Abstract
Telomerase is a ribonucleoprotein enzyme that adds repetitive sequences to the ends of linear chromosomes, thereby counteracting nucleotide loss due to incomplete replication. A short region of the telomerase RNA subunit serves as template for nucleotide addition onto the telomere 3′ end. Although Saccharomyces cerevisiae contains only one telomerase RNA gene, telomere repeat sequences are degenerate in this organism. Based on a detailed analysis of the telomere sequences specified by wild-type and mutant RNA templates in vivo, we show that the divergence of telomere repeats is due to abortive reverse transcription in the 3′ and 5′ regions of the template and due to the alignment of telomeres in multiple registers within the RNA template. Through the interpretation of wild-type telomere sequences, we identify nucleotides in the template that are not accessible for base pairing during substrate annealing. Rather, these positions become available as templates for reverse transcription only after alignment with adjacent nucleotides has occurred, indicating that a conformational change takes place upon substrate binding. We also infer that the central part of the template region is reverse transcribed processively. The inaccessibility of certain template positions for alignment and the processive polymerization of the central template portion may serve to reduce the possible repeat diversification and enhance the incorporation of binding sites for Rap1p, the telomere binding protein of budding yeast.
Telomeres protect the ends of the linear eukaryotic chromosomes from end-to-end fusions and serve as buffer zones against sequence loss due to incomplete replication (1). They are maintained by the ribonucleoprotein enzyme telomerase, a cellular reverse transcriptase that uses a specific region of its RNA subunit as template for DNA synthesis (16, 17, 32, 58). The template region of the RNA is copied repeatedly onto the 3′ ends of the chromosomes, thus specifying the telomere repeats. The ability to maintain telomeres is a prerequisite to undergo unlimited rounds of replication (33), and reactivation of telomerase is seen in more than 80% of human tumors (23). In the yeast Saccharomyces cerevisiae, the RNA subunit of telomerase is encoded by TLC1 (49) and the catalytic protein subunit is encoded by EST2 (8, 32). EST1 and EST3 encode other telomerase-associated proteins essential for telomere maintenance in vivo but dispensable for telomerase activity in vitro (4, 22, 28, 31, 33, 45).
Several organisms, including some protozoa, fungi, slime molds, and plants, have irregular telomere repeat sequences (56). The degeneracy is most pronounced in S. cerevisiae and Schizosaccharomyces pombe with the telomere consensus sequences (TG)1–4G2-3 (5, 48, 52, 54) and GGTTACA(G)1–4, respectively (21). Several models have been put forward to explain the synthesis of variable telomeric repeats with only one RNA template (6). Slippage of the template in a stretch of four G/C base pairs was proposed to account for the synthesis of poly(dG) observed in vitro with Tetrahymena thermophila telomerase, whereas a high frequency of nucleotide misincorporation at a specific template position appears responsible for the mixed synthesis of T2G4 and T3G3 repeats in Paramecium tetraurelia (39). The diversity of S. cerevisiae telomere repeats was proposed to be due to multiple possible alignment registers between telomere and template as well as abortive reverse transcription (49). This hypothesis is tested here.
Telomerases from different species differ in their repeat addition processivity, i.e., their ability to add multiple telomere repeats to the substrate in a single binding event. Enzymes isolated from human and hamster cells, T. thermophila, Euplotes aediculatus, and Saccharomyces castellii have processive polymerization characteristics, whereas the telomerases from S. cerevisiae, Kluyveromyces lactis, and S. pombe add maximally one telomere repeat per binding event in vitro (4, 10, 15, 18, 19, 27, 35, 51). The mechanisms causing the observed differences remain to be elucidated. A Tetrahymena telomerase catalytic subunit mutant with a leucine-to-tyrosine substitution close to the active site showed increased processivity, suggesting an important role for the catalytic core in processive synthesis (2).
The telomerase RNA serves as a passive template for substrate annealing and reverse transcription but also appears to play an active role in catalysis (6). Certain nucleotides in the template region can be substituted without affecting enzymatic activity in vitro (11, 42, 44, 55) or in vivo (24, 38–40, 43, 49, 50, 58), whereas other substitutions lead to loss of telomerase activity (39, 43, 57) or repeat addition processivity (11, 12, 55). In addition, S. cerevisiae telomerase RNAs functionally interact in enzyme multimers containing two different template RNA molecules (42). The molecular basis for this interaction is not understood.
Telomere length in yeast is negatively regulated by the double-stranded telomere binding protein Rap1p (7, 20, 26, 34, 37). Short telomeres that have few Rap1 molecules bound are more efficiently extended by telomerase than are longer telomeres that have reached their equilibrium length (36). Upon clonal expansion, the sequence of a given telomere remains constant in the centromere-proximal region but diverges within the last 80 to 100 nucleotides (nt) at its distal end (9, 54). Divergence is dependent on telomerase activity (9), and therefore, this region demarcates the dynamic zone where telomere shortening and telomerase-mediated extension occur. Since in the absence of telomerase only 3 to 5 nt are lost per generation, which is considerably less than the region of repeat divergence, telomerase does not extend a given telomere in every cell cycle. Instead, S. cerevisiae telomeres appear to be elongated during short periods of efficient extension separated by a number of replication cycles with gradual telomere shortening. Alternatively, extensive nucleolytic processing of the telomere may depend on the presence of telomerase.
The length of artificially shortened telomeres increased initially by, on average, 15 nt in the first generation (36). Taking into account the sequence losses due to incomplete replication, this suggests that S. cerevisiae telomerase can add at least 18 to 20 nt to a telomere in one cell cycle. Since the entire TLC1 template region contains only 16 nt, either multiple repeats are added in a processive fashion by a single telomerase enzyme or single repeats are added in successive binding events by multiple telomerases during telomere elongation in vivo. In vitro, S. cerevisiae telomerase does not show repeat addition processivity. It adds maximally the number of nucleotides present between the position of alignment and the 5′ boundary of the template. The extended substrate oligonucleotide then stays associated with the telomerase enzyme in a stable manner (42).
In this study, we elucidate the mechanisms by which yeast telomerase specifies divergent telomere repeats in vivo. To this end, we transformed tlc1-Δ cells with plasmids encoding mutant TLC1 RNA templates and subsequently analyzed the induced changes in the telomere sequence patterns. This analysis indicated that redundant alignment possibilities within the template RNA and abortive reverse transcription events both contribute to the synthesis of divergent repeats as hypothesized previously (49). The analysis of the mutants allowed us to deduce the template utilization by yeast telomerase and in turn to interpret wild-type (WT) telomere sequences, which serve as written traces of the enzyme's action in vivo. We determined the alignment probabilities along the WT template region and found that positions 479C to 477C of the template are inaccessible for substrate binding but subsequently become available for base pairing during reverse transcription. In addition, we infer that the enzyme shows abortive reverse transcription in the 3′ and 5′ parts of the template region but does not allow product dissociation during copying of the central part of the template.
MATERIALS AND METHODS
TLC1 mutagenesis.
