Abstract
Zearalenone (ZEN) is an estrogenic mycotoxin produced by the Fusarium species and induces severe reproductive disorders in animals thus a major concern in the livestock industry. Probiotic bacteria treatments have been shown to inactivate mycotoxins, therefore, in this study, we investigated the effect of two commercial probiotic feed additives on the sequestration of ZEN. Commercial probiotic blends containing clay-based binder with Aspergillus niger, Bacillus licheniformis, Bacillus pumilus, and Bacillus subtilis at various proportions from BioMatrix International were incubated with ZEN in a time-dependent manner and then analyzed by Enzyme-Linked Immunosorbent Assay (ELISA) to quantify unbound ZEN. Sequestration of ZEN was further verified by using MCF-7 cell-based cytotoxicity and/or cell proliferation assays. ZEN, or probiotic mix, was nontoxic to MCF-7 cells. Probiotic blends decreased ZEN concentration by 45% (∼100 μg L−1) and prevented ZEN from inducing MCF-7 cell proliferation (20%–28% reduction). The probiotic feed supplements tested show a potential utility in ZEN neutralization.
Keywords: zearalenone, probiotics, mycotoxins, feed additives, livestock health
Significance and impact of the study.
Mycotoxins in animal feed can significantly impact livestock health. Zearalenone (ZEN) is an estrogenic mycotoxin produced by the Fusarium species that induces severe reproductive disorders resulting in huge economic loss. Probiotic bacteria treatments have been shown to inactivate mycotoxins, therefore, in this study, we demonstrate the sequestration of ZEN by probiotics blend, which was further confirmed by MCF-7 cell-based cytotoxicity and/or cell proliferation assays. The results show the probiotic blend as feed supplements can substantially neutralize ZEN thus reducing the risk of reproductive disorders in livestock.
Introduction
Zearalenone (ZEN) is an estrogenic mycotoxin produced as a secondary metabolite by the Fusarium species. The mycotoxin can contaminate several different grains mainly maize (corn), barley, rice, and wheat, which are typically used as feed for livestock (Mahato et al. 2021). Corn being the major ingredient of animal feed, opportunities for contamination with ZEN are very high. Consequently, ZEN contamination can significantly affect the reproductive health of livestock (Velayudhan et al. 2015). In cattle and swine, ZEN induces decreased fertility, an abnormal estrus cycle, and swollen reproductive organs, including mammary glands. ZEN activity is regulated by its ability to bind to estrogen receptors, which are present in reproductive organs such as the uterus and mammary glands (Hueza et al. 2014). Thus, animals that consume ZEN experience reproductive disorders (Lei et al. 2014) leading to decreased livestock yield and economic losses. The fiscal effect of ZEN and other mycotoxins cost billions of dollars annually as crop yield decreases, reduced animal performance, and increased spending on fungicides (Loi et al. 2017). Therefore, there is a dire need for an intervention that can reduce the effects of ZEN to benefit the livestock industry and increase feed safety.
Recently, biological detoxification of these secondary metabolites has been favored over chemical or physical methods due to efficiency and environmental safety (Zhu et al. 2017). Microbial methods such as the usage of bacteria, enzymes, and other fungi are promising approaches for detoxification due to their specificity and a smaller number of toxic residues (Ji et al. 2016, Aalipanah et al. 2022). Probiotics have garnered attention in swine nutrition as feed additives since they can help maintain a healthy gut and improve growth performance by upregulating swine immune systems as well as increasing nutrient utilization (Liao and Nyachoti 2017). Therefore, their usage provides alternate benefits along with alleviating the toxicity of mycotoxins, proving their superiority in treatment options.
In the present research, a study was conducted to test the efficacy of two commercial probiotic blends (BioMatrix International, Princeton, MN, USA) in inhibiting ZEN under physiological conditions. The findings would allow the team to analyze the probiotic strain, which most efficiently disables ZEN. Furthermore, the data will serve as a stepping stone for carrying out the study in vivo. Here, we found both probiotic blends significantly reduced free ZEN and associated estrogenic properties.
