Abstract
OBJECTIVES
The authors estimated changes of stressed blood volume (SBV) induced by splanchnic nerve block (SNB) in patients with either decompensated or ambulatory heart failure with reduced ejection fraction (HFrEF).
BACKGROUND
The splanchnic vascular capacity is a major determinant of the SBV, which in turn determines cardiac filling pressures and may be modifiable through SNB.
METHODS
We analyzed data from 2 prospective, single-arm clinical studies in decompensated HFrEF (splanchnic HF-1; resting hemodynamics) and ambulatory heart failure (splanchnic HF-2; exercise hemodynamics). Patients underwent invasive hemodynamics and short-term SNB with local anesthetics. SBV was simulated using heart rate, cardiac output, central venous pressure, pulmonary capillary wedge pressure, systolic and diastolic systemic arterial and pulmonary artery pressures, and left ventricular ejection fraction. SBV is presented as ml/70 kg body weight.
RESULTS
Mean left ventricular ejection fraction was 21 ± 11%. In patients with decompensated HFrEF (n = 11), the mean estimated SBV was 3,073 ±251 ml/70 kg. At 30 min post-SNB, the estimated SBV decreased by 10% to 2,754 ± 386 ml/70 kg (p = 0.003). In ambulatory HFrEF (n = 14) patients, the mean estimated SBV was 2,664 ± 488 ml/70 kg and increased to 3,243 ± 444 ml/70 kg (p < 0.001) at peak exercise. The resting estimated SBV was lower in ambulatory patients with HFrEF than in decompensated HFrEF (p = 0.019). In ambulatory patients with HFrEF, post-SNB, the resting estimated SBV decreased by 532 ± 264 ml/70 kg (p < 0.001). Post-SNB, with exercise, there was no decrease of estimated SBV out of proportion to baseline effects (p = 0.661).
CONCLUSIONS
The estimated SBV is higher in decompensated than in ambulatory heart failure. SNB reduced the estimated SBV in decompensated and ambulatory heart failure. The reduction in estimated SBV was maintained throughout exercise. (Splanchnic Nerve Anesthesia in Heart Failure, NCT02669407; Abdominal Nerve Blockade in Chronic Heart Failure, NCT03453151) (J Am Coll Cardiol HF 2021;9:293–300) © 2021 by the American College of Cardiology Foundation.
Graphical Abstract
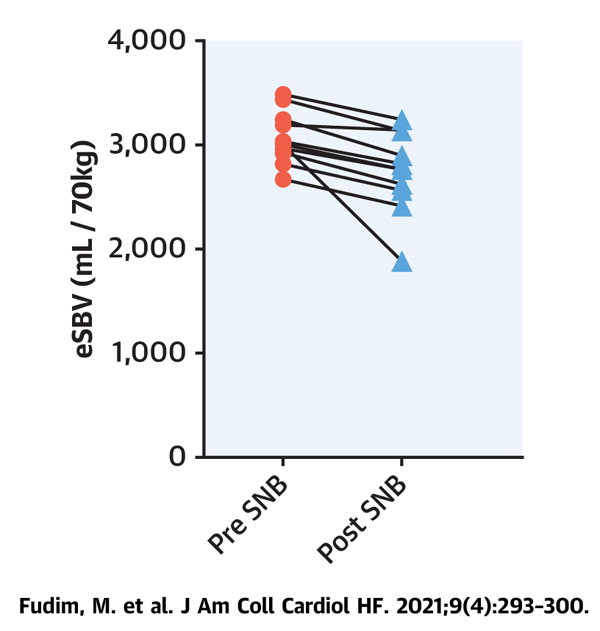
CENTRAL ILLUSTRATION Stressed Blood Volume in Decompensated Heart Failure
Estimated stressed blood volume (eSBV) and effects of splanchnic nerve blockade (SNB) in decompensated heart failure (HF).