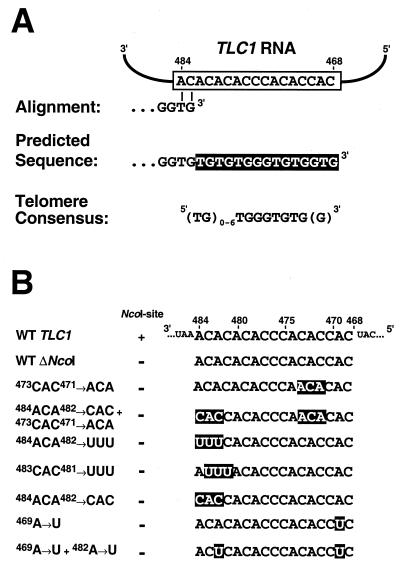
The NcoI-NsiI fragment of TLC1 containing the template region was PCR amplified from pSD107 (derived from pRS314 by inserting a genomic fragment containing the TLC1 gene; obtained from D. Gottschling) with a sense primer containing a BspHI site instead of the NcoI site and an antisense primer. Ligation of the BspHI-NsiI-digested PCR product into the compatible ends of NcoI-NsiI-digested pSD107 led to destruction of the original NcoI site in the TLC1 gene, thus simplifying identification of mutant clones. The sequence of the sense primer was 5′-TAATTATCATGAGAAGCCTACCATCCATCACCACACCCACACACAAATGTTAC-3′. The underlined sequence corresponds to the TLC1 template region. It was changed according to Fig. 1B to introduce the various mutations. The sequence of the antisense primer was 5′-TATCTAAATGCATCGAAGGCATTAG-3′ with the NsiI site indicated in italics. All constructs were sequenced to confirm the presence of the desired mutations. The plasmids coding for the different mutations were named pKF5 (WT template but ΔNcoI, i.e., 455G→A), pKF6 (484ACA482→UUU + 455G→A), pKF7 (483CAC481→UUU + 455G→A), pKF8 (469A→U + 455G→A), pKF9 (469A→U + 482A→U + 455G→A), pKF10 (484ACA482→CAC + 455G→A), pKF11 (473CAC471→ACA + 455G→A), and pKF12 (484ACA482→CAC + 473CAC471→ACA + 455G→A).
FIG. 1.
(A) The telomere consensus sequence does not correspond to perfect repeats of the sequence specified by the TLC1 template RNA. (B) TLC1 template mutants used in this study. Mutant nucleotides are highlighted. In order to facilitate the cloning, the NcoI restriction site 3′ of the template was destroyed in the mutants by ligation with compatible BspHI ends. This results in a single 455A→G nucleotide change.
Telomere mutagenesis and sequence analysis.
The plasmids pKF5 to pKF12 and pSD107 were transformed into yeast strain YKF19 (ade2 his3-11 can1Δ leu2 trp1 ura3-52 DIA5-1 [ADE2 telomere VR] tlc1::HIS3 rad52::LEU2) as the cells underwent senescence. The colonies obtained after rescue were restreaked once, and telomere VR was amplified, cloned, and sequenced as described previously (9). The following telomere clones were obtained: pKF5, pktel 56, 57, 58, 59, 61, 62, 63, 159b, 160, 161, 162, and 163 (12 clones); pKF6, pktel 103, 104, 106, 107, 151, 153, 174, 175, and 176 (9 clones); pKF7, pktel 166, 169, 170, 171, 172, 176, 177, 178, 179, and 180 (10 clones); pKF8, pktel 83, 84, 86, 135, 136, and 138 (6 clones); pKF9, pktel 81, 95, 96, 100, 101, 131, and 134 (7 clones); pKF10, pktel 159a, 178, and 179 (3 clones); pKF11, pktel 55 and 56 (2 clones); and pKF12, pktel 51, 52, and 148 (3 clones).
To identify newly incorporated sequences, the telomeres were aligned with a telomere cloned from an earlier passage of strain YKF19 (pktel120) with the GCG software and sequences distal to the point of divergence from pktel120 were analyzed as described in Results. The χ2 analysis for the spacer length distributions was performed with Microsoft Excel. Telomere sequences from the Saccharomyces Genome Database (http://genome-www.stanford.edu/Saccharomyces/) were retrieved for telomeres IL, IR, IIR, IIIL, IIIR, IVL, VIR, VIIL, VIIIL, VIIIR, IXL, IXR, XL, XR, XIL, XIR, XIIL, XIIIL, XIIIR, XIVR, XVL, and XVR.
Telomerase preparation and in vitro assay.
S. cerevisiae telomerase was prepared essentially as described previously (4, 43). Briefly, cells from 500-ml overnight cultures in synthetic medium lacking tryptophan were harvested at an optical density at 600 nm of 1 and lysed in 2 ml of buffer L (20 mM Tris-HCl [pH 8.0], 500 mM NaAc, 1.1 mM MgCl2, 0.1 mM EDTA, 1.5 mM dithiothreitol [DTT], 0.1% Triton X-100, 0.2% NP-40, 10% glycerol, 1 mM phenymethylsulfonyl fluoride [PMSF], 60 U of RNAguard [Pharmacia]) by grinding them for 5 min with dry ice in a coffee grinder (MioStar) as described previously (41). After centrifugation at 5,500 × g for 2 min, the supernatant was collected and the protein concentration was adjusted to 10 mg/ml with TMG-500 (10 mM Tris-HCl [pH 8.0], 500 mM NaAc, 1.1 mM MgCl2, 0.1 mM EDTA, 1.5 mM DTT, 0.1% Triton X-100, 10% glycerol, 0.1 mM PMSF). The extract (20 to 50 mg of total protein) was batch adsorbed during 30 min at 4°C to 1 ml of DEAE-Sepharose Fast Flow (Pharmacia) equilibrated in TMG-500. The resin was pelleted by centrifugation at 400 × g for 20 s and washed three times with 13 ml of TMG-500. After the last wash, 2 ml of TMG-900 (10 mM Tris-HCl [pH 8.0], 900 mM NaAc, 1.1 mM MgCl2, 0.1 mM EDTA, 1.5 mM DTT, 0.1% Triton X-100, 10% glycerol, 0.1 mM PMSF) was added to the resin. After 15 min of agitation at 4°C, the resin was pelleted by centrifugation at 5,500 × g for 5 min. The supernatant was loaded onto a Sephadex G-25 desalting column (2-ml bed volume) equilibrated with TMG-30 (10 mM Tris-HCl [pH 8.0], 30 mM NaAc, 1.1 mM MgCl2, 0.1 mM EDTA, 1.5 mM DTT, 0.1% Triton X-100, 10% glycerol, 0.1 mM PMSF) and eluted into a centrifugal microconcentrator device (Ultrafree 4; Millipore; molecular mass cutoff, 30 kDa). The telomerase preparation was concentrated to 40 μl, mixed with an equal volume of glycerol, and stored in aliquots at −70°C. The amount of TLC1 RNA contained in the different preparations was determined by Northern hybridization.
Telomerase reactions were carried out in 10-μl reaction mixtures with final concentrations of 20 mM Tris-HCl (pH 8.0); 25 mM NaCl; 1 mM DTT; 1 mM spermidine; 1 mM MgCl2; 1 U of RNAguard; 50 μM dATP, dCTP, and dGTP; 5 μM dTTP; 10 μCi of [α-32P]dTTP (Amersham; 3,000 mCi/mmol); and up to 50% (vol/vol) of telomerase fraction. The reaction mixture was incubated at 30°C for 45 min and stopped by addition of 200 μl of proteinase K buffer (20 mM Tris-HCl [pH 8.0], 1 mM EDTA, 0.5% sodium dodecyl sulfate, 250 ng of proteinase K/μl). Trace amounts of a labeled oligonucleotide (32 nt) were added to control for precipitation efficiency and gel loading. After digestion at 30°C for 60 min, the samples were extracted with phenol-chloroform and precipitated with ethanol in the presence of 2.5 M NH4Ac and 30 μg of glycogen (Roche Molecular Biochemicals) as carrier. After two washes with 70% ethanol, the pellets were dried and subsequently dissolved in 5 μl of formamide loading buffer. The reaction products were analyzed on 14% acrylamide-urea sequencing gels. Quantification of the bands was performed on a Fuji BAS PhosphorImager.
Nucleotide sequence accession number.
The telomere clone sequences were submitted to GenBank, and their accession numbers are AF371374 to AF371439.
RESULTS
Repeating sequence in baker's yeast telomeres.
