Abstract
Background and Objectives
Olfactory function declines with aging, and olfactory deficits are one of the earliest features of neurodegenerative diseases, such as Parkinson disease and Alzheimer disease. Previous studies have shown that olfaction is associated with brain volumes and cognitive function, but data are exclusively cross-sectional. We aimed to examine longitudinal associations of olfaction with changes in brain volumes and neuropsychological function.
Methods
In the Baltimore Longitudinal Study of Aging, we chose the first assessment of olfaction to examine the associations with retrospective and prospective changes in neuropsychological performance and brain volumes in participants aged 50 years or older using linear mixed-effects models, adjusted for demographic variables and cardiovascular disease. Olfaction was measured as odor identification scores through the 16-item Sniffin' Sticks.
Results
We analyzed data from 567 (58% women, 42% men, 27% Black, 66% White, and 7% others) participants who had data on odor identification scores and brain volumetric MRI (n = 420 with retrospective repeats over a mean of 3.7 years, n = 280 with prospective repeats over a mean of 1.2 years). We also analyzed data from 754 participants (56% women, 44% men, 29% Black, 65% White, and 6% others) with neuropsychological assessments (n = 630 with retrospective repeats over a mean of 6.6 years, n = 280 with prospective repeats over a mean of 1.5 years). After adjustment, higher odor identification scores were associated with prior and subsequent slower brain atrophy in the entorhinal cortex (β ± SE = 0.0093 ± 0.0031, p = 0.0028 and β ± SE = 0.0176 ± 0.0073, p = 0.0169, respectively), hippocampus (β ± SE = 0.0070 ± 0.0030, p = 0.0192 and β ± SE = 0.0173 ± 0.0066, p = 0.0089, respectively), and additional frontal and temporal areas (all p < 0.05). Higher odor identification scores were also associated with prior slower decline in memory, attention, processing speed, and manual dexterity and subsequent slower decline in attention (all p < 0.05). Some associations were attenuated after exclusion of data points at and after symptom onset of cognitive impairment or dementia.
Discussion
In older adults, olfaction is related to brain atrophy of specific brain regions and neuropsychological changes in specific domains over time. The observed associations are driven, in part, by those who developed cognitive impairment or dementia. Future longitudinal studies with longer follow-ups are needed to understand whether olfactory decline precedes cognitive decline and whether it is mediated through regionally specific brain atrophy.
The sense of smell is essential in our daily lives. The ability to smell has an impact on flavor, taste, and appetite and on detecting environmental hazards. As people age, the sense of smell declines, and the prevalence of loss of smell (i.e., anosmia) substantially increases after age 65 years.1 The olfactory deficit in aging is associated with future cognitive decline and impairment, and impaired smell is one of the earliest features of neurodegenerative diseases, such as Alzheimer (AD) and Parkinson disease. Because of its predictive value in cognitive impairment and risks of neurodegenerative diseases, it is considered to be an early and cost-effective biomarker.2 Olfactory processing primarily involves olfactory receptors in the olfactory epithelium, olfactory bulb, primary olfactory cortices, and secondary areas, including the piriform cortex, entorhinal cortex, and orbital cortex.3 It has been proposed that mechanisms underlying olfactory deficits in aging and neurodegeneration may be different.4 Impairments in both peripheral and central olfactory systems and other factors, such as olfactory receptor cell damage, respiratory tract inflammation, brain abnormalities, and exposures to smoking and airborne pollutants, may contribute to the olfactory deficit in aging. In AD, studies have suggested that amyloid and tau deposition in olfactory-related brain areas and accumulation over time underlie the relationship between olfactory deficits and AD.5,6
Although contributing factors to olfactory deficits may differ in aging and neurodegenerative diseases, regional brain atrophy may be a shared mechanism. Existing neuroimaging studies through brain MRI have consistently shown that lower olfactory function, commonly measured as odor identification, is associated with smaller brain volumes in temporal areas, such as the hippocampus, parahippocampal gyrus, entorhinal cortex, and amygdala in cognitively normal older individuals and those with cognitive impairment.7-14 However, several limitations to prior studies are worth noting. First, previous brain MRI studies are exclusively cross-sectional and do not reflect within-person changes over time. Second, previous studies primarily focused on one or a small number of regions of interest, such as the medial temporal lobe. Thus, the spatial distribution of associations between olfactory function and brain atrophy across the whole brain is less clear. Understanding neural correlates across the whole brain with olfaction may provide insights into mechanisms underlying reported associations of olfactory function with impaired cognition and motor function,2,15,16 Third, not all previous studies have accounted for the cognitive status of participants, including mild cognitive impairment (MCI) or dementia. As olfactory function is related to cognitive impairment and dementia, it is essential to determine whether the association between olfaction and brain atrophy is evident in samples limited to cognitively normal individuals and samples of mixed cognitive status.
Previous studies examining neuropsychological function in older adults are mostly cross-sectional and have reported that olfactory function, specifically odor identification, is associated with global cognitive function, verbal memory, attention, executive function, fluency, psychomotor speed, and manual dexterity.2,17-23 Data on the relation between olfaction and longitudinal changes in domain-specific neuropsychological function are limited, especially in domains involving a motor component, such as psychomotor speed and manual dexterity.24,25
To address prior limitations, in this study we examined associations of olfactory function with longitudinal changes in brain volumes and neuropsychological function over time in a sample of well-characterized community-dwelling adults from the Baltimore Longitudinal Study of Aging (BLSA). We further examined whether these longitudinal associations were driven by persons who were cognitively impaired or had dementia.
