Abstract
Adhesion of cells to extracellular matrix is mediated by integrin family receptors. The process of receptor-ligand binding is dependent on metabolic energy and is regulated by intracellular signals, termed inside-out signals. The strength of the initial α5β1-mediated adhesion of v-src-transformed chicken embryo fibroblasts (v-srcCEF) was similar to that of normal CEF. A chemically cross-linked fibronectin substrate was able to restore cell spreading and the ability of v-srcCEF to assemble a fibronectin matrix. Over time, v-srcCEF showed decreased adhesion due to the reduction of α5β1-fibronectin bonds consequent on the reduction of substrate-bound fibronectin due to the secretion of proteases by v-srcCEF. Excess synthesis of hyaluronic acid by v-srcCEF also reduced the α5β1-fibronectin bonds and contributed to cell detachment at later times in culture. Thus, the adhesion defects were not due to a failure of α5β1 function and adhesion of the v-srcCEF was α5β1 dependent. Integrin-mediated adhesion also produces signals that affect cell proliferation and cell differentiation. An early consequence of these “outside-in” signals was the phosphorylation of FAK Y397 in direct proportion to the number of α5β1-fibronectin bonds formed. In contrast, v-srcCEF had an increased level of phosphorylation on five different tyrosines in FAK, and none of these phosphorylation levels were sensitive to the number of α5β1-fibronectin bonds. In the absence of serum, CEF proliferation was sensitive to changes in α5β1-mediated adhesion levels. Transformation by v-src increased the serum-free proliferation rate and made it insensitive to α5β1-mediated adhesion. Thus, the v-srcCEF were insensitive to the normal outside-in signals from α5β1 integrin.
Despite the role of v-src as the prototype oncogene, analysis of the mechanism by which v-src transforms cells has lagged behind that for other oncogenes. One school of thought linked src to the growth factor signaling pathways through its potential to interact with the receptors for epidermal growth factor and platelet-derived growth factor (36, 61). The alternative hypotheses centered on the initial discovery that v-src was localized to focal adhesions (48). Using a screen for proteins which showed increased tyrosine phosphorylation in v-src transformed cells, Parsons and coworkers identified a series of adhesion and cytoskeletal associated proteins that were overphosphorylated in v-src-transformed cells, suggesting a role for v-src in the regulation of cell adhesion and cytoskeletal assembly (26, 31). More recently, work with src family knockout mice has provided further support to the idea that c-src controls cell adhesion-mediated signals and suggested that it does not play an essential role in the growth factor-mediated signals (32, 33).
Integrins are heterodimeric cell surface receptors that serve to attach cells to extracellular ligands. The conformation of the extracellular cell binding domain of the hererodimer can be regulated by intracellular signals (16, 55). This control of integrin-ligand interactions by intracellular signals has been called inside-out signaling, and it regulates the affinity of the integrin for its ligand, adhesion to extracellular matrix, and extracellular matrix assembly (29, 64). Transformation of cells by v-src results in a less-spread, fusiform, or round cell morphology, which appears to be less adherent to the substrate. One way to achieve this morphologic change would be to reduce the adhesion of the cells to their extracellular matrix substrate by the modulation of integrin inside-out signaling. Ligand binding by integrins can also act to control cell differentiation and cell proliferation (21, 68) mediated by intracellular signaling systems that appear to include FAK, cas, paxillin, and the mitogen-activated protein kinase (MAPK) cascade (4, 26, 51). The cytoplasmic domains of both β1 and β3 integrins contain a membrane-proximal tyrosine in an NPXY motif that represents a known v-src phosphorylation target and a more distal tyrosine that also is in a similar motif and hence a potential target for v-src (57). Both β1 and β3 tyrosine phosphorylation are increased in cells transformed by v-src (28, 58) (A. Datta, unpublished results). Mutation of this tyrosine in β1 altered the adhesion of cells to laminin but showed little effect on adhesion to either fibronectin or vitronectin substrates (50). Analysis of β1 integrin mutants and differential detergent extraction of β1 integrin from v-src transformed cells suggested that phosphorylation of the proximal tyrosine in β1 tended to redistribute the β1 away from the adhesion structures and therefore make it nonfunctional (25, 50, 58). This led to the hypothesis that integrin function may be controlled by phosphorylation of the β-cytoplasmic domain and that v-src could result in a disregulation of integrin function. Phosphorylation of FAK is increased either by adhesion of the cells to extracellular matrix or by transformation with v-src (8, 24, 34, 38). Phosphorylation of FAK is linked to numerous downstream signaling pathways and thus may govern the specific alterations in growth control that are central to the transformed phenotype.
Analysis of integrin function in v-src-transformed cells is complicated by the large increase in secretion of both plasminogen activator-type and metalloproteases (2, 56). Some of these secreted proteases are specifically localized to the focal contact regions of chicken embryo fibroblasts (CEF) (6). Since the number of integrin-ligand bonds would be reduced by the proteolytic cleavage and removal of the ligand, this would have the effect of reducing integrin-mediated adhesion independently of integrin activation and inside-out signals. Protease inhibitors have been shown to suppress morphologic aspects of v-src transformation (62). However, given the multiplicity of extracellular matrix molecules available from the serum and synthesized by the cells, it was not clear which matrix molecules were responsible for the protease effects. High concentrations of fibronectin also caused some morphologic reversion, but again the mechanism was not analyzed (1, 65). The experiments described here employ a chemically cross-linked fibronectin matrix to reduce the effects of protease cleavage and to provide an analysis of integrin function without the confounding simultaneous effects of the proteases that are upregulated in the process of cell transformation.
MATERIALS AND METHODS
Cell culture and transformation.
Primary CEF were isolated from 11-day-old chicken embryos (B&E Eggs, Inc., Stevens, Pa.) and cultured in Dulbecco modified Eagle medium supplemented with 1% chicken serum (Life Technologies, Inc.), 4% fetal calf serum (Sigma), l-glutamine, penicillin, and streptomycin (Life Technologies, Inc.). For transformation by v-src, cells were trypsinized and replated. Two hours after the replating, the culture medium was removed and the cells were infected with the Rous sarcoma virus strain Schmidt-Ruppin subtype A (SRA), which expresses the v-src oncogene. Normal medium was replaced 2 h later. Transformation-associated morphologic changes were usually observable within 48 h after infection. Cells were used between the second and fifth passages.
Deposition and cross-linking of fibronectin.
Cover glasses were coated with 10 μg of human plasma fibronectin (Life Technologies, Grand Island, N.Y.)/ml for 30 min and washed with Dulbecco phosphate-buffered saline (PBS) three times. After absorption to the glass surface, some cover glasses were treated with 0.1% glutaraldehyde in PBS for 10 min to cross-link the deposited fibronectin. The cover glasses were incubated in Tris-dextrose (25 mM Tris, 135 mM NaCl, 0.4 mM Na2HPO4, 5 mM KCl, 5 mM dextrose) for 10 min to quench the cross-linking reaction and washed twice with Tris-dextrose. Cells were plated at low density on normal and cross-linked fibronectin in the presence or absence of hyaluronidase (20 μg/ml) in Dulbecco modified Eagle medium without serum for up to 7 days.
Immunofluorescence.
Cells were seeded on fibronectin-coated cover glasses in serum-free medium for 48 h. The cover glasses were then washed twice with Dulbecco PBS. Cells were fixed with 3.7% formaldehyde, washed twice with PBS and permeabilized with 0.5% Triton X-100 for 15 min. The cover glasses were blocked with 0.1% bovine serum albumin (BSA). HFN7.1 monoclonal antibody (American Type Culture Collection, Rockville, Md.) hybridoma supernatant against human fibronectin was added at a 1:5 dilution, or B3D6 monoclonal antibody (Developmental Studies Hybridoma Bank, Iowa City, Iowa [submitted by D. M. Fambrough]) purified ascites fluid against chick fibronectin was added at 1:500. The primary antibody was detected by fluorescein-conjugated secondary anti-mouse antibody (Jackson ImmunoResearch, West Grove, Pa.). For actin staining, cells were prepared as described above and stained with fluorescein-conjugated phalloidin (Molecular Probes).
Fibronectin enzyme-linked immunosorbent assay (ELISA).
Cover glasses (25 mm in diameter) were coated with human plasma fibronectin at different concentrations ranging from 0 to 10 μg/ml with or without cross-linking as described earlier. A total of 5 × 105 cells were seeded per cover glass in the absence of serum and incubated for 48 h. The cover glasses were washed twice with PBS and treated briefly with 0.1% sodium dodecyl sulfate (SDS) in PBS to remove the cells. The cover glasses were then washed twice in PBS and blocked with blocking buffer (0.05% Tween 20, 0.25% BSA, and 1 mM EDTA in PBS [pH 7.4]). Residual fibronectin was detected with either HFN7.1 or rabbit anti-fibronectin antibody (Cappel, West Chester, Pa.) and then with fluorescein isothiocyanate (FITC)-conjugated secondary antibody. The cover glasses were scanned in a Storm fluorescent imager (Molecular Dynamics, Sunnyvale, Calif.) at a photomultiplier tube voltage of 900 V. The relative fluorescence intensities were determined by densitometry using ImageQuant software (Molecular Dynamics).
