Abstract
It is widely known that GnRH plays a role in facilitating reproductive function via the HPG axis, and this was once believed to be its only function. However, over the last several decades important neuromodulatory roles of GnRH in multiple brain functions have been elucidated. Multiple GnRH isoforms and receptors have been detected outside the HPG-axis across different species. In this review, we focus on the human CNS where GnRH I and II isoforms and a functional GnRH I receptor have been isolated. We first describe the traditional understanding of GnRH within the hypothalamus and the pituitary and current clinical use of GnRH analogues. We then review the location and function of GnRH-producing neurons and receptors located outside the HPG axis. We next review the GnRH I and II neuron location and quantity and GnRH I receptor gene expression throughout the human brain, using the Allen Brain Map Atlas. This analysis demonstrates a wide expression of GnRH throughout the brain, including prominent expression in the basal forebrain and cerebellum. Lastly, we examine the potential role of GnRH in aging and inflammation and its therapeutic potential for neurodegenerative disease and spinal cord lesions.
Keywords: GnRH I, GnRH II, GnRH receptor, Aging, Neurodegenerative disease, Alzheimer’s disease
1. Introduction
Gonadotropin-releasing hormone (GnRH), formerly referred to as the luteinizing hormone-releasing hormone (LHRH), is produced and secreted primarily by the hypothalamus in a pulsatile fashion, and acts on the anterior pituitary gland to release luteinizing hormone (LH) and follicle stimulating hormone (FSH). These two gonadotropins have downstream effects on the peripheral reproductive organs essential for maturation and fertility (reviewed in Veldhuis, 1996). In addition to this traditional understanding of GnRH functioning in reproduction, roles for GnRH in CNS functioning are beginning to be appreciated (Jones, 1987; Casoni et al., 2016; Skrapits et al., 2021; Martínez-Moreno et al., 2018). It has been discovered that GnRH receptors and GnRH producing neurons, originally thought to be concentrated within the hypothalamic-pituitary-gonadal (HPG) axis, are present in many other regions of the central nervous system (CNS) and the periphery (Jones, 1987; Wilson et al., 2006; Casoni et al., 2016; Skrapits et al., 2021).
In this review we will focus only on the human brain unless stated otherwise. We will review the traditional understanding of GnRH in regulating sex hormones through the HPG axis along with current clinical uses of GnRH analogue medications. We will then look at GnRH neurons and receptors external to the pituitary and the hypothalamus including a brain-wide quantification of GnRH gene expression, with a focus on their potential function. Lastly, we will explore the therapeutic potential of GnRH modulation in terms of neurodegenerative disease such as Alzheimer’s disease (AD) and on spinal cord lesions.
2. GnRH molecule
GnRH is a decapeptide without a carboxy-end-terminal cleaved from an 89-amino acid preprohormone (Fernald and White, 1999). It was one of the first hypothalamic-pituitary hormones to be found and sequenced. The neuropeptide was initially discovered in 1971 when it was isolated from porcine and subsequently in the ovine hypothalamus (Matsuo et al., 1976; Burgus et al., 1972). Since then over 25 different isoforms of GnRH have been isolated. The first two isoforms of GnRH are called GnRH I (pGlu-His-Trp-Ser-Tyr-Gly-Leu-Arg-Pro-Gly·NH2) and GnRH II (pGln-His-Trp-Ser-His-Gly-Trp-Tyr-Pro-Gly.NH2) which are encoded by a gene on chromosome 8p11 and 20p13, respectively, and differ from each other by three amino acids at positions 5, 7 and 8 (White et al., 1998). These two forms are most commonly found in primates including humans. The neurons that produce GnRH I also differ from those that produce GnRH II. GnRH I is predominantly found within the CNS while GnRH II is expressed more within the periphery especially within the kidney, prostate, ovaries and bone marrow (Cheng and Leung, 2005; White et al., 1998). Both hormones are believed to have important autocrine and/or paracrine regulatory functions. They both produced an increase in human chorionic gonadotropin (HCG) in first semester trophoblasts and placental tissue, with GnRH I having a more significant increase compared to GnRH II (Islami, 2001).
Similar to GnRH I, it was discovered in the brain of the rhesus macaque that GnRH II is also effective in stimulating the release of gonadotropes both in vitro and in vivo (Densmore and Urbanski, 2003; reviewed in Urbanski, 2012). One interesting contrast is that the neuronal response to estradiol differs: GnRH I gene expression is inhibited by estradiol. However, GnRH II gene expression is stimulated as the GnRH II gene promoter contains estradiol response elements. Thus, estradiol elicits a negative feedback on GnRH I neurons while it produces a positive feedback towards GnRH II neurons. It has been suggested based on work in animal models that GnRH I neurons likely play a role in the maintenance and modulation of pulsatile LH, while GnRH II neurons likely play a role in the generation of the preovulatory LH surge (Urbanski, 2012). However, it is unclear if the same feedback expression occurs in the human brain or if it holds true for all GnRH producing cells (Urbanski, 2012). GnRH II also has anti-proliferative tumor effects, especially in ovarian carcinomas (Choi et al., 2001; Cheng and Leung, 2005).
3. GnRH producing neurons within the hypothalamus
The hypothalamus was considered the only location for GnRH neurons for many years. GnRH I is the most common isoform found within the hypothalamus. At the embryonic level, GnRH neurons of the HPG axis originate at the olfactory placode and migrate to the hypothalamic region and eventually the median eminence (Schwanzel-Fukuda and Pfaff, 1989; Casoni et al., 2016; Whitlock et al., 2003; Whitlock, 2005). By 9 weeks gestation GnRH neurons are detectable in the hypothalamus, and the HPG axis is functional at 11.5 weeks gestation (Whitlock, 2005).
Within the hypothalamus, GnRH is produced in the medial preoptic area and the infundibular/arcuate nucleus from 1500 to 2000 neurons which are dispersed and not segregated into nuclei, but are functionally interconnected forming what is referred to as the GnRH pulse generator (Whitlock, 2005). The neuropeptide then descends into the median eminence through neural projections, established approximately at 16 weeks gestation, where it is released into the capillary bed of the hypophyseal portal system in a pulsatile manner (Antunes et al., 1978).
In humans, GnRH pulsatile activity was first detected during pituitary surgery in adults (Antunes et al., 1978). Currently, due to the ethical implications and technical difficulties of sampling hypophyseal blood, and the ~4 minute half-life of GnRH, measurement of LH, with a half-life of 23 hours, is generally regarded as a surrogate for GnRH pulse activity (Redding et al., 1973; Marques et al., 2000).
While there is evidence for GnRH neuron spontaneous/intrinsic autorhythmicity mediated by factors such as oscillatory intracellular calcium release, astroglial growth-hormone signaling, neuronal gap junctional communication and multiple neurotransmitters (Grumbach, 2002) there is now very strong evidence from animal models that GnRH pulsatile release is controlled by kisspeptin neurons projecting to GnRH neurons serving as an extrinsic pulse generator.
Kisspeptin is coded by the KiSS1 gene on the chromosome 1q32 and is a crucial regulator of puberty, sex hormone mediated gonadotropin release and fertility (Pinilla et al., 2012; Skorupskaite et al., 2014). The role of kisspeptin in control of gonadotropins was first discovered in 2003 by two independent research groups who found mutations in the kisspeptin gene to underlie idiopathic hypogonadotropic hypogonadism (De Roux et al., 2003) Kisspeptin neurons are located within the hypothalamus in the rostral preoptic area and the infundibular nucleus, but more upstream from the GnRH releasing neurons (Skorupskaite et al., 2014; Rometo et al., 2007; Hrabovszky et al., 2010; Hrabovszky et al., 2021). Kisspeptin is also found in other locations in lower concentrations, especially within the dentate gyrus of the hippocampus and the amygdala, where it may play a role in regulating memory and emotions. Kisspeptin neurons are sensitive to sex steroid feedback and metabolic cues which promotes its release when required. These neurons co-express neurokinin B and dynorphin (KNDy neurons), which via neurokinin B receptor and kappa opioid peptide receptor autosynaptically regulate pulsatile kisspeptin release, with neurokinin B being stimulatory and dynorphin inhibitory (Skorupskaite et al., 2014; Skrapits et al., 2015). Kisspeptin neurons make multiple connections within the GnRH neurons of the human infundibular stalk, the site of GnRH neurosecretion: axo-somatic, axo-dendritic and axo-axonal contacts (Skorupskaite et al., 2014; Hrabovszky et al., 2010). Kisspeptin, when released, binds to the KiSS1 receptor (KiSS1R), a G-protein coupled receptor (GPCR), located on the GnRH neurons, which activates phospholipase C thereby activating the secondary intracellular messengers, inositol trisphosphate and diacylglycerol. This in turn mediates calcium release and protein kinase C activation to mediate kisspeptin’s function and GnRH release from its neurons at the median eminence (Muir et al., 2001; Liu et al., 2008; Skorupskaite et al., 2014).