Methods
Study Population
Participants were from the BLSA, a prospective longitudinal study with continuous enrollment that began in 1958.26 BLSA participants are community-dwelling adult volunteers. At enrollment, eligible participants are free of cognitive impairment, functional limitations, chronic diseases, and cancer within the past 10 years. Participants visit the National Institute on Aging Clinical Research Unit for 3 days and receive comprehensive health, cognitive, and functional assessments. The visit schedule is as follows: every 4 years for age less than 60 years, every 2 years for age 60–79 years, and annually for persons aged 80 years or older.
The timing of the initial assessment for specific neuropsychological measures has varied over time. Here, we focused on data collected from 2005 and onward, when all neuropsychological measures of interest were available. Starting in 2009, all eligible BLSA participants received brain MRI scans at each visit. The olfactory assessment began in 2015. In this study, we used only the first assessment of olfaction to investigate the association with retrospective and prospective changes in neuropsychological performance measures and brain volumes. Note that data on olfaction in this study were collected between 2015 and February 2020, which was before the COVID-19 pandemic.
Standard Protocol Approvals, Registrations, and Patient Consents
The BLSA protocol was approved by the Institutional Review Board of the NIH. Participants provide written informed consent at each visit.
Olfaction
The validated 16-item Sniffin' Sticks Identification Test was used to measure odor identification.27 Participants were presented with 16 common odors. For each odor, participants had to select among 4 choices (1 correct odor). Odor identification test scores ranged between 0 and 16, with higher scores reflecting better olfactory function. Two alternate versions of the 16-item Sniffin' Sticks were used and randomized at the initial assessment to minimize potential learning effects.
Brain MRI
Magnetization-prepared rapid gradient-echo (MPRAGE: repetition time = 6.8 ms, echo time = 3.2 ms, flip angle = 8°, image matrix = 256 × 256 × 170, and voxel size = 1 × 1 × 1.2 mm3) scans were acquired on a 3 T Philips Achieva MRI scanner. Anatomical labels and regional brain volumes were computed from MPRAGE scans using MUlti-atlas region Segmentation using Ensembles (MUSE) of registration algorithms and parameters.28 In brief, “multiple atlases with semiautomatically extracted ground‐truth ROI labels are first warped individually to the target image using a nonlinear registration method. The ensemble is fused into a final consensus segmentation. This workflow for segmenting the brain into a set of anatomical ROIs has been previously validated extensively in the BLSA MRI dataset.29 Notably, the MUSE anatomically labeling approach is robust and accurate, owing to its use of multiple atlases and multiple registration methods. This ensemble approach has consistently outperformed segmentations using individual warping methods alone and has achieved high accuracy in several benchmark datasets.28 The MUSE methodology has been used for processing thousands of scans from various datasets, producing robust and consistent results. MUSE is available through the image processing portal: ipp.cbica.upenn.edu.”
In this study, we aimed to confirm prior cross-sectional findings in the medial temporal area and to examine longitudinal changes in the medial temporal area and other areas of interest across the brain including both gray and white matter and the cerebellum. For gray matter volume, we examined specific regions in the frontal (medial, middle, superior, inferior, orbitofrontal, insula, supplementary motor, and precentral), parietal (postcentral, superior, precuneus, supramarginal, and angular), temporal (entorhinal, parahippocampal, fusiform, and amygdala), occipital (middle, superior, inferior, and occipital pole), limbic (anterior, middle, and posterior cingulate), and subcortical (hippocampus, putamen, caudate, pallidum, and thalamus) areas. For white matter volume, we examined volumes of white matter in frontal, parietal, temporal, and occipital lobes as well as the corpus callosum.
Neuropsychological Function
We examined a number of neuropsychological domains, including mental status, memory, language, attention, executive function, processing speed, visuospatial ability, and manual dexterity. Mental status was measured using the Mini-Mental State Examination (MMSE).30 Memory was measured using the California Verbal Learning Test (CVLT) immediate recall (sum of 5 learning trials) and long-delay free recall,31 and language was measured using the Boston Naming Test.32 Letter (F, A, and S)33 and Category (fruits, animals, and vegetables) Fluency measured fluent production.34 Visuospatial ability was measured using a modified version35 of the Educational Testing Service Card Rotations Test.36 Attention was measured using the Trail Making Test part A (TMT-A).37 Executive function was measured using the Trail Making Test part B.37 Processing speed was measured using the Digit Symbol Substitution Test (DSST).38 Manual dexterity was measured using the Purdue Pegboard Test.39
Diagnoses of Mild Cognitive Impairment and Dementia
Procedures for the determination of cognitive status have been described previously.6 “Clinical and selected neuropsychological data from BLSA participants were reviewed at a consensus conference if participants screened positive on the Blessed Information-Memory-Concentration Test score (i.e., score ≥4), if their Clinical Dementia Rating score was ≥0.5 using subject or informant report, or if concerns were raised about their cognitive status. MCI was determined using the Petersen criteria and diagnosed when (1) cognitive impairment was evident for a single domain (typically memory) or (2) cognitive impairment in multiple domains occurred without significant functional loss in activities of daily living.40 In BLSA, diagnoses of dementia and AD have continued to follow the DSM-III-R and the National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA) criteria, respectively. The date of symptom onset was estimated for MCI/AD and was considered the date of onset of MCI.”
Data Availability
Data from the BLSA will be available on request by proposal submission through the BLSA website (blsa.nih.gov). All requests are reviewed by the BLSA Data Sharing Proposal Review Committee and are also subject to approval from the NIH Institutional Review Board.
