lipids in lipid vesicles. Biochemistry. 1997;36:8840–8848. doi: 10.1021/bi970145r. [DOI] [PubMed] [Google Scholar]
- 3.Bogin L, Papa M Z, Polak-Charcon S, Degani H. TNF-induced modulations of phospholipid metabolism in human breast cancer cells. Biochim Biophys Acta. 1998;1392:217–232. doi: 10.1016/s0005-2760(98)00035-6. [DOI] [PubMed] [Google Scholar]
- 4.Bossy-Wetzel E, Green D R. Caspases induce cytochrome c release from mitochondria by activating cytosolic factors. J Biol Chem. 1999;274:17484–17490. doi: 10.1074/jbc.274.25.17484. [DOI] [PubMed] [Google Scholar]
- 5.Chakraborty T R, Vancura A, Balija V, Haldar D. Phosphatidic acid synthesis in mitochondria. J Biol Chem. 1999;274:29786–29790. doi: 10.1074/jbc.274.42.29786. [DOI] [PubMed] [Google Scholar]
- 6.Chao D T, Korsmeyer S J. Bcl-2 family: regulators of cell death. Annu Rev Immunol. 1998;16:395–419. doi: 10.1146/annurev.immunol.16.1.395. [DOI] [PubMed] [Google Scholar]
- 7.Degli Esposti M, Avitabile E, Barilli M, Schiavo G, Montecucco C, Lenaz G. Comparative biochemistry of the ubiquinol-cytochrome c reductase (EC 1.10.2.2) isolated from different heart mitochondria. Comp Biochem Physiol. 1986;B85:543–552. doi: 10.1016/0305-0491(86)90044-1. [DOI] [PubMed] [Google Scholar]
- 8.De Kroon A I P M, Dolis D, Mayer A, Lill R, de Kruijff B. Phospholipid composition of highly purified mitochondrial outer membranes of rat liver and Neurospora crassa. Is cardiolipin present in the outer mitochondrial membrane? Biochim Biophys Acta. 1997;1325:108–116. doi: 10.1016/s0005-2736(96)00240-4. [DOI] [PubMed] [Google Scholar]
- 9.Eskes R, Desangher S, Antonsson B, Martinou J M. Bid induces oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol Cell Biol. 2000;20:929–935. doi: 10.1128/mcb.20.3.929-935.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Footz T K, Birren B, Minoshima S, Asakawa S, Shimizu N, Riazi M A, McDermid H E. The gene for death agonist Bid maps to the region of human 22q11.2 duplicated in cat eye syndrome chromosomes and mouse chromosome 6. Genomics. 1998;51:472–475. doi: 10.1006/geno.1998.5392. [DOI] [PubMed] [Google Scholar]
- 11.Frisch S M. Evidence for a function of death-receptor-related, death-domain-containing proteins in anoikis. Curr Biol. 1999;9:1047–1049. doi: 10.1016/s0960-9822(99)80455-2. [DOI] [PubMed] [Google Scholar]
- 12.Gomar J, Petit M C, Sodano P, Sy D, Marion D, Kader J C, Vovelle F, Ptak M. Solution structure and lipid binding of a non-specific lipid transfer protein extracted from maize seeds. Protein Sci. 1996;5:565–577. doi: 10.1002/pro.5560050402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomez B, Robinson N C. Quantitative determination of cardiolipin in mitochondrial electron transferring complexes by silicic acid high performance liquid chromatography. Anal Biochem. 1999;267:565–577. doi: 10.1006/abio.1998.2998. [DOI] [PubMed] [Google Scholar]
- 14.Green D R. Apoptotic pathways: paper wraps stone blunts scissors. Cell. 2000;102:1–4. doi: 10.1016/s0092-8674(00)00003-9. [DOI] [PubMed] [Google Scholar]
