Objective:
An understanding of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) transmission in schools is important. It is often difficult, using epidemiological information alone, to determine whether cases associated with schools represent multiple introductions from the community or transmission within the school. We describe the use of whole genome sequencing (WGS) in multiple schools to investigate outbreaks of SARS-CoV-2 in the pre-Omicron period.
Study Design:
School outbreaks were identified for sequencing by local public health units based on multiple cases without known epidemiological links. Cases of SARS-CoV-2 from students and staff from 4 school outbreaks in Ontario underwent WGS and phylogenetic analysis. The epidemiological clinical cohort data and genomic cluster data are described to help further characterize these outbreaks.
Results:
A total of 132 positive SARS-CoV-2 cases among students and staff from 4 school outbreaks were identified with 65 (49%) of cases able to be sequenced with high-quality genomic data. The 4 school outbreaks consisted of 53, 37, 21 and 21 positive cases; within each outbreak there were between 8 and 28 different clinical cohorts identified. Among the sequenced cases, between 3 and 7 genetic clusters, defined as different strains, were identified in each outbreak. We found genetically different viruses within several clinical cohorts.
Conclusions:
WGS, together with public health investigation, is a useful tool to investigate SARS-CoV-2 transmission within schools. Its early use has the potential to better understand when transmission may have occurred, can aid in evaluating how well mitigation interventions are working and has the potential to reduce unnecessary school closures when multiple genetic clusters are identified.
Keywords: SARS, CoV, 2, school, genomic sequencing
Over the course of the coronavirus disease 2019 (COVID-19) pandemic, schools were recognized as critical for the overall health and wellbeing of children. For schools to remain open for in-person learning, enhanced health and safety measures were recommended, in addition to robust case and contact management.1,2 Together, these measures have been generally successful at preventing widespread transmission of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) in Ontario schools, with a median of 2 cases per outbreak (interquartile range 2–4).3 Furthermore, of the 94 outbreaks that occurred in Ontario during the 2020 to 2021 school year, which included in-person learning between August 30, 2020, and April 24, 2021, 93.1% consisted of <10 cases.3 This limited secondary transmission is consistent with the experience in other jurisdictions.4–16
When outbreaks do occur in schools, it can be difficult to determine if they reflect transmission within the school or multiple separate introductions from the community. Coupling epidemiologic information with SARS-CoV-2 whole genome sequencing (WGS) has the potential to better characterize school outbreaks and potential transmission events within schools and may inform public health responses if multiple cases within a school may be proven to be genetically different. This would demonstrate the transmission did not occur within the school. Given that SARS-CoV-2 mutations occur at a relatively low rate (1–3 mutations per month),17 if significant variations in the SARS-CoV-2 genomic sequence is present, this suggests that transmission did not occur between cases.
In this study, we compared SARS-CoV-2 sequences among students and staff in 4 school outbreaks to evaluate the utility of WGS and phylogenetic analysis, coupled with the clinical epidemiologic data, as a mechanism to better characterize whether spread of SARS-CoV-2 occurred within schools with a declared outbreak.
METHODS
We performed a retrospective description and analysis of 4 SARS-CoV-2 elementary school (includes kindergarten to grade 8) outbreaks in Ontario, Canada. All outbreaks occurred in the urban setting. At the time of collection, the preventative measures in place in school included the exclusion of positive cases from school for 10 days from symptom onset, testing offered to asymptomatic close contacts, and the mandated use of face masks.