Statistical Analysis
We examined the associations of odor identification scores with both retrospective and prospective longitudinal trajectories of brain volumes and neuropsychological performance using linear mixed-effects (LME) models. In LME models, the olfaction assessment was used as the anchor point, that is, time 0. Data points prior were time in years before time 0, that is, −1 year and −2 years. Data points after were time in years after time 0, that is, 1 year and 2 years (data collection is demonstrated in eFigure 1, links.lww.com/WNL/C513). For both retrospective and prospective analyses, we included fixed effects of odor identification scores (independent variable), age (at the olfaction assessment), sex, race (Black vs non-Black), years of education, the olfactory test version, time (in years from the olfaction assessment), and all the 2-way interactions of odor identification scores and covariates with time. Covariates of age, sex, race, education, and cardiovascular disease including stroke are important for outcomes, and the olfactory test version is related to odor identification scores. For brain volume outcomes, we additionally included intracranial volume estimated at age 70 years as a covariate. We also adjusted for APOE ε4 carrier status in subsets of participants who had available data (n = 516 in the MRI sample; n = 697 in the neuropsychological sample). From this model specification, the main effect of odor identification scores estimates the cross-sectional associations between odor identification scores with brain volume and neuropsychological performance. The odor identification scores and time interaction term estimates the effect of odor identification scores on changes in brain volume and neuropsychological performance. The random effect included intercept and time with unstructured covariance. The cross-sectional associations with brain volume and neuropsychological performance were similar in both retrospective and prospective analyses, and we report cross-sectional associations from the retrospective analysis. Based on the most recent participants' status over the prospective period, in the brain MRI sample, 92.6% remained active cohort (n = 525), 2.8% died during follow-up (n = 16), and 4.6% were lost to follow-up or dropped out (n = 26). In the neuropsychological sample over the prospective period, 91.8% remained active cohort (n = 692), 3.2% died (n = 24), and 5.0% were lost to follow-up or dropped out (n = 38). As the number of loss to follow-up is extremely low, the LME models treat missing data as missing at random (number of missing is listed in eTable 1, links.lww.com/WNL/C514). To interpret results meaningfully, we reported all associations and effect sizes based on per interquartile range change in odor identification scores.
To test the strength of these associations, we repeated analyses excluding data points at and after the symptom onset of cognitive impairment or dementia. Participants at the remaining visits were considered cognitively normal by our adjudication process and did not have deficits that met the criteria for cognitive impairment at these visits. We, however, recognize that subsequently impaired individuals may show subtle cognitive decline before symptom onset. We additionally adjusted for smoking status as sensitivity analyses.
In this exploratory analysis, we presented significant associations at a 2-tailed p value of <0.05. We also adjusted for multiple comparisons using the false discovery rate (FDR) correction. All the LME models were fit using the PROC MIXED procedure with the restricted maximum likelihood estimation method in SAS 9.4 (Cary, NC).
Results
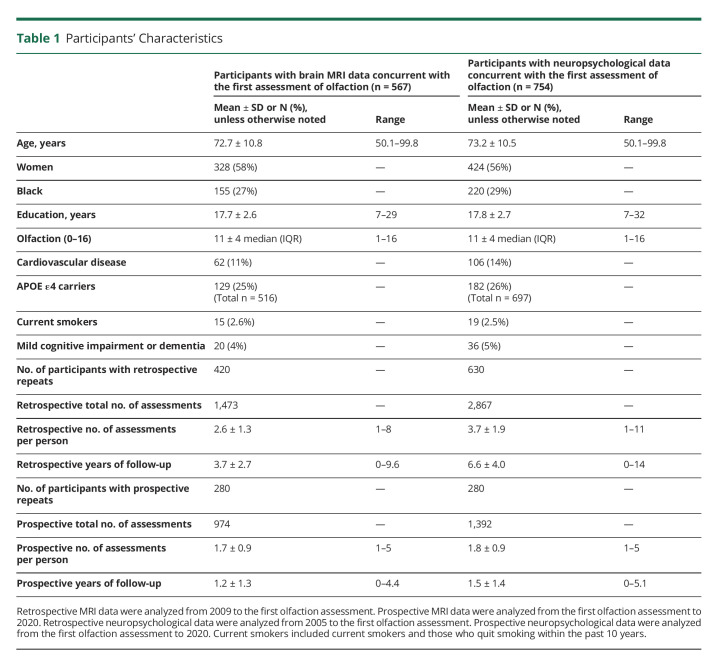
Participants' characteristics are presented in Table 1. For both brain MRI and neuropsychological outcomes, the retrospective period is relatively longer with a greater number of assessments than the prospective period. Specifically, we identified 567 participants (58% women, 42% men, 27% Black, 66% White, and 7% others) aged 50 years or older who had concurrent data on both olfaction and brain MRI. Of the 567 individuals, 420 had retrospective repeated measures of brain MRI over a mean of 3.7 years, and 280 had prospective repeated measures of brain MRI over a mean of 1.2 years. We identified 754 participants who had concurrent olfactory and neuropsychological data (56% women, 44% men, 29% Black, 65% White, and 6% others). Of the 754, 630 had retrospective repeated measures of neuropsychological data over a mean of 6.6 years, and 280 had prospective repeated measures of neuropsychological data over a mean of 1.5 years. In both brain MRI and neuropsychological samples, those who developed cognitive impairment or dementia had worse odor identification scores than those who remained cognitively normal (both p < 0.05).
Table 1.