Materials and methods
Sample preparation
Two proprietary probiotic sample blends (dried powder) containing a clay-based binder with Aspergillus niger, Bacillus licheniformis, Bacillus pumilus, and Bacillus subtilis at various proportions designated probiotic mixture-1 (Mix-1) and probiotic mixture-2 (Mix-2) with recommended commercial use of 2 and 0.2 lb ton−1 of feed, respectively, were obtained from BioMatrix International (Princeton, MN, USA). Both preparations were suspended in phosphate-buffered saline, pH 7.4 (PBS) at a stock concentration of 10 and 1 mg ml−1, respectively.
ZEN (Z2125-25MG) was purchased from Sigma–Aldrich (St. Louis, MO, USA) and reconstituted in dimethyl sulfoxide (DMSO; Fisher Scientific, Hampton, NH, USA) to a stock concentration of 25 mg ml−1. β-Estradiol (E2) from Sigma–Aldrich (St. Louis, MO, USA) was used as a positive control in the cytotoxicity and cell proliferation assays.
Effect of probiotic blends on ZEN
Probiotics blends (Mix-1 or Mix-2) were suspended in PBS and 100 μl of each was mixed with 100 μl ZEN and adjusted to a final volume of 1 ml with PBS. Samples were kept under constant agitation in a 37°C incubator. After 24, 48, 96, and 120 h incubation, samples were centrifuged (13 548 x g for 15 min) and supernatants (designated ZEN-Sup) were tested by ELISA and cell culture assays. In a separate experiment, 100 μl of ZEN (25 mg ml−1) suspended in 900 μl of PBS without probiotic blends was held at 37°C for 24–96 h with constant agitation as control and tested by ELISA.
Mycotoxin quantification by ELISA
An ELISA Kit (Veratox Max for ZEN, a direct competitive ELISA) was purchased from Neogen (Lansing, MI, USA) to quantify the amount of free ZEN in Mix-1 or Mix-2-treated samples that were incubated for 24 and 96 h (see "Effect of probiotic blends on ZEN" section). ZEN-Sup samples (100 μl well−1) were added to the wells of ELISA as per the manufacturer's instruction (Neogen) and ZEN concentration in each sample was calculated based on the standard curve generated using pure ZEN. Percent ZEN binding to probiotic blend was calculated as follows: the amount of toxin retained in the ZEN-Sup (unbound) ÷ amount of toxin in the standard × 100 = % unbound toxins. 100–% unbound toxin = % bound toxin.
Cytotoxicity assay
To determine if the probiotic supplements or ZEN-Sup have any cytotoxic effects, an MCF-7 cell line (breast cancer cell line) sensitive to ZEN was used (Tatay et al. 2018). Actively growing MCF-7 cells were suspended in Roswell Park Memorial Institute medium (RPMI-1640) (Thermo Fisher Scientific, Waltham, MA, USA) without fetal bovine serum and seeded on 96-well cell culture plates (Techno Plastic Products, Trasadingen, Switzerland) and grown in a humidified cell culture incubator at 37°C with a constant supply of 5% CO2 for 24–48 h or until 80% confluency is achieved. Filter sterilized (passed through 0.2 μm membrane filter; Amicon), ZEN-Sup diluted 1:10 in RPMI-1640 and incubated with the MCF cells for up to 120 h. MCF-7 cell supernatants were collected from each well and measured for lactate dehydrogenase (LDH) activity using an LDH Cytotoxicity Assay Kit (Cayman Chemical Company; Ann Arbor, MI, USA). A 1% Triton X-100 Surfact-Amps Detergent Solution (Thermo Fisher Scientific) capable of completely lysing MCF-7 monolayers for maximum LDH release served as a positive control.
Cell proliferation assay
As an estrogen analog, ZEN is known to induce cell proliferation. To assess whether the probiotics could mitigate the proliferative effects of ZEN, a BrdU (5-Bromo-2′-deoxyuridine) Cell Proliferation Assay Kit (Cell Signaling; Danvers, MA, USA) was used. MCF-7 cell monolayers were treated with ZEN-sup for 24–120 h in a humidified cell culture incubator at 37°C. The scheduled termination of the experiment 24 h before, BrdU reagent (10 μmol L−1) was added to each well, and the absorbance (450 nm) was measured according to manufacturer protocols.