Setting and Specimen Collection
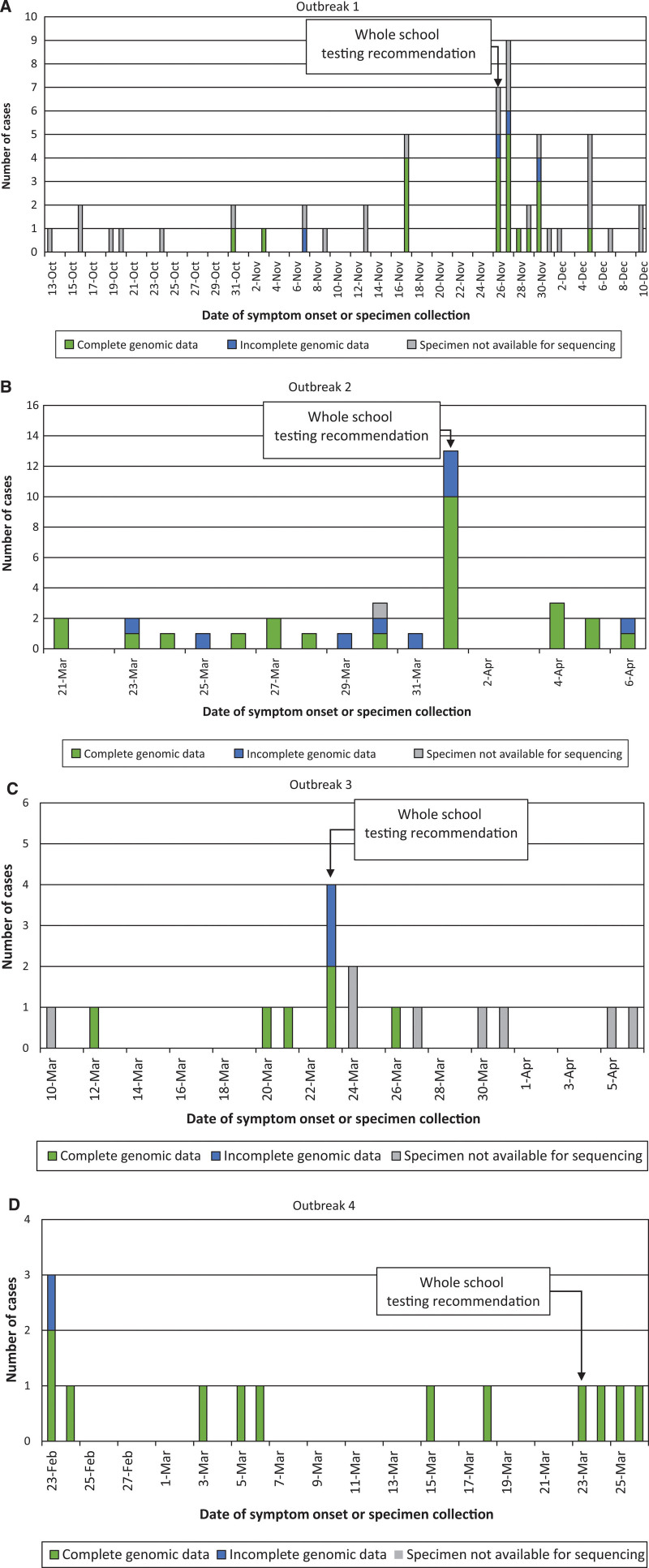
Respiratory tract specimens, including nasoph aryngeal swabs, oral-nasal swabs and saliva, were collected for clinical detection of SARS-CoV-2 from students and school staff and included in the analysis. Samples were collected from individuals based on the presence of symptoms or local public health guidance for whole-school asymptomatic testing (as indicated on Fig. 1) as part of outbreak investigations. Positive cases in schools were routinely investigated through local public health units with additional case management occurring at their direction. Specimen collection occurred between October 13, 2020, and April 4, 2021.
FIGURE 1.
Epidemic curves. Molecular-confirmed cases of SARS-CoV-2 are shown for each school outbreak (A–D). Complete genomic data indicates a genome coverage ≥90%, while incomplete/no genomic data indicates that either <90% coverage, or the specimen was unavailable for genomic testing.
Molecular testing occurred in multiple laboratory sites across an Ontario laboratory network. Positive specimens identified at these sites were directed to The Hospital for Sick Children (SickKids) for SARS-CoV-2 genomic analysis at the request of the public health unit to supplement clinical epidemiologic data collected as part of local public health intervention. Outbreak investigations were chosen for genomic analysis based on request from the public health unit. At the time of testing around these outbreaks, SARS-CoV-2 vaccination was unable to students and prevention measures included the use of masks for staff and students. Individuals testing positive were required to isolate for 10 days. The retrospective analysis was approved by the SickKids Research Ethics Board (REB#1000076653).