The S. cerevisiae telomerase RNA is predicted to specify the synthesis of the sequence 5′-TGTGTGGGTGTGGTG-3′ if a substrate were to anneal with its 3′ end at position 483 and if reverse transcription occurred from position 482 to 468 (Fig. 1A). However, yeast telomeres do not consist of perfect tandem arrays of this sequence. Rather, a consensus can be defined as (TG)1–4G2-3 (48, 52, 54). Examination of yeast telomere sequences determined in this study and present in the public database (Table 1) revealed additional constraints that could be included in the consensus definition. The heptanucleotide 5′-TGGGTGT-3′ sequence is present at an average spacing of 11 nt. With this core sequence, approximately 90% of the telomere sequences can be broken up into individual repeats [Table 1, columns TGGGTGTGGT and TGGG(TG)nT]. Most of the remaining sequences can be attributed to three additional repeat types [Table 1, columns TGGTGGGT, GGGG, and TGG(TG)nTGGT]. The analysis revealed that the [TGGGTGT] core sequence is preceded by a variable number of TG dinucleotides (Table 2) and followed in 50% of all cases by a GG dinucleotide as predicted from the telomerase RNA template [Table 1, columns TGGGTGTGGT and TGGG(TG)n T]. Thus, a more precise consensus for S. cerevisiae telomere sequences is 5′-[(TG)0–6TGGGTGTG(G)]n-3′ (Fig. 1A).
TABLE 1.
Occurrence of repeat types in S. cerevisiae telomere sequences
| TLC1 allele | Total nt evaluated (avg telomere length ± SD) | Total no. of telomeric repeats identified (repeat length in nt) | % Frequency (absolute no.) of repeat type:
|
|||||
|---|---|---|---|---|---|---|---|---|
| TGGGTGTGGT | TGGG(TG)nT | TGGTGGGT | GGGG | TGG (TG)n TGGT | Other | |||
| Genome sequence | 2,407 (NAa) | 215 (11.2) | 49 (105) | 35 (75) | 1 (3) | 1 (2) | 12 (26) | 2 (4) |
| WT | 727 (222 ± 38) | 68 (10.7) | 51 (35) | 41 (28) | 1 (1) | 0 | 4 (3) | 1 (1) |
| WT ΔNcoI | 1,333 (244 ± 26) | 121 (11.0) | 58 (70) | 32 (39) | 2 (2) | 0 | 5 (6) | 3 (4) |
| 473CAC471→ACA | 160 (268 ± 13) | 14 (11.4) | 0 (0)b | 100 (14)c | 0 | 0 | 0 | 0 |
| 484ACA482→CAC + 473CAC471→ACA | 291 (208 ± 28) | 30 (9.7) | 13 (4)b | 83 (25)c | 0 | 0 | 0 | 3 (1) |
| 484ACA482→UUU | 1,371 (247 ± 60) | 125 (11.0) | 51 (64) | 33 (41) | 1 (1) | 2 (2) | 10 (12) | 4 (5) |
| 483CAC481→UUU | 511 (175 ± 13) | 48 (10.6) | 31 (15) | 44 (21) | 0 | 2 (1) | 21 (10) | 3 (1) |
| 484ACA482→CAC | 323 (261 ± 81) | 34 (9.5) | 56 (19) | 41 (14) | 0 | 3 (1) | 0 | 0 |
| 469A→U | 884 (212 ± 35) | 85 (10.4) | 35 (30)d | 63 (54) | 0 | 0 | 0 | 2 (1) |
| 469A→U + 482A→U | 532 (242 ± 39) | 73 (7.3) | 18 (13) | 81 (59) | 0 | 0 | 0 | 1 (1) |
NA, not applicable.
Sequence GGGTT(GT)nGGT.
Sequence GGGTT(GT)n.
Contains also GGGTGTGGA sequences.
TABLE 2.
Distance in TG dinucleotides between -GGGTGTGG and the next -TGGG element
| Sequence | % Frequency (absolute no.) of spacing:
|
Distribution like that of genome sequence (P) | ||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||
| Genome sequence | 3 (3) | 25 (24) | 39 (37) | 19 (18) | 14 (13) | 1.00 |
| WT | 3 (1) | 25 (9) | 36 (13) | 22 (8) | 14 (5) | 0.99 |
| WT ΔNcoI | 3 (2) | 30 (18) | 34 (21) | 23 (14) | 10 (6) | 0.88 |
| 484ACA482→UUU | 2 (1) | 31 (17) | 44 (24) | 15 (8) | 7 (4) | 0.65 |
| 483CAC481→UUU | 0 (0) | 60 (6) | 20 (2) | 20 (2) | 0 (0) | 0.17 |
| 484ACA482→CAC | 0 (0) | 89 (17) | 5 (1) | 5 (1) | 0 (0) | <0.01 |
In the following, we first define the templating function of various nucleotides and present a model for the molecular events responsible for S. cerevisiae telomere sequence divergence. Second, we will use the model to interpret WT telomere sequences. We infer substrate alignment probabilities for most template positions and identify template regions where product dissociation can occur as well as template portions that are reverse transcribed processively.
Incorporation of GG dinucleotides and GGG trinucleotides depends on reverse transcription of specific template positions.
To elucidate the templating function of TLC1, several mutant tlc1 alleles (Fig. 1B) were generated on centromeric plasmids and introduced at the onset of senescence into yeast cells carrying a deletion of the chromosomal TLC1 and RAD52 genes. All plasmids rescued the cells from senescence with comparable efficiency (data not shown). Telomeres were amplified and cloned by telomere PCR (9). The newly incorporated sequences were identified by their divergence from the original WT telomere sequence.
Telomeres cloned from cells carrying a 473CAC471→ACA mutant tlc1 allele (Fig. 1B), which lacks a CC dinucleotide in the template sequence, were devoid of GG dinucleotides (Table 1). On the other hand, in the mutant 482ACA480→CAC + 473CAC471→ACA, where a CC dinucleotide is present at positions 480 to 479, GG dinucleotides were again incorporated into telomeres (Table 1). These results indicate that the telomeric GG sequence is specified by the CC dinucleotide, corresponding to positions 470C and 471C in the WT template. It is not generated by product dissociation during the reverse transcription of the 477CCC475 trinucleotide.
In cells expressing 469A→U mutant tlc1 or the 469A→U + 482A→U double mutant, the incorporated adenine nucleotides were found only adjacent to GG dinucleotides and never adjacent to a GGG trinucleotide. Thus, the incorporation of GGG trinucleotides is most likely generated by reverse transcription of template positions 477CCC475 rather than through slippage during the copying of positions 471CC470. This validates the use of the TGGGTGT core sequence to define individual telomeric repeats, as the GGG trinucleotide can be incorporated only once per substrate binding event.
Multiple alignment registers contribute to the length variability of telomeric repeats.
The 3′ portion of the template region consists of a stretch of CA dinucleotides (Fig. 1A), and it has been proposed elsewhere that it may allow multiple alignment possibilities for telomeres ending with -GTG-3′, the predicted product of a complete extension cycle (49) (see also Fig. 3). Such variable alignment may give rise to the variable length of the (TG)n spacer found between either a GG dinucleotide and the following GGG trinucleotide or two adjacent GGG trinucleotides. To test this hypothesis, we constructed mutant tlc1 alleles (Fig. 1B) that were predicted to reduce the number of possible alignments within the 3′ region of the template (484ACA482→UUU, 483CAC481→UUU, and 484ACA482→CAC) and determined the sequence of the specified telomeric repeats.
FIG. 3.
