Abstract
Purpose of review:
Time-restricted eating (TRE) entails consuming energy intake within a 4–10-hour window, with the remaining time spent fasting. While studies have reported health benefits from TRE, little is known about the impact of TRE on common chronic diseases such as type 2 diabetes, cancer, and cardiovascular disease. This review summarizes and critically evaluates the most recent TRE research findings relevant to managing and treating these chronic diseases.
Recent findings:
Most recent TRE studies have been in populations with overweight/obesity or metabolic syndrome; two have been in populations with diabetes, three in cancer survivors, and none in populations with cardiovascular disease. Collectively, these studies showed that participants could adhere to TRE and TRE is safe. These studies also showed preliminary efficacy for improved glucose regulation and insulin sensitivity, a reduction in body fat and blood pressure, reduced cardiovascular risk scores, and increased quality of life. More research is required to define the most effective TRE protocol (i.e., length and timing of eating window, intervention duration).
Summary:
TRE has demonstrated benefits on cardiovascular, metabolic, and clinical outcomes relevant to the underlying pathophysiology, but there are limited data on TRE implemented specifically within populations with diabetes, cancer, or cardiovascular disease.
Keywords: fasting, circadian rhythm, behavioral intervention, nutrition, oncology, heart disease
Introduction
Time-restricted eating (TRE) is a chrono-nutrition strategy and a format of intermittent fasting where individuals consume all energy intake within a specified time window, typically 4–10 hours during the day, with the remainder of the 24-hour period consisting of fasting (i.e., no caloric intake). While ad libitum TRE often results in spontaneous energy restriction, TRE without changes to energy intake also induces health effects through an impact on the timing of intake and repeated fasting periods.
Most of the cells and tissues in the body operate according to 24-hour circadian clocks that are responsible for physical, mental, and behavioral changes. By skewing energy intake away from nighttime, TRE promotes regulation of the “master clock” in the suprachiasmatic nucleus of the hypothalamus as well as peripheral “clocks” in liver, adipose tissue, muscle, and other metabolically active tissues [1]. Circadian clocks are also integral to the regulation of cardiovascular growth, renewal, repair, and remodeling [2] and regulate the expression of numerous genes of tissues, and when disrupted, can promote cancer [3].
TRE promotes daily periods of feeding and fasting, where repeated fasting elicits adaptive cell signaling pathways that promote intrinsic defenses against oxidative and metabolic stress, including reduction of inflammation and enhancement of repair and removal pathways (DNA repair, protein quality control, autophagy) [4].
Type 2 diabetes (T2D), cancer, and cardiovascular disease (CVD) are leading causes of death worldwide. In addition to their independent impact on morbidity and mortality, these conditions share a “common soil” of several risk factors and biobehavioral mechanisms of development and progression. Obesity, physical inactivity, poor dietary quality, overconsumption, chronic inflammation, oxidative stress, and resulting metabolic dysfunction are all implicated in the development and progression of T2D, cancer, and CVD [5–7].
The primary health effects of TRE studied to-date have been primarily related to body composition, obesity, and metabolism. However, we propose that TRE, through its pleiotropic effects, has the potential to modulate several shared risk factors and alter the shared biology among T2D, cancer, and CVD (Figure 1), and thus may have a positive impact on the development and progression of all three conditions. TRE is a relatively new field of research, and much of the literature has focused on metabolically unhealthy individuals (e.g., obesity, metabolic syndrome, impaired fasting glucose) without overt T2D, cancer, or CVD. The purpose of this narrative review is to summarize and critically evaluate the recent evidence for TRE with a focal point of its utility in the prevention and treatment of T2D, cancer, and CVD in humans. We also provide suggestions on future directions and outstanding questions relevant to these populations.
Figure 1:
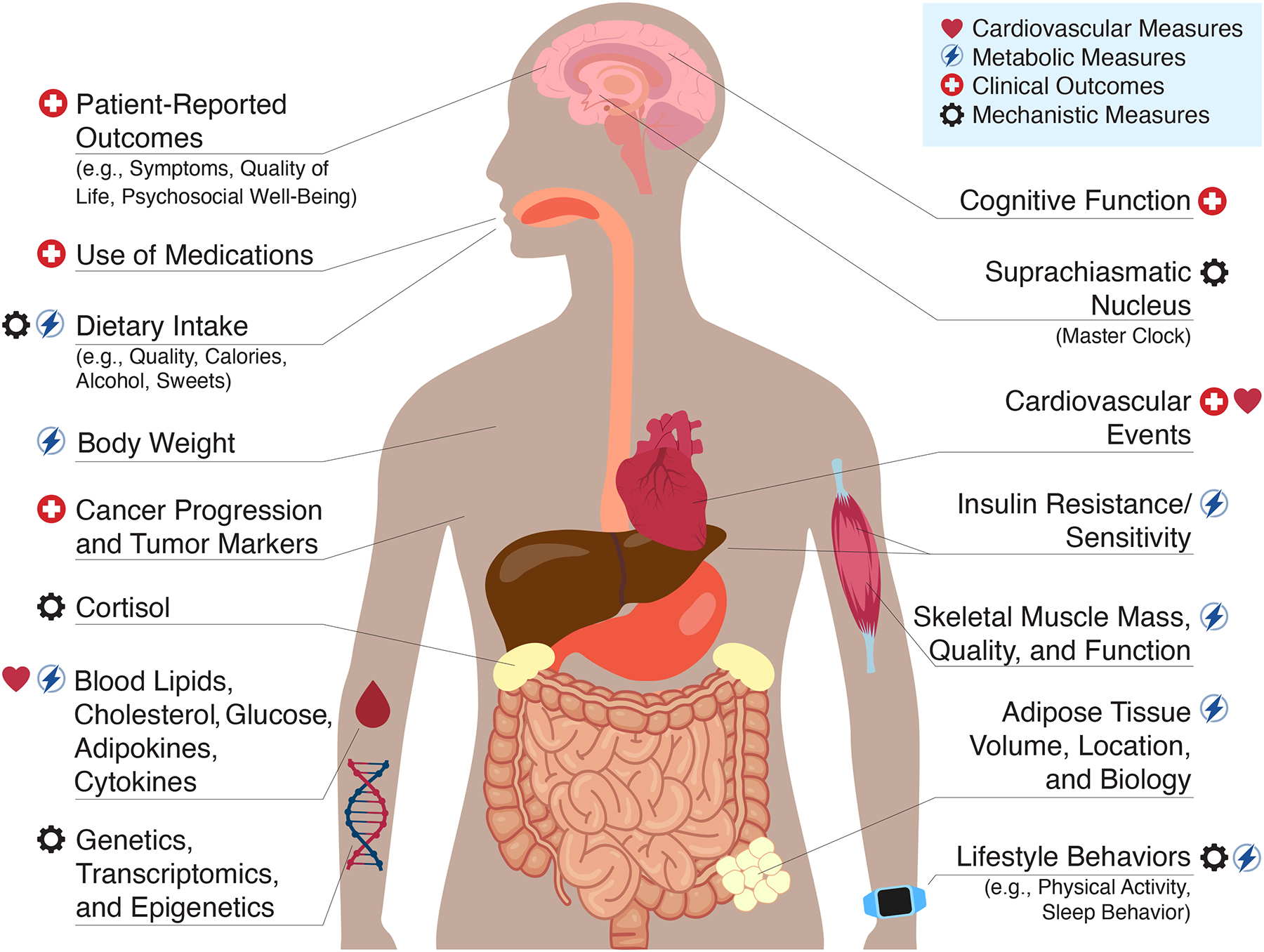
