Abstract
Chemical modifications of RNAs, known as the epitranscriptome, are emerging as widespread regulatory mechanisms underlying gene regulation. The field of epitranscriptomics advances recently due to improved transcriptome-wide sequencing strategies for mapping RNA modifications and intensive characterization of writers, erasers, and readers that deposit, remove, and recognize RNA modifications, respectively. Herein, we review recent advances in characterizing plant epitranscriptome and its regulatory mechanisms in post-transcriptional gene regulation and diverse physiological processes, with main emphasis on N6-methyladenosine (m6A) and 5-methylcytosine (m5C). We also discuss the potential and challenges for utilization of epitranscriptome editing in crop improvement.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13059-023-02872-6.
Introduction
A variety of naturally occurring chemical modifications on cellular RNAs, collectively termed as epitranscriptome, add an additional layer of regulatory information to RNAs. To date, there are over 170 distinct RNA modifications discovered, with information stored in the MODOMICS database [1]. Over the last decade, advances in techniques in detecting RNA modifications and technologies coupling the next generation sequencing with antibody, chemical, and enzymatic approaches in mapping RNA modification sites have profoundly improved our understanding of the complexity and function of epitranscriptome, especially in messenger RNAs (mRNAs). Thus far, diverse mRNA modifications have been discovered and mapped in eukaryotic cells, including N7-methylguanosine (m7G) and nicotinamide adenine diphosphate (NAD+) modifications [2] at the 5′-cap, and other modifications occurring internally, such as N6-methyladenosine (m6A) [3, 4], N1-methyladenosine (m1A) [5, 6], 5-methylcytosine (m5C) [7], N4-acetylcytidine (Ac4C) [8], 5-hydroxymethylcytosine (hm5C) [9], m7G [10], and pseudouridine (ψ) [11, 12]. Accumulating evidence suggests that these modifications with great chemical and structure diversities provide extraordinary regulatory potential in modulating RNA metabolism, thus affecting gene expression. Moreover, extensive characterization of their effector proteins, including writers, erasers, and readers that perform respective functions in installing, removing, and decoding mRNA modifications, has also significantly advanced our knowledge in epitranscriptome regarding its fundamental regulatory roles in diverse and dynamic cellular processes.
In plant epitranscriptome, m6A represents the most prevalent and best characterized internal modification on mRNA. Its landscape and the relevant effectors have been revealed across a variety of plant species [13–16]. Several other internal modifications, including m5C, m1A, and ψ, have been mapped on a transcriptome-wide scale in some plant species along with identification of their writers (Fig. 1a) [17–19]. In addition to these internal modifications, plant mRNAs are also modified at their 5′ end, such as the canonical m7G or non-canonical NAD+ caps (Fig. 1a) [20, 21]. In this review, we highlight recent advances in our understanding of plant epitranscriptome and its regulatory mechanisms in post-transcriptional gene regulation and physiological processes, with main emphasis on m6A and m5C. We also highlight the outstanding questions pertaining to plant epitranscriptome and discuss the potential and challenges of future crop improvement through epitranscriptome editing.
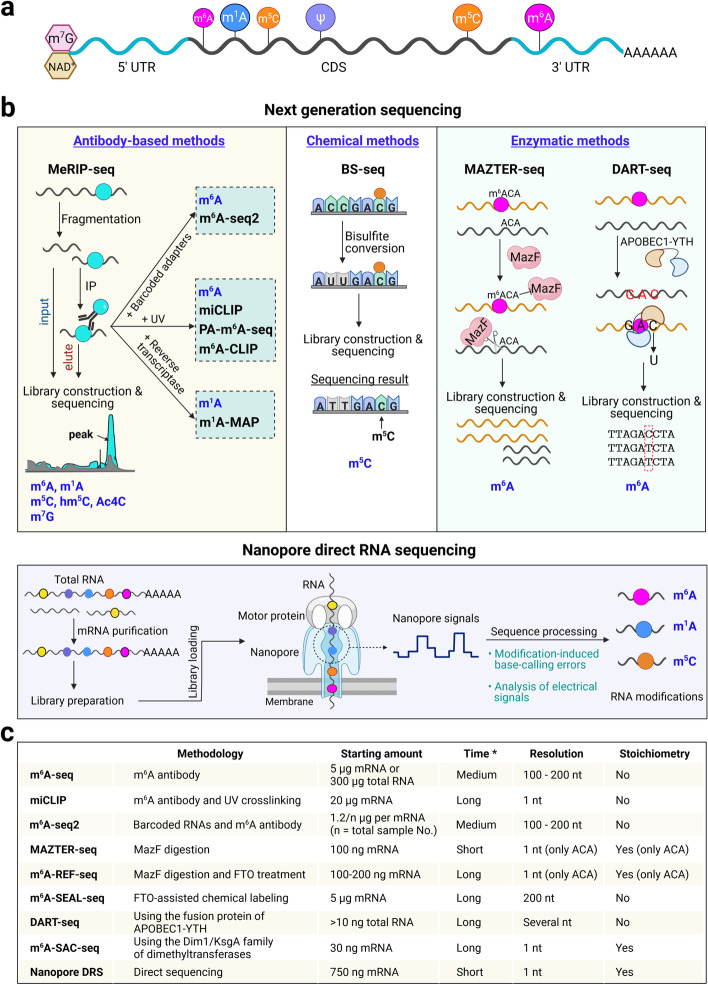
Fig. 1.
An overview of the plant epitranscriptome and techniques for mapping epitranscriptome. a Known RNA modification in plants. Predominant locations of various RNA modifications are illustrated in a transcript. The mRNAs are capped with N7-methylguanosine (m7G) or nicotinamide adenine diphosphate (NAD+) and contain internal modifications, including N6-methyladenosine (m6A), N1-methyladenosine (m1A), 5-methylcytosine (m5C), and pseudouridine (ψ), in plants. b Approaches for mapping RNA modifications. Techniques coupling next generation sequencing with antibody-, chemical-, and enzyme-based approaches are shown in the upper panels, while nanopore direct RNA sequencing is illustrated in the lower panel. c Comparison of the key features of different m6A profiling techniques. The asterisk indicates the time required for sample preparation before library construction and sequencing. Created with Biorender.com
Advances in epitranscriptome profiling technologies
The breakthrough of epitranscriptome studies in the past decade is largely attributed to advancement in epitranscriptome detecting and profiling technologies. Biochemical methods based on physicochemical properties like liquid chromatography-tandem mass spectrometry (LC-MS/MS) [22, 23] allow precise detection and quantification of overall levels of multiple epitranscriptomic marks in plants, including m6A [24], m1A [17], m5C, and hm5C [19]. Dot blot analysis using antibodies recognizing specifically modified nucleotides also detects overall RNA modification levels with low sensitivity and precision [25]. However, these approaches are unable to profile the context-dependent transcriptome-wide pattern of RNA modifications. To fill this gap, a variety of high-throughput transcriptome-wide profiling technologies have been developed, including those coupling short-read based sequencing with antibody, chemical, and enzymatic methodologies and Nanopore long-read direct RNA sequencing.
Antibody-, chemical-, and enzyme-based epitranscriptome profiling
Methods coupling next generation sequencing with antibody immunoprecipitation of modified RNAs, including m6A-seq [3, 4], m1A-seq [6], m5C-seq [26], hm5C-seq [9], Ac4C-seq [8], and m7G-seq [10] (Fig. 1b), are most commonly used so far for mapping various RNA modifications. These methods have significantly contributed to our current knowledge of the location and distribution of epitranscriptome marks. For instance, m6A-seq has revealed the prevalence of m6A methylation in thousands of transcripts with unique and conserved distribution preferentially around stop codons and in 3′ untranslated regions (UTRs) in eukaryotic organisms [3, 4, 27–29]. Nevertheless, these approaches suffer from poor resolution (100–200 nt) on positional information of epitranscriptome marks. Additional steps have been introduced to improve these methods for detecting RNA modifications at single-base resolution. First, incorporating a UV crosslinking step to RNA immunoprecipitation as shown in m6A individual-nucleotide-resolution crosslinking and immunoprecipitation (miCLIP) [30, 31], m6A crosslinking immunoprecipitation (m6A-CLIP) [32], and photo-crosslinking-assisted m6A sequencing strategy (PA-m6A-seq) [33] improves m6A detection to the exact or + 1 site. However, these methods are unable to quantify differential modifications among samples without utilizing methylated spike-in controls or an input library for correction or normalization [34]. Second, specific reverse transcriptases are applied to improve the detection resolution for RNA modifications that induce misincorporations during reverse transcription. These methods include m1A-MAP using the reverse transcriptase TGIRT for high-resolution profiling of m1A methylomes [35]. In addition, m6A-seq2 using barcoded adaptors ligated to RNAs from different samples has been developed for simultaneously interrogating m6A dynamics across different samples [36]. This approach could be extended to detect other RNA modifications by using their specific antibodies.
Despite the versatility of antibody-based approaches, their applications have to depend on high-quality antibodies and large quantities of starting RNAs. Alternatively, antibody-independent methods, including bisulfite sequencing (BS-seq) based on chemical-induced signature to detect m5C RNA modifications (Fig. 1b) [7, 37], have been developed for epitranscriptome mark detection. Sodium bisulfite treatment converts an unmethylated cytosine (C) instead of m5C into a uracil (U), creating a signature that allows the mapping of m5C at single-nucleotide resolution. However, a major disadvantage of BS-seq is an unavoidable false positive detection because of incomplete C-U conversion caused by variation of bisulfite treatment, double-stranded RNA structures, or disruption from other modifications such as hm5C [38–41]. In plants, BS-seq has been applied to detect m5C in tRNA, rRNA and mRNA [42–44].
Recent development of enzymatic techniques offers another useful alternative for quantitatively mapping exact locations of m6A in an antibody-independent manner. These techniques either take advantage of m6A-sensitive RNA-cleaving restriction enzymes, such as MAZTER-seq [45] and m6A-REF-seq [46], or utilize m6A erasers/readers, such as m6A-SEAL [47] and DART-seq [48], or make use of dimethyltransferases like MjDim1 for converting m6A to m62A in m6A-seletive allyl chemical labeling and sequencing (m6A-SAC-seq) [49]. In MAZTER-seq/m6A-REF-seq, the m6A-sensitive enzyme MazF cleaves RNA at ACA rather than m6ACA, thus inferring quantitative site-specific m6A profiles (Fig. 1b). However, as MazF only recognizes the ACA motif in a subset of m6A sites, further engineering of the current enzymes or exploring other m6A-sensitive enzymes is required for expanding the toolbox. Another antibody-free approach DART-seq adopts the fusion protein of the cytidine deaminase APOBEC1 and the m6A-binding YTH domain APOBEC1-YTH, which induces C to U deamination at sites adjacent to m6As [48] (Fig. 1b). Notably, these antibody-free approaches could work on limited RNAs, as little as nanograms or even picograms, promising m6A profiling in rare materials or single cells. Moreover, many of them are capable of estimating the m6A stoichiometry across different samples. As so far these approaches have been mostly tested in mammalian systems, future optimization and application of these methods in plants will be helpful to explore context-dependent dynamics of plant m6A methylomes.
Nanopore direct RNA sequencing to locate epitranscriptome marks
Although the above-mentioned epitranscriptome profiling approaches have provided unprecedented insights into the distribution and regulation of RNA modifications, challenges remain for quantitative mapping of RNA modification landscape in multiple samples. Moreover, these approaches largely depend on short-read cDNA-based sequencing which requires the conversion of RNA to cDNA. In contrast, the nanopore long-read direct RNA sequencing (DRS) platform is emerging as a promising approach to quantitatively locate and compare RNA modifications at single-nucleotide resolution across different conditions (Fig. 1b) [50, 51]. In this approach, when an RNA molecule traverses a protein nanopore, its modifications cause changes in intensity levels of the electric current, thus permitting prediction of modified bases in a quantitative manner by computational methods [52, 53]. Currently, the algorithms for predicting RNA modifications are built based on either characteristic base-calling error signatures, such as EpiNano [53] and DiffErr [31], or machine-learning methods to capture differences in raw current signals, such as Tombo [54], Nanocompore [55], and xPore [56]. Nanopore DRS and its associated algorithms have been used to profile RNA modifications, such as m6A, m5C, and ψ, in multiple organisms [56–58]. In plants, differential and comparative m6A methylomes at high-resolution have been generated by nanopore DRS for Arabidopsis mutants defective in two m6A writers [31, 59]. Additionally, nanopore DRS and Tombo have been used to identify m5C peaks in Arabidopsis [58], with an overall pattern similar to that identified by m5C-seq [19]. Future development of algorithms with a focus on improving the accuracy of detecting a broad spectrum of RNA modifications will certainly strengthen epitranscriptome studies.