4. GnRH receptors
The anterior pituitary was considered the main location of GnRH receptors for many years, though it is now recognized that GnRH receptors are widespread throughout the brain. There are two types of GnRH receptors: GnRH I receptors are found in humans with a high affinity to both GnRH I and II ligands. The GnRH II receptor gene also continues to be expressed in humans, however, there is a frameshift in exon 1 and a premature stop codon in exon 2 (Pawson et al., 2003). Therefore, humans lack the appropriate coding sequence to produce a full-length GnRH II receptor protein due to these gene coding errors (Desaulniers et al., 2017). Other species, including chickens, amphibians, and fish, continue to express functional GnRH II receptors which have a high affinity for the GnRH II ligand compared to GnRH I. Due to this, GnRH II receptors are believed to have been evolutionarily silenced in humans since GnRH I receptors can carry out the effects of both GnRH I and II (Millar et al., 2004; Pawson et al., 2003; Desaulniers et al., 2017).
All GnRH receptors are rhodopsin-like G-protein coupled 7-transmembrane domain receptors that couples with the Gq/11 family, but unlike other GPCRs, they lack the intracellular carboxy terminal domain. In humans the gene that encodes the GnRH receptor I is located on chromosome 4, which is the only functional GnRH receptor (Kakar et al., 1992; Chi et al., 1993; Rispoli and Nett, 2005).
When GnRH is released into the hypophyseal portal system it acts on the extracellular domain of GnRH receptors in the anterior pituitary inducing a conformational change into an active state. This active conformation propagates signal transduction through the activation of phospholipase C resulting in the hydrolysis of membrane-bound phosphatidylinositol 4,5-bisphosphate (PIP2) to inositol 1,4,5-triphosphate (IP3) and diacylglycerol, which in turn mobilize intracellular calcium and activate protein kinase C, respectively. This then stimulates the biosynthesis and secretion of LH and FSH from the anterior pituitary (Pawson and McNeilly, 2005).
There are several ways these receptor activities are modulated. First of all, N-linked glycosylation may occur through the downstream effects of estradiol and inhibin exposure. Also, prolonged exposure to estradiol leads to more glycosylation. Glycosylation sites are believed to play a role in the stability of receptors, with fewer number of receptors being found upon hyperglycosylation (Davidson et al., 1995). Secondly, the density of GnRH receptors on the anterior pituitary modulates the concentration of gonadotropes released. This density is highest before ovulation as it is important for the preovulatory surge of LH. What regulates the density of GnRH receptors is unclear but it is essential in understanding the changes to pituitary sensitivity (Rispoli and Nett, 2005). Thirdly, sustained GnRH binding to its receptor causes desensitization, mediated by phosphorylation of the cytoplasmic carboxyl tail by GPCR kinases. A receptor modifier referred to as β-arrestin then binds to the phosphorylated domain and uncouples the G protein from its receptor leading to the receptor being clathrin-coated and subsequently internalized for degradation by the lysosomes or recycling back to the plasma membrane (Hislop et al., 2001). Since mammalian GnRH receptors lack an intracellular carboxy terminal, this internalization process occurs slower than in non-mammalian species, which also leads to slower desensitization (Rispoli and Nett, 2005; Flanagan and Manilall, 2017). Lastly, GnRH neurons contain their own neuromodulatory negative feedback mechanism through the autoreceptors located at the presynapse, which allows the neuropeptide to bind and downregulate its release which indirectly affects the stimulation of GnRH receptors within the anterior pituitary (Xu et al., 2008).
5. Current clinical applications of GnRH analogues
Due to the essential role GnRH I plays in reproductive function, GnRH analogues have a wide-array of uses in medicine including prostate cancer, breast/ovarian cancer, endometriosis and leiomyoma, central precocious puberty, hyperandrogenism/hirsutism, menorrhagia, chemical castration to reduce paraphilias in sex offenders, and in vitro fertilization (Wilson et al., 2007). Table 1 summarizes the current clinical applications of GnRH. Most of these effects are through chronic GnRH agonist treatment except for in vitro fertilization, which uses GnRH agonists acutely to produce a surge of FSH and LH to increase estrogen levels and facilitate ovulation (Wilson et al., 2007).
Table 1.
Current clinical applications of GnRH analogues.
| Clinical applications | Mechanism of action | References |
|---|---|---|
| Prostate cancer | Downregulate GnRH receptor expression in the pituitary → lowers FSH and LH → lowers testosterone levels and suppresses tumor growth | Huhtaniemi et al., 1985; Labrie et al., 1986; Ortmann et al., 2002; Wilson et al., 2007 |
| Breast/ovarian cancer | Downregulate GnRH receptor expression in the pituitary → lowers FSH and LH → reduce estrogen production → improves prognosis/hinders further growth | Nicholson and Walker, 1989; Ortmann et al., 2002; Wilson et al., 2007; |
| Endometriosis and uterine leiomyoma | Downregulate GnRH receptor expression in the pituitary → lowers FSH and LH → lowers estrogen → reduces the size of endometriomas and leiomyomas | Broekmans, 1996; Erickson and Ory, 1989; Ortmann et al., 2002; Wilson et al., 2007 |
| Central precocious puberty | Downregulate GnRH receptor expression in the pituitary → lowers FSH and LH → lowers estrogen and testosterone levels → offsets the early sexual development in females and males, respectively | Oostdijk et al., 1990; Mul and Hughes, 2008; Ortmann et al., 2002; Wilson et al., 2007 |
| Hyperandrogenism/hirsutism | Downregulate GnRH receptor expression in the pituitary → lowers FSH and LH → lowers testosterone levels → decreases signs and symptoms of hyperandrogenism/hirsutism | Wallach and Adashi, 1990; Ortmann et al., 2002; Wilson et al., 2007 |
| Menorrhagia | Downregulate GnRH receptor expression in the pituitary → lowers FSH and LH → lowers estrogen and progesterone levels → thins uterine layer | Candiani et al., 1990; Ortmann et al., 2002; Wilson et al., 2007 |
| Chemical castration for paraphilias in sex offenders | Downregulate GnRH receptor expression in the pituitary → lowers FSH and LH → lowers testosterone levels → reduce male libido and sexual activity | Rousseau et al., 1988; Ortmann et al., 2002; Wilson et al., 2007; |
| In vitro fertilization techniques (acute use) | Increased LH and FSH release by acute administration of GnRH agonist → increase estrogen and progesterone levels → facilitates ovulation | Gonen et al., 1990; Ortmann et al., 2002; Wilson et al., 2007; |
6. GnRH-producing neurons outside the HPG axis: prior studies
While GnRH producing neurons were once considered to be limited to the hypothalamus, it is now recognized that they are present throughout the brain, and recent evidence suggests there may actually be more GnRH neurons outside the hypothalamus than within it (Skrapits et al., 2021; Casoni et al., 2016; Martínez-Moreno et al., 2018). Although the GnRH neurons of the hypothalamus originated from the olfactory placode, Casoni et al. (2016) identified approximately 10,000 GnRH I neurons in the human fetal brain, only 2000 of which followed the migratory ventral route to the hypothalamus, while 8000 followed a previously unknown dorsal route. It is theorized that some of the GnRH neurons external to the HPG axis may originate from these neurons (Casoni et al., 2016).
The majority of GnRH neurons in the human CNS produce GnRH I. GnRH II neurons are also found within CNS of the human brain, but to a lesser degree compared to the periphery. Other than the structural difference between GnRH I and II, the functional similarities or differences remain unclear, with the exception of gonadotrope stimulation seen from both GnRH I and II (Urbanski, 2012). White et al. (1998) reported that within the human brain GnRH II is distinctly found in the amygdala, caudate, hippocampus and the thalamus.
Recently, it was found that approximately 150,000 to 200,000 GnRH I cell bodies were scattered throughout the basal ganglia and basal forebrain, with most of them being found within the putamen along with others being located at the nucleus accumbens, caudate, globus pallidus, the ventral pallidum, nucleus of the stria terminalis, and a few also in the nucleus basalis of Meynert. Due to the large number of GnRH I neurons they are suspected not to have originated from the olfactory placode region (Skrapits et al., 2021).
