Abstract
Background and Objectives
Epilepsy and depression share a bidirectional relationship; however, its magnitude and long-term temporal association remain to be elucidated. This study investigates the magnitude and long-term association between epilepsy and depression, comparing with the risks of the 2 disorders after another chronic medical illness (asthma).
Methods
In a nationwide register-based matched cohort study, we identified all individuals who received a first diagnosis of epilepsy, depression, and asthma from January 1, 1980, to December 31, 2016. We used a Cox regression model to estimate the risk of epilepsy after depression and vice versa and the risk of epilepsy or depression after asthma, compared with healthy references matched on age and sex, adjusting for medical comorbidity, substance abuse, and calendar time. Results were stratified by epilepsy subtype. We furthermore investigated the risk of admission with acute seizures for persons with epilepsy who became depressed.
Results
In a population of 8,741,955 individuals, we identified 139,014 persons with epilepsy (54% males, median age at diagnosis 43 years [inter quartile range (IQR) 17–65 years]), 219,990 persons with depression (37% males, median age at diagnosis 43 years [IQR 29–60 years]), and 358,821 persons with asthma (49% males, median age at diagnosis 29 years [IQR 6–56 years]). The adjusted hazard ratio (aHR) of depression after epilepsy was 1.88 (95% CI 1.82–1.95), and the aHR of epilepsy after depression was 2.35 (95% CI 2.25–2.44). The aHR of depression after asthma was 1.63 (95% CI 1.59–1.67) and that of epilepsy after asthma, 1.48 (95% CI 1.44–1.53). The risk of depression was highest in the few years preceding and after an epilepsy diagnosis, and vice versa, but remained elevated during the entire follow-up period for both directions of the association. There was no evidence of a stronger association with depression for any epilepsy subtype. Receiving a diagnosis of depression subsequent to an epilepsy diagnosis was associated with a 1.20-fold (95% CI 1.07–1.36) increased HR of acute hospital admission with seizures.
Discussion
We identified a long-term bidirectional relationship between depression and epilepsy in a large-scale cohort study. Risk estimates were higher than those of epilepsy or depression after asthma.
Depression is the most frequent psychiatric comorbidity in people with epilepsy1 with prevalence of active depression ranging from 13% to 37%,2-4 an estimate 2–5 times higher than in the background population.5 The clinical and therapeutic implications of this comorbidity are significant; for example, comorbid mood disorders are among the strongest predictors of poor quality of life,6,7 suicidal ideation, and premature death8-10 in people with epilepsy and has been associated with higher frequency and severity of seizures and poor response and adherence to antiseizure medication.11-14
Historically, the view has been widely held that epilepsy and depression had a cause-consequence relationship; that is, depressive symptoms were adaptive phenomena reactive to the seizure disorder. However, over the past 2 decades, epidemiologic studies have established that not only are persons with epilepsy at a greater risk of experiencing depression, but patients with primary depression also have a 1.45- to 7-fold higher risk of developing epilepsy.13,15-18 Theories of common pathogenic mechanisms operant in both conditions have been suggested and are supported by shared features in the principal theories of epilepsy and depression pathogenesis, including imbalances of common neurotransmitters19; structural and functional disturbances of common brain regions, such as the hippocampus and amygdala20; and endocrine and inflammatory disturbances.21
Yet, studies on the co-occurrence of epilepsy and depression have shown substantial disparity, possibly owing to heterogeneity in study designs and methods (e.g., differences in study populations and case definitions). Few large-scale, population-based studies have been conducted on this matter, and most have compared risks to healthy individuals rather than to individuals with other chronic diseases.4,13,15,16 The latter is relevant to address the facts that experiencing a chronic disabling disease may increase the risk of depression per se and inherently increase the frequency of medical contacts and thereby the likelihood of obtaining a second diagnosis. Thus, among references, there may be more cases of unidentified depression because these persons are less often screened by a doctor—and the association may be overestimated when merely comparing the risk of, for example, depression between persons with epilepsy and persons without epilepsy. This form of selection bias is known as the Berkson bias.22
Although the association between epilepsy and depression has been compellingly established and is currently the subject of massive attention of researchers, its magnitude, consequences at a population level, and temporal aspects need further elucidation. We therefore used data from nationwide registers to study the relationship between depression and epilepsy and addressed the potential impact from the Berkson bias by comparing the risks in patients with another chronic medical illness, asthma. Furthermore, we assessed whether specific epilepsy subtypes are particularly associated with depression, whether severity of depression is associated with risk of epilepsy, and whether comorbid depression is associated with risk of treatment failure in patients with epilepsy.
Methods
Study Design and Data Sources
Data were obtained by linking the following Danish nationwide registers23: the Danish National Patient Registry (DNPR)24 with information on all patients treated at somatic hospitals (i.e., all hospitals that primarily take care of patient with nonpsychiatric illnesses) in Denmark since 1977 (outpatient and emergency room contacts since 1995), the Danish Central Psychiatric Register (DCPR)25 with information on all psychiatric admissions in Denmark since 1970 (outpatient and emergency room contacts since 1995), the Danish Civil Registration System,26 containing information on vital and emigration status on all individuals living in Denmark since 1969, and the Income Statistics Register,27 containing information on the income composition of the entire Danish population since 1970. From 1977 to 1993, diagnostic information in the DNPR and the DCPR was based on the Danish version of the International Classification of Diseases, eighth revision (ICD-8) and from 1994 on the International Classification of Diseases, 10th revision (ICD-10).24,25 This study was designed as a nationwide register-based matched cohort study.
Study Population
We identified all persons who were alive and resident in Denmark at some point during the study period from January 1, 1980, to December 31, 2016. From this population, we defined 3 cohorts with first-time diagnoses of epilepsy, major depression, and asthma. Persons diagnosed with epilepsy or asthma were identified from the DNPR, and persons with major depression were identified from the DCPR. Persons with epilepsy were subdivided into the following categories of epilepsy subtypes: epilepsy with focal-onset seizures, epilepsy with generalized seizures, and other/unspecified epilepsy. Persons diagnosed with major depression were subdivided into the following categories of severity28: mild, moderate, severe, or unspecified. Main and auxiliary diagnoses were used for the identification of patients. Diagnostic codes of all disorders and subclassifications are listed in eTable 1 (links.lww.com/WNL/C491).