Besides these experimental approaches, prediction methods, such as RAM-NPPS [60], BERMP [61], and PEA [62], have also been developed to predict m6A in plants. Among these methods, PEA predicts m6A at over 70% sensitivity and specificity in Arabidopsis. Together, all these detection approaches bear their own intrinsic advantages and weaknesses. For instance, different m6A profiling techniques require greatly varied amount of starting materials ranging from 100 ng to 20 μg of mRNA with different detection resolutions and abilities to infer stoichiometric information (Fig. 1c). These characteristics should be considered together with the biological questions to be addressed when designing an epitranscriptome profiling study. In addition, most antibody-free sequencing techniques are yet to be adopted in plant epitranscriptome research despite an increasing trend of using Nanopore DRS to locate the precise RNA modification sites in various plant species. Future applications of these profiling approaches will undoubtedly contribute to our understanding of the dynamics of plant epitranscriptome.
Advances in characterizing epitranscriptome players in plants
m6A writers and recruiters
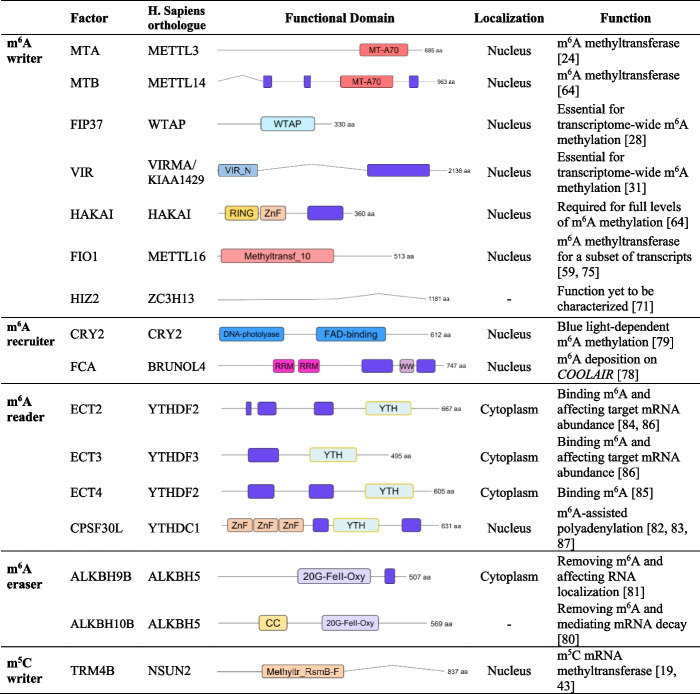
m6A deposition to target transcripts requires an evolutionarily conserved multicomponent m6A writer/methyltransferase complex. In Arabidopsis, this complex consists of two core methyltransferases, mRNA adenosine methylase (MTA; ortholog of METTL3) and MTB (ortholog of METTL14), and several accessory proteins including FKBP12 INTERACTING PROTEIN 37KD (FIP37; ortholog of WTAP), VIRILIZER (VIR; ortholog of VIRMA), and HAKAI (Fig. 2a and Table 1) [24, 28, 63–67]. Studies in mammals have suggested that the m6A methyltransferase complex could be divided into two subcomplexes, termed m6A-METTL Complex (MAC) and m6A-METTL Associated Complex (MACOM) [68]. MAC formed by MTA and MTB constitutes the catalytic core of the m6A methyltransferase complex, while MACOM containing the accessory subunits including FIP37, VIR, and HAKAI is required for the full activity of MAC. Several additional factors, such as RNA-binding motif protein 15 (RBM15)/RBM15B and zinc finger CCCH domain-containing protein 13 (ZC3H13), are other major components of the mammalian MACOM [69, 70]. In Arabidopsis, a recent study suggests that a HAKAI-interacting zinc finger protein HIZ2 might be the plant equivalent of ZC3H13 [71], but its biological function in the m6A writer complex needs further investigation. Additionally, although FLOWERING LOCUS PA (FPA) represents the closest ortholog of RBM15/RBM15B and co-purifies with m6A writers, it does not influence global m6A levels when it is defective or overexpressed [72], implying a limited effect of FPA on m6A modifications in Arabidopsis. Thus, whether plant m6A requires RBM15/RBM15B equivalents needs further exploration.
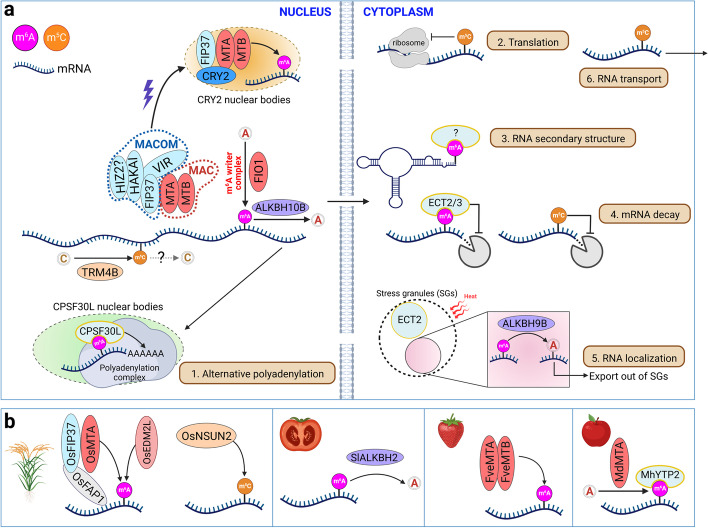
Fig. 2.
An overview of the effector proteins and molecular functions of m6A and m5C. a Effector proteins and molecular functions of m6A and m5C in Arabidopsis. m6A is deposited to its target transcripts mainly by a multicomponent m6A methyltransferase complex in the nucleus. This complex could be divided into two subcomplexes, namely the m6A-METTL Complex (MAC) and the m6A-METTL Associated Complex (MACOM). Upon blue light treatment, MTA, MTB, and FIP37 are recruited to the CRY2 nuclear bodies for m6A methylation of several central oscillator genes. Another known m6A methyltransferase FIO1 acts separately to deposit m6A in a subset of transcripts. m6A is removed by ALKBH10B in the nucleus or by ALKBH9B in stress granules (SGs) in the cytoplasm. m6A is recognized by CPSF30L in the nucleus or ECT2/3 in the cytoplasm. m5C is catalyzed by TRM4B. RNA modifications affect RNA metabolism in many aspects, including (1) alternative polyadenylation, (2) translation, (3) RNA secondary structure, (4) RNA stability, (5) RNA localization, and (6) RNA transport. b Some known effector proteins of m6A and m5C in crops. Created with Biorender.com
Table 1.
Functional domain, subcellular localization, and function of m6A and m5C effectors in Arabidopsis [19, 24, 28, 31, 43, 59, 64, 71, 75, 78–87]
In the “Functional domain” column, blue represents low complexity region as predicated by “Prion-like Amino Acid Composition” (PLACC) [88]; CC, coiled-coil domain; ZnF, zinc finger domain; RRM; RNA recognition motif
Within this m6A methyltransferase complex, mutual regulation among different subunits takes place especially at the post-translational level. For instance, WTAP is required for recruiting METLL3-METTL14 to nuclear speckles and mRNA targets in mammalian cells [66], and for stabilizing the METTL3-METTL14 interaction in Drosophila [73]. ZC3H13 facilitates nuclear localization of other m6A writers in mouse embryonic stem (mES) cells [69]. By contrast, the exact roles of different Arabidopsis m6A writers remain completely obscure. In particular, whether the accessory subunits, FIP37, VIR, and HAKAI, play regulatory functions in mediating m6A methyltransferases, MTA and MTB, awaits further examination. Nevertheless, each individual m6A writer, such as MTA, MTB, FIP37, and VIR, is indispensable for m6A deposition [28, 31, 71, 74], indicating their functional interdependence and mutual regulation for maintaining the functionality of the m6A methyltransferase complex in Arabidopsis.
This Arabidopsis m6A methyltransferase complex, like its mammalian counterpart, installs m6A on mRNAs preferentially near stop codons and in 3′UTRs in a major sequence context of RRACH (R = A/G; H = A/C/U) [13, 28, 31], accounting for the majority of total m6A levels in Arabidopsis. In contrast, another known methyltransferase FIONA1 (FIO1; ortholog to METTL16) acts separately to deposit m6A modifications on a subset of transcripts, contributing modestly to overall m6A levels (Fig. 2a and Table 1) [59, 75]. Unlike METLL16 association with a UACm6AGAGAA sequence embedded in a stem-loop structure [76, 77], FIO1-mediated m6A methylation is enriched in a YHAGA (Y = C/U) motif in coding sequences peaked near stop codons [59] or motifs resembling RRACH in 3′UTRs [75]. Despite these differences, both METTL16 and FIO1 deposit m6A to the noncoding U6 spliceosomal small nuclear RNA [59, 75, 77].
These two distinct m6A-depositing machineries seem to have both common and distinct targets [59, 75], raising intriguing questions regarding how m6A writers select their targets in response to developmental and environmental signals. Recent advances in plants suggest that target-specificity of m6A could be achieved through recruiting m6A writers to specific transcripts by RNA binding proteins (RBPs) and other writer-associated proteins, which are tentatively named m6A recruiters. The RBP FCA co-purifies with MTA, MTB, and FIP37 and facilitates m6A deposition on the noncoding antisense transcript COOLAIR during Arabidopsis flowering [78]. Another RBP OsFIP37-assocated protein 1 (OsFAP1) recruits the m6A writer OsFIP37 for adding m6A on OsYUCCA3 transcripts during male meiosis in rice (Fig. 2b) [89]. Other characterized m6A recruiters include cryptochrome 2 (CRY2), which undergoes liquid-liquid phase separation (LLPS) to form CRY2-nuclear bodies in response to blue light and interacts with MTA, MTB, and FIP37 to mediate m6A installation on transcripts of central circadian clock oscillator genes (Fig. 2a) [79]. These studies exemplify how various factors are engaged in m6A-depositing machineries to achieve transcript-specific m6A methylation. Specific m6A recruiters may act at different developmental stages or under various environmental stimuli to guide m6A writers to distinct sets of transcripts, generating development- or stimulus-dependent m6A methylomes. Moreover, chromatin or epigenetic signatures also affect m6A deposition in mammalian cells, represented by H3K36me3 that guides m6A methylation co-transcriptionally through METTL14 [90]. Likewise, m6A sites are correlated to H3K36me2 marks in Arabidopsis [91], implying a potential mechanism of m6A deposition mediated by epigenetic signatures in plants.
Another recent study in rice has suggested the presence of a third m6A methyltransferase ENHANCED DOWNY MILDEW 2-like (OsEDM2L), which contains a highly conserved N6-adenine methyltransferase-like (MTL) domain (Fig. 2b) [92]. Total m6A levels and transcriptome-wide m6A enrichment are significantly reduced in osedm2l mutants, suggesting that OsEDM2L is indispensable for the m6A methylation landscape. As OsEDM2L is specifically expressed in anthers, context-dependent m6A methylation could be modulated by organ-specific m6A methyltransferases.
m6A erasers
m6A has been known as a reversible modification since the discovery of m6A erasers Fat mass and obesity-associated protein (FTO) and AlkB homolog 5 (ALKBH5) [93, 94]. Although there are no plant orthologs of FTO, multiple copies of ALKBH5 orthologs have been found in various plant species [95], including six of them in Arabidopsis, namely ALKBH9A/9B/9C/10A/10B/10C [96]. Among ALKBH5 plant orthologs, ALKBH9B/10B in Arabidopsis and SlALKBH2 in tomato have been characterized to mediate m6A demethylation (Fig. 2 and Table 1) [80, 97, 98]. ALKBH9B not only demethylates m6A-containing viral RNAs and modulates viral infection [98] but also mediates demethylation of a heat-activated retroelement Onsen in stress granules (SGs) [81]. So far, the mechanisms by which ALKBHs select their targets for demethylation are yet to be elucidated.
m6A readers
Recognition and interpretation of m6A by its readers affect the fate of methylated mRNAs in various mRNA metabolism processes. To date, three groups of m6A readers have been found to recognize m6A-modified transcripts through different mechanisms [99]. YTH domain-containing proteins directly bind m6A through highly conserved YTH domains [100, 101], while heterogeneous nuclear ribonucleoproteins (HNRNPs) recognize m6A-containing RNAs through m6A-dependent RNA structure remodeling [102–105]. Several other RBPs, such as insulin-like growth factor 2 mRNA binding protein, are associated with m6A-modified transcripts via unknown mechanisms [106]. By now, characterized m6A readers in plants all belong to the YTH domain protein family, including EVOLUTIONARILY CONSERVED C-TERMINAL REGION 2-4 (ECT2-4) and CPSF30L in Arabidopsis and MhYTP2 in apple (Fig. 2 and Table 1) [82–85, 107, 108].