Descriptive Epidemiology
Epidemiologic data were collected by the public health unit as part of routine investigation. Data included the date of specimen collection, presence of symptoms, clinical cohort (eg, a defined classroom within a school), and whether the specimen was collected from a staff member, including teacher, principal, office staff or student. Individuals may have been part of 2 clinical cohorts and only clinical cohorts with molecular-confirmed cases of SARS-CoV-2 were included in analysis. The date of asymptomatic whole-school SARS-CoV2 molecular testing was also captured. A school outbreak was defined as all cases associated with a school until a 14-day period with no identified cases.18
SARS-CoV-2 Sequencing
All specimens identified with a cycle threshold (Ct) below 30 for the SARS-CoV-2 molecular test at the primary clinical testing site, and with adequate specimen available for sequencing, were included in the analysis. Briefly, RNA was extracted on the bioMeurieux easyMag (bioMeurieux, France) or Seegene STARlet (Seegene, Korea) extraction system. Following extraction, RNA was converted to cDNA and SARS-CoV-2 amplification using primers based on the ARTIC nCoV-2 protocol version 3 with primers purchased from Integrated DNA Technologies. Sequencing occurred on a R9.4.1 flow cell on a MinION instrument (Oxford Nanopore Technologies, Oxford, United Kingdom). Bioinformatics analysis used Artic 1.1.3 pipeline (nanopolish) for reference based (GenBank accession number MN908947.3) assembly and variant calling (https://artic.network/ncov-2019).19 A threshold of ≥90% consensus genome completeness was used for quality control. The distribution of specimens that met this quality control standard are shown in Figure 1.
Consensus fasta sequence were aligned with mafft 7.310. A maximum-likelihood phylogenetic tree was constructed by IQ Tree (COVID-edition) with fconst used to specify the number of invariant sites as calculated by snp-sites and MN908947.3 as the outgroup.20 Consensus sequences were uploaded to GISAID (www.gisaid.org/) with accession numbers in Table, Supplemental Digital Content 1, http://links.lww.com/INF/E921.
Genetic and Epidemiologic Analysis
Sequences that met a ≥90% genome completeness quality control standard were included in genomic analysis. A maximum-likelihood phylogenetic tree was constructed using IQ Tree (2.1.3 COVID-edition) and included all specimens meeting this standard within each outbreak grouped by genetic cluster or clinical cohort. The phylogenetic tree was labeled with case number, where the case number was sequentially assigned from the index case in the outbreak and included both sequenced and nonsequenced cases.
Lineages were assigned with Phylogenetic Assignment of Named Global Outbreak Lineages (pangolin) version 3.0.3.21 Within Pango lineages, genetic clusters were assigned to each case based on the presence of ≤3 SARS-CoV-2 mutations. Viruses within a genetic cluster were related. Viral sequences with >3 mutations were considered sufficiently genetically unrelated and were assigned to different genetic clusters based on the mutation rate described by Duchene et al.17 Any ambiguous base calls were not included in the determination of relatedness among cases, and the positive was excluded from analysis.
RESULTS
Four SARS-CoV-2 school outbreaks, consisting of students and staff, were included in analysis. A total of 132 individuals were positive for SARS-CoV-2 by molecular testing: 110 (83%) from students and 13 (10%) from staff (missing, n = 9). Of these, 61 (46%) were symptomatic at the time of collection (missing, n = 20). Within each outbreak, between 8 and 28 different clinical cohorts were identified (Fig. 2).