The time of disease onset was defined as the first day of the first hospital contact with each of these diagnoses (index date). To minimize inclusion of prevalent cases, we excluded individuals diagnosed before January 1, 1980. We sampled a reference population for each cohort, by matching 5 persons to each person with the disorder by sex and age at diagnosis, among those who were alive and did not have neither the exposure nor the outcome disorder at the index date (i.e., the day the index person was diagnosed with the index diagnosis). Exposure density sampling was used for the matching process.29 According to the diagnostic hierarchy in ICD-8 and ICD-10, schizophrenia and bipolar disorder rank higher than depression; that is, if diagnosed with either of the former, any cases of depression would be considered to fall under the higher ranked disease and the person would not be considered to experience depression or be at risk of depression. Therefore, in analyses with depression as exposure or outcome, persons were excluded if diagnosed with schizophrenia or bipolar disorder before index date and censored if diagnosed with these disorders after the index date (for diagnostic codes, see eTable 1, links.lww.com/WNL/C491).
The cohorts of persons with newly diagnosed epilepsy, major depression, or asthma and their respective reference cohorts were followed up from the index date until a diagnosis of interest (epilepsy or major depression), death, emigration, censoring, or the end of follow-up on December 31, 2016, whichever came first.
Statistical Analysis
We used a Cox regression model to estimate the hazard ratio (HR) and corresponding 95% CI of depression after an epilepsy diagnosis, compared with the matched reference group without epilepsy. Time since the index date was used as the underlying time scale. Persons in the reference groups were censored in case of diagnosis with the index diagnosis. We performed the analyses for epilepsy diagnoses combined and by epilepsy subtype (at first registered diagnosis of epilepsy) in a stratified analysis. In addition, we performed the analysis for the entire study period and population combined and analyses stratified by time since diagnosis and by age groups (age at index date 0–19 years, 20–39 years, 40–59 years, 60–79 years, and 80+ years).
In a similar manner, we estimated the HR of epilepsy in persons with diagnosis of depression compared with matched persons without depression. This was performed for the depression diagnoses combined and by depression severity (at first registered diagnosis) for the subset of persons diagnosed from 1994 and onward. Finally, we estimated HRs of depression and epilepsy after an asthma diagnosis, compared with matched persons without asthma, in a manner similar to that described earlier.
In all analyses, a multivariable Cox regression model was performed with full adjustment for Charlson comorbidity index (CCI) score on the index date, substance abuse, and calendar year. The ICD-8 and ICD-10 codes used to define CCI score are listed in eTable 2 (links.lww.com/WNL/C491). Substance abuse was defined as having a main or auxiliary diagnosis of alcohol or drug abuse in the DNPR or the DCPR (for diagnostic codes, see eTable 1) on or before the index date. Calendar year was included in the model as a time-varying covariate (divided into 8 periods: 1980–1984, 1985–1989, 1990–1994, 1995–1999, 2000–2004, 2005–2009, 2010–2014, and 2015–2016).
We estimated the cumulative incidences of depression in the epilepsy group and the matched population without epilepsy, and vice versa, using competing risk regression with death and emigration as competing events and time since index date as the underlying time scale. To visualize the temporal correlation between epilepsy and depression onset, we plotted the incidence rate of major depression diagnoses over time in people with epilepsy, and vice versa, against the rate in matched references, on time axes from 20 years before to 20 years past index date.
To evaluate whether comorbid depression affected the risk of treatment failure in persons with epilepsy, we examined the risk of an acute hospital admission with seizures (for diagnostic codes, see eTable 1, links.lww.com/WNL/C491) as a proxy for treatment failure in people with and without comorbid depression, after allowing an initial 6-month stabilization period after epilepsy diagnosis. To account for socioeconomic status, we performed a sensitivity analysis in which we included equated household income from the Income Statistics Register (disposable income of a household weighted by number of household members, available in the register from 1987 and onward) as a covariate, on a subset of cohorts with index dates from 1987 and onward. In another sensitivity analysis, we performed the main analyses of risk of depression after epilepsy and vice versa, without censoring for bipolar disorder or schizophrenia.
A p value of less than 5% indicated significance throughout all the analyses (2-sided). Interaction was tested by including interaction terms and using likelihood ratio tests. The proportional hazard assumption for the Cox regression model fits was tested with the cox.zph function in R's “survival package”30; the assumption was violated for the main exposures of the main analyses; when stratified by follow-up time, proportionality was fulfilled within each stratum. All analyses were performed with statistical software programs (SAS version 9.4; R version 4.0.4).
Standard Protocol Approvals, Registrations, and Patient Consents
The study was based on pseudoanonymized data, and the study involved no individual patient contact. The study was therefore exempt form ethical review and approval according to Danish law. The study was approved by the Danish Data Protection Agency.
Data Availability
The raw data that support the findings of this study are not publicly available due to the legislation of Statistics Denmark and the Danish Act on Processing of Personal Data.