Increased intracardiac pressures at rest and more so during exercise are key hallmarks of heart failure (HF). Patients with HF are characterized by a heightened sympathetic and vascular tone. Unstressed blood volume (UBV) and stressed blood volume (SBV) are terms used to describe the functional distribution of the total blood volume (TBV) and its contribution to right and left ventricular preload. UBV (70% to 75% of TBV) is the volume required to fill blood vessels to the point beyond which tension arises in the elastic walls of vascular structures. SBV (20% to 25% of TBV) is the volume above UBV that stretches vessel walls and creates pressure within the vessels. An increase in SBV as a result of venoconstriction has been proposed as an important driving mechanism of cardiac filling pressure elevations in both acute and chronic HF (1–3).
The splanchnic vascular compartment contains a large portion of intravascular blood volume. Active and passive changes, primarily in the venous compartment, can induce changes in the distribution of blood between UBV and SBV within seconds (4,5). More specifically, a large proportion of the UBV resides in the splanchnic venous bed. Sympathetically mediated increases of splanchnic venous blood vessel tone can therefore induce rapid and potent functional shifts blood from UBV to SBV components. Such increases of SBV also result in physical shifts of blood from the splanchnic bed to the thorax (6,7).
Splanchnic nerve blockade (SNB) was tested as a novel therapy for heart failure with reduced ejection fraction (HFrEF) (8–10). The proposed mode of action is splanchnic vasodilation with a functional shift of SBV to UBV with return of blood to the splanchnic reservoir. Short-term SNB was tested in the Splanchnic HF-1 (decompensated HFrEF) and Splanchnic HF-2 (ambulatory HFrEF) studies. In Splanchnic HF-1, SNB reduced resting filling pressures (central venous pressure [CVP], pulmonary arterial pressure, and pulmonary capillary wedge pressure [PCWP]) with a parallel increase in cardiac output (CO). In Splanchnic HF-2, SNB decreased resting and exercise induced filling pressures with parallel improvement in CO and trend towards improved exercise function.
Investigations of blood volume shifts in humans during exercise are limited given the complexity of indicator dilution, radiodilution techniques, or plethysmography techniques required to measure and image the blood pool. While the technical success of SNB can be measured via arterial and intracardiac pressure changes, these measures are only surrogates of SBV and its changes. The objective of this study was to use a previously validated model to estimate the changes of SBV induced by SNB in both Splanchnic HF-1 and HF-2 studies.
METHODS
STUDY DESIGN AND POPULATION.
The present study included data obtained from 2 prior studies investigating the effects of SNB in HFrEF. Splanchnic HF-1 was a prospective, single-arm, open-label study of patients hospitalized for decompensated HFrEF between April 2017 and May 2018 (NCT02669407) (9,10). Eligible patients had persistent New York Heart Association functional class III/IV symptoms, and a mean PCWP >15 mm Hg (>12 mm Hg if on inotropes) on baseline right heart catheterization during the hospitalization. Patients underwent short-term bilateral SNB by direct injection of lidocaine. The primary outcome was a change in resting, supine cardiac filling pressures (9,10).
Splanchnic HF-2 was a prospective open label, single-arm study of patients with ambulatory HFrEF. Eligible patients were enrolled from May 2018 through June 2019 (NCT03453151) (8). Patients enrolled in the study were on guideline-directed medical therapy with persistent New York Heart Association functional class II/III symptoms. To qualify for SNB, patients had to have a resting mean PCWP ≥15 mm Hg and/or ≥25 mm Hg at peak exercise during an initial (pre-SNB) invasive cardiopulmonary exercise test. Patients underwent short-term SNB with ropivacaine. The first 5 patients had bilateral SNB and given observed orthostatic response to the block the subsequent 10 patients had unilateral SNB only. Patients underwent an invasive cardiopulmonary exercise test before and after SNB. The primary outcome was a change in exercise-induced filling pressures (mean pulmonary arterial pressure and PCWP) and exercise capacity as measured by peak oxygen consumption (VO2) (8).
In both studies, patients with chronic kidney disease stage V, known coagulopathies, and those on oral anticoagulants or oral antiplatelet agents other than aspirin were excluded. Morning medications were withheld before the study. Both study protocols were approved by the local institutional review board, and all patients provided written informed consent.
INVASIVE HEMODYNAMIC TESTING.