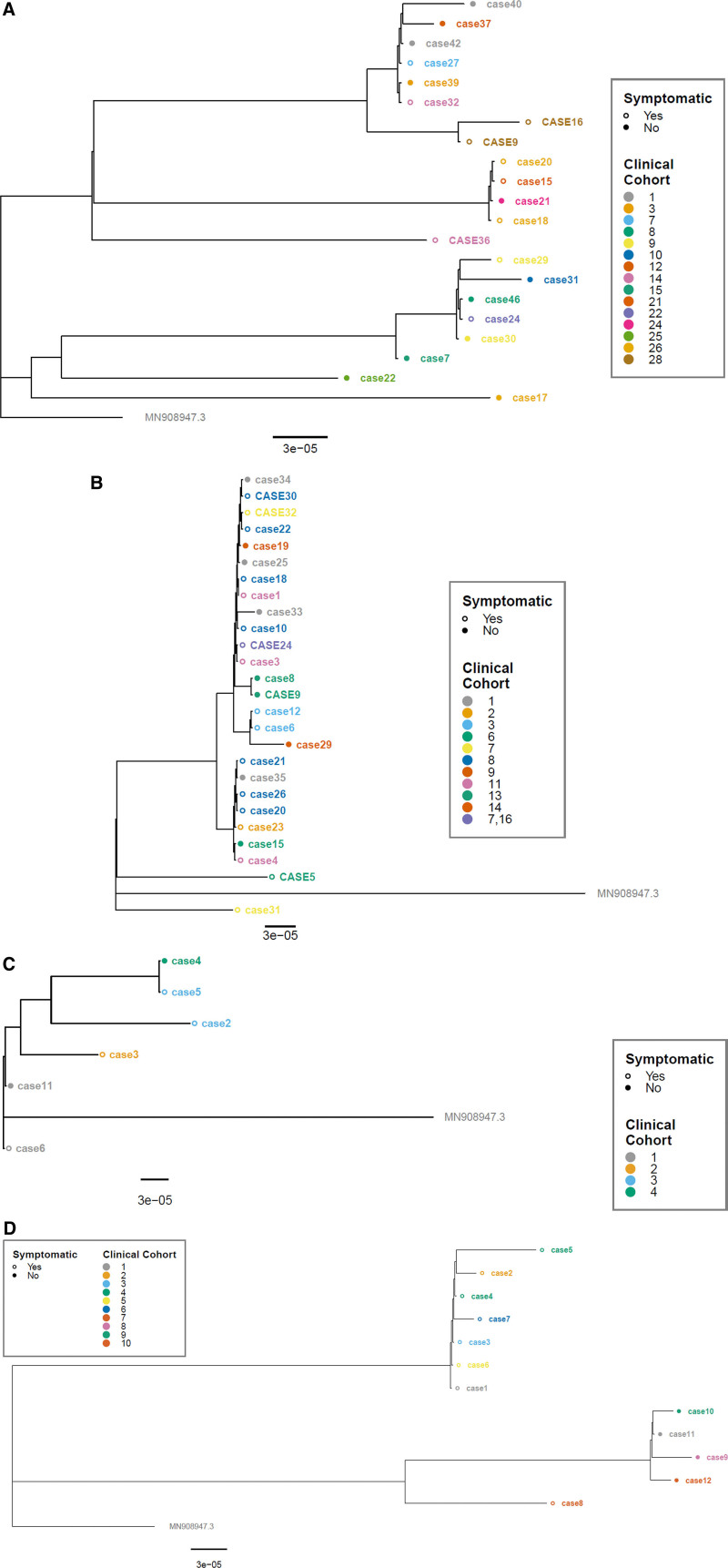
FIGURE 2.
Phylogenetic trees. Phylogenetic analysis of 4 school SARS-CoV-2 outbreaks (A–D). Staff cases are indicated in all upper-case letters, while student cases are in lowercase letters. Individuals that were symptomatic at the time of collection have open circles, and cases asymptomatic at the time of collection have closed circles. All trees are rooted to the reference genome MN908947.3 with the scale representing nucleotide substitutions per site based on the full viral genome.
Of the cases, a total of 79 (60%) underwent SARS-CoV-2 WGS (Table 1). Sixty-five specimens met our threshold for high-quality genomic data for inclusion in genomic analysis. Of these, 11 different SARS-CoV-2 PANGO lineages were identified, with alpha (B.1.1.7) being most frequent (n = 36 cases, 55%). Each school outbreak had between 3 and 7 genetic clusters (Fig. 2).
TABLE 1.