Importantly, Skrapits et al. (2021) discovered that a majority of extrahypothalamic GnRH neurons, including those in the basal forebrain are cholinergic and contains the cholinergic marker enzyme choline acetyltransferase (ChAT). Therefore, they have the capabilities to co-release both GnRH and the neurotransmitter acetylcholine (Arendt et al., 1983; Skrapits et al., 2021). They represent the following proportion of the adult human forebrain that was sampled post-mortem: 34.9 % of all cholinergic neurons in the putamen, 6.3 % in the nucleus accumbens, 1.8 % in the head of the caudate, 3.6 % in the nucleus basalis meynert, and 28.4 % in the globus pallidus. Upon further analysis they found that GnRH receptors were expressed selectively in cholinergic interneurons. They proposed that GnRH may act locally on these autoreceptors modulating the activity of cholinergic neurons, and alteration to this modulatory system may play a role in the progression of neurodegenerative such as AD (Skrapits et al., 2021). These findings were only seen in human brains and are not found on rat brains (Skrapits et al., 2021). Cholinergic GnRH neurons are not limited to the basal forebrain and striatum, as approximately 34.6 % of hypothalamic GnRH neurons also contained ChAT (Skrapits et al., 2021). Therefore, cholinergic GnRH neurons are widespread though their function remains unclear.
7. GnRH-producing neurons: brain-wide gene expression
To complement individual studies of GnRH neuron location and function we utilized the Allen Brain Atlas to quantify GnRH I and II mRNA expression across the entire brain. As detailed in Fig. 1 and the subregions presented in Appendix A, the Allen Brain Atlas provides microarray data from multiple adult control brains. To evaluate GnRH 1 and 2 mRNA expression, we extrapolated data from the Allen Brain and compared GnRH I and GnRH II gene expression across multiple brain regions using microarray data. Three different genetic probes targeting distinct exons were used to measure the amount of GnRH I or GnRH II cRNA in each brain region. The amount of detected GnRH I or GnRH II cRNA for each brain region was normalized to the average detected level of GnRH I or GnRH II cRNA across the entire brain in the form of a normalized z-score for each genetic probe. The normalized z-score across all three genetic probes and across all six donor brains was averaged to produce an average z-score for GnRH I and GnRH II genetic expression in each brain region, providing a measure of GnRH gene expression within each brain region relative to the whole brain mean.
Fig. 1.
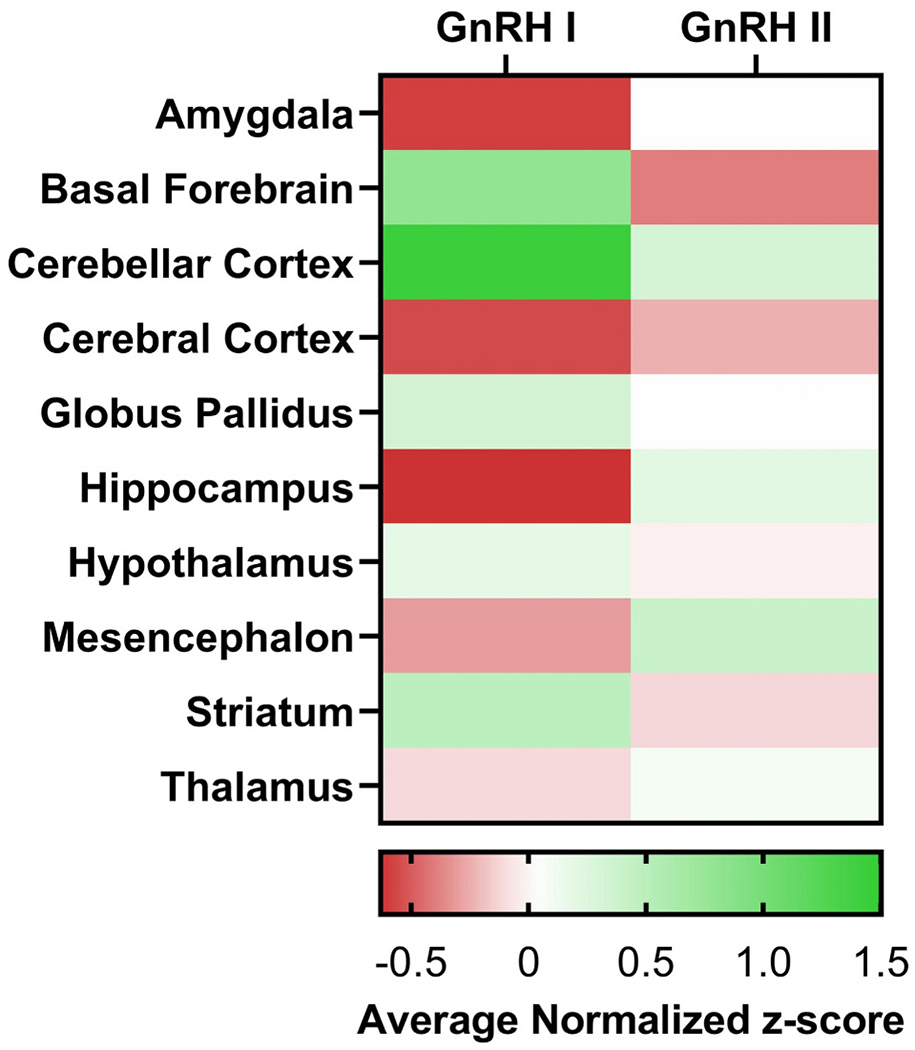
Expression of GnRH neuronal isoforms in the adult human brain. The data is presented in z-scores. Value of 0 means average expression, negative means underexpression, and positive numbers means overexpression, compared to the brain as a whole.
Overall, both GnRH I and II are widely expressed within the human brain. As expected based on traditional understanding of the role of GnRH in the HPG axis, GnRH I is highly expressed in the hypothalamus, especially the arcuate nucleus. GnRH I is also expressed in basal forebrain and striatum, in accord with recent studies summarized above (Skrapits et al., 2021). In addition, there is prominent GnRH I and II expression in the cerebellum. While cerebellar GnRH I has been reported previously (Martínez-Moreno et al., 2018), such high expression, exceeding that found in hypothalamus, has not to our knowledge been reported. GnRH I expression in the choroid plexus of the lateral ventricle may relate to GnRH I found in the CSF, discussed below. Increased GnRH I expression in dentate gyrus of hippocampus in humans may be a novel finding, and is potentially of great interest given recent appreciation of the role of hippocampal GnRH receptors in memory and other functions (Skrapits et al., 2021; Wilson et al., 2006). It has been noted previously that the endogenous source of GnRH acting on hippocampal neurons was unknown (Skrapits et al., 2021); current findings suggest the dentate gyrus as a possible local source. In this respect, it is relevant that, like hypothalamic neural progenitor cells, hippocampal neural progenitor cells are capable of differentiating into GnRH neurons in vitro, though to our knowledge this has not been demonstrated in vivo (Markakis, 2004). Also notable is that GnRH II expression is highest in the Edinger–Westphal nucleus and oculomotor nucleus. The significance of this finding is uncertain, but it may relate to interplay between autonomic and reproductive function. Other regions of above average GnRH I expression are the epithalamus, hippocampus, and the pons (please see the GnRH I and II expressions of the subregions in Appendix A).
8. GnRH receptors outside the HPG axis: prior studies
Over the last four decades widespread expression of GnRH receptors external to the HPG axis have been investigated (Cheung and Wong, 2008; Millar et al., 2004). Expression of the GnRH receptors external to the HPG axis was initially suspected when GnRH depolarized the sympathetic ganglia of a bullfrog (Jones, 1987). Since then the use of immunohistochemistry has detected GnRH receptors in different areas of the human brain. Table 2 summarizes these findings and its potential function (Martínez-Moreno et al., 2018; Skrapits et al., 2021).
Table 2.