All invasive hemodynamic measurements were recorded in the supine position. Right heart catheterization through the internal jugular vein (8-F sheath, and 7-F pulmonary artery catheter) and a radial arterial catheterization (5-F sheath) were used to assess central hemodynamics, arterial pressures, and for blood gas analysis. In Splanchnic HF-1, CO was estimated with the indirect Fick method. Hemodynamics were recorded every 15 min, with an observed peak effect at 30 min after SNB. In Splanchnic HF-2, CO was calculated via direct Fick method (VO2 ÷ AVO2-diff) using breath-by-breath oxygen consumption (Vmax e29, Vyaire Medical, Loma Linda, California). Hemodynamics were recorded in the supine position with feet down, feet up in the ergometer pedals, 20 W, peak exercise, and after 2-min recovery. Intracardiac pressures were obtained as the average end-expiratory values across multiple respiratory cycles over a 10-s period. Pressure tracings were evaluated in a blinded fashion. Full details on exercise protocol and hemodynamic testing can be found in the original publication (8).
STUDY INTERVENTION.
In both studies, the SNB was performed following baseline hemodynamic testing. All patients underwent a fluoroscopic-guided SNB by anesthesiologists with subspecialty training in interventional pain medicine. A 22-g spinal needle was guided to the anterolateral edge of the thoracolumbar spine at the T12/L1 level. Once the needle was in final position, iodinated contrast followed by a “test” dose of 3 ml of 1.5% lidocaine with 1:200,000 epinephrine was injected to confirm extravascular location of the needle. In Splanchnic HF-1, we used 15 ml of 1% lidocaine (with an expected duration of action of <90 min); and in Splanchnic HF-2 we used 12 ml of 0.5% ropivacaine (expected duration of action <24 h). Patients remained in a supine position throughout the study, except during the SNB when the patient was prone on his/her abdomen.
STRESSED BLOOD VOLUME ESTIMATION.
As detailed above, TBV is functionally divided into UBV and SBV pools: TBV = UBV + SBV. Direct measurement UBV and SBV requires complex experimental preparations and maneuvers that are not readily applicable to humans and are especially not applicable to studies of exercise physiology. Accordingly, we used a previously described simulation-based method for estimating SBV based on widely used models of the cardiovascular system (11). In brief, the systemic and pulmonary circulations are represented by series of resistors and capacitors (12) and the heart chambers are represented by individual time-varying elastances (11,13–15) (Supplemental Figure 1). The nonlinear, time-varying differential equations governing this model can be solved by numeric integration. For estimation of SBV, the measured values of heart rate, CO, CVP, PCWP, systolic and diastolic arterial and pulmonary artery pressures, and left ventricular ejection fraction for a given patient and condition are provided to the model. Aside from heart rate, each of the other 8 variables represents a measured parameter that must be matched by the output of the model on a patient-by-patient basis. This is accomplished by a custom-developed fitting algorithm that performs an unbiased search of the multidimensional model parameter space, adjusting parameter model values to optimize the fit between model-predicted and measured cardiovascular variables. Model parameter values that are optimized include right and left ventricular end-systolic elastances and diastolic stiffness constants, systemic and pulmonary arterial compliances and characteristic impedances and, finally, SBV. For a given patient, ventricular stiffness constants were assumed to be the same at rest and during exercise. In addition, based on preclinical studies showing that venous compliance is not influenced by sympathetic tone or infusions of exogenous catecholamines, venous compliance was also assumed to be the same at rest and during exercise so that change in estimated SBV (eSBV) would be the result of shift of the venous pressure-volume relationship and not related to changes in its slope (16,17). Because SBV determined by this method is not a direct measurement, it will be designated as eSBV. The parameter fitting algorithm has been implemented and used in prior studies (12,18). One key feature of this approach is that nonlinear venous compliance curves have been incorporated into the model to account for the demonstrated nonlinearities identified experimentally at high venous pressures such as those encountered during exercise in patients with HF.
To account for differences in patient sizes, all eSBV values are presented as ml/70 kg body weight.
STATISTICAL ANALYSIS.