Characterization of the 4 SARS-CoV-2 School Outbreaks in Ontario, Canada, Occurring Between November 2020 to April 2021
| Outbreak Number | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Total no. cases (n) | 53 | 37 | 21 | 21 |
| Staff (%) | 5 (9) | 8 (22) | 0 | 0 |
| Student (%) | 48 (91) | 29 (37) | 21 (100) | 12 (57) |
| Unknown (%) | 0 | 0 | 0 | 9 (43) |
| Symptom status | ||||
| Symptomatic (%) | 23 (43) | 13 (35) | 13 (62) | 8 (38) |
| Asymptomatic (%) | 30 (57) | 22 (60) | 3 (14) | 4 (19) |
| Unknown (%) | 0 | 2 (5) | 5 (24) | 9 (43) |
| No. cases sequenced | 25 | 34 | 8 | 12 |
| Complete genomic data* | 21 | 26 | 6 | 12 |
| Incomplete genomic data† | 4 | 8 | 2 | 0 |
| Clinical cohorts‡ | 28 | 16 | 8 | 10 |
| Median cases (range) | 1.5 (1–4) | 1.5 (1–8) | 2 (1–4) | 1 (1–2) |
| Genetic clusters§ | 7 | 3 | 3 | 4 |
Complete genomic data indicated by a SARS-CoV-2 genome coverage ≥90%.
Incomplete genomic data indicated by a SARS-CoV-2 genome coverage <90%.
Clinical cohorts are based on epidemiologic data (eg, classroom).
Genetic clusters are based on SARS-CoV-2 sequence data, where the presence of ≤3 mutations, regardless of the identified Pango lineage, represents 1 genetic cluster.
Outbreak One
The largest school outbreak consisted of 48 students and 5 staff, with specimens collected over a 9-week period (Fig. 1A). Most cases were asymptomatic (n = 30, all students) and were primarily identified through whole-school testing (Fig. 2A). Twenty-eight different clinical cohorts were affected. Despite the large number of affected cohorts (Figure, Supplemental Digital Content 2a, http://links.lww.com/INF/E922), the median number of cases per cohort was only 1.5 (range 1–4), with <4 cases occurring in 24 of 28 cohorts.
There were 25 specimens (47%) that met criteria (Ct value < 30) and were available for sequencing; of those, 21 specimens generated sequence data of high enough quality to be included in genetic analysis (Table 1). Of the 21 specimens with genetic sequencing data (students = 18, staff = 3), 11 were from asymptomatic cases and 10 were from symptomatic cases.
Seven different SARS-CoV-2 lineages were identified (Fig. 2A). The most common lineage was AE.8 (n = 8) and, within this lineage, 2 genetic clusters were identified across cases from 6 different clinical cohorts. The next most common lineage was B.1.349 (n = 6), which had 1 genetic cluster across 5 clinical cohorts. When the largest clinical cohorts (those with 4 cases, n = 4 clinical cohorts) were reviewed in relation to genetic clusters, 3 of 4 samples were able to be sequenced in one instance (clinical cohort 26), 2 of 4 in another (clinical cohort 28), 1 of 4 in the third instance (clinical cohort 12) and none in the fourth (clinical cohort 27). Of the 3 cases in clinical cohort 26, 2 were genetically identical, while the third belonged to a different genetic cluster. In the second clinical cohort with 2 sequenced cases (clinical cohort 28), the sequences were identical.
Outbreak Two
This outbreak consisted of 29 students and 8 staff, with specimens collected over a 3-week period (Fig. 1B). Most cases were detected among symptomatic individuals (n = 22, 59%) (Fig. 2B). There were 16 different clinical cohorts identified (data missing for 2 cases). The median number of cases per cohort was 1.5 (range 1–8), with the largest (clinical cohort 8) consisting of 8 cases, including 6 students and 2 staff (Figure, Supplemental Digital Content 2b, http://links.lww.com/INF/E922); one staff (case 24) was included in 2 clinical cohorts.
There were 34 specimens (92%) available for sequencing, with 26 specimens generating sequence data of high enough quality to be included in genetic analysis (Table 1). Of the 26 specimens with genetic sequencing data (students = 21, staff = 5), 17 were from symptomatic cases (Fig. 2B).