Prior reports of GnRH receptors possibly functioning outside the HPG axis in humans and rat models.
| Brain regions | Description | References |
|---|---|---|
| Basal ganglia/basal forebrain structures | GnRH autoreceptors found in cholinergic GnRH neurons may modify emotions, motor functions and cognition. | Skrapits et al., 2021 |
| Cerebral cortex | Widespread expression of receptors alludes to the broad neuromodulatory role of GnRH. | Martínez-Moreno et al., 2018; Skinner et al., 1995; Quintanar et al., 2009 |
| Cerebellum | Receptors within the middle lobe of the human cerebellum may play a role in modulating motor behavior and cognition. | Skinner et al., 2009; Martínez-Moreno et al., 2018 |
| Hippocampus | Receptors within the CA1-CA3 regions may modify memory, emotions and reproduction. | Yang et al., 1999; Prange-Kiel et al., 2008; Martínez-Moreno et al., 2018 |
| Spinal cord | Receptors within the gray matter of the motor neurons may play a role in modulating motor function. | Guzmän-Soto et al., 2012; Quintanar et al., 2009; Quintanar et al., 2015; Quintanar et al., 2018; Díaz-Galindo et al., 2020; Martínez-Moreno et al., 2018 |
GnRH receptors in the hippocampus are of particular interest. A recent study revealed that the effects of hippocampal GnRH administered through cannulation enhanced estradiol secretion, caused mRNA overexpression of GnRH receptors and increased CA1 pyramidal cell excitability all within the hippocampal area of the amyloid-b (AB) induced rodent brains (Marbouti et al., 2020). Clinically cognitive benefits such as the prevention of AB-induced working memory decline, reduced anxiogenic behavior and better novel object recognition were observed in rats (Marbouti et al., 2020).
9. GnRH found within the cerebrospinal fluid
As shown in Fig. 1, Table 2 and previously reported, not all GnRH receptor areas produce GnRH, which initiated the theory that GnRH from other brain areas may travel through the CSF to brain regions with GnRH receptors to elicit a response (Martínez-Moreno et al., 2018). Although no human studies have been conducted, it was discovered in ewes that GnRH is also released from the hypothalamic median eminence, in addition to entering the hypophyseal portal system circulation, and is present in significant quantities in the third ventricle. In addition, as noted above, GnRH is expressed by the choroid plexus of the ventricle and would be expected to be released into CSF, allowing it to act on areas of the brain distant from its production source (Skinner et al., 2009; Skinner et al., 1995).
10. GnRH secretion changes over the lifespan
The GnRH pulse generator of the HPG axis is functional 12 days after birth. GnRH induced LH and FSH episodic discharge lasts 6 months for male infants and approximately 12 months for female infants. Afterwards it tapers off, becoming quiescent till puberty (Grumbach, 2002). This time period is referred to as the prepubertal hiatus which lasts almost a decade and is due to the intrinsic-CNS inhibitory mechanism of the GnRH I pulse generator mediated through the neurotransmitter GABA (Waldhauser et al., 1981; Zegher et al., 1992; Veldhuis, 1996; Grumbach, 2002).
During the onset of puberty this inhibition wanes resulting in the disinhibition of the GnRH I pulse generator. The amplitude and frequency of GnRH I nocturnal pulsation increase exponentially when compared to the prepubertal stage, leading to LH and FSH pulsations. In males, this leads to testicular secretion of androgens and enlargement in the size of testicles. In females, production of ovarian secretion of estradiol, progesterone, and ovarian androgens occurs. After puberty, females undergo monthly fluctuations in GnRH, LH and FSH that regulate ovulatory menstrual cycles (Veldhuis, 1996).
Although the precise mechanism which reactivates the HPG axis during puberty with pulsatile release of GnRH, LH and FSH is unknown, the peptide hormones kisspeptin, is part of the hypothalamic neuronal system believed to play a role in facilitating and triggering this process (Hrabovszky et al., 2010; Hrabovszky et al., 2021). Kisspeptin is an endogenous ligand of the GPCR expressed GPR54 gene (De Roux et al., 2003). It has been shown in human studies that a mutation at the receptor can produce hypogonadotropic hypogonadism, otherwise known as Kallman syndrome. These findings suggest that kisspeptin is essential for the onset of puberty and regulates the reproductive system in humans (Uenoyama et al., 2019; De Roux et al., 2003).
11. GnRH and reproductive aging physiology
With reproductive senescence the normal physiologic pulsatile nature of GnRH, LH and FSH secretions are disrupted in both men and women (Hall et al., 2000). Andropause in men leads to a gradual loss of testicular hormone production and reproductive function, with a corresponding progressive increase in circulating gonadotropin concentrations with the loss of testicular feedback inhibition on the hypothalamus and pituitary. In women, menopause drastically reduces the negative feedback of ovarian hormones (estrogens, progestagens, inhibins) on the HPG axis resulting in significant elevations in circulating LH and FSH concentrations. Menopause is associated with hot flashes, sleep problems and/or mood changes (Sturdee, 2008). Reports of GnRH I alterations in menopause include description of long-lasting increased GnRH production and release, though some studies reported declining GnRH I concentrations in post-menopausal women compared to the start of menopause (Hall et al., 2000). This declining trend in GnRH I secretion may be attributed to age-related senescence of hypothalamic astrocytes which contain high peroxidase activity, a marker of apoptosis, and microglia. Astrocytes, as mentioned, are responsible for facilitating GnRH release in the hypothalamus, however, age-related astrocytic dysfunction may impair its release (Dai et al., 2020). Dysfunctional regulation of GnRH release by kisspeptin neurons, which also project to vasomotor center, has been shown to cause the menopausal symptoms of hot flashes; drugs to treat hot flashes based on this mechanism are currently undergoing evaluation in clinical trials with promising early results.
12. GnRH, aging and inflammation
It has recently been shown that GnRH neurons in the hypothalamus may be key mediators of aging. Hypothalamic GnRH neurons and hypothalamic stem cells destined to become GnRH neurons appear to be extremely sensitive to the effects of inflammation mediated through hypothalamic microglia (Li et al., 2012). Microglial-mediated inflammation, through IκB kinase-β (IKK-β) and nuclear factor κB (NF-κB), specifically downregulates GnRH-1 neurons resulting in low levels of GnRH I and signs of brain and body aging including cognitive decline. Hypothalamic microglial inflammation may play a role in human menopause (Butler et al., 2022). When hypothalamic inflammation is prevented by blocking these inflammatory mediators, or when exogenous GnRH is administered, significant slower progression of aging, and lifespan extension, were observed in mice, suggesting that hypothalamic GnRH I neuron death and dysfunction and decreased secretion of GnRH over time may be a critical process during aging (Zhang et al., 2013; Gabuzda and Yankner, 2013; Shi et al., 2020).
13. GnRH and Alzheimer’s disease
Alzheimer’s disease (AD) is an age-related neurodegenerative disease that affects approximately 15 % of the population over the age of 65. It is marked by progressive cognitive decline with early and prominent memory deficits. Neuropathologically, AD is defined by the presence of amyloid plaques and tau tangles, with early atrophy of basal forebrain and hippocampus visible on clinical neuroimaging (Raji et al., 2009). After menopause, the risk of AD rises significantly. This effect has been attributed to the dysregulation of the HPG axis (Atwood and Bowen, 2015) with loss of estrogen considered to play a major role. There is strong evidence from animal models that estrogen is neuroprotective, by maintaining neurons within the basal forebrain, cortex and especially hippocampus (McEwen, 2002; Gibbs and Aggarwal, 1998) However, there is also strong evidence that post-reproduction, elevated levels of luteinizing hormone (LH) and FSH - which are controlled by GnRH - promote neurodegeneration (Atwood and Bowen, 2015), and gonadotropin elevation may be more important than loss of estrogen in mediating neurodegeneration. LH and FSH are elevated in serum and CSF of AD patients as compared to controls, and cortical and hippocampal neurons with LH receptors follow a degenerative pattern after menopause (Rao, 2016). Decreasing LH improves cognitive performance and decreases amyloid deposition and tau phosphorylation in multiple animal models of AD (Bowen et al., 2004; Wilson et al., 2007). LH is considered toxic to the brain because it serves as a signal for post-mitotic neurons to attempt to enter the cell cycle, which leads to cell death. This theory, termed the Cell Cycle Theory of Aging and AD is reviewed by Herrup (2012) and Atwood and Bowen (2015). GnRH agonists/antagonists are one method for decreasing circulating gonadotropin concentrations in humans, and this motivated a clinical trial of GnRH analogues in AD (Bowen et al., 2015; Butler et al., 2021).