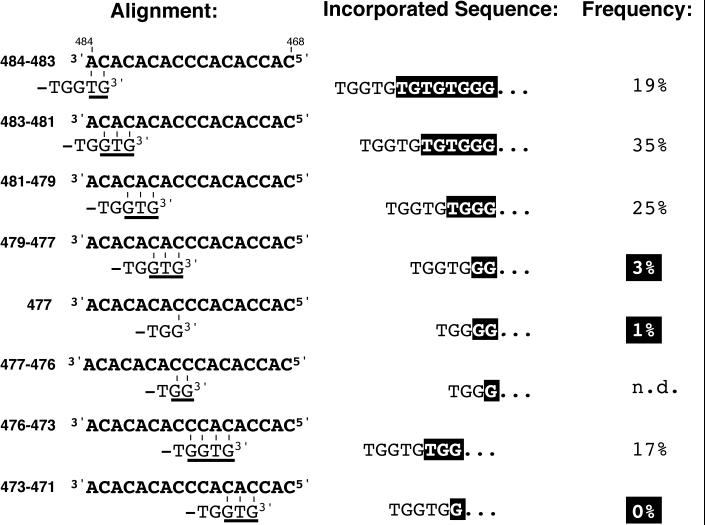
Alignment possibilities for telomeric 3′ ends containing a GG dinucleotide along the WT template region. The alignment positions within TLC1 are indicated on the left. Underlined nucleotides indicate positions of ambiguity for the alignment (see text). Incorporated telomere sequences resulting from the alignment indicated on the left are highlighted in the middle. Relative frequencies of the indicated telomere sequence in the pooled WT telomeres (genome sequence, WT TLC1, and tlc1 ΔNcoI) are indicated on the right. n.d., not determined.
Reverse transcription of template positions 471C470C is responsible for the incorporation of GG dinucleotides into the telomeres. The (TG)n spacer between a GG dinucleotide and the following GGG trinucleotide therefore contains maximally one TG dinucleotide contributed by the 5′ end of the template region (positions 469A468C). Telomere repeats that do not contain the GG dinucleotide are caused by product dissociation before reverse transcription of template position 470C (see below). The (TG)n spacer between adjacent GGG trinucleotides can therefore contain up to two TG dinucleotides contributed by the region 5′ of 475C (positions 474A to 471C). To avoid this ambiguity of the origin of the TG dinucleotides, we restricted our analysis to telomeric repeats that contain the GG dinucleotide. Spacer lengths of more than four TG dinucleotides occurred at a frequency of only 3% in WT telomeres and were therefore omitted.
Restricting the predicted alignment possibilities by changing 484ACA482 into UUU resulted in a trend toward shorter spacing of telomeric repeats (Table 2; not significant at the present sample size). Telomeres cloned from 483CAC481→UUU mutant cells showed a pronounced shift toward a closer spacing of telomeric repeats (Table 2). The most dramatic effect on the spacer length was achieved with the 484ACA482→CAC mutation. In a strain carrying this mutant tlc1 allele, the number of base pairs formed between the GG dinucleotide containing telomeric 3′ ends and the template RNA is increased compared to that in the WT. Telomere sequences recovered from 484ACA482→CAC mutant cells showed an almost complete loss of spacer length variability, with 17 of 19 telomere repeats showing a spacing of one TG dinucleotide as predicted from the most stable alignment (Table 2). In summary, the data strongly support the notion that multiple alignment registers in the 3′ part of the TLC1 template region contribute to the length heterogeneity of the (TG)n spacer.
Abortive reverse transcription of the template 5′ region in vivo.
In WT cells, only half of the telomeric TGGGTGT core sequences were followed by a GG dinucleotide as predicted from TLC1 template positions 471C470C (Table 1 and Fig. 1A). The frequent absence of GG dinucleotides from telomeric repeats could be explained by premature abortion of reverse transcription, degradation of longer primary products, or frequent slippage of the telomerase enzyme while copying the template positions C471C470, thus converting the GG sequence into a GGG sequence (already ruled out above). In order to address this issue, we directly measured the incorporation efficiency of the 5′-terminal region of the template in vivo. To this end, a mutant tlc1 allele carrying a 469A→U substitution was generated. This mutant was predicted to specify adenosine adjacent to a GG dinucleotide. The tlc1 469A→U allele encoded on a plasmid rescued senescing tlc1-Δ cells as efficiently as did WT TLC1 (data not shown) and gave rise to a similar telomere length (Table 1). However, in telomeres recovered from 469A→U cells, we found that only 12% (10 of 86) of the telomeric repeats contained the specified adenosine nucleotide (WT 0 of 68). Therefore, the two most 5′-terminal template positions were rarely reverse transcribed by the mutant telomerase into telomeric DNA in vivo.
Since telomeres with an incorporated adenine nucleotide should be compromised in their ability to base pair with the template in subsequent elongation cycles, we also tested a 469A→U + 482A→U double mutant that was predicted to partially restore the base pairing in the 3′ region of the template. However, the incorporation frequency of adenine nucleotides increased only slightly, to 16% (12 of 73). This suggests that abortive reverse transcription rather than degradation due to impaired alignment is responsible for the low incorporation rate of the mutant nucleotides. Unexpectedly, about half of the telomeric adenosines incorporated via the 469A→U mutation were found in the sequence GGATGT (6 of 10 in 469A→U mutants and 5 of 12 in 469A→U + 482A→U mutants), which indicates the occurrence of 3′-terminal mismatches or template skipping upon incorporation of an adenosine base. Such events were not detected with WT telomerase, as the sequence GGTTGT was never observed.
Abortive reverse transcription of the template 3′ region in vivo.
As indicated in Fig. 1A, alignment of a telomeric 3′ end with the sequence -TGGTG-3′ with positions 484A483C in WT TLC1 will result in the incorporation of three TG dinucleotides. This is, therefore, the maximal spacer length that can be generated with a single alignment event. Strikingly, in WT cells as well as in cells expressing mutant tlc1 alleles, we detected spacer lengths that exceed this maximal length (Table 2). This indicates that dissociation of partially extended products and realignment occurred before reverse transcription of positions 477C to 475C.
Nonabortive reverse transcription in the central part of the template.
The prevalence of the 5′-TGGGTGT-3′ telomeric core sequence could be explained by continuous reverse transcription from template positions 478A to 472A. The telomere sequence analysis suggests that, during reverse transcription of at least part of this region, product dissociation never occurs. This conclusion is based on the observation that telomeres cloned from cells carrying the 473CAC471→ACA mutant tlc1 allele (which lacks a CC template sequence) lacked GG dinucleotides. If product dissociation occurred after reverse transcription of template position 476C, GG dinucleotides should have become incorporated.
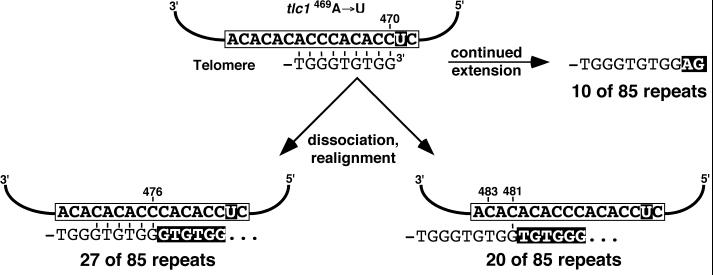
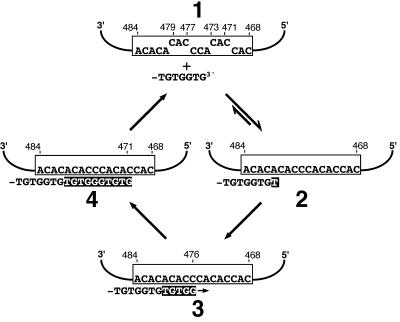
Alternatively, if after dissociation at position 476C, which generates a telomeric 3′ end with the sequence -TGG-3′, realignment always occurred again at position 476C, dissociation at this position may be undetectable in the incorporated telomere sequences (see Fig. 3). However, in 469A→U mutant telomerase, reverse transcription up to position 470C also generates a telomeric 3′ end with the sequence -GTGTGG-3′ (Fig. 2). At this point, extension may be either continued (Fig. 2, upper right) or aborted, followed by product dissociation and realignment at different positions (Fig. 2, bottom left and right). The different events can be distinguished in 469A→U mutant cells based on the incorporated sequences (indicated in Fig. 2). Reannealing of the sequence -GTGTGG-3′ at position 476C is predicted to form six base pairs and to convert the GG dinucleotide into a GGG trinucleotide after reverse transcription of position 475C (Fig. 2, bottom left). However, alignment often occurred at other positions (e.g., Fig. 2, bottom right) because a substantial number of GG dinucleotides were incorporated into the telomeric repeats that were not followed by adenine as specified by the 469A→U mutation. This indicates that telomeres ending in -TGTGG-3′ do not necessarily anneal at template position 476C.