Statistical analysis
Experimental data were analyzed using GraphPad Prism (La Jolla, CA, USA) software. Comparisons between treatments and controls were performed using the one-way or two-way analysis of variance with Tukey's multiple-comparison test. Unless otherwise indicated, data for all experiments are presented as the mean ± standard error of the mean (SEM) of three independent experiments.
Results and discussion
Immunoassay showed sequestration of ZEN by probiotic blends
This study aimed to determine the sequestration capabilities of various probiotic feed additives as a functional supplement for the biological detoxification of ZEN. Probiotics have merit as detoxifying agents in the gut due to bile tolerance and adhesion capabilities (Hsu et al. 2018). Thus, these microorganisms have recently gained traction as the preferred method of choice to eliminate mycotoxins, as they can survive the harsh conditions of the gut to inhibit toxins while concurrently strengthening the intestinal epithelial barrier (Wang et al. 2019, Drolia et al. 2020). In this study, we tested probiotics blend Mix-1 and Mix-2 (BioMatrix International) containing proprietary combinations of A. niger, B. licheniformis, B. pumilus, and B. subtilis at various concentrations for their ability to sequester ZEN.
ZEN-specific ELISA (competitive ELISA) produced a linear curve (R2 = 0.8375), when tested with a purified ZEN sample (data not shown)). Furthermore, ZEN samples suspended in PBS maintained their immunoreactivity when suspended at 37°C for 24–96 h (data not shown). ELISA results of probiotic blend samples showed a significant (P < 0.05) sequestration of ZEN by Mix-1 and Mix-2 treatments. At 24 h, the Mix-1 treatment showed a 40% reduction of ZEN (345.0 μg L−1) while the Mix-2 treatment achieved a 45% reduction of ZEN (306.6 μg L−1) when compared with the samples that contained ZEN only (497.6 μg L−1) (Fig. 1a). At 96 h, the Mix-1 treatment showed a 45% reduction of ZEN (262.8 μg L−1) while the Mix-2 treatment achieved a 40% reduction of ZEN (312.8 μg L−1) when compared with the sample that contained ZEN only (476.7μg L−1) (Fig. 1b). Taken together, these data indicate both probiotic blends are capable of sequestering ZEN.
Figure 1.
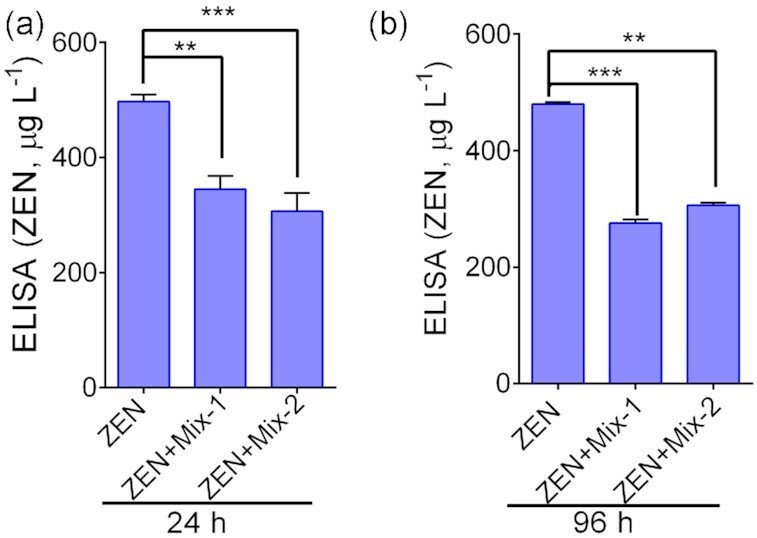