ECT2/3/4 recognize m6A via their aromatic cages and function redundantly in regulating the timing of organ initiation and leaf morphology [85, 109]. In agreement with their genetic redundancy, ECT3 shares most overlapping target sites with ECT2 and modulates mRNA abundance in the cytoplasm [86]. However, there are conflicting views on functional mechanisms of ECT2. An early study suggests that ECT2 is localized both in the nucleus and cytoplasm to affect 3′UTR length and mRNA stability, respectively [84], whereas a recent study shows that ECT2 is exclusively localized in the cytoplasm to regulate its target abundance but has little direct effect on alterative polyadenylation (APA) [86]. Another m6A reader CPSF30L forms phase-separated nuclear bodies to influence APA of m6A-containing mRNAs (Fig. 2a) [82, 83, 87]. Disruption of CPSF30L results in global poly(A) site shifts and transcriptional readthrough in recently rearranged gene pairs in Arabidopsis [82, 87]. Additionally, the apple m6A reader MhYTP2 plays dual functions in mediating transcript stability and translation efficiency via unknown mechanisms [108]. Surprisingly, overexpression of MhYTP2 leads to a transcriptome-wide increase in m6A levels possibly via affecting expression levels of multiple m6A writers and erasers, implying possible crosstalk among m6A effectors to maintain appropriate cellular m6A levels.
Notably, plant genomes encode more YTH domain proteins than other eukaryotes. For instance, there are 13 in Arabidopsis, 12 in rice, and 39 in wheat [95]. These genes may exhibit diverse expression patterns under different developmental and stress conditions, thus conferring functional diversities. Further exploration of their biological roles and functional modes are critical for better interpreting m6A epitranscriptome in plants.
m5C writers
Like m6A, m5C deposition, removal, and interpretation in animals require the respective roles of writers including NOL1/NOP2/sun (NSUN) family and DNA methyltransferase homolog DNMT2, erasers such as ten-eleven translocation proteins, and readers including Aly/REF export factor and Y-box binding protein 1 [110]. However, only m5C writers so far have been characterized in plants and include the NSUN orthologs, such as tRNA-specific methyltransferase 4 (TRM4B), TRM4C/NOP2A, TRM4D/NOP2B, and TRM4H in Arabidopsis and OsNSUN2 in rice, and the DNMT2 ortholog tRNA aspartic acid methyltransferase 1 (TRDMT1) in Arabidopsis [19, 42, 43, 111, 112]. Among them, the Arabidopsis TRM4B and rice OsNSUN2 have been shown to mediate m5C methylation in mRNAs (Fig. 2 and Table 1).
Despite sequence homology among plant m5C writer proteins, m5C distribution patterns and targets are less conserved in various plants. For example, as revealed by BS-seq, m5C is evenly distributed in coding sequences (CDSs) and highly enriched in 3′UTRs in Arabidopsis siliques, seedling shoots and roots [43], whereas m5C is mostly enriched immediately after the start codon in rice seedling shoots [42]. These observations imply functional divergence of m5C writers in selecting their targets in different organisms. Notably, so far different m5C profiling approaches have revealed different m5C distribution patterns. For example, in contrast to those revealed by BS-seq mentioned above [43], a m5C-RIP-seq study uncovers strong enrichment of m5C in CDSs with a small peak just after the start codon and a high peak before the stop codon in Arabidopsis seedlings [19], while another m5C-RIP-seq study with a different m5C antibody shows enrichment of m5C in CDSs with a high peak after the start codon and a less pronounced peak before the stop codon [113]. Thus, it is necessary to use other approaches like miCLIP-seq [114] and Aza-IP-seq [115] to cross-check bona fide m5C sites in plants. In addition, as functional redundancies among members of NSUN families (e.g. eight in Arabidopsis) [44] confound the characterization of m5C writers in plants, further elucidation of their biological functions will partly rely on generation and characterization of high-order mutants.
Advances in landscape and regulation of plant m6A dynamics
Characteristics of m6A landscape across plant species
Transcriptome-wide m6A targets and distribution have been extensively profiled with m6A-seq or nanopore DRS in multiple plant species, including pear, apple, strawberry, soybean, pak-choi, Arabidopsis, tomato, rice, maize, etc. [27–29, 92, 97, 108, 116–134]. m6A methylation ratios range from 29% in common wheat to 51% in earthmoss across 13 plant species [27]. The m6A distribution preference around stop codons and in 3′UTRs is highly conserved across most plant species from green algae to higher land plants (Fig. 3a), indicating that m6A is an evolutionarily conserved RNA modification. Despite their low abundance, m6A marks in CDSs have been consistently observed in several plant species, such as pear, strawberry, sea-buckthorn, pak-choi, and rice [92, 116–118, 122, 127]. In the rare case, m6A is highest enriched in CDSs in apple leaf [108]. These observations indicate that m6A in CDS might be a previously neglected but important feature with functional significance. For instance, there is an increase in m6A peaks in CDSs during strawberry fruit ripening [117], implying that m6A in CDS might be associated with changing developmental contexts. In addition, m6A enrichment is also observable around the start codon in Arabidopsis, pear, and sea-buckthorn [28, 116, 118] and within 5’UTRs in apple, pak-choi and sweet sorghum [122, 128, 135].
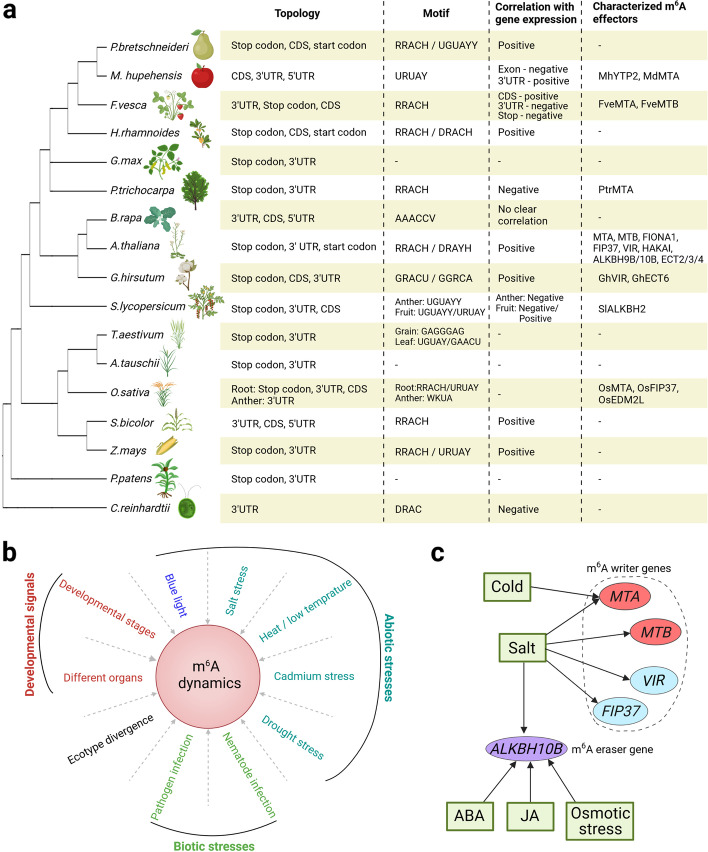
Fig 3.
Features of m6A RNA methylation in plants. a Summary of key features of m6A methylation in plants. Plant species with known m6A profiles on a global scale are shown in a phylogenetic tree (left). The key m6A features (right) are summarized based on the studies in Pyrus bretschneideri (pear) [116], Malus hupehensis (apple) [108], Fragaria vesca (strawberry) [117], Hippophae rhamnoides (sea-buckthorn) [118], Glycine max (soybean) [27], Populus trichocarpa (populus) [119–121], Brassica rapa (pak-choi) [122], Arabidopsis thaliana (Arabidopsis) [28, 29], Gossypium hirsutum (cotton) [133, 136], Solanum lycopersicum (tomato) [97, 123, 124], Triticum aestivum (common wheat) [125, 126], Aegilops tauschii (rough-spike hard grass) [27], Oryza sativa (rice) [92, 127], Sorghum bicolor (sorghum) [128], Zea mays (maize) [134], Physcomitrella patens (earthmoss) [27], and Chlamydomonas reinhardtii (green algae) [130]. Among these features, the “correlation with gene expression” is shown at the transcriptome-wide basis. R = A/G; W = A/U; K = G/U; Y = C/U; D = A/G/U; H = A/C/U; V = A/G/C. b m6A modifications are influenced by various endogenous and environmental signals as shown in the following studies: developmental stages (strawberry [117], wheat [125]); different organs (Arabidopsis [137]); ecotype divergence (Arabidopsis [138]); blue light (Arabidopsis [79]); salt stress (Arabidopsis [139, 140], sweet sorghum [128], rice [141], sugar beet [142], cotton [133]); heat/low temperature (pak-choi [122], Arabidopsis [143], tomato [124]); cadmium stress (rice [127], barley [132]); drought stress (sea-buckthorn [118], populus [119], apple [135, 144]); pathogen infection (apple [108], rice [145], wheat [126], watermelon [131], pear [116]); and nematode infection (soybean [146]). c Expression of m6A writer and eraser genes are modulated by multiple external stimuli in Arabidopsis. Arrows indicate positive regulation. Created with Biorender.com
m6A mainly falls into two sequence motifs in various plant species, including the conserved RRACH motif and the plant-specific URUAY (Y = C/U) motif, whereas a distinct AAACCV (V = A/G/C) motif has only been reported in pak-choi (Fig. 3a) [122]. Interestingly, m6A could occur in divergent sequence contexts at different developmental stages of the same plant species. For instance, in common wheat, the GAGGGAG and UGUAY motifs are found in m6A peaks in grains and leaves, respectively [125, 126].
Dynamic regulation of m6A distribution
m6A methylomes are dynamically changed at different developmental stages and in response to environmental stimuli in diverse plant species (Fig. 3b). Among different Arabidopsis tissues including roots, rosette leaves, and flowers, the fraction of transcripts displaying differential m6A modifications is significantly larger than that showing different transcript levels [137], indicating that m6A may contribute to organ differentiation.
m6A modifications are also dynamically affected by various stresses. Abiotic stresses, such as salt, drought, and heat, or biotic stresses, such as virus and fungal diseases, do not significantly influence the overall m6A distribution pattern in the 3′UTR and around stop codons but greatly induce dynamic m6A redistribution on selected transcripts [79, 108, 117–119, 122, 124–128, 131–133, 135, 137–147]. Salt stress significantly increases m6A methylation in Arabidopsis seedlings [139] and rice shoots but not in rice roots [141]. It induces dynamic deposition of m6A to salt-stress-related transcripts to protect them from degradation in Arabidopsis [140] and also increases m6A methylation on some salt-resistant-related transcripts to enhance their RNA stability in sweet sorghum [128]. Drought stress causes changes in m6A levels of drought-responsive genes, thereby affecting their expression levels in apple [135]. Cadmium (Cd) induces a transcriptome-wide m6A hypermethylation in barley roots [132] and alters methylation levels of a large number of transcripts in rice [127]. Together, these observations imply a prominent role of stress-induced m6A redistribution in stress adaptation.
Although many studies have revealed dynamic m6A methylations in organ-, age-, and stress-dependent manners in plants, the underlying mechanisms so far remain elusive. Such dynamic m6A regulation in various contexts could be partially achieved through titration of levels of m6A writers and erasers, resulting in global m6A redistribution. In Arabidopsis, salt stress increases m6A methylation likely through upregulating the expression of the m6A writer genes, MTA, MTB, VIR, and FIP37 (Fig. 3c) [139]. Interestingly, ALKBH10B expression is also upregulated under salt stress [148], indicating that salt stress-induced m6A dynamics is cooperatively sculpted by increased levels of m6A writers and erasers. Drought stress reduces m6A methylation in sea-buckthorn, which is associated with a significantly increased expression of m6A demethylase genes HrALKBH10B/10C/10D [118]. Besides transcriptional regulation, post-transcriptional modification of m6A effectors or deployment of m6A recruiters under various conditions may also contribute to m6A dynamics. For instance, upon blue light treatment, MTA, MTB, and FIP37 are recruited to the CRY2 nuclear bodies for selective m6A methylation on central oscillator transcripts [79]. Further exploring the mechanisms underlying dynamic m6A alterations on selective transcripts under various conditions will advance our mechanistic understanding of context-dependent regulation of m6A dynamics.
Advances in plant epitranscriptome in gene regulation
RNA modifications determine plant mRNA fate through influencing various aspects of mRNA metabolism, including alternative splicing (AS), APA, folding, translation, localization, transport, and decay (Figs. 2a and 4) [13, 15, 16]. These effects on mRNA metabolism ultimately impact a wide range of physiological processes in plant development and stress responses, as demonstrated by characterization of a collection of the mutants defective in RNA modification effectors.