Continuous variables are presented using the mean ±SD, median (25th and 75th percentiles), and the minimum and maximum, as appropriate based on the underlying distribution. Statistical comparisons between variables assessed only once post-SNB used the paired Student’s t-test. Repeated measures models with compound symmetry variance structure were used to evaluate the change of eSBV from pre-SNB tests (legs down, legs elevated, 20 W, peak, 2-min recovery) using a simulation method to adjust for multiple pairwise comparisons of each post-procedure time point to pre-SNB values. Each model included the test, pre-SNB test value, time (pre- or post-SNB), and test-by-time interaction. Statistical analyses were completed by the Duke Department of Biostatistics and Bioinformatics (Durham, North Carolina) using SAS version 9.4 (SAS, Institute, Inc., Cary, North Carolina). A p < 0.05 was considered statistically significant.
RESULTS
eSBV.
All patients originally included in the Splanchnic HF-1 (n = 11) and 14 of 15 patients in the Splanchnic HF-2 study were included in the analysis of eSBV. One patient from the Splanchnic HF-2 study was excluded due to absent measurement of CVP with exercise, which precluded the analysis of eSBV. The majority of the patients were men with ischemic cardiomyopathy and a high burden of comorbid disease. Mean (min-max) left ventricular ejection fraction was 18% (<15% to 45%) (Table 1). The hemodynamic responses SNB at rest and during exercise have been detailed in the original papers and are summarized in Tables 2 and 3. In general, SNB was associated with decreases of CVP, pulmonary arterial pressure, PCWP and arterial pressure and increases of cardiac output (CO) at rest and during exercise. In the Splanchnic HF-2 study of stable HFrEF outpatients, SNB was also associated with a decrease of systemic vascular resistance (SVR).
TABLE 1.
Baseline Characteristics
| Splanchnic HF-1 (n = 11) |
Splanchnic HF-2 (n = 14) |
|
|---|---|---|
| Male | 8 (73) | 8 (57) |
| Age, yrs | 64 ± 13 | 58 ± 13 |
| Ischemic cardiomyopathy | 7 (64) | 9 (64) |
| History of hypertension | 6 (55) | 4 (29) |
| History of diabetes | 7 (64) | 4 (29) |
| History of atrial fibrillation | 7 (64) | 7 (50) |
| LVEF, % | 18 ± 11 | 21 ± 12 |
| BMI, kg/m2 | 32 (22–43) | 31 (22–56) |
| Implantable cardioverter-defibrillator | 10 (91) | 14 (100) |
| Creatinine, mg/dl | 2 ± 0.7 | 1.2 ± 0.4 |
| BUN, mg/dl | 58 ± 20 | 20 ± 10 |
| NT-proBNP, pmol/l | 8,134 (1,221–38,364) | 2,234 (112–9,319) |
| Inotrope | 6 (55) | 0 (0) |
| Beta blockers | 5 (45) | 14 (100) |
| ACE-I/ARB | 3 (27) | 10 (71) |
| Mineralocorticoid receptor antagonists | 8 (73) | 10 (71) |
Values are n (%) mean SD, or mean (min-max).
ACE-I = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; BMI = body mass index; BUN = blood urea nitrogen; LVEF = left ventricular ejection fraction; NT-proBNP = N-terminal pro-B-type natriuretic peptide.
TABLE 2.
Hemodynamic Parameters Pre- and Post-Splanchnic Nerve Blockade in Decompensated Heart Failure
| Pre-SNB | Post-SNB | |
|---|---|---|
| Heart rate, beats/min | 89.8 ± 12.4 | 90.3 ± 14.9 |
| Cardiac output, l/min | 4.5 ± 1.5 | 5.4 ± 1.6 |
| Central venous pressure, mm Hg | 18.7 ± 5.6 | 14.4 ± 4.6 |
| PA systolic, mm Hg | 65.2 ± 9.9 | 53.9 ± 12.5 |
| PA diastolic, mm Hg | 33.8 ± 6.2 | 25.7 ± 4.5 |
| PCWP, mm Hg | 29.5 ± 6.5 | 22.3 ± 7.1 |
| Systolic BP, mm Hg | 122.6 ± 19.8 | 111.6 ± 16.3 |
| Diastolic BP, mm Hg | 69.3 ± 12 | 56.7 ± 9.4 |
| PVR, Woods U | 3.6 ± 1.4 | 2.9 ± 1.3 |
| SVR, dynes/s/cm5 | 886 ± 306 | 913 ± 308 |
Values are mean SD.