Spinning disk assay.
The spinning disk assay was performed as described by Garcia et al. (19). Briefly, the cells were detached by trypsinization, and the trypsin neutralized by treatment with 0.05 mg of soybean trypsin inhibitor (Sigma)/ml. The cells were either pretreated with CSAT function-blocking monoclonal antibody to chick β1 integrin (a gift from A. F. Horwitz) for 15 min or left untreated. The cells were then allowed to adhere to the deposited extracellular matrix on a coverslip placed on the spinning disk for 15 min. The chamber containing the disk was then filled with buffer, and the disk was spun at a constant angular velocity. After the spinning step, the cells were fixed with ice-cold 95% ethanol and stained with ethidium homodimer. Cell numbers at different radial positions were determined by using a motorized stage and Phase 3 image analysis software version 3.0, and the shear stress corresponding to 50% cell detachment (τ50) was calculated by fitting the data to a sigmoid curve by using SigmaPlot software.
Cross-linking of bound integrins.
A total of 2 × 106 cells were seeded on fibronectin-coated dishes (10 μg/ml) for 1 h and cross-linked with 1 mM Sulfo-BSOCOES (Pierce, Rockford, Ill.) in PBS for 30 min. The cells were extracted with 0.1% SDS in PBS containing protease inhibitors. After detergent extraction, the extracted proteins were quantified by a BCA Assay (Pierce). The cross-links were reversed by adding carbonate buffer (50 mM Na2CO3, 0.1% SDS; pH 11.6) for 2 h at 37°C. The cross-linked pool of integrins was detected by Western blotting using a polyclonal antibody against β1 integrin (10) and monoclonal antibodies A21F7 and D71E2 against avian α5 integrin (Developmental Studies Hybridoma Bank, Iowa City, Iowa [submitted by A. F. Horwitz]).
Analysis of FAK and general tyrosine phosphorylation.
Normal and v-src-transformed CEF were serum starved overnight, trypsinized, neutralized with soybean trypsin inhibitor, washed, and plated on fibronectin at densities of 0, 15, 100, 250, and 350 ng/cm2 blocked with 1% heat-inactivated BSA. Cells were incubated for 1 h at room temperature in PBS with 1 mM Ca2+–1 mM Mg2+ and 2 μg of dextrose/ml (adhesion buffer). Cells were extracted with ice-cold lysis buffer (100 mM NaCl, 10 mM Tris-HCl, 1 mM EDTA, 1 mM EGTA, 20 mM Na2P2O7, 1 mM NaF, 1% Triton X-100, 0.1% SDS, 2 mM Na3VO4, 350 μg of phenylmethylsulfonyl fluoride/ml, 10 μg of leupeptin/ml, and 10 μg of aprotinin/ml). Total cellular protein was quantified by the BCA Assay. Equal amounts of total protein were loaded in each lane, and the proteins were resolved by SDS-polyacrylamide gel electrophoresis. The levels of phosphorylation of FAK(Y397), FAK(Y407), FAK(Y577), FAK(Y861), and FAK(Y925) were detected by phosphorylation state-specific polyclonal antibodies (Biosource International, Camarillo, Calif.). Total FAK was detected by a monoclonal antibody from Transduction Laboratories (Lexington, Ky.). Primary antibody was detected by using horseradish peroxidase-conjugated secondary antibodies (Calbiochem, San Diego, Calif.), followed by incubation with enhanced chemiluminescence reagent (Amersham Pharmacia Biotech, Piscataway, N.J.). The bands were visualized by Fuji LAS-1000plus luminescent image analyzer and analyzed with ScienceLab v2.5 software.
Analysis of cell proliferation.
Cells were plated on fibronectin (350 ng/cm2), cross-linked fibronectin (350 ng/cm2), or polylysine coated at 100 μg/ml in serum-free medium for 48 h and 10 μg of bromodeoxyuridine (BrdU)/ml was added for the next 24 h. At 72 h, the cells were washed with PBS and stained as described by Foster et al. (15). The cells were treated with 2 N HCl for 20 min and washed three times for 20 min with 100 mM Tris–50 mM NaCl (pH 7.6). The cover glasses were blocked with 1% BSA and 5% fetal calf serum in PBS for 15 min and then incubated with anti-BrdU monoclonal antibody G3G4. The primary antibody was detected with FITC-conjugated anti-mouse antibody. Nuclei were counterstained with ethidium homodimer. The anti-BrdU-stained and total nuclei were counted by fluorescence microscopy.
RESULTS
Adhesion of v-src-transformed CEF is dependent on α5β1 integrin.
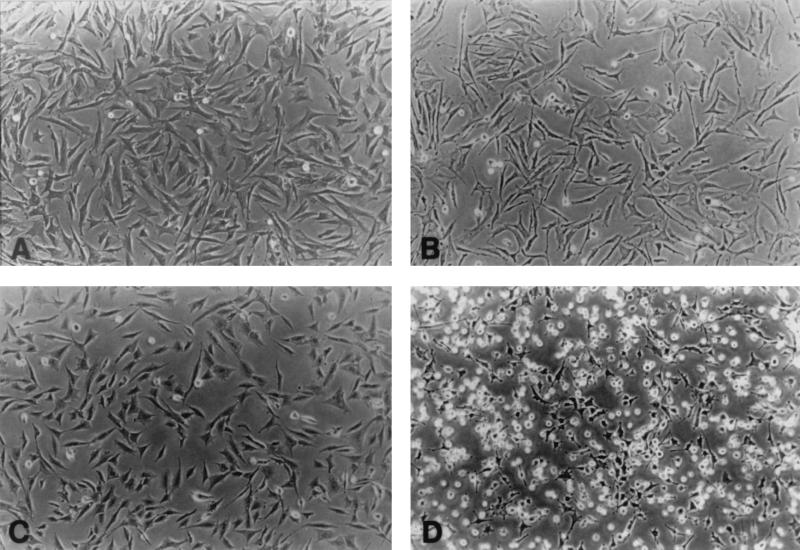
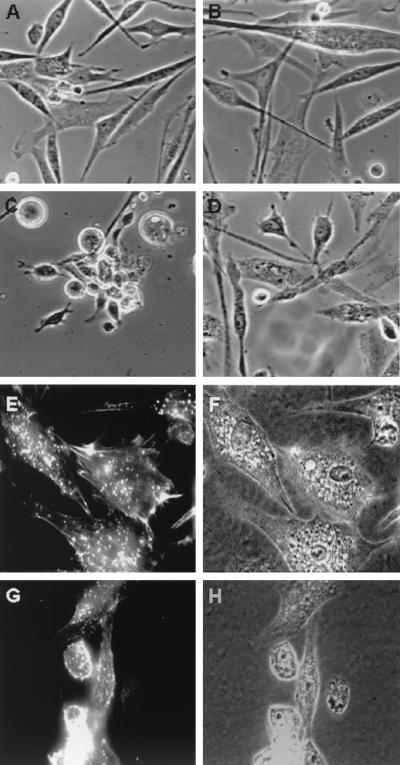
From the early observations, oncogenic transformation of cells in culture has been recognized by altered cell morphology (60). The transformed cells appear to be rounder and more refractile, suggesting a reduction in their adhesion to the culture dish. To analyze the receptors involved in these adhesion differences, normal CEF and cells transformed by Rous sarcoma virus encoding the v-src oncogene were plated and allowed to spread. CSAT monoclonal antibody that blocks β1 integrin was added for 6 h, and the effect on the spread cells was examined. Figure 1 shows that CSAT caused a slight retraction of the normal cells but they remained largely spread, whereas the transformed cells were dramatically retracted and many cells detached. This demonstrates that the adhesion of the transformed cells was dependent on β1 integrins while the normal cells appear to have additional adhesion mechanisms that operate when β1 integrin-mediated adhesion is blocked.
FIG. 1.
Cells were allowed to spread and then were treated for 6 h with CSAT monoclonal antibody at 30 μg/ml. CSAT is a function-blocking anti-chicken β1 antibody. (A) Normal CEF. (B) Normal CEF treated with CSAT. (C) v-src-transformed CEF. (D) v-src-transformed CEF treated with CSAT.