Participants' Characteristics
Relationships Between Olfaction and Brain Volumes
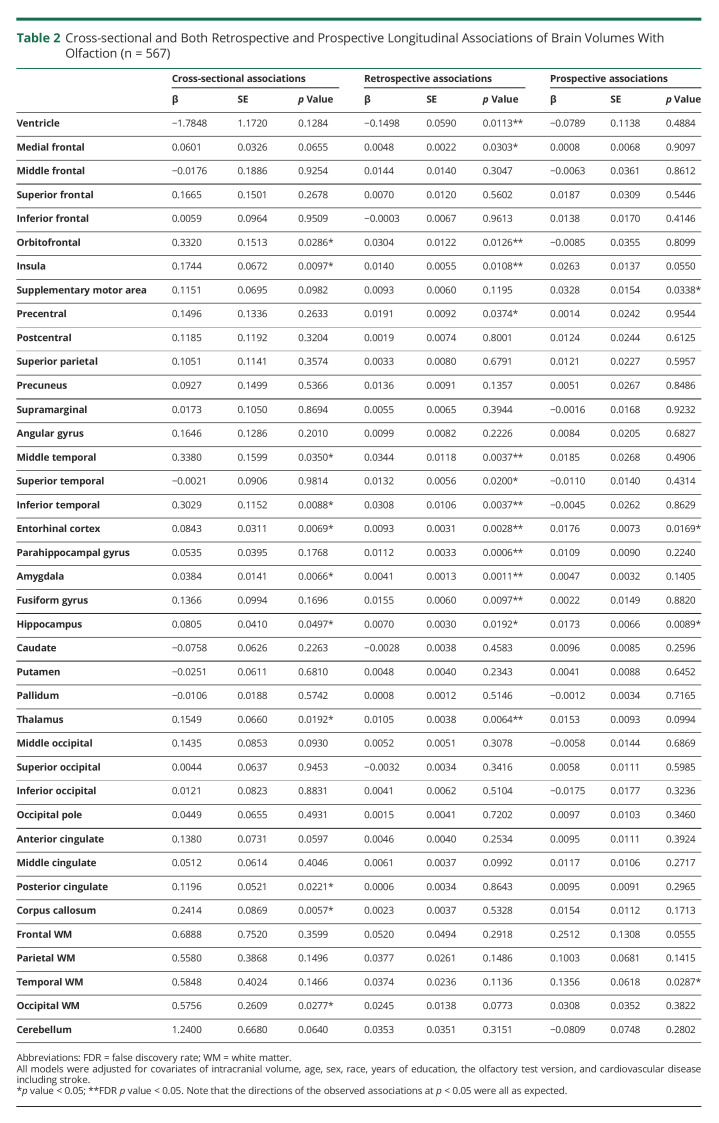
In the overall sample of mixed cognitive status, higher odor identification scores were cross-sectionally associated with greater brain volumes mostly in specific frontal (orbitofrontal and insula), temporal (middle, inferior, entorhinal cortex, amygdala, and hippocampus), and other (thalamus, posterior cingulate, corpus callosum, and occipital white matter) areas (Table 2). After exclusion of data points at and after the symptom onset of cognitive impairment or dementia, most of these cross-sectional associations remained statistically significant (eTable 2, links.lww.com/WNL/C514).
Table 2.
Cross-sectional and Both Retrospective and Prospective Longitudinal Associations of Brain Volumes With Olfaction (n = 567)
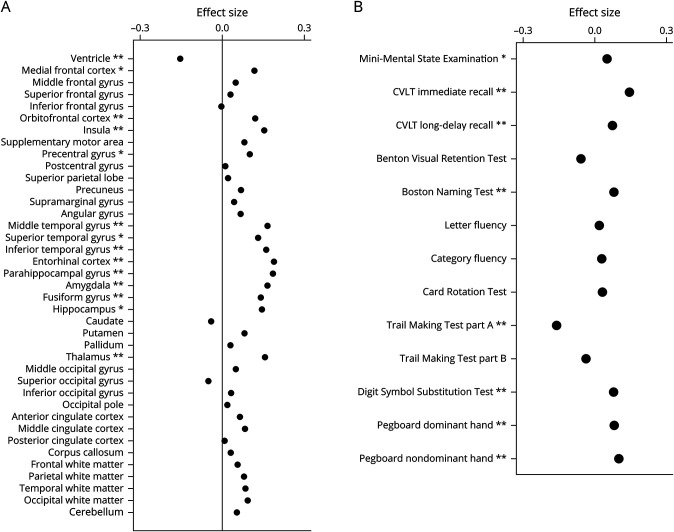
Retrospectively, higher odor identification scores were significantly associated with prior slower rates of increase in ventricular volume and slower rates of brain atrophy in specific frontal (medial frontal, orbitofrontal, insula, and precentral) and temporal areas (middle, superior, inferior, entorhinal cortex, parahippocampal, amygdala, fusiform, and hippocampus), and thalamus. Most of these associations survived FDR-adjusted p < 0.05 (Table 2 and Figure A). After exclusion of data points at and after the symptom onset of cognitive impairment or dementia, some retrospective longitudinal associations were attenuated, but associations with rates of increase in ventricular volume and atrophy in specific temporal areas (entorhinal cortex, parahippocampal gyrus, and amygdala) and the thalamus remained statistically significant (eTable 2, links.lww.com/WNL/C514). Prospectively, higher odor identification scores were associated with slower rates of atrophy in the supplementary motor area, entorhinal cortex, hippocampus, and temporal lobe white matter volume (Table 2). These prospective associations remained statistically significant or similar after exclusion of data points at and after the symptom onset of cognitive impairment or dementia (eTable 2).