FIG. 2.
At template position 470C, the reverse transcription can be continued (upper right) or aborted (bottom). With the 469A→U mutant telomerase, the different possibilities can be distinguished based on the incorporated telomere sequence (indicated below the TLC1 template sequence; newly incorporated nucleotides are highlighted). For reasons of simplicity, all 5′-TGGGTGTGGGT-3′ sequences were attributed to alignment at position 476C (bottom left). However, product dissociation at position 473C followed by realignment with 479C would also result in the incorporation of the same telomeric sequence. Therefore, the number given on the bottom left is probably an overestimate of the alignment frequency at 476C. The number of events indicated for alignment 3′ of position 479C (bottom right) comprises both position 483C and position 481C. Only the latter is represented in the scheme. Repeats not listed in the figure resulted from product dissociation 3′ of position 470C.
Positions 479C to 477C and 473C to 471C are inaccessible for substrate alignment.
Above, we have presented evidence that GG dinucleotides in telomeric repeats result from reverse transcription of template positions 471C470C and that the consecutive number of TG dinucleotides is principally specified by the position of alignment. Thus, the length of the TG dinucleotide spacer between a GG dinucleotide and the subsequent GGG element (Table 2) can be used to infer the approximate position of alignment between 484A and 479C in vivo (Fig. 3). Ambiguity for the exact alignment positions, however, remained (underlined in Fig. 3) because, for GG dinucleotide-containing telomeric repeats, reverse transcription may have aborted at 470C, 469A, or 468C. The different theoretical alignment possibilities along the WT TLC1 template and the resulting telomere sequences are shown in Fig. 3, along with the inferred alignment probability from all pooled WT telomere sequences (genome database, WT TLC1 and tlc1 ΔNcoI telomeres, 205 interpretable repeats). While these values represent only the GG dinucleotide-containing telomeric 3′ ends, we detected no major differences from non-GG dinucleotide-containing repeats (data not shown). Alignment events that took place 5′ of template position 471C remained undetectable and could therefore not be included in the calculation. Also, the alignment of substrates ending in -TGG-3′ at position 476C leads to conversion of the GG dinucleotide into a GGG trinucleotide (see above). Therefore, we could not determine the alignment probability for position 476C.
Alignment occurred in 79% of all interpretable cases 3′ of position 479C (Fig. 2). Strikingly, the sequence 5′-TGGTGGG-3′ (Fig. 3), which is predicted to be generated by annealing at positions 479C to 477C, was almost completely absent. Also, the sequence 5′-TGGGG-3′, predicted to be generated by annealing of telomeres ending in -TGG-3′ with position 477C, is found at a very low frequency (Fig. 3). Thus, base pairing is suppressed for substrates ending in -TGG-3′ with 479C or 477C, for substrates ending in -TGGT-3′ with 479C and 478A, and for substrates ending in -TGGTG-3′ with 479C478A477C. It appears that these three bases are shielded during substrate binding. The sequence TGGTGGT was never observed, indicating that a second portion of the template (473CAC471) is masked for alignment (Fig. 3).
Abortive reverse transcription of WT and mutant telomerases in vitro.
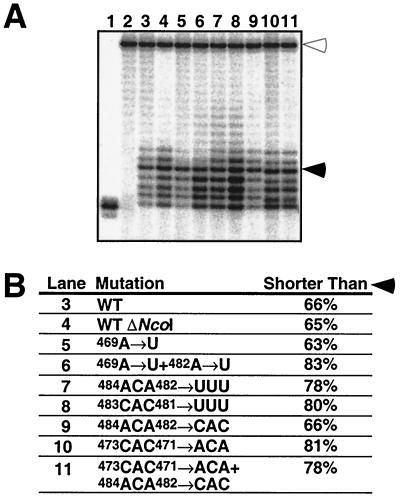
Since the catalytic properties of telomerase can be influenced by the template RNA sequence (11, 12, 43, 55), we examined whether our mutations grossly perturbed the in vitro properties of the corresponding telomerases. We employed an oligonucleotide substrate that ended with the sequence -TGGG-3′ to ensure that annealing of the 3′ end always occurred with template position 475C. Thus, 7 nt could be added before the template 5′ boundary was reached. All mutant telomerases were active in vitro (Fig. 4A) and catalyzed extension of the primer substrate up to the 5′ boundary of the template. Like WT telomerase, none of the mutant enzymes showed significant addition of more than one repeat to the substrate primer. Consistent with earlier studies that described frequent stalling of the telomerase enzyme in vitro (4, 32, 43), we detected stalling of the WT and mutant telomerase enzyme at every position. It was most pronounced at position 470C (Fig. 4A, filled arrowhead). This was not due to limiting dTTP concentrations or a specific product length (data not shown).
FIG. 4.
Telomerase assays using WT and mutant telomerases in vitro. Reaction mixtures contained equal amounts of TLC1 RNA. 5′-GTGTGTGTGGG-3′ was used as a substrate. (A) Lane 1, substrate extended with [α-32P]ddATP using terminal deoxynucleotidyltransferase; lane 2, telomerase reaction from WT cells pretreated with RNase A; and lanes 3 to 11, reactions performed with mutant telomerases as indicated in panel B. The open arrowhead indicates the position of a labeled 32-mer oligonucleotide that served as control for precipitation efficiency and gel loading. The filled arrowhead indicates extension to position 470C. (B) Relative amounts of +1 to +4 products. The different numbers of incorporated radioactive nucleotides were taken into account.
Reverse transcription has to proceed at least to position 470C in order to generate a GGGTGTGG sequence. Therefore, the premature stalling observed in vitro could explain the in vivo incorporation rate of this repeat type of only 50% (Table 1, column TGGGTGTGGT). For a quantitative comparison, the band intensities of the in vitro reactions were corrected for the specific activity of the products (number of incorporated labeled nucleotides), and the sum of +1 to +4 products, corresponding to stalling events before template position 470C, was calculated (Fig. 4B). Certain mutants (WT ΔNcoI, 469A→U, and 484ACA482→CAC) showed no increased stalling relative to WT, whereas other mutants (469A→U + 482A→U, 484ACA482→UUU, 483CAC481→UUU, 473CAC471→ACA, and 484ACA482→UUU + 473CAC471→ACA) had an increased tendency for premature termination. However, a higher rate of abortive reverse transcription in vitro did not always cause a decreased incorporation rate of GG dinucleotide-containing repeats in vivo. Furthermore, even for WT TLC1, the frequency of products stalled before template position 470C was higher than the incorporation rate of telomere repeats lacking GG dinucleotides (Table 1). Thus, telomerase has a higher nucleotide addition processivity in vivo than in vitro.