Proposed metabolic and cardiovascular measures, clinical outcomes, and mechanisms to evaluate the effects of time-restricted eating.
Summary of Recent Evidence
Health Effects of TRE on Individuals with Overweight/Obesity
The initial interest in TRE as a health intervention among humans was based on the hypothesis that shorter eating duration and more consistent timing of eating (especially with variability introduced by weekends, i.e., “social jetlag”) would help regulate circadian rhythm, improve metabolism, and ultimately reduce body weight. This hypothesis was tested by Gill and Panda [8] in a seminal study of 8 individuals with overweight/obesity in 2015. Overweight/obesity are well-established risk factors for T2D, several cancer diagnoses, and CVD. Within the recent TRE literature, weight loss and fat loss continue to be the most consistently reported positive health effects (Table 1). Numerous recent studies in individuals with overweight/obesity have also reported improvements to each component of the metabolic syndrome (waist circumference, fasting glucose, triglycerides, HDL, and blood pressure), while others have failed to find any effect (Table 1). Moreover, growing evidence suggests that TRE may have auxiliary lifestyle benefits, including improved sleep and quality of life, no negative effect on physical activity, and reduced calorie intake without calorie counting. These studies demonstrate proof-of-principle for TRE as a strategy to improve metabolic health outcomes relevant to the primary prevention of T2D, cancer, and CVD. However, the accumulated results to date are not necessarily generalizable to individuals with these conditions.
Table 1:
Summary of recent time-restricted eating (TRE) studies and major findings
| Study | Study Cohort | Condition Characteristics | Duration, Design | TRE Protocol (duration, time, intake prescription) | Major Findings |
|---|---|---|---|---|---|
| Type 2 Diabetes | |||||
| Che et al., 2021 [14] | n=104 46% F 48±10 y 26±2 kg/m2 100% with overweight |
Type 2 diabetes HbA1c ~8.6%, 5±1 y since diagnosis, managed via diet, OHA and/or insulin | 12 weeks RCT |
10 hours 0800 – 1600 h ad libitum vs control (usual eating pattern, ~15 hours, ~0600 – 1830 h) |
Adherence: >6 d/wk, “compliance was excellent” Safety: no adverse events including hypoglycemia; Differences vs control: ↓ HbA1c (−0.8%), fasting glucose, body weight, BMI, HOMA-IR, medication effects score, triglycerides, total cholesterol, LDL; ↑ HOMA-β, QoL; Concurrent lifestyle behaviors: −28% EI (vs +5% control), ↔ PA. |
| Parr et al., 2020 [13] | n=19 53% F 50±9 y 34±5 kg/m2 100% with overweight |
Type 2 diabetes, HbA1c ~7.6%, 3±3 y since diagnosis, current medication of up to 2 OHAs excluding insulin, sulfonylurea, GLP-1 agonists | 4 weeks pre-post |
9 hours 1000 – 1900 h ad libitum |
Adherence: 72% (range: 4–100%); Safety: ↔ lean mass; no hypoglycemia or major adverse events; Changes from baseline: ↔ body weight, fat mass, HbA1c, fasting glucose; Concurrent lifestyle behaviors: ↔ EI or macronutrient intake; ↓ EI when adherent (↓ CHO, alcohol). |
| Cancer | |||||
| Kirkham et al., 2022 [15] | n=22 100% F 66±5 y 31±5 kg/m2 100% with overweight/obesity |
Breast cancer survivors aged ≥60 y with overweight, 3±1 years since anthracycline chemotherapy treatment | 8 weeks pre-post |
8 hours 1200 – 2000 h weekdays ad libitum |
Adherence: median 98%, range: 85–100%; Safety: ↔ fat-free mass, thigh muscle volume; minor symptoms (headache, irritability) lasting 5 minutes to 3 hours; no major adverse events; Changes from baseline: ↓ Framingham 10-year CVD risk, body weight and whole-body fat (bioelectrical impedance), visceral fat (magnetic resonance imaging); ↔ total cholesterol, HDL, SBP, BMI; Concurrent lifestyle behaviors: −22% (median) EI. |
| Kleckner et al., 2022 [17] | n=39 92% F 62±12 y 32±7 kg/m2 80% with overweight/obesity |
Any cancer diagnosis and fatigue ≥3 on 0–10 scale, 2±1 years since treatment | 2 weeks pre-post |
10 hours self-selected ad libitum |
Adherence: average 90% Safety: n=1 grade 1 headache; n=1 grade 1 insomnia; Changes from baseline: ↓ fatigue, drowsiness; ↑ QoL Concurrent lifestyle behaviors: ↔ sleep problems; −11±35% EI. |
| O’Donnell et al., 2022 [16] | n=40 100% F 60 y (34–76) 60% with overweight/obesity |
Breast cancer survivors completed treatment, median 4.5 y since diagnosis | 12 weeks pre-post | 11 hours timing NR ad libitum |
Adherence: median 93%, range 0–100%; Safety: NR; Changes from baseline: ↓ BMI, anxiety, depression, fatigue; ↔ lipid profile, HbA1c, leptin, adiponectin, C-Reactive Protein, IL-6, TNF-α, QoL Concurrent lifestyle behaviors: ↔ PA. |