Direct competitive ELISA results showing sequestration of ZEN by probiotic blends. (a) Sequestration of ZEN by Mix-1 and Mix-2 at 24 h, and (b) at 96 h at 37°C posttreatments. The data are an average of three independent trials. ***, P < 0.001 and **P < 0.01
Probiotics blend-treated ZEN samples were nontoxic
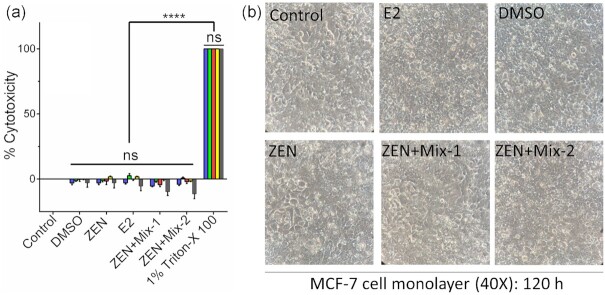
The Cytotoxic effects, if any, of ZEN-Sup or free ZEN samples were evaluated on the MCF-7 cell line. As shown in Fig. 2, both Mix-1 and Mix-2-treated ZEN samples (ZEN-Sup) did not show any cytotoxic response on MCF-7 cell monolayers as measured by LDH release. Furthermore, ZEN alone (250 ng ml−1) or β-Estradiol (E2) (10 nmol L−1) also did not show any cytotoxicity. DMSO as a vehicle had no cytotoxic effect (Fig. 2a). Likewise, microscopic images of MCF-7 cell monolayers also did not reveal any cell damage or changes in cell morphology (Fig. 2b). These data indicate that both ZEN and E2 are nontoxic, and even probiotic blend treatment did not affect ZEN's response to MCF-7 cells. Based on these results, the working concentration of 10 nmol L−1E2 and 250 ng mL−1 ZEN was chosen for subsequent cell proliferation experiments
Figure 2.
Cytotoxic effect of probiotic blends (Mix-1 and Mix-2) treated ZEN samples on the MCF-7 cell lines exposed for 24–120 h. (a) Percent LDH releases at ( ) 24, (
) 24, ( ) 48, (
) 48, ( ) 72, (
) 72, ( ) 96, and (
) 96, and ( ) 120 h, (b) light microscopic photographs. The data are an average of 4 samples. ****, P < 0.0001. E2, Estradiol, DMSO.
) 120 h, (b) light microscopic photographs. The data are an average of 4 samples. ****, P < 0.0001. E2, Estradiol, DMSO.
Probiotics blend treatment reduced ZEN-induced MCF-7 cell proliferation
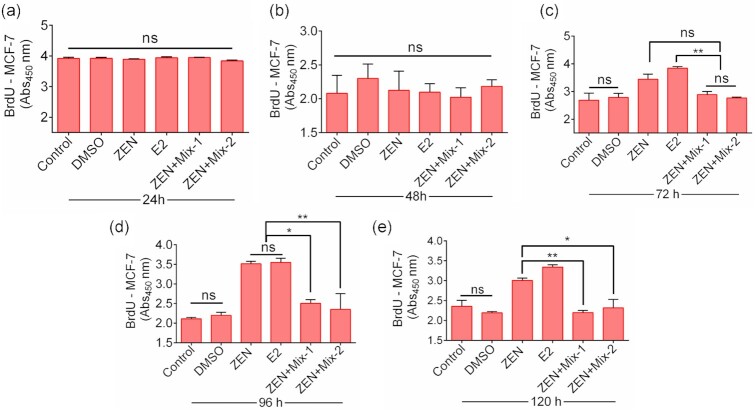
Both ZEN and β-Estradiol (E2, as positive control) promote the proliferation of cells in reproductive tissues. Thus, we assessed whether treatment with probiotics blends (Mix-1 and Mix-2) could mitigate the cell proliferation associated with phytoestrogen compounds (ZEN and E2) using BrdU incorporation assays. MCF-7 cells (breast cancer cell line) were chosen as the ideal model, as this cell line exhibits estrogen receptors and thus responds well in the presence of phytoestrogens (Tatay et al. 2018). Zen alone does not induce any cytotoxic effect on these cells when treated for up to 120 h (Fig. 2); therefore, we analyzed the cell proliferative response of MCF-7 cells for 120 h (Fig. 3).