Figure. Effect Sizes for the Retrospective Longitudinal Associations of Brain Volumes (A) and Neuropsychological Performance (B) With Olfaction in the Overall Sample of Mixed Cognitive Status.
Effect sizes are based on per interquartile range change in odor identification scores (i.e., 4). ** indicates associations at p < 0.01; * indicates associations at 0.01 < p < 0.05. CVLT = California Verbal Learning Test.
These results remained similar after additional adjustment for smoking status and APOE ε4 carrier status (data not shown).
Relationships Between Olfaction and Neuropsychological Performance
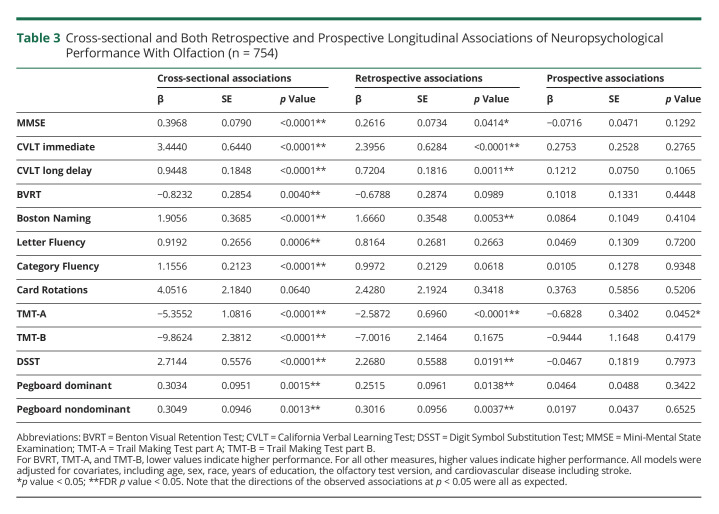
In the overall sample of mixed cognitive status, higher odor identification scores were cross-sectionally associated with higher performance on all neuropsychological measures of interest except the Card Rotations Test (Table 3). These significant associations also survived FDR-adjusted p < 0.05 and remained significant after exclusion of data points at and after the symptom onset of cognitive impairment or dementia (Table 3).
Table 3.
Cross-sectional and Both Retrospective and Prospective Longitudinal Associations of Neuropsychological Performance With Olfaction (n = 754)
Retrospectively, higher odor identification scores were associated with prior slower rates of decline in measures of mental status (MMSE), memory (CVLT immediate recall and long-delay free recall), language (Boston Naming Test), attention (TMT-A), processing speed (DSST), and manual dexterity (pegboard dominant and nondominant hand performance) (Table 3 and Figure B). These associations survived FDR-adjusted p < 0.05 except for the MMSE. After exclusion of data points at and after the symptom onset of cognitive impairment or dementia, associations with rates of change in CVLT immediate recall, TMT-A, and pegboard nondominant hand performance remained statistically significant, and associations with changes in CVLT immediate recall and pegboard nondominant hand performance survived FDR-adjusted p < 0.05 (eTable 3, links.lww.com/WNL/C514). Prospectively, higher odor identification scores were associated with a slower rate of decline in attention (TMT-A) in the overall sample of mixed cognitive status (β ± SE = −0.6828 ± 0.3402, p = 0.0452) and remained significant after exclusion of data points at and after the symptom onset of cognitive impairment or dementia (β ± SE = −0.8264 ± 0.3392, p = 0.0151) (eTable 3 links.lww.com/WNL/C514).
These results remained similar after additional adjustment for smoking status and APOE ε4 carrier status (data not shown).
Discussion
We investigated associations of olfactory function with cognition and brain volumes, extending prior neuroimaging studies by examining relationships with both retrospective and prospective changes over time. Our longitudinal study of a community-dwelling adult population established 3 important findings. First, we found that higher olfactory function is associated with slower rates of atrophy over time in specific brain areas, mainly frontal and temporal regions. Second, higher olfaction is associated with slower declines in several domains of neuropsychological function over time, including verbal memory, attention, and manual dexterity. Furthermore, attenuation of findings after exclusion of data points after the diagnosis of cognitive impairment suggests that the observed associations are driven, in part, by those who developed cognitive impairment or dementia.
Our neuroimaging findings add to the existing literature and further support the notion that olfaction is related to cognitive impairment. First, the specific gray matter regions identified in our longitudinal study belong to the central olfactory system and are also part of brain areas that typically show atrophy in AD, that is, AD signature regions. In both retrospective and prospective associations, atrophy of the hippocampus and entorhinal cortex is associated with olfaction, both of which are affected early in the AD pathologic process. Identified temporal areas, as well as frontal regions, such as the orbitofrontal cortex and insula, are also in line with prior cross-sectional findings of the associations between olfaction and MRI volumes in older adults.8,10,11,14,41 Second, prospectively, we also observe the association between olfaction and change in white matter volume in the temporal area. Previous studies have not examined the associations between olfaction and white matter volumes in specific lobes. Third, these findings are somewhat attenuated after exclusion of data points after cognitive impairment. Those subsequently impaired participants also have lower olfactory function than those who remained cognitively normal. This was in line with our previous report that lower odor identification scores were associated with incident MCI.6
Our neuroimaging findings also provide new insights into mechanisms underlying the previously reported relationship between olfaction and motor function.15,16 Possible mechanisms that connect olfactory deficits and motor impairment may include neurovascular burden, neuronal loss, and neuropathology, such as microglial dysfunction and amyloid and tau deposition, especially in brain areas important for both olfaction and motor function. Brain volumes in the temporal area, such as the hippocampus and entorhinal cortex, are found to be related to mobility decline and gait disturbance. The hippocampus is also shown as a shared neural substrate of slow gait and cognitive impairment.42-44 We also observed other areas, such as the thalamus in retrospective analysis and the supplementary motor area in prospective analysis, both of which play key roles in motor function and may also be involved in olfaction. Although olfactory processing bypasses the thalamus, the orbitofrontal cortex and the primary olfactory cortex are indirectly connected through the mediodorsal thalamus, which may modulate olfactory processing.45 Data on the role of the supplementary motor area in olfaction are limited; recent brain fMRI data have shown that brain activation in the supplementary motor area in response to food odor stimuli is related to subsequent BMI change.46 Mitochondrial dysfunction may also be one of the mechanisms, which contributes to the loss of dopaminergic neurons in the olfactory bulb and also affects mobility decline.47,48 Future research is needed to further understand mechanisms that connect motor function and olfaction.