| Overweight/Obesity | |||||
| Cienfuegos et al. 2022 [21] | n=49 91% F 47±3 y 37±1 kg/m2 |
Adults with obesity | 8 weeks RCT |
4 hours 1500 – 1900 h ad libitum (n=19) vs 6 hours 1300 – 1900 h ad libitum (n=20) vs control (n=19) |
Adherence: 89±2%; Safety: mild adverse effects, such as dizziness, nausea, headaches, constipation, diarrhea, and dry mouth in both TRE groups vs control; no major adverse events; ↔ lean mass; Differences vs control: ↓ body weight and fat mass, ↔ VAT; Concurrent lifestyle behaviors: −29–30% EI in both TRE groups; ↔ PA, sleep quality, or insomnia severity in any group. |
| Crose et al., 2021 [22] | n=20 85% F 46±12 y 34±8 kg/m2 |
Adults with overweight | 12 weeks RCT |
8 hours self-selected window ad libitum (n=11) vs control (n=9) |
Adherence: 95% of eating events in 10±2 h; Safety: NR; Changes from baseline: ↓ body weight (-3.7%); ↑ health transition QoL; Differences vs control: ↑ health transition QoL, emotional health QoL; Concurrent lifestyle behaviors: NR. |
| Karras et al., 2021 [23] | n=45 76% F ~48±9 y 29±6 kg/m2 |
Adults with overweight | 7 weeks non-RCT follow up at 13 weeks |
8 hours 0800 – 1600 h plus CR (F: 1200–1500 kcal/d M: 1500–1800 kcal/d) (n=16) vs Orthodox fasting (dawn-dusk) plus same CR (n=29) |
Adherence: NR; Safety: ↔ lean mass; Changes from baseline: ↓ body weight (7, 13 weeks), BMI, WC; ↑ HDL; ↔ body fat mass, total cholesterol, LDL, triglycerides, fasting glucose; Differences vs Orthodox fasting: NR; Concurrent lifestyle behaviors: ~-250 kcal/d EI, ↓ fiber intake. |
| Karras et al., 2022 [24] | n=97 100% F ~47±7 y 29±6 kg/m2 |
Premenopausal women with overweight | 7 weeks (48 d) non-RCT follow up at 12 weeks |
8 hours 0800 – 1600 h plus CR (F: 1200–1500 kcal/d M: 1500–1800 kcal/d) (n=42) vs Orthodox fasting (dawn- dusk) plus same CR (n=55) |
Adherence: NR; Safety: NR; Changes from baseline: ↓ BMI, WC (7, 12 wks); Differences vs Orthodox fasting: ↔ BMI, WC Concurrent lifestyle behaviors: ~-30% EI, ↓ monounsaturated and total fat intake, ↓ carbohydrate and protein intake. |
| Kotarsky et al, 2021 [25] | n=21 86% F 44±7 y 30±3 kg/m2 |
Adults with overweight/obesity | 8 weeks RCT |
8 hours 1200–2000 h ad libitum plus aerobic and resistance exercise training (n=11) vs aerobic and resistance exercise training (n=10) |
Adherence: “only two instances of non-compliance reported among two participants”; Safety: n=1 headache; no major adverse events; Changes from baseline: ↓ body weight, BMI, WC; Differences vs control: ↓ body weight, BMI; ↔ WC; Concurrent lifestyle behaviors: −15% EI in both groups; ↔ sedentary time or light PA in either group; ↔ group difference in moderate-vigorous PA. |
| Li et al., 2021 [28] | n=15 100% F 18–31 y (range) 30±4 kg/m2 |
Women (18–40 y) with polycystic ovarian syndrome and BMI ≥24 kg/m2 | 5 weeks pre-post |
8 hours 0800 – 1600 h ad libitum |
Adherence: NR; Safety: ↔ lean mass; Changes from baseline: ↓ body weight (−1.7%), BMI, fat mass, body fat percentage, VAT, fasting insulin, HOMA-IR, insulin response to oral glucose tolerance test, C-reactive Protein, alanine aminotransferase; ↑ menstrual cycle regularity, insulin-like growth fator-1; Concurrent lifestyle behaviors: ↔ eating behaviors. |
| Lin et al., 2021 [27] | n=63 100% F ~52±8 y 26±4 kg/m2 |
Women, aged 40–65 y with BMI ≥24 kg/m2 or WC >80 cm | 8 weeks RCT |
8 hours 1000 – 1800 h or 1200 – 2000 h plus CR (1400 kcal/d) (n=30) vs CR alone (1400 kcal/d) alone (n=33) |
Adherence: “around 84%”; Safety: NR; Changes from baseline: ↓ body weight (−4.5% TRE+CR; −2.4% CR alone), BMI, WC, body fat percentage, VAT; ↓ DBP (TRE+CR); ↑ fasting glucose, HOMA-IR (TRE+CR) Changes vs CR alone: ↓ body weight, BMI, DBP; Concurrent lifestyle behaviors: −9–11% EI; 13–19% carbohydrate intake. |
| Liu et al., 2022 [26] | n=139 (n=118 completed) 48% F 32±9 y 32±3 kg/m2 |
Adults with BMI of 28–45 kg/m2 | 12 months RCT |
8 hours 0800 – 1600 h plus 25% CR (n=69) vs 25% CR alone (n=70) |
Adherence: 84±16% (TRE+CR) vs 84±13% (CR alone); Safety: ↓ lean mass (↔ between groups), ↔ in adverse events between groups; Changes from baseline: ↓ body weight (-8.0 kg), fat mass (−5.9 kg), VAT, abdominal subcutaneous fat, liver fat, SBP, DBP; Differences vs control: ↔ body weight or any composition variables, SBP, DBP, lipid profile, markers of glucose or insulin control; Concurrent lifestyle behaviors: ↔ physical activity from baseline or between groups; ↔ group difference in EI. |