Figure 3.
Cell proliferative effect of probiotic blends (Mix-1 and Mix-2)-treated ZEN samples on the MCF-7 cell line exposed for 24 (a), 48 (b), 72 (c), 96 (d), and 120 h (e) analyzed by BrdU incorporation assay. E2, estradiol, DMSO. The data are the average absorbance values from three independent trials. ** P < 0.001 and * P < 0.05.
ZEN or E2 treatment for 24 or 48 h did not show any noticeable cell proliferation (Fig. 3ab) while a sharp decrease in incorporation of BrdU was detected after 72 h (20% reduction) (Fig. 3c) of ZEN-Sup treatment compared to ZEN and E2, which continued until 120 h (27%–28% reduction) posttreatment (Fig. 3de). These data confirm the cell proliferative response of ZEN on MCF-7 cells, can be significantly (P < 0.05) reduced by pretreatment of ZEN by probiotics blends (Mix-1 and Mix-2).
ZEN is known to accelerate the proliferation of MCF-7 cells due to its estrogenic properties (Yip et al. 2017). Thus, we expected reduced incorporation of BrdU when ZEN-exposed MCF-7 cells were supplemented with probiotic feed additives. Here, probiotic blends Mix-1 and Mix-2 decreased cell proliferation by 20%–28% compared to ZEN and estradiol in a time-dependent fashion (Fig. 3c–e).
The mechanism of action for neutralizing ZEN can vary from biotransformation to degradation; however, not all biotransformation products are free from estrogenic activity (Wang et al. 2019). Surprisingly, we found probiotic feed additives Mix-1 and Mix-2 were able to reduce ZEN activity as demonstrated by ELISA. Several different mechanisms of inactivation are being proposed, such as the interaction between mycotoxin and teichoic acid on the probiotic cell wall, allowing mycotoxin sequestration (Hernandez‐Mendoza et al. 2009), and enzymatic degradation (Ji et al. 2016). Reduced interaction of ZEN with MCF-7 cells led to lower cell proliferation as demonstrated by reduced BrdU incorporation.
As the use of probiotics to control mycotoxin contamination increases, novel strains will continue to be discovered and optimized. These biological agents will no doubt see an increased application in animal feed safety. In this study, the probiotic blends were able to successfully reduce the harmful effects of ZEN contamination in an in vitro approach, thus providing a viable option for controlling ZEN contamination in feed thus benefiting the livestock industry.
ACKNOWLEDGEMENTS
The authors acknowledge the technical assistance of Raghu Vishweswaraiah, Nicholas Gallina, Dongqi Liu, and Manalee Samaddar.
Contributor Information
Vignesh B Nathan, Molecular Food Microbiology Laboratory, Department of Food Science, Purdue University, West Lafayette, IN 47907, USA.
Hang Lu, Research and Development, United Animal Health, Sheridan, IN 46069, USA.
Nathan L Horn, Research and Development, United Animal Health, Sheridan, IN 46069, USA.
Rishi Drolia, Molecular Food Microbiology Laboratory, Department of Food Science, Purdue University, West Lafayette, IN 47907, USA; Department of Biological Science, Eastern Kentucky University, Richmond, KY 40475, USA; Purdue Institute of Inflammation, Immunology and Infectious Disease, Purdue University, West Lafayette, IN 47907, USA.
Arun K Bhunia, Molecular Food Microbiology Laboratory, Department of Food Science, Purdue University, West Lafayette, IN 47907, USA; Purdue Institute of Inflammation, Immunology and Infectious Disease, Purdue University, West Lafayette, IN 47907, USA; Department of Comparative Pathobiology, College of Veterinary Medicine, Purdue University, West Lafayette, IN 47907, USA.
Conflict of interest
The authors, H.L. and N.L.H., were previously employed by BioMatrix International.
Funding
This research was supported by funds from the USDA National Institute of Food and Agriculture (Hatch accession no. 1016249), BioMatrix, Inc., and NIGMS grant # P20GM103436.