13.1. Therapeutic potential of GnRH modulation
13.1.1. GnRH analogues to treat Alzheimer’s disease
GnRH agonists initially have a stimulatory effect on LH and FSH along with subsequent estrogen and/or testosterone release, however, the continuous administration of the drug leads to it becoming a potent antagonist that downregulates GnRH receptors on the anterior pituitary and thereby LH and FSH and sex hormone secretion (Wilson et al., 2007). Reduction or alteration of gonadotropin concentrations is the basis for clinical uses of GnRH analogues listed in Table 1. In postmenopausal woman, who already have low estrogen levels, the main effect of GnRH analogues is to lower LH and FSH. As noted above, lowering LH and FSH reduces amyloid, tau and neurodegeneration in animal models of AD. To investigate this effect in humans, a 48-week randomized double-blind placebo-controlled clinical trial was conducted to test the effect of the GnRH analogue leuprolide acetate (LA) in women (n = 109) with mild to moderate AD (Bowen et al., 2015). Upon primary analysis, although no significant differences were observed between the high-dose LA (22.5 mg/3 months), low-dose (11.5 mg/3 months) LA and the placebo group, a trend towards clinical improvement was noted with the high dose LA. In a planned subgroup analysis involving the 70 % of women who were also taking the acetylcholinesterase inhibitor donepezil, a standard therapy for AD, the effect of high dose LA was significant, and the progression of cognitive decline was halted. A potential mechanism of synergy between a GnRH analogue and a cholinesterase inhibitor - unknown at the time the trial was conducted - can now be understood in the context of the very recent demonstration that in humans but not rodents, the majority of GnRH neurons are also cholinergic (Skrapits et al., 2021). A follow-up trial called LUCINDA (Leuprolide + Cholinesterase Inhibition to reduce Neurologic Decline In Alzheimer’s) is currently being conducted to confirm a synergistic effect of combined GnRH and cholinergic modulation in women with AD (Butler et al., 2021).
13.1.2. Neurotrophic effects of GnRH on spinal cord injuries
GnRH also may have neurotrophic effects on spinal cord lesions. GnRH and mRNA receptor expression have been found more recently in human spinal cords with 90 % of receptor expression on the gray matter of motor neurons (Martínez-Moreno et al., 2018; Díaz-Galindo et al., 2020). GnRH agonist administered to rats with induced spinal cord injury (SCI) showed increased recovery of locomotor activity including gait, improved urinary dysfunction, decreased histopathological damage with increased number and caliber of neuronal axons at the level of the SCI (Calderón-Vallejo and Quintanar, 2012; Quintanar et al., 2009; Calderón-Vallejo et al., 2015). Another study reported improvement in external sphincter activity in neurogenic bowel for rats with induced SCI (Altamira-Camacho et al., 2019). In a human clinical trial administering GnRH agonists for individuals with chronic SCI improved sensitivity, motor activity and independence (Quintanar et al., 2018). Overall, these studies suggest the potential neurotrophic role GnRH has on the spinal cord. However, although the exact mechanism of action is yet to be deduced it has been proposed that GnRH agonist upregulates LH which is the basis of the neurotrophic effect observed (Martínez-Moreno et al., 2018; Quintanar et al., 2018; Calderón-Vallejo and Quintanar, 2012; Calderón-Vallejo et al., 2015).
14. Conclusion
In conclusion, as observed GnRH and its receptor gene expression are widespread within human central nervous systems and are multifaceted neuromodulators that not only play a pivotal role in reproduction but also in the aging processes, cognition and motor functions. Their roles within the human brain should be further investigated. A clinical trial of a GnRH analogue is underway as a potential treatment for AD. Additional study of GnRH systems is likely to advance therapeutic options in other human brain disorders.
Funding
This research was supported by NIH grant [R01AG05768].
Appendix A
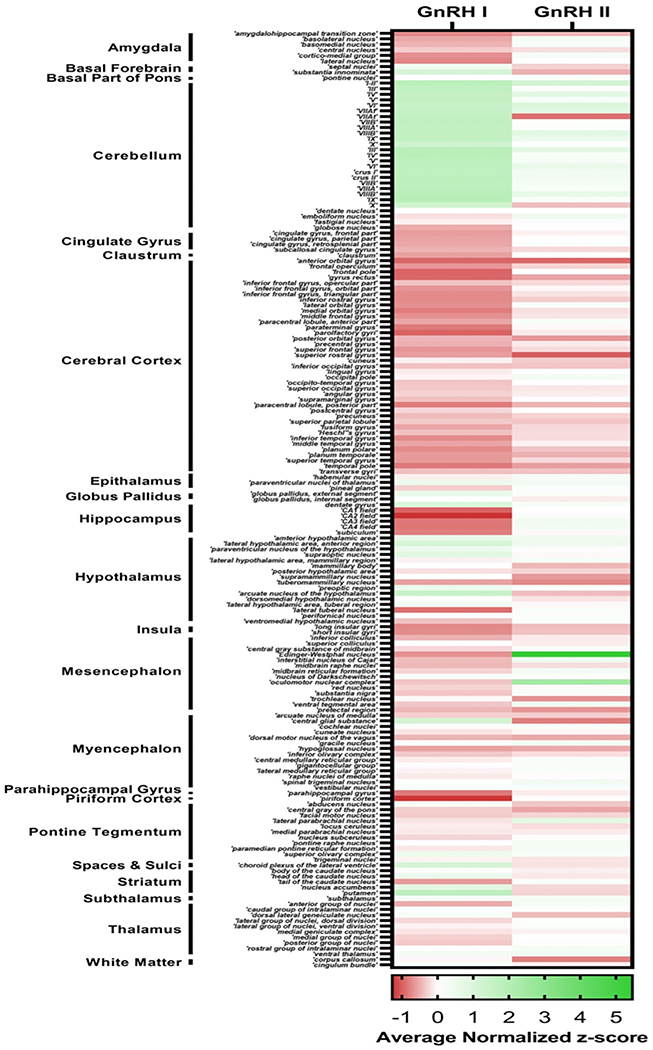
Footnotes
Declaration of competing interest
None.
References
- Altamira-Camacho M, Medina-Aguiñaga D, Cruz Y, Calderón-Vallejo D, Kovacs K, Rotondo F, Quintanar JL, 2019. Leuprolide acetate, a GnRH agonist, improves the neurogenic bowel in ovariectomized rats with spinal cord injury. Dig. Dis. Sci 65 (2), 423–430. 10.1007/s10620-019-05783-4. [DOI] [PubMed] [Google Scholar]
- Antunes JL, Carmel PW, Housepian EM, Ferin M, 1978. Luteinizing hormone-releasing hormone in human pituitary blood. J. Neurosurg 49 (3), 382–386. 10.3171/jns.1978.49.3.0382. [DOI] [PubMed] [Google Scholar]
- Arendt T, Bigl V, Arendt A, Tennstedt A, 1983. Loss of neurons in the nucleus basalis of meynert in Alzheimer’s disease, paralysis agitans and Korsakoff’s disease. Acta Neuropathol. 61 (2), 101–108. 10.1007/bf00697388. [DOI] [PubMed] [Google Scholar]
- Atwood CS, Bowen RL, 2015. The endocrine dyscrasia that accompanies menopause and andropause induces aberrant cell cycle signaling that triggers re-entry of post-mitotic neurons into the cell cycle, neurodysfunction, neurodegeneration and cognitive disease. Horm. Behav 76, 63–80. 10.1016/j.yhbeh.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen RL, Verdile G, Liu T, Parlow AF, Perry G, Smith MA, Martins RN, Atwood CS, 2004. Luteinizing hormone, a reproductive regulator that modulates the processing of amyloid-β precursor protein and amyloid-β deposition. J. Biol. Chem 279 (19), 20539–20545. 10.1074/jbc.m311993200. [DOI] [PubMed] [Google Scholar]
- Bowen RL, Perry G, Xiong C, Smith MA, Atwood CS, 2015. A clinical study of lupron depot in the treatment of women with Alzheimer’s disease: preservation of cognitive function in patients taking an acetylcholinesterase inhibitor and treated with high dose lupron over 48 weeks. J. Alzheimers Dis 44 (2), 549–560. 10.3233/jad-141626. [DOI] [PubMed] [Google Scholar]
- Broekmans FJ, 1996. GnRH agonists and uterine leiomyomas. Hum. Reprod 11 (Suppl. 3), 3–25. 10.1093/humrep/11.suppl_3.3. [DOI] [PubMed] [Google Scholar]
- Burgus R, Butcher M, Amoss M, Ling N, Monahan M, Rivier J, Fellows R, Blackwell R, Vale W, Guillemin R, 1972. Primary structure of the ovine hypothalamic luteinizing hormone-releasing factor (LRF). Proc. Natl. Acad. Sci 69 (1), 278–282. 10.1073/pnas.69.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler T, Goldberg JD, Galvin JE, Maloney T, Ravdin L, Glodzik L, de Leon MJ, Hochman T, Bowen RL, Atwood CS, 2021. Rationale, study design and implementation of the LUCINDA trial: leuprolide plus cholinesterase inhibition to reduce neurologic decline in Alzheimer’s. Contemp. Clin. Trials 107, 106488. 10.1016/j.cct.2021.106488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler T, Glodzik L, Wang XH, Xi K, Li Y, Pan H, Zhou L, Chiang G, Morim S, Wickramasuriya N, Tanzi E, Maloney T, Harvey P, Mao X, Razlighi RQ, Rusinek H, Shungu D, de Leon M, Atwood CS, Mozley PD, 2022. Greater hypothalamic microglial activation with aging in women as compared to men: potential relevance to sex and species differences in reproductive and biologic aging. Res. Square. 10.21203/rs.3.rs-1538708/v1. [DOI] [Google Scholar]