| Lobene et al., 2021 [29] | n=20 85% F 46±3 y 34±2 kg/m2 |
Adults with overweight | 12 weeks RCT |
8 hours self-selected window ad libitum (n=11) vs control (n=9) |
Adherence: 56±22%; Safety: ↓ lean mass relative to baseline and control; Changes from baseline: ↓ body weight (−3.7%), fat mass (-4.0%), VAT (-11.1%); ↔ bone mineral density; Differences vs control: ↓ body weight, VAT; ↑ bone mineral content; ↔ bone mineral density or bone turnover markers; Concurrent lifestyle behaviors: NR. |
| Peeke et al., 2021 [30] | n=60 88% F 44±11 y 38±9 kg/m2 |
Adults with obesity | 8 weeks RCT |
10 hours self-selected window with start 0700–1000 h/stop 1700–2000 h plus CR by −500–1000 kcal/d, with “fasting snack” (200 kcal ~0500–0800 h) vs 12 hours + same CR |
Adherence: NR; Safety: no adverse events; Changes from baseline: ↓ body weight (−10.7 kg, TRE+CR; −8.9 kg CR), fasting glucose Differences vs CR alone: ↓ body weight (−1.9 kg, −1.4%), ↔ fasting glucose; Concurrent lifestyle behaviors: NR. |
| Prasad et al., 2021 [31] | n=16 82% F 51±12 y 31±11 kg/m2 |
Adults with overweight/obesity | 3 months pre-post |
10 hours self-selected window ad libitum |
Adherence: 47±19%; Safety: NR; Changes from baseline: ↓ body weight (−2%), BMI, WC, SBP; Concurrent lifestyle behaviors: ↔ weight efficacy lifestyle questionnaire, morning-eveningness questionnaire. |
| Przulj et al., 2021 [32] | n=50 74% F 50±12 y 35±4 kg/m2 |
Adults with BMI ≥30 kg/m2 or BMI ≥28 kg/m2 with comorbidities | 12 weeks, pre-post |
8 hours self-selected window ad libitum |
Adherence: 5±2 d/wk; Safety: NR; Changes from baseline: ↓ body weight (-2.6 kg), rating of hunger; ↔ lipid profiles, SBP, DBP; Concurrent lifestyle behaviors: NR. |
| Querioz et al., 2022 [33] | n=37 84% F 30±6 y 31±3 kg/m2 |
Adults with BMI ≥25-≤34.9 kg/m2 | 8 weeks RCT |
8 hours 0800 – 1600 h plus −25% CR vs 8 hours 1200 – 2000 h plus −25% CR vs −25% CR alone |
Adherence: 85% and 73 % of participants in earlier and later TRE + CR group, respectively, were fully adherent; Safety: ↓ lean mass (↔ groups), ↓ RMR; Changes from baseline: ↓ body weight (−4.2 kg, −4.8 kg, −4.0 kg for 3 groups, fat mass, fasting glucose, insulin, total cholesterol, LDL, HDL; Concurrent lifestyle behaviors: ↔ PA, ↑ sleep quality. |
| Schroder et al., 2021 [20] | n=32 100% F 37±2 y 33±1 kg/m2 |
Women with obesity | 3 months non-RCT | 8 hours 1200 – 2000 h ad libitum (n=20) vs control (n=12) |
Adherence: NR; Safety: NR; Differences vs control: ↓ body weight, BMI, WC, SBP, DBP, Framingham 30-year CVD risk; ↑ QoL; Concurrent lifestyle behaviors: NR. |
| Thomas et al, 2022 [34] | n=81 85% F 38±8 y 34±6 kg/m2 |
Adults with BMI of 27–45 kg/m2 | 12 weeks RCT follow up at 39 weeks |
10 hours window starting within 3 h of waking plus −35% CR (n=32) vs −35% CR alone (n=31) |
Adherence: 5±1 days to TRE+CR vs 4±2 to CR alone; Safety: ↓ lean mass (↔ between groups); Changes from baseline: ↓ body weight, fat mass Differences between groups: ↔ body weight, fat mass Concurrent lifestyle behaviors: −500 kcal/d EI and ↑ health eating index in TRE+CR, ↔ PA. |
| Zhao et al., 2022 [35] | n=15 0% F 63±4 years 31±2 kg/m2 |
Men with WC ≥94 cm | 8 weeks pre-post |
10 hours window finished by 1930 h ad libitum |
Adherence: 88±10%; Safety: NR; Changes from baseline: ↓ body weight, WC, VAT, percentage body fat, fasting glucose, HbA1c; ↔ SBP, DBP, fasting insulin, total cholesterol, HDL, triglycerides, NEFAs; Concurrent lifestyle behaviors: ↔ EI or sleep duration; ↓ PA and total energy expenditure; |
Key: arrows indicate significant reductions (↓), increases (↑), or no significant changes/differences (↔). Abbreviations: BMI, body mass index; CR, calorie restriction; CVD, cardiovascular disease; DBP, diastolic blood pressure; EI, energy intake; F, females; HbA1c, glycated hemoglobin; HDL, high density lipoprotein; HOMA-IR, Homeostatic Model Assessment for Insulin Resistance; LDL, low density lipoprotein; NR, not reported; OHA, oral hypoglycemic agent; PA, physical activity; QoL, quality of life; RCT, randomized controlled trial; RMR, resting metabolic rate; SBP, systolic blood pressure; TRE, time-restricted eating; VAT, visceral adipose tissue; WC, waist circumference.
The Evidence for TRE to Improve Glucose Metabolism in Individuals with T2D