Among several domains of neuropsychological function examined, we observed widespread cross-sectional associations in almost all domains except visuospatial ability. Our cross-sectional findings for global mental status, attention, executive function, memory, fluency, psychomotor speed, and manual dexterity are consistent with the previous literature.2,17-23 Longitudinally, we only found associations in specific domains, including changes in memory, attention, psychomotor speed, and manual dexterity. Atrophy in specific brain areas, such as frontal and temporal areas, may be a shared mechanism underlying the relationships between olfaction and domain-specific neuropsychological changes over time. Previous research has also suggested that cholinergic neurons in the basal forebrain may explain the relationship between olfaction and attentional ability.17 A line of research has suggested that the impairment of the cholinergic basal forebrain is related to both olfactory dysfunction and cognitive impairment, especially attention and memory.49 Use of anticholinergic medication has been associated with greater brain atrophy and cognitive decline among cognitively normal older adults.50 Notably, prospective longitudinal associations were not as strong as retrospective associations. The lack of prospective longitudinal associations may be due to a relatively shorter follow-up time with fewer visits compared with the data available for retrospective analysis. Note, however, that we found prospective associations with brain atrophy in specific frontal and temporal areas. As neuroimaging measures are more stable over time, they may be more sensitive than neuropsychological performance measures. It is also possible that changes in neuroimaging markers are evident before performance changes.
This study has limitations. The BLSA sample tends to be healthier than the general older population because of its inclusion and exclusion criteria at the study entry and voluntary participation. Second, the prospective follow-up is relatively shorter than the retrospective period because of the more recent introduction of olfactory testing. This study also has several strengths. The study population is well characterized with rigorous prospective adjudication of cognitive impairment and dementia. This allows us to investigate the role of cognitive status in the relationship between olfaction and brain outcomes. Second, we examined regional gray matter and white matter across the 4 lobes and the cerebellum, providing information on the spatial distribution of associations of MRI volumes with olfactory function. Furthermore, we examined various domains of neuropsychological function, including global mental status, cognition, and manual dexterity.
In conclusion, among community-dwelling older adults including cognitively impaired and normal individuals, olfactory function was related to brain atrophy of specific areas and neuropsychological changes in specific domains over time. Future longitudinal studies with longer follow-ups are needed to understand whether reduced olfactory function precedes cognitive changes and whether these associations are mediated through brain atrophy.
Acknowledgment
This study was supported by the Intramural Research Program of the National Institute on Aging, Baltimore, MD.
Glossary
- AD
Alzheimer disease
- BLSA
Baltimore Longitudinal Study of Aging
- CVLT
California Verbal Learning Test
- DSM-III-R
Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised
- DSST
Digit Symbol Substitution Test
- FDR
false discovery rate
- LME
linear mixed effect
- MCI
mild cognitive impairment
- MMSE
Mini-Mental State Examination
- MPRAGE
magnetization-prepared rapid gradient echo
- MUSE
MUlti-atlas region Segmentation using Ensembles
- TMT-A
Trail Making Test part A
- TMT-B
Trail Making Test part B
Appendix. Authors
Study Funding
The authors report no targeted funding.
