Abstract
Nitric oxide plays a central role in the regulation of skeletal homeostasis. In cells of the osteoblastic lineage, NO is generated in response to mechanical stimulation and estrogen exposure. Via activation of soluble guanylyl cyclase (sGC) and cGMP-dependent protein kinases (PKGs), NO enhances proliferation, differentiation, and survival of bone-forming cells in the osteoblastic lineage. NO also regulates the differentiation and activity of bone-resorbing osteoclasts; here the effects are largely inhibitory and partly cGMP-independent. We review the skeletal phenotypes of mice deficient in NO synthases, sGC, or PKGs, and the effects of NO and cGMP on bone formation and resorption. We examine the roles of NO and cGMP in bone adaptation to mechanical stimulation. Finally, we discuss preclinical and clinical data showing that NO donors and NO-independent sGC activators may protect against estrogen deficiency-induced bone loss. sGC represents an attractive target for the treatment of osteoporosis.
Keywords: Nitric oxide, cGMP, protein kinase G, bone, osteoporosis, osteoblasts, osteoclasts
Introduction
The appendicular skeleton and vertebrae develop via a process called endochondral ossification, where mesenchymal cells differentiate into chondroblasts to form a cartilaginous “template” that is replaced by osteoblasts producing mineralizing matrix (1). After birth, endochondral ossification continues in the growth plates, where it governs longitudinal bone growth. Throughout the organismal lifespan, mineralized bone undergoes constant remodeling to maintain skeletal homeostasis and strength: osteoclasts initiate the remodeling cycle by resorbing mineralized matrix, while osteoblasts form new matrix (Fig. 1). An imbalance in bone remodeling –caused by excess bone resorption or decreased bone formation relative to resorption– results in a loss of bone mass and quality, leading to osteoporosis and an increased risk of bone fractures (2).
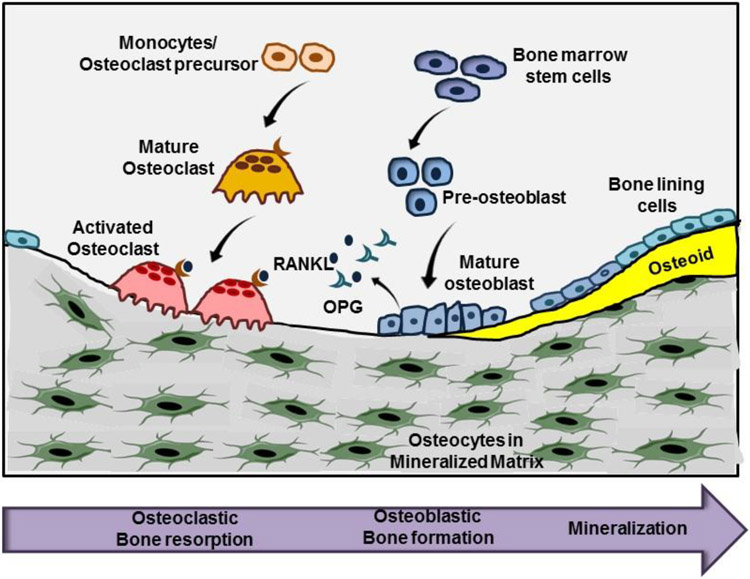
Fig. 1: Bone remodeling by osteoblasts and osteoclasts:
Osteoclasts differentiate from hematopoietic precursors under the influence of RANKL, which is secreted by osteoblasts together with its antagonist OPG. Osteoclasts resorb calcified bone matrix and recruit osteoblasts to fill the defect with new bone. Osteoblasts differentiate from mesenchymal stem cells and secrete extracellular matrix to form osteoid. Osteoid calcifies to form mature bone and surrounds mature osteocytes.
Bone-resorbing osteoclasts are multi-nucleated cells derived from hematopoietic precursors of the monocyte/macrophage lineage, and their differentiation and function is controlled by cytokines such as receptor of activated nuclear factor kappa-B ligand (RANKL)1 and its antagonist osteoprotegerin (OPG) (2). Bone-forming osteoblasts differentiate from bone marrow stromal cells in response to Wnts and other growth factors; they secrete extracellular matrix proteins to produce osteoid, which becomes calcified to form mature bone (2). During this process, some osteoblasts differentiate into osteocytes, which are completely embedded in mineralized matrix, but interconnected via cytoplasmic processes extending through micro-canaliculi. Osteocytes are long-lived and highly active cells, which sense mechanical stress and produce regulatory peptides, including sclerostin, a potent inhibitor of Wnt signaling (3). Bone remodeling is controlled by systemic hormones, such as estrogens and parathyroid hormone, by locally-produced factors, such as RANKL/OPG and Wnts/sclerostin, and by small signaling molecules such as NO and prostaglandins (2,3). Nitric oxide in bone has been the subject of several reviews in the past (4-7); here we concentrate on NO functions in bone that are mediated via activation of soluble guanylyl cyclase (sGC) and production of cGMP.
Expression of NO synthases and regulation of NO synthesis in bone cells
All three NO synthase (NOS) forms have been identified in bone and in isolated osteoblasts and osteoclasts by RT-PCR and immunohistochemistry (8-15). NOS-1 and -3 are constitutively expressed in primary osteoblasts from humans and rodents, with NOS activity stimulated by increases in intracellular calcium concentrations. Mechanical stimulation significantly increases NO production in osteoblasts and osteocyte-like cells, and this increase is blocked by calcium chelation, but the source(s) of mechanically-stimulated NO synthesis in osteoblastic cells remain controversial (11,16-19). Estrogens rapidly stimulate NOS-3 activation via a membrane-bound estrogen receptor; this requires an increase in intracellular calcium and Akt phosphorylation of NOS-3 in endothelial cells, with similar mechanisms operative in osteoblasts and osteocytes (20-24). In addition, estrogen treatment increases NOS-3 mRNA expression over 24 h, likely via nuclear estrogen receptor binding to the NOS-3 promoter (25). Thyroid hormone treatment of osteoblasts increases NO production via a membrane-bound thyroid receptor; thyroid hormone-induced NO synthesis is coupled to an increase in intracellular calcium concentrations and is abolished in NOS-3-deficient osteoblasts (26).
NOS-2 mRNA expression is regulated primarily at the transcriptional level, and is induced by inflammatory cytokines such as TNF-α, interferon-γ, and interleukin-1; NOS-2 expression in osteoblasts is also up-regulated by mechanical stimulation (9,10). NOS-2 expression in differentiating osteoclasts is induced by RANKL, and exerts a negative feedback to restrict differentiation (13) (described below).
The bone phenotype of NOS-deficient mice
NOS-1 knockout mice.
NOS-1-deficient mice have an osteosclerotic phenotype with high bone mass, at least partly due to decreased osteoclastic bone resorption (27). NOS-1-deficient mice demonstrate increased trabecular and cortical bone mineral density, and histomorphometry shows decreased osteoclast and osteoblast numbers, with reduced bone remodeling, reflected in low mineral apposition and bone formation rates (27). Reduced osteoclast number and activity in NOS-1-deficient mice was confirmed in an in vivo model of inflammation-induced bone resorption (28). Unexpectedly, more osteoclasts are formed from NOS-1-deficient compared to wild type bone marrow in the presence of RANKL and M-CSF in vitro, but the NOS-1-deficient osteoclasts are abnormally large and have reduced bone resorptive capacity (27,28) (Table 1). The high bone mass, low bone turnover phenotype of globally NOS-1-deficient mice may not be solely explained by defects of NOS-1 deficient bone cells, but indirect effects, e.g. hormonal changes due to NOS-1 deficiency in the nervous system, may contribute.
Table 1:
Bone Phenotypes of NO Synthase-deficient mice
| Global Knockout |
Bone Phenotype | Histomorphometry | In vitro Studies | References |
|---|---|---|---|---|
| NOS-1 −/− | Osteosclerosis, increased BMD | Increased BV/TV, trabecular and cortical thickness, OC number Decreased OB number, MAR & BFR |
Increased OC size & number, decreased resorption | 27,28 |
| NOS-2 −/− | No obvious bone phenotype at baseline, Decreased reloading-induced osteogenesis |
Normal BV/TV, BFR, OC number | OC: enhanced RANKL-induced differentiation | 13,29 |
| NOS-3 −/− | Reduced bone length & bone volumes, reduced BMD (only up to 8-10 weeks of age?) Decreased osteogenesis in response to fluid shear (venous ligation) More bone loss after OVX and blunted response to estrogens |
Reduced BV/TV, trabecular number, BFR & MAR, OB number (normal OC number) | Defects in chondrocyte and OB proliferation, defects in OB differentiation | 12,30-36 |
NOS-2 knockout mice.