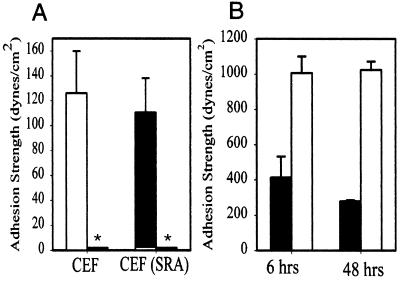
To dissect this problem, we developed a system for the quantitative analysis of cell adhesion. The spinning disk analysis allows the measurement of adhesion strength for intact cells, and hence the assay is sensitive to inside-out signaling (18, 22, 29), which is likely to differ between the normal and the transformed cells. In addition, this remains the only adhesion assay in which a direct quantitative relationship between the number of receptor-ligand bonds and the strength of cell adhesion has been demonstrated. Because of this direct relationship the differences in adhesion strength represent the product of differences in the strengths of the integrin-ligand bonds and the number of those bonds (19). Figure 2A shows that there was no significant difference in the strength of adhesion for v-src-transformed CEF compared to normal CEF after 15 min of plating on a fibronectin substrate. This adhesion was dependent on β1 integrin since CSAT antibody reduced the adhesion to background levels for both cell populations. This demonstrates that the β1 integrin receptors for fibronectin, principally α5β1, are functioning reasonably normally in the v-src-transformed cells. The spinning disk also provides the unique ability to measure the strength of adhesion for cells which have been plated for several hours or days (20). Figure 2B shows a separate experiment in which normal and v-src-transformed CEF were allowed to adhere to fibronectin for 6 or 48 h before analysis in the spinning disk device. At these times, the v-src-transformed CEF were only 3- to 4-fold more adherent than they were at 15 min, whereas the normal CEF were 10-fold more adherent. Thus, although the cells still demonstrate reasonable strength of adhesion, the transformed cell adhesion was compromised at the later time points.
FIG. 2.
Adhesion assays for normal and v-src-transformed CEF obtiained by using the spinning disk assay. (A) Mean adhesion strength for normal CEF and v-src-transformed CEF [CEF(SRA)] for adhesion to fibronectin (160 ng/cm2) for 15 min in the absence or presence of CSAT antibody (∗; <2 dynes/cm2). Error bars indicate the standard deviation (n = 3). (B) Mean adhesion strength for normal CEF (white bars) and v-src-transformed CEF (black bars) for cells were allowed to adhere to fibronectin for 6 or 48 h before analysis by using the spinning disk. Error bars indicate the standard deviation (n = 3).
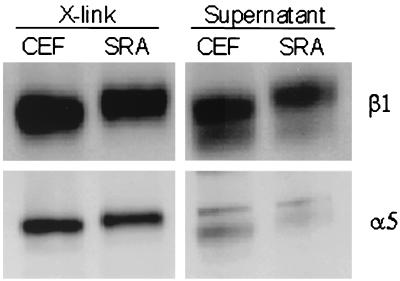
Another approach to the analysis of the integrins involved in adhesion to substrate involves the use of cell-impermeant chemical cross-linkers to cross-link the bound receptors to their substrate-adsorbed ligands (11). Only active integrins can be cross-linked, and cross-linking requires the availability of the correct ligand (11, 21). For α5β1 integrin, there is a direct linear relationship between the proportion of α5 and β1 cross-linked and the number of α5β1-fibronectin bonds (17) (Q. Shi and D. E. Boettiger, unpublished results). The proportion of α5 and β1 integrin that could be cross-linked to fibronectin 1 h after plating was examined for normal and v-src-transformed cells (Fig. 3). The lower band represents intracellular forms of α5 and β1 integrin, which are not fully processed and hence were seen only for the supernatant pool which includes the intracellular integrin. Quantification of the data show that the cross-linked α5β1 was reduced by ca. 20% for the v-src-transformed cells.
FIG. 3.
Chemical cross-linking of fibronectin-bound α5β1. Level of substrate-bound β1 and α5 integrins after 1 h of attachment of cells to fibronectin was detected by Western blot of β1 and α5 integrins. X-link, recovered integrin subunits after cleavage of the cross-linker; Supernatant, extract of non-cross-linked integrins; CEF, normal cells; SRA, v-src-transformed cells.
These analyses demonstrate that, at least for early times after plating on a fibronectin substrate, the adhesion of the v-src-transformed CEF is very similar to that for normal CEF. Differences in the multiplicity of adhesion mechanisms and in the strength of adhesion were observed for later times. Since the initial interaction between integrin and fibronectin requires inside-out signals to activate binding to fibronectin, this implies that these inside-out signals are still functional in the presence of v-src. The data point to the importance of β1 integrins in mediating substrate adhesion for v-src-transformed CEF. Unfortunately, specific function-blocking antibodies are not available to distinguish between different avian β1 integrin types, but the cross-linking data supplied here and previously (11), and similar analyses of primary fibroblasts of human origin, strongly implicate α5β1 (Datta and Boettiger, unpublished).
Reduced adhesion of v-src-transformed CEF is caused by reduced fibronectin on the substrate and reduced accessibility to the fibronectin.
The analyses performed focused on the α5β1-fibronectin bond because this appears to be the dominant mechanism of adhesion of v-src-transformed CEF in culture. Since the strength of integrin-mediated adhesion is determined by the number of receptor-ligand bonds, changes in the surface density of fibronectin would alter the overall strength of the adhesion. Oncogenically transformed cells in general, and v-src transformed cells in particular, produce increased levels of both metalloproteases and plasminogen activator-type proteases (2, 56). To test the hypothesis that the digestion of fibronectin by proteases led to a decreased density of fibronectin on the substrate and hence to a decrease in integrin-mediated adhesion, a model was developed using chemically cross-linked fibronectin to reduce the ability of proteases to remove it from the substrate.
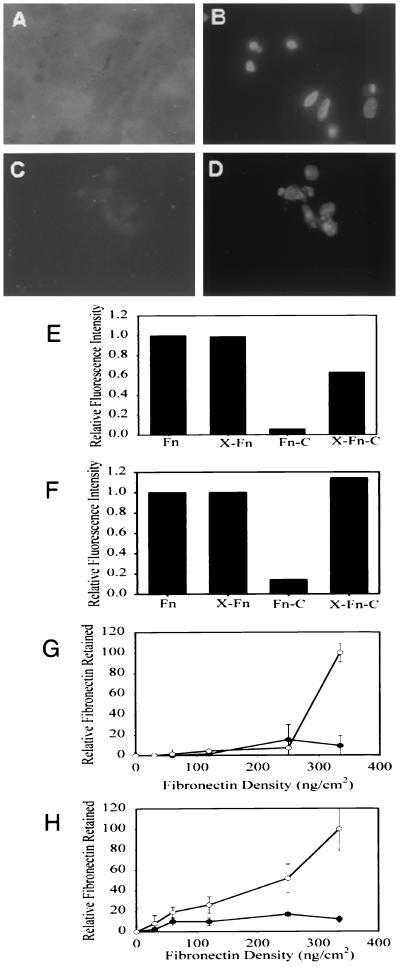
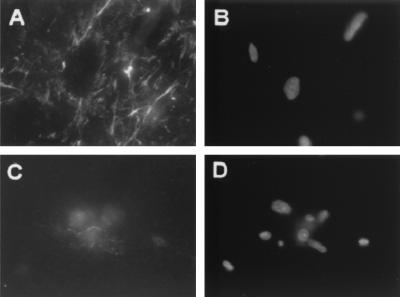
v-src-transformed cells were plated on normal or chemically cross-linked fibronectin in the absence of serum. The adsorbed fibronectin appears as a diffuse staining and was not assembled into fibrils. The presence of fibronectin on the surface was analyzed after 48 h by using a monoclonal antibody, HFN7.1, which recognizes the cell-binding domain of human fibronectin (52). Figure 4A to D shows that chemical cross-linking of the fibronectin led to its retention, whereas the bulk of the non-cross-linked fibronectin was removed. To quantify this removal of fibronectin, densitometric analysis of the remaining fibronectin was performed by using both the HFN7.1 monoclonal antibody and a rabbit polyclonal anti-fibronectin antibody (Fig. 4E and F). By both antibodies, >90% of the non-cross-linked fibronectin was removed. After cross-linking, HFN7.1 detected 63% of the control level, whereas the polyclonal antibody showed no decrease relative to the control. The difference between these two measurements could be due to the presence of newly synthesized chick fibronectin deposited on the surface and/or to a preferential loss of the HFN7.1 epitope in the presence of transformed cell proteases.
FIG. 4.
Chemical cross-linking inhibits proteolytic removal of fibronectin. v-src-transformed CEF were plated on fibronectin-coated or chemically cross-linked fibronectin-coated cover glasses. (A) Cells plated for 48 h on cross-linked fibronectin and stained with HFN7.1 monoclonal antibody showing diffuse staining of the initial human fibronectin. (B) Same field as panel A but stained with ethidium homodimer. (C) Cells plated on normal fibronectin for 48 h and stained with HFN7.1 showing that most of the fibronectin has been removed by the cells. (D) Same field as panel C but stained with ethidium homodimer. (E and F) Quantification of residual fibronectin levels after 48 h by using HFN7.1 antibody (E) or rabbit anti-fibronectin (F). Fn, fibronectin only; X-Fn, cross-linked fibronectin only; Fn-C, fibronectin plus transformed cells; X-Fn-C, cross-linked fibronectin plus transformed cells. (G and H) Modified enzyme-linked immunosorbent assay showing the removal of normal (●) or cross-linked (○) fibronectin by transformed cells as a function of fibronectin density by using HFN7.1 antibody (G) and rabbit anti-fibronectin antibody (H). Error bars indicate the standard deviation (n = 3).
