Abstract
Glypican 3 (GPC3) is a family of glycosylphosphatidylinositol-anchored, cell-surface heparan sulfate proteoglycans. Loss-of-function mutations of GPC3 cause Simpson-Golabi-Behmel syndrome characterized by overgrowth of multiple organs, including liver. Our previous study showed that in GPC3 transgenic (TG) mice, hepatocyte-targeted overexpression of GPC3 suppresses hepatocyte proliferation and liver regeneration after partial hepatectomy and alters gene expression profiles and potential cell cycle-related proteins. This study investigates the role of GPC3 in hepatocyte proliferation and hepatomegaly induced by the xenobiotic mitogens phenobarbital (PB) and TCPOBOP (1, 4-bis [2-(3, 5-dichloropyridyloxy)] benzene). Wildtype (WT) and GPC3 TG mice were given 0.1% PB in drinking water for 10 days or a single dose of TCPOBOP (3 mg/kg) by oral gavage. At day 5 the WT mice showed a 2.2- and 3.0-fold increase in liver weight, whereas the GPC3 TG mice showed a 1.3- and 1.6-fold increase in liver weight after PB and TCPOBOP administration, respectively. There was a significant suppression of proliferative response in the GPC3 TG mice, as assessed by percent of Ki67-positive hepatocyte nuclei. Moreover, gene array analysis showed a panel of changes in the gene expression profile of TG mice, both before and after administration of the xenobiotic mitogens. Expression of cell cycle-related genes in the TG mice was also decreased compared to the WT mice.
Conclusion:
Our results indicate that in GPC3 TG mice, hepatocyte-targeted overexpression of GPC3 plays an important role for regulation of liver size and termination of hepatocyte proliferation induced by the xenobiotic mitogens PB and TCPOBOP, comparable to the effects seen in the GPC3 TG mice during liver regeneration after partial hepatectomy.
Glypican 3 (GPC3) is a member of glypican family of heparan sulfate proteoglycans that is attached to the cell surface by a glycosylphosphatidylinositol anchor.1,2 GPC3 has been reported to be highly expressed during embryogenesis and organogenesis.3–5 The stage- and tissue-specific pattern of expression suggests that GPC3 is involved in morphogenesis and development.4 A loss-of-function mutation of the GPC3 gene results in Simpson-Golabi-Behmel syndrome (SGBS), which is an X-linked disorder characterized by prenatal and postnatal overgrowth, numerous visceral and skeletal anomalies, and an increased risk of development of embryonic tumors during early childhood.6–8 Filmus and colleagues9 confirmed the involvement of GPC3 in SGBS by GPC3-deficient mice (GPC3−/−), which display many of the phenotypic features of SGBS. Because the functional loss of GPC3 leads to overgrowth of multiple organs (including the liver) in mice and humans, it is reasonable to speculate that GPC3 may play an important role in the negative regulation of organ growth. On the other hand, our previous study showed GPC3 is highly up-regulated in hepatocellular carcinoma (HCC) and hepatoblastoma but not in the normal liver.10 The soluble form of GPC3 was also elevated in the serum of a large proportion of patients with HCC, and can be used as a serologic marker for the diagnosis of HCC.11,12 These findings suggest that GPC3 plays a major role for regulation of normal liver growth and tumorigenesis. However, the exact manner of GPC3 function in liver is not well characterized.
The liver responds to specific xenobiotics by inducing members of the nuclear hormone receptor superfamily, particularly the constitutive androstane receptor (CAR) and the pregnane X receptor.13–15 Previous study has shown that the hepatic enlargement and hepatocyte proliferation induced by xenobiotic chemical mitogens proceeds through different signaling mechanisms compared to liver regeneration.16 Two strong xenobiotic chemical mitogens, phenobarbital (PB) and 1,4-bis [2-(3,5-dichoropyridyloxy)] benzene (TCPOBOP), activate CAR.14 PB induces translocation of CAR by a ligand-independent process and CAR’s constitutive activity is sufficient to induce gene expression.14 The halogenated hydrocarbon TCPOBOP is the strongest hepatic chemical mitogen and also an ideal candidate for studying xenobiotic metabolism. TCPOBOP is both a nongenotoxic carcinogen on its own and a potent tumor promoter when combined with genotoxic agents.17–20 It potently induces genes associated with hepatocyte proliferation and xenobiotic detoxification in a CAR-dependent manner.13–15 PB and TCPOBOP-induced hepatic enlargement and hepatocyte proliferation involves both hypertrophy and hyperplasia. However, the mechanisms involved in modulation of the liver size and termination of hepatocyte proliferation following treatment with PB and TCPOBOP-induced proliferative and growth response of hepatocyte are not well understood.
Our previous study has shown that extracellular matrix (ECM)-mediated signaling by way of integrin-like kinase (ILK) is essential in proper termination of liver regeneration after partial hepatectomy (PHx)21 and hepatocyte proliferation induced by PB and TCPOBOP.22,23 Moreover, we have demonstrated that GPC3 played a role as a negative regulator of liver regeneration and hepatocyte proliferation in rat hepatocyte cultures.24 Recently, we generated GPC3 transgenic (TG) mice with hepatocyte-targeted GPC3 overexpression under the control of the albumin promoter.25 We demonstrated that after PHx, liver regeneration and hepatocyte proliferation were suppressed in GPC3 TG mice. Gene expression profile was shown to be significantly different between TG and WT mice.25 These findings highlighted that GPC3 was a suppressor of hepatocyte proliferation and GPC3 may play a role in regulation of both liver regeneration and its termination after PHx. Thus, we wanted to investigate the mechanism of termination of hepatocyte proliferation mediated by two strong chemical mitogens using chronic administration of PB and an acute stimulus of TCPOBOP in GPC3 TG mice and their nontransgenic (WT) littermates. Based on the previous studies, we hypothesized that hepatocyte-targeted overexpression of GPC3 TG mice would also suppress proliferative response of hepatocyte to PB and TCPOBOP resulting in smaller liver size. As expected, we found that liver size and hepatocyte proliferation induced by the chemical mitogens were significantly suppressed in GPC3 TG mice. Gene array analysis revealed a panel of changes in the gene expression profile in TG mice; expression of growth factor and cell cycle-related genes decreased in the GPC3 TG mice. Western blotting demonstrated changes in multiple specific pathways. The overall findings suggest that GPC3 suppresses hepatocyte growth and that it may play a major role in the regulation of processes causing the termination of hepatocyte proliferation induced by chemical mitogens, such as PB and TCPOBOP. In view of the fact that similar effects related to termination of hepatocyte proliferation and final liver weight are seen in hepatocyte targeted ILK-deficient and GPC3 TG mice with both PHx and chemical mitogens, our findings suggest that these two different modes of inducing hepatocyte proliferation in vivo may have similar termination pathways.
Materials and Methods
For the Materials and Methods section, please see the Supporting Information.
Results
Suppressed Hepatocyte Proliferation in GPC3 TG Mice.
Ki67 immunohistochemical results (Fig. 1A) showed that in WT mice there was a peak of hepatocyte proliferation at day 3 after PB administration, as shown by the percentage of Ki67-positive hepatocyte nuclei. In TG mice the hepatocyte proliferation was significantly suppressed at day 3 after PB administration, with no significant difference at days 5 and 10 as compared to WT mice, suggesting a suppression of proliferative response (Fig. 1B). Western blotting (Fig. 1C) also revealed that the protein levels of proliferative cell nuclear antigen assay (PCNA) were lower at days 1 and 3 after PB administration in TG mice than WT mice. On the other hand, there was a peak of hepatocyte proliferation at day 2 after TCPOBOP administration in both WT and TG mice, demonstrated by the percentage of Ki67-positive hepatocyte nuclei (Fig. 1D). The number of Ki67-positive hepatocyte nuclei is significantly lower at all timepoints after TCPOBOP administration in the livers of GT mice as compared to the livers of WT mice, suggesting a sustained suppression of proliferative response in TG mice (Fig. 1E). Western blotting (Fig. 1F) revealed the protein levels of PCNA were lower at days 2, 5, and 7 after TCPOBOP administration in TG mice than WT mice.