NOS-2-deficient mice do not have obvious bone abnormalities, with normal femur lengths, trabecular bone volume fraction, bone formation rate, and osteoclast surface (29). However, NOS-2-deficient mice show altered responses to mechanical loading (discussed below, and Table 1).
NOS-3 knockout mice.
Different strains of NOS-3-deficient mice have been generated with some differences in bone phenotypes, perhaps due to different genetic backgrounds (30-35). NOS-3-deficient mice show defects in endochondral bone formation causing abnormal pre-natal and post-natal bone development, including fetal growth restriction, limb malformations, reduced longitudinal bone growth with hypocellular growth plates, and increased perinatal fatality (30-32). Young (6-9 week old) NOS-3−/− mice demonstrate marked retardation in post-natal bone formation, not only reduced longitudinal growth —a function of chondroblast growth and differentiation in growth plates— but also reduced bone volumes associated with defects in osteoblast maturation and activity (32,33). These NOS-3−/− mice show markedly reduced osteoblast numbers and profound defects in bone formation and mineral apposition rates, with decreased trabecular bone volume and cortical thickness compared to wild type mice (32,33). Osteoclasts appear to be unaffected by NOS-3 deficiency (32,33). Bone densitometry scanning and micro-CT analysis show reduced femoral and spinal bone mineral density in 8 week-old NOS-3-deficient compared to wild type mice, with some investigators reporting that these abnormalities persisted at 20 weeks (33), whereas others found that the differences diminished and normalized by 12-18 weeks (32,35,36). Altered responses of NOS-3-deficient mice to fluid shear stress or estrogen treatment after ovariectomy are discussed below (and Table 1).
In vitro, NOS-3-deficient osteoblasts proliferate slower and differentiate less well compared to wild type cells; both defects can be restored by the addition of exogenous NO (32,34). NOS-3-deficient osteoblasts form less mineralized nodules, show reduced alkaline phosphatase activity, and express lower amounts of runx-2 (a master transcription factor for osteoblast lineage cells) and osteocalcin (an extracellular matrix protein important for mineralization) (32,33,36).
Cyclic GMP synthesis in bone cells
NO-stimulated cGMP synthesis and/or sGC expression have been documented in (pre)osteoblasts and osteoclasts (19,37-40). Transcripts for the common, heme-containing β1 subunit of sGC are easily detectable in bone, but the distribution of sGC α1 and α2 subunits has not been determined.
Cyclic GMP is also generated by receptor guanylyl cyclases in bone, especially by the C-type natriuretic peptide (CNP)-activated GC-B receptor (41-43). GC-B is expressed in chondroblasts, osteoblasts, and osteocytes, and has important functions in endochondral ossification – with deficiencies in CNP or GC-B leading to dwarfism, whereas over-expression or activating mutations in the receptor lead to skeletal over-growth in humans and rodents (43-47). This system has been the subject of previous reviews and is not further discussed here (43,48).
Mice deficient in sGC and mice with NO-unresponsive sGC
Mice with a global deletion of the β1 subunit of sGC, or “Apo-sGC” mice with a H105F point mutation in the β1 subunit —resulting in a heme-free, NO-unresponsive enzyme—are viable but have a reduced lifespan (49,50). These mice display gastrointestinal abnormalities and growth retardation, which is not entirely explained by malnutrition or malabsorption (49,50). However, the skeletal phenotype of these mice has not been examined in detail.
Cyclic GMP targets in bone: PKG1 and 2 and Phosphodiesterases
The main cGMP effector proteins in bone are cGMP-dependent protein kinases (PKG1 and PKG2). The largely cytoplasmic PKG1 has two isoforms (PKG1α and 1β) which are generated by differential splicing of the first exon and differ only in the N-terminal ~100 amino acids; this unique N-terminal domain mediates dimerization and docking to specific target proteins (51). (Pre)osteoblasts and osteocytes express both PKG1 isoforms as well as PKG2, with all three enzymes displaying different functions (19,24,52). For example, the pro-proliferative effects of cGMP in osteoblasts require PKG2 activation of Src and Erk-1/2, whereas the anti-apoptotic effects of cGMP in osteocytes are mediated by PKG1α and PKG2 via distinct mechanisms discussed below (Fig. 3) (19,24,52). Osteoclasts express primarily PKG1α and 1β (53,54).
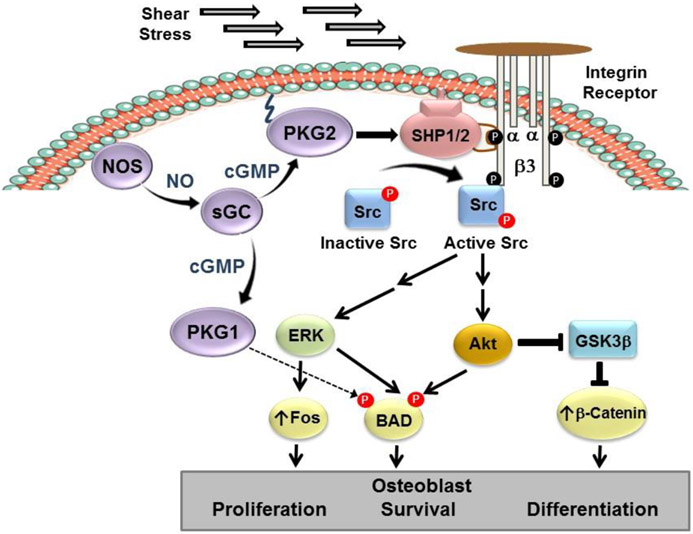
Fig. 3: NO/cGMP signaling in fluid shear-stressed osteoblasts.
Shear stress activates NOS via increased calcium. Membrane-bound PKG2 activates integrin-bound Src to stimulate Erk and Akt, which regulate osteoblast proliferation, survival, and differentiation via BAD phosphorylation and increased fos- and β-catenin-controlled genes. PKG1α directly phosphorylates and inactivates BAD to promote survival.
Phosphodiesterases, which degrade cAMP and/or cGMP and can be allosterically regulated by cGMP binding, have not been studied extensively in bone cells (51). Osteoblasts contain multiple phosphodiesterases, but the cGMP-regulated PDE-5 was not detected (55).
PKG1- or PKG2-deficient mice
Mice globally deficient in PKG1 have a short life span due to severe intestinal dysfunction, and their growth retardation is primarily due to malnutrition (56). PKG1 knockout mice with smooth muscle-specific expression of either PKG1α or 1β have a much improved life expectancy, but their skeletal phenotype was not reported (57).
Mice globally deficient in PKG2 are dwarfs due to severe defects in endochondral ossification (58). PKG2 deficiency also causes dwarfism in rats, cattle, and humans, indicating a central function of PKG2 in longitudinal bone growth across multiple species (59-61). The skeletal phenotype of PKG2 knockout mice mimics that of CNP- or GC-B-deficient mice, and PKG2 acts downstream of CNP in chondroblasts (58, 62). Cartilage-specific CNP transgene expression rescues the dwarfism phenotype of CNP-deficient mice, but has no effect on the skeletal phenotype of PKG2−/− mice (62). In addition to the CNP/GC-B pathway, the NOS-3/sGC pathway likely contributes to PKG2 activation in growth plate chondroblasts, since NOS-3-deficient mice show at least transient growth retardation in long bones (described above) (30-32). To examine the functions of PKG1 and PKG2 in adult skeletal homeostasis, we are presently characterizing osteoblast-specific PKG1- and PKG2-knockout mice.
In vitro effects of NO and cGMP in cells of osteoblastic lineage
Nitric oxide has biphasic effects on (pre)osteoblasts and osteocytes in vitro. Low NO concentrations promote proliferation, differentiation, and survival, whereas high NO concentrations have opposite effects (37,38,63). The positive effects of low NO concentrations on osteoblast proliferation are mediated by sGC and PKG2, because they are mimicked by cell-permeable cGMP analogs and prevented by pharmacological inhibition of sGC or PKG, or siRNA-mediated knock-down of PKG2 (37,52,64). The anti-apoptotic effects of low NO concentrations in bone marrow stromal cells and osteocytes are also mediated by cGMP, and require both PKG1 and PKG2; they are reduced by siRNA-mediated knock-down of PKG2 or PKG1α (24,37,65). PKG2 exerts its anti-apoptotic and pro-proliferative functions in osteoblasts via activation of Src, Erk-1/2 and Akt; the latter two kinases directly phosphorylate and inactivate the pro-apoptotic protein BAD (24). Akt also phosphorylates and inactivates glycogen synthase kinase-3β, which leads to stabilization and nuclear translocation of β-catenin and activation of Wnt-pathway genes (Fig. 3, below) (37,66). Wnt signaling plays an essential role in (pre)osteoblast differentiation, proliferation, and survival and drives bone formation in vivo (2).