To determine whether intramolecular cross-linking was sufficient to render the fibronectin resistant to protease removal, fibronectin was deposited at different densities ranging from 5 to 350 ng/cm2 and then cross-linked. Cells were seeded on the cross-linked fibronectin and on corresponding densities of non-cross-linked fibronectin. After 48 h of cell culture, cells were removed and the remaining human fibronectin was detected with both HFN7.1 and rabbit anti-fibronectin antibodies (Fig. 4G and H). Retention of the HFN7.1 α5β1 integrin-binding epitope was seen only at the highest coating density. This shows that intermolecular cross-linking was particularly important for the retention of the cell-binding domain of fibronectin. Retention of the wider range of epitopes recognized by the polyclonal antibody was less sensitive to fibronectin density, suggesting that intramolecular cross-linking also plays a role in immobilization of the fibronectin.
The ability of the chemical-cross-linking procedure to reverse this reduction of adhesion by the v-src-transformed cells was analyzed. To reduce the complicating effects of serum factors and the presence of proteases and protease precursors in serum, these experiments were all performed serum free. v-src cells were able to survive periods of at least a week in the complete absence of serum without observable effects of apoptosis. After 12 h, v-src-transformed cells plated on normal or cross-linked fibronectin remained well adherent and spread; however, by 48 h the cells on normal fibronectin had retracted into rounded, loosely adherent cell clusters, whereas the cells on cross-linked fibronectin remained largely spread (Fig. 5A to D). After more than 72 h, even the cells on the cross-linked fibronectin retracted into cell clusters.
FIG. 5.
Effects of fibronectin cross-linking and hyaluronidase on the morphology of v-src transformed CEF. (A) Plated for 12 h in the absence of serum on cover glasses coated with fibronectin. (B) Plated for 12 h in the absence of serum on cover glasses coated with glutaraldehyde cross-linked fibronectin. (C) Plated for 48 h on fibronectin. (D) Plated for 48 h of glutaraldehyde cross-linked fibronectin. (E) Plated for 7 days on glutaraldehyde cross-linked fibronectin in the presence of hyaluronidase and phalloidin stained. (F) Corresponding phase micrograph (i.e., for panel E). (G) Plated for 7 days on fibronectin in the presence of hyaluronidase and phalloidin stained. (H) Corresponding phase micrograph (i.e., for panel G).
In addition to the secretion of proteases by transformed cells, there are changes in the proteoglycans produced (54) and an increase in the synthesis of hyaluronic acid (30). The biological effects of these differences had not been investigated. To determine whether the synthesis of hyaluronic acid and altered proteoglycans affected adhesion, hyaluronidase was added to the cultures. Figure 5E and F shows v-src-transformed cells after 7 days in serum-free medium plated on cross-linked fibronectin in the presence of hyaluronidase. These cells maintained their spread appearance and show actin staining in podosomes, a finding characteristic of v-src-transformed cells, and some filamentous actin staining near the tips of the cells. The actin staining pattern was not reverted to the intense actin cables seen in normal CEF (3). Figure 5G and H shows the parallel staining for cells plated on non-cross-linked fibronectin. The majority of the cells had retracted into small clusters. Shown here is one of the more spread clusters to reveal the peripheral actin staining seen most prominently in the round cell. Whether one expects the induced spreading of the transformed cells to restore actin stress fibers depends on whether this assembly is part of the activation process, i.e., inside-out signaling, or part of the response, i.e., “outside-in” signaling. Note that there still is assembled actin at the cell periphery that could fulfill the actin assembly requirements associated with integrin activation (67). Since stress fibers appear relatively late in the spreading process and accumulate with time, it would appear more likely that the failure to restore these structures is a consequence of defective outside-in signaling.
Thus, the analysis of integrin-mediated adhesion for transformed cells is confounded both by the secretion of proteases which removes the substrate-bound ligands necessary for integrin-mediated adhesion and by the secretion of proteoglycans and hyaluronic acid which can act to insulate the integrin receptors from the substrate-bound ligands, as shown previously for normal chondroblasts (10).
Fibronectin assembly by v-src-transformed cells.
In tissues, most of the fibronectin is assembled into fibrils in a process that requires activated integrins (63, 64). In contrast, transformed cells in culture tend to have very little assembled fibronectin and this has been interpreted as a failure of integrin activation or function (3, 49). Since the results presented above failed to find a problem with the ability of α5β1 expressed on v-src-transformed cells to be activated and to mediate strong adhesion to fibronectin, we hypothesized that the failure to observe assembled fibronectin by transformed cells was due to its digestion by the secreted proteases rather than due to a failure of integrin function. v-src-transformed cells plated on cross-linked fibronectin were able to assemble an extensive fibrillar fibronectin matrix, whereas v-src-transformed cells plated on normal fibronectin show only a diffuse fibronectin in the region of the cells (Fig. 6). Thus, the previous failures to observe fibronectin assembly by the v-src-transformed cells (49) are likely to be due to the specific proteases secreted by the transformed cells and not to a failure of integrin function in fibronectin assembly.
FIG. 6.
v-src-transformed cells can assemble a fibronectin matrix. v-src-transformed CEF were incubated for 48 h on coated cover glasses. (A) Cross-linked fibronectin stained with anti-chicken fibronectin monoclonal antibody B3D6. (B) Same field as panel A but stained with ethidium homodimer. (C) Non-cross-linked fibronectin stained with B3D6. (D) Same field as panel C but stained with ethidium homodimer.
How does v-src affect integrin?
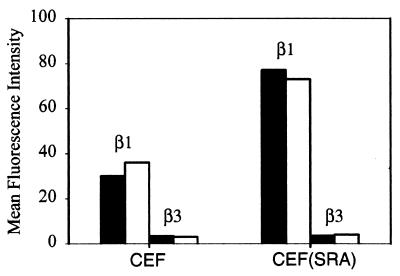
The data presented above show that the reduced adhesion and spreading of v-src-transformed cells can be explained by effects of protease and extracellular matrix secretion, which is altered in the transformed cells. However, changes in the level of integrin expression could also contribute to the reduced adhesion. Control and v-src-transformed CEF were examined by flow cytometry to determine whether there were differences in the level of expression of β1 and β3 integrin and whether these levels were affected by culture on cross-linked fibronectin substrates. Figure 7 shows that cross-linked substrates had no effect on integrin surface expression levels, but the v-src-transformed cells had about twice as much surface β1 compared to normal CEF. Unfortunately, antibodies to determine whether this also results in an increase in α5 were not available. It has been reported that v-src-transformed cells have increased α3β1 and reduced α5β1 (45), so the increase in β1 integrin may not reflect an increase in α5β1 on the surface of the transformed cells. α3β1 is primarily a receptor for laminin 5. Given the quantitative results presented above that show very little difference in total adhesion, this raises the possibility that a portion of the α5β1 on the v-src-transformed cells could be in an inactive form.
FIG. 7.
Mean fluorescence index (calculated as geometric mean integrin – geometric mean control/geometric mean control) as determined by flow cytometry for expression of β1 and β3 integrin after 48 h on normal fibronectin (black bars) or glutaraldehyde cross-linked fibronectin (white bars).
v-src blocks outside-in signals.
In addition to its function in cell adhesion, integrin also provides signals to cells that affect normal cellular processes including differentiation and proliferation (41, 69). While many downstream signals that result from the plating of cells on a fibronectin substrate have been investigated, the actual link between integrin and these signaling processes remains to be defined. To address this issue, we have taken a kinetic approach. The basic assumption in this analysis is that a linear range of signals will generate a similar range of responses or an accelerated range of responses (ultrasensitivity) as it is passed down a signaling pathway. Ultimately, the cell will have to integrate the signals and provide a single response, usually an “all-or-none” response. We have shown that the strength of adhesion is directly related to the number of α5β1 receptors bound (17, 19). Thus, providing a range of fibronectin densities will result in a similar range of α5β1 bound and initiate a range of integrin signaling.