BP = arterial blood pressure; PA = pulmonary arterial pressure; PCWP = pulmonary capillary wedge pressure; PVR = pulmonary vascular resistance; SNB = splanchnic nerve block; SVR = systemic vascular resistance.
TABLE 3.
Hemodynamic Parameters Pre- and Post-Splanchnic Nerve Blockade in Ambulatory Heart Failure
| Pre-SNB |
Post-SNB |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rest | Legs Up | 20 W | Peak | Recovery 2 min | Rest | Legs Up | 20 W | Peak | Recovery 2 min | |
| Heart rate, beats/min | 75.5 ± 15.9 | 77.7 ± 16 | 94.4 ± 21.7 | 95.8 ± 23.8 | 81.2 ± 16.0 | 80.6 ± 18.2 | 80.1 ± 18.4 | 99.8 ± 23.5 | 104.8 ± 26.4 | 83.8 ± 16.8 |
| Cardiac output, l/min | 3.9 ± 1.0 | 3.8 ± 1.3 | 5.8 ± 2.0 | 6.7 ± 2.4 | 6.6 ± 3.1 | 4.7 ± 1.7 | 4.2 ± 1.1 | 6.5 ± 1.7 | 7.4 ± 2.1 | 6.8 ± 2.1 |
| Central venous pressure, mm Hg | 13.6 ± 4.1 | 15.9 ± 5.0 | 23.1 ± 5.8 | 23.2 ± 6.2 | 15.6 ± 3.6 | 8.4 ± 4.1 | 10.3 ± 4.9 | 15.3 ± 8.3 | 15.4 ± 7.8 | 9.9 ± 4.6 |
| PA systolic, mm Hg | 54 ± 16.1 | 59.9 ± 18.1 | 71.2 ± 15.5 | 76.5 ± 18.6 | 64.1 ± 18.3 | 43.5 ± 16.3 | 46.6 ± 19.6 | 55.5 ± 25.0 | 65.9 ± 23 | 52.1 ± 21.8 |
| PA diastolic, mm Hg | 24.4 ± 9.9 | 27.8 ± 10.9 | 27.3 ± 15.1 | 31.3 ± 11.9 | 28.2 ± 11.6 | 19 ± 11.0 | 21.1 ± 11.5 | 21.3 ± 12.4 | 23.0 ± 12.7 | 20.7 ± 11.5 |
| PCWP, mm Hg | 27.5 ± 7.3 | 30.7 ± 8.3 | 34.3 ± 10.6 | 34.3 ± 10.1 | 26.9 ± 8.3 | 19.1 ± 8.4 | 20.7 ± 10.3 | 22.7 ± 10 | 24.4 ± 10.7 | 17.7 ± 10.2 |
| Systolic BP, mm Hg | 131.6 ± 19.2 | 135.4 ± 18.5 | 151.6 ± 20.8 | 149.6 ± 24.4 | 141.9 ± 21.3 | 120.7 ± 20.3 | 126.1 ± 18.0 | 127.0 ± 22.5 | 134.4 ± 28.1 | 128.6 ± 28 |
| Diastolic BP, mm Hg | 74.7 ± 10.8 | 79.1 ± 12.1 | 78.9 ± 16.9 | 77.6 ± 20.3 | 72.4 ± 14.0 | 66.5 ± 11.9 | 69.5 ± 10.3 | 64.6 ± 16.0 | 68.6 ± 17.6 | 65.2 ± 15.4 |
| PVR, WU | 3.2 ± 2 | 3.6 ± 1.8 | 2.9 ± 1.8 | 2.9 ± 2.2 | 3.1 ± 2.3 | 2.6 ± 1.4 | 3.2 ± 1.7 | 2.5 ± 1.9 | 2.8 ± 1.3 | 3.0 ± 1.3 |
| SVR, dynes/s/cm5 | 1,676 ± 692 | 1,728 ± 768 | 1,136 ± 559 | 973 ± 573 | 1,004 ± 992 | 1,306 ± 584 | 1,488 ± 487 | 863 ± 334 | 827 ± 320 | 911 ± 406 |
| eSBV, ml/70 kg | 2,664 ± 488 | 2,845 ± 416 | 3,260 ± 362 | 3,243 ± 352 | 2,762 ± 444 | 2,132 ± 570 | 2,266 ± 613 | 2,581 ± 640 | 2,662 ± 656 | 2,221 ± 679 |
Values are mean ± SD.