Results
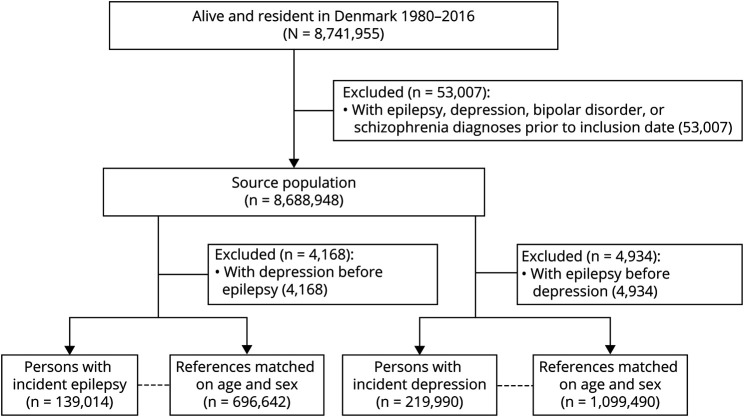
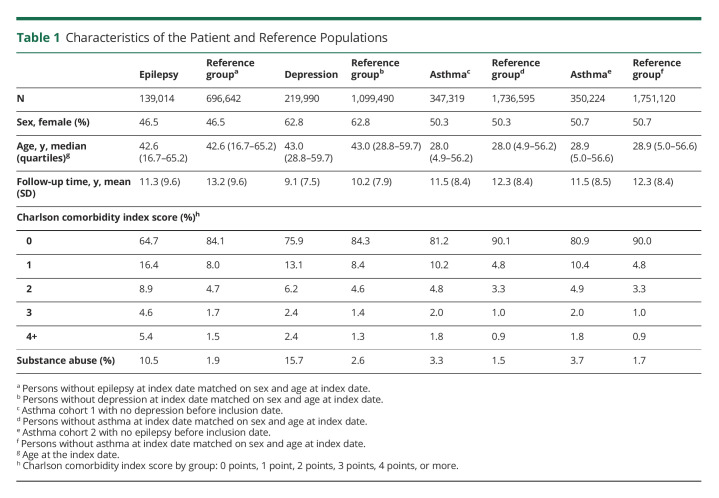
We identified 8,741,955 individuals alive and resident in Denmark at some point between January 1, 1980, and December 31, 2016 (Figure 1). We excluded 53,007 persons diagnosed with epilepsy, major depression, bipolar disorder, or schizophrenia before January 1, 1980, leaving 8,688,948 persons in the source population, from which the epilepsy, depression, and matched reference cohorts were identified (Figure 1; for the generation of the asthma cohorts see eFigure 1, links.lww.com/WNL/C491). Characteristics of the 3 patient cohorts and their respective reference groups are summarized in Table 1. Persons with asthma had a lower median age at index date (28.0–28.9 years) than those with index diagnoses of epilepsy (42.6 years) or depression (43.0 years), p < 0.001. Persons with epilepsy generally experienced more comorbid disorders (CCI score ≥2: 18.9% vs 11.0% in the depression and 8.6%–8.7% in the asthma cohorts, p < 0.001), while persons with index diagnoses of depression were at a greater risk of being substance abusers (15.7% vs 10.5% in the epilepsy and 3.3%–3.7% in the asthma cohorts, p < 0.001) (Table 1).
Figure 1. Generation of the Source and Study Populations.
Table 1.
Characteristics of the Patient and Reference Populations
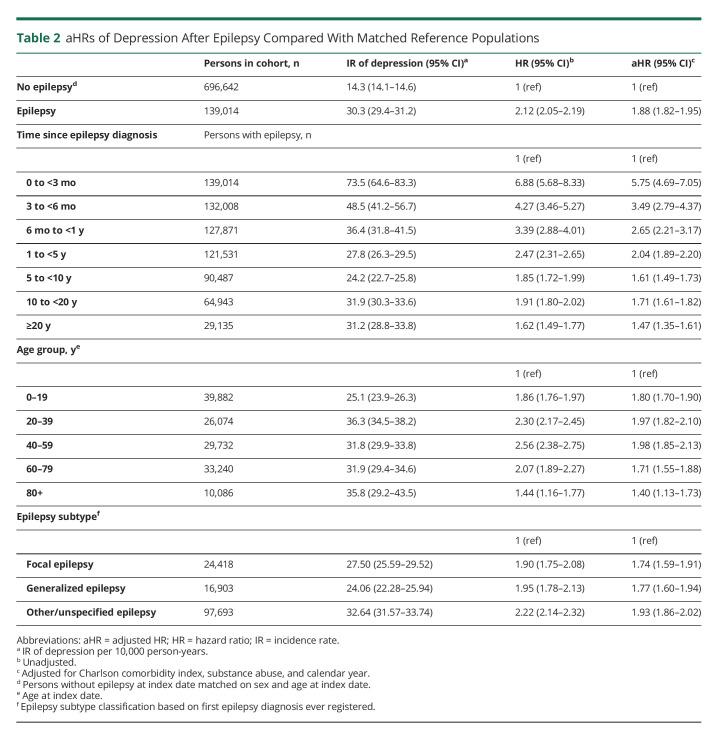
The rate of developing depression was nearly 2-fold increased in persons with epilepsy compared with the matched population without epilepsy (adjusted HR [aHR] 1.88, 95% CI 1.82–1.95) (Table 2). The HRs were highest in the first months and years after primary diagnosis, declined over time, yet remained significantly elevated compared with those for the matched references throughout the observation period. The highest rate of depression after epilepsy was found in those aged 40–59 years (aHR 1.98, 95% CI 1.85–2.13) and lowest for those aged 0–19 years (aHR 1.80, 95% CI 1.70–1.90) at first epilepsy diagnosis (Table 2). In the analysis stratified by epilepsy subtype, there was no evidence of a stronger association with depression for any epilepsy subtype because all CIs overlapped; the highest rate of depression was found in the “other/unspecified” group (aHR 1.93, 95% CI 1.86–2.02) (Table 2).
Table 2.