Fig. 1.
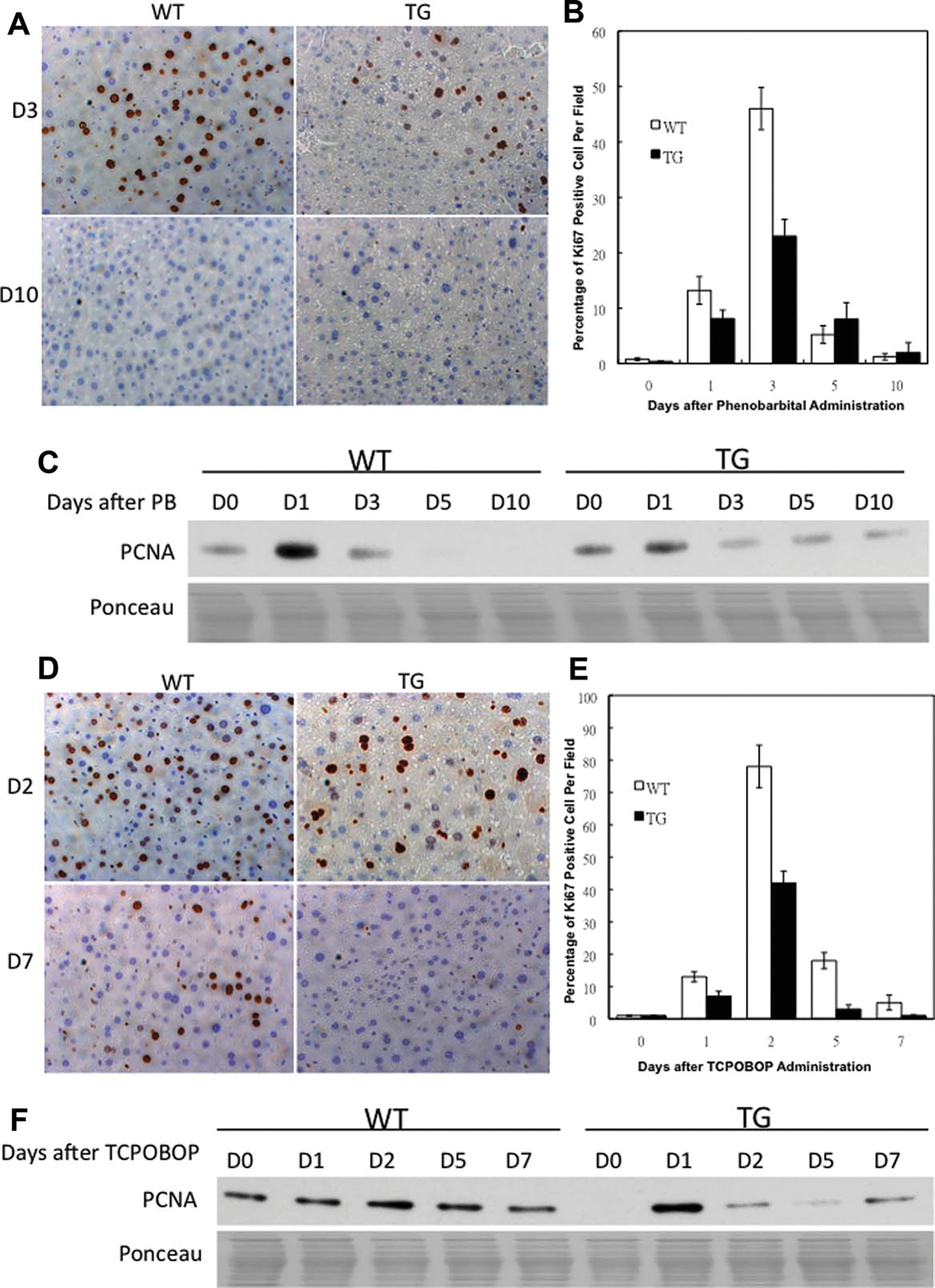
Decreased liver growth after PB and TCPOBOP administration in GPC3 TG mice. (A,B) Statistical analysis of the liver weight normalized to day 0 (D0) liver weight in WT and GPC3 TG mice after PB and TCPOBOP administration. There were no statistically significant differences in D0 liver weight between WT and TG mice. Each data point is the mean ± standard derivation (SD) from more than three measurements per point. Asterisk indicates significantly different data between GPC3 TG and WT mice (P < 0.01).
Decreased Liver Growth After PB and TCPOBOP Administration in GPC3 TG Mice.
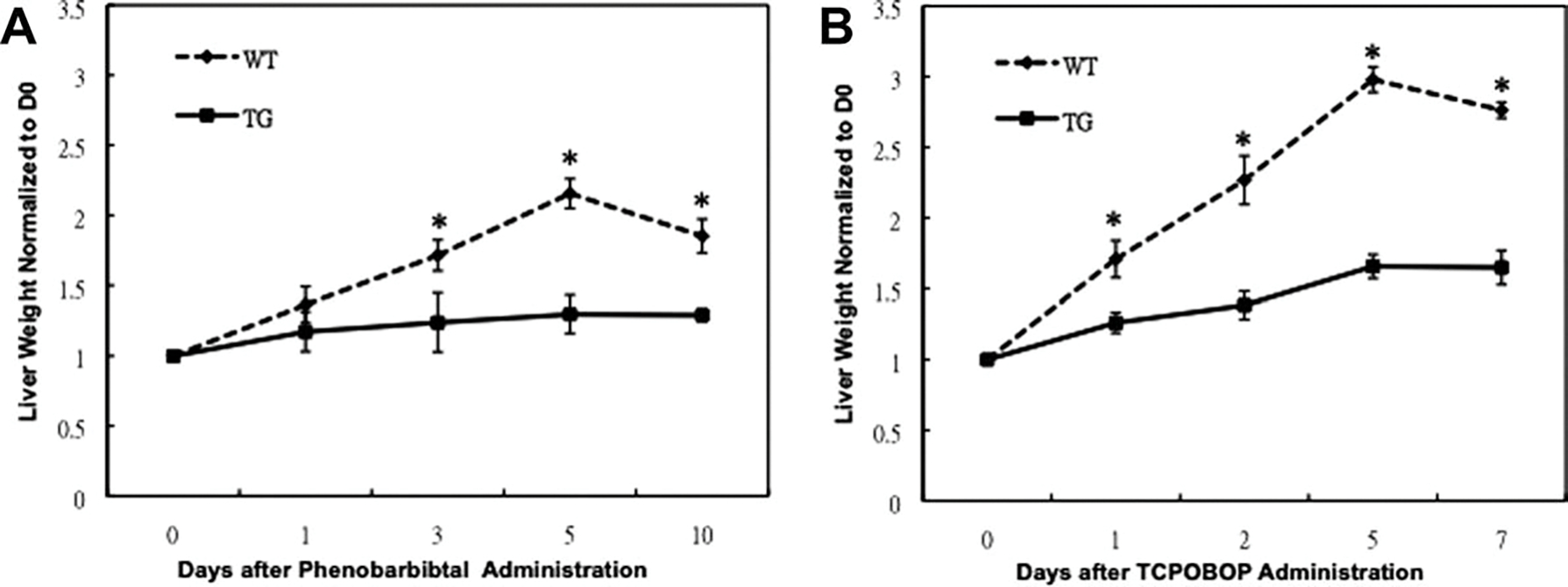
The liver weight and liver to body weight ratio were measured in both GPC3 TG and WT male mice before and after PB and TCPOBOP administration (Table 1). At least three mice for each group were sacrificed and analyzed in parallel. The liver weight was normalized to day 0 liver weight in each group. The data are presented in Fig. 2. There was no statistically significant difference in the liver weight and liver to body weight ratio at day 0 between WT and TG mice.