DISCUSSION
Telomere sequences have been previously exploited to predict a template sequence for S. cerevisiae telomerase RNA before it was cloned (25). Also, telomere sequences were previously characterized in vivo upon expression of tlc1 mutant alleles in the context of TLC1/tlc1 diploids (43). This earlier study delineated the telomerase RNA template, but because the analysis was done in WT-mutant heterozygotes, the mechanism of telomere repeat divergence could not be systematically addressed. In this paper, we present evidence that repeat diversity in S. cerevisiae telomere sequences arises from the use of multiple alignment registers between the telomere 3′ end and the telomerase RNA template as well as from frequent incomplete reverse transcription of the RNA template, supporting a previously proposed model (49). In addition, we present evidence for the masking of template positions 479C to 477C and 473C to 471C during substrate annealing. Finally, we show that processive nucleotide addition occurs during the reverse transcription of the central part of the template (minimally position 476C).
A framework for the interpretation of S. cerevisiae telomere sequences.
Results gained from in vitro assays of budding yeast telomerase are difficult to extend to the in vivo situation. Three gene products known to be essential for telomere maintenance are dispensable in vitro (4, 31), and the nucleotide addition processivity is lower in vitro than in vivo (this study). Based on the identification of individual template positions for the specification of certain nucleotides in the telomeric repeat (i.e., 470CC471 for GG and 475CCC478 for GGG), we were able to analyze WT telomere sequences and derive functional characteristics of telomerase, such as the alignment probabilities along the template region and the enzyme's propensity to abort reverse transcription at certain positions. Telomere sequence analysis can therefore contribute to the functional analysis of S. cerevisiae telomerase.
Structural changes of the template RNA occur during the telomerase reaction cycle.
Strikingly, sequences resulting from alignment of the substrate at positions 479C to 477C and 473C to 471C were almost completely absent from the recovered telomeres. This indicates that these bases are not available for alignment. However, after substrate annealing has occurred 3′ of position 479C, base pairing with 479C to 477C and 473C to 471C becomes possible during reverse transcription. We propose that a conformational change of these positions is induced upon substrate binding. For example, formation of a helix between the template RNA and the substrate may induce a strain on the RNA backbone, thus forcing the bases out of a shielded position and allowing them to serve as templates for reverse transcription.
As an alternative to the inaccessibility of positions 479C to 477C during the alignment, the low rate of incorporation of GGTGGG and GGGG sequences into telomeres could be caused by specific sequence loss, for example, due to an inability of these sequences to recruit telomerase. This appears unlikely, since in a previous study (25) de novo telomere repeat addition in vivo was found adjacent to various GT-rich elements, including the sequence GGTGGG. Furthermore, GGTGGG-containing sequences are bound by Cdc13p with the same affinity as are natural yeast telomeric repeats (30).
The central portion of the RNA template is reverse transcribed processively in vivo.
The occurrence of TG spacer lengths that cannot be explained by a single substrate binding event suggests that some dissociation of partially extended products occurs during the reverse transcription of the template 3′ region in vivo. This abortive mode of polymerization is also found in the template 5′ region as evidenced by the absence of the GG dinucleotide from 50% of all telomeric repeats. However, since the telomeres cloned from 473CAC471→ACA mutant cells did not contain any GG dinucleotides, it appears that product dissociation does not occur after reverse transcription of position 476C.
Certain template mutations adversely affect telomerase function in vivo.
A straightforward extrapolation from telomerases with mutant templates to the WT enzyme is not possible in all cases. For example, the 469A→U mutation also led to the incorporation of 5′-GGATG-3′ sequences, indicating that the alignment and/or translocation capacities of the mutant enzyme were changed relative to those of the WT enzyme. On the other hand, yeast cells carrying the 469A→U mutation grew normally and maintained their telomeres at lengths similar to that of the WT (Table 1). Reverse transcription of the template 5′ end is therefore not essential in budding yeast. This is clearly different in P. tetraurelia. Mutation of the template position adjacent to the 5′ boundary abolished telomerase activity in this organism unless it was combined with a compensating mutation in the 3′ part of the template (57). A second functional alteration was detected in telomeres recovered from 473CAC471→ACA and 484ACA482→CAC + 473CAC471→ACA mutant cells. In addition to the specified TGGGTT(GT)n repeats, they occasionally contained TGGGTTT(GT)n repeats (not listed in Table 1). These repeats may arise due to template slippage at the mutant position 473A. The corresponding event in WT telomerase should lead to the incorporation of 5′-GGGTGGT-5′ sequences, which were never detected. Slippage of the mutant enzyme is most likely limited to position 473A, since expansions of the GGG triplet were not detected.
The possibilities for repeat divergence are restricted.
The function of telomere repeat divergence has not been elucidated. It is pronounced in both fission yeast and budding yeast, organisms in which homologous recombination pathways are very efficient. We propose that the divergence of telomere sequences may protect the organism from rampant telomere-telomere recombination events, which would lead to stochastic telomere length changes by intra- and intertelomeric recombination (29). The recent finding that homologous recombination between the divergent telomeres in WT cells is inhibited by the mismatch repair machinery (47) supports a role of telomere repeat divergence for suppressing unwanted recombination events.
However, telomere repeat divergence must be limited to allow the efficient binding of proteins involved in end protection and end replication. The masking of positions 479C to 477C for substrate annealing and the processive synthesis of the following GGG trinucleotide reduce the degeneracy of budding yeast telomeric repeats and enhance the incorporation of 5′-TGTGGGT-3′ sequences into telomeres. This sequence is part of telomeric Rap1p consensus binding sites (3, 13, 14), and the GGG trinucleotide was recently shown to be involved in multiple base-specific protein-DNA interactions in cocrystals of Rap1p with telomeric DNA (53). Thus, the synthesis of variable repeat sequences is restrained by two mechanisms which together enhance the incorporation of Rap1p binding sites into telomeres. This may represent the best compromise between the need to let the telomere sequences diverge in order to prevent recombination and the opposing need to ensure the binding of the proteins that recognize and protect the telomere.
While this paper was in review, Ray and Runge (46) published a study of the occurrence of different theoretically possible Rap1 binding sites in telomeres from WT, yku70-Δ, and tel1-Δ cells. They concluded that certain motifs are found at lower frequencies than would be expected if the sequences were generated randomly with the same nucleotide composition as the TLC1 template region. Furthermore, they also found an incorporation rate of the GG dinucleotide of around 50%.
The S. cerevisiae telomerase reaction cycle.
Based on the results presented in this study, we propose the following working model for S. cerevisiae telomerase (Fig. 5). The telomere 3′ end aligns at one of several possible sites in the 3′ part of the TLC1 template region (Fig. 5, state 1). Positions 479C to 477C and 473C to 471C are inaccessible for alignment. Upon substrate binding, a conformational change occurs which enables these positions to serve as templates for reverse transcription (Fig. 5, state 2). Dissociation of partially extended products may occur during reverse transcription of positions 484A to 478A. However, since stretches of four and more TG dinucleotides are found at moderate frequency, processive polymerization is favored over product dissociation. Once the active site has reached position 476C, product dissociation is prevented and reverse transcription occurs at least to position 475C (Fig. 5, state 3). In most cases, polymerization may continue processively until position 472A to guarantee synthesis of the telomeric 5′-TGGGTGT-3′ core sequence. As reverse transcription approaches the 5′ end of the template, telomerase has the tendency to abort the reaction and only 50% of the products are extended to position 470C or beyond (Fig. 5, state 4). At the end of an extension cycle, the rate-limiting step in vivo may be the unwinding of the DNA product from the RNA template. Upon unwinding (Fig. 5, from state 4 to 1 or from state 2 to 1), the template RNA reverses the conformational switch that occurred during substrate binding. Then, the telomeric substrate may either translocate back to the template 3′ end or dissociate from the telomerase enzyme and allow access and extension by a different active site (42).