aHRs of Depression After Epilepsy Compared With Matched Reference Populations
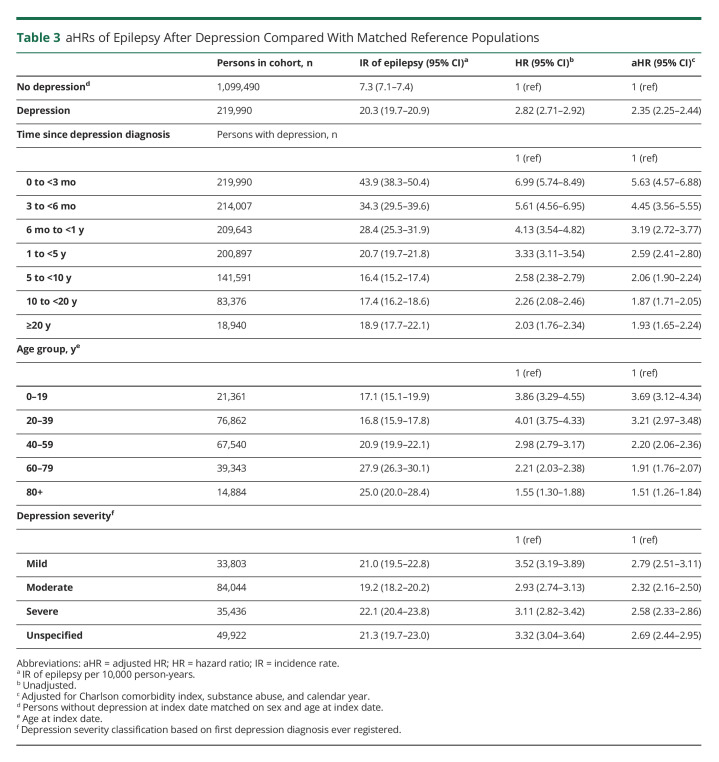
The rate of epilepsy was more than 2-fold increased in persons with incident depression compared with the matched population without depression (aHR 2.35, 95% CI 2.25–2.44) (Table 3). As in the opposite analysis, the HRs were highest in the first months and years after depression diagnosis and declined over time. The rate of epilepsy was highest among the youngest, that is, those aged 0–19 years (aHR 3.69, 95% CI 3.12–4.34) at time of first depression diagnosis (Table 3), whereas the lowest rate was found among those aged 80 years or more (aHR 1.51, 95% CI 1.26–1.84) at first depression diagnosis (Table 3). In the analysis stratified for depression severity, the highest rate of epilepsy was found for those diagnosed with “mild” depression at first ever depression diagnosis (aHR 2.79, 95% CI 2.51–3.11) (Table 3) and the lowest rate of epilepsy was found for those diagnosed with “moderate” depression (aHR 2.32, 95% CI 2.16–2.50), although all CIs overlapped.
Table 3.
aHRs of Epilepsy After Depression Compared With Matched Reference Populations
The rates of depression and epilepsy in persons with asthma were 1.63-fold (95% CI 1.59–1.67) and 1.48-fold (95% CI 1.44–1.53) increased respectively, compared with matched populations of persons without asthma (eTables 3 and 4, links.lww.com/WNL/C491). Additional analyses on the reverse associations are summarized in eTable 5.
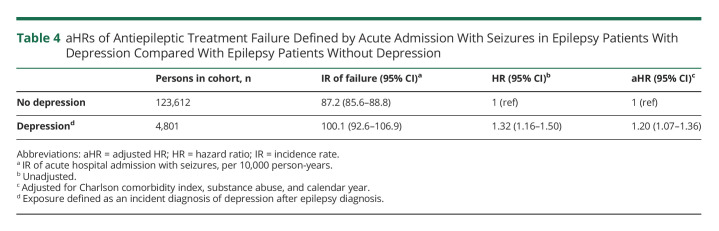
For the epilepsy cohort, there was a higher rate of an acute admission with seizures (aHR 1.20, 95% CI 1.07–1.36) in those who received a depression diagnosis after the onset of epilepsy compared with those who did not (Table 4).
Table 4.
aHRs of Antiepileptic Treatment Failure Defined by Acute Admission With Seizures in Epilepsy Patients With Depression Compared With Epilepsy Patients Without Depression
We analyzed whether the risk of epilepsy and depression varied with sex, comorbidity, substance abuse, and calendar year and summarized interaction parameters in eTables 6 and 7 (links.lww.com/WNL/C491). There was no significant interaction with sex in the rate of depression after epilepsy (p = 0.47) or vice versa (p = 0.68). There was significant interaction with comorbidity score (p = 0.007), substance abuse (p < 0.001), and calendar year (p < 0.001) for the rate of depression after epilepsy and for epilepsy after depression (p < 0.001 for all 3 interaction parameters). The elevated rate of depression after epilepsy and vice versa held significant across all strata of the covariates.
Sensitivity analyses with additional adjustment for socioeconomic status yielded an aHR of 1.76 (95% CI 1.68–1.83) (eTable 8, links.lww.com/WNL/C491) for depression after epilepsy compared with matched references without epilepsy and an aHR of 2.30 (95% CI 2.20–2.41) (eTable 8) for epilepsy after depression, compared with matched individuals without depression.
Sensitivity analyses without censoring for bipolar disorder or schizophrenia yielded slightly higher aHRs for depression after epilepsy compared with matched references without epilepsy (aHR 1.91, 95% CI 1.85–1.98) and for epilepsy after depression (aHR 2.41, 95% CI 2.31–2.50) compared with matched individuals without depression (eTable 9, links.lww.com/WNL/C491).
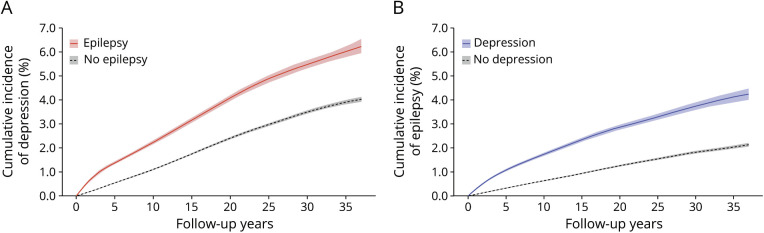
Figure 2A illustrates the cumulative incidence of depression after epilepsy diagnosis compared with the matched population without epilepsy. The cumulative incidence of depression at 5 and 35 years of follow-up in the epilepsy cohort was 1.37% (95% CI 1.33–1.41) and 6.05% (95% CI 5.81–6.19) respectively, compared with 0.59% (95% CI 0.58–0.61) and 3.92% (95% CI 3.84–4.00) at 5 and 35 years of follow-up in the reference population (Gray test for group difference: p < 0.001). The curve had a slightly steeper slope over the first few years, leveling off to a steady increase with increasing follow up-time. Similarly, the cumulative incidence of epilepsy after a major depression diagnosis was increased compared with the matched population without depression (Figure 2B), with a cumulative incidence of epilepsy in the depression cohort of 1.10% (95% CI 1.08–1.12) at 5 years of follow-up and 4.19% (95% CI 3.95–4.38) at 35 years of follow-up, compared with 0.32% (95% CI 0.31–0.33) and 2.06% (95% CI 1.99–2.12) at the same time points in the reference population (Gray test for group difference: p < 0.001).