Table 1.
Liver Weight and Liver to Body Weight Ratio (%) in WT and GPC3 TG Mice After Phenobarbital (PB) and TCPOBOP Administration
| Liver weight |
Liver to body weight ratio (%) |
|||
|---|---|---|---|---|
| WT | TG | WT | TG | |
|
| ||||
| Prior to PB | 1.17 ± 0.05 | 1.55 ± 0.27 | 4.09 ± 0.07 | 4.32 ± 0.30 |
| 1 Day after PB | 1.60 ± 0.15 | 1.81 ± 0.21 | 5.41 ± 0.17 | 4.89 ± 0.53 |
| 3 Days after PB | 2.01 ± 0.13 | 1.91 ± 0.32 | 7.01 ± 0.36 | 5.81 ± 0.22* |
| 5 Days after PB | 2.52 ± 0.12 | 2.00 ± 0.21* | 8.32 ± 0.59 | 6.15 ± 0.49* |
| 10 Days after PB | 2.17 ± 0.14 | 1.99 ± 0.06 | 7.23 ± 0.33 | 5.89 ± 0.37* |
| Prior to TCPOBOP | 1.17 ± 0.05 | 1.55 ± 0.27 | 4.09 ± 0.07 | 4.32 ± 0.30 |
| 1 Day after TCPOBOP | 2.00 ± 0.15 | 1.95 ± 0.05 | 6.42 ± 0.38 | 5.58 ± 0.23# |
| 2 Days after TCPOBOP | 2.62 ± 0.20 | 2.14 ± 0.05* | 7.75 ± 0.12 | 6.60 ± 0.50* |
| 5 Days after TCPOBOP | 3.49 ± 0.11 | 2.56 ± 0.05* | 10.30 ± 0.85 | 7.53 ± 0.46* |
| 7 Days after TCPOBOP | 3.23 ± 0.04 | 2.52 ± 0.14* | 9.89 ± 0.68 | 7.28 ± 0.32* |
Each data point is the mean ± standard derivation (SD) from more than three measurements per point. Significantly different from WT mice at that same timepoint (#P < 0.05 and *P < 0.01).
Fig. 2.
Suppression of hepatocyte proliferation after PB and TCPOBOP administration in GPC3 TG mice. Immunohistochemical staining for Ki67 (brown), a proliferation marker, in paraffin sections of WT and TG mouse livers at days 3 and 10 after PB administration (A) and at days 2 and 7 after TCPOBOP administration (D). Percentage of Ki67-positive nuclei in hepatocyte was counted in low-power field (200×) in 15 random sections from at least three different WT and TG mice. A significant decrease in Ki-67-positive hepatocyte nuclei was observed in TG mice at day 3 after PB administration (B) and at day 2 after TCPOBOP administration (E). Western blotting analysis of PCNA. Pooled liver samples from at least three mice were used for protein analysis after PB (C) and TCPOBOP (F) administration. Ponceau staining was used as loading control for nuclear lysates.
At day 5 after PB administration the liver weight of TG mice was significantly lower than WT mice (Table 1). At day 5 the WT mice showed a maximum f 2.2-fold increase in liver weight as compared to day 0, whereas the TG mice showed a maximum 1.3-fold increase (Fig. 2A). When normalized in terms of body weight, the WT mice showed a maximum 2.0-fold increase in liver to body weight ratio as compared to day 0, whereas the TG mice showed a maximum 1.4-fold increase at day 5 after PB administration (Table 1).
For TCPOBOP, at days 2, 5, and 7 after TCPOBOP administration, the liver weight of TG mice was significantly lower than WT mice (Table 1). At day 5 the WT mice showed a maximum 3.0-fold increase in liver weight as compared to day 0, whereas the TG mice showed a maximum 1.6-fold increase in liver weight as compared to 0 day (Fig. 2B). Furthermore, the WT mice showed 2.5-fold increases in liver to body weight ratio, whereas the TG mice showed a 1.6-fold increase at day 5 after TCPOBOP administration (Table 1).
GPC3 TG Mice Show Sustained and Increased Induction of GPC3.
To study the changes in GPC3 protein levels in TG and WT mice, total liver protein extracts from at least three mice at each timepoint were examined and analyzed individually (per mouse) with western blotting. There was an induction of GPC3 protein in WT and TG mice but a slight reduction at days 5 and 10 in WT mice after PB administration (Fig. 3A). On the other hand, there was a strong induction of GPC3 protein but a slight reduction at days 2, 5, and 7 in WT and TG mice after TCPOBOP administration (Fig. 3B). In addition, the expression of GPC3 protein was higher in TG mice as compared to WT mice at all timepoints before and after mitogenic stimulus, as expected from the transgenic regulation of GPC3 expression. Moreover, there was an induction of GPC3 messenger RNA (mRNA) with a slight uptrend in WT and TG mice after PB and TCPOBOP administration (Fig. 3E,F). Interestingly, CD81 (binding partner of GPC3),24 protein levels were down-regulated in TG mice, with a downtrend after PB and TCPOBOP administration (Fig. 3C,D). The observed small changes in the expression of GPC3 in the transgene probably reflect changes in activation of the albumin promoter following PB or TCPOBOP administration.
Fig. 3.