All 26 analyzed sequences were identified as alpha (B.1.1.7), with 3 genetic clusters identified; one with 24 cases, and 2 genetic clusters each with 1 case. In the largest clinical cohort with 8 cases (clinical cohort 8), 6 had adequate sequencing data, and all belonged to the same genetic cluster. There was also evidence that transmission did not occur within a clinical cohort. Thus, while case 31 (student) and case 32 (staff) (Fig. 2B), were both identified as having alpha (B.1.1.7) strains, in-depth genetic analysis demonstrated >3 single nucleotime polymorphism (SNP) difference between them. This was also seen within another cohort (clinical cohort 13) where case 5 (staff) was genetically different than the other staff (case 9) and student (case 8) (Fig. 2B).
Outbreak Three
This school outbreak consisted of 21 cases (all students). Specimens were collected over a 4-week period (date unknown for 5 cases) (Fig. 1C). Of the cases identified in this outbreak, 13 (62%) were asymptomatic at the time of collection (Fig. 2C). Cases were distributed across 8 different clinical cohorts (Figure, Supplemental Digital Content 2c, http://links.lww.com/INF/E922), with a median of 2 cases per cohort (range 1–4).
There were 8 specimens (38%) that met criteria and were available for sequencing; of these, 6 (29% of all cases) generated high-quality sequence data that could be included in the analysis (Table 1). Four of the sequenced cases were symptomatic at the time of collection and 2 were asymptomatic.
The majority of cases sequenced (n = 5, 83%) were of alpha (B.1.1.7) lineage, with 4 genetic clusters identified, and one case of the B.1 lineage, for a total of 5 genetic clusters. Of the samples that sequenced to the B.1.1.7 lineage, only 2 of the cases sequenced had a genetically related virus (cases 1 and 5); the other 3 cases each had a virus with >3 mutations between each other. The 2 cases with genetically similar virus were across 2 different clinical cohorts.
Outbreak Four
This school outbreak included 21 cases over a 5-week period (collection date unknown, n = 9); 12 were students (missing, n = 9) (Fig. 1D). For the 12 student cases, 8 were symptomatic and 4 were asymptomatic at the time of collection (Fig. 2D); these cases were distributed across 10 clinical cohorts (Figure, Supplemental Digital Content 2d, http://links.lww.com/INF/E922).
These 12 cases each had a specimen available for sequencing and sequencing generating high-quality sequence data in all instances (Table 1). The lineage most commonly identified was B.1.1.519 (n = 7, 58%), with two genetic clusters, one with 6 cases and the other a single case. Of these 7 cases of B.1.1.519, only 2 belonged to the same clinical cohort (case 4 and case 5), but they were in different genetic clusters, indicating they did not transmit SARS-CoV-2 within the clinical cohort. The remaining 5 cases were identified as alpha (B.1.1.7). These 5 alpha cases (cases 8–12) belonged to 5 different clinical cohorts, but 4 were part of the same genetic cluster.
DISCUSSION
WGS is helpful in understanding patterns of SARS-CoV-2 transmission in various settings, including long-term care homes and hospitals.22–24 In this study, we show that WGS can also be helpful in evaluating outbreaks within schools. The genetic sequencing analysis we conducted, between October 2020 and April 2021, demonstrated that most within-school clinical clusters were associated with multiple distinct SARS-CoV-2 genetic strains, suggesting multiple separate introductions, likely reflective of the surrounding school community, rather than transmission within the school. In contrast, in one of the clusters that included >4 individuals, within-school transmission could not be excluded as only one genetic cluster was identified (outbreak 2). In this latter circumstance, a detailed evaluation of the infection control and public health measures would be particularly important.
An important finding was that lineage alone was insufficient to determine if transmission occurred between individuals. This was evident in outbreaks 3 and 4, where there were multiple cases with the same lineage, but WGS demonstrated sufficient sequence differences to indicate that transmission likely did not occur between these individuals. This is consistent with other studies that have demonstrated that real-time SARS-CoV-2 sequencing may enable rapid identification and exclusion of transmission events.23 This is an important consideration as it would not be uncommon for multiple cases within a school to be the same lineage based on community prevalence, where one lineage tends to dominate at any given time.3 Community genomic data were not compared in the current study, but this study was undertaken when there was no predominate community lineage and only captured the early introduction of Alpha in the community.