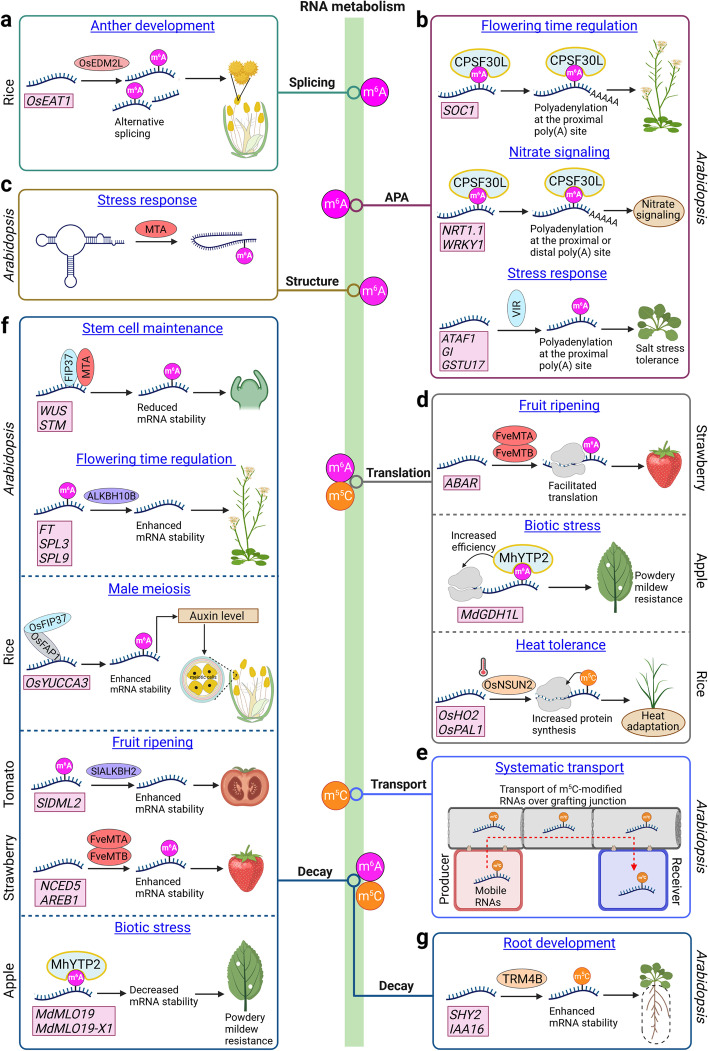
Fig. 4.
Epitranscriptome-mediated RNA metabolism and its effects on plant development, cellular processes, and stress responses. a OsEDM2L mediates m6A modification of OsEAT1, resulting in proper alternative splicing of OsEAT1 in rice anther development. b m6A modification affects alternative polyadenylation (APA) in Arabidopsis. Binding of CPSF30L to m6A-modified SOC1 mRNA regulates its APA and results in relatively stable SOC1 transcripts with a shorter 3′UTR to promote flowering. CPSF30L also mediates nitrate signaling through regulating the APA of several m6A-modified transcripts, including NRT1.1 and WRKY1, in the nitrate signaling pathway. VIR mediates m6A modification and APA of several stress-related transcripts in salt stress response. c m6A deposition on salt-stress-responsive transcripts by MTA is associated with a decrease in RNA secondary structures, causing increased RNA stability. d m6A modification affects protein translation in several crops. In strawberry fruit ripening, FveMTA- and FveMTB-mediated m6A modification of ABAR facilitates its translation. In apple, binding of MhYTP2 to m6A-modified MdGDH1L promotes its translation to confer powdery mildew resistance. In rice, OsNSUN2-dependent m5C modification increased protein synthesis to enhance rice adaptation to heat stress. e m5C RNA modification regulates RNA transport over the grafting junction in Arabidopsis. f m6A modification affects RNA stability in various plants. In Arabidopsis, m6A modification of WUS and STM mediated by FIP37 and MTA reduces their mRNA stability to maintain normal stem cell activity. The m6A eraser ALKBH10B demethylates FT, SPL3, and SPL9, thus enhancing their mRNA stability to promote flowering in Arabidopsis. In rice, OsFIP37 interacts with OsFAP1 to deposit m6A modification on OsYUCCA3 transcripts to promote auxin biosynthesis required for male meiosis. In tomato fruits, SlALKBH2 demethylates and enhances the stability of SlDML2 to accelerate fruit ripening. In strawberry, FveMTA and FveMTB deposit m6A modification on NCED5 and AREB1 transcripts, thus enhancing their RNA stability to promote fruit ripening. In apple, MhYTP2 binding to m6A-modified transcripts of MdMLO19 and MdMLO19-X1 destabilizes their transcripts to promote resistance to powdery mildew. g TRM4B-dependent m5C modification enhances RNA stability of its target transcripts in Arabidopsis root development. Created with Biorender.com
m6A function in the nucleus
m6A affects the pre-mRNA maturation processes, including AS and APA, to different extents in plants. Although decreased m6A methylation in FIP37-, VIR-, or FIO1-defective Arabidopsis mutants [28, 31, 59, 75] or OsFIP37-defective rice [149] has a mild effect on transcriptome-wide AS, OsEDM2L-mediated m6A methylation on ETERNAL TAPETUM 1 (OsEAT1) affects AS of OsEAT1, thereby modulating rice anther development (Fig. 4a) [92]. These observations indicate that instead of having a general effect on AS, m6A may regulate AS in specific transcripts or tissues. In contrast, m6A influences APA on a transcriptome-wide scale. Loss of VIR-mediated m6A results in defective 3′end formation of RNAs, which is mainly a shift to usage of proximal poly(A) sites [31]. CPSF30L regulates global poly(A) site choice through recognizing m6A-modified far-upstream sequence but does not show clear preference to proximal or distal poly(A) sites [82]. This m6A-assisted polyadenylation by CPSF30L also restricts transcription readthrough and chimeric mRNA formation at rearranged genomic loci, thus protecting transcriptome integrity [87]. A correlation of m6A sites with APA has also been observed in maize and populus [119, 120], implying a general role of m6A in regulating APA in plants. Moreover, APA at specific transcripts has been linked with plant physiological processes (Fig. 4b). For instance, CPSF30L modulates APA of several transcripts, including SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 (SOC1) and NRT1.1 to influence floral transition and nitrate signaling [82, 83]. VIR-mediated m6A methylation affects 3′UTR lengthening via APA of several salt stress regulators including ATAF1, GI, and GSTU17 in stress adaptation [139].
Besides pre-mRNA maturation, accumulating evidence suggests that m6A exerts important roles in regulating chromatin accessibility and transcription in mammalian cells [150, 151], whereas the similar evidence has just begun to emerge in plants. m6A affects dynamics of FCA nuclear condensates, further influencing the DNA:RNA hybrid (R-loop) formation at the COOLAIR locus in Arabidopsis [78], which indicates a possible role of m6A in chromatin regulation. Moreover, overexpression of the human FTO in rice increases chromatin accessibility, resulting in a more open chromatin state [152]. Whether m6A mediated by endogenous m6A effectors in plants modulates chromatin states needs further exploration.
m6A function in the cytoplasm
Compared with its nuclear roles in pre-mRNAs, cytoplasmic roles of m6A in affecting mature mRNA metabolism, including folding, translation, localization, and decay, are better understood (Figs. 2a and 4). m6A deposition on transcripts in salt-dependent manner is negatively correlated with RNA folding and the resulting secondary structure, which further affects RNA stability (Fig. 4c) [153]. The m6A-mediated change in RNA secondary structure has also been observed in primary microRNAs (pri-miRNAs), in which MTA-mediated m6A deposition induces their secondary structures [154]. These observations imply that m6A has both stimulatory and inhibitory effects on RNA folding, which may be target- or context-dependent. Structural change in RNAs induced by m6A may affect their interactions with RNA-binding proteins [104], thus influencing other RNA metabolism steps.
Recent studies have also shown m6A effect on mRNA translation in plants. Combination of m6A-seq and polysome profiling analyses in maize has revealed complex correlations between m6A and translation efficiency, in which m6A is negatively correlated with translation status on the global scale, whereas m6A close to the start codon tends to facilitate translation [155]. Similarly, a m6A site in the 5’UTR of Md4CL3 transcripts in apple has been shown to promote translation [135], which is similar to the observation in HeLa cells [156]. The promotive effect of m6A on translation has also been observed in apple transcripts containing m6A in the 3′UTR and the transcripts in strawberry fruit [108, 117]. Moreover, m6A-mediated translation seems to have important functions in different physiological processes (Fig. 4d). FveMTA/FveMTB-mediated m6A methylation on the putative ABA receptor (ABAR) facilitates translation of ABAR, thereby regulating strawberry fruit ripening [117], while the apple m6A reader MhYTP2 enhances translation of MdGDH1L, conferring resistance to powdery mildew [108]. Despite these observations, the mechanisms underlying m6A role in translation remain unknown in plants.
So far, the effect on mRNA stability is the best characterized role of m6A in various plant species and influences multiple aspects of developmental processes and stresses responses. From a transcriptome-wide perspective, m6A displays both promotive and inhibitory effects on mRNA stability in plants. In Arabidopsis, loss of MTA-, VIR-, and FIP37-dependent m6A methylation leads to a global reduction of transcript abundance [28, 31, 140], indicating a stabilizing role of m6A in mRNA. Likewise, m6A readers ECT2/3 stabilize transcripts as most target transcripts of ECT2/3 exhibit reduced abundance upon loss of ECT2/3/4 [86]. These effects on increasing RNA stability likely result from the inhibition of ribonucleolytic cleavage by m6A [140]. Effects of m6A on gene expression have also been examined in various crops via combining m6A-seq and RNA-seq (Fig. 3a). Positive correlation between m6A and transcript abundance has been similarly observed in pear [116], sea buckthorn [118], cotton [133], salt-resistant related transcripts in sweet sorghum [128], and maize genes bearing 2,4-D-induced m6A peaks [129], whereas negative correlation has been shown in populus [120] and green alga [130]. Interestingly, m6A and transcript abundance are generally negatively associated in anthers and during fruit ripening [97, 124] but positively correlated during fruit expansion in tomato [123]. Moreover, m6A near the stop codon and within the 3′UTR is negatively related to transcript abundance, whereas m6A in the CDS tends to stabilize mRNA in strawberry fruit [117]. These complex relationships between m6A and transcript abundance reflect distinct cellular fates of m6A-modified mRNAs in different contexts, implying that mRNA decay mediated by m6A is likely affected by species, developmental stage, cell type, and stress. However, as previous studies have been mostly focused on entire seedlings or organs [157], the heterogeneity of m6A dynamics and its effect on transcript abundance so far remain elusive. Thus, further analyses, including measuring the half-life of RNA at the transcriptome-wide scale, in various tissues and developmental stages of defective mutants of m6A writers, erasers, or readers will be necessary to disclose the complex and dynamic effects of m6A on transcript stability.
It has also been shown that m6A exerts promotive and inhibitory roles on stability of specific transcripts, which is of great importance in regulating many biological processes in plants (Fig. 4f). In Arabidopsis, FIP37-dependent m6A deposition on WUSCHEL (WUS) and SHOOTMERISTEMLESS (STM) mRNAs accelerates their mRNA decay, which is crucial for maintaining the normal function of shoot apical meristem [28]. The m6A demethylase ALKBH10B demethylates m6A-containing transcripts of FLOWERING LOCUS T (FT) and SQUAMOUSA PROMOTER BINDING PROTEIN-LIKE 3/9 (SPL3/9), thereby increasing their mRNA stability to promote flowering [80]. Likewise, the tomato SlALKBH2 demethylates the m6A-modified SlDML2 transcript and increases its stability, contributing to fruit ripening [97]. Overexpression of an apple m6A reader MhYTP2 promotes the decay of MdMLO19 and MdMLO19-X1, thus promoting apple resistance to powdery mildew [108]. These studies exemplify how m6A destabilizes specific transcripts to regulate various developmental processes and stress responses. By contrast, m6A also stabilizes specific transcripts in multiple physiological processes. In rice, m6A methylation on OsYUCCA3 mediated by the OsFIP37-OsFAP1 complex stabilizes OsYUCCA3 transcripts, thereby promoting local auxin biosynthesis in anthers to secure successful male meiosis and fertility [89]. In strawberry, FveMTA-mediated m6A modifications on NCED5 and AREB1 enhance their transcript stability to ensure normal fruit ripening [117].
While these pieces of evidence clearly demonstrate that m6A affects transcript stability, the underlying regulatory modes await further examination. Recognition of m6A marks by different m6A readers is possibly important for sorting mRNAs for stabilization or destabilization. It has been suggested that in mammalian cells, YTHDF2 (ortholog of ECT2/3/4) mediates degradation of m6A-containing transcripts via two mechanisms: directly recruiting the CCR4-NOT deadenylase complex for deadenylation of m6A-containing transcripts or interacting with endoribonucleases for endoribonucleolytic cleavage [158–160]. On the contrary, expression levels of a majority of ECT2/3-target RNAs are decreased in ect2/3/4 triple mutants, indicating the roles of ECT2/3 in stabilizing transcripts [86]. Intriguingly, upon heat exposure, ECT2 is relocated to SGs through which ECT2 may affect transcript stability [107]. Similarly, the complexes of YTHDFs-m6A-marked mRNAs are partitioned into phase-separated compartments including SGs under stress conditions in mES cells [161]. Many plant ECTs contain low complexity regions or prion-like domains (PrLDs), which could drive LLPS to facilitate formation of membraneless condensates (Table 1) [82, 162], implying that interaction between ECTs and their associated m6A-modified RNAs in distinct cellular compartments might be important for determining the stability of these RNAs.