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Attems J, Walker L, Jellinger KA. Olfaction and aging: a mini-review. Gerontology. 2015;61(6):485-490. doi: 10.1159/000381619 [DOI] [PubMed] [Google Scholar]
- 2.Windon MJ, Kim SJ, Oh ES, Lin SY. Predictive value of olfactory impairment for cognitive decline among cognitively normal adults. Laryngoscope. 2020;130(4):840-847. doi: 10.1002/lary.28166 [DOI] [PubMed] [Google Scholar]
- 3.Benarroch EE. Olfactory system: functional organization and involvement in neurodegenerative disease. Neurology. 2010;75(12):1104-1109. doi: 10.1212/wnl.0b013e3181f3db84 [DOI] [PubMed] [Google Scholar]
- 4.Parvand M, Rankin CH. Is there a shared etiology of olfactory impairments in normal aging and neurodegenerative disease? J Alzheimer's Dis. 2020;73:1-21. doi: 10.3233/jad-190636 [DOI] [PubMed] [Google Scholar]
- 5.Tu L, Lv X, Fan Z, Zhang M, Wang H, Yu X. Association of odor identification ability with amyloid-beta and tau burden: a systematic Review and meta-analysis. Front Neurosci. 2020;14:586330. doi: 10.3389/fnins.2020.586330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tian Q, Bilgel M, Moghekar AR, Ferrucci L, Resnick SM. Olfaction, cognitive impairment, and PET biomarkers in community-dwelling older adults. J Alzheimers Dis. 2022;86(3):1275-1285. doi: 10.3233/JAD-210636 [DOI] [PubMed] [Google Scholar]
- 7.Baek MS, Cho H, Lee HS, Lee JH, Ryu YH, Lyoo CH. Effect of A/T/N imaging biomarkers on impaired odor identification in Alzheimer's disease. Sci Rep. 2020;10(1):11556. doi: 10.1038/s41598-020-68504-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dintica CS, Marseglia A, Rizzuto D, et al. Impaired olfaction is associated with cognitive decline and neurodegeneration in the brain. Neurology. 2019;92(7):e700–e709. doi: 10.1212/wnl.0000000000006919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Growdon ME, Schultz AP, Dagley AS, et al. Odor identification and Alzheimer disease biomarkers in clinically normal elderly. Neurology. 2015;84(21):2153-2160. doi: 10.1212/wnl.0000000000001614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vassilaki M, Christianson TJ, Mielke MM, et al. Neuroimaging biomarkers and impaired olfaction in cognitively normal individuals. Ann Neurol. 2017;81(6):871-882. doi: 10.1002/ana.24960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshii F, Onaka H, Kohara S, Ryo M, Takahashi W. Association of smell identification deficit with Alzheimer's disease assessment scale-cognitive subscale, Japanese version scores and brain atrophy in patients with dementia. Eur Neurol. 2019;81(3-4):145-151. doi: 10.1159/000501311 [DOI] [PubMed] [Google Scholar]
- 12.Kjelvik G, Saltvedt I, White LR, et al. The brain structural and cognitive basis of odor identification deficits in mild cognitive impairment and Alzheimer's disease. BMC Neurol. 2014;14(1):168. doi: 10.1186/s12883-014-0168-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smitka M, Puschmann S, Buschhueter D, et al. Is there a correlation between hippocampus and amygdala volume and olfactory function in healthy subjects? Neuroimage. 2012;59(2):1052-1057. doi: 10.1016/j.neuroimage.2011.09.024 [DOI] [PubMed] [Google Scholar]
- 14.Devanand DP, Tabert MH, Cuasay K, et al. Olfactory identification deficits and MCI in a multi-ethnic elderly community sample. Neurobiol Aging. 2010;31(9):1593-1600. doi: 10.1016/j.neurobiolaging.2008.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tian Q, Resnick SM, Studenski SA. Olfaction is related to motor function in older adults. J Gerontol A Biol Sci Med Sci. 2017;72(8):1067-1071. doi: 10.1093/gerona/glw222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan Y, Li C, Luo Z, Simonsick EM, Shiroma EJ, Chen H. Olfaction and physical functioning in older adults: a longitudinal study. J Gerontol A Biol Sci Med Sci. 2022;77(8):1612-1619. doi: 10.1093/gerona/glab233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uchida S, Shimada C, Sakuma N, Kagitani F, Kan A, Awata S. The relationship between olfaction and cognitive function in the elderly. J Physiol Sci. 2020;70(1):48. doi: 10.1186/s12576-020-00777-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi JS, Hur K, Chow M, Shen J, Wrobel B. Olfactory dysfunction and cognition among older adults in the United States. Int Forum Allergy Rhinol. 2018;8(5):648-654. doi: 10.1002/alr.22078 [DOI] [PubMed] [Google Scholar]
- 19.Schubert CR, Cruickshanks KJ, Fischer ME, et al. Odor identification and cognitive function in the Beaver Dam Offspring Study. J Clin Exp Neuropsychol. 2013;35(7):669-676. doi: 10.1080/13803395.2013.809701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Makizako M, Makizako H, Doi T, et al. Olfactory identification and cognitive performance in community-dwelling older adults with mild cognitive impairment. Chem Senses. 2014;39(1):39-46. doi: 10.1093/chemse/bjt052 [DOI] [PubMed] [Google Scholar]
- 21.Yang X, Si J, Lin S, et al. Olfactory function, genetic predisposition, and cognitive performance in Chinese adults. Curr Alzheimer Res. 2021;18(14):1093-1103. doi: 10.2174/1567205019666211222151851 [DOI] [PubMed] [Google Scholar]
- 22.Tebrugge S, Winkler A, Gerards D, et al. Olfactory function is associated with cognitive performance: results of the Heinz Nixdorf Recall Study. J Alzheimer's Dis. 2018;63(1):319-329. doi: 10.3233/jad-170863 [DOI] [PubMed] [Google Scholar]
- 23.Yahiaoui-Doktor M, Luck T, Riedel-Heller SG, Loeffler M, Wirkner K, Engel C. Olfactory function is associated with cognitive performance: results from the population-based LIFE-Adult-Study. Alzheimer's Res Ther. 2019;11(1):43. doi: 10.1186/s13195-019-0494-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sohrabi HR, Bates KA, Weinborn MG, et al. Olfactory discrimination predicts cognitive decline among community-dwelling older adults. Transl Psychiatry. 2012;2(5):e118. doi: 10.1038/tp.2012.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swan GE, Carmelli D. Impaired olfaction predicts cognitive decline in nondemented older adults. Neuroepidemiology. 2002;21(2):58-67. doi: 10.1159/000048618 [DOI] [PubMed] [Google Scholar]