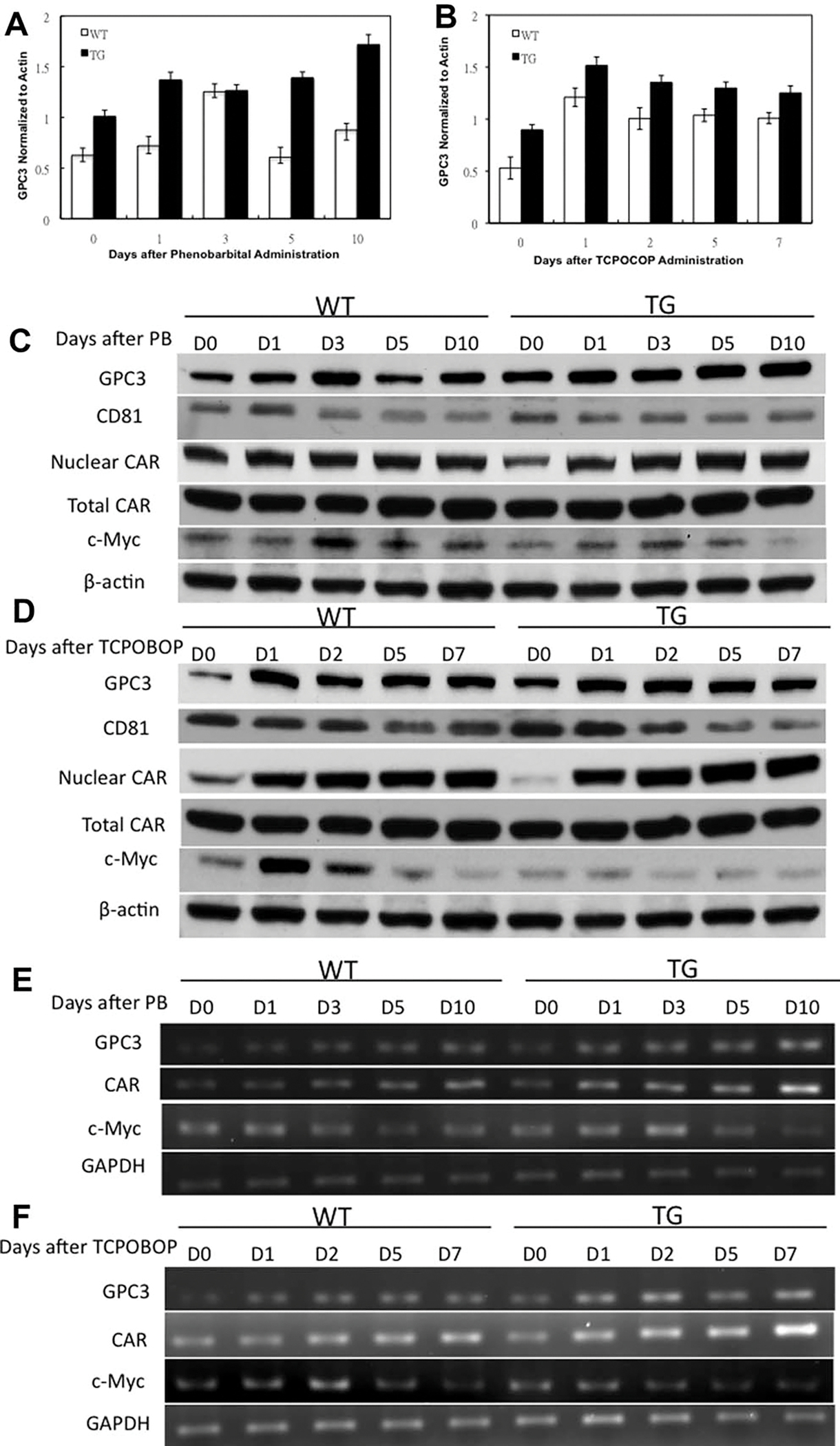
Western blotting analysis of protein levels of several signaling molecules in WT and GPC3 TG mice. The protein levels were individually examined by western blotting after PB (A) and TCPOBOP (B) administration. The signal intensity of each protein band was quantitated with ImageJ software and was statistically analyzed. Each bar signifies the mean and standard deviation of measurements from at least three separate animals. Each measurement involved the intensity of GPC3 bands normalized to β-actin. Western blotting showing GPC3, CD81, total CAR, nuclear CAR, and nuclear c-Myc levels in pooled liver samples after PB (C) and TCPOBOP (D) administration. β-Actin was used as loading control for total cell lysates. Semi quantitative reverse-transcriptase polymerase chain analysis (RT-PCR) analysis of mRNA levels of GPC3, CAR, and c-Myc in pooled liver samples after PB (E) and TCPOBOP (F) administration. GAPDH was used as loading control for mRNA.
GPC3 TG Mice Show Sustained and Increased Induction of CAR.
It is well documented that PB causes nuclear translocation of CAR without acting as a CAR ligand.26 We looked at CAR protein levels in total cell lysates and nuclear fractions after PB administration (Fig. 3C). In total cell lysates, we did not observe any change in CAR expression between WT and TG mice after PB administration. In the nuclear fraction, we saw an increase in CAR in both WT and TG mice. The levels of CAR were higher in TG mice as compared to WT mice at days 3, 5, and 10 after PB administration.
TCPOBOP is a CAR agonist and its binding to CAR leads to nuclear localization of CAR.13–15 In total cell lysates, we also did not observe any change in CAR expression in either WT and TG mice after TCPOBOP administration. In the nuclear fraction, we saw an induction in the expression of CAR in both WT and TG mice. The expression of CAR was higher in TG mice than WT mice at days 1, 2, 5, and 7 after TCPOBOP administration (Fig. 3D). There was an induction of CAR mRNA level in both WT and TG mice and the levels of CAR mRNA were higher in TG mice as compared to as WT mice at all timepoints after PB and TCPOBOP administration (Fig. 3E,F), suggesting a sustained and increased expression of CAR in TG mice.
Signaling Pathways Leading to Decreased Proliferation in GPC3 TG Mice.
C-Myc is known to be key mediator of TCPOBOP-CAR-induced direct liver hyperplasia.13 Thus, we examined whether c-Myc levels were differently expressed in TG mice. The protein levels of c-Myc increased at days 1 and 3 but decreased at days 5 and 10 after PB administration in both TG and WT mice (Fig. 3C). For TCPOBOP, the levels of c-Myc increased at day 1 but decreased at days 2, 5, and 7 in both TG and WT mice (Fig. 3D). The expression of c-Myc was lower at all timepoints in TG mice as compared to WT mice before and after PB and TCPOBOP administration. Moreover, the expression of c-Myc mRNA was generally lower in TG mice as compared to WT mice after PB and TCPOBOP administration (Fig. 3E,F), suggesting a decreased expression of c-Myc in TG mice.
GPC3 TG Mice Have Decreased Expression of Hepatocyte Growth Factor.
To further analyze signaling pathways involved in suppression of proliferative response to PB and TCPOBOP in TG mice, we investigated the levels of growth factors. The protein levels of hepatocyte growth factor (HGF) went up at day 1 but went down at days 3, 5, and 10 after PB administration in both TG and WT mice (Fig. 4A). On the other hand, the levels of HGF went up at days 1 and 2 and did not change at days 5 and 7 after TCPOBOP administration in both TG and WT mice (Fig. 4B). Although the HGF protein levels as well as mRNA were induced after PB and TCPOBOP administration in both TG and WT mice, the HGF levels were lower in TG mice at all timepoints as compared to WT mice before and after PB and TCPOBOP administration (Fig. 4A,B), suggesting a sustained decreased expression of HGF in GPC3 TG mice. Moreover, the levels of MET (HGF receptor) were higher at all timepoints in TG mice as compared to WT mice before and after PB administration (Fig. 4A). The MET levels were higher in TG mice at days 0, 1, and 2 as compared to WT mice after TCPOBOP administration (Fig. 4B).
Fig. 4.