Certain caveats should be kept in mind when interpreting the findings of genomic analysis. First, genomic analysis is most useful in excluding transmission event within a defined timeframe based on detection of sequences that differ significantly between individuals. Second, detecting identical or near-identical sequences is more difficult to interpret as it does not prove that transmission occurred between specific individuals.25 Isolates with >3 SNPs were considered to be genetically different, while isolates that had <3 SNP difference were considered similar regardless of time of collection between cases. This assumption further limits the ability to demonstrate transmission or relatedness but does not affect the ability to prove transmission did not occur, which was the primary objective. The chosen mutation rate to consider cases different would also affect the interpretation. Thus, it is important to combine the genomic analysis with a careful epidemiologic evaluation of potential links between individuals in outbreaks. However, even when these processes are in place, uncertainty may remain when evaluating possible transmission events. Nevertheless, data derived from genomic analysis may have significant public health utility when evaluating the effectiveness of school mitigation interventions, and potentially in reducing the risk of school closures. Viral genomic data may be most useful when obtained in a timely manner as it could contribute to changes in public health interventions.
Limitations
The selection of outbreaks represents a limitation. Outbreaks were selected based on external review and request and may not represent the typical distribution. Often outbreaks with multiple cases at a school were selected. The community rates of SARS-CoV-2 were not assessed and may contribute to the diversity or selection of a school for analysis. A limitation of this analysis is that only specimens with a Ct value <30 were included due to WGS technical limitations. Additionally, only individuals who were tested for SARS-CoV-2 were included, as testing was recommended but not mandatory. Some asymptomatic individuals or those who did not present for testing, would not be captured, potentially underestimating the number of cases in a school outbreak or within an individual cohort, and limiting our ability to link cases epidemiologically both clinically and through WGS. This risk was partially mitigated by offering asymptomatic whole-school molecular-based testing, which may be able to identify potential asymptomatic reservoirs. Finally, the application of WGS during periods of other SARS-CoV-2 variants, such as Delta (B.1.617.2) or Omicron (B.1.1.529), requires ongoing review.
The use of WGS for SARS-CoV-2 school cases can help understand the source of transmission and impact health and safety measures in place in schools. Implementation of a rapid sequencing program could also potentially aid in preventing school closures if the evidence supported a lack of or only limited transmission within the school.
Supplementary Material
Footnotes
Initial funding was provided through a McLaughlin Centre grant from the University of Toronto.
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com).
Contributor Information
Allison Chris, Email: allison.chris@toronto.ca.
Julia Orkin, Email: julia.orkin@sickkids.ca.
Lynette Lau, Email: lynette.lau@sickkids.ca.
Christian Marshall, Email: crm@sickkids.ca.
Ari Bitnun, Email: ari.bitnun@sickkids.ca.
Sarah A Buchan, Email: sarah.buchan@oahpp.ca.
Liane MacDonald, Email: liane.macdonald@oahpp.ca.
Nisha Thampi, Email: nthampi@cheo.on.ca.
Janine McCready, Email: janine.mccready@tehn.ca.
Peter Juni, Email: peter.juni@utoronto.ca.
Rulan S Parekh, Email: Rulan.Parekh@wchospital.ca.
Michelle Science, Email: michelle.science@sickkids.ca.
REFERENCES
- 1.(CDC) CfDCaP. COVID-19 guidance for schools Kindergarten to Grade 12. 2021. Available at: https://www.cdc.gov/coronavirus/2019-ncov/community/schools-childcare/schools.html. Accessed June 9, 2021.
- 2.Table OS. School operation for the 2021-2022 academic year in the context of the COVID-19 pandemic. 10.47326/ocsat.2021.02.38.1.0.