Figure 2. Cumulative Incidences of Depression After Incident Epilepsy and Vice Versa, Compared With Matched Reference Populations.
(A) Cumulative incidence of depression in people with epilepsy vs a reference population without epilepsy, matched on sex and age during epilepsy diagnosis. (B) Cumulative incidence of epilepsy in people with depression vs a reference population without depression, matched on sex and age during depression diagnosis.
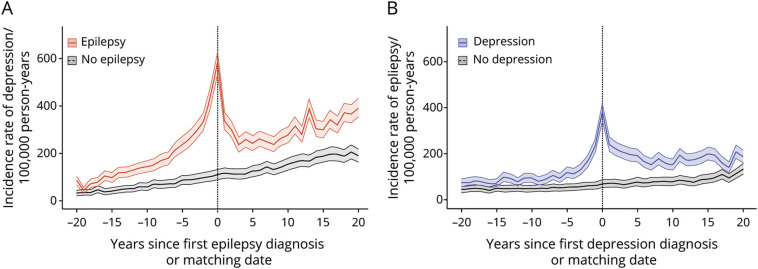
The incidence rate of depression peaked around the time of epilepsy diagnosis in individuals with epilepsy (Figure 3A), with an elevated rate in the years before and after epilepsy diagnosis, when compared with the matched reference group. Similarly, in individuals with depression, the incidence rate of epilepsy peaked around the time of depression diagnosis (Figure 3B) and remained elevated in the following years, when compared with that in the reference group.
Figure 3. Incidence Rate of Depression in a Cohort With Epilepsy and Matched References and Incidence Rate of Epilepsy in a Cohort With Depression and Matched References.
(A). Incidence rate of depression from 20 years before to 20 years after diagnosis of epilepsy (red line) or matching date in the reference population (black line). (B). Incidence rate of epilepsy from 20 years before to 20 years after diagnosis of depression (blue line) or matching date in the reference population (black line).
Discussion
In this study, we used Danish nationwide registers to demonstrate the increased risk of depression after incident epilepsy and increased risk of epilepsy after incident depression. The association between the 2 disorders was robust and persisted after adjusting for medical comorbidity, calendar year, substance abuse, and socioeconomic status. Disease rates peaked in the years just before and after the primary diagnosis but remained elevated throughout follow-up time. Using admission with seizures as a proxy for treatment failure, we found increased risks of treatment failure in persons with epilepsy who were diagnosed with depression.
Overall, we found relative risk estimates that were in line with that found in population-based studies of comparable design and sample size. In a recent, large-scale Canadian study,13 incident epilepsy was associated with an increased HR for depression of 2.04 (95% CI 1.97–2.09), and incident depression was associated with an increased HR for developing epilepsy of 2.55 (95% CI 2.49–2.60). In a recent meta-analysis31 of 4,195 participants, the overall odds ratio (OR) of lifetime depression in persons with epilepsy compared with that in healthy individuals was 2.20 (95% CI 1.07–4.51). In a community-based Swedish study16 from 1990, cases of incident epilepsy had 7-fold increased odds of having a history of depression compared with sex-matched and age-matched controls (OR 7.03, p = 0.003). This study had a small sample size (n = 83), and information on prior depression was obtained through retrospective interviews, with risk of recall bias. In another small-scale (n = 435) matched case-control study,15 a 4-fold increased risk of incident, unprovoked seizures was found for those with a history of major depression (adjusted OR [aOR] 3.7, 95% CI 0.8–17.0). This study was limited to adults older than 54 years, and risk was not adjusted for alcohol or drug abuse, a considerable confounder for the risk of epilepsy after depression.32 Indeed, our estimates were substantially attenuated after adjustment for substance abuse.
Our results add to existing evidence by reliably confirming the bidirectional association between epilepsy and depression with high precision estimates, provided by a large study sample, a long and complete follow-up of the entire Danish population, and detailed studies of the association across a broad range of analysis types.
A major strength of our study was the inclusion of a reference group with another chronic disease. The correlation between epilepsy and depression may partly be explained by the fact that a chronic disorder in itself increases frequency of medical visits, thus increasing chances of any additional disorders being caught, compared with healthy persons who have less medical contacts. By taking this issue, known as the Berkson bias, into account, we strengthen the reliability of the association found. Asthma was chosen as a reference because it shares features with both epilepsy and depression—being fluctuating in intensity and affecting people at a wide distribution of ages—and similar to epilepsy, it is associated with reduced quality of life, emotional stress, sudden unpredictable exacerbations, and fear of fatal episodes.33 Thus, the analyses of comorbidity with asthma serve dual purposes, indicating that neither the Berkson bias nor the theory of depression being purely reactive to experiencing a chronic encumbering disorder fully explains the associations found. Of note, however, for the majority, the impact of an epilepsy diagnosis on life quality is probably graver than that of a diagnosis with asthma,34 and in addition, physicians may be more aware of the need for screening for depression in patients diagnosed with the former; thus, residual bias may persist.
The median age at onset was lower in the asthma cohort than in the epilepsy and depression cohorts, a difference that was necessary to take into account because risks of both epilepsy and depression are age dependent.35,36 We addressed this by presenting HRs stratified by index age group for all main analyses. The estimates for depression after epilepsy were higher than those for depression after asthma across all age groups, although for some age groups (index age groups 0–19, 20–39, and 80+ years), there were overlapping CIs. Similarly, the risk of epilepsy after depression was higher across all age groups than that of epilepsy after asthma, but with overlapping confidence intervals among those oldest (older than 80 years) at index time.