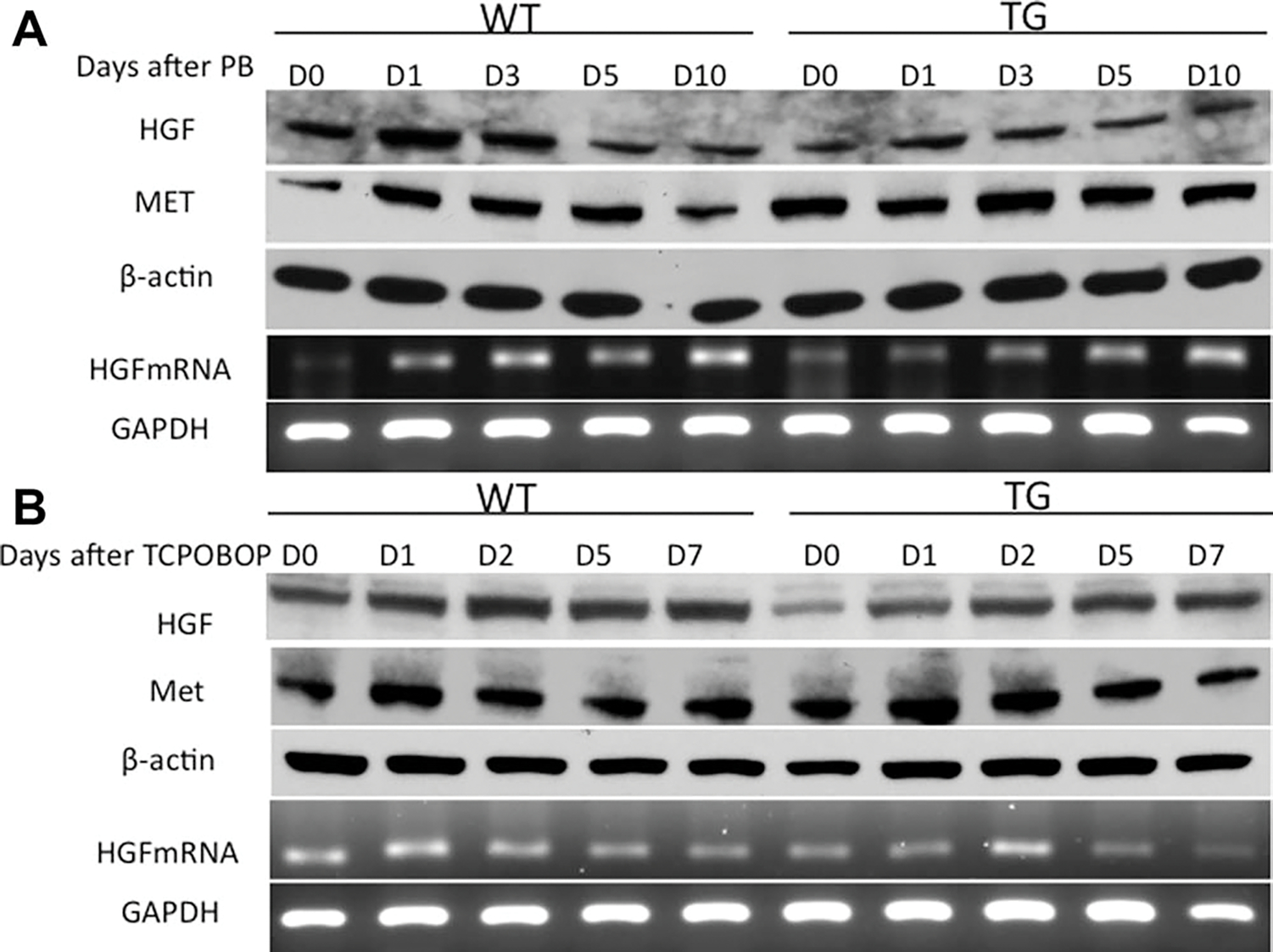
Protein levels of HGF and MET and mRNA levels of HGF after PB (A) and TCPOBOP (B) administration. Pooled liver samples from at least three mice were used preparing total protein lysates and mRNA. β-Actin and GAPDH were used as loading controls for total cell lysates and mRNA.
Sustained Suppression of Expression in Cell Cycle Genes in GPC3 TG Mice.
We looked into the key genes that are involved in hepatocyte proliferation. There was an induction of cyclin B1 protein at days 1 and 3 but a reduction at days 5 and 10 after PB administration in both WT and TG mice (Fig. 5A). For TCPOBOP there was an induction of cyclin B1 protein levels at days 1 and 2 but a reduction at days 5 and 7 in both WT and TG mice (Fig. 5B). The levels of cyclin B1 were lower in TG mice as compared to the WT mice at all timepoints after PB and TCPOBOP administration, suggesting a sustained suppression of mitosis. Cyclin D1 has been shown to play an important role in hepatocyte proliferation.27 The expression of cyclin D1 increased at days 1 and 3 but disappeared at days 5 and 10 after PB administration in WT mice (Fig. 5A). The expression of cyclin D1 increased at day 1 but decreased at days 3, 5, and 10 after PB administration in TG mice. The expression of cyclin D1 was higher in WT mice as compared to TG mice at days 0, 1, and 3 after PB administration, suggesting an early induction of cyclin D1 in WT mice. For TCPOBOP, the expression of cyclin D1 increased at day 1 but decreased at day 2, 5, and 7 after TCPOBOP administration in WT mice (Fig. 5B). The expression of cyclin D1 increased at days 1 and 2 but decreased at days 5 and 7 after TCPOBOP administration in TG mice. The expression of cyclin D1 was higher in WT mice as compared to the TG mice at days 0 and 1 after TCPOBOP administration, suggesting an early induction of cyclin D1 in WT mice.
Fig. 5.
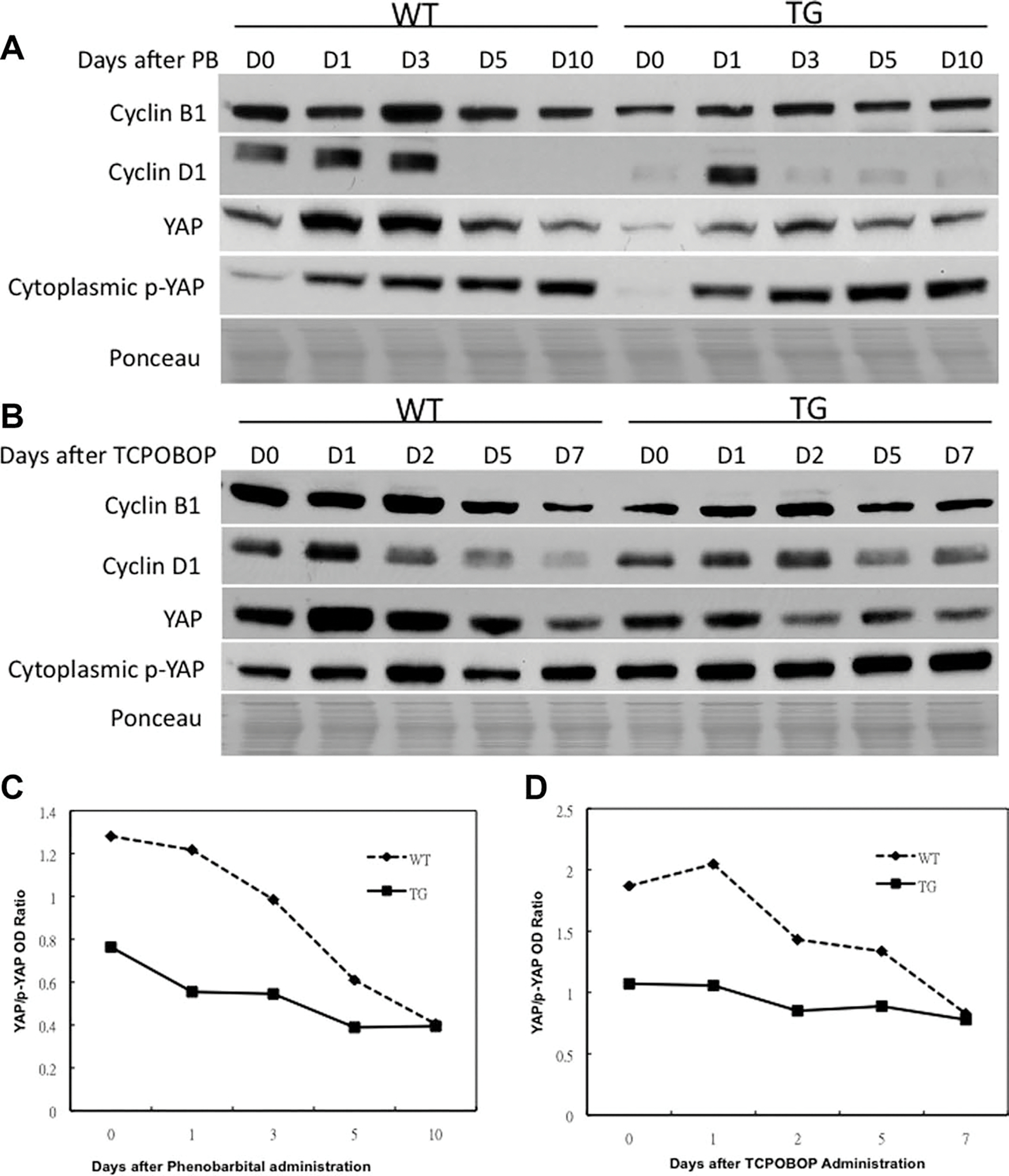
Western blotting analysis of protein levels of various cell cycle-related molecules after PB (A) and TCPOBOP (B) administration. Graphical representation of YAP/p-YAP ratio after PB (C) and TCPOBOP (D) administration. Pooled liver samples from at least three mice were used preparing cytoplasmic and nuclear lysates. Ponceau staining was used as loading control for nuclear lysates.