Abbreviations as in Table 2.
We confirmed that the model optimization approach with nonlinear vascular compliances we used to estimate SBV accurately fit CVP, PCWP, and CO (Supplemental Figure 2); these 3 parameters are key in the determination of SBV as will be detailed in the Discussion section (19).
In patients hospitalized for decompensated HFrEF, the mean eSBV was 3,073 ± 251 ml/70 kg. At 30 min post-SNB (the peak hemodynamic effect of SNB), the eSBV decreased to 2,754 ± 386 ml/70 kg (p = 0.003) (Central Illustration). This equates to an average reduction of 319 ± 278 ml/70 kg.
In ambulatory patients with HFrEF, eSBV averaged 2,664 ± 488 ml/70 kg, which was 409 ± 1,362 ml/70 kg lower than the decompensated patients with HFrEF in Splanchnic HF-1 (p = 0.019). In these ambulatory patients with HFrEF, eSBV increased by 181 ± 169 ml/70 kg when legs were elevated to 2,845 ± 416 ml/70 kg (p = 0.001) and by an additional 579 ± 427 ml/70 kg to 3,243 ± 444 ml/70 kg (p < 0.001) at peak exercise. Post-SNB, eSBV at rest decreased by 532 ± 264 ml/70 kg (p < 0.001). With leg elevation, eSBV increased 134 ± 276 ml/70 kg (p = 0.104 for absolute values pre- and post-SNB). With peak exercise, the eSBV increased by 530 ± 312 ml/70 kg to 2,662 ± 656 ml/70 kg (p < 0.001). Whereas the absolute value of eSBV was reduced post-SNB under all conditions, the absolute exercise-induced increase of eSBV did not differ significantly between pre- and post-SNB (p = 0.661) (Figure 1).
FIGURE 1. Stressed Blood Volume in Ambulatory Heart Failure.
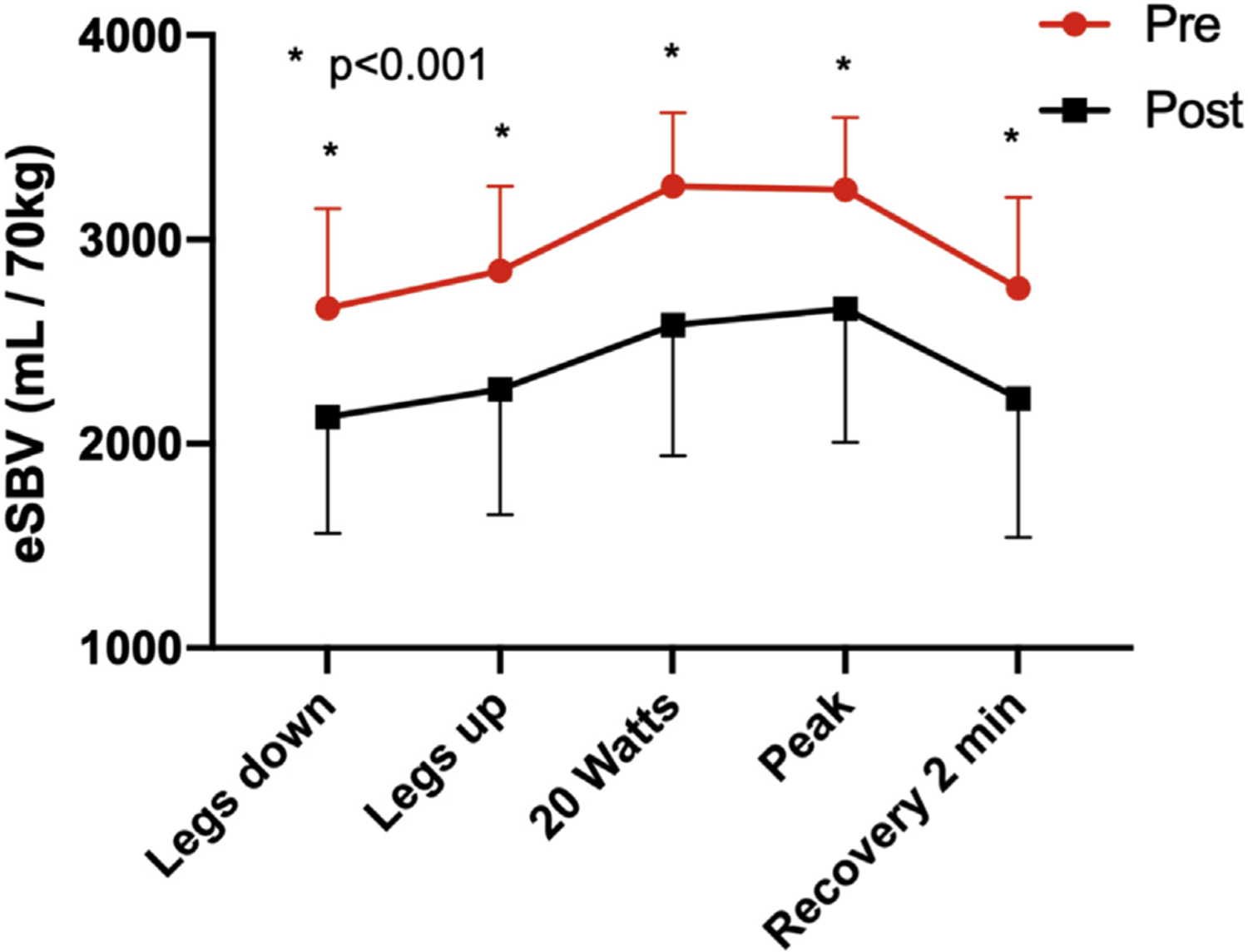
Estimated stressed blood volume and effects of splanchnic nerve blockade in ambulatory heart failure. Asterisks indicate an adjusted p < 0.001 for a pairwise comparison with the pre-SNB value. HF = heart failure; eSBV = estimated stressed blood volume; SNB = splanchnic nerve block.