Several organs with peripheral clocks (e.g., pancreas, liver, muscle, adipose tissue) interact to regulate blood glucose concentrations in a diurnal pattern, such that insulin resistance is greatest in the evening in individuals without metabolic disease but greatest in the morning in those with T2D [9]. Based on this integral role of the feed-fast cycle on glucose metabolism, the timing of intake and length of the eating window are highly modifiable behaviors with potential for biologic impact on glycemic regulation. Both late-night intake and shorter overnight fasting have been associated with impaired glucose control in individuals with and without T2D [10, 11].
There have been several studies of TRE in individuals with overweight/obesity or prediabetes that indicate that glycemic control may be favorably impacted by TRE [12]. Thus, there is the potential for TRE to be a simple alternative strategy to making dietary changes for individuals with T2D to improve their blood glucose management. To-date, two studies of TRE in individuals with T2D have been published. In 2020, Parr et al. [13] published a 4-week feasibility study of TRE in 19 individuals with T2D (glycated hemoglobin A1c [HbA1c] of 6.5–10%) that found that participants adhered to a 9-hour mid-day TRE (i.e., delayed breakfast at 1000 h, earlier dinner finished by 1900 h) protocol on ~5 of 7 prescribed days per week, without adverse events, including incidence of hypoglycemia. There was a trend for improved HbA1c, but no change to fasting glucose concentrations. On adherent days (i.e., intake confined to 1000 to 1900 h), participants reduced their energy intake, including intake of both carbohydrates and alcohol. These findings support the notion that TRE may improve dietary quality toward a profile more likely to contribute to improved glycemic management. Most recently, Che et al. [14] randomized 120 individuals with T2D to either follow a 10-hour 0800 to 1600 h early TRE protocol or to continue their habitual eating pattern (~15-hour eating window) for 12 weeks. The TRE group followed the protocol on ~6 of 7 prescribed days/week without adverse events, including incidence of hypoglycemia. The TRE protocol resulted in a 28% average decrease in energy intake, and 1.54% absolute (8% relative) reduction in HbA1c, 14% reduction in Homeostatic Model Assessment for Insulin Resistance (HOMA-IR), and a 24% improvement in HOMA β-cell function, which were all significant relative to the control group. Thus, this latest randomized controlled trial (RCT) provides strong preliminary evidence to suggest that TRE may be a feasible dietary strategy for T2D and warrants further study.
The Evidence for TRE on Clinical and Supportive Care Outcomes in Individuals with Cancer
TRE is a potential supportive care strategy that may reduce acute and long-term cancer treatment side effects including cancer-related fatigue, and among survivors, could reduce the risk of recurrence and competing risks through positive effects on body composition and cardiovascular risk factors. To-date, there have been three single-arm TRE studies published among individuals with a history of cancer. In the first, Kirkham et al. [15] assessed an 8-hour (1200 to 2000 h) weekday-only TRE protocol for 8 weeks among 22 breast cancer survivors who had completed anthracycline-based chemotherapy 3±1 year prior. Adherence to this protocol was 98% and the Framingham 10-year cardiovascular disease risk, total body fat mass, and visceral adipose tissue all decreased by end of intervention. O’Donnell et al. [16] tested the feasibility of an 11-hour eating window (13-hour overnight fast) for 12 weeks in 40 breast cancer survivors who were a median of 4.5 years since diagnosis. Their feasibility criterion was met in that 95% of participants reported adherence to TRE at least 70% of prescribed days (i.e., 5 of 7 days per week). They also showed preliminary efficacy for reduced anxiety at 6 weeks and improvements in body mass index, anxiety, depression, and fatigue at 12 weeks. Kleckner et al. [17] evaluated the feasibility of 2 weeks of TRE following a 10-hour self-selected eating window among 39 cancer survivors 2±1 years since treatment with self-reported cancer-related fatigue (≥3 on 10-point scale). On average, participants adhered to the 10-hour eating window on 90% of the days in the 14-day period. Cancer-related fatigue decreased after just two weeks of TRE. Importantly, all of these studies reported no major adverse events among cancer survivors following TRE, yet no studies have evaluated TRE during active cancer treatment, which would be require careful study of potential interactions with treatment and cancer-related adverse events.