- 3.(Ontario) OAfHPaPPH. COVID-19 in Ontario: Elementary and Secondary School Outbreaks and Related Cases, August 30, 2020 to April 24, 2021. Queen’s Printer for Ontario; 2021. [Google Scholar]
- 4.(NCIRS) NCfIRaS. COVID-19 in Schools—the Experience in NSW. National Centre for Immunisation Research and Surveillance (NCIRS); 2020. [Google Scholar]
- 5.Environment NIfPHat. Children, School and COVID-19. Ministry of Health WaS; 2020. [Google Scholar]
- 6.Macartney K, Quinn HE, Pillsbury AJ, et al. Transmission of SARS-CoV-2 in Australian educational settings: a prospective cohort study. Lancet Child Adolesc Health. 2020;4:807–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ehrhardt J, Ekinci A, Krehl H, et al. Transmission of SARS-CoV-2 in children aged 0 to 19 years in childcare facilities and schools after their reopening in May 2020, Baden-Württemberg, Germany. Euro Surveill. 2020;25:2001587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Control ECfDPa. COVID-19 in Children and the Role of School Settings in COVID-19 Transmission. ECDC; 2020. [Google Scholar]
- 9.Fontanet A, Tondeur L, Grant R, et al. SARS-CoV-2 infection in schools in a northern French city: a retrospective serological cohort study in an area of high transmission, France, January to April 2020. Euro Surveill. 2021;26:2001695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heavey L, Casey G, Kelly C, et al. No evidence of secondary transmission of COVID-19 from children attending school in Ireland, 2020. Euro Surveill. 2020;25:2000903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scottish Government. Coronavirus (COVID-19) in schools. 2020. Available at: https://www.gov.scot/news/coronavirus-covid-19-in-schools/. Accessed December 24, 2020.
- 12.Surveillance NCfIRa. COVID-19 in Schools and Early Childhood Education Care Services—the Term 3 Experience in NSW. NSW Government; 2020. [Google Scholar]
- 13.Ismail SA, Saliba V, Lopez Bernal J, et al. SARS-CoV-2 infection and transmission in educational settings: a prospective, cross-sectional analysis of infection clusters and outbreaks in England. Lancet Infect Dis. 2021;21:344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Otte Im Kampe E, Lehfeld AS, Buda S, et al. Surveillance of COVID-19 school outbreaks, Germany, March to August 2020. Euro Surveill. 2020;25:2001645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tools NCCfMa. Living rapid review update 14: what is the specific role of daycares and schools in COVID-19 transmission? 2021.
- 16.Zimmerman KO, Akinboyo IC, Brookhart MA, et al. Incidence and Secondary Transmission of SARS-CoV-2 Infections in Schools. Pediatrics. 2021;147:e2020048090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duchene S, Featherstone L, Haritopoulou-Sinanidou M, et al. Temporal signal and the phylodynamic threshold of SARS-CoV-2. Virus Evol. 2020; 6:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ontario Science Table. School Operation for the 2021-2022 Academic Year in the Context of the COVID-19 Pandemic. 2021. Available at: 10.47326/ocsat.2021.02.38.1.0. Accessed September 29, 2021. [DOI]
- 19.N L. artic-ncov2019 primer schemes. Available at: https://artic.network/ncov-2019. Accessed June 9, 2021.
- 20.Minh BQ, Schmidt HA, Chernomor O, et al. IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Mol Biol Evol. 2020;37:24611530–24612461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rambaut A, Holmes EC, O’Toole A, et al. A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology. Nat Microbiol. 2020;5:1403–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murti M, Goetz M, Saunders A, et al. Investigation of a severe SARS-CoV-2 outbreak in a long-term care home early in the pandemic. Can Med Assoc J. 2021;193:E681–E688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meredith LW, Hamilton WL, Warne B, et al. Rapid implementation of SARS-CoV-2 sequencing to investigate cases of health-care associated COVID-19: a prospective genomic surveillance study. Lancet Infect Dis. 2020;20:1263–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.San JE, Ngcapu S, Kanzi AM, et al. Transmission dynamics of SARS-CoV-2 within-host diversity in two major hospital outbreaks in South Africa. Virus Evol. 2021;7:veab041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Villabona-Arenas CJ, Hanage WP, Tully DC. Phylogenetic interpretation during outbreaks requires caution. Nat Microbiol. 2020;5:876–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.