FIG. 5.
Model of the S. cerevisiae telomerase reaction cycle. (State 1) Telomerase before substrate binding. Template positions 479CAC477 and 473CAC471 are not accessible for alignment. (State 2) Upon substrate binding, a conformational change occurs and all template positions are available for base pairing during reverse transcription. Dissociation of a partially extended telomere occurs with moderate frequency. (State 3) The central part of the TLC1 template region is reverse transcribed processively. The results presented in this paper exclude product dissociation at position 476C. However, the region of processive reverse transcription may cover template positions 478A to 472A to favor incorporation of the telomeric 5′-TGGGTGT-3′ core sequence. (State 4) Template positions close to the 5′ boundary are reverse transcribed with moderate frequency. Only half of the products are extended beyond template position 471C.
ACKNOWLEDGMENTS
We thank Pierre Page for sequencing telomeres, members of the Lingner and Nabholz labs for helpful discussions and critical reading of the manuscript, Arthur Zaug for suggesting the TLC1 mutagenesis strategy, and Dan Gottschling for sharing reagents.
This work was supported by the Swiss National Science Foundation, the Human Frontier Science Program, and a Ph.D. fellowship of the Boehringer Ingelheim Fonds awarded to K.F.
REFERENCES
- 1.Bertuch A, Lundblad V. Telomeres and double-strand breaks—trying to make ends meet. Trends Cell Biol. 1998;8:339–342. doi: 10.1016/s0962-8924(98)01331-2. [DOI] [PubMed] [Google Scholar]
- 2.Bryan T M, Goodrich K J, Cech T R. A mutant of Tetrahymena telomerase reverse transcriptase with increased processivity. J Biol Chem. 2000;275:24199–24207. doi: 10.1074/jbc.M003246200. [DOI] [PubMed] [Google Scholar]
- 3.Buchman A, Lue N, Kornberg R. Connections between transcriptional activators, silencers, and telomeres as revealed by functional analysis of a yeast DNA-binding protein. Mol Cell Biol. 1988;8:5086–5099. doi: 10.1128/mcb.8.12.5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohn M, Blackburn E H. Telomerase in yeast. Science. 1995;269:396–400. doi: 10.1126/science.7618104. [DOI] [PubMed] [Google Scholar]
- 5.Cohn M, McEachern M J, Blackburn E H. Telomeric sequence diversity within the genus Saccharomyces. Curr Genet. 1998;33:83–91. doi: 10.1007/s002940050312. [DOI] [PubMed] [Google Scholar]
- 6.Collins K. Ciliate telomerase biochemistry. Annu Rev Biochem. 1999;68:187–218. doi: 10.1146/annurev.biochem.68.1.187. [DOI] [PubMed] [Google Scholar]
- 7.Conrad M, Wright J, Wolf J, Zakian V. RAP1 protein interacts with yeast telomeres in vivo: overproduction alters telomere structure and decreases chromosome stability. Cell. 1990;63:739–750. doi: 10.1016/0092-8674(90)90140-a. [DOI] [PubMed] [Google Scholar]
- 8.Counter C M, Meyerson M, Eaton E N, Weinberg R A. The catalytic subunit of yeast telomerase. Proc Natl Acad Sci USA. 1997;94:9202–9207. doi: 10.1073/pnas.94.17.9202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forstemann K, Hoss M, Lingner J. Telomerase-dependent repeat divergence at the 3′ ends of yeast telomeres. Nucleic Acids Res. 2000;28:2690–2694. doi: 10.1093/nar/28.14.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fulton T B, Blackburn E H. Identification of Kluyveromyces lactis telomerase: discontinuous synthesis along the 30-nucleotide-long templating domain. Mol Cell Biol. 1998;18:4961–4970. doi: 10.1128/mcb.18.9.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilley D, Blackburn E H. Specific RNA residue interactions required for enzymatic functions of Tetrahymena telomerase. Mol Cell Biol. 1996;16:66–75. doi: 10.1128/mcb.16.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilley D, Lee M S, Blackburn E H. Altering specific telomerase RNA template residues affects active site function. Genes Dev. 1995;9:2214–2226. doi: 10.1101/gad.9.18.2214. [DOI] [PubMed] [Google Scholar]
- 13.Gilson E, Roberge M, Giraldo R, Rhodes D, Gasser S. Distortion of the DNA double helix by RAP1 at silencers and multiple telomeric binding sites. J Mol Biol. 1993;231:293–310. doi: 10.1006/jmbi.1993.1283. [DOI] [PubMed] [Google Scholar]
- 14.Graham I, Chamber A. Use of selection technique to identify the diversity of binding sites for the yeast RAP1 transcription factor. Nucleic Acids Res. 1994;22:124–130. doi: 10.1093/nar/22.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greider C W. Telomerase is processive. Mol Cell Biol. 1991;11:4572–4580. doi: 10.1128/mcb.11.9.4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greider C W, Blackburn E H. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985;43:405–413. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- 17.Greider C W, Blackburn E H. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature. 1989;337:331–337. doi: 10.1038/337331a0. [DOI] [PubMed] [Google Scholar]
- 18.Haering C H, Nakamura T M, Baumann P, Cech T R. Analysis of telomerase catalytic subunit mutants in vivo and in vitro in Schizosaccharomyces pombe. Proc Natl Acad Sci USA. 2000;97:6367–6372. doi: 10.1073/pnas.130187397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hammond P W, Cech T R. dGTP-dependent processivity and possible template switching of euplotes telomerase. Nucleic Acids Res. 1997;25:3698–3704. doi: 10.1093/nar/25.18.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hardy C F, Sussel L, Shore D. A RAP1-interacting protein involved in transcriptional silencing and telomere length regulation. Genes Dev. 1992;6:801–814. doi: 10.1101/gad.6.5.801. [DOI] [PubMed] [Google Scholar]
- 21.Hiraoka Y, Henderson E, Blackburn E H. Not so peculiar: fission yeast telomere repeats. Trends Biochem Sci. 1998;23:126. doi: 10.1016/s0968-0004(98)01176-1. [DOI] [PubMed] [Google Scholar]
- 22.Hughes T R, Evans S K, Weilbaecher R G, Lundblad V. The est3 protein is a subunit of yeast telomerase. Curr Biol. 2000;10:809–812. doi: 10.1016/s0960-9822(00)00562-5. [DOI] [PubMed] [Google Scholar]
- 23.Kim N W, Piatyszek M A, Prowse K R, Harley C B, West M D, Ho P L, Coviello G M, Wright W E, Weinrich S L, Shay J W. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 24.Kirk K E, Harmon B P, Reichardt I K, Sedat J W, Blackburn E H. Block in anaphase chromosome separation caused by a telomerase template mutation. Science. 1997;275:1478–1481. doi: 10.1126/science.275.5305.1478. [DOI] [PubMed] [Google Scholar]
- 25.Kramer K M, Haber J E. New telomeres in yeast are initiated with a highly selected subset of TG1-3 repeats. Genes Dev. 1993;7:2345–2356. doi: 10.1101/gad.7.12a.2345. [DOI] [PubMed] [Google Scholar]