In addition to the above cytoplasmic roles, m6A may also function in regulating RNA localization. m6A-demethylation of the heat-activated retroelement Onsen mediated by SG-localized ALKBH9B releases Onsen from SG, thus altering its localization (Fig. 2a) [81]. Taken together, current studies have suggested multifaceted nuclear and cytoplasmic roles of m6A in post-transcriptional gene regulation and also raised intriguing questions about the fundamental nature of the relevant mechanisms.
m5C function
As another abundant internal mRNA modification, m5C has also been studied, to a lesser extent, in recent years, which uncovered its important roles in mediating RNA translation, transport, and stability. m5C is associated with mRNAs with low translational activity in Arabidopsis [19], whereas m5C mediated by OsNSUN2 facilitates protein synthesis in rice [42], indicating the functional divergence of m5C in different plant species. In particular, OsNSUN2-mediated m5C on several targets, including OsHO2 and OsPAL1, promotes their protein synthesis, thus enhancing rice adaptation to heat stress (Fig. 4d) [42]. Interestingly, heat stress leads to increased m5C methylation on mRNAs involved in photosynthesis and detoxification systems [42], implying that m5C is dynamically modulated during stress conditions.
In Arabidopsis, the m5C methyltransferase TRM4B promotes root growth by enhancing mRNA stability of its methylation targets, such as SHORT HYPOCOTYL 2 (SHY2) and INDOLEACETIC ACID-INDUCED PROTEIN 16 (IAA16) (Fig. 4g) [19, 43]. In addition, m5C is highly enriched in mobile mRNAs [58, 113]. TRM4B- and TRDMT1-mediated m5C modifications on mobile mRNAs are essential for systematic mRNA transport from producer to receiver cells over graft junctions (Fig. 4e) [113].
Outlook
The last decade has witnessed rapid advances in plant epitranscriptome with respect to m6A dynamics under stresses in different plant species and mechanistic understanding of m6A and m5C modifications in the model plant Arabidopsis and rice. Current evidence strongly suggests that epitranscriptomic marks constitute an essential layer of post-transcriptional gene regulation that determines mRNA fate and ultimately influences plant development and adaptation to various environmental stresses. However, our understanding of plant epitranscriptome is still in its infancy. Many open questions regarding the target selectivity and functional modes of epitranscriptome marks remain to be explored. For example, how do writers and erasers select their targets in different physiological contexts? How are mRNA modifications dynamically regulated in response to environmental stimuli? How do reader proteins recognize their targets and exert their roles in subsequent RNA metabolic processes? Furthermore, since RNA modifications are highly dependent on cellular contexts, the relevant regulatory pathways may differ in different tissues and organs, at distinct developmental stage, or under different stresses. Thus, it is necessary to analyze RNA modification dynamics from tissue levels to cellular levels at single-nucleotide resolution via newly developed profiling techniques. This will advance and expand our knowledge in the plant epitranscriptome.
Multifaced roles of epitranscriptome in developmental processes and stress adaptations in diverse plant species underpin that editing epitranscriptome is a promising strategy for crop improvement. Indeed, introducing the human m6A demethylase in rice and potato not only increases yield but also enhances drought tolerance [152], demonstrating empirically the great potential of epitranscriptome editing in boosting agricultural production. In particular, advanced DNA/RNA editing techniques in recent years facilitate the development of multiple strategies for epitranscriptome editing of crops [63, 163]. First, modulation of expression or activity of RNA modification-related proteins (RMPs) could be achieved through CRISPR/Cas9-mediated gene editing, thus affecting traits mediated by RNA modifications. Second, RNA modification sites could be directly mutated on specific targets through precise base editors, such as the adenine base editor composed of the catalytically inactive CRISPR/Cas9 protein and an engineered adenosine deaminase causing A to G substitution [164, 165]. Third, RNA modifications could be specifically created or removed on specific target sites through the catalytically inactivated Cas13 (dCas13)-RMPs. For examples, in mammalian cells, dCas13 fused with a truncated methyltransferase domain of METTL3 guides site-specific m6A methylation in target transcripts [166], while dCas13 fused to m6A demethylases, such as ALKBH5, results in targeted RNA demethylation [167]. Intriguingly, targeted RNA demethylation or methylation can also be achieved in spatiotemporal manners using dCas13 fused with the light-sensitive protein CBIN and its adaptor CRY2 linked to FTO or essential domains of METTL3/14, respectively [168].
It is noteworthy that the application of epitranscriptome editing in crop biotechnology should at least address the following bottlenecks. First, as m6A sites at single-base resolution are largely unknown in crops, it is necessary to apply techniques, such as miCLIP, MAZTER-seq, m6A-SAC-seq, and Nanopore DRS, to precisely interrogate m6A at single-nucleotide resolution in crops. Second, biological effects of removing/adding epitranscriptome mark at a specific transcript are mostly unknown in plants. Third, although writers, erasers, and readers of RNA modifications have been identified in diverse crops [95], their endogenous roles and functional mechanisms remain largely elusive. Fourth, DNA/RNA-editing systems and their target specificities and editing efficiencies have yet to be established and examined in most crops. Thus, precise identification of RNA modification sites through advanced profiling approaches, mechanistic understanding of epitranscriptome marks, and development of efficient plant DNA/RNA editors should constitute integral parts of epitranscriptome editing to maximize its potential for crop improvement.
Supplementary Information
Acknowledgements
We apologize to those colleagues whose excellence work could not be cited due to space restrictions.
Peer review information
Wenjing She was the primary editor of this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Review history
The review history is available as Additional file 1.
Authors’ contributions
All authors wrote and approved the final version of the manuscript.
Funding
This work was supported by the National Research Foundation Competitive Research Programme (NRF-CRP22-2019-0001), Singapore Food Story R&D Programme (SFS_RND_SUFP_001_04), and the intramural research support from National University of Singapore and Temasek Life Sciences Laboratory.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Lisha Shen, Email: lisha@tll.org.sg.
Hao Yu, Email: dbsyuhao@nus.edu.sg.
References
- 1.Boccaletto P, Stefaniak F, Ray A, Cappannini A, Mukherjee S, Purta E, et al. MODOMICS: a database of RNA modification pathways. 2021 update. Nucleic Acids Res. 2022;50(D1):D231–5. [DOI] [PMC free article] [PubMed]
- 2.Cahova H, Winz ML, Hofer K, Nubel G, Jaschke A. NAD captureSeq indicates NAD as a bacterial cap for a subset of regulatory RNAs. Nature. 2015;519:374–377. doi: 10.1038/nature14020. [DOI] [PubMed] [Google Scholar]
- 3.Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR. Comprehensive analysis of mRNA methylation reveals enrichment in 3' UTRs and near stop codons. Cell. 2012;149:1635–1646. doi: 10.1016/j.cell.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485:201–206. doi: 10.1038/nature11112. [DOI] [PubMed] [Google Scholar]
- 5.Li X, Xiong X, Wang K, Wang L, Shu X, Ma S, et al. Transcriptome-wide mapping reveals reversible and dynamic N1-methyladenosine methylome. Nat Chem Biol. 2016;12:311. doi: 10.1038/nchembio.2040. [DOI] [PubMed] [Google Scholar]
- 6.Dominissini D, Nachtergaele S, Moshitch-Moshkovitz S, Peer E, Kol N, Ben-Haim MS, et al. The dynamic N1-methyladenosine methylome in eukaryotic messenger RNA. Nature. 2016;530:441–446. doi: 10.1038/nature16998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Squires JE, Patel HR, Nousch M, Sibbritt T, Humphreys DT, Parker BJ, et al. Widespread occurrence of 5-methylcytosine in human coding and non-coding RNA. Nucleic Acids Res. 2012;40:5023–5033. doi: 10.1093/nar/gks144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arango D, Sturgill D, Alhusaini N, Dillman AA, Sweet TJ, Hanson G, et al. Acetylation of cytidine in mRNA promotes translation efficiency. Cell. 2018;175(1872-1886):e1824. doi: 10.1016/j.cell.2018.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delatte B, Wang F, Ngoc LV, Collignon E, Bonvin E, Deplus R, et al. Transcriptome-wide distribution and function of RNA hydroxymethylcytosine. Science. 2016;351:282–285. doi: 10.1126/science.aac5253. [DOI] [PubMed] [Google Scholar]
- 10.Zhang LS, Liu C, Ma H, Dai Q, Sun HL, Luo G, et al. Transcriptome-wide mapping of internal N7-methylguanosine methylome in mammalian mRNA. Mol Cell. 2019;74(1304-1316):e1308. doi: 10.1016/j.molcel.2019.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz S, Bernstein DA, Mumbach MR, Jovanovic M, Herbst RH, Leon-Ricardo BX, et al. Transcriptome-wide mapping reveals widespread dynamic-regulated pseudouridylation of ncRNA and mRNA. Cell. 2014;159:148–162. doi: 10.1016/j.cell.2014.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carlile TM, Rojas-Duran MF, Zinshteyn B, Shin H, Bartoli KM, et al. Pseudouridine profiling reveals regulated mRNA pseudouridylation in yeast and human cells. Nature. 2014;515:143–146. doi: 10.1038/nature13802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shao Y, Wong CE, Shen L, Yu H. N6-methyladenosine modification underlies messenger RNA metabolism and plant development. Curr Opin Plant Biol. 2021;63:102047. doi: 10.1016/j.pbi.2021.102047. [DOI] [PubMed] [Google Scholar]
- 14.Zhou L, Gao G, Tang R, Wang W, Wang Y, Tian S, et al. m6A-mediated regulation of crop development and stress responses. Plant Biotechnol J. 2022;20:1447–1455. doi: 10.1111/pbi.13792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhatia G, Prall W, Sharma B, Gregory BD. Covalent RNA modifications and their budding crosstalk with plant epigenetic processes. Curr Opin Plant Biol. 2022;69:102287. doi: 10.1016/j.pbi.2022.102287. [DOI] [PubMed] [Google Scholar]
- 16.Yu X, Sharma B, Gregory BD. The impact of epitranscriptomic marks on post-transcriptional regulation in plants. Brief Funct Genom. 2021;20:113–124. doi: 10.1093/bfgp/elaa021. [DOI] [PubMed] [Google Scholar]
- 17.Yang W, Meng J, Liu J, Ding B, Tan T, Wei Q, et al. The N1-methyladenosine methylome of petunia mRNA. Plant Physiol. 2020;183:1710–1724. doi: 10.1104/pp.20.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun L, Xu Y, Bai S, Bai X, Zhu H, Dong H, et al. Transcriptome-wide analysis of pseudouridylation of mRNA and non-coding RNAs in Arabidopsis. J Exp Bot. 2019;70:5089–5600. doi: 10.1093/jxb/erz273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cui X, Liang Z, Shen L, Zhang Q, Bao S, Geng Y, et al. 5-methylcytosine RNA methylation in Arabidopsis thaliana. Mol Plant. 2017;10:1387–1399. doi: 10.1016/j.molp.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 20.Yu X, Willmann MR, Vandivier LE, Trefely S, Kramer MC, Shapiro J, et al. Messenger RNA 5' NAD(+) capping is a dynamic regulatory epitranscriptome mark that is required for proper response to abscisic acid in Arabidopsis. Dev Cell. 2021;56(125-140):e126. doi: 10.1016/j.devcel.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Li S, Zhao Y, You C, Le B, Gong Z, et al. NAD(+)-capped RNAs are widespread in the Arabidopsis transcriptome and can probably be translated. Proc Natl Acad Sci. 2019;116:12094–12102. doi: 10.1073/pnas.1903682116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarkar A, Gasperi W, Begley U, Nevins S, Huber SM, Dedon PC, et al. Detecting the epitranscriptome. Wiley Interdiscip Rev RNA. 2021;12:e1663. doi: 10.1002/wrna.1663. [DOI] [PubMed] [Google Scholar]
- 23.Thüring K, Schmid K, Keller P, Helm M. LC-MS Analysis of methylated RNA. Methods Mol Biol. 2017;1562:3–18. doi: 10.1007/978-1-4939-6807-7_1. [DOI] [PubMed] [Google Scholar]
- 24.Zhong SL, Li HY, Bodi Z, Button J, Vespa L, Herzog M, et al. MTA is an Arabidopsis messenger RNA adenosine methylase and interacts with a homolog of a sex-specific splicing factor. Plant Cell. 2008;20:1278–1288. doi: 10.1105/tpc.108.058883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen L, Liang Z, Yu H. Dot Blot Analysis of N6-methyladenosine RNA modification levels. Bio-protocol. 2017;7:e2095. doi: 10.21769/BioProtoc.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edelheit S, Schwartz S, Mumbach MR, Wurtzel O, Sorek R. Transcriptome-wide mapping of 5-methylcytidine RNA modifications in bacteria, archaea, and yeast reveals m5C within archaeal mRNAs. PLoS Genet. 2013;9:e1003602. doi: 10.1371/journal.pgen.1003602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miao Z, Zhang T, Xie B, Qi Y, Ma C. Evolutionary implications of the RNA N6-methyladenosine methylome in plants. Mol Biol Evol. 2022;39:msab299. [DOI] [PMC free article] [PubMed]