- 15.Griffiths G J, Dubrez L, Morgan C P, Jones N A, Whitehouse J, Corfe B M, Dive C, Hickman J A. Cell damage-induced conformational changes of the pro-apoptotic protein Bak in vivo precede the onset of apoptosis. J Cell Biol. 1999;144:903–914. doi: 10.1083/jcb.144.5.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gross A, Yin X, Wang K, Wei M C, Jockel J, Milliman C, Erdjument-Bromage H, Tempst P, Korsmeyer S J. Caspase cleaved BID targets mitochondria and is required for cytochrome c release, while BCL-XL prevents this release but not tumor necrosis factor death. J Biol Chem. 1999;274:1156–1163. doi: 10.1074/jbc.274.2.1156. [DOI] [PubMed] [Google Scholar]
- 17.Hacki J, Egger L, Monney L, Conus S, Rossé T, Fellay I, Borner C. Apoptotic crosstalk between the endoplasmic reticulum and mitochondria controlled by Bcl-2. Oncogene. 2000;19:2286–2895. doi: 10.1038/sj.onc.1203592. [DOI] [PubMed] [Google Scholar]
- 18.Hatch G M. Cardiolipin: biosynthesis, remodeling and trafficking in the heart of mammalian cells. Int J Mol Med. 1998;1:33–41. doi: 10.3892/ijmm.1.1.33. [DOI] [PubMed] [Google Scholar]
- 19.Jattela M, Benedict M, Tewari M, Shayman J A, Dixit V M. Bcl-x and Bcl-2 inhibit TNF and Fas-induced apoptosis ad activation of phospholipase A2 in breast carcinoma cells. Oncogene. 1995;10:2907–2305. [PubMed] [Google Scholar]
- 20.Jiang F, Ryan M T, Schlame M, Zhao M, Gu Z, Klingenberg M, Pfanner N, Greenberg M L. Absence of cardiolipin in the crd1 null mutant results in decreased mitochondrial membrane potential and reduced mitochondrial function. J Biol Chem. 2000;275:22387–22394. doi: 10.1074/jbc.M909868199. [DOI] [PubMed] [Google Scholar]
- 21.Khanna S J, Brown R, Whetton A D, Ball K L, Dive C. v-Abl protein tyrosine kinase upregulates p21WAF-1 in cell cycle arrested and proliferating myeloid cells. J Biol Chem. 2001;276:11143–11150. doi: 10.1074/jbc.M007073200. [DOI] [PubMed] [Google Scholar]
- 22.Kluck R, Degli Esposti M, Perkins G, Renken C, Kuwana T, Bossy-Wetzel E, Goldberg M, Allen T, Barber M J, Green D R, Newmeyer D D. The pro-apoptotic proteins, Bid and Bax, cause a limited permeabilization of the mitochondrial outer membrane that is enhanced by cytosol. J Cell Biol. 1999;147:809–822. doi: 10.1083/jcb.147.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kudla G, Montessuit S, Eskes R, Berrier C, Martinou J C, Ghazi A, Antonsson B. The destabilisation of lipid membranes induced by the C-terminal fragment of caspase 8-cleaved Bid is inhibited by the N-terminal fragment. J Biol Chem. 2000;275:22713–22718. doi: 10.1074/jbc.M003807200. [DOI] [PubMed] [Google Scholar]
- 24.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 25.Lutter M, Fang M, Luo X, Nishijima M, Xie X, Wang X. Cardiolipin provides specificity for targeting of tBid to mitochondria. Nat Cell Biol. 2000;2:754–756. doi: 10.1038/35036395. [DOI] [PubMed] [Google Scholar]
- 26.McDonnell J M, Fushman D, Milliman C, Korsmeyer S J, Cowburn D. Solution structure of the proapoptotic molecule BID: a structural basis for apoptotic agonists and antagonists. Cell. 1999;96:625–634. doi: 10.1016/s0092-8674(00)80573-5. [DOI] [PubMed] [Google Scholar]