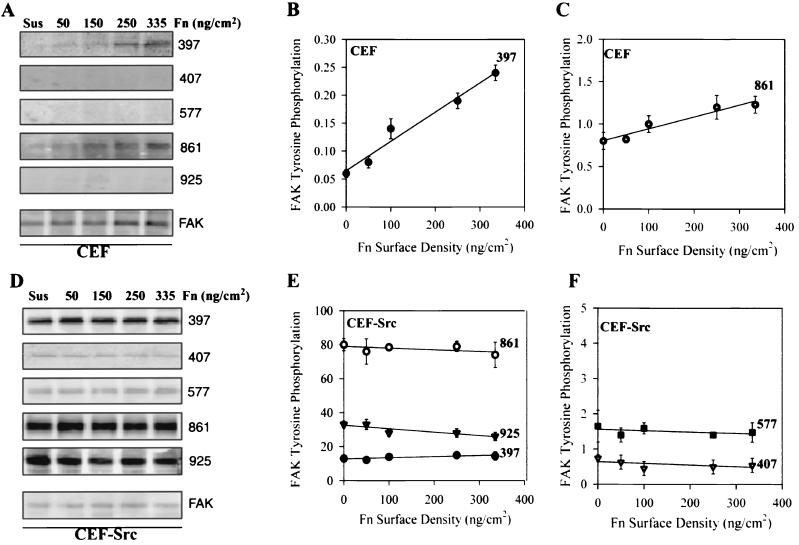
To look for the next step in this chain, we analyzed the level of phosphorylation of FAK at tyrosines 397, 407, 577, 861, and 925 in normal CEF plated for 1 h at room temperature on different densities of fibronectin (Fig. 8A to C). In the absence of pretreatment of CEF with vanadate to block phosphatases, significant phosphorylation of only Y397 and Y861 could be detected. The phosphorylation levels were normalized to total FAK in each sample to derive the phosphorylation plots. Y397 showed a linear increase in phosphorylation as a function of fibronectin density with almost a 1:1 ratio of phosphorylation level to fibronectin density, suggesting that each α5β1 bound to fibronectin produced one pY397. The phosphorylation of Y861 showed a weaker dependence on fibronectin density rising only twofold for a sevenfold increase in fibronectin density. According to the experimental rationale, phosphorylation of Y397 is likely to be a close downstream response to α5β1-fibronectin binding and signaling. In contrast, for v-src-transformed CEF, phosphorylation of all five tyrosines was detected, but there was no significant change in the level of phosphorylation of FAK at any of these sites as a function of fibronectin density (Fig. 8D to F). Thus, while FAK was able to sense integrin-mediated adhesion in normal cells, it was not able to do so in the transformed cells. At this point, phosphorylation levels on FAK are the only reported markers that show this type of a dose response.
FIG. 8.
Specific induction of FAK phosphorylation by α5β1-mediated adhesion to fibronectin. Cells were serum starved overnight, trypsinized, neutralized with soybean trypsin inhibitor, and plated on fibronectin-coated plates at densities of 0, 50, 100, 150, and 350 ng/cm2 for 60 min at room temperature (without phosphatase inhibitors). (A) Normal CEF extracts were analyzed by for phosphorylation of FAK(Y397), FAK(Y407), FAK(Y577), FAK(Y861), FAK(Y925), and total FAK protein. (B) Quantification of relative phosphorylation of Y397 in CEF; phosphorylated pY397/total FAK for each point. (C) Similar quantification of relative phosphorylation of Y861. (D) v-src-transformed CEF extracts were analyzed for specific phosphorylation of FAK(Y397), FAK(Y407), FAK(Y577), FAK(Y861), FAK(Y925), and total FAK. (E) Quantification of relative phosphorylation of Y397, Y861, and Y925 normalized for total FAK in each extract. (F) Similar quantification of Y407 and Y577. Error bars indicate the standard deviations (n = 3). Symbols: ▪, pY397; ▾, pY407; ▪, pY577; ○, pY861; ▾, pY925.
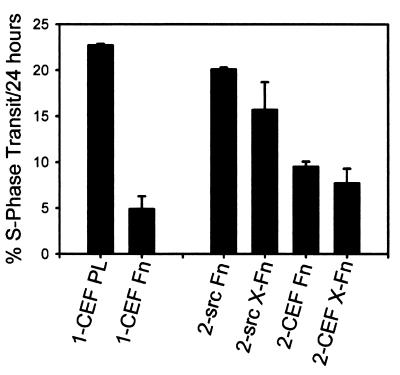
In the presence of serum, or growth factors, normal fibroblasts require adhesion to a substrate for proliferation (39, 69). Since v-src-transformed cells proliferate in suspension, they appear to have bypassed the requirement for integrin-mediated adhesion. The adhesion-mediated control of proliferation of cells in the absence of serum is less well studied. Myoblasts do proliferate in the absence of serum, and their proliferation was reduced as adhesion was increased (21). Normal CEF showed a similar adhesion control of cell proliferation. Figure 9 shows two experiments that analyze the effect of adhesion on the growth of normal and v-src-transformed CEF under serum-free conditions. The cells were plated on different substrates in the absence of serum and incubated for 2 days to minimize the effects of trypsinization and residual serum and then labeled with BrdU for 24 h. The first experiment shows that polylysine increased the rate of proliferation of CEF ∼4-fold compared to fibronectin, demonstrating that normal CEF do respond to differences in their adhesive substrate and that this can control the rate of proliferation. In the second experiment, normal and v-src-transformed CEF were plated on normal or cross-linked fibronectin. It was expected that the transformed cells would remove the fibronectin substrate during the initial 2-day incubation period as shown above. Nevertheless, the transformed cells maintained the higher proliferation rate that is characteristic of the transformed cells and showed no response to the differences in fibronectin. As expected, the normal CEF also showed no difference, but these cells do not remove their fibronectin matrix even in the absence of cross-linking. Thus, the transformed cells appear to be insensitive to differences in integrin-mediated adhesion for regulation of their proliferation rate. This may explain why the transformed cells are able to proliferate in suspension.
FIG. 9.
Adhesion-dependent control of cell growth rate in the absence of serum. Cells were plated on substrates for 48 h in serum-free medium. BrdU was added from 48 to 72 h, the cells were stained with anti-BrdU, and the proportion of BrdU positive nuclei was counted. In experiment 1, normal CEF were plated on polylysine (1-CEF PL) or fibronectin (1-CEF-Fn). In experiment 2, normal CEF were plated on normal (2-CEF-Fn) or cross-linked (2-CEF-X-Fn) fibronectin, and v-src-transformed CEF were plated on normal (2-src-Fn) or cross-linked (2-src-X-Fn) fibronectin. Error bars indicate the standard deviations (n = 3).
DISCUSSION
The change in cell morphology from a flattened, spread shape to a more rounded, less spread, or fusiform shape is a widely used indicator of in vitro oncogenic transformation (60). While many of the elements that contribute to the altered phenotype have been previously identified, analysis of the role of integrin receptor function provides a missing link that now allows the elements to be assembled into a consistent model. The experiments described here employ the original avian CEF cell model and focus on the role for α5β1 integrin because that is the dominant fibronectin receptor in these cells. In contrast to previous work, we find that the activation of α5β1 and its ability to function in cell adhesion and fibronectin assembly were not significantly impaired by v-src-mediated cell transformation. However, control of downstream events in cell signaling and control of cell proliferation is no longer linked to cell adhesion.
Inside-out signaling to α5β1 in v-src-transformed cells.
On normal fibroblasts α5β1, integrin is expressed in a form that does not bind significantly to soluble fibronectin, and α5β1-mediated adhesion of these cells to a fibronectin substrate is dependent on signaling and metabolic energy (12, 20). The intracellular events that contribute to the conformational change in the integrin to allow or induce strong adhesion has been referred to as inside-out integrin signaling (22). A number of indirect assays of integrin adhesion function have been applied, such as cell migration, cell spreading, and formation of focal adhesions, but these assays depend on aspects of cytoskeleton assembly in addition to adhesion functions. The more direct assays of integrin function are adhesion strength and assembly of a fibronectin matrix (19, 64). The experiments described here showed no significant difference in adhesion after transformation by v-src in short-term assays. The adhesion data are supported by chemical cross-linking data showing that α5β1 can be cross-linked to a similar extent in normal and v-src-transformed cells. Previous experiments have shown that only activated, fibronectin-bound α5β1 could be cross-linked in this assay (11, 19). The absence of a difference in adhesion for v-src-transformed cells in the short term is in agreement with the data of Plantefaber and Hynes, who showed no temperature dependence of adhesion for cells carrying a temperature-sensitive v-src mutant (45). Sakai et al. (49) used β1-integrin-transfected GD25 cells to analyze the effect of v-src on β1 integrin and also show very little difference in adhesion for the normal and v-src-transformed cells to fibronectin, although it is not clear which integrin is functioning in the assays.
In contrast with the short-term assays, v-src-transformed cells do become less adherent at longer times after plating. Unlike the earlier assays, the spinning disk assay can apply sufficient force to measure long-term adhesion (20). Data are presented showing that the v-src-transformed cells have a reduced adhesion by 6 h after plating. This is consistent with the accumulation of cellular products accounting for the difference. Here we define two factors that contribute to this reduced adhesion over time. First, v-src-transformed CEF have increased production of both metalloproteinase and plasminogen activator-type protease (2, 6, 56). These proteases remove the fibronectin ligand required for cell adhesion. A reduction in ligand causes a proportionate reduction in the number of integrin-ligand bonds and consequently a proportionate reduction in the strength of adhesion (19). The removal of fibronectin could be inhibited by chemical cross-linking of the fibronectin, and this partially reversed the transformed morphology, permitting the cells to remain spread. Second, v-src-transformed cells have altered glycosaminoglycans and secrete high levels of hyaluronic acid (30, 54). Chicken chondroblasts in culture also synthesize high levels of glycosaminoglycans which accumulate on the cell surface and block α5β1-mediated adhesion to fibronectin. Removal of these glycosaminoglycans with hyaluronidase restored α5β1-mediated adhesion (10, 43). In a similar manner, addition of hyaluronidase was necessary to retain the spread morphology of v-src-transformed cells on a cross-linked fibronectin matrix for longer than 2 days. Thus, increased, or altered, secretion of proteases and glycosaminoglycans presents a confounding factor in assigning the reduced adhesion of transformed cells to differences in integrin receptor function.