- Calderón-Vallejo D, Quintanar JL, 2012. Gonadotropin-releasing hormone treatment improves locomotor activity, urinary function and neurofilament protein expression after spinal cord injury in ovariectomized rats. Neurosci. Lett 515 (2), 187–190. 10.1016/j.neulet.2012.03.052. [DOI] [PubMed] [Google Scholar]
- Calderón-Vallejo D, Quintanar-Stephano A, Hernández-Jasso I, Jiménez-Hernández V, Ruiz-Ornelas J, Jiménez I, Quintanar JL, 2015. Functional and structural recovery of the injured spinal cord in rats treated with gonadotropin-releasing hormone. Neurochem. Res 40 (3), 455–462. 10.1007/s11064-014-1486-9. [DOI] [PubMed] [Google Scholar]
- Candiani GB, Vercellini P, Fedele L, Arcaini L, Bianchi S, Candiani M, 1990. Use of goserelin depot, a gonadotropin-releasing hormone agonist, for the treatment of menorrhagia and severe anemia in women with leiomyomata uteri. Acta Obstet. Gynecol. Scand 69 (5), 413–415. 10.3109/00016349009013304. [DOI] [PubMed] [Google Scholar]
- Casoni F, Malone SA, Belle M, Luzzati F, Collier F, Allet C, Hrabovszky E, Rasika S, Prevot V, Chédotal A, Giacobini P, 2016. Development of the neurons controlling fertility in humans: new insights from 3D imaging and transparent fetal brains. Development 143 (21), 3969–3981. 10.1242/dev.139444. [DOI] [PubMed] [Google Scholar]
- Cheng CK, Leung PCK, 2005. Molecular biology of gonadotropin-releasing hormone (GnRH)-I, GnRH-II, and their receptors in humans. Endocr. Rev 26 (2), 283–306. 10.1210/er.2003-0039. [DOI] [PubMed] [Google Scholar]
- Cheung LW, Wong AS, 2008. Gonadotropin-releasing hormone: GnRH receptor signaling in extrapituitary tissues. FEBS J. 275 (22), 5479–5495. 10.1111/j.1742-4658.2008.06677.x. [DOI] [PubMed] [Google Scholar]
- Chi L, Zhou W, Prikhozhan A, Flanagan C, Davidson J, Golembo M, Illing N, Millar R, Sealfon S, 1993. Cloning and characterization of the human GnRH receptor. Mol. Cell. Endocrinol 91 (1–2), R1–R6. 10.1016/0303-7207(93)90278-r. [DOI] [PubMed] [Google Scholar]
- Choi KC, Auersperg N, Leung PCK, 2001. Expression and antiproliferative effect of a second form of gonadotropin-releasing hormone in normal and neoplastic ovarian surface epithelial cells. J. Clin. Endocrinol. Metab 86 (10), 5075. 10.1210/jcem.86.10.8100. [DOI] [PubMed] [Google Scholar]
- Dai X, Hong L, Shen H, Du Q, Ye Q, Chen X, Zhang J, 2020. Estradiol-induced senescence of hypothalamic astrocytes contributes to aging-related reproductive function declines in female mice. Aging 12 (7), 6089–6108. 10.18632/aging.103008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson JS, Flanagan CA, Zhou W, Becker II, Elario R, Emeran W, Sealfon SC, Millar RP, 1995. Identification of N-glycosylation sites in the gonadotropin-releasing hormone receptor: role in receptor expression but not ligand binding. Mol. Cell. Endocrinol 107 (2), 241–245. 10.1016/0303-7207(94)03449-4. [DOI] [PubMed] [Google Scholar]
- De Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E, 2003. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc. Natl. Acad. Sci 100 (19), 10972–10976. 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Densmore VS, Urbanski HF, 2003. Relative effect of gonadotropin-releasing hormone (GnRH)-I and GnRH-II on gonadotropin release. J. Clin. Endocrinol. Metab 88 (5), 2126–2134. 10.1210/jc.2002-021359. [DOI] [PubMed] [Google Scholar]
- Desaulniers AT, Cederberg RA, Lents CA, White BR, 2017. Expression and role of gonadotropin-releasing hormone 2 and its receptor in mammals. Front. Endocrinol 8 10.3389/fendo.2017.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Galindo C, Calderón-Vallejo D, Hernández-Jasso I, Cervantes-García D, Martínez-Díaz D, Ibarra-Martínez D, Muñoz-Ortega M, Quintanar JL, 2020. Gonadotropin-releasing hormone receptor expression in human spinal cord. Neurochem. Res 46 (2), 165–170. 10.1007/s11064-020-03178-w. [DOI] [PubMed] [Google Scholar]
- Erickson LD, Ory SJ, 1989. GnRH analogues in the treatment of endometriosis. Obstet. Gynecol. Clin. N. Am 16 (1), 123–145. 10.1016/s0889-8545(21)00142-x. [DOI] [PubMed] [Google Scholar]
- Fernald RD, White RB, 1999. Gonadotropin-releasing hormone genes: phylogeny, structure, and functions. Front. Neuroendocrinol 20 (3), 224–240. 10.1006/frne.1999.0181. [DOI] [PubMed] [Google Scholar]
- Flanagan CA, Manilall A, 2017. Gonadotropin-releasing hormone (GnRH) receptor structure and GnRH binding. Front. Endocrinol 8 10.3389/fendo.2017.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabuzda D, Yankner BA, 2013. Inflammation links ageing to the brain. Nature 497 (7448), 197–198. 10.1038/nature12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB, Aggarwal P, 1998. Estrogen and basal forebrain cholinergic neurons: implications for brain aging and Alzheimer’s disease-related cognitive decline. Horm. Behav 34 (2), 98–111. 10.1006/hbeh.1998.1451. [DOI] [PubMed] [Google Scholar]
- Gonen Y, Balakier H, Powell W, Casper RF, 1990. Use of gonadotropin-releasing hormone agonist to trigger follicular maturation for in vitro fertilization. * Journal of Clinical Endocrinology & Metabolism 71 (4), 918–922. 10.1210/jcem-71-4-918. [DOI] [PubMed] [Google Scholar]
- Grumbach MM, 2002. The neuroendocrinology of human puberty revisited. Horm. Res. Paediatr 57 (2), 2–14. 10.1159/000058094. [DOI] [PubMed] [Google Scholar]
- Guzmán-Soto I, Salinas E, Hernández-Jasso I, Quintanar JL, 2012. Leuprolide acetate, a GnRH agonist, improves experimental autoimmune encephalomyelitis: a possible therapy for multiple sclerosis. Neurochem. Res 37 (10), 2190–2197. 10.1007/s11064-012-0842-x. [DOI] [PubMed] [Google Scholar]
- Hall JE, Lavoie HB, Marsh EE, Martin KA, 2000. Decrease in gonadotropin-releasing hormone (GnRH) pulse frequency with aging in postmenopausal women. J. Clin. Endocrinol. Metab 85 (5), 1794–1800. 10.1210/jcem.85.5.6612. [DOI] [PubMed] [Google Scholar]
- Herrup K, 2012. The contributions of unscheduled neuronal cell cycle events to the death of neurons in Alzheimer’s disease. Front. Biosci E4 (6), 2101–2109. 10.2741/e527. [DOI] [PubMed] [Google Scholar]
- Hislop JN, Everest HM, Flynn A, McArdle CA, 2001. Internalization of mammalian and non-mammalian GnRH receptors: uncoupling of dynamin-dependent internalization from MAP kinase signalling. Biochem. Soc. Trans 29 (3), A84. 10.1042/bst029a084. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Ciofi P, Vida B, Horvath MC, Keller E, Caraty A, Bloom SR, Ghatei MA, Dhillo WS, Liposits Z, Kallo I, 2010. The kisspeptin system of the human hypothalamus: sexual dimorphism and relationship with gonadotropin-releasing hormone and neurokinin B neurons. Eur. J. Neurosci 31 (11), 1984–1998. 10.1111/j.1460-9568.2010.07239.x. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Takäcs S, Rumpler É, Skrapits K, 2021. The human hypothalamic kisspeptin system: functional neuroanatomy and clinical perspectives. Handb. Clin. Neurol 180, 275–296. 10.1016/B978-0-12-820107-7.00017-3. [DOI] [PubMed] [Google Scholar]