The Evidence for TRE on CVD Measures
The circadian clock governs heart rate, blood pressure, cardiac metabolism, contractility, and coagulation. An increasing number of epidemiological and basic science studies demonstrate that disruptions to the circadian rhythm are associated with increased CVD risk and worse outcomes [18]. However, to our knowledge, no study has been published that implemented a TRE intervention in patients with any type of CVD, though the feasibility and safety of combining it with cardiac rehabilitation among patients with coronary artery disease is currently in progress by Kirkham et al. (clinicaltrials.gov: NCT05075317). Yet, the impact of TRE has been studied on several CVD risk factors including body composition and glucose control as discussed earlier, as well as blood pressure and plasma lipid profile (i.e., total cholesterol, high and low-density lipoprotein, triglycerides). In a recent review, Gabel et al. [19] noted a general inconsistency of effects among studies, with some reporting positive effects of TRE on systolic and diastolic blood pressure, low-density and high-density lipoprotein (HDL), and triglycerides, with others finding no effect. There may also be interindividual variability in the effect of TRE on individual CVD risk factors. Kirkham et al. [15] found a significant decrease in 10-year cardiovascular disease risk (a composite score based on modifiable risk factors of total cholesterol, HDL, and systolic blood pressure), despite no overall change in any of the individual variables, suggesting the origin of the reduced CVD risk differed among participants. Similarly, Schroder et al. [20] found that the Framingham 30-year CVD risk was reduced by 3 months of 1200 – 2000 h TRE among 20 women with obesity. These preliminary findings illustrate that TRE holds promise as a CVD risk reduction strategy and that future studies should continue to evaluate changes in composite outcomes to allow for interindividual variability in responses.
Future Directions in TRE research
While most TRE studies have demonstrated positive health changes with high levels of safety, many questions require further investigation to optimize TRE as a dietary strategy for specific outcomes in specific populations.
TRE protocol
A variety of TRE protocols have been shown to have positive health benefits, thus the parameters of the optimal TRE intervention have yet to be defined. The important parameters requiring further study include the length and timing of the eating window and their required consistency, the duration of intervention, the sustainability of positive TRE health effects, and the frequency of TRE (i.e., planned “cheat days” or weekends off). Further, the sustainability of adherence to long-term TRE is of major importance, as this is primary limitation among other dietary interventions.
Mechanisms of TRE
While impact on circadian rhythm, benefits of repeated fasting, and incidental reductions in energy intake might all play a role in the noted metabolic and cardiovascular health effects, quantifying the biobehavioral mediators for how TRE exerts its effects on these outcomes will help to define the optimal TRE protocol. For example, the role of potential concurrent lifestyle behavior changes in diet (quality, macronutrient ratio), physical activity (volume, intensity), total energy balance, sleep, or perceived stress is not well quantified. The biological impact of TRE on adipose tissue, adipokines, insulin sensitivity, inflammation, or oxidative stress also require further study.
Primary Prevention
While the evidence is not yet strong enough to dedicate the resources required for a RCT evaluating the effects of TRE on disease incidence, ongoing large cohort studies that incorporate food logs with timestamps can be leveraged to further understand the role of TRE in primary prevention. Smartphone apps and online data collection tools that facilitate real-time collection of dietary intake with time-stamps can be used in clinical trials and the free-living population to gather this information.
Secondary and Tertiary Prevention
The health benefits of TRE demonstrated to-date have the potential to reduce the impact or slow the progression of T2D, cancer, and CVD, and to manage the long-term impact of these chronic diseases, but the available literature has not adequately investigated the role of TRE as a therapeutic secondary or tertiary prevention strategy among individuals with these conditions. The clinical trials required to do so must include health or clinical outcomes that are relevant to the condition (Figure 1), an intervention duration and long-term follow-up adequate to demonstrate clinical impact, and systematically collect adverse events to demonstrate safety.
Another important step toward evaluation of TRE as a primary, secondary, or tertiary prevention therapy is phase III clinical trials that compare the adherence, safety, and efficacy of TRE to established behavioral and pharmacological therapeutic interventions and evaluate potential synergy and safety of their combination. Relevant lifestyle interventions for comparison include those that focus on the quantity of food (e.g., energy restriction without time-restriction), composition of food (e.g., Mediterranean Diet, low glycemic index diet), and timing of food (e.g., other TRE and intermittent fasting protocols), as well as those that promote sleep hygiene, circadian rhythm alignment (e.g., bright light therapy), or pleiotropic effects on metabolism (e.g., exercise). The potential for TRE to affect pharmacological treatment dose requirements, intake timing (i.e., chronotherapy), efficacy, or need (e.g., as a first line therapy for blood pressure or cholesterol) also warrant further study. In the context of cancer treatment, TRE could be evaluated as a potential strategy to promote enhanced chemotherapy efficacy while also reducing side effects on healthy tissues. In the context of T2D, the combination and potential interactions of TRE with metformin require further evaluation prior to implementation in this population. The study of TRE is most limited currently in cardiovascular conditions, despite the well-established importance of circadian rhythm and metabolic health in those with CVD.