Yes-associated protein (YAP) also plays a role in hepatocyte proliferation, liver size adjustment, and cancer development.28,29 YAP is a nuclear protein, whose phosphorylation causes its nuclear export and degradation, which is associated with a reduction of cell proliferation. We investigated whether GPC3 regulates YAP expression during PB and TCPOBOP-induced hepatic enlargement. We found an induction of YAP protein levels at days 1 and 3 after PB administration in both WT and TG mice (Fig. 5A), suggesting an induction at the time of proliferation. At days 5 and 10 the YAP levels decreased. The levels of YAP were lower at all timepoints in TG mice as compared to WT mice, suggesting a sustained suppression of proliferation in TG mice. For TCPOBOP, we found an induction of YAP levels at day 1 in WT mice but reduced at subsequent timepoints after TCPOBOP administration in both WT and TG mice (Fig. 5B). The TG mice had overall lower levels of YAP, consistent with overall suppression of hepatocyte proliferation. A serine phosphorylated form of YAP reflects active removal from cell nuclei and shows an inverse correlation with cell proliferation. The changes observed in p-YAP are consistent with the overall decrease in hepatocyte proliferation and liver size in TG mice.
We also looked into levels of p21, transforming growth factor beta type I (TGF-β1), and runt-related transcription factor 3 (Runx3), which are known mito-inhibitors.30–33 We have also shown in our previous studies with GPC3 TG mice an enhanced expression of Runx3 in TG mice. Although there is variation in expression of these proteins from day to day, there was an overall enhanced expression of all of them in GPC3 TG mice as compared to WT mice (Fig. 6A,B).
Fig. 6.
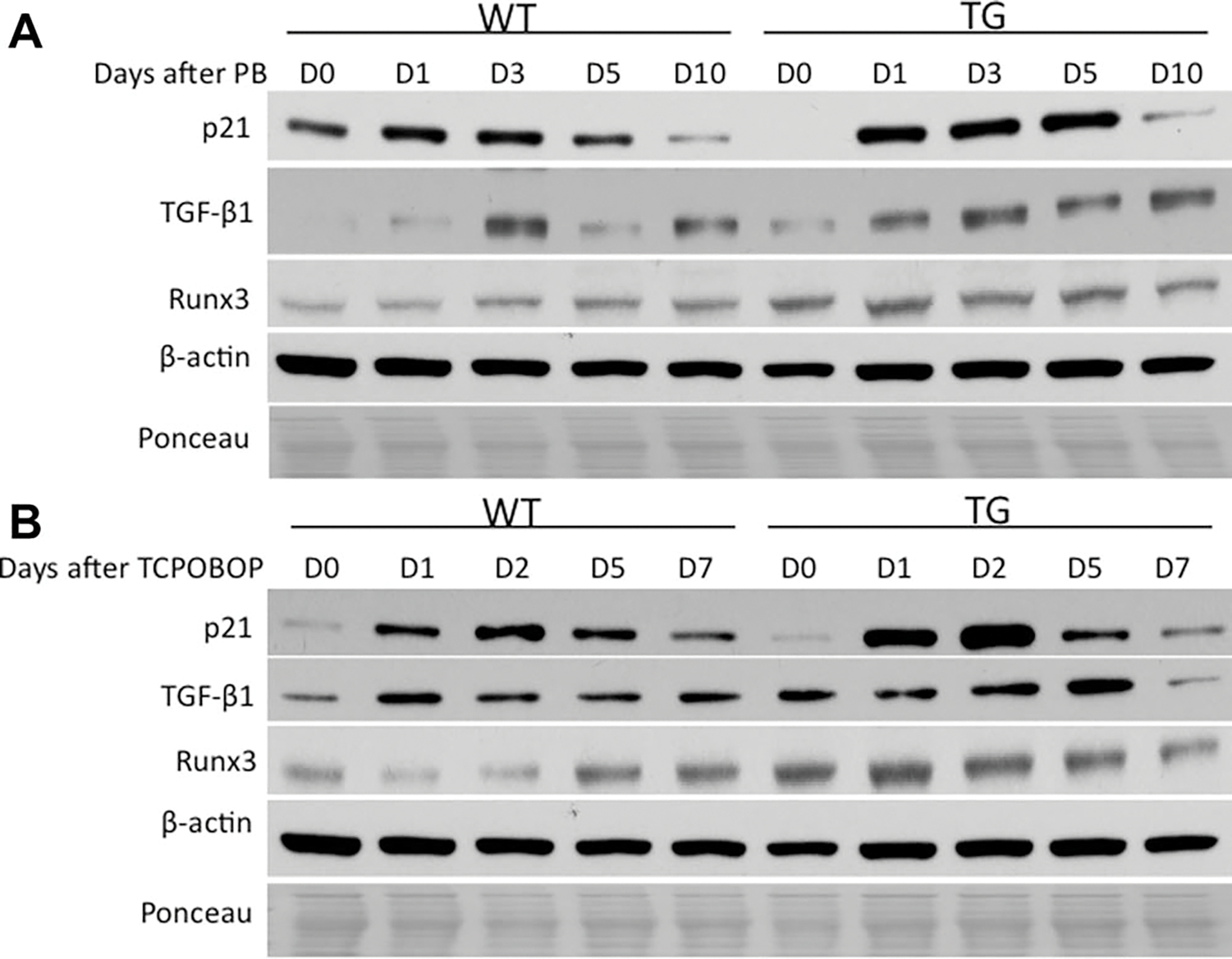
Western blotting analysis of protein levels of various molecules with known suppressing effects on cell cycle after PB (A) and TCPOBOP (B) administration. Pooled liver samples from at least three mice were used preparing total cell and nuclear lysates. β-Actin and Ponceau staining were used as loading controls for total cell and nuclear lysates.
Comparison of Gene Expression Patterns Between GPC3 TG and WT Mice Following PB or TCPOBOP Administration.
A comprehensive microarray analysis was conducted using liver mRNA collected at 0, 1, 3, 5, and 10 days after PB administration and at 0, 1, 2, 5, and 7 days after TCPOBOP administration. The Mouse Genome 430 2.0 GeneChip array is a single array analysis the expression levels of more than 45,000 transcripts and variants, including more than 35,000 well-characterized mouse genes (Affymetrix, Santa Clara, CA). We compared the expression of the 150 most-expressed genes at day 0 in each of the two categories of mice and followed their expression levels at different days after PB and TCPOBOP administration (Supporting Fig. 1). In WT mice, levels of expression of the top 150 most-expressed genes at day 0 were slightly affected. In TG mice there was substantial increase in the expression of many of the same top 150 genes at day 3 after PB administration. For TCPOBOP, in WT mice there was an increased expression of some of the top 150 most-expressed genes at day 0 but decrease following TCPOBOP treatment. In TG mice there was an increase in the expression of many of the same top 150 genes at day 7. Moreover, a direct comparison of TG and WT mouse expression patterns showed up-regulated and down-regulated in TG mice with a more than 1.5-fold change (Supporting Table 1). In addition, a panel of important genes known to be involved in hepatocyte growth arrest and cell cycle was examined and analyzed at day 3 after PB administration and day 2 after TCPOBOP administration (Supporting Tables 2, 3).