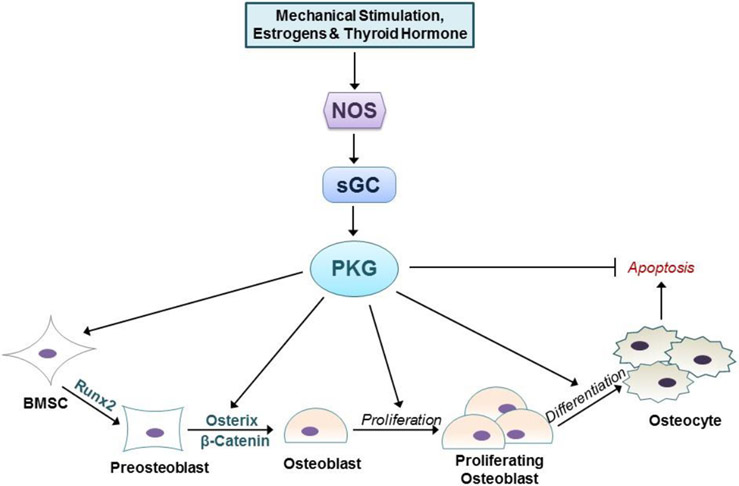
Low doses of NO donors enhance osteogenic differentiation of bone marrow stromal cells and (pre)osteoblasts in vitro, by stimulating mRNA expression of osteoblastic genes, e.g. alkaline phosphatase, osteocalcin, and collagen-1, and increasing bone matrix synthesis and mineralization (38,63,67,68). Again, these NO effects are blocked by sGC inhibitors and mimicked by cell-permeable cGMP analogs or NO-independent stimulators of sGC, suggesting that NO works via cGMP (42,63,64,69). The osteoblast differentiation-promoting effects of NO/cGMP likely involve stimulation of Wnt/β-catenin pathway activity (see above), and increased expression and activity of the osteoblast master transcription factor Runx2, and of Fos-related proteins which cooperate with Runx2 (19,36,68,70,71). Thus, NO activation of sGC exerts positive effects on cells of the osteoblastic lineage at all stages of differentiation, from immature bone marrow stromal cells (BMSCs) to mature osteocytes (Fig. 2).
Fig. 2: NO/cGMP regulation of osteoblastic cells.
NO is synthesized in response to mechanical stimulation, or treatment with estrogen and thyroid hormones. NO-stimulated cGMP synthesis results in PKG activation. PKG regulates all stages of osteoblastic cells, promoting proliferation and differentiation, and inhibiting apoptosis.
In vitro effects of NO and cGMP on osteoclasts
Similar to its actions in the osteoblastic lineage, low concentrations of NO may promote, while higher concentrations inhibit osteoclast differentiation and survival (27,37,72). In vivo, NOS-1 appears to be required for normal osteoclast differentiation and/or survival, since NOS-1-deficient mice have decreased osteoclast numbers, and in vitro, NOS-1-deficient bone marrow monocytes produced poorly-functioning osteoclasts, as described above (Table 1) (27,28). The effects of pharmacological NOS inhibition on osteoclast differentiation in vitro are varied and perhaps depend on the time of addition and the NOS isoform targeted (13,14,73-75). RANKL induces NOS-2 expression in osteoclast precursors, and the high NO concentrations produced by NOS-2 inhibit osteoclastic differentiation in a cGMP-independent fashion (13). Correspondingly, osteoclast precursors isolated from NOS-2-deficient mice show enhanced RANKL-induced differentiation and bone pit formation in vitro (Table 1) (13). NOS-2 induction by interleukin-1 and interferon-γ also suppresses osteoclast formation, activity and survival (14,74-76).
NO donors, NO-independent sGC activators (YC-1), and cell-permeable cGMP analogs inhibit osteoclast differentiation and bone pit formation in vitro (37,40,77). YC-1 induces osteoclast apoptosis via caspases-3 and -8 activation (77). NO induces detachment of mature osteoclasts from bone and down-regulates their acid secretion, thereby inhibiting bone resorption (40,78-80). These effects appear to be mediated by endogenous NO production and require cGMP and PKG, since NOS or PKG inhibition increase attachment and acid secretion (40,79,80). NO stimulates osteoclast motility and detachment via PKG1 phosphorylation of the vasodilator-stimulated protein (VASP) and the inositol-1,4,5-triphosphate receptor-associated protein (IRAG), events that lead to cytoskeletal rearrangement and calcium activation of the proteinase μ-calpain, respectively (53,54,81). Thus, the effects of NO and cGMP on osteoclast differentiation and function in vitro are largely inhibitory.
In vivo effects of NO and cGMP on bone formation and resorption
The low bone volume/low bone formation phenotype of NOS-3-deficient mice suggests that NOS-3-derived NO is important for osteoblast differentiation and function in vivo, as described above (Table 1) (32,33). Administration of non-isoform-specific NOS inhibitors, such as L-NAME or aminoguanidine, to adult rats causes a significant decrease in bone formation and bone mineral density, again suggesting that basal NO production is required for skeletal homeostasis (82-84).
Consistent with a positive role of NO in bone formation, administration of the NO donor nitrosyl-cobinamide (NO-Cbi) to adult mice increases mineral apposition and bone formation rates, as well as trabecular bone volume fraction and bone mineral density (37). NO-Cbi-treated mice demonstrate increased osteoblast numbers and osteocalcin mRNA expression, while osteoclast numbers and osteoclast-specific gene expression (cathepsin-K and tartrate-resistant acid phosphatase) are decreased. The negative effect of NO-Cbi on osteoclasts may be partly due to reduced RANKL production in mice treated with the NO donor (37).
Administration of sGC-stimulating agents to young rats –at high doses that cause profound hypotension– increases bone formation and resorption with increased numbers of osteoblasts and osteoclasts, leading to a striking increase in bone turnover within a short period of time (7 d), which is slowly reversible (after 35 d) (85). In contrast, administration of the heme-independent sGC activator cinaciguat to adult mice –at a low dose that did not significantly lower systolic blood pressure– increases osteoblast number, mineral apposition and bone formation rates, without significantly affecting osteoclast numbers (69). Thus, NO –via activation of sGC– increases osteoblastic bone formation in rodents, but the effect on osteoclasts may require higher doses and depend on the age of the animals.
NO/cGMP functions in bone adaptation to mechanical stimulation
Mechanical stimulation is a primary determinant of bone growth and remodeling, with fluid flow through the bone canalicular system generating shear stress that stimulates osteoblasts and osteocytes and enhances their anabolic activity (3,6,86-89). Bone marrow stromal cells, osteoblasts, and osteocyte-like cells all respond to fluid shear stress in vitro with increasing proliferation and survival (6,88). These anabolic responses require increased NO production, which occurs acutely from calcium-mediated NOS-3 activation, and longer-term from increased NOS-2 and NOS-3 mRNA expression (6,10,19,52,90). We found that the NO/cGMP/PKG2 signaling pathway activates Src in mechanically stimulated osteoblasts to initiate Erk-1/2 activation and a proliferative response (Fig. 3) (52). Fluid shear stress triggers the recruitment of PKG2, Src, and SHP-1 into an integrin β3-containing membrane complex (a “mechanosome”, which translates mechanical stimuli into biochemical responses), and PKG2 directly phosphorylates and stimulates the phosphatase SHP-1 to de-phosphorylate Src on an inhibitory site (52). The anti-apoptotic effects of fluid shear stress in osteoblasts and osteocytes require Akt activation, which occurs through two parallel pathways: one via calcium activating NOS and NO/cGMP/PKG2 activating Src, the other via calcium-dependent activation of focal adhesion kinase and Src, independent of NO (66,90).
Unloading of bone causes rapid bone loss due to reduced bone formation and increased resorption (88). In contrast, (re)loading of bone stimulates osteogenesis via increased Wnt signaling (due to suppression of sclerostin) and inhibits bone resorption via suppression of RANKL signaling (due to upregulation of the RANKL antagonist OPG) (3,88). (Re)loading of bone increases NOS-2, NOS-3, and c-fos mRNA expression in osteoblasts and osteocytes, and NOS inhibitors block mechanically-induced bone formation and c-fos mRNA induction (10,29,82,83,91-95).