Earlier studies suggested that in persons with both epilepsy and depression, the 2 disorders may often occur in temporal proximity of each other. In a study from Sweden,17 risk of new onset seizures was presented from >5 years before (OR 2.2, 95% CI: 1.3–3.7) to >2 years after (OR 1.7, 95% CI: 1.0–2.8) debut of depression. In another study of 1,000 adults with epilepsy,37 HRs of developing psychiatric disorders, compared with controls, were highest in the first year after epilepsy diagnosis (HR 11.4, 95% CI 9.88–13.2), gradually diminishing to nonsignificance after 4 years. In a cohort study of 3,800 epilepsy cases 10–60 years of age,38 IRRs for depression were 2-fold increased compared with those for controls, in all years from 3 years before to 3 years after epilepsy diagnosis. We were able to add to the existing evidence in both detail and temporal range, showing that the increased risks of depression after epilepsy and vice versa were sustained over a time span from 20 years before to 20 years after index diagnosis, with results presented year by year, across all patient ages. We found that the incidence of depression peaked around the time of epilepsy diagnosis and vice versa. These marked peaks probably partly reflect patients being extra carefully screened by medical professionals in the course of being diagnosed; however, in both directions, the peak extended to several years before and after index time, compellingly giving the impression of a temporal coherence.
We used rate of admission with seizures as a proxy for failure of epilepsy management and found that incident depression was associated with an increased risk of acute admission with seizures. This approach is likely to underestimate the true rate of treatment failure–related events because many patients experience seizures that may not lead to hospital admission. Our result is, however, in accordance with prior findings of decreased adherence to antiseizure medication,14 increased pharmacoresistance,11,12 and increased risk of failing to achieve 1-year seizure freedom for epilepsy patients with depression.13 Thus, many factors may affect chances of successful epilepsy management in those with comorbid depression; our result supports these findings.
Some studies have found certain epilepsy subtypes to be associated with an increased risk of depression, most often focal epilepsies,15,16,39 whereas others found no difference between subtypes.40 In this study, we failed to detect any differences in the risk of depression between epilepsy subtypes. Our finding may be partly due to a relatively low predictive value for epilepsy subtypes in the DNPR, estimated at 35%–60%.41 In addition, the stratification was based on first registered diagnosis with epilepsy, thus disregarding potential further subclassification of patients at later admissions.
Of important note, our study sample was limited to hospitalized inpatients or outpatients including emergency room contacts. Persons with epilepsy in Denmark are diagnosed and treated in hospital settings; but for 85%–90% of patients with depression in a Danish study, the first medical treatment took place outside of psychiatric hospitals and three-quarters of persons treated for depression received no hospital care within 5 years.42 Thus, we might primarily catch those at the severe end of the depression spectrum, which has previously been related to a higher risk of epilepsy,13 and the result may thus not generalize to cases of depression treated in primary care. The results presented in this study, however, suggest no higher risk of epilepsy in the more severely depressed. Contrarily, there was some indication of a higher risk of epilepsy for the mildly depressed than for those categorized with moderate or severe depression at first registered depression diagnosis; however, it was not statistically significant.
A noteworthy limitation of the study is the somewhat lacking validity of epilepsy, major depression, and asthma diagnoses in the Danish registries, with positive predictive values (PPVs) estimated at 81%,41 75%,43 and 65%,44 respectively; notably, the PPV of asthma may have been underestimated in the latter study because this was based on conscription records, which may underascertain asthma diagnoses if another medical condition has already been recorded as the reason for exemption, or conscripts wanting to serve may underreport asthma.44 Nonetheless, misclassification of the registered disorders, especially in the asthma analyses, may cloud the associations. Furthermore, the clinical presentation of depression in persons with epilepsy often does not correspond to the criteria in operationalized classification systems such as ICD-10,45 and thus, failure to detect depression may occur at a higher rate in those with underlying epilepsy. Such a differential misclassification could further bias the estimates of the association toward the null.
Another limitation is the diagnostic delay in both epilepsy and depression. In a Spanish study, the mean diagnostic delay from onset of symptoms to a major depression diagnosis was approximately 10 weeks,46 and hospital-based identification of patients with depression is likely longer. Likewise, in a validation study of the epilepsy diagnosis in the DNPR, delay from index seizure to epilepsy diagnosis was more than a year for 36% of patients.41 Thus, the capability to address the temporal aspects of the association with the present data is limited. Finally, we did not have access to data on prescriptions or other treatments for this study, preventing us from adjusting for this in the main analyses or inferring on their potential contribution to the correlation.
At present, the nature of the bidirectional relationship between epilepsy and depression remains unclear. Theories of etiologies span from common neurobiological properties, hereditary and psychosocial aspects, negative psychotropic effects of antiseizure medication, and proconvulsant properties of antidepressants.19,20,45 The latter has been a widely held concern, adding to a reluctance to treat depression in persons with epilepsy,3 but this has been largely disproven by studies concluding that nearly all antidepressants are safe for persons with epilepsy when administered in proper doses,47 and the increased risk of epilepsy after depression has been proven robust to adjustment for antidepressant treatment.15 The present results add to the idea that concurrent depression seems to be associated with worse seizure outcomes for persons with epilepsy, but whether this finding is related to the depression in itself or its treatment cannot be inferred using this study design.
In conclusion, we found a robust association between epilepsy and depression in nationwide Danish registers, strongly supporting previous observations of a bidirectional association between these 2 brain disorders. The results add to the growing amount of evidence, indicating the need for increased clinical awareness of this association and prompt further research to better understand the nature of this association and its consequences.
Acknowledgment
This study was conducted as a part of the BrainDrugs research alliance.48
Glossary
- aHR
adjusted HR
- aOR
adjusted OR
- CCI
Charlson comorbidity index
- DCPR
Danish Central Psychiatric Register
- DNPR
Danish National Patient Registry
- HR
hazard ratio
- ICD-8
International Classification of Diseases, eighth revision
- ICD-10
International Classification of Diseases, 10th revision
- OR
odds ratio
- PPV
positive predictive value
Appendix. Authors
Footnotes
Patient Page e995
Infographic NPub.org/ig1009
Study Funding
This study was funded by the Lundbeck Foundation (R279-2018-1145, BrainDrugs), the Danish Epilepsy Association, and the Novo Nordisk Foundation (NNF16OC0019126).