It has been reported that transformation of cells by v-src blocks their ability to assemble fibronectin into fibrils (49). We were able to restore this defect by cross-linking the substrate fibronectin before plating the cells. The assembly of fibronectin is thought to involve the physical stretching of the fibronectin molecules to provide an open conformation and reveal sites in the first type III repeat necessary for the intermolecular assembly (42, 44). In order to generate the force on the fibronectin by the pulling of integrin linked to the actin cytoskeleton, it is necessary to restrain one end of the fibronectin to provide the stress (66). Based on immunofluorescence localization, it has been suggested that this is the function of αvβ3 integrin retained at the tips of spreading cells (44). Our data offer a simpler explanation. Fibronectin adsorbed to the surface binds to the newly synthesized fibronectin and provides the necessary anchor. Removal of this fibronectin by proteases secreted selectively at these adhesion points by the v-src-transformed cells (5) would remove the anchor point, and thus the failure to assemble fibronectin fibrils would be due to the protease action and not to a failure of integrin function. Here we show that, after the removal of confounding factors, all of the direct assays demonstrate that α5β1 is functioning normally in the v-src-transformed cells.
Nevertheless, several laboratories have demonstrated an increase in the level of phosphorylation of β1 integrin in v-src-transformed cells (25, 49, 58). Mutational analysis of β1 integrin demonstrates that substitution of phenylalanines for tyrosines in the cytoplasmic domain tends to retain β1 integrins in focal adhesions and to retain β1 function. In contrast, mutation to glutamate to mimic the phosphorylated state tends to keep β1 out of focal adhesions (46, 50). Biochemical analysis of the distribution of phosphorylated β1 in v-src-transformed cells demonstrated that the increase in phosphorylated β1 occurred in cellular fractions which were not associated with cell adhesion (25). These data are consistent with a model in which the phosphorylation of β1 causes it to leave the focal adhesion or in which phosphorylation of β1 occurs outside the focal adhesion due to the large excess of v-src kinase in the cells. If this is the case, increased phosphorylation would have the effect of reducing the pool of β1 available to join focal adhesions. The increase in β1 integrin phosphorylation in the v-src-transformed cells would thus reduce the pool of β1 available for cell adhesion. However, for most cells in culture, β1 integrin is produced in excess, and there are multiple α-chain species which can form heterodimers with β1 integrin. Thus, reducing the pool of usable β1 integrin would have only a modest effect on cell adhesion.
A second, intracellular factor that can affect the strength of adhesion is the assembly of actin (19, 67). Even the v-src-transformed cells, which retained a spread morphology being plated on cross-linked fibronectin in the presence of hyaluronidase, showed very few actin stress fibers, although shorter actin filaments were detected at the periphery of the cell and in podosomes (59). While it is not known which specific actin structures contribute to strong integrin-mediated adhesion, one would expect that the reduction of actin filaments would contribute to a weaker adhesion. This reduced assembly of actin filaments would be expected to have a later and cumulative effect on integrin-mediated adhesion because they assemble later during cell spreading and in spread cells. Hence, this would also contribute the relative reduction in adhesion of the v-src-transformed cells with time.
Outside-in signaling by v-src-transformed cells.
The earliest demonstration of the involvement of integrin in cell signaling was the reversible block to myogenic differentiation by an antibody that blocked β1 integrin function (41). This effect of blocking β1 integrin paralleled the effect of expressing a temperature-sensitive v-src in these avian myogenic cells (14). Subsequent studies have revealed that many cellular signaling pathways are dependent on integrin-mediated adhesion (7, 37, 53). However, the critical link between the adhesion mediated by integrin and intracellular signals has remained elusive. We have used a signaling kinetic approach based on the original work of Ferrel and coworkers (13, 23). In this model, cells detect extracellular signals through the binding of specific ligands to surface receptors. The initial signaling responses in the cell are proportionate to the number of bound receptors. Within the individual cell these signals are converted to an all-or-none response based on an increasing Hill coefficient (sharper sigmoid dose-response curve) as the message is passed down the signaling pathway. This model was applied to the α5β1 integrin-mediated signals by varying the fibronectin density and thus the number of α5β1 integrins bound.
Attachment of fibroblasts to fibronectin or the aggregation of fibronectin receptors with antibodies resulted in the phosphorylation of FAK (24, 35). The initial phosphorylation appears to be an autophosphorylation of Y397 by FAK itself, which provides a binding site for the SH2 domain of src. This phosphorylation event was dependent on ligand binding, and the level of phosphorylation was proportional to the number of α5β1-fibronectin bonds formed (17). The proportion of FAK phosphorylated on Y397 provides a direct readout of integrin binding. In normal CEF, phosphorylated Y397 provides a binding site for c-src, which can then provide the next stage in signal transduction by phosphorylation of additional sites on FAK that bind downstream targets (8). In one system, the activation of v-src resulted in a reduction of phosphorylated Y397, although other sites on FAK remained phosphorylated (40). Since the function of phosphorylation of Y397 is to form complexes between FAK and src, underphosphorylation of Y397 can be compensated for either by the high level of v-src expression or by the ability to form complexes between FAK and v-src that are not dependent on Y397. v-src isolated from several strains of Rous sarcoma virus contains mutations in the RT loop in the SH3 domain, which provides an alternate mechanism for binding between the v-src and FAK that involves the v-src SH3 domain and the proline-rich domain in the C-terminal domain of FAK (27). Although these mutations in v-src may contribute to cell transformation, they are not essential since the single Y527F mutation in the C terminus of c-src is sufficient to activate transforming potential (47).
Several elements contribute to the adhesion-independent phosphorylation of FAK that in turn can provide constitutive signals to activate cell proliferation. The increased level of v-src over that of the normal c-src leads to an increase in v-src–FAK complex formation by increasing the background level of phosphorylation of Y397 and by driving the binding of v-src to FAK to stabilize the phosphorylated Y397. v-src could also bind to the proline-rich region of FAK to further increase complex formation. Complex formation leads to increased, src-mediated, phosphorylation of other tyrosines on FAK. The high level of v-src and the increased kinase activity lead to an inability to reset the system, and thus the cells become insensitive to integrin outside-in signaling.
The effect of adhesion signals on cellular behavior is complex. For example, the rate of cell migration shows a biphasic dependence on adhesion, with maximum rates of cell movement occurring at intermediate adhesion levels (9). Similarly, cell proliferation can be either stimulated or inhibited by integrin-mediated adhesion (21, 69). It has been challenging to separate out longer-term effects that depend on adhesion from those that depend on growth factors, in part because cells can produce autocrine growth factors and in part because the intracellular signaling pathways contain many common elements. One of the clearer results is the dependence of myogenic differentiation on the α5β1-fibronectin binding (21). For the case of v-src transformation of CEF, it is clear that the transformed cells are able to proliferate in suspension, whereas the normal CEF do not, even in the presence of serum. This illustrates a difference in adhesion dependence, but it is not very informative about the role of specific adhesion mechanisms and the reasons for the independence. The negative regulation of proliferation of CEF by increased or specific adhesion to fibronectin compared to polylysine provides an alternative model system. We have tried numerous approaches in addition to the use of cross-linked fibronectin to modify the fibronectin substrate to reduce the proliferation rate of the v-src-transformed cells without success. This provides an additional demonstration of the failure of the v-src-transformed cells to respond to fibronectin-integrin-mediated signals.
In summary, α5β1 integrin activation, adhesion function, and the ability to assemble a fibronectin matrix are not strongly affected by v-src-mediated cell transformation. A minor portion of β1 integrin does show increased phosphorylation and the phosphorylated β1 integrin may not participate in these functions due to its diffuse location and failure to concentrate in adhesion structures. In contrast, the v-src-transformed cells show complete insensitivity to integrin-mediated outside-in signals both at the level of FAK phosphorylation and in the specific adhesion-mediated events involved in myogenic differentiation and inhibition of cell proliferation in the absence of serum. These data demonstrate a functional and mechanistic separation of the inside-out and outside-in signaling pathways for β1 integrin.
