Fig. 2 |. Mechanism of proton coupling.
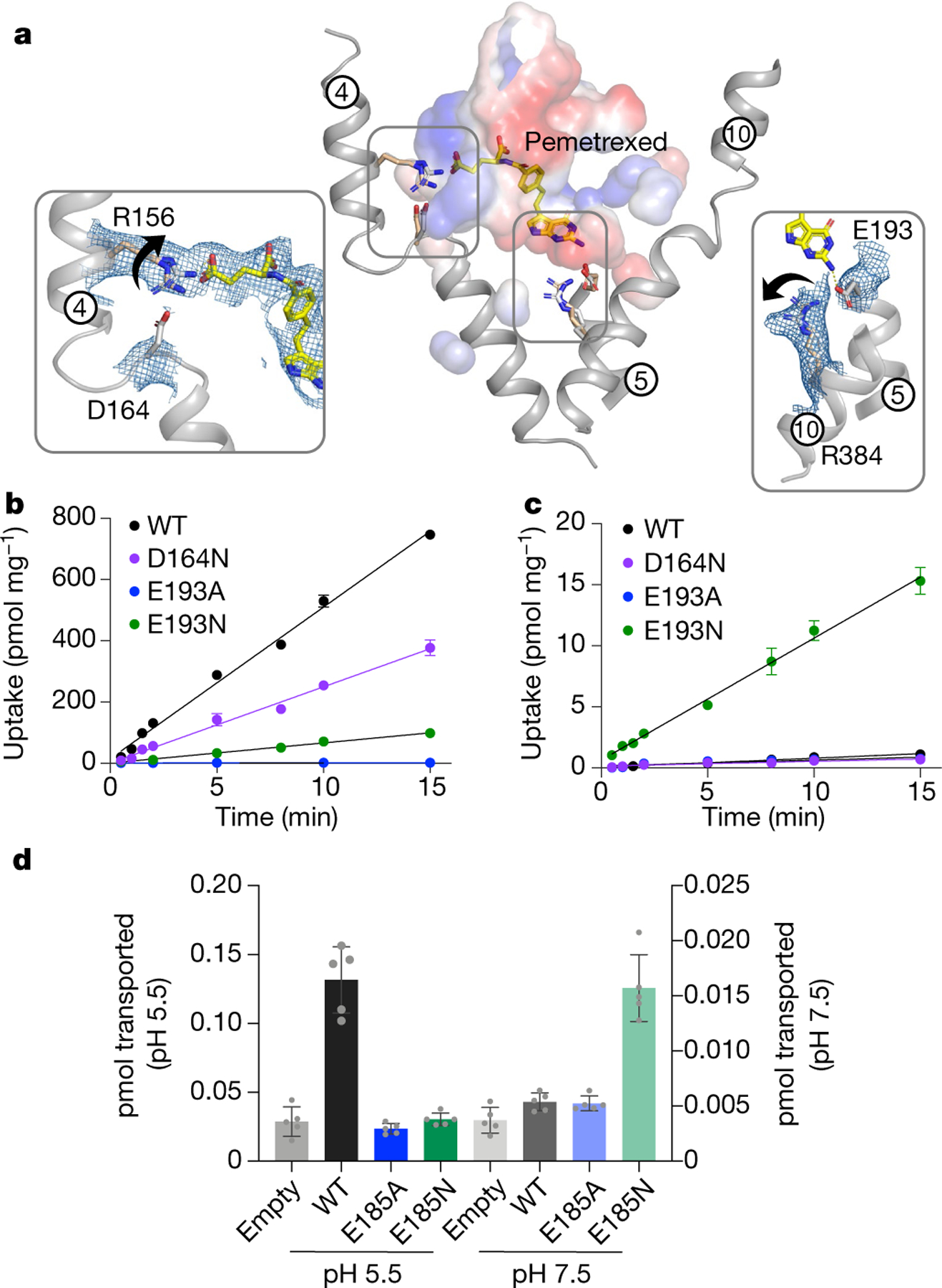
a, Structural comparison of the pemetrexed-bound and apo structure. Key side-chain rearrangements are observed after pemetrexed and proton binding. View is a direct comparison to Fig. 1d. Insets show the cryo-EM density for the two salt-bridge interactions, which are broken in the presence of substrate. Arrows indicate direction of side-chain movement. b, Initial rates of folic acid uptake into liposomes containing wild-type (WT) and variant forms of PCFT at pH 5.5. n = 3 independent experiments. c, As in b, but performed at pH 7.5 and highlighting the role of Glu193 in proton coupling. Internal pH of liposomes was maintained at 7.5. n = 3 independent experiments. d, Cell based transport assays for wild-type and variant human PCFT. n = 5 biologically independent experiments. In b–d, data are mean ± s.d.