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Kanner AM, Balabanov A. Depression and epilepsy: how closely related are they? Neurology. 2002;58(8 suppl 5):S27-S39. doi: 10.1212/wnl.58.8_suppl_5.s27. [DOI] [PubMed] [Google Scholar]
- 2.Patel RS, Elmaadawi A, Mansuri Z, Kaur M, Shah K, Nasr S. Psychiatric comorbidities and outcomes in epilepsy patients: an insight from a nationwide inpatient analysis in the United States. Cureus. 2017;9(9):e1686. doi: 10.7759/cureus.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ajinkya S, Fox J, Lekoubou A. Trends in prevalence and treatment of depressive symptoms in adult patients with epilepsy in the United States. Epilepsy Behav. 2020;105:106973. doi: 10.1016/j.yebeh.2020.106973. [DOI] [PubMed] [Google Scholar]
- 4.Gaitatzis A, Carroll K, Majeed A, Sander JW. The epidemiology of the comorbidity of epilepsy in the general population. Epilepsia. 2004;45(12):1613-1622. doi: 10.1111/j.0013-9580.2004.17504.x. [DOI] [PubMed] [Google Scholar]
- 5.Bromet E, Andrade LH, Hwang I, et al. Cross-national epidemiology of DSM-IV major depressive episode. BMC Med. 2011;9(1):90. doi: 10.1186/1741-7015-9-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGee RE, Sajatovic M, Quarells RC, et al. Depression and quality of life among African Americans with epilepsy: findings from the Managing Epilepsy Well (MEW) Network integrated database. Epilepsy Behav. 2019;94:301-306. doi: 10.1016/j.yebeh.2019.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cramer JA, Blum D, Reed M, Fanning K. The influence of comorbid depression on quality of life for people with epilepsy. Epilepsy Behav. 2003;4(5):515-521. doi: 10.1016/j.yebeh.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Fazel S, Wolf A, Långström N, Newton CR, Lichtenstein P. Premature mortality in epilepsy and the role of psychiatric comorbidity: a total population study. Lancet. 2013;382(9905):1646-1654. doi: 10.1016/s0140-6736(13)60899-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin CY, Harnod T, Lin CL, Shen WC, Kao CH. Differences in incidence and risks of suicide attempt and suicidal drug overdose between patients with epilepsy with and without comorbid depression. Int J Environ Res Public Health. 2019;16(22):4533. doi: 10.3390/ijerph16224533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mainio A, Alamäki K, Karvonen K, Hakko H, Särkioja T, Räsänen P. Depression and suicide in epileptic victims: a population-based study of suicide victims during the years 1988-2002 in northern Finland. Epilepsy Behav. 2007;11(3):389-393. doi: 10.1016/j.yebeh.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Nogueira MH, Yasuda CL, Coan AC, Kanner AM, Cendes F. Concurrent mood and anxiety disorders are associated with pharmacoresistant seizures in patients with MTLE. Epilepsia. 2017;58(7):1268-1276. doi: 10.1111/epi.13781. [DOI] [PubMed] [Google Scholar]
- 12.Hitiris N, Mohanraj R, Norrie J, Sills GJ, Brodie MJ. Predictors of pharmacoresistant epilepsy. Epilepsy Res. 2007;75(2-3):192-196. doi: 10.1016/j.eplepsyres.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Josephson CB, Lowerison M, Vallerand I, et al. Association of depression and treated depression with epilepsy and seizure outcomes. JAMA Neurol. 2017;74(5):533. doi: 10.1001/jamaneurol.2016.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ettinger AB, Good MB, Manjunath R, Edward Faught R, Bancroft T. The relationship of depression to antiepileptic drug adherence and quality of life in epilepsy. Epilepsy Behav. 2014;36:138-143. doi: 10.1016/j.yebeh.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Hesdorffer DC, Hauser WA, Annegers JF, Cascino G. Major depression is a risk factor for seizures in older adults. Ann Neurol. 2000;47(2):246-249. doi:. [DOI] [PubMed] [Google Scholar]
- 16.Forsgren L, Nyström L. An incident case-referent study of epileptic seizures in adults. Epilepsy Res. 1990;6(1):66-81. doi: 10.1016/0920-1211(90)90010-s. [DOI] [PubMed] [Google Scholar]
- 17.Adelow C, Andersson T, Ahlbom A, Tomson T. Hospitalization for psychiatric disorders before and after onset of unprovoked seizures/epilepsy. Neurology. 2012;78(6):396-401. doi: 10.1212/wnl.0b013e318245f461. [DOI] [PubMed] [Google Scholar]
- 18.Martin RC, Faught E, Richman J, et al. Psychiatric and neurologic risk factors for incident cases of new-onset epilepsy in older adults: data from U.S. Medicare beneficiaries. Epilepsia. 2014;55(7):1120-1127. doi: 10.1111/epi.12649. [DOI] [PubMed] [Google Scholar]
- 19.Kanner AM. Can neurochemical changes of mood disorders explain the increased risk of epilepsy or its worse seizure control? Neurochem Res. 2017;42(7):2071-2076. doi: 10.1007/s11064-017-2331-8. [DOI] [PubMed] [Google Scholar]
- 20.Hećimović H, Goldstein JD, Sheline YI, Gilliam FG. Mechanisms of depression in epilepsy from a clinical perspective. Epilepsy Behav. 2003;4:25-30. doi: 10.1016/j.yebeh.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 21.Kanner AM. Psychiatric comorbidities and epilepsy: is it the old story of the chicken and the egg? Ann Neurol. 2012;72(2):153-155. doi: 10.1002/ana.23679. [DOI] [PubMed] [Google Scholar]
- 22.Westreich D. Berksonʼs bias, selection bias, and missing data. Epidemiology. 2012;23(1):159-164. doi: 10.1097/ede.0b013e31823b6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thygesen LC, Daasnes C, Thaulow I, Brønnum-Hansen H. Introduction to Danish (nationwide) registers on health and social issues: structure, access, legislation, and archiving. Scand J Public Health. 2011;39(7_suppl):12-16. doi: 10.1177/1403494811399956. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt M, Schmidt SAJ, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449-490. doi: 10.2147/clep.s91125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mors O, Perto GP, Mortensen PB. The Danish Psychiatric Central Research Register. Scand J Public Health. 2011;39(7_suppl):54-57. doi: 10.1177/1403494810395825. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt M, Pedersen L, Sørensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol. 2014;29(8):541-549. doi: 10.1007/s10654-014-9930-3. [DOI] [PubMed] [Google Scholar]
- 27.Baadsgaard M, Quitzau J. Danish registers on personal income and transfer payments. Scand J Public Health. 2011;39(7_suppl):103-105. doi: 10.1177/1403494811405098. [DOI] [PubMed] [Google Scholar]
- 28.Kessing LV. Severity of depressive episodes according to ICD-10: prediction of risk of relapse and suicide. Br J Psychiatry. 2004;184(2):153-156. doi: 10.1192/bjp.184.2.153. [DOI] [PubMed] [Google Scholar]
- 29.Ohneberg K, Beyersmann J, Schumacher M. Exposure density sampling: dynamic matching with respect to a time-dependent exposure. Stat Med. 2019;38(22):4390-4403. doi: 10.1002/sim.8305. [DOI] [PubMed] [Google Scholar]
- 30.R survival (version 3.3-1). Accessed May 25, 2021. rdocumentation.org/packages/survival/versions/3.3-1/topics/cox.zph.