In a murine hind limb suspension model, bone loss occurs within 7-14 d and is reversed by reloading; bone loss in this model can be prevented by increasing interstitial fluid flow via venous ligation (35). Mice deficient in NOS-2 or NOS-3 experience the same bone loss in unloaded limbs as wild type mice, but NOS-3-deficient animals are not protected by venous ligation, and NOS-2-deficient animals fail to increase bone formation after reloading (29,35). While a role of cGMP/PKG signaling downstream of NO in osteoblast/osteocyte mechano-transduction has been established in vitro (52,66), the role of cGMP/PKG in vivo awaits experiments in osteoblast-specific PKG2 knockout mice.
NO/cGMP effects in estrogen-deficient rodents
Ovariectomy-induced estrogen deficiency in rodents causes marked bone loss with associated changes in bone architecture and turnover that recapitulate those observed in post-menopausal women, including increased bone resorption, an inadequate bone formation response, and enhanced osteocyte apoptosis (96). Estrogens normally limit osteoclast survival by transcriptionally upregulating Fas ligand via nuclear estrogen receptor (independent of NO) (97,98). In contrast, the beneficial effects of estrogens on cells of the osteoblastic lineage are largely mediated via a membrane-bound estrogen receptor-α, which couples to NOS-3 (20-22,99,100). Estrogens promote the survival of osteoblasts and osteocytes via NO/cGMP-dependent phosphorylation and inactivation of BAD (directly, by PKG1, and indirectly, by PKG2 stimulation of Erk and Akt, as described above) (23,24). NO also mediates estrogen stimulation of (pre)osteoblast proliferation and differentiation (23,32).
Data in rodents are consistent with a requirement for NOS-3 activation downstream of the membrane-bound estrogen receptor for at least some of the bone-protective effects of estrogens: (i) bone formation induced by high doses of estrogens in vivo, in intact mice or in ovariectomized rats, is blocked by administration of the NOS inhibitor L-NAME (101,102); (ii) NOS-3 knockout mice loose more bone after ovariectomy than wild type mice, and their response to exogenous estrogens is blunted (12,33); and (iii) NO-generating agents can at least partly substitute for estrogens’ bone protective effects in vivo (37,102,103). In fact, treating ovariectomized rats with NO-generating nitrates (e.g., nitroglycerin) preserves bone mineral density and improves mechanical bone properties to a similar degree as estrogen replacement (102,103). On the other hand, in ovariectomized mice with a point mutation which abolishes membrane localization of the estrogen receptor the estrogen’s bone-protective effects are partly abolished, suggesting an important role of the NOS-3-coupled estrogen membrane receptor in bone (99,100).
While organic nitrates are the only NO donors currently FDA-approved for long-term use in humans, their clinical benefits are limited by development of tolerance and induction of oxidative stress (104-107). Nitrates require enzymatic activation to release NO, and during the activation, reactive oxygen species are generated (106,108). Novel NO donors circumvent this problem; for example, nitrosyl-cobinamide (NO-Cbi) releases NO directly, without biotransformation or generation of reactive oxygen species (109). NO-Cbi increases trabecular bone mass in both intact and ovariectomized mice, at a dose that does not significantly affect systolic blood pressure (37). NO-Cbi increases intracellular cGMP concentrations, Wnt/β-catenin signaling, proliferation, and osteoblastic gene expression in murine primary osteoblasts, and protects cells from apoptosis (37). Correspondingly, in intact and ovariectomized mice, NO-Cbi increases serum cGMP concentrations, bone formation, and osteoblastic gene expression (37). In ovariectomized mice, NO-Cbi prevents estrogen deficiency-induced osteocyte apoptosis (37). NO-Cbi also reduces osteoclast numbers in intact mice and prevents the ovariectomy-induced increase in osteoclasts, in part due to a reduction in the RANKL/OPG gene expression ratio, and in part due to direct inhibition of osteoclast differentiation, observed in vitro in the presence of excess RANKL (37). Although the positive NO effects in osteoblasts are mediated by cGMP/PKG, the osteoclast-inhibitory effects are partly cGMP-independent (37).
Ovariectomized mice have lower serum cGMP concentrations compared to sham-operated animals, suggesting that estrogen deficiency causes a state of systemic NO/cGMP deficiency due to defective NOS-3 activation in endothelial cells (20,21,37,69). NO’s bone-protective effects appear to be largely mediated by cGMP, because treating ovariectomized mice with the NO-independent sGC activator cinaciguat improves trabecular bone microarchitecture and enhances osteocyte survival, with effect sizes similar to those obtained with estrogen replacement therapy (69). However, compared to 17β-estradiol or NO-Cbi, which completely prevent the ovariectomy-induced increase in osteoclast number, cinaciguat has a lesser effect on osteoclasts (37,69). On the other hand, the NO-independent sGC activator YC-1 effectively prevents the increase in bone resorption and improves bone architecture in ovariectomized rats (77).
Thus, NO donors and sGC activators show anabolic effects in pre-clinical models of estrogen-deficiency osteoporosis.
NO effects on bone in human studies
Similar to rodents, estrogens also appear to induce NO production in humans. Plasma concentrations of stable nitric oxide derivatives (nitrites and nitrates) correlate positively with estrogen concentrations in humans, and are decreased in post-menopausal women and increased with estrogen supplementation (110-112). Several epidemiological studies have reported a bone-protective effect of NO-generating organic nitrates (nitroglycerin and isosorbide mononitrate), which are clinically used for treatment of heart failure and coronary insufficiency (113-115). Two case control studies compared nitrate use in individuals who sustained a fracture to that in age- and sex-matched controls without a fracture, and found a 10-15% reduction in fracture risk in nitrate users after adjusting for confounding factors (113,114). The greatest benefit appeared to be among patients using low doses of short-acting nitrates on an intermittent basis (113,115).
A positive effect of nitrates on bone, at doses lower than those used for vasodilation, has also been demonstrated in several independent, prospective, randomized clinical trials: (i) nitroglycerin was as effective as estrogen replacement in preventing bone loss in young women after ovariectomy (116); (ii) in post-menopausal women with established osteoporosis, subjects randomized to isosorbide mononitrate treatment showed similar improvement in bone mineral density as subjects who received a bisphosphonate (a proven anti-resorptive therapy for osteoporosis) (117); and (iii) in healthy post-menopausal women, isosorbide mononitrate treatment for 12 weeks increased a bone formation marker (bone-specific alkaline phosphatase) and decreased a bone resorption marker (N-telopeptide) compared to placebo (118). However, a three-year trial in early post-menopausal women failed to show an effect of once-daily nitroglycerin ointment on bone mineral density, albeit with poor treatment adherence (119). A second trial testing transdermal nitroglycerin versus placebo in post-menopausal women was completed by a different group, but the report was retracted.
Thus, while there is a substantial amount of data in vitro and in vivo indicating bone-anabolic effects of NO via activation of sGC and PKG, more work is required to test the clinical usefulness of this approach. Organic nitrates are clearly suboptimal NO donors because of tolerance development and oxidative stress (104,106). Novel classes of NO donors are under clinical development (37,107). However, estrogen-deficiency and aging cause excess oxidative stress, which may affect NO bioavailability and render sGC insensitive to NO (107,120). Therefore, cinaciguat and newer, NO-independent sGC activators may be more ideally suited to restore NO/cGMP signaling in post-menopausal and aging bone, and may represent a new paradigm for the treatment of osteoporosis (69,121).
Acknowledgments:
This work was supported by NIH grants R21-AR065658 and R01-AR068601 to RBP.
Footnotes
Conflicts of Interest: None of the authors have any conflicts of interest to declare.
The following abbreviations were used: BFR, bone formation rate; BMD, bone mineral density; BMSC, bone marrow stromal cell; BV/TV, bone volume fraction; CNP, C-type natriuretic peptide, GC-B, guanylyl cyclase-B; GSK, glycogen synthase kinase; MAR, mineral apposition rate; NO, nitric oxide; NOS, NO synthase; OB, osteoblast; OC, osteoclast; OPG, osteoprotegerin; OVX, ovariectomy; PKG, cGMP-dependent protein kinase; RANKL, receptor of activated nuclear factor-KB ligand; sGC, soluble guanylyl cyclase.
