Extended Data Fig. 4 |. Analysis of PCFT–pemetrexed complex.
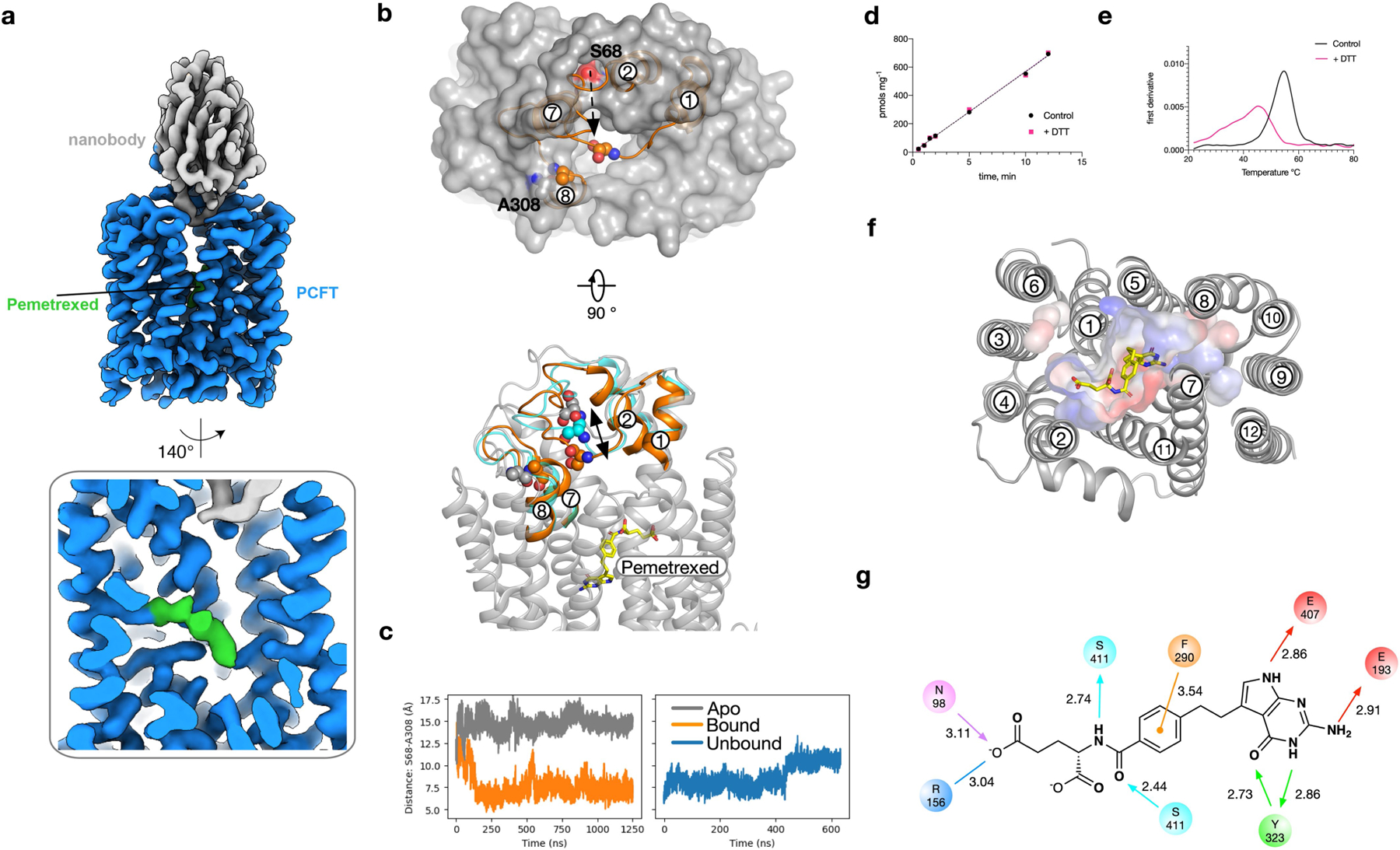
a, Cryo-EM density of PCFT in complex with pemetrexed and nanobody. b, In molecular dynamics simulations of the apo structure (grey), the TM1–TM2 loop is positioned such that the entrance to the binding pocket is accessible. In simulations with pemetrexed bound (orange), the loop closes quickly (within 250 ns) as measured by the relative position of Ser68 on the TM1–TM2 loop and Ala308 on the TM7–TM8 loop. c, Time course of the distance between the Cα atoms of Ser68 and Ala308 for both apo (grey) and pemetrexed-bound (orange) simulations. The closure event observed with pemetrexed bound occurs around 250 ns, and this closed conformation remains stable for the remainder of the simulation. On removal of pemetrexed from the end of the simulation shown in this panel (orange), the TM1–TM2 moves away from the TM7–TM8 loop (blue). The movement is shown in b (cyan). d, The presence of 2 mM DTT does not alter the uptake of folic acid into liposomes by PCFT, consistent with previous studies on the human transporter35. e, The presence of 2 mM DTT does lead to a destabilization of the protein as determined by differential scanning fluorimetry. f, View of pemetrexed within the binding cavity, with surface charge highlighted. g, Schematic of the binding pose observed for pemetrexed. Hydrogen-bond donors and acceptors are highlighted by directional arrows, the sole charge–charge interaction by a solid line and the π–π interaction in orange. Values indicate distance in Å.