- Huhtaniemi I, Nikula H, Rannikko S, 1985. Treatment of prostatic cancer with a gonadotropin-releasing hormone agonist analog: acute and long term effects on endocrine functions of testis tissue. * J. Clin. Endocrinol. Metab 61 (4), 698–704. 10.1210/jcem-61-4-698. [DOI] [PubMed] [Google Scholar]
- Islami D, 2001. Comparison of the effects of GnRH-I and GnRH-II on HCG synthesis and secretion by first trimester trophoblast. Mol. Hum. Reprod 7 (1), 3–9. 10.1093/molehr/7.1.3. [DOI] [PubMed] [Google Scholar]
- Jones SW, 1987. A muscarine-resistant M-current in C cells of bullfrog sympathetic ganglia. Neurosci. Lett 74 (3), 309–314. 10.1016/0304-3940(87)90315-6. [DOI] [PubMed] [Google Scholar]
- Kakar SS, Musgrove LC, Devor DC, Sellers JC, Neill JD, 1992. Cloning, sequencing, and expression of human gonadotropin releasing hormone (GnRH) receptor. Biochem. Biophys. Res. Commun 189 (1), 289–295. 10.1016/0006-291x(92)91556-6. [DOI] [PubMed] [Google Scholar]
- Labrie F, Dupont A, Bélanger A, St-Arnaud R, Giguère M, Lacourcière Y, MonfettE G, 1986. Treatment of prostate cancer with gonadotropin-releasing hormone agonists. Endocr. Rev 7 (1), 67–74. 10.1210/edrv-7-1-67. [DOI] [PubMed] [Google Scholar]
- Li J, Tang Y, Cai D, 2012. IKKβ/NF-κB disrupts adult hypothalamic neural stem cells to mediate a neurodegenerative mechanism of dietary obesity and pre-diabetes. Nat. Cell Biol 14 (10), 999–1012. 10.1038/ncb2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Lee K, Herbison AE, 2008. Kisspeptin excites gonadotropin-releasing hormone neurons through a phospholipase C/calcium-dependent pathway regulating multiple ion channels. Endocrinology 149 (9), 4605–4614. 10.1210/en.2008-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marbouti L, Zahmatkesh M, Riahi E, Shafiee Sabet M, 2020. GnRH protective effects against amyloid β-induced cognitive decline: a potential role of the 17β-estradiol. Mol. Cell. Endocrinol 518, 110985 10.1016/j.mce.2020.110985. [DOI] [PubMed] [Google Scholar]
- Markakis EA, 2004. Novel neuronal phenotypes from neural progenitor cells. J. Neurosci 24 (12), 2886–2897. 10.1523/jneurosci.4161-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques P, Skorupskaite K, George JT, et al. , 2000. Physiology of GNRH and gonadotropin secretion. [Updated 2018 Jun 19]. Available from:. In: Feingold KR, Anawalt B, Boyce A, et al. (Eds.), Endotext [Internet]. MDText.com, Inc., South Dartmouth (MA) https://www.ncbi.nlm.nih.gov/books/NBK279070/. [Google Scholar]
- Martínez-Moreno C, Calderón-Vallejo D, Harvey S, Arämburo C, Quintanar J, 2018. Growth hormone (GH) and gonadotropin-releasing hormone (GnRH) in the central nervous system: a potential neurological combinatory therapy? Int. J. Mol. Sci 19 (2), 375. 10.3390/ijms19020375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo H, Baba Y, Nair RM, Arimura A, Schally AV, 1976. THE structure of the porcine LH- and FSH-releasing hormone. The proposed amino acid sequence. Am. J. Obstet. Gynecol 125 (8), 1141. 10.1016/0002-9378(76)90821-8. [DOI] [PubMed] [Google Scholar]
- McEwen B, 2002. Estrogen actions throughout the brain. Recent Prog. Horm. Res 57 (1), 357–384. 10.1210/rp.57.1.357. [DOI] [PubMed] [Google Scholar]
- Microarray Data: Allen Brain Atlas: Human Brain. (n.d.). Allen Brain Atlas. Retrieved September 16, 2021, from https://human.brain-map.org.
- Millar RP, Lu ZL, Pawson AJ, Flanagan CA, Morgan K, Maudsley SR, 2004. Gonadotropin-releasing hormone receptors. Endocr. Rev 25 (2), 235–275. 10.1210/er.2003-0002. [DOI] [PubMed] [Google Scholar]
- Muir AI, Chamberlain L, Elshourbagy NA, Michalovich D, Moore DJ, Calamari A, Szekeres PG, Sarau HM, Chambers JK, Murdock P, Steplewski K, Shabon U, Miller JE, Middleton SE, Darker JG, Larminie CG, Wilson S, Bergsma DJ, Emson P, Harrison DC, 2001. AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J. Biol. Chem 276 (31), 28969–28975. 10.1074/jbc.m102743200. [DOI] [PubMed] [Google Scholar]
- Mul D, Hughes IA, 2008. The use of GnRH agonists in precocious puberty. Eur. J. Endocrinol 159 (suppl_1), S3–S8. 10.1530/eje-08-0814. [DOI] [PubMed] [Google Scholar]
- Nicholson RI, Walker KJ, 1989. Gn-RH agonists in breast and gynaecologic cancer treatment. J. Steroid Biochem 33 (4), 801–804. 10.1016/0022-4731(89)90496-2. [DOI] [PubMed] [Google Scholar]
- Oostdijk W, Hümmelink R, Odink RJH, Partsch CJ, Drop SLS, Lorenzen F, Sippell WG, van der Velde EA, Schultheiss H, 1990. Treatment of children with central precocious puberty by a slow-release gonadotropin-releasing hormone agonist. Eur. J. Pediatr 149 (5), 308–313. 10.1007/bf02171554. [DOI] [PubMed] [Google Scholar]
- Ortmann O, Weiss J, Diedrich K, 2002. Gonadotrophin-releasing hormone (GnRH) and GnRH agonists: mechanisms of action. Reprod. BioMed. Online 5, 1–7. 10.1016/s1472-6483(11)60210-1. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, McNeilly AS, 2005. The pituitary effects of GnRH. Animal Reproduction Science 88 (1–2), 75–94. 10.1016/j.anireprosci.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Pawson A, Morgan K, Maudsley S, Millar R, 2003. Type II gonadotrophin-releasing hormone (GnRH-II) in reproductive biology. Reproduction 271–278. 10.1530/rep.0.1260271. [DOI] [PubMed] [Google Scholar]
- Pinilla L, Aguilar E, Dieguez C, Millar RP, Tena-Sempere M, 2012. Kisspeptins and reproduction: physiological roles and regulatory mechanisms. Physiol. Rev 92 (3), 1235–1316. 10.1152/physrev.00037.2010. [DOI] [PubMed] [Google Scholar]
- Prange-Kiel J, Jarry H, Schoen M, Kohlmann P, Lohse C, Zhou L, Rune GM, 2008. Gonadotropin-releasing hormone regulates spine density via its regulatory role in hippocampal estrogen synthesis. Journal of Cell Biology 180 (2), 417–426. 10.1083/jcb.200707043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintanar JL, Salinas E, González R, 2009. Gonadotropin-releasing hormone receptor in spinal cord neurons of embryos and adult rats. Neurosci. Lett 461 (1), 21–24. 10.1016/j.neulet.2009.06.028. [DOI] [PubMed] [Google Scholar]
- Quintanar J, Díaz-Galindo C, Gómez-González B, Salinas E, Calderón-Vallejo D, Hernändez-Jasso I, Bautista E, 2015. Leuprolide acetate induces structural and functional recovery of injured spinal cord in rats. Neural Regen. Res 10 (11), 1819. 10.4103/1673-5374.170311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintanar JL, Díaz-Galindo C, Calderón-Vallejo D, Hernändez-Jasso I, Rojas F, Medina-Aguiñaga D, Olvera-Sandoval C, 2018. Neurological improvement in patients with chronic spinal cord injury treated with leuprolide acetate, an agonist of GnRH. Acta Neurobiol. Exp 78 (4), 352–357. 10.21307/ane-2018-034. [DOI] [PubMed] [Google Scholar]