Reference List
- 1.Kobayashi T, and Kronenberg HM 2014. Overview of skeletal development. Methods Mol. Biol 1130:3–12. [DOI] [PubMed] [Google Scholar]
- 2.Kawai M, Modder UI, Khosla S, and Rosen CJ 2011. Emerging therapeutic opportunities for skeletal restoration. Nat. Rev. Drug Discov 10:141–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dallas SL, Prideaux M, and Bonewald LF 2013. The osteocyte: an endocrine cell … and more. Endocr. Rev 34:658–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalyanaraman H, Ramdani G, and Pilz RB 2016. Targeting NO signaling for the treatment of osteoporosis. Current Medicinal Chemistry 23:1–8. [Google Scholar]
- 5.Wimalawansa SJ 2010. Nitric oxide and bone. Ann. N. Y. Acad. Sci 1192:391–403. [DOI] [PubMed] [Google Scholar]
- 6.Klein-Nulend J, van Oers RF, Bakker AD, and Bacabac RG 2014. Nitric oxide signaling in mechanical adaptation of bone. Osteoporos. Int 25:1427–1437. [DOI] [PubMed] [Google Scholar]
- 7.van't Hof RJ, and Ralston SH 2001. Nitric oxide and bone. Immunology 103:255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hukkanen MV, Platts LA, Fernandez DM, I, O'Shaughnessy M, MacIntyre I, and Polak JM 1999. Developmental regulation of nitric oxide synthase expression in rat skeletal bone. J. Bone Miner. Res 14:868–877. [DOI] [PubMed] [Google Scholar]
- 9.Helfrich MH, Evans DE, Grabowski PS, Pollock JS, Ohshima H, and Ralston SH 1997. Expression of nitric oxide synthase isoforms in bone and bone cell cultures. J. Bone Miner. Res 12:1108–1115. [DOI] [PubMed] [Google Scholar]
- 10.Basso N, and Heersche JN 2006. Effects of hind limb unloading and reloading on nitric oxide synthase expression and apoptosis of osteocytes and chondrocytes. Bone 39:807–814. [DOI] [PubMed] [Google Scholar]
- 11.Bakker AD, Huesa C, Hughes A, Aspden RM, van't Hof RJ, Klein-Nulend J, and Helfrich MH 2013. Endothelial nitric oxide synthase is not essential for nitric oxide production by osteoblasts subjected to fluid shear stress in vitro. Calcif. Tissue Int 92:228–239. [DOI] [PubMed] [Google Scholar]
- 12.Grassi F, Fan X, Rahnert J, Weitzmann MN, Pacifici R, Nanes MS, and Rubin J. 2006. Bone re/modeling is more dynamic in the endothelial nitric oxide synthase(−/−) mouse. Endocrinology 147:4392–4399. [DOI] [PubMed] [Google Scholar]
- 13.Zheng H, Yu X, Collin-Osdoby P, and Osdoby P 2006. RANKL stimulates inducible nitric-oxide synthase expression and nitric oxide production in developing osteoclasts. An autocrine negative feedback mechanism triggered by RANKL-induced interferon-beta via NF-kappaB that restrains osteoclastogenesis and bone resorption. J. Biol. Chem 281:15809–15820. [DOI] [PubMed] [Google Scholar]
- 14.Brandi ML, Hukkanen M, Umeda T, Moradi-Bidhendi N, Bianchi S, Gross SS, Polak JM, and MacIntyre I 1995. Bidirectional regulation of osteoclast function by nitric oxide synthase isoforms. Proc. Natl. Acad. Sci. U. S. A 92:2954–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacPherson H, Noble BS, and Ralston SH 1999. Expression and functional role of nitric oxide synthase isoforms in human osteoblast-like cells. Bone 24:179–185. [DOI] [PubMed] [Google Scholar]
- 16.McAllister TN, and Frangos JA 1999. Steady and transient fluid shear stress stimulate NO release in osteoblasts through distinct biochemical pathways. J Bone Miner. Res 14:930–936. [DOI] [PubMed] [Google Scholar]
- 17.Vatsa A, Smit TH, and Klein-Nulend J. 2007. Extracellular NO signalling from a mechanically stimulated osteocyte. J. Biomech 40 Suppl 1:S89–S95. [DOI] [PubMed] [Google Scholar]
- 18.Klein-Nulend J, Helfrich MH, Sterck JG, MacPherson H, Joldersma M, Ralston SH, Semeins CM, and Burger EH 1998. Nitric oxide response to shear stress by human bone cell cultures is endothelial nitric oxide synthase dependent. Biochem. Biophys. Res. Commun 250:108–114. [DOI] [PubMed] [Google Scholar]
- 19.Rangaswami H, Marathe N, Zhuang S, Chen Y, Yeh JC, Frangos JA, Boss GR, and Pilz RB 2009. Type II cGMP-dependent protein kinase mediates osteoblast mechanotransduction. J. Biol. Chem 284:14796–14808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Russell KS, Haynes MP, Sinha D, Clerisme E, and Bender JR 2000. Human vascular endothelial cells contain membrane binding sites for estradiol, which mediate rapid intracellular signaling. Proc. Natl. Acad. Sci. U. S. A 97:5930–5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chambliss KL, Yuhanna IS, Mineo C, Liu P, German Z, Sherman TS, Mendelsohn ME, Anderson RG, and Shaul PW 2000. Estrogen receptor alpha and endothelial nitric oxide synthase are organized into a functional signaling module in caveolae. Circ. Res 87:E44–E52. [DOI] [PubMed] [Google Scholar]
- 22.Mendelsohn ME, and Karas RH 2010. Rapid progress for non-nuclear estrogen receptor signaling. J. Clin. Invest 120:2277–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Shaughnessy MC, Polak JM, Afzal F, Hukkanen MV, Huang P, MacIntyre I, and Buttery LD 2000. Nitric oxide mediates 17beta-estradiol-stimulated human and rodent osteoblast proliferation and differentiation. Biochem. Biophys. Res. Commun 277:604–610. [DOI] [PubMed] [Google Scholar]
- 24.Marathe N, Rangaswami H, Zhuang S, Boss GR, and Pilz RB 2012. Pro-survival Effects of 17beta-Estradiol on Osteocytes Are Mediated by Nitric Oxide/cGMP via Differential Actions of cGMP-dependent Protein Kinases I and II. J. Biol. Chem 287:978–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Armour KE, and Ralston SH 1998. Estrogen upregulates endothelial constitutive nitric oxide synthase expression in human osteoblast-like cells. Endocrinology 139:799–802. [DOI] [PubMed] [Google Scholar]
- 26.Kalyanaraman H, Schwappacher R, Joshua J, Zhuang S, Scott BT, Klos M, Casteel DE, Frangos JA, Dillmann W, Boss GR et al. 2014. Nongenomic thyroid hormone signaling occurs through a plasma membrane-localized receptor. Sci. Signal 7:ra48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van't Hof RJ, Macphee J, Libouban H, Helfrich MH, and Ralston SH 2004. Regulation of bone mass and bone turnover by neuronal nitric oxide synthase. Endocrinology 145:5068–5074. [DOI] [PubMed] [Google Scholar]
- 28.Jung JY, Lin AC, Ramos LM, Faddis BT, and Chole RA 2003. Nitric oxide synthase I mediates osteoclast activity in vitro and in vivo. J. Cell Biochem 89:613–621. [DOI] [PubMed] [Google Scholar]
- 29.Watanuki M, Sakai A, Sakata T, Tsurukami H, Miwa M, Uchida Y, Watanabe K, Ikeda K, and Nakamura T 2002. Role of inducible nitric oxide synthase in skeletal adaptation to acute increases in mechanical loading. J. Bone Miner. Res 17:1015–1025. [DOI] [PubMed] [Google Scholar]
- 30.Yan Q, Feng Q, and Beier F 2010. Endothelial nitric oxide synthase deficiency in mice results in reduced chondrocyte proliferation and endochondral bone growth. Arthritis Rheum. 62:2013–2022. [DOI] [PubMed] [Google Scholar]
- 31.Hefler LA, Reyes CA, O'Brien WE, and Gregg AR 2001. Perinatal development of endothelial nitric oxide synthase-deficient mice. Biol. Reprod 64:666–673. [DOI] [PubMed] [Google Scholar]