- 28.Shen L, Liang Z, Gu X, Chen Y, Teo ZW, Hou X, et al. N6-methyladenosine RNA modification regulates shoot stem cell fate in Arabidopsis. Dev Cell. 2016;38:186–200. doi: 10.1016/j.devcel.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo GZ, MacQueen A, Zheng G, Duan H, Dore LC, Lu Z, et al. Unique features of the m6A methylome in Arabidopsis thaliana. Nat Commun. 2014;5:5630. doi: 10.1038/ncomms6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Linder B, Grozhik AV, Olarerin-George AO, Meydan C, Mason CE, Jaffrey SR. Single-nucleotide-resolution mapping of m6A and m6Am throughout the transcriptome. Nat Methods. 2015;12:767–772. doi: 10.1038/nmeth.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parker MT, Knop K, Sherwood AV, Schurch NJ, Mackinnon K, Gould PD, et al. Nanopore direct RNA sequencing maps the complexity of Arabidopsis mRNA processing and m6A modification. eLife. 2020;9:e49658. doi: 10.7554/eLife.49658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ke SD, Alemu EA, Mertens C, Gantman EC, Fak JJ, Mele A, et al. A majority of m6A residues are in the last exons, allowing the potential for 3' UTR regulation. Genes Dev. 2015;29:2037–2053. doi: 10.1101/gad.269415.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen K, Lu Z, Wang X, Fu Y, Luo GZ, Liu N, et al. High-resolution N6-methyladenosine (m6A) map using photo-crosslinking-assisted m6A sequencing. Angew Chem Int Ed Eng. 2015;54:1587–1590. doi: 10.1002/anie.201410647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koh CWQ, Goh YT, Goh WSS. Atlas of quantitative single-base-resolution N6-methyl-adenine methylomes. Nat Commun. 2019;10:5636. doi: 10.1038/s41467-019-13561-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X, Xiong X, Zhang M, Wang K, Chen Y, Zhou J, et al. Base-resolution mapping reveals distinct m1A methylome in nuclear- and mitochondrial-encoded transcripts. Mol Cell. 2017;68(993-1005):e1009. doi: 10.1016/j.molcel.2017.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dierks D, Garcia-Campos MA, Uzonyi A, Safra M, Edelheit S, Rossi A, et al. Multiplexed profiling facilitates robust m6A quantification at site, gene and sample resolution. Nat Methods. 2021;18:1060–1067. doi: 10.1038/s41592-021-01242-z. [DOI] [PubMed] [Google Scholar]
- 37.Schaefer M, Pollex T, Hanna K, Lyko F. RNA cytosine methylation analysis by bisulfite sequencing. Nucleic Acids Res. 2009;37:e12. doi: 10.1093/nar/gkn954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huber SM, van Delft P, Mendil L, Bachman M, Smollett K, Werner F, et al. Formation and abundance of 5-hydroxymethylcytosine in RNA. ChemBioChem. 2015;16:752–755. doi: 10.1002/cbic.201500013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Legrand C, Tuorto F, Hartmann M, Liebers R, Jacob D, Helm M, et al. Statistically robust methylation calling for whole-transcriptome bisulfite sequencing reveals distinct methylation patterns for mouse RNAs. Genome Res. 2017;27:1589–1596. doi: 10.1101/gr.210666.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang X, Liu M, Li M, Zhang S, Hiju H, Sun J, et al. Epigenetic modulations of noncoding RNA: a novel dimension of cancer biology. Mol Cancer. 2020;19:64. doi: 10.1186/s12943-020-01159-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen LS, Liang Z, Wong CE, Yu H. Messenger RNA modifications in plants. Trends Plant Sci. 2019;24:328–341. doi: 10.1016/j.tplants.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 42.Tang Y, Gao CC, Gao Y, Yang Y, Shi B, Yu JL, et al. OsNSUN2-mediated 5-methylcytosine mRNA modification enhances rice adaptation to high temperature. Dev Cell. 2020;53(272-286):e277. doi: 10.1016/j.devcel.2020.03.009. [DOI] [PubMed] [Google Scholar]
- 43.David R, Burgess A, Parker B, Li J, Pulsford K, Sibbritt T, et al. Transcriptome-wide mapping of RNA 5-methylcytosine in Arabidopsis mRNAs and noncoding RNAs. Plant Cell. 2017;29:445–460. doi: 10.1105/tpc.16.00751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burgess AL, David R, Searle IR. Conservation of tRNA and rRNA 5-methylcytosine in the kingdom Plantae. BMC Plant Biol. 2015;15:199. doi: 10.1186/s12870-015-0580-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garcia-Campos MA, Edelheit S, Toth U, Safra M, Shachar R, Viukov S, et al. Deciphering the "m6A Code" via antibody-independent quantitative profiling. Cell. 2019;178(731-747):e716. doi: 10.1016/j.cell.2019.06.013. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Z, Chen LQ, Zhao YL, Yang CG, Roundtree IA, Zhang Z, et al. Single-base mapping of m6A by an antibody-independent method. Sci Adv. 2019;5:eaax0250. doi: 10.1126/sciadv.aax0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Y, Xiao Y, Dong S, Yu Q, Jia G. Antibody-free enzyme-assisted chemical approach for detection of N6-methyladenosine. Nat Chem Biol. 2020;16:896–903. doi: 10.1038/s41589-020-0525-x. [DOI] [PubMed] [Google Scholar]
- 48.Meyer KD. DART-seq: an antibody-free method for global m6A detection. Nat Methods. 2019;16:1275–1280. doi: 10.1038/s41592-019-0570-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu L, Liu S, Peng Y, Ge R, Su R, Senevirathne C, et al. m6A RNA modifications are measured at single-base resolution across the mammalian transcriptome. Nat Biotechnol. 2022;40:1210–1219. doi: 10.1038/s41587-022-01243-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garalde DR, Snell EA, Jachimowicz D, Sipos B, Lloyd JH, Bruce M, et al. Highly parallel direct RNA sequencing on an array of nanopores. Nat Methods. 2018;15:201–206. doi: 10.1038/nmeth.4577. [DOI] [PubMed] [Google Scholar]
- 51.Anreiter I, Mir Q, Simpson JT, Janga SC, Soller M. New twists in detecting mRNA modification dynamics. Trends Biotechnol. 2020;39:72–89. doi: 10.1016/j.tibtech.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lorenz DA, Sathe S, Einstein JM, Yeo GW. Direct RNA sequencing enables m6A detection in endogenous transcript isoforms at base-specific resolution. RNA. 2020;26:19–28. doi: 10.1261/rna.072785.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu H, Begik O, Lucas MC, Ramirez JM, Mason CE, Wiener D, et al. Accurate detection of m6A RNA modifications in native RNA sequences. Nat Commun. 2019;10:4079. doi: 10.1038/s41467-019-11713-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stoiber M, Quick J, Egan R, Eun Lee J, Celniker S, Neely RK, et al. De novo identification of DNA modifications enabled by genome-guided nanopore signal processing. BioRxiV. 2016;094672.
- 55.Leger A, Amaral PP, Pandolfini L, Capitanchik C, Capraro F, Miano V, et al. RNA modifications detection by comparative Nanopore direct RNA sequencing. Nat Commun. 2021;12:7198. doi: 10.1038/s41467-021-27393-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pratanwanich PN, Yao F, Chen Y, Koh CWQ, Wan YK, Hendra C, et al. Identification of differential RNA modifications from nanopore direct RNA sequencing with xPore. Nat Biotechnol. 2021;39:1394–1402. doi: 10.1038/s41587-021-00949-w. [DOI] [PubMed] [Google Scholar]
- 57.Begik O, Lucas MC, Pryszcz LP, Ramirez JM, Medina R, Milenkovic I, et al. Quantitative profiling of pseudouridylation dynamics in native RNAs with nanopore sequencing. Nat Biotechnol. 2021;39:1278–1291. doi: 10.1038/s41587-021-00915-6. [DOI] [PubMed] [Google Scholar]
- 58.Zhang S, Li R, Zhang L, Chen S, Xie M, Yang L, et al. New insights into Arabidopsis transcriptome complexity revealed by direct sequencing of native RNAs. Nucleic Acids Res. 2020;48:7700–7711. doi: 10.1093/nar/gkaa588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu T, Wu X, Wong CE, Fan S, Zhang Y, Zhang S, et al. FIONA1-mediated m6A modification regulates the floral transition in Arabidopsis. Adv Sci. 2022;9:e2103628. doi: 10.1002/advs.202103628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xing P, Su R, Guo F, Wei L. Identifying N6-methyladenosine sites using multi-interval nucleotide pair position specificity and support vector machine. Sci Rep. 2017;7:46757. doi: 10.1038/srep46757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang Y, He N, Chen Y, Chen Z, Li L. BERMP: a cross-species classifier for predicting m6A sites by integrating a deep learning algorithm and a random forest approach. Int J Biol Sci. 2018;14:1669–1677. doi: 10.7150/ijbs.27819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhai J, Song J, Cheng Q, Tang Y, Ma C. PEA: an integrated R toolkit for plant epitranscriptome analysis. Bioinformatics. 2018;34:3747–3749. doi: 10.1093/bioinformatics/bty421. [DOI] [PubMed] [Google Scholar]
- 63.Shen L, Yu H. Epitranscriptome engineering in crop improvement. Mol Plant. 2021;14:1418–1420. doi: 10.1016/j.molp.2021.08.006. [DOI] [PubMed] [Google Scholar]
- 64.Růžička K, Zhang M, Campilho A, Bodi Z, Kashif M, Saleh M, et al. Identification of factors required for m6A mRNA methylation in Arabidopsis reveals a role for the conserved E3 ubiquitin ligase HAKAI. New Phytol. 2017;215:157–172. doi: 10.1111/nph.14586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schwartz S, Mumbach MR, Jovanovic M, Wang T, Maciag K, Bushkin GG, et al. Perturbation of m6A writers reveals two distinct classes of mRNA methylation at internal and 5' sites. Cell Rep. 2014;8:284–296. doi: 10.1016/j.celrep.2014.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ping XL, Sun BF, Wang L, Xiao W, Yang X, Wang WJ, et al. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 2014;24:177–189. doi: 10.1038/cr.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu JZ, Yue YN, Han DL, Wang X, Fu Y, Zhang L, et al. A METTL3–METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol. 2014;10:93–95. doi: 10.1038/nchembio.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Knuckles P, Lence T, Haussmann IU, Jacob D, Kreim N, et al. Zc3h13/Flacc is required for adenosine methylation by bridging the mRNA-binding factor Rbm15/Spenito to the m6A machinery component Wtap/Fl(2)d. Genes Dev. 2018;32:415–429. doi: 10.1101/gad.309146.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wen J, Lv RT, Ma HH, Shen HJ, He CX, Wang JH, et al. Zc3h13 regulates nuclear RNA m6A methylation and mouse embryonic stem cell self-renewal. Mol Cell. 2018;69:1028–1038. doi: 10.1016/j.molcel.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Patil DP, Chen CK, Pickering BF, Chow A, Jackson C, Guttman M, et al. m6A RNA methylation promotes XIST-mediated transcriptional repression. Nature. 2016;537:369–373. doi: 10.1038/nature19342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang M, Bodi Z, Mackinnon K, Zhong S, Archer N, Mongan NP, et al. Two zinc finger proteins with functions in m6A writing interact with HAKAI. Nat Commun. 2022;13:1127. doi: 10.1038/s41467-022-28753-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Parker MT, Knop K, Zacharaki V, Sherwood AV, Tomé D, Yu X, et al. Widespread premature transcription termination of Arabidopsis thaliana NLR genes by the spen protein FPA. eLife. 2021;10:e65537. doi: 10.7554/eLife.65537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lence T, Akhtar J, Bayer M, Schmid K, Spindler L, Ho CH, et al. m6A modulates neuronal functions and sex determination in Drosophila. Nature. 2016;540:242–247. doi: 10.1038/nature20568. [DOI] [PubMed] [Google Scholar]
- 74.Bodi Z, Zhong SL, Mehra S, Song J, Graham N, Li HY, et al. Adenosine methylation in Arabidopsis mRNA is associated with the 3' end and reduced levels cause developmental defects. Front Plant Sci. 2012;3:1–10. doi: 10.3389/fpls.2012.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang C, Yang J, Song P, Zhang W, Lu Q, Yu Q, et al. FIONA1 is an RNA N6-methyladenosine methyltransferase affecting Arabidopsis photomorphogenesis and flowering. Genome Biol. 2022;23:40. doi: 10.1186/s13059-022-02612-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Warda AS, Kretschmer J, Hackert P, Lenz C, Urlaub H, Hobartner C, et al. Human METTL16 is a N6-methyladenosine (m6A) methyltransferase that targets pre-mRNAs and various non-coding RNAs. EMBO Rep. 2017;18:2004–2014. doi: 10.15252/embr.201744940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pendleton KE, Chen B, Liu K, Hunter OV, Xie Y, Tu BP, et al. The U6 snRNA m6A methyltransferase METTL16 regulates SAM synthetase intron retention. Cell. 2017;169(824-835):e814. doi: 10.1016/j.cell.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu C, Wu Z, Duan HC, Fang X, Jia G, Dean C. R-loop resolution promotes co-transcriptional chromatin silencing. Nat Commun. 2021;12:1790. doi: 10.1038/s41467-021-22083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang X, Jiang B, Gu L, Chen Y, Mora M, Zhu M, et al. A photoregulatory mechanism of the circadian clock in Arabidopsis. Nat Plants. 2021;7:1397–1408. doi: 10.1038/s41477-021-01002-z. [DOI] [PubMed] [Google Scholar]
- 80.Duan HC, Wei LH, Zhang C, Wang Y, Chen L, Lu ZK, et al. ALKBH10B is an RNA N6-methyladenosine demethylase affecting Arabidopsis floral transition. Plant Cell. 2017;29:2995–3011. doi: 10.1105/tpc.16.00912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fan W, Wang L, Lei Z, Chu J, Cho J. Suppression of transposon mobilization by m6A-mediated RNA sequestration in stress granules. BioRxiV. 2022;2022-03.