Discussion
Hepatocyte proliferation induced by xenobiotic mitogens such as PB and TCPOBOP is complicated and involves many factors and signaling pathways.16 Our previous studies have shown that GPC3 inhibits hepatocyte proliferation in culture and liver regeneration after PHx.24,25 Despite the new evidence that demonstrates that one aspect of GPC3 function suppresses growth, the molecular mechanism responsible for its actions remains largely unknown. The overall growth regulatory effects of GPC3 may extend beyond growth suppression and cover lineage determination, because increased expression of GPC3 has been shown in hepatic progenitor cells.34 The current study further demonstrates that the effects of GPC3 on hepatocyte proliferation and growth are primarily inhibitory not only in liver regeneration after PHx, but also after administration of chemical mitogens. Our results reveal overexpression of GPC3 in hepatocytes is associated with suppression of proliferation and a concomitant sustained decrease in the liver size in GPC3 TG mice.
PB and TCPOBOP are two strong chemical mitogens for the liver.14–16,19 Many studies have shown that liver size, although highly susceptible to nutritional and hormonal responses, is overall adjusted to proper level for the size of the body of the animal.35 Our recent studies have implicated pericellular and ECM as involved in this process.21–25 We used the term “hepatostat” to summarize the processes associated with adjustment of the liver size to the body weight and physiologic status. Overexpression of the pericellular protein GPC3 in hepatocytes resulted in a lower “hepatostat” in three different models of growth, such as liver regeneration after PHx,24,25 PB, and TCPOBOP. In addition, interference with integrin/ECM signaling by elimination of hepatocyte ILK resulted in a higher hepatostat in the same three different models.21–23 Our present studies highlight the important role of GPC3 protein, one of the ECM components, as a regulator of the hepatostat for the final liver size. These findings are important from the perspective of comparison of the mechanistic pathways leading to hepatocyte proliferation in these two very different processes (i.e., liver regeneration after PHx and augmentative hepatomegaly induced by chemical mitogens). The preponderance of studies done in these experimental models has shown that the pathways leading to initiation of hepatocyte proliferation are almost totally different. PHx leads to a cascade of activation of growth factor receptors (MET and EGFR), signaling by nonmitogenic cytokines tumor necrosis factor (TNF) and interleukin 6 (IL6), involvement of Notch, norepinephrine, bile acids, leptin, etc.30 On the other hand, hepatomegaly induced by chemical mitogens does not follow these pathways and signaling systems.16 CAR effects after its migration to the nucleus appear to be central in the signaling leading to hepatomegaly from chemical mitogens. Both signaling pathways converge on cyclin D1, causing activation and migration to the nucleus.19 Whereas the pathways of initiation of hepatocyte proliferation in these two models are different, the pathways leading to termination of hepatocyte proliferation appear to be similar. Both ILK hepatocyte-targeted elimination and GPC3 transgenic expression in hepatocytes lead to the same results in both models.
PB induces nuclear translocation of CAR by a ligand-independent process,14 whereas effects of TCPOBOP are known to be dependent on binding to CAR.13,14 We found that there was CAR activation in both WT and TG mice after PB and TCPOBOP administration. The nuclear levels of CAR were higher in TG mice at all timepoints after PB and TCPOBOP administration, suggesting a sustained and increased expression of CAR in TG mice. This finding is counterintuitive, and the mechanism by which overexpression of GPC3 leads to higher activation of CAR is worthy of further study. These results, however, suggest that GPC3 effects are independent of CAR nuclear levels and they are exercised on signaling components downstream of CAR nuclear effects.
We investigated changes in specific signaling molecules behind the suppression of hepatocyte proliferation in TG mice. There was overall a decrease in HGF expression in TG mice. C-Myc plays a very important role in cell proliferation and is known to be a key mediator of TCPOBOP-CAR-induced direct liver hyperplasia.13 We found sustained reduction of c-Myc in TG mice as well. It is possible that the decreased proliferation in TG mice is in part c-Myc-dependent. The changes associated with cell cycle-related genes after hepatomitogens are easier to correlate with the suppression of hepatocyte proliferation, with respect to cyclin D1, known to be associated with the entry of hepatocytes into the S-phase of the cycle, and cyclin B1 associated with M-phase of cell cycle.36,37 It is also important to notice the decrease in YAP nuclear levels in TG mice, which is associated with increase in p-YAP cytoplasmic levels. YAP plays a role in hepatocyte proliferation and liver size regulation.28,29 Phosphorylation of YAP is associated with nuclear export and degradation, which decreases in cell proliferation. TG mice had a much less YAP/p-YAP ratio as compared to WT mice (Fig. 5C,D), suggesting more degradation of nuclear YAP and suppression of proliferation in TG mice. p21, TGF-β1, and Runx3 are known mito-inhibitors. Previous studies have reported that p21 is not affected by TCPOBOP.19,39 Our results show that p21 was induced in WT and TG mice after chemical mitogens, and was further up-regulated in TG mice. Moreover, the TG mice showed higher expression of TGF-β1 and Runx3 as compared to WT mice after PB and TCPOBOP administration, correlating with a sustained suppression of proliferation. Taken together, the TG mice have sustained reduction of mitogenic signaling and persistent induction of mito-inhibitors. It is not easy to assign the termination of chemical mitogen-induced hepatocyte proliferation to any specific single signaling system. However, overexpression of GPC3 in hepatocytes indeed results in hypoproliferation due to effects in multiple signaling pathways.
In summary, these results demonstrate that overexpression of GPC3 in hepatocytes results in suppression of proliferative response to PB and TCPOBOP. Our current work further expands the evidence that GPC3 has negative effects on hepatocyte growth. These studies provide overall critical information on the multiple signaling pathways which GPC3 controls in relation to hepatocyte proliferation.
Supplementary Material
Acknowledgments
Supported by National Institutes of Health (NIH) grants CA30241 and CA35373 (to G.K.M.).