- 32.Aguirre J, Buttery L, O'Shaughnessy M, Afzal F, de Marticorena IF, Hukkanen M, Huang P, Maclntyre I, and Polak J 2001. Endothelial nitric oxide synthase gene-deficient mice demonstrate marked retardation in postnatal bone formation, reduced bone volume, and defects in osteoblast maturation and activity. Am. J. Pathol 158:247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Armour KE, Armour KJ, Gallagher ME, Godecke A, Helfrich MH, Reid DM, and Ralston SH 2001. Defective bone formation and anabolic response to exogenous estrogen in mice with targeted disruption of endothelial nitric oxide synthase. Endocrinology 142:760–766. [DOI] [PubMed] [Google Scholar]
- 34.Lagumdzija A, Ou G, Petersson M, Bucht E, Gonon A, and Pernow Y 2004. Inhibited anabolic effect of insulin-like growth factor-1 on stromal bone marrow cells in endothelial nitric oxide synthase-knockout mice. Acta Physiol. Scand 182: 29–35. [DOI] [PubMed] [Google Scholar]
- 35.Bergula AP, Haidekker MA, Huang W, Stevens HY, and Frangos JA 2004. Venous ligation-mediated bone adaptation is NOS III-dependent. Bone 34:562–569. [DOI] [PubMed] [Google Scholar]
- 36.Afzal F, Polak J, and Buttery L 2004. Endothelial nitric oxide synthase in the control of osteoblastic mineralizing activity and bone integrity. J. Pathol 202:503–510. [DOI] [PubMed] [Google Scholar]
- 37.Kalyanaraman H, Ramdani G, Joshua J, Schall N, Boss GR, Cory E, Sah RL, Casteel DE, and Pilz RB 2017. A Novel, Direct NO Donor Regulates Osteoblast and Osteoclast Functions and Increases Bone Mass in Ovariectomized Mice. J. Bone Miner. Res 32:46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Otsuka E, Hirano K, Matsushita S, Inoue A, Hirose S, Yamaguchi A, and Hagiwara H 1998. Effects of nitric oxide from exogenous nitric oxide donors on osteoblastic metabolism. Eur. J. Pharmacol 349:345–350. [DOI] [PubMed] [Google Scholar]
- 39.Korkmaz Y, Baumann MA, Schroder H, Behrends S, Addicks K, Raab WH, and Bloch W 2004. Localization of the NO-cGMP signaling pathway molecules, NOS III-phosphorylation sites, ERK1/2, and Akt/PKB in osteoclasts. J. Periodontol 75:1119–1125. [DOI] [PubMed] [Google Scholar]
- 40.Dong SS, Williams JP, Jordan SE, Cornwell T, and Blair HC 1999. Nitric oxide regulation of cGMP production in osteoclasts. J. Cell Biochem 73:478–487. [PubMed] [Google Scholar]
- 41.Yasoda A, Ogawa Y, Suda M, Tamura N, Mori K, Sakuma Y, Chusho H, Shiota K, Tanaka K, and Nakao K 1998. Natriurenic peptide regulation of endochondral ossification. J. Biol. Chem 273:11695–11700. [DOI] [PubMed] [Google Scholar]
- 42.Hagiwara H, Inoue A, Yamaguchi A, Yokose S, Furuya M, Tanaka S, and Hirose S 1996. cGMP produced in response to ANP and CNP regulates proliferation and differentiation of osteoblastic cells. Am. J. Physiol 270:C1311–C1318. [DOI] [PubMed] [Google Scholar]
- 43.Kuhn M 2016. Molecular Physiology of Membrane Guanylyl Cyclase Receptors. Physiol Rev. 96:751–804. [DOI] [PubMed] [Google Scholar]
- 44.Chusho H, Tamura N, Ogawa Y, Yasoda A, Suda M, Miyazawa T, Nakamura K, Nakao K, Kurihara T, Komatsu Y et al. 2001. Dwarfism and early death in mice lacking C-type natriuretic peptide. Proc. Natl. Acad. Sci. USA 98:4016–4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yasoda A, Komatsu Y, Chusho H, Miyazawa T, Ozasa A, Miura M, Kurihara T, Rogi T, Tanaka S, Suda M et al. 2004. Overexpression of CNP in chondrocytes rescues achondroplasia through a MAPK-dependent pathway. Nat. Med 10:80–86. [DOI] [PubMed] [Google Scholar]
- 46.Miura K, Namba N, Fujiwara M, Ohata Y, Ishida H, Kitaoka T, Kubota T, Hirai H, Higuchi C, Tsumaki N et al. 2012. An overgrowth disorder associated with excessive production of cGMP due to a gain-of-function mutation of the natriuretic peptide receptor 2 gene. PLoS. ONE 7:e42180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bocciardi R, Giorda R, Buttgereit J, Gimelli S, Divizia MT, Beri S, Garofalo S, Tavella S, Lerone M, Zuffardi O et al. 2007. Overexpression of the C-type natriuretic peptide (CNP) is associated with overgrowth and bone anomalies in an individual with balanced t(2;7) translocation. Hum. Mutat 28:724–731. [DOI] [PubMed] [Google Scholar]
- 48.Teixeira CC, Agoston H, and Beier F 2008. Nitric oxide, C-type natriuretic peptide and cGMP as regulators of endochondral ossification. Dev. Biol 319:171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Friebe A, Mergia E, Dangel O, Lange A, and Koesling D 2007. Fatal gastrointestinal obstruction and hypertension in mice lacking nitric oxide-sensitive guanylyl cyclase. Proc. Natl. Acad. Sci U. S. A 104:7699–7704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thoonen R, Cauwels A, Decaluwe K, Geschka S, Tainsh RE, Delanghe J, Hochepied T, De CL, Rogge E, Voet S et al. 2015. Cardiovascular and pharmacological implications of haem-deficient NO-unresponsive soluble guanylate cyclase knock-in mice. Nat. Commun 6:8482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Francis SH, Busch JL, Corbin JD, and Sibley D 2010. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol. Rev 62:525–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rangaswami H, Schwappacher R, Marathe N, Zhuang S, Casteel DE, Haas B, Chen Y, Pfeifer A, Kato H, Shattil S et al. 2010. Cyclic GMP and protein kinase G control a Src-containing mechanosome in osteoblasts. Sci. Signal 3:ra91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yaroslavskiy BB, Zhang Y, Kalla SE, Garcia P,V, Sharrow AC, Li Y, Zaidi M, Wu C, and Blair HC 2005. NO-dependent osteoclast motility: reliance on cGMP-dependent protein kinase I and VASP. J. Cell Sci 118:5479–5487. [DOI] [PubMed] [Google Scholar]
- 54.Yaroslavskiy BB, Turkova I, Wang Y, Robinson LJ, and Blair HC 2010. Functional osteoclast attachment requires inositol-1,4,5-trisphosphate receptor-associated cGMP-dependent kinase substrate. Lab Invest 90:1533–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wakabayashi S, Tsutsumimoto T, Kawasaki S, Kinoshita T, Horiuchi H, and Takaoka K 2002. Involvement of phosphodiesterase isozymes in osteoblastic differentiation. J. Bone Miner. Res 17:249–256. [DOI] [PubMed] [Google Scholar]
- 56.Pfeifer A, Klatt P, Massberg S, Ny L, Sausbier M, Hirneib C, Wang G-X, Korth M, Aszòdi A, Andersson K-E et al. 1998. Defective smooth muscle regulation in cGMP kinase I-deficient mice. EMBO J. 17:3045–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weber S, Bernhard D, Lukowski R, Weinmeister P, Worner R, Wegener JW, Valtcheva N, Feil S, Schlossmann J, Hofmann F et al. 2007. Rescue of cGMP Kinase I Knockout Mice by Smooth Muscle Specific Expression of Either Isozyme. Circ. Res [DOI] [PubMed] [Google Scholar]
- 58.Pfeifer A, Aszòdi A, Seidler U, Ruth P, Hofmann F, and Fässler R 1996. Intestinal secretory defects and dwarfism in mice lacking cGMP-dependent protein kinase II. Science 274:2082–2086. [DOI] [PubMed] [Google Scholar]
- 59.Chikuda H, Kugimiya F, Hoshi K, Ikeda T, Ogasawara T, Kamekura S, Ogata N, Nakamura K, Chung UI, and Kawaguchi H 2005. Mutation in cGMP-dependent protein kinase II causes dwarfism in a rat mutant KMI through uncoupling of proliferation and differentiation of chondrocytes. J. Bone Miner. Metab 23:200–204. [DOI] [PubMed] [Google Scholar]