- 82.Song PZ, Yang JB, Wang CL, Lu Q, Shi LQ, Tayier S, et al. Arabidopsis N6-methyladenosine reader CPSF30-L recognizes FUE signals to control polyadenylation site choice in liquid-like nuclear bodies. Mol Plant. 2021;14:571–587. doi: 10.1016/j.molp.2021.01.014. [DOI] [PubMed] [Google Scholar]
- 83.Hou YF, Sun J, Wu BX, Gao YY, Nie HB, Nie ZT, et al. CPSF30-L-mediated recognition of mRNA m6A modification controls alternative polyadenylation of nitrate signaling-related gene transcripts in Arabidopsis. Mol Plant. 2021;14:688–699. doi: 10.1016/j.molp.2021.01.013. [DOI] [PubMed] [Google Scholar]
- 84.Wei LH, Song PZ, Wang Y, Lu ZK, Tang Q, Yu Q, et al. The m6A reader ECT2 controls trichome morphology by affecting mRNA stability in Arabidopsis. Plant Cell. 2018;30:968–985. doi: 10.1105/tpc.17.00934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Arribas-Hernández L, Bressendorff S, Hansen MH, Poulsen C, Erdmann S, Brodersen P. An m6A-YTH module controls developmental timing and morphogenesis in Arabidopsis. Plant Cell. 2018;30:952–967. doi: 10.1105/tpc.17.00833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Arribas-Hernandez L, Rennie S, Schon M, Porcelli C, Enugutti B, Andersson R, et al. The YTHDF proteins ECT2 and ECT3 bind largely overlapping target sets and influence target mRNA abundance, not alternative polyadenylation. Elife. 2021;10:e72377. doi: 10.7554/eLife.72377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pontier D, Picart C, El Baidouri M, Roudier F, Xu T, Lahmy S, et al. The m6A pathway protects the transcriptome integrity by restricting RNA chimera formation in plants. Life Sci Alliance. 2019;2:e201900393. doi: 10.26508/lsa.201900393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lancaster AK, Nutter-Upham A, Lindquist S, King OD. PLAAC: a web and command-line application to identify proteins with prion-like amino acid composition. Bioinformatics. 2014;30:2501–2502. doi: 10.1093/bioinformatics/btu310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cheng P, Bao S, Li C, Tong J, Shen L, Yu H. RNA N6-methyladenosine modification promotes auxin biosynthesis required for male meiosis in rice. Dev Cell. 2022;57(246-259):e244. doi: 10.1016/j.devcel.2021.12.014. [DOI] [PubMed] [Google Scholar]
- 90.Huang HL, Weng HY, Zhou KR, Wu T, Zhao BS, Sun ML, et al. Histone H3 trimethylation at lysine 36 guides m6A RNA modification co-transcriptionally. Nature. 2019;567:414–419. doi: 10.1038/s41586-019-1016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shim S, Lee HG, Lee H, Seo PJ. H3K36me2 is highly correlated with m6A modifications in plant. J Integr Plant Biol. 2020;62:1455–1460. doi: 10.1111/jipb.12917. [DOI] [PubMed] [Google Scholar]
- 92.Ma K, Han J, Zhang Z, Li H, Zhao Y, Zhu Q, et al. OsEDM2L mediates m6A of EAT1 transcript for proper alternative splicing and polyadenylation regulating rice tapetal degradation. J Integr Plant Biol. 2021;63:1982–1994. doi: 10.1111/jipb.13167. [DOI] [PubMed] [Google Scholar]
- 93.Jia GF, Fu Y, Zhao X, Dai Q, Zheng GQ, Yang Y, et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol. 2011;7:885–887. doi: 10.1038/nchembio.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zheng G, Dahl JA, Niu Y, Fedorcsak P, Huang CM, Li CJ, et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell. 2013;49:18–29. doi: 10.1016/j.molcel.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu M, Nie F, Liu H, Zhang T, Li M, Song X, et al. The evolution of N6-methyladenosine regulators in plants. Methods. 2022;203:268–275. doi: 10.1016/j.ymeth.2021.11.013. [DOI] [PubMed] [Google Scholar]
- 96.Liang Z, Riaz A, Chachar S, Ding YK, Du H, Gu XF. Epigenetic modifications of mRNA and DNA in plants. Mol Plant. 2020;13:14–30. doi: 10.1016/j.molp.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 97.Zhou LL, Tian SP, Qin GZ. RNA methylomes reveal the m6A-mediated regulation of DNA demethylase gene SlDML2 in tomato fruit ripening. Genome Biol. 2019;20:156. doi: 10.1186/s13059-019-1771-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Martínez-Pérez M, Aparicio F, López-Gresa MP, Bellés JM, Sánchez-Navarro JA, Pallás V. Arabidopsis m6A demethylase activity modulates viral infection of a plant virus and the m6A abundance in its genomic RNAs. Proc Natl Acad Sci. 2017;114:10755–10760. doi: 10.1073/pnas.1703139114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shi H, Wei J, He C. Where, when, and how: context-dependent functions of RNA methylation writers, readers, and erasers. Mol Cell. 2019;74:640–650. doi: 10.1016/j.molcel.2019.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang X, Zhao BS, Roundtree IA, Lu ZK, Han DL, Ma HH, et al. N6-methyladenosine modulates messenger RNA translation efficiency. Cell. 2015;161:1388–1399. doi: 10.1016/j.cell.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang X, Lu ZK, Gomez A, Hon GC, Yue Y, et al. m6A-dependent regulation of messenger RNA stability. Nature. 2014;505:117–120. doi: 10.1038/nature12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhou KI, Shi H, Lyu R, Wylder AC, Matuszek Z, Pan JN, et al. Regulation of co-transcriptional pre-mRNA splicing by m6A through the low-complexity protein hnRNPG. Mol Cell. 2019;76:70–81. doi: 10.1016/j.molcel.2019.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sun L, Fazal FM, Li P, Broughton JP, Lee B, Tang L, et al. RNA structure maps across mammalian cellular compartments. Nat Struct Mol Biol. 2019;26:322–330. doi: 10.1038/s41594-019-0200-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu N, Dai Q, Zheng GQ, He C, Parisien M, Pan T. N6-methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature. 2015;518:560–564. doi: 10.1038/nature14234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Alarcón CR, Goodarzi H, Lee H, Liu XH, Tavazoie S, et al. HNRNPA2B1 is a mediator of m6A-dependent nuclear RNA processing events. Cell. 2015;162:1299–1308. doi: 10.1016/j.cell.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Huang HL, Weng HY, Sun WJ, Qin X, Shi HL, Wu HZ, et al. Recognition of RNA N6-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat Cell Biol. 2018;20:285–295. doi: 10.1038/s41556-018-0045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Scutenaire J, Deragon JM, Jean V, Benhamed M, Raynaud C, Favory JJ, et al. The YTH domain protein ECT2 is an m6A reader required for normal trichome branching in Arabidopsis. Plant Cell. 2018;30:986–1005. doi: 10.1105/tpc.17.00854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Guo T, Liu C, Meng F, Hu L, Fu X, Yang Z, et al. The m6A reader MhYTP2 regulates MdMLO19 mRNA stability and antioxidant genes translation efficiency conferring powdery mildew resistance in apple. Plant Biotechnol J. 2022;20:511–525. doi: 10.1111/pbi.13733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Arribas-Hernández L, Simonini S, Hansen MH, Paredes EB, Bressendorff S, Dong Y, et al. Recurrent requirement for the m6A-ECT2/ECT3/ECT4 axis in the control of cell proliferation during plant organogenesis. Development. 2020;147:dev189134. doi: 10.1242/dev.189134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xue C, Zhao Y, Li L. Advances in RNA cytosine-5 methylation: detection, regulatory mechanisms, biological functions and links to cancer. Biomarker Res. 2020;8:43. doi: 10.1186/s40364-020-00225-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wu H, Qu X, Dong Z, Luo L, Shao C, Forner J, et al. WUSCHEL triggers innate antiviral immunity in plant stem cells. Science. 2020;370:227–231. doi: 10.1126/science.abb7360. [DOI] [PubMed] [Google Scholar]
- 112.Pavlopoulou A, Kossida S. Phylogenetic analysis of the eukaryotic RNA (cytosine-5)-methyltransferases. Genomics. 2009;93:350–357. doi: 10.1016/j.ygeno.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 113.Yang L, Perrera V, Saplaoura E, Apelt F, Bahin M, Kramdi A, et al. m5C methylation guides systemic transport of messenger RNA over graft junctions in plants. Curr Biol. 2019;29(2465-2476):e2465. doi: 10.1016/j.cub.2019.06.042. [DOI] [PubMed] [Google Scholar]
- 114.Hussain S, Sajini Abdulrahim A, Blanco S, Dietmann S, Lombard P, et al. NSun2-mediated cytosine-5 methylation of vault noncoding RNA determines its processing into regulatory small RNAs. Cell Rep. 2013;4:255–261. doi: 10.1016/j.celrep.2013.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Khoddami V, Cairns BR. Identification of direct targets and modified bases of RNA cytosine methyltransferases. Nat Biotechnol. 2013;31:458–464. doi: 10.1038/nbt.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Han C, Zhang F, Qiao X, Zhao Y, Qiao Q, Huang X, et al. Multi-omics analysis reveals the dynamic changes of RNA N6-methyladenosine in pear (Pyrus bretschneideri) defense responses to Erwinia amylovora pathogen infection. Front Microbiol. 2021;12:803512. doi: 10.3389/fmicb.2021.803512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhou L, Tang R, Li X, Tian S, Li B, Qin G. N6-methyladenosine RNA modification regulates strawberry fruit ripening in an ABA-dependent manner. Genome Biol. 2021;22:168. doi: 10.1186/s13059-021-02385-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang G, Lv Z, Diao S, Liu H, Duan A, He C, et al. Unique features of the m6A methylome and its response to drought stress in sea buckthorn (Hippophae rhamnoides Linn.) RNA Biol. 2021;18:794–803. doi: 10.1080/15476286.2021.1992996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gao Y, Liu X, Jin Y, Wu J, Li S, Li Y, et al. Drought induces epitranscriptome and proteome changes in stem-differentiating xylem of Populus trichocarpa. Plant Physiol. 2022;190:459–479. doi: 10.1093/plphys/kiac272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gao YB, Liu XQ, Wu BZ, Wang HH, Xi FH, Kohnen MV, et al. Quantitative profiling of N6-methyladenosine at single-base resolution in stem-differentiating xylem of Populus trichocarpa using Nanopore direct RNA sequencing. Genome Biol. 2021;22:22. doi: 10.1186/s13059-020-02241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lu L, Zhang Y, He Q, Qi ZX, Zhang G, Xu WC, et al. MTA, an RNA m6A methyltransferase, enhances drought tolerance by regulating the development of trichomes and roots in poplar. Int J Mol Sci. 2020;21:2462. doi: 10.3390/ijms21072462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu GF, Wang J, Hou XL. Transcriptome-wide N6-methyladenosine (m6A) methylome profiling of heat stress in Pak-choi (Brassica rapa ssp. chinensis) Plants. 2020;9:1080. doi: 10.3390/plants9091080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hu J, Cai J, Umme A, Chen Y, Xu T, Kang H. Unique features of mRNA m6A methylomes during expansion of tomato (Solanum lycopersicum) fruits. Plant Physiol. 2022;188:2215–2227. doi: 10.1093/plphys/kiab509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yang D, Xu H, Liu Y, Li M, Ali M, Xu X, et al. RNA N6-methyladenosine responds to low-temperature stress in tomato anthers. Front Plant Sci. 2021;12:687826. doi: 10.3389/fpls.2021.687826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li W, Yu Y, Chen X, Fang Q, Yang A, Chen X, et al. N6-methyladenosine dynamic changes and differential methylation in wheat grain development. Planta. 2022;255:125. doi: 10.1007/s00425-022-03893-4. [DOI] [PubMed] [Google Scholar]