- 27.Moreau F, Davy de Virville J, Hoffelt M, Guerbette F, Kader J C. Use of a fluorimetric method to assay the binding and transfer of phospholipids by lipid transfer proteins from maize seedlings and Arabidopsis leaves. Plant Cell Physiol. 1994;35:267–274. [Google Scholar]
- 28.Nagata S. Biddable death. Nat Cell Biol. 1999;1:E143–E145. doi: 10.1038/14094. [DOI] [PubMed] [Google Scholar]
- 29.Nichols J W, Pagano R E. Kinetics of soluble lipid monomer diffusion between vesicles. Biochemistry. 1981;20:2783–2789. doi: 10.1021/bi00513a012. [DOI] [PubMed] [Google Scholar]
- 30.Schelling J R, Cleveland R P. Involvement of Fas-dependent apoptosis in renal tubular epithelial cell deletion in chronic renal failure. Kidney Int. 1999;56:1313–1316. doi: 10.1046/j.1523-1755.1999.00684.x. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt A, Wolde M, Thiele C, Fest W, Kratzin H, Podtelejnikov A V, Witke W, Huttner W B, Soling H. Endophilin I mediates synaptic vesicle formation by transfer of arachidonate to lysophosphatidic acid. Nature. 1999;401:133–141. doi: 10.1038/43613. [DOI] [PubMed] [Google Scholar]
- 32.Tassin S, Broekaert W F, Marion D, Acland D P, Ptak M, Vovelle F, Sodano P. Solution structure of Ace-AMP1, a potent antimicrobial protein extracted from onion seeds. Structural analogies with plant nonspecific lipid transfer proteins. Biochemistry. 1998;37:3623–3637. doi: 10.1021/bi9723515. [DOI] [PubMed] [Google Scholar]
- 33.Tremblay J M, Helmkamp G M, Yarbrough L R. Limited proteolysis of rat phosphatidylinositol transfer protein by trypsin cleaves the C terminus, enhances binding to lipid vesicles, and reduces phospholipid transfer activity. J Biol Chem. 1996;271:21075–21080. doi: 10.1074/jbc.271.35.21075. [DOI] [PubMed] [Google Scholar]
- 34.Vander Heiden M G, Thompson C B. Bcl-2 proteins: regulators of apoptosis or of mitochondrial homeostasis? Nat Cell Biol. 1999;1:E209–E215. doi: 10.1038/70237. [DOI] [PubMed] [Google Scholar]
- 35.Wang K, Yin X M, Chao D T, Milliman C L, Korsmeyer S J. BID: a novel BH3 domain-only death agonist. Genes Dev. 1996;10:2859–2869. doi: 10.1101/gad.10.22.2859. [DOI] [PubMed] [Google Scholar]
- 36.Wei M C, Lindsen T, Mootha V K, Eiler S, Gross A, Ashiya A, Thompson C B, Korsmeyer S J. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 2000;14:2060–2071. [PMC free article] [PubMed] [Google Scholar]
- 37.Yin X M, Wang K, Gross A, Zhao Y, Zinkel S, Klocke B, Roth K A, Korsmeyer S J. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature. 1999;400:886–891. doi: 10.1038/23730. [DOI] [PubMed] [Google Scholar]
- 38.Zha J, Weiler S, Oh K, Wei M C, Korsmeyer S J. Posttranslational N-myristoylation of BID as a molecular switch for targeting to mitochondria and apoptosis. Science. 2000;290:1761–1765. doi: 10.1126/science.290.5497.1761. [DOI] [PubMed] [Google Scholar]
- 39.Zheng T S, Hunot S, Kuida K, Momoi T, Srninivasan A, Nicholson D W, Lazebnik Y, Flavell R A. Deficiency in caspase-9 or caspase-3 induces compensatory caspase activation. Nat Med. 2000;6:1241–1247. doi: 10.1038/81343. [DOI] [PubMed] [Google Scholar]