- 60.Koltes JE, Mishra BP, Kumar D, Kataria RS, Totir LR, Fernando RL, Cobbold R, Steffen D, Coppieters W, Georges M et al. 2009. A nonsense mutation in cGMP-dependent type II protein kinase (PRKG2) causes dwarfism in American Angus cattle. Proc. Natl. Acad. Sci. U. S. A 106:19250–19255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bonnet C, Andrieux J, Beri-Dexheimer M, Leheup B, Boute O, Manouvrier S, Delobel B, Copin H, Receveur A, Mathieu M et al. 2010. Microdeletion at chromosome 4q21 defines a new emerging syndrome with marked growth restriction, mental retardation and absent or severely delayed speech. J. Med. Genet 47:377–384. [DOI] [PubMed] [Google Scholar]
- 62.Miyazawa T, Ogawa Y, Chusho H, Yasoda A, Tamura N, Komatsu Y, Pfeifer A, Hofmann F, and Nakao K 2002. Cyclic GMP-dependent protein kinase II plays a critical role in C-type natriuretic peptide-mediated endochondral ossification. Endocrinology 143:3604–3610. [DOI] [PubMed] [Google Scholar]
- 63.Mancini L, Moradi-Bidhendi N, Becherini L, Martineti V, and MacIntyre I 2000. The biphasic effects of nitric oxide in primary rat osteoblasts are cGMP dependent. Biochem. Biophys. Res. Commun 274:477–481. [DOI] [PubMed] [Google Scholar]
- 64.Inoue A, Hiruma Y, Hirose S, Yamaguchi A, and Hagiwara H 1995. Reciprocal regulation by cyclic nucleotides of the differentiation of rat osteoblast-like cells and mineralization of nodules. Biochem. Biophys. Res. Commun 215:1104–1110. [DOI] [PubMed] [Google Scholar]
- 65.Wong JC, and Fiscus RR 2011. Essential roles of the nitric oxide (no)/cGMP/protein kinase G type-Ialpha (PKG-Ialpha) signaling pathway and the atrial natriuretic peptide (ANP)/cGMP/PKG-Ialpha autocrine loop in promoting proliferation and cell survival of OP9 bone marrow stromal cells. J. Cell Biochem 112:829–839. [DOI] [PubMed] [Google Scholar]
- 66.Rangaswami H, Schwappacher R, Tran T, Chan GC, Zhuang S, Boss GR, and Pilz RB 2012. Protein Kinase G and Focal Adhesion Kinase Converge on Src/Akt/beta-Catenin Signaling Module in Osteoblast Mechanotransduction. J. Biol. Chem 287:21509–21519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hikiji H, Shin WS, Oida S, Takato T, Koizumi T, and Toyo-oka T 1997. Direct action of nitric oxide on osteoblastic differentiation. FEBS Lett. 410:238–242. [DOI] [PubMed] [Google Scholar]
- 68.Lin IC, Smartt JM Jr., Nah HD, Ischiropoulos H, and Kirschner RE 2008. Nitric oxide stimulates proliferation and differentiation of fetal calvarial osteoblasts and dural cells. Plast. Reconstr. Surg 121:1554–1566. [DOI] [PubMed] [Google Scholar]
- 69.Joshua J, Schwaerzer GK, Kalyanaraman H, Cory E, Sah RS, Li M, Vaida F, Boss GR, and Pilz RB 2014. Soluble guanylate cyclase as a novel treatment target for osteoporosis. Endocrinology 155:4720–4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zaragoza C, Lopez-Rivera E, Garcia-Rama C, Saura M, Martinez-Ruiz A, Lizarbe TR, Martin-de-Lara F, and Lamas S 2006. Cbfa-1 mediates nitric oxide regulation of MMP-13 in osteoblasts. J. Cell Sci 119:1896–1902. [DOI] [PubMed] [Google Scholar]
- 71.D'Alonzo RC, Selvamurugan N, Karsenty G, and Partridge NC 2002. Physical interaction of the activator protein-1 factors c-Fos and c-Jun with Cbfa1 for collagenase-3 promoter activation. J. Biol. Chem 277:816–822. [DOI] [PubMed] [Google Scholar]
- 72.Lee SK, Huang H, Lee SW, Kim KH, Kim KK, Kim HM, Lee ZH, and Kim HH 2004. Involvement of iNOS-dependent NO production in the stimulation of osteoclast survival by TNF-a. Exp. Cell Res 298:359–368. [DOI] [PubMed] [Google Scholar]
- 73.Wimalawansa SM, Shankar VS, Simmins DJ, and Wimalawansa SJ 2000. The mechanism of bone resorption by cyclosporin: involvement of the NO-cGMP pathway. J. Musculoskelet. Neuronal. Interact 1:141–143. [PubMed] [Google Scholar]
- 74.van't Hof RJ, and Ralston SH 1997. Cytokine-induced nitric oxide inhibits bone resorption by inducing apoptosis of osteoclast progenitors and suppressing osteoclast activity. J. Bone Miner. Res 12:1797–1804. [DOI] [PubMed] [Google Scholar]
- 75.Lowik CW, Nibbering PH, van de RM, and Papapoulos SE 1994. Inducible production of nitric oxide in osteoblast-like cells and in fetal mouse bone explants is associated with suppression of osteoclastic bone resorption. J. Clin. Invest 93:1465–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ralston SH, Ho LP, Helfrich MH, Grabowski PS, Johnston PW, and Benjamin N 1995. Nitric oxide: a cytokine-induced regulator of bone resorption. J. Bone Miner. Res 10:1040–1049. [DOI] [PubMed] [Google Scholar]
- 77.Wang JW, Yeh CB, Chou SJ, Lu KC, Chu TH, Chen WY, Chien JL, Yen MH, Chen TH, and Shyu JF 2018. YC-1 alleviates bone loss in ovariectomized rats by inhibiting bone resorption and inducing extrinsic apoptosis in osteoclasts. J. Bone Miner. Metab, in press. [DOI] [PubMed] [Google Scholar]
- 78.Mancini L, Moradi-Bidhendi N, Brandi ML, and MacIntyre I 1998. Nitric oxide and peroxynitrite modulate osteoclast activity. Biochem. Biophys. Res. Com 243: 785–790. [DOI] [PubMed] [Google Scholar]
- 79.Yaroslavskiy BB, Li Y, Ferguson DJ, Kalla SE, Oakley JI, and Blair HC 2004. Autocrine and paracrine nitric oxide regulate attachment of human osteoclasts. J. Cell Biochem 91:962–972. [DOI] [PubMed] [Google Scholar]
- 80.Fuller K, Kirstein B, and Chambers TJ 2006. Murine osteoclast formation and function: differential regulation by humoral agents. Endocrinology 147:1979–1985. [DOI] [PubMed] [Google Scholar]
- 81.Yaroslavskiy BB, Sharrow AC, Wells A, Robinson LJ, and Blair HC 2007. Necessity of inositol (1,4,5)-trisphosphate receptor 1 and mu-calpain in NO-induced osteoclast motility. J. Cell Sci 120:2884–2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tsukahara H, Miura M, Tsuchida S, Hata I, Hata K, Yamamoto K, Ishii Y, Muramatsu I, and Sudo M 1996. Effect of nitric oxide synthase inhibitors on bone metabolism in growing rats. Am. J. Physiol 270:E840–E845. [DOI] [PubMed] [Google Scholar]
- 83.Turner CH, Owan I, Jacob DS, McClintock R, and Peacock M 1997. Effects of nitric oxide synthase inhibitors on bone formation in rats. Bone 21:487–490. [DOI] [PubMed] [Google Scholar]
- 84.Musumeci G, Loreto C, Clementi G, Fiore CE, and Martinez G 2011. An in vivo experimental study on osteopenia in diabetic rats. Acta Histochem. 113:619–625. [DOI] [PubMed] [Google Scholar]
- 85.Homer BL, Morton D, Bagi CM, Warneke JA, Andresen CJ, Whiteley LO, Morris DL, and Tones MA 2015. Oral administration of soluble guanylate cyclase agonists to rats results in osteoclastic bone resorption and remodeling with new bone formation in the appendicular and axial skeleton. Toxicol. Pathol 43:411–423. [DOI] [PubMed] [Google Scholar]
- 86.Papachroni KK, Karatzas DN, Papavassiliou KA, Basdra EK, and Papavassiliou AG 2009. Mechanotransduction in osteoblast regulation and bone disease. Trends Mol. Med 15:208–216. [DOI] [PubMed] [Google Scholar]
- 87.Riddle RC, and Donahue HJ 2009. From streaming-potentials to shear stress: 25 years of bone cell mechanotransduction. J. Orthop. Res 27:143–149. [DOI] [PubMed] [Google Scholar]
- 88.Ozcivici E, Luu YK, Adler B, Qin YX, Rubin J, Judex S, and Rubin CT 2010. Mechanical signals as anabolic agents in bone. Nat. Rev. Rheumatol 6:50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Santos A, Bakker AD, and Klein-Nulend J, 2009. The role of osteocytes in bone mechanotransduction. Osteoporos. Int 20:1027–1031. [DOI] [PubMed] [Google Scholar]