- 26.Kyrion G, Boake K, Lustig J. C-terminal truncation of RAP1 results in the deregulation of telomere size, stability, and function in Saccharomyces cerevisiae. Mol Cell Biol. 1992;12:5159–5173. doi: 10.1128/mcb.12.11.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee M S, Blackburn E H. Sequence-specific DNA primer effects on telomerase polymerization activity. Mol Cell Biol. 1993;13:6586–6599. doi: 10.1128/mcb.13.10.6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lendvay T S, Morris D K, Sah J, Balasubramanian B, Lundblad V. Senescence mutants of Saccharomyces cerevisiae with a defect in telomere replication identify three additional EST genes. Genetics. 1996;144:1399–1412. doi: 10.1093/genetics/144.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li B, Lustig A J. A novel mechanism for telomere size control in Saccharomyces cerevisiae. Genes Dev. 1996;10:1310–1326. doi: 10.1101/gad.10.11.1310. [DOI] [PubMed] [Google Scholar]
- 30.Lin Y C, Hsu C L, Shih J W, Lin J J. Specific binding of single-stranded telomeric DNA by Cdc13p of Saccharomyces cerevisiae. J Biol Chem. 2001;276:24588–24593. doi: 10.1074/jbc.M101642200. [DOI] [PubMed] [Google Scholar]
- 31.Lingner J, Cech T R, Hughes T R, Lundblad V. Three Ever Shorter Telomere (EST) genes are dispensable for in vitro yeast telomerase activity. Proc Natl Acad Sci USA. 1997;94:11190–11195. doi: 10.1073/pnas.94.21.11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lingner J, Hughes T R, Shevchenko A, Mann M, Lundblad V, Cech T R. Reverse transcriptase motifs in the catalytic subunit of telomerase. Science. 1997;276:561–567. doi: 10.1126/science.276.5312.561. [DOI] [PubMed] [Google Scholar]
- 33.Lundblad V, Szostak J W. A mutant with a defect in telomere elongation leads to senescence in yeast. Cell. 1989;57:633–643. doi: 10.1016/0092-8674(89)90132-3. [DOI] [PubMed] [Google Scholar]
- 34.Lustig A, Kurtz S, Shore D. Involvement of the silencer and UAS binding protein Rap1 in regulation of telomere length. Science. 1990;250:549–553. doi: 10.1126/science.2237406. [DOI] [PubMed] [Google Scholar]
- 35.Maine I P, Chen S F, Windle B. Effect of dGTP concentration on human and CHO telomerase. Biochemistry. 1999;38:15325–15332. doi: 10.1021/bi991596+. [DOI] [PubMed] [Google Scholar]
- 36.Marcand S, Brevet V, Gilson E. Progressive cis-inhibition of telomerase upon telomere elongation. EMBO J. 1999;18:3509–3519. doi: 10.1093/emboj/18.12.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marcand S, Gilson E, Shore D. A protein-counting mechanism for telomere length regulation in yeast. Science. 1997;275:986–990. doi: 10.1126/science.275.5302.986. [DOI] [PubMed] [Google Scholar]
- 38.Marusic L, Anton M, Tidy A, Wang P, Villeponteau B, Bacchetti S. Reprogramming of telomerase by expression of mutant telomerase RNA template in human cells leads to altered telomeres that correlate with reduced cell viability. Mol Cell Biol. 1997;17:6394–6401. doi: 10.1128/mcb.17.11.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCormick-Graham M, Haynes W J, Romero D P. Variable telomeric repeat synthesis in Paramecium tetraurelia is consistent with misincorporation by telomerase. EMBO J. 1997;16:3233–3242. doi: 10.1093/emboj/16.11.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McEachern M J, Blackburn E H. Runaway telomere elongation caused by telomerase RNA gene mutations. Nature. 1995;376:403–409. doi: 10.1038/376403a0. [DOI] [PubMed] [Google Scholar]
- 41.Peterson S, Stellwagen A, Diede S, Singer M, Haimberger Z, Johnson C, Tzoneva M, Gottschling D. The function of a stem-loop in telomerase RNA is linked to the DNA repair protein Ku. Nat Genet. 2001;27:64–67. doi: 10.1038/83778. [DOI] [PubMed] [Google Scholar]
- 42.Prescott J, Blackburn E H. Functionally interacting telomerase RNAs in the yeast telomerase complex. Genes Dev. 1997;11:2790–2800. doi: 10.1101/gad.11.21.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prescott J, Blackburn E H. Telomerase RNA mutations in Saccharomyces cerevisiae alter telomerase action and reveal nonprocessivity in vivo and in vitro. Genes Dev. 1997;11:528–540. doi: 10.1101/gad.11.4.528. [DOI] [PubMed] [Google Scholar]
- 44.Prescott J C, Blackburn E H. Telomerase RNA template mutations reveal sequence-specific requirements for the activation and repression of telomerase action at telomeres. Mol Cell Biol. 2000;20:2941–2948. doi: 10.1128/mcb.20.8.2941-2948.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qi H, Zakian V A. The Saccharomyces telomere-binding protein Cdc13p interacts with both the catalytic subunit of DNA polymerase alpha and the telomerase-associated Est1 protein. Genes Dev. 2000;14:1777–1788. [PMC free article] [PubMed] [Google Scholar]
- 46.Ray A, Runge K W. Yeast telomerase appears to frequently copy the entire template in vivo. Nucleic Acids Res. 2001;29:2382–2394. doi: 10.1093/nar/29.11.2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Riziki A, Lundblad V. Defects in mismatch repair promote telomerase-independent proliferation. Nature. 2001;411:713–716. doi: 10.1038/35079641. [DOI] [PubMed] [Google Scholar]
- 48.Shampay J, Szostak J, Blackburn E. DNA sequences of telomeres maintained in yeast. Nature. 1984;310:154–157. doi: 10.1038/310154a0. [DOI] [PubMed] [Google Scholar]
- 49.Singer M S, Gottschling D E. TLC1: template RNA component of Saccharomyces cerevisiae telomerase. Science. 1994;266:404–409. doi: 10.1126/science.7545955. [DOI] [PubMed] [Google Scholar]
- 50.Smith C D, Blackburn E H. Uncapping and deregulation of telomeres lead to detrimental cellular consequences in yeast. J Cell Biol. 1999;145:203–214. doi: 10.1083/jcb.145.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun D, Lopez-Guajardo C C, Quada J, Hurley L H, Von Hoff D D. Regulation of catalytic activity and processivity of human telomerase. Biochemistry. 1999;38:4037–4044. doi: 10.1021/bi982249n. [DOI] [PubMed] [Google Scholar]
- 52.Szostak J, Blackburn E. Cloning yeast telomeres on linear plasmid vectors. Cell. 1982;29:245–255. doi: 10.1016/0092-8674(82)90109-x. [DOI] [PubMed] [Google Scholar]
- 53.Taylor H O, O'Reilly M, Leslie A G, Rhodes D. How the multifunctional yeast Rap1p discriminates between DNA target sites: a crystallographic analysis. J Mol Biol. 2000;303:693–707. doi: 10.1006/jmbi.2000.4161. [DOI] [PubMed] [Google Scholar]
- 54.Wang S, Zakian V. Sequencing of Saccharomyces telomeres cloned using T4 DNA polymerase reveals two domains. Mol Cell Biol. 1990;10:4415–4419. doi: 10.1128/mcb.10.8.4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ware T L, Wang H, Blackburn E H. Three telomerases with completely non-telomeric template replacements are catalytically active. EMBO J. 2000;19:3119–3131. doi: 10.1093/emboj/19.12.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wellinger R J, Sen D. The DNA structures at the ends of eukaryotic chromosomes. Eur J Cancer. 1997;33:735–749. doi: 10.1016/S0959-8049(97)00067-1. [DOI] [PubMed] [Google Scholar]
- 57.Ye A, Haynes W, Romero D. Expression of mutated Paramecium telomerase RNAs in vivo leads to templating errors that resemble those made by retroviral reverse transcriptase. Mol Cell Biol. 1999;19:2887–2894. doi: 10.1128/mcb.19.4.2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu G L, Bradley J D, Attardi L D, Blackburn E H. In vivo alteration of telomere sequences and senescence caused by mutated Tetrahymena telomerase RNAs. Nature. 1990;344:126–132. doi: 10.1038/344126a0. [DOI] [PubMed] [Google Scholar]