- 126.Zhang TY, Wang ZQ, Hu HC, Chen ZQ, Liu P, Gao SQ, et al. Transcriptome-wide N6-methyladenosine (m6A) profiling of susceptible and resistant wheat varieties reveals the involvement of variety-specific m6A modification involved in virus-host interaction pathways. Front Microbiol. 2021;12:656302. doi: 10.3389/fmicb.2021.656302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cheng Q, Wang P, Wu G, Wang Y, Tan J, Li C, et al. Coordination of m6A mRNA methylation and gene transcriptome in rice response to cadmium stress. Rice. 2021;14:62. doi: 10.1186/s12284-021-00502-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zheng H, Sun X, Li J, Song Y, Song J, Wang F, et al. Analysis of N6-methyladenosine reveals a new important mechanism regulating the salt tolerance of sweet sorghum. Plant Sci. 2021;304:110801. doi: 10.1016/j.plantsci.2020.110801. [DOI] [PubMed] [Google Scholar]
- 129.Du XM, Fang T, Liu Y, Wang M, Zang MS, Huang LY, et al. Global profiling of N6-methyladenosine methylation in maize callus induction. Plant Genome. 2020;13:e20018. doi: 10.1002/tpg2.20018. [DOI] [PubMed] [Google Scholar]
- 130.Lv Y, Han F, Liu M, Zhang T, Cui G, Wang J, et al. Characteristics of N6-methyladenosine modification during sexual reproduction of Chlamydomonas reinhardtii. Genom Proteom Bioinform. 2022;S1672-0229;40-7. [DOI] [PubMed]
- 131.He Y, Li L, Yao Y, Li Y, Zhang H, Fan M. Transcriptome-wide N6-methyladenosine (m6A) methylation in watermelon under CGMMV infection. BMC Plant Biol. 2021;21:516. doi: 10.1186/s12870-021-03289-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Su T, Fu L, Kuang L, Chen D, Zhang G, Shen Q, et al. Transcriptome-wide m6A methylation profile reveals regulatory networks in roots of barley under cadmium stress. J Hazard Mater. 2022;423:127140. doi: 10.1016/j.jhazmat.2021.127140. [DOI] [PubMed] [Google Scholar]
- 133.Wang W, Li W, Cheng Z, Sun J, Gao J, Li J, et al. Transcriptome-wide N6-methyladenosine profiling of cotton root provides insights for salt stress tolerance. Environ Exp Bot. 2022;194:104729. doi: 10.1016/j.envexpbot.2021.104729. [DOI] [Google Scholar]
- 134.Miao Z, Zhang T, Qi Y, Song J, Han Z, Ma C. Evolution of the RNA N6-methyladenosine methylome mediated by genomic duplication. Plant Physiol. 2020;182:345–360. doi: 10.1104/pp.19.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hou N, Li C, He J, Liu Y, Yu S, Malnoy M, et al. MdMTA-mediated m6A modification enhances drought tolerance by promoting mRNA stability and translation efficiency of genes involved in lignin deposition and oxidative stress. New Phytol. 2022;234:1294–1314. doi: 10.1111/nph.18069. [DOI] [PubMed] [Google Scholar]
- 136.Huang X, Abuduwaili N, Wang X, Tao M, Wang X, Huang G. Cotton (Gossypium hirsutum) VIRMA as an N6-methyladenosine RNA methylation regulator participates in controlling chloroplast-dependent and independent leaf development. Int J Mol Sci. 2022;23:9887. doi: 10.3390/ijms23179887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wan Y, Tang K, Zhang D, Xie S, Zhu X, Wang Z, et al. Transcriptome-wide high-throughput deep m6A-seq reveals unique differential m6A methylation patterns between three organs in Arabidopsis thaliana. Genome Biol. 2015;16:272. doi: 10.1186/s13059-015-0839-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Xu Z, Shi X, Bao M, Song X, Zhang Y, Wang H, et al. Transcriptome-wide analysis of RNA m6A methylation and gene expression changes among two Arabidopsis ecotypes and their reciprocal hybrids. Front Plant Sci. 2021;12:685189. doi: 10.3389/fpls.2021.685189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hu J, Cai J, Park SJ, Lee K, Li Y, Chen Y, et al. N6-methyladenosine mRNA methylation is important for salt stress tolerance in Arabidopsis. Plant J. 2021;106:1759–1775. doi: 10.1111/tpj.15270. [DOI] [PubMed] [Google Scholar]
- 140.Anderson SJ, Kramer MC, Gosai SJ, Yu X, Vandivier LE, Nelson ADL, et al. N6-methyladenosine inhibits local ribonucleolytic cleavage to stabilize mRNAs in Arabidopsis. Cell Rep. 2018;25:1146–1157. doi: 10.1016/j.celrep.2018.10.020. [DOI] [PubMed] [Google Scholar]
- 141.Wang Y, Du F, Li Y, Wang J, Zhao X, Li Z, et al. Global N6-methyladenosine profiling revealed the tissue-specific epitranscriptomic regulation of rice responses to salt stress. Int J Mol Sci. 2022;23:2091. doi: 10.3390/ijms23042091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cui J, Liu J, Li J, Cheng D, Dai C. Genome-wide sequence identification and expression analysis of N6-methyladenosine demethylase in sugar beet (Beta vulgaris L.) under salt stress. PeerJ. 2022;10:e12719. doi: 10.7717/peerj.12719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Govindan G, Sharma B, Li YF, Armstrong CD, Merum P, Rohila JS, et al. mRNA N6-methyladenosine is critical for cold tolerance in Arabidopsis. Plant J. 2022;111:1052–1068. doi: 10.1111/tpj.15872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mao X, Hou N, Liu Z, He J. Profiling of N6-methyladenosine (m6A) modification landscape in response to drought stress in apple (Malus prunifolia (Willd.) Borkh) Plants. 2021;11:103. doi: 10.3390/plants11010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang K, Zhuang X, Dong Z, Xu K, Chen X, Liu F, et al. The dynamics of N6-methyladenine RNA modification in interactions between rice and plant viruses. Genome Biol. 2021;22:189. doi: 10.1186/s13059-021-02410-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Han X, Shi Q, He Z, Song W, Chen Q, Qi Z. Transcriptome-wide N6-methyladenosine (m6A) methylation in soybean under Meloidogyne incognita infection. Abiotech. 2022;3:197-211. [DOI] [PMC free article] [PubMed]
- 147.Han X, Wang J, Zhang Y, Kong Y, Dong H, Feng X, et al. Changes in the m6A RNA methylome accompany the promotion of soybean root growth by rhizobia under cadmium stress. J Hazard Mater. 2023;441:129843. [DOI] [PubMed]
- 148.Tang J, Yang J, Duan H, Jia G. ALKBH10B, an mRNA m6A demethylase, modulates ABA response during seed germination in Arabidopsis. Front Plant Sci. 2021;12:712713. doi: 10.3389/fpls.2021.712713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang F, Zhang YC, Liao JY, Yu Y, Zhou YF, Feng YZ, et al. The subunit of RNA N6-methyladenosine methyltransferase OsFIP regulates early degeneration of microspores in rice. PLoS Genet. 2019;15:e1008120. doi: 10.1371/journal.pgen.1008120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Selmi T, Lanzuolo C. Driving chromatin organisation through N6-methyladenosine modification of RNA: what do we know and what lies ahead? Genes. 2022;13:340. doi: 10.3390/genes13020340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kan RL, Chen J, Sallam T. Crosstalk between epitranscriptomic and epigenetic mechanisms in gene regulation. Trends Genet. 2022;38:182–193. doi: 10.1016/j.tig.2021.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yu Q, Liu S, Yu L, Xiao Y, Zhang S, Wang X, et al. RNA demethylation increases the yield and biomass of rice and potato plants in field trials. Nat Biotechnol. 2021;39:1581–1588. doi: 10.1038/s41587-021-00982-9. [DOI] [PubMed] [Google Scholar]
- 153.Kramer MC, Janssen KA, Palos K, Nelson ADL, Vandivier LE, Garcia BA, et al. N6-Methyladenosine and RNA secondary structure affect transcript stability and protein abundance during systemic salt stress in Arabidopsis. Plant Direct. 2020;00:1–22. doi: 10.1002/pld3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bhat SS, Bielewicz D, Gulanicz T, Bodi Z, Yu X, Anderson SJ, et al. mRNA adenosine methylase (MTA) deposits m6A on pri-miRNAs to modulate miRNA biogenesis in Arabidopsis thaliana. Proc Natl Acad Sci. 2020;117:21785–21795. doi: 10.1073/pnas.2003733117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Luo JH, Wang Y, Wang M, Zhang LY, Peng HR, Zhou YY, et al. Natural variation in RNA m6A methylation and its relationship with translational status. Plant Physiol. 2020;182:332–344. doi: 10.1104/pp.19.00987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Meyer KD, Patil DP, Zhou J, Zinoviev A, Skabkin MA, Elemento O, et al. 5' UTR m6A promotes cap-independent translation. Cell. 2015;163:999–1010. doi: 10.1016/j.cell.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Arribas-Hernandez L, Brodersen P. Occurrence and functions of m6A and other covalent modifications in plant mRNA. Plant Physiol. 2020;182:79–96. doi: 10.1104/pp.19.01156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lee Y, Choe J, Park OH, Kim YK. Molecular mechanisms driving mRNA degradation by m6A modification. Trends Genet. 2020;36:177–188. doi: 10.1016/j.tig.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 159.Park OH, Ha H, Lee Y, Boo SH, Kwon DH, Song HK, et al. Endoribonucleolytic cleavage of m6A-containing RNAs by RNase P/MRP complex. Mol Cell. 2019;74:494–507. doi: 10.1016/j.molcel.2019.02.034. [DOI] [PubMed] [Google Scholar]
- 160.Du H, Zhao Y, He J, Zhang Y, Xi H, Liu M, et al. YTHDF2 destabilizes m6A-containing RNA through direct recruitment of the CCR4-NOT deadenylase complex. Nat Commun. 2016;7:12626. doi: 10.1038/ncomms12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ries RJ, Zaccara S, Klein P, Olarerin-George A, Namkoong S, Pickering BF, et al. m6A enhances the phase separation potential of mRNA. Nature. 2019;571:424–428. doi: 10.1038/s41586-019-1374-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lee HG, Kim J, Seo PJ. N6-methyladenosine-modified RNA acts as a molecular glue that drives liquid-liquid phase separation in plants. Plant Signal Behav. 2022;17:2079308. doi: 10.1080/15592324.2022.2079308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zheng HX, Sun X, Zhang XS, Sui N. m6A editing: new tool to improve crop quality? Trends Plant Sci. 2020;25:859–867. doi: 10.1016/j.tplants.2020.04.005. [DOI] [PubMed] [Google Scholar]
- 164.Kim JS. Precision genome engineering through adenine and cytosine base editing. Nat Plants. 2018;4:148–151. doi: 10.1038/s41477-018-0115-z. [DOI] [PubMed] [Google Scholar]
- 165.Kang BC, Yun JY, Kim ST, Shin Y, Ryu J, Choi M, et al. Precision genome engineering through adenine base editing in plants. Nat Plants. 2018;4:427–431. doi: 10.1038/s41477-018-0178-x. [DOI] [PubMed] [Google Scholar]
- 166.Wilson C, Chen PJ, Miao Z, Liu DR. Programmable m6A modification of cellular RNAs with a Cas13-directed methyltransferase. Nat Biotechnol. 2020;38:1431–1440. doi: 10.1038/s41587-020-0572-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Li J, Chen Z, Chen F, Xie G, Ling Y, Peng Y, et al. Targeted mRNA demethylation using an engineered dCas13b-ALKBH5 fusion protein. Nucleic Acids Res. 2020;48:5684–5694. doi: 10.1093/nar/gkaa269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhao J, Li B, Ma J, Jin W, Ma X. Photoactivatable RNA N6-methyladenosine editing with CRISPR-Cas13. Small. 2020;16:e1907301. doi: 10.1002/smll.201907301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.