- 90.Tan SD, Bakker AD, Semeins CM, Kuijpers-Jagtman AM, and Klein-Nulend J 2008. Inhibition of osteocyte apoptosis by fluid flow is mediated by nitric oxide. Biochem. Biophys. Res. Commun 369:1150–1154. [DOI] [PubMed] [Google Scholar]
- 91.Inoue D, Kido S, and Matsumoto T 2004. Transcriptional induction of FosB/DFosB gene by mechanical stress in osteoblasts. J. Biol. Chem 279:49795–49803. [DOI] [PubMed] [Google Scholar]
- 92.Chow JW, Fox SW, Lean JM, and Chambers TJ 1998. Role of nitric oxide and prostaglandins in mechanically induced bone formation. J. Bone Miner. Res 13:1039–1044. [DOI] [PubMed] [Google Scholar]
- 93.Fox SW, Chambers TJ, and Chow JW 1996. Nitric oxide is an early mediator of the increase in bone formation by mechanical stimulation. Am. J. Physiol 270:E955–E960. [DOI] [PubMed] [Google Scholar]
- 94.Lean JM, Mackay AG, Chow JW, and Chambers TJ 1996. Osteocytic expression of mRNA for c-fos and IGF-I: an immediate early gene response to an osteogenic stimulus. Am. J Physiol 270:E937–E945. [DOI] [PubMed] [Google Scholar]
- 95.Turner CH, Takano Y, Owan I, and Murrell GA 1996. Nitric oxide inhibitor L-NAME suppresses mechanically induced bone formation in rats. Am. J. Physiol 270:E634–E639. [DOI] [PubMed] [Google Scholar]
- 96.Khosla S, Melton LJ III, and Riggs BL 2011. The unitary model for estrogen deficiency and the pathogenesis of osteoporosis: is a revision needed? J. Bone Miner. Res 26:441–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nakamura T, Imai Y, Matsumoto T, Sato S, Takeuchi K, Igarashi K, Harada Y, Azuma Y, Krust A, Yamamoto Y et al. 2007. Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell 130:811–823. [DOI] [PubMed] [Google Scholar]
- 98.Krum SA, Miranda-Carboni GA, Hauschka PV, Carroll JS, Lane TF, Freedman LP, and Brown M 2008. Estrogen protects bone by inducing Fas ligand in osteoblasts to regulate osteoclast survival. EMBO J. 27:535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gustafsson KL, Farman H, Henning P, Lionikaite V, Moverare-Skrtic S, Wu J, Ryberg H, Koskela A, Gustafsson JA, Tuukkanen J et al. 2016. The role of membrane ERa signaling in bone and other major estrogen responsive tissues. Sci Rep 6:29473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vinel A, Hay E, Valera MC, Buscato M, Adlanmerini M, Guillaume M, Cohen-Solal M, Ohlsson C, Lenfant F, Arnal JF et al. 2016. Role of ERa in the Effect of Estradiol on Cancellous and Cortical Femoral Bone in Growing Female Mice. Endocrinology 157:2533–2544. [DOI] [PubMed] [Google Scholar]
- 101.Samuels A, Perry MJ, Gibson RL, Colley S, and Tobias JH 2001. Role of endothelial nitric oxide synthase in estrogen-induced osteogenesis. Bone 29:24–29. [DOI] [PubMed] [Google Scholar]
- 102.Wimalawansa SJ, De MG, Gangula P, and Yallampalli C 1996. Nitric oxide donor alleviates ovariectomy-induced bone loss. Bone 18:301–304. [DOI] [PubMed] [Google Scholar]
- 103.Hukkanen M, Platts LA, Lawes T, Girgis SI, Konttinen YT, Goodship AE, MacIntyre I, and Polak JM 2003. Effect of nitric oxide donor nitroglycerin on bone mineral density in a rat model of estrogen deficiency-induced osteopenia. Bone 32:142–149. [DOI] [PubMed] [Google Scholar]
- 104.Thomas GR, DiFabio JM, Gori T, and Parker JD 2007. Once daily therapy with isosorbide-5-mononitrate causes endothelial dysfunction in humans: evidence of a free-radical-mediated mechanism. J. Am. Coll. Cardiol 49:1289–1295. [DOI] [PubMed] [Google Scholar]
- 105.Munzel T, Wenzel P, and Daiber A 2007. Do we still need organic nitrates? J. Am. Coll. Cardiol 49:1296–1298. [DOI] [PubMed] [Google Scholar]
- 106.Parker JD 2004. Nitrate tolerance, oxidative stress, and mitochondrial function: another worrisome chapter on the effects of organic nitrates. J. Clin. Invest 113:352–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lundberg JO, Gladwin MT, and Weitzberg E 2015. Strategies to increase nitric oxide signalling in cardiovascular disease. Nat. Rev. Drug Discov 14:623–641. [DOI] [PubMed] [Google Scholar]
- 108.Sydow K, Daiber A, Oelze M, Chen Z, August M, Wendt M, Ullrich V, Mulsch A, Schulz E, Keaney JF Jr. et al. 2004. Central role of mitochondrial aldehyde dehydrogenase and reactive oxygen species in nitroglycerin tolerance and cross-tolerance. J. Clin. Invest 113:482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Broderick KE, Alvarez L, Balasubramanian M, Belke DD, Makino A, Chan A, Woods VL Jr., Dillmann WH, Sharma VS, Pilz RB et al. 2007. Nitrosyl-cobinamide, a new and direct nitric oxide-releasing drug effective in vivo. Exp. Biol. Med. (Maywood.) 232:1432–1440. [DOI] [PubMed] [Google Scholar]
- 110.Hayashi T, Ito I, Kano H, Endo H, and Iguchi A 2000. Estriol (E3) replacement improves endothelial function and bone mineral density in very elderly women. J. Gerontol. A Biol. Sci. Med. Sci 55:B183–B190. [DOI] [PubMed] [Google Scholar]
- 111.Stacey E, Korkia P, Hukkanen MV, Polak JM, and Rutherford OM 1998. Decreased nitric oxide levels and bone turnover in amenorrheic athletes with spinal osteopenia. J. Clin. Endocrinol. Metab 83:3056–3061. [DOI] [PubMed] [Google Scholar]
- 112.Rosselli M, Imthurn B, Keller PJ, Jackson EK, and Dubey RK 1995. Circulating nitric oxide (nitrite/nitrate) levels in postmenopausal women substituted with 17 beta-estradiol and norethisterone acetate. A two-year follow-up study. Hypertension 25:848–853. [DOI] [PubMed] [Google Scholar]
- 113.Rejnmark L, Vestergaard P, and Mosekilde L 2006. Decreased fracture risk in users of organic nitrates: a nationwide case-control study. J. Bone Miner. Res 21:1811–1817. [DOI] [PubMed] [Google Scholar]
- 114.Pouwels S, Lalmohamed A, van ST, Cooper C, Souverein P, Leufkens HG, Rejnmark L, de BA, Vestergaard P, and de VF 2010. Use of organic nitrates and the risk of hip fracture: a population-based case-control study. J. Clin. Endocrinol. Metab 95:1924–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jamal SA, Browner WS, Bauer DC, and Cummings SR 1998. Intermittent use of nitrates increases bone mineral density: the study of osteoporotic fractures. J. Bone Miner. Res 13:1755–1759. [DOI] [PubMed] [Google Scholar]
- 116.Wimalawansa SJ 2000. Nitroglycerin therapy is as efficacious as standard estrogen replacement therapy (Premarin) in prevention of oophorectomy-induced bone loss: a human pilot clinical study. J. Bone Miner. Res 15:2240–2244. [DOI] [PubMed] [Google Scholar]
- 117.Nabhan AF, and Rabie NH 2008. Isosorbide mononitrate versus alendronate for postmenopausal osteoporosis. Int. J. Gynaecol. Obstet 103:213–216. [DOI] [PubMed] [Google Scholar]
- 118.Jamal SA, Cummings SR, and Hawker GA 2004. Isosorbide mononitrate increases bone formation and decreases bone resorption in postmenopausal women: a randomized trial. J. Bone Miner. Res 19:1512–1517. [DOI] [PubMed] [Google Scholar]
- 119.Wimalawansa SJ, Grimes JP, Wilson AC, and Hoover DR 2009. Transdermal nitroglycerin therapy may not prevent early postmenopausal bone loss. J. Clin. Endocrinol. Metab 94:3356–3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Manolagas SC 2010. From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr. Rev 31:266–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schmidt HH, Schmidt PM, and Stasch JP 2009. NO- and haem-independent soluble guanylate cyclase activators. Handb. Exp. Pharmacol 309–339. [DOI] [PubMed] [Google Scholar]