Authors contribution
Vignesh B. Nathan (Conceptualization, Formal analysis, Methodology, Writing – original draft), Hang Lu (Methodology, Project administration), Nathan L. Horn (Conceptualization, Methodology, Project administration), Rishi Drolia (Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Supervision), and Arun K. Bhunia (Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Project administration, Supervision, Writing – original draft, Writing – review & editing).
Data availability
All major data are presented in the manuscript and unpublished data are available upon request.
References
- Aalipanah S, Fazeli MR, Akhavan Sepahi A et al. Synergistic effects of probiotic bifidobacterium isolated from chicken's intestine in combination with polyvinylpyrrolidone on reduction of aflatoxin B1. Lett Appl Microbiol. 2022;75:1160–70.. 10.1111/lam.13783 [DOI] [PubMed] [Google Scholar]
- Drolia R, Amalaradjou MAR, Ryan V et al. Receptor-targeted engineered probiotics mitigate lethal Listeria infection. Nat Commun. 2020;11:6344. 10.1038/s41467-020-20200-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Mendoza A, Guzman-de-Peña D, Garcia HS. Key role of teichoic acids on aflatoxin B1 binding by probiotic bacteria. J Appl Microbiol. 2009;107:395–403.. 10.1111/j.1365-2672.2009.04217.x [DOI] [PubMed] [Google Scholar]
- Hsu T-C, Yi P-J, Lee T-Y et al. Probiotic characteristics and zearalenone-removal ability of a Bacillus licheniformis strain. PLoS One. 2018;13:e0194866. 10.1371/journal.pone.0194866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hueza IM, Raspantini PCF, Raspantini LER et al. Zearalenone, an estrogenic mycotoxin, is an immunotoxic compound. Toxins. 2014;6:1080–95.. 10.3390/toxins6031080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji C, Fan Y, Zhao L. Review on biological degradation of mycotoxins. Anim Nutr. 2016;2:127–33.. 10.1016/j.aninu.2016.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei YP, Zhao LH, Ma QG et al. Degradation of zearalenone in swine feed and feed ingredients by Bacillus subtilis ANSB01G. World Mycotoxin J. 2014;7:143–51.. 10.3920/WMJ2013.1623 [DOI] [Google Scholar]
- Liao SF, Nyachoti M. Using probiotics to improve swine gut health and nutrient utilization. Anim Nutr. 2017;3:331–43.. 10.1016/j.aninu.2017.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loi M, Fanelli F, Liuzzi VC et al. Mycotoxin biotransformation by native and commercial enzymes: present and future perspectives. Toxins. 2017;9:111. 10.3390/toxins9040111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahato DK, Devi S, Pandhi S et al. Occurrence, impact on agriculture, human health, and management strategies of zearalenone in food and feed: a review. Toxins. 2021;13:92. 10.3390/toxins13020092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatay E, Espín S, García-Fernández A-J et al. Estrogenic activity of zearalenone, α-zearalenol and β-zearalenol assessed using the E-screen assay in MCF-7 cells. Toxicol Mech Methods. 2018;28:239–42.. 10.1080/15376516.2017.1395501 [DOI] [PubMed] [Google Scholar]
- Velayudhan DE, Kim IH, Nyachoti CM. Characterization of dietary energy in swine feed and feed ingredients: a review of recent research results. Asian-Australasian J Anim Sci. 2015;28:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Wu W, Pan J et al. Detoxification strategies for zearalenone using microorganisms: a review. Microorganisms. 2019;7:208. 10.3390/microorganisms7070208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip KY, Wan MLY, Wong AST et al. Combined low-dose zearalenone and aflatoxin B1 on cell growth and cell-cycle progression in breast cancer MCF-7 cells. Toxicol Lett. 2017;281:139–51.. 10.1016/j.toxlet.2017.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Hassan YI, Lepp D et al. Strategies and methodologies for developing microbial detoxification systems to mitigate mycotoxins. Toxins. 2017;9:130. 10.3390/toxins9040130 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All major data are presented in the manuscript and unpublished data are available upon request.