- 31.Fiest KM, Dykeman J, Patten SB, et al. Depression in epilepsy: a systematic review and meta-analysis. Neurology. 2013;80(6):590-599. doi: 10.1212/wnl.0b013e31827b1ae0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nilsson F. On the increased risk of developing late-onset epilepsy for patients with major affective disorder. J Affect Disord. 2003;76(1-3):39-48. doi: 10.1016/s0165-0327(02)00061-7. [DOI] [PubMed] [Google Scholar]
- 33.Panicker N, Sharma P, Al-Duwaisan A. Psychological distress and associated risk factors in bronchial asthma patients in Kuwait. Indian J Med Sci. 2008;62(1):1. doi: 10.4103/0019-5359.38915. [DOI] [PubMed] [Google Scholar]
- 34.Austin JK, Huster GA, Dunn DW, Risinger MW. Adolescents with active or inactive epilepsy or asthma: a comparison of quality of life. Epilepsia. 1996;37(12):1228-1238. doi: 10.1111/j.1528-1157.1996.tb00558.x. [DOI] [PubMed] [Google Scholar]
- 35.Christensen J, Vestergaard M, Pedersen MG, Pedersen CB, Olsen J, Sidenius P. Incidence and prevalence of epilepsy in Denmark. Epilepsy Res. 2007;76(1):60-65. doi: 10.1016/j.eplepsyres.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 36.Olsen LR, Mortensen EL, Bech P. Prevalence of major depression and stress indicators in the Danish general population. Acta Psychiatr Scand. 2004;109(2):96-103. doi: 10.1046/j.0001-690x.2003.00231.x. [DOI] [PubMed] [Google Scholar]
- 37.Chang HJ, Liao CC, Hu CJ, Shen WW, Chen TL. Psychiatric disorders after epilepsy diagnosis: a population-based retrospective cohort study. PLoS One. 2013;8(4):e59999. doi: 10.1371/journal.pone.0059999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hesdorffer DC, Ishihara L, Mynepalli L, Webb DJ, Weil J, Hauser WA. Epilepsy, suicidality, and psychiatric disorders: a bidirectional association. Ann Neurol. 2012;72(2):184-191. doi: 10.1002/ana.23601. [DOI] [PubMed] [Google Scholar]
- 39.Li Q, Chen D, Zhu LN, et al. Depression in people with epilepsy in West China: status, risk factors and treatment gap. Seizure. 2019;66:86-92. doi: 10.1016/j.seizure.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 40.Berg AT, Caplan R, Hesdorffer DC. Psychiatric and neurodevelopmental disorders in childhood-onset epilepsy. Epilepsy Behav. 2011;20(3):550-555. doi: 10.1016/j.yebeh.2010.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Christensen J, Vestergaard M, Olsen J, Sidenius P. Validation of epilepsy diagnoses in the Danish National Hospital Register. Epilepsy Res. 2007;75(2-3):162-170. doi: 10.1016/j.eplepsyres.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 42.Musliner KL, Liu X, Gasse C, Christensen KS, Wimberley T, Munk-Olsen T. Incidence of medically treated depression in Denmark among individuals 15-44 years old: a comprehensive overview based on population registers. Acta Psychiatr Scand. 2019;139(6):548-557. doi: 10.1111/acps.13028. [DOI] [PubMed] [Google Scholar]
- 43.Bock C, Bukh J, Vinberg M, Gether U, Kessing L. Validity of the diagnosis of a single depressive episode in a case register. Clin Pract Epidemiol Ment Health. 2009;5(1):4. doi: 10.1186/1745-0179-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jensen AØ, Nielsen GL, Ehrenstein V. Validity of asthma diagnoses in the Danish National Registry of Patients, including an assessment of impact of misclassification on risk estimates in an actual dataset. Clin Epidemiol. 2010;2:67-72. doi: 10.2147/clep.s6875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prueter C, Norra C. Mood disorders and their treatment in patients with epilepsy. J Neuropsychiatry Clin Neurosci. 2005;17(1):20-28. doi: 10.1176/jnp.17.1.20. [DOI] [PubMed] [Google Scholar]
- 46.Huerta-Ramírez R, Bertsch J, Cabello M, Roca M, Haro JM, Ayuso-Mateos JL. Diagnosis delay in first episodes of major depression: a study of primary care patients in Spain. J Affect Disord. 2013;150(3):1247-1250. doi: 10.1016/j.jad.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 47.Kanner AM. Most antidepressant drugs are safe for patients with epilepsy at therapeutic doses: a review of the evidence. Epilepsy Behav. 2016;61:282-286. doi: 10.1016/j.yebeh.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 48.BrainDrugs. Accessed June 3, 2020. nru.dk/index.php/braindrugs.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data that support the findings of this study are not publicly available due to the legislation of Statistics Denmark and the Danish Act on Processing of Personal Data.