Safety of TRE
While no major adverse events have been reported during TRE interventions within the populations studied to-date (Table 1), the safety of TRE has not yet been adequately established to be included into any clinical guidelines. TRE is not advised for individuals who are at risk for hypoglycemia, with a history of eating disorders, at risk for cachexia or sarcopenia, or on medications where fasting is contraindicated (e.g., insulin, sulfonylureas). It is important that individuals with or at risk for any of these conditions seek guidance and supervision from their physician and ideally a registered dietitian prior to initiating TRE or other dietary strategies. A requirement for establishing the safety of TRE among populations with chronic disease, especially those associated with risk for cachexia or sarcopenia, is more data on changes in lean mass relative to total body mass.
Conclusion
TRE is an intervention with demonstrated health benefits for body weight and composition, and growing evidence for an impact on glycemic control, blood pressure, and plasma lipid profile. While the available evidence suggests it could play a role in risk factor management in the primary prevention of T2D, cancer, and CVD, the evidence for the impact of TRE in individuals with T2D, CVD or cancer are limited. Nevertheless, given the demonstrated proof-of-principle of pleiotropic biobehavioral effects on relevant health metrics and the high safety profile, future research of TRE in individuals with T2D, cancer, and CVD is warranted.
Key points.
Time-restricted eating has demonstrated feasibility and safety in populations with overweight/obesity, metabolic syndrome, type 2 diabetes, and cancer.
Time-restricted eating has demonstrated preliminary efficacy to improve glucose regulation and insulin sensitivity, reduce body fat, blood pressure, cardiovascular risk, fatigue, and improve quality of life in clinical populations.
Larger definitive studies are needed before TRE can be incorporated into clinical guidelines.
More research is needed to determine the optimal parameters of time-restricted eating protocols (i.e., length and timing of eating window, length of intervention) for health outcomes, to understand the underlying mechanisms of TRE on metabolism, and to determine its utility for primary or secondary prevention.
Financial support and sponsorship
This publication was supported in part by the Heart and Stroke Foundation of Canada (A.A.K), the University of Maryland Baltimore, Institute for Clinical & Translational Research via grant no. UL1TR003098 (pilot funds to A.S.K.), the Maryland Department of Health’s Cigarette Restitution Fund Program (A.S.K), and a Diabetes Australia Research Program Grant (Y20G-PARE; to E.B.P).
Footnotes
Conflicts of interest: None.
References
- [1].Wehrens SMT, Christou S, Isherwood C, et al. Meal Timing Regulates the Human Circadian System. Curr Biol 2017; 27: 1768–1775.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Xu W, Jain MK, Zhang L. Molecular link between circadian clocks and cardiac function: a network of core clock, slave clock, and effectors. Curr Opin Pharmacol 2021; 57: 28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Fekry B, Eckel-Mahan K. The circadian clock and cancer: links between circadian disruption and disease Pathology. J Biochem 2022; 171: 477–486. [DOI] [PubMed] [Google Scholar]
- [4].Cabo R de Mattson MP. Effects of intermittent fasting on health, aging, and disease. New Engl J Med 2019; 381: 2541–2551. [DOI] [PubMed] [Google Scholar]
- [5].Caussy C, Aubin A, Loomba R. The Relationship Between Type 2 Diabetes, NAFLD, and Cardiovascular Risk. Curr Diabetes Rep 2021; 21: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lau ES, Paniagua SM, Liu E, et al. Cardiovascular Risk Factors Are Associated With Future Cancer. Jacc Cardiooncology 2021; 3: 48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lees B, Hampton JM, Trentham-Dietz A, et al. A population-based study of causes of death after endometrial cancer according to major risk factors. Gynecol Oncol 2021; 160: 655–659. [DOI] [PubMed] [Google Scholar]
- [8].Gill S, Panda S. A Smartphone App Reveals Erratic Diurnal Eating Patterns in Humans that Can Be Modulated for Health Benefits. Cell Metab 2015; 22: 789–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Peng F, Li X, Xiao F, et al. Circadian clock, diurnal glucose metabolic rhythm, and dawn phenomenon. Trends Neurosci 2022; 45: 471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sakai R, Hashimoto Y, Ushigome E, et al. Late-night-dinner is associated with poor glycemic control in individuals with type 2 diabetes: The KAMOGAWA-DM cohort study. Endocr J 2018; 65: 395–402. [DOI] [PubMed] [Google Scholar]
- [11].Marinac CR, Natarajan L, Sears DD, et al. Prolonged Nightly Fasting and Breast Cancer Risk: Findings from NHANES (2009–2010). Cancer Epidemiol Biomarkers Prev 2015; 24: 783–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Parr EB, Devlin BL, Hawley JA. Perspective: Time-Restricted Eating—Integrating the What with the When. Adv Nutr 2022; 13: 699–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Parr E, Devlin B, Lim K, et al. Time-restricted eating as a nutrition strategy for individuals with type 2 diabetes: A feasibility study. Nutrients 2020; 12: 3228–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **[14].Che T, Yan C, Tian D, et al. Time-restricted feeding improves blood glucose and insulin sensitivity in overweight patients with type 2 diabetes: a randomised controlled trial. Nutr Metabolism 2021; 18: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is a rigorously designed and executed study of early TRE in individuals with type 2 diabetes. This study will set the stage for future work in this population.