- Raji CA, Lopez OL, Kuller LH, Carmichael OT, Becker JT, 2009. Age, alzheimer disease, and brain structure. Neurology 73 (22), 1899–1905. 10.1212/wnl.0b013e3181c3f293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao CV, 2016. Involvement of luteinizing hormone in Alzheimer disease development in elderly women. Reprod. Sci 24 (3), 355–368. 10.1177/1933719116658705. [DOI] [PubMed] [Google Scholar]
- Redding TW, Kastin AJ, Gonzalez-Barcena D, Coy DH, Coy EJ, Schalch DS, Schally AV, 1973. The half-life, metabolism and excretion of tritiated luteinizing hormone-releasing hormone (LH-RH) in man. J. Clin. Endocrinol. Metab 37 (4), 626–631. 10.1210/jcem-37-4-626. [DOI] [PubMed] [Google Scholar]
- Rispoli L, Nett T, 2005. Pituitary gonadotropin-releasing hormone (GnRH) receptor: structure, distribution and regulation of expression. Anim. Reprod. Sci 88 (1–2), 57–74. 10.1016/j.anireprosci.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Rometo AM, Krajewski SJ, Lou Voytko M, Rance NE, 2007. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J. Clin. Endocrinol. Metab 92 (7), 2744–2750. 10.1210/jc.2007-0553. [DOI] [PubMed] [Google Scholar]
- Rousseau L, Dupont A, Labrie F, Couture M, 1988. Sexuality changes in prostate cancer patients receiving antihormonal therapy combining the antiandrogen flutamide with medical (LHRH agonist) or surgical castration. Arch. Sex. Behav 17 (1), 87–98. 10.1007/bf01542054. [DOI] [PubMed] [Google Scholar]
- Schwanzel-Fukuda M, Pfaff DW, 1989. Origin of luteinizing hormone-releasing hormone neurons. Nature 338 (6211), 161–164. 10.1038/338161a0. [DOI] [PubMed] [Google Scholar]
- Shi C, Li G, Guo H, Liu X, 2020. Forkhead transcription factor FOXO1 is involved in hypoxia/reoxygenation-induced gonadotropin-releasing hormone decline. NeuroReport 31 (18), 1296–1301. 10.1097/wnr.0000000000001548. [DOI] [PubMed] [Google Scholar]
- Skinner DC, Malpaux B, Delaleu B, Caraty A, 1995. Luteinizing hormone (LH)-releasing hormone in third ventricular cerebrospinal fluid of the ewe: correlation with LH pulses and the LH surge. Endocrinology 136 (8), 3230–3237. 10.1210/endo.136.8.7628356. [DOI] [PubMed] [Google Scholar]
- Skinner DC, Albertson AJ, Navratil A, Smith A, Mignot M, Talbott H, Scanlan-Blake N, 2009. Effects of gonadotrophin-releasing hormone outside the hypothalamic-pituitary-reproductive axis. J. Neuroendocrinol 21 (4), 282–292. 10.1111/j.1365-2826.2009.01842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skorupskaite K, George JT, Anderson RA, 2014. The kisspeptin-GnRH pathway in human reproductive health and disease. Hum. Reprod. Update 20 (4), 485–500. 10.1093/humupd/dmu009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrapits K, Borsay B, Herczeg L, Ciofi P, Liposits Z, Hrabovszky E, 2015. Neuropeptide co-expression in hypothalamic kisspeptin neurons of laboratory animals and the human. Front. Neurosci 9 10.3389/fnins.2015.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrapits K, Sárvári M, Farkas I, Göcz B, Takács S, Rumpler V, Váczi V, Vastagh C, Rácz G, Matolcsy A, Solymosi N, Póliska S, Tóth B, Erdélyi F, Szabó G, Culler MD, Allet C, Cotellessa L, Prévot V, Hrabovszky E, 2021. The cryptic gonadotropin-releasing hormone neuronal system of human basal ganglia, elife 10. 10.7554/elife.67714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturdee DW, 2008. The menopausal hot flush—anything new? Maturitas 60 (1), 42–49. 10.1016/j.maturitas.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Uenoyama Y, Inoue N, Nakamura S, Tsukamura H, 2019. Central mechanism controlling pubertal onset in mammals: a triggering role of kisspeptin. Front. Endocrinol 10 10.3389/fendo.2019.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanski HF, 2012. Differential roles of GnRH-I and GnRH-II neurons in the control of the primate reproductive axis. Front. Endocrinol 3 10.3389/fendo.2012.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuis JD, 1996. Neuroendocrine mechanisms mediating awakening of the human gonadotropic axis in puberty. Pediatr. Nephrol 10 (3), 304–317. 10.1007/bf00866767. [DOI] [PubMed] [Google Scholar]
- Waldhauser F, Weisenbacher G, Frisch H, Pollak A, 1981. Pulsatile secretion of gonadotropins in early infancy. Eur. J. Pediatr 137 (1), 71–74. 10.1007/bf00441173. [DOI] [PubMed] [Google Scholar]
- Wallach EE, Adashi EY, 1990. Potential utility of gonadotropin-releasing hormone agonists in the management of ovarian hyperandrogenism. Fertil. Steril 53 (5), 765–779. 10.1016/s0015-0282(16)53508-0. [DOI] [PubMed] [Google Scholar]
- White RB, Eisen JA, Kasten TL, Fernald RD, 1998. Second gene for gonadotropin-releasing hormone in humans. Proc. Natl. Acad. Sci 95 (1), 305–309. 10.1073/pnas.95.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock KE, 2005. Origin and development of GnRH neurons. Trends Endocrinol. Metab 16 (4), 145–151. 10.1016/j.tem.2005.03.00. [DOI] [PubMed] [Google Scholar]
- Whitlock K, Wolf C, Boyce M, 2003. Gonadotropin-releasing hormone (gnrh) cells arise from cranial neural crest and adenohypophyseal regions of the neural plate in the zebrafish, Danio rerio. Dev. Biol 257 (1), 140–152. 10.1016/s0012-1606(03)00039-3. [DOI] [PubMed] [Google Scholar]
- Wilson AC, Salamat MS, Haasl RJ, Roche KM, Karande A, Meethal SV, Terasawa E, Bowen RL, Atwood CS, 2006. Human neurons express type I GnRH receptor and respond to GnRH I by increasing luteinizing hormone expression. Journal of Endocrinology. 191 (3), 651–663. 10.1677/joe.1.07047. [DOI] [PubMed] [Google Scholar]
- Wilson AC, Vadakkadath Meethal S, Bowen RL, Atwood CS, 2007. Leuprolide acetate: a drug of diverse clinical applications. Expert Opin. Investig. Drugs 16 (11), 1851–1863. 10.1517/13543784.16.11.1851. [DOI] [PubMed] [Google Scholar]
- Xu C, Roepke TA, Zhang C, Rønnekleiv OK, Kelly MJ, 2008. Gonadotropin-releasing hormone (GnRH) activates the M-current in GnRH neurons: an autoregulatory negative feedback mechanism? Endocrinology 149 (5), 2459–2466. 10.1210/en.2007-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Lu F, Wu J, Liu D, Hsieh W, 1999. Activation of gonadotropin-releasing hormone receptors induces a long-term enhancement of excitatory postsynaptic currents mediated by ionotropic glutamate receptors in the rat hippocampus. Neuroscience Letters 260 (1), 33–36. 10.1016/s0304-3940(98)00939-2. [DOI] [PubMed] [Google Scholar]
- Zegher FD, Devlieger H, Veldhuis JD, 1992. Pulsatile and sexually dimorphic secretion of luteinizing hormone in the human infant on the day of birth. Pediatr. Res 32 (5), 605–607. 10.1203/00006450-199211000-00025. [DOI] [PubMed] [Google Scholar]
- Zhang G, Li J, Purkayastha S, Tang Y, Zhang H, Yin Y, Li B, Liu G, Cai D, 2013. Hypothalamic programming of systemic ageing involving IKK-β, NF-κB and GnRH. Nature 497 (7448), 211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]