REFERENCES
- 1.Ali I U, Maunter V, Lanza R, Hynes R O. Restoration of normal morphology, adhesion and cytoskeleton in transformed cells by addition of a transformation-sensitive surface protein. Cell. 1977;11:115–126. doi: 10.1016/0092-8674(77)90322-1. [DOI] [PubMed] [Google Scholar]
- 2.Bell S M, Brackenbury R W, Leslie N D, Degen J L. Plasminogen activator gene expression is induced by the src oncogene product and tumor promoters. J Biol Chem. 1990;265:1333–1338. [PubMed] [Google Scholar]
- 3.Boschek C B, Jockusch B M, Friis R R, Back R, Grundmann E, Bauer H. Early changes in the distribution and organization of microfilament proteins during cell transformation. Cell. 1981;24:175–184. doi: 10.1016/0092-8674(81)90513-4. [DOI] [PubMed] [Google Scholar]
- 4.Burridge K, Turner C E, Romer L H. Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. J Cell Biol. 1992;119:893–903. doi: 10.1083/jcb.119.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen W-T, Chen J-M, Parsons S J, Parsons J T. Local degradation of fibronectin at sites of expression of the transforming gene product pp60src. Nature. 1985;316:156–158. doi: 10.1038/316156a0. [DOI] [PubMed] [Google Scholar]
- 6.Chen W-T, Olden K, Bernard B A, Chu F-F. Expression of transformation-associated protease(s) that degrade fibronectin at cell contact sites. J Cell Biol. 1984;98:1546–1555. doi: 10.1083/jcb.98.4.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark E A, Brugge J S. Integrins and signal transduction pathways: the road taken. Science. 1995;268:5208. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- 8.Cobb B S, Schaller M D, Leu T H, Parsons J T. Stable association of pp60src and pp59fyn with the focal adhesion-associated protein tyrosine kinase, pp125FAK. Mol Cell Biol. 1994;14:147–155. doi: 10.1128/mcb.14.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DiMilla P A, Stone J A, Quinn J A, Albelda S M, Lauffenburger D A. Maximal migration of human smooth muscle cells on fibronectin and type IV collagen occurs at an intermediate attachment strength. J Cell Biol. 1993;122:729–737. doi: 10.1083/jcb.122.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Enomoto M, Leboy P S, Menko A S, Boettiger D. Beta 1 integrins mediate chondrocyte interaction with type I collagen, type II collagen, and fibronectin. Exp Cell Res. 1993;205:276–285. doi: 10.1006/excr.1993.1087. [DOI] [PubMed] [Google Scholar]
- 11.Enomoto-Iwamoto M, Menko A S, Philp N, Boettiger D. Evaluation of integrin molecules involved in substrate adhesion. Cell Adhes Commun. 1993;1:191–202. doi: 10.3109/15419069309097253. [DOI] [PubMed] [Google Scholar]
- 12.Faull R J, Kovach N L, Harlan J M, Ginsberg M H. Affinity modulation of integrin α5β1: regulation of the functional response by soluble fibronectin. J Cell Biol. 1993;121:155–162. doi: 10.1083/jcb.121.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrell J E., Jr How regulated protein translocation can produce switch-like responses. Trends Biochem Sci. 1998;23:461–465. doi: 10.1016/s0968-0004(98)01316-4. [DOI] [PubMed] [Google Scholar]
- 14.Fiszman M Y, Fuchs P. Temperature-sensitive expression of differentiation in transformed myoblasts. Nature. 1975;254:429–431. doi: 10.1038/254429a0. [DOI] [PubMed] [Google Scholar]
- 15.Foster R F, Thompson J M, Kaufman S J. A laminin substrate promotes myogenesis in rat skeletal muscle cultures: analysis of replication and development using antidesmin and anti-BrdUrd monoclonal antibodies. Dev Biol. 1987;122:11–20. doi: 10.1016/0012-1606(87)90327-7. [DOI] [PubMed] [Google Scholar]
- 16.Frelinger A L, Du X P, Plow E F, Ginsberg M H. Monoclonal antibodies to ligand-occupied conformers of integrin αIIbβ 3 (glycoprotein IIb-IIIa) alter receptor affinity, specificity, and function. J Biol Chem. 1991;266:17106–17111. [PubMed] [Google Scholar]
- 17.Garcia A J, Boettiger D. Integrin-fibronectin interactions at the cell-material interface: initial integrin binding and signaling. Biomaterials. 1999;20:2427–2433. doi: 10.1016/s0142-9612(99)00170-2. [DOI] [PubMed] [Google Scholar]
- 18.Garcia A J, Ducheyne P, Boettiger D. Quantification of cell adhesion using a spinning disc device and application to surface-reactive materials. Biomaterials. 1997;18:1091–1098. doi: 10.1016/s0142-9612(97)00042-2. [DOI] [PubMed] [Google Scholar]
- 19.Garcia A J, Huber F, Boettiger D. Force required to break α5β 1 integrin-fibronectin bonds in intact adherent cells is sensitive to integrin activation state. J Biol Chem. 1998;273:10988–10993. doi: 10.1074/jbc.273.18.10988. [DOI] [PubMed] [Google Scholar]
- 20.Garcia A J, Takagi J, Boettiger D. Two-stage activation for alpha5beta1 integrin binding to surface-adsorbed fibronectin. J Biol Chem. 1998;273:34710–34715. doi: 10.1074/jbc.273.52.34710. [DOI] [PubMed] [Google Scholar]
- 21.Garcia A J, Vega M D, Boettiger D. Modulation of cell proliferation and differentiation through substrate-dependent changes in fibronectin conformation. Mol Biol Cell. 1999;10:785–798. doi: 10.1091/mbc.10.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ginsberg M H, Du X, Plow E F. Inside-out integrin signalling. Curr Opin Cell Biol. 1992;4:766–771. doi: 10.1016/0955-0674(92)90099-x. [DOI] [PubMed] [Google Scholar]
- 23.Guadagno T M, Ferrell J E., Jr Requirement for MAPK activation for normal mitotic progression in Xenopus egg extracts. Science. 1998;282:1312–1315. doi: 10.1126/science.282.5392.1312. [DOI] [PubMed] [Google Scholar]
- 24.Guan J L, Trevithick J E, Hynes R O. Fibronectin/integrin interaction induces tyrosine phosphorylation of a 120-kDa protein. Cell Regul. 1991;2:951–964. doi: 10.1091/mbc.2.11.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haimovich B, Aneskievich B, Boettiger D. Cellular partitioning of β1 integrins and their phosphorylated forms is altered after transformation by Rous sarcoma virus or treatment with cytochalaisin D. Cell Regul. 1991;2:271–283. doi: 10.1091/mbc.2.4.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harte M T, Hildebrand J D, Burnham M R, Bouton A H, Parsons J T. p130Cas, a substrate associated with v-Src and v-Crk, localizes to focal adhesions and binds to focal adhesion kinase. J Biol Chem. 1996;271:13649–13655. doi: 10.1074/jbc.271.23.13649. [DOI] [PubMed] [Google Scholar]
- 27.Hauck C R, Hunter T, Schlaepfer D D. The v-src SH3 domain facilitates a cell adhesion-independent association with focal adhesion kinase. J Biol Chem. 2001;276:17653–17662. doi: 10.1074/jbc.M009329200. [DOI] [PubMed] [Google Scholar]
- 28.Hirst R, Horwitz A F, Buck C A, Rohrschneider L. Phosphorylation of the fibronectin receptor complex in cells transformed by oncogenes that encode tyrosine kinases. Proc Natl Acad Sci USA. 1986;83:6470–6474. doi: 10.1073/pnas.83.17.6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hynes R O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 30.Ishimoto N, Temin H M, Strominger J L. Studies of carcinogenesis by avian sarcoma viruses. II. Virus-induced increase in hyaluronic acid synthetase in chicken fibroblasts. J Biol Chem. 1966;241:2052–2057. [PubMed] [Google Scholar]
- 31.Kanner S B, Reynolds A B, Vines R R, Parsons J T. Monoclonal antibodies to individual tyrosine-phosphorylated protein substrates of oncogene-encoded tyrosine kinases. Proc Natl Acad Sci USA. 1990;87:3328–3332. doi: 10.1073/pnas.87.9.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaplan K B, Swedlow J R, Morgan D O, Varmus H E. c-Src enhances the spreading of src−/− fibroblasts on fibronectin by a kinase-independent mechanism. Genes Dev. 1995;9:1505–1517. doi: 10.1101/gad.9.12.1505. [DOI] [PubMed] [Google Scholar]
- 33.Klinghoffer R A, Sachsenmaier C, Cooper J A, Soriano P. Src family kinases are required for integrin but not PDGFR signal transduction. EMBO J. 1999;18:2459–2471. doi: 10.1093/emboj/18.9.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kornberg L, Earp H S, Parsons J T, Schaller M, Juliano R L. Cell adhesion or integrin clustering increases phosphorylation of a focal adhesion-associated tyrosine kinase. J Biol Chem. 1992;267:23439–23442. [PubMed] [Google Scholar]
- 35.Kornberg L J, Earp H S, Turner C E, Prockop C, Juliano R L. Signal transduction by integrins: increased protein tyrosine phosphorylation caused by clustering of β1 integrins. Proc Natl Acad Sci USA. 1991;88:8392–8396. doi: 10.1073/pnas.88.19.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kypta R M, Goldberg Y, Ulug E T, Courtneidge S A. Association between the PDGF receptor and members of the src family of tyrosine kinases. Cell. 1990;62:481–492. doi: 10.1016/0092-8674(90)90013-5. [DOI] [PubMed] [Google Scholar]