Abbreviations:
- CAR
constitutive androstane receptor
- ECM
extracellular matrix
- GPC3
glypican 3
- HCC
hepatocellular carcinoma
- HGF
hepatcyte growth factor
- ILK
integrin-like kinase
- PB
phenobarbital
- PCNA
proliferating cell nuclear antigen
- PHx
partial hepatectomy
- Runx3
runt-related transcription factor 3
- SGBS
Simpson-Golabi-Behmel syndrome
- TCPOBOP
1,4-bis [2-(3,5-dichoropyridyloxy)] benzene
- TG
transgenic
- TGF-β1
transforming growth factor beta type I
- WT
widetype
- YAP
yes-associated protein
Footnotes
Potential conflict of interest: Nothing to report.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Fransson LA. Glypicans. Int J Biochem Cell Biol 2003;35:125–129. [DOI] [PubMed] [Google Scholar]
- 2.Filmus J, Selleck SB. Glypicans: proteoglycans with a surprise. J Clin Invest 2001;108:497–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song HH, Filmus J. The role of glypicans in mammalian development. Biochim Biophys Acta 2002;1573:241–246. [DOI] [PubMed] [Google Scholar]
- 4.Filmus J Glypicans in growth control and cancer. Glycobiology 2001;11:19R–23R. [DOI] [PubMed] [Google Scholar]
- 5.Iglesias BV, Centeno G, Pascuccelli H, Ward F, Peters MG, Filmus J, et al. Expression pattern of glypican-3 (GPC3) during human embryonic and fetal development. Histol Histopathol 2008;23:1333–1340. [DOI] [PubMed] [Google Scholar]
- 6.Pilia G, Hughes-Benzie RM, MacKenzie A, Baybayan P, Chen EY, Huber R, et al. Mutations in GPC3, a glypican gene, cause the Simpson-Golabi-Behmel overgrowth syndrome. Nat Genet 1996;12:241–247. [DOI] [PubMed] [Google Scholar]
- 7.Neri G, Gurrieri F, Zanni G, Lin A. Clinical and molecular aspects of the Simpson-Golabi-Behmel syndrome. Am J Med Genet 1998;79:279–283. [DOI] [PubMed] [Google Scholar]
- 8.Pellegrini M, Pilia G, Pantano S, Lucchini F, Uda M, Fumi M, et al. Gpc3 expression correlates with the phenotype of the Simpson-Golabi-Behmel syndrome. Dev Dyn 1998;213:431–439. [DOI] [PubMed] [Google Scholar]
- 9.Cano-Gauci DF, Song HH, Yang H, McKerlie C, Choo B, Shi W, et al. Glypican-3-deficient mice exhibit developmental overgrowth and some of the abnormalities typical of Simpson-Golabi-Behmel syndrome. J Cell Biol 1999;146:255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo JH, Ren B, Keryanov S, Tseng GC, Rao UN, Monga SP, et al. Transcriptomic and genomic analysis of human hepatocellular carcinomas and hepatoblastomas. Hepatology 2006;44:1012–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Capurro M, Wanless IR, Sherman M, Deboer G, Shi W, Miyoshi E, et al. Glypican-3: a novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology 2003;125:89–97. [DOI] [PubMed] [Google Scholar]
- 12.Filmus J, Capurro M. Glypican-3 and alphafetoprotein as diagnostic tests for hepatocellular carcinoma. Mol Diagn 2004;8:207–212. [DOI] [PubMed] [Google Scholar]
- 13.Blanco-Bose WE, Murphy MJ, Ehninger A, Offner S, Dubey C, Huang W, et al. C-Myc and its target FoxM1 are critical downstream effectors of constitutive androstane receptor (CAR) mediated direct liver hyperplasia. Hepatology 2008;48:1302–1311. [DOI] [PubMed] [Google Scholar]
- 14.Qatanani M, Moore DD. CAR, the continuously advancing receptor, in drug metabolism and disease. Curr Drug Metab 2005;6:329–339. [DOI] [PubMed] [Google Scholar]
- 15.Wei P, Zhang J, Egan-Hafley M, Liang S, Moore DD. The nuclear receptor CAR mediates specific xenobiotic induction of drug metabolism. Nature 2000;407:920–923. [DOI] [PubMed] [Google Scholar]
- 16.Columbano A, Shinozuka H. Liver regeneration versus direct hyperplasia. FASEB J 1996;10:1118–1128. [DOI] [PubMed] [Google Scholar]
- 17.Diwan BA, Lubet RA, Ward JM, Hrabie JA, Rice JM. Tumor-promoting and hepatocarcinogenic effects of 1,4-bis[2-(3,5-dichloropyridyloxy)] benzene (TCPOBOP) in DBA/2NCr and C57BL/6NCr mice and an apparent promoting effect on nasal cavity tumors but not on hepatocellular tumors in F344/NCr rats initiated with N-nitrosodiethylamine. Carcinogenesis 1992;13:1893–1901. [DOI] [PubMed] [Google Scholar]
- 18.Columbano A, Ledda-Columbano GM. Mitogenesis by ligands of nuclear receptors: an attractive model for the study of the molecular mechanisms implicated in liver growth. Cell Death Differ 2003;10 Suppl 1:S19–21. [DOI] [PubMed] [Google Scholar]
- 19.Ledda-Columbano GM, Pibiri M, Loi R, Perra A, Shinozuka H, Columbano A. Early increase in cyclin-D1 expression and accelerated entry of mouse hepatocytes into S phase after administration of the mitogen 1, 4-bis[2-(3,5-dichloropyridyloxy)] benzene. Am J Pathol 2000;156:91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Locker J, Tian J, Carver R, Concas D, Cossu C, Ledda-Columbano GM, et al. A common set of immediate-early response genes in liver regeneration and hyperplasia. Hepatology 2003;38:314–325. [DOI] [PubMed] [Google Scholar]
- 21.Apte U, Gkretsi V, Bowen WC, Mars WM, Luo JH, Donthamsetty S, et al. Enhanced liver regeneration following changes induced by hepatocyte-specific genetic ablation of integrin-linked kinase. Hepatology 2009;50:844–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donthamsetty S, Bowen W, Mars W, Bhave V, Luo JH, Wu C, et al. Liver-specific ablation of integrin-linked kinase in mice results in enhanced and prolonged cell proliferation and hepatomegaly after phenobarbital administration. Toxicol Sci 2010;113:358–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donthamsetty S, Bhave VS, Kliment CS, Bowen WC, Mars WM, Bell AW, et al. Excessive hepatomegaly of mice with hepatocyte-targeted elimination of integrin linked kinase following treatment with 1,4-bis [2-(3,5-dichaloropyridyloxy)] benzene. Hepatology 2011;53:587–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu B, Paranjpe S, Bowen WC, Bell AW, Luo JH, Yu YP, et al. Investigation of the role of glypican 3 in liver regeneration and hepatocyte proliferation. Am J Pathol 2009;175:717–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu B, Bell AW, Paranjpe S, Bowen WC, Khillan JS, Luo JH, et al. Suppression of liver regeneration and hepatocyte proliferation in hepatocyte-targeted glypican 3 transgenic mice. Hepatology 2010;52:1060–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ross PK, Woods CG, Bradford BU, Kosyk O, Gatti DM, Cunningham ML, et al. Time-course comparison of xenobiotic activators of CAR and PPARalpha in mouse liver. Toxicol Appl Pharmacol 2009;235:199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seewaldt VL, Dietze EC, Johnson BS, Collins SJ, Parker MB. Retinoic acid-mediated G1-S-phase arrest of normal human mammary epithelial cells is independent of the level of p53 protein expression. Cell Growth Differ 1999;10:49–59. [PubMed] [Google Scholar]
- 28.Saucedo LJ, Edgar BA. Filling out the Hippo pathway. Nat Rev Mol Cell Biol 2007;8:613–621. [DOI] [PubMed] [Google Scholar]
- 29.Zhao B, Lei QY, Guan KL. The Hippo-YAP pathway: new connections between regulation of organ size and cancer. Curr Opin Cell Biol 2008;20:638–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michalopoulos GK. Liver regeneration. J Cell Physiol 2007;213:286–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D. p21 is a universal inhibitor of cyclin kinases. Nature 1993;366:701–704. [DOI] [PubMed] [Google Scholar]
- 32.Gao J, Chen Y, Wu KC, Liu J, Zhao YQ, Pan YL, et al. RUNX3 directly interacts with intracellular domain of Notch1 and suppresses Notch signaling in hepatocellular carcinoma cells. Exp Cell Res 2010;316:149–157. [DOI] [PubMed] [Google Scholar]
- 33.Kohler C, Bell AW, Bowen WC, Monga SP, Fleig W, Michalopoulos GK. Expression of Notch-1 and its ligand Jagged-1 in rat liver during liver regeneration. Hepatology 2004;39:1056–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grozdanov PN, Yovchev MI, Dabeva MD. The oncofetal protein glypican-3 is a novel marker of hepatic progenitor/oval cells. Lab Invest 2006;86:1272–1284. [DOI] [PubMed] [Google Scholar]
- 35.Michalopoulos GK. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. Am J Pathol 2010;176:2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mullany LK, White P, Hanse EA, Nelsen CJ, Goggin MM, Mullany JE, et al. Distinct proliferative and transcriptional effects of the D-type cyclins in vivo. Cell Cycle 2008;7:2215–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morgan DO. Principles of CDK regulation. Nature 1995;374:131–134. [DOI] [PubMed] [Google Scholar]
- 38.Columbano A, Ledda-Columbano GM, Pibiri M, Concas D, Reddy JK, Rao MS. Peroxisome proliferator-activated receptor-alpha mice show enhanced hepatocyte proliferation in response to the hepatomitogen 1,4-bis [2-(3,5-dichloropyridyloxy)] benzene, a ligand of constitutive androstane receptor. Hepatology 2001;34:262–266. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