- *[15].Kirkham AA, Ford KL, Topolnyski J, et al. Time-Restricted Eating to Reduce Cardiovascular Risk Among Older Breast Cancer Survivors: A Single-arm Feasibility Study. JACC: Cardio-Oncology; accepted Mar 7, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first TRE study conducted in a cancer population and also the first to employ a weekday-only TRE protocol without weekend restrictions in humans.
- [16].O’Donnell E, Shapiro Y, Comander A, et al. Pilot study to assess prolonged overnight fasting in breast cancer survivors (longfast). Breast Cancer Res Tr 2022; 193: 579–587. [DOI] [PubMed] [Google Scholar]
- [17].Kleckner AS, Altman BJ, Reschke JE, et al. Time-restricted eating to address cancer-related fatigue among cancer survivors: A single-arm pilot study,” Journal of Integrative Oncology 11(4):379. doi: 10.37421/2329-6771.2022.11.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lecour S, Pré BCD, Bøtker HE, et al. Circadian rhythms in ischaemic heart disease. Key aspects for preclinical and translational research: Position paper of the ESC Working Group on Cellular Biology of the Heart. Cardiovasc Res 2021; cvab293. [DOI] [PubMed] [Google Scholar]
- [19].Gabel K, Cienfuegos S, Kalam F, et al. Time-Restricted Eating to Improve Cardiovascular Health. Curr Atheroscler Rep 2021; 23: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Schroder JD, Falqueto H, Mânica A, et al. Effects of time-restricted feeding in weight loss, metabolic syndrome and cardiovascular risk in obese women. J Transl Med 2021; 19: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cienfuegos S, Gabel K, Kalam F, et al. The effect of 4-h versus 6-h time restricted feeding on sleep quality, duration, insomnia severity and obstructive sleep apnea in adults with obesity. Nutrition Heal 2022; 28: 5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Crose A, Alvear A, Singroy S, et al. Time-Restricted Eating Improves Quality of Life Measures in Overweight Humans. Nutrients 2021; 13: 1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Karras SN, Koufakis T, Adamidou L, et al. Effects of Christian Orthodox Fasting Versus Time-Restricted Eating on Plasma Irisin Concentrations Among Overweight Metabolically Healthy Individuals. Nutrients 2021; 13: 1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Karras SN, Koufakis T, Adamidou L, et al. Implementation of Christian Orthodox fasting improves plasma adiponectin concentrations compared with time-restricted eating in overweight premenopausal women. Int J Food Sci Nutr 2021; 73: 1–11. [DOI] [PubMed] [Google Scholar]
- [25].Kotarsky CJ, Johnson NR, Mahoney SJ, et al. Time‐restricted eating and concurrent exercise training reduces fat mass and increases lean mass in overweight and obese adults. Physiological Reports 2021; 9: e14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **[26].Liu D, Huang Y, Huang C, et al. Calorie Restriction with or without Time-Restricted Eating in Weight Loss. New Engl J Med 2022; 386: 1495–1504. [DOI] [PubMed] [Google Scholar]; This is a rigorously designed and conducted randomized controlled trial of early TRE combined with calorie restriction versus calorie restriction alone among over 100 individuals with obesity. It showed that adherence was >80% for 12 months in both groups but that TRE did not significantly enhance the effects of calorie restriction on weight loss and fat mass loss.
- [27].Lin Y-J, Wang Y-T, Chan L-C, et al. Effect of time-restricted feeding on body composition and cardio-metabolic risk in middle-aged women in Taiwan. Nutrition 2022; 93: 111504. [DOI] [PubMed] [Google Scholar]
- [28].Li C, Xing C, Zhang J, et al. Eight-hour time-restricted feeding improves endocrine and metabolic profiles in women with anovulatory polycystic ovary syndrome. J Transl Med 2021; 19: 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lobene AJ, Panda S, Mashek DG, et al. Time-Restricted Eating for 12 Weeks Does Not Adversely Alter Bone Turnover in Overweight Adults. Nutrients 2021; 13: 1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Peeke PM, Greenway FL, Billes SK, et al. Effect of time restricted eating on body weight and fasting glucose in participants with obesity: results of a randomized, controlled, virtual clinical trial. Nutrition & diabetes 2021; 11: 1048–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Prasad M, Fine K, Gee A, et al. A Smartphone Intervention to Promote Time Restricted Eating Reduces Body Weight and Blood Pressure in Adults with Overweight and Obesity: A Pilot Study. Nutrients 2021; 13: 2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Przulj D, Ladmore D, Smith KM, et al. Time restricted eating as a weight loss intervention in adults with obesity. Plos One 2021; 16: e0246186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Queiroz J do N, Macedo RCO, Santos GC dos, et al. Cardiometabolic Effects of Early vs. Delayed Time-Restricted Eating Plus Caloric Restriction in Adults with Overweight and Obesity: An Exploratory Randomized Clinical Trial. Brit J Nutr 2022; 1–36. [DOI] [PubMed] [Google Scholar]
- [34].Thomas EA, Zaman A, Sloggett KJ, et al. Early time-restricted eating compared with daily caloric restriction: A randomized trial in adults with obesity. Obesity 2022; 30: 1027–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Zhao L, Hutchison AT, Liu B, et al. Time-restricted eating improves glycemic control and dampens energy-consuming pathways in human adipose tissue. Nutrition 2022; 96: 111583. [DOI] [PubMed] [Google Scholar]