- 37.Lee R J, Albanese C, Stenger R J, Watanabe G, Inghirami G, Haines G K, Webster M, Muller W J, Brugge J S, Davis R J, Pestell R G. pp60v-src induction of cyclin D1 requires collaborative interactions between the extracellular signal-regulated kinase, p38, and Jun kinase pathways: a role for cAMP response element-binding protein and activating transcription factor-2 in pp60v-src signaling in breast cancer cells. J Biol Chem. 1999;274:7341–7350. doi: 10.1074/jbc.274.11.7341. [DOI] [PubMed] [Google Scholar]
- 38.Lipfert L, Haimovich B, Schaller M D, Cobb B S, Parsons J T, Brugge J S. Integrin-dependent phosphorylation and activation of the protein tyrosine kinase pp125FAK in platelets. J Cell Biol. 1992;119:905–912. doi: 10.1083/jcb.119.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Macpherson I, Montagnier L M. Agar suspension culture for selective assay of cells transformed by polyoma virus. Virology. 1964;23:291. doi: 10.1016/0042-6822(64)90301-0. [DOI] [PubMed] [Google Scholar]
- 40.McLean G W, Fincham V J, Frame M C. v-Src induces tyrosine phosphorylation of focal adhesion kinase independently of tyrosine 397 and formation of a complex with Src. J Biol Chem. 2000;275:23333–23339. doi: 10.1074/jbc.M909322199. [DOI] [PubMed] [Google Scholar]
- 41.Menko A S, Boettiger D. Occupation of the extracellular matrix receptor integrin is a control point for myogenic differentiation. Cell. 1987;51:51–57. doi: 10.1016/0092-8674(87)90009-2. [DOI] [PubMed] [Google Scholar]
- 42.Morla A, Ruoslahti E. A fibronectin self-assembly site involved in matrix assembly: reconstruction in a synthetic peptide. J Cell Biol. 1992;118:421. doi: 10.1083/jcb.118.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pacifici M, Soltesz R, Thal G, Shanley D J, Boettiger D, Holtzer H. Immunological characterization of the major chick cartilage proteoglycan and its intracellular localization in cultured chondroblasts: a comparison with Type II procollagen. J Cell Biol. 1983;97:1724–1736. doi: 10.1083/jcb.97.6.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pankov R, Cukierman E, Katz B Z, Matsumoto K, Lin D C, Lin S, Hahn C, Yamada K M. Integrin dynamics and matrix assembly: tensin-dependent translocation of α5β1 integrins promotes early fibronectin fibrillogenesis. J Cell Biol. 2000;148:1075–1090. doi: 10.1083/jcb.148.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Plantefaber L C, Hynes R O. Changes in integrin receptors on oncogenically transformed cells. Cell. 1989;56:281–290. doi: 10.1016/0092-8674(89)90902-1. [DOI] [PubMed] [Google Scholar]
- 46.Reszka A A, Hayashi Y, Horwitz A F. Identification of amino acid sequences in the integrin β1 cytoplasmic domain implicated in cytoskeletal association. J Cell Biol. 1992;117:1321–1330. doi: 10.1083/jcb.117.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reynolds A B, Vila J, Lansing T J, Potts W M, Weber M J, Parsons J T. Activation of the oncogenic potential of the avian cellular src protein by specific structural alteration of the carboxy terminus. EMBO J. 1987;6:2359–2364. doi: 10.1002/j.1460-2075.1987.tb02512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rohrschneider L. Adhesion plaques of Rous sarcoma virus-transformed cells contain the src gene product. Proc Natl Acad Sci USA. 1980;77:3514–3518. doi: 10.1073/pnas.77.6.3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sakai T, Jove R, Fassler R, Mosher D F. Role of the cytoplasmic tyrosines of beta 1A integrins in transformation by v-src. Proc Natl Acad Sci USA. 2001;98:3808–3813. doi: 10.1073/pnas.240456398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sakai T, Zhang Q, Fassler R, Mosher D F. Modulation of beta1A integrin functions by tyrosine residues in the beta1 cytoplasmic domain. J Cell Biol. 1998;141:527–538. doi: 10.1083/jcb.141.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schlaepfer D D, Hanks S K, Hunter T, van der Geer P. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature. 1994;372:786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- 52.Schoen R C, Bentley K L, Klebe R J. Monoclonal antibody against human fibronectin inhibits cell attachment. Hybridoma. 1982;1:99–108. doi: 10.1089/hyb.1.1982.1.99. [DOI] [PubMed] [Google Scholar]
- 53.Schwartz M A, Schaller M D, Ginsberg M H. Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- 54.Shanley D J, Cossu G, Boettiger D, Holtzer H, Pacifici M. Transformation by Rous sarcoma virus induces similar patterns of glycosaminoglycan synthesis in chick embryo skin fibroblasts and vertebral chondroblasts. J Biol Chem. 1983;258:810–816. [PubMed] [Google Scholar]
- 55.Sims P J, Ginsberg M H, Plow E F, Shattil S J. Effect of platelet activation on the conformation of the plasma membrane glycoprotein IIb-IIIa complex. J Biol Chem. 1991;266:7345–7352. [PubMed] [Google Scholar]
- 56.Sullivan L M, Quigley J P. An anticatalytic monoclonal antibody to avian plasminogen activator: its effect on behavior of RSV-transformed chick fibroblasts. Cell. 1986;45:905–915. doi: 10.1016/0092-8674(86)90565-9. [DOI] [PubMed] [Google Scholar]
- 57.Tamkun J W, DeSimone D W, Fonda D, Patel R S, Buck C A, Horwitz A F, Hynes R O. Structure of integrin, a glycoprotein involved in the transmembrane linkage between fibronectin and actin. Cell. 1986;46:271–282. doi: 10.1016/0092-8674(86)90744-0. [DOI] [PubMed] [Google Scholar]
- 58.Tapley P, Horwitz A, Buck C, Duggan K, Rohrschneider L. Integrins isolated from Rous sarcoma virus-transformed chicken embryo fibroblasts. Oncogene. 1989;4:325–333. [PubMed] [Google Scholar]
- 59.Tarone G, Cirillo D, Giancotti F G, Comoglio P M, Marchisio P C. Rous sarcoma virus transformed fibroblasts adhere primarily at discrete protrusions of the ventral membrane called podosomes. Exp Cell Res. 1985;159:141–157. doi: 10.1016/s0014-4827(85)80044-6. [DOI] [PubMed] [Google Scholar]
- 60.Temin H M, Rubin H. Characteristics of an assay for Rous sarcoma virus and Rous sarcoma cells in tissue culture. Virology. 1958;6:669–688. doi: 10.1016/0042-6822(58)90114-4. [DOI] [PubMed] [Google Scholar]
- 61.Wasilenko W J, Payne D M, Fitzgerald D L, Weber M J. Phosphorylation and activation of epidermal growth factor receptors in cells transformed by the src oncogene. Mol Cell Biol. 1991;11:309–321. doi: 10.1128/mcb.11.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weber M J. Inhibition of protease activity in cultures of Rous sarcoma virus transformed cells: effect on the transformed phenotype. Cell. 1975;5:253–261. doi: 10.1016/0092-8674(75)90100-2. [DOI] [PubMed] [Google Scholar]
- 63.Wennerberg K, Lohikangas L, Gullberg D, Pfaff M, Johansson S, Fassler R. Beta 1 integrin-dependent and -independent polymerization of fibronectin. J Cell Biol. 1996;132:227–238. doi: 10.1083/jcb.132.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu C, Keivens V M, O'Toole T E, MacDonald J A, Ginsberg M H. Integrin activation and cytoskeletal interaction are essential for the assembly of a fibronectin matrix. Cell. 1995;83:715–724. doi: 10.1016/0092-8674(95)90184-1. [DOI] [PubMed] [Google Scholar]
- 65.Yamada K M, Yamada S S, Pastan I. Cell surface protein partially restores morphology, adhesiveness and contact inhibition of movement to transformed fibroblasts. Proc Natl Acad Sci USA. 1976;73:1217–1221. doi: 10.1073/pnas.73.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhong C, Chrzanowska-Wodnicka M, Brown J, Shaub A, Belkin A M, Burridge K. Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. J Cell Biol. 1998;141:539–551. doi: 10.1083/jcb.141.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhong C, Kinch M S, Burridge K. Rho-stimulated contractility contributes to the fibroblastic phenotype of Ras-transformed epithelial cells. Mol Biol Cell. 1997;8:2329–2344. doi: 10.1091/mbc.8.11.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhu X, Assoian R K. Integrin-dependent activation of MAP kinase: a link to shape-dependent cell proliferation. Mol Biol Cell. 1995;6:273–282. doi: 10.1091/mbc.6.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhu X, Ohtsubo M, Bohmer R M, Roberts J M, Assoian R K. Adhesion-dependent cell cycle progression linked to the expression of cyclin D1: activation of cyclin E-cdk2, and phosphorylation of the retinoblastoma protein. J Cell Biol. 1996;133:391–403. doi: 10.1083/jcb.133.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]