- 26.Shock NW, Gruelich R, Andres R, et al. Normal Human Aging: The Baltimore Longitudinal Study of Aging. : U.S. Government Printing Office; 1984. [Google Scholar]
- 27.Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G. ‘Sniffin’ Sticks': olfactory performance assessed by the combined testing of odour identification, odor discrimination and olfactory threshold. Chem Senses. 1997;22(1):39-52. doi: 10.1093/chemse/22.1.39 [DOI] [PubMed] [Google Scholar]
- 28.Doshi J, Erus G, Ou Y, et al. MUSE: MUlti-atlas region Segmentation utilizing Ensembles of registration algorithms and parameters, and locally optimal atlas selection. Neuroimage. 2016;127:186-195. doi: 10.1016/j.neuroimage.2015.11.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erus G, Doshi J, An Y, Verganelakis D, Resnick SM, Davatzikos C. Longitudinally and inter-site consistent multi-atlas based parcellation of brain anatomy using harmonized atlases. Neuroimage. 2018;166:71-78. doi: 10.1016/j.neuroimage.2017.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. J Psychiatr Res. 1975;12(3):189-198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 31.Delis D, Kramer J, Kaplan E, Ober B. California Verbal Learning Test, Research edition. Psychological Corporation; 1987. [Google Scholar]
- 32.Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test, 2nd ed. Lea & Febiger; 1983. [Google Scholar]
- 33.Benton AL. Differential behavioral effects in frontal lobe disease. Neuropsychologia. 1968;6(1):53-60. doi: 10.1016/0028-3932(68)90038-9 [DOI] [Google Scholar]
- 34.Newcombe F. Missile Wounds of the Brain: A Study of Psychological Deficits. Oxford University Press; 1969. [Google Scholar]
- 35.Wilson JR, De Fries JC, McClearn GE, Vandenberg SG, Johnson RC, Rashad MN. Cognitive abilities: use of family data as a control to assess sex and age differences in two ethnic groups. Int J Aging Hum Dev. 1975;6(3):261-276. doi: 10.2190/bbjp-xkug-c6ew-kyb7 [DOI] [PubMed] [Google Scholar]
- 36.Ekstrom RB, French JW, Harman HH, Dermen D. Manual for Kit of Factor Referenced Cognitive Tests. Educational Testing Service; 1976. [Google Scholar]
- 37.Reitan RM. Trail Making Test: Manual for Administration and Scoring. Reitan Neuropsychology Laboratory; 1992. [Google Scholar]
- 38.Wechsler D. Wechsler Adult Intelligence Scale-Revised, Vol 1. Psychological Corporation.; 1981. [Google Scholar]
- 39.Tiffin J. Purdue Pegboard Examiner Manual. Science Research Associates; 1968. [Google Scholar]
- 40.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Kokmen E, Tangelos EG. Aging, memory, and mild cognitive impairment. Int Psychogeriatr. 1997;9(S1):65-69. doi: 10.1017/s1041610297004717 [DOI] [PubMed] [Google Scholar]
- 41.Segura B, Baggio HC, Solana E, et al. Neuroanatomical correlates of olfactory loss in normal aged subjects. Behav Brain Res. 2013;246:148-153. doi: 10.1016/j.bbr.2013.02.025 [DOI] [PubMed] [Google Scholar]
- 42.Rosso AL, Verghese J, Metti AL, et al. Slowing gait and risk for cognitive impairment: the hippocampus as a shared neural substrate. Neurology. 2017;89(4):336-342. doi: 10.1212/wnl.0000000000004153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tian Q, Resnick SM, Davatzikos C, et al. A prospective study of focal brain atrophy, mobility and fitness. J Intern Med. 2019;286(1):88-100. doi: 10.1111/joim.12894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sakurai R, Bartha R, Montero-Odasso M. Entorhinal cortex volume is associated with dual-task gait cost among older adults with MCI: results from the Gait and Brain Study. J Gerontol Ser A. 2019;74(5):698-704. doi: 10.1093/gerona/gly084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tham WWP, Stevenson RJ, Miller LA. The role of the mediodorsal thalamic nucleus in human olfaction. Neurocase. 2011;17(2):148-159. doi: 10.1080/13554794.2010.504728 [DOI] [PubMed] [Google Scholar]
- 46.Han P, Chen H, Hummel T. Brain responses to food odors associated with BMI change at 2-year follow-up. Front Hum Neurosci. 2020;14:574148. doi: 10.3389/fnhum.2020.574148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tian Q, Mitchell BA, Zampino M, Fishbein KW, Spencer RG, Ferrucci L. Muscle mitochondrial energetics predicts mobility decline in well-functioning older adults: The Baltimore Longitudinal Study of Aging. Aging Cell. 2022;21(2):e13552. doi: 10.1111/acel.13552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paß T, Aßfalg M, Tolve M, et al. The impact of mitochondrial dysfunction on dopaminergic neurons in the olfactory bulb and odor detection. Mol Neurobiol. 2020;57(9):3646-3657. doi: 10.1007/s12035-020-01947-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ballinger EC, Ananth M, Talmage DA, Role LW. Basal forebrain cholinergic circuits and signaling in cognition and cognitive decline. Neuron. 2016;91(6):1199-1218. doi: 10.1016/j.neuron.2016.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Risacher SL, McDonald BC, Tallman EF, et al. Association between anticholinergic medication use and cognition, brain metabolism, and brain atrophy in cognitively normal older adults. JAMA Neurol. 2016;73(6):721-732. doi: 10.1001/jamaneurol.2016.0580 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data from the BLSA will be available on request by proposal submission through the BLSA website (blsa.nih.gov). All requests are reviewed by the BLSA Data Sharing Proposal Review Committee and are also subject to approval from the NIH Institutional Review Board.