To help define the relative contributions of the reduction of eSBV and reduction of SVR to the SNB-induced decreases of filling pressures and increased CO noted during exercise in Splanchnic HF-2, we modeled the impact of sequentially changing these 2 parameters in a simulation of a patient with an average hemodynamic profile at 20 W of exercise (values summarized in Table 3). As shown in Supplemental Figure 2, the observed reduction of SVR increased CO but did not detectably impact filling pressures or arterial and pulmonary pressures. However, adding the estimated reduction of eSBV had little impact on CO but fully accounted for the reductions of filling pressures in both ventricles.
DISCUSSION
The key findings of this study are: 1) decompensated patients with HFrEF had a higher eSBV than chronic ambulatory patients; 2) SNB resulted in an acute reduction of eSBV by 319 to 532 ml/70 kg in both decompensated and chronic ambulatory patients with HFrEF; and 3) in chronic ambulatory patients with HFrEF, the reduction in eSBV was maintained throughout exercise, but there was no effect on changes of eSBV from the starting resting condition.
Reduced splanchnic vascular capacity has been considered to contribute to both symptoms of exercise intolerance and the more insidious development of cardiac decompensation (2,3,10,20,21–24). The evidence for reduced splanchnic vascular capacity has been shown in preclinical models of HF and in humans with HF (20,25,26). Evidence in humans is limited to a report of 12 patients from the 1950s showing a decreased splanchnic blood volume in a state of TBV excess (20). In HF, it is common that patients have normal resting hemodynamics but an abnormal hemodynamic response to exercise characterized by rapid and marked elevations of filling pressures (22,23,27). Redistribution of splanchnic blood volume towards the central arteries and veins can lead to a rapid elevation in right- and left-sided cardiac pressures in HF, which may be further amplified by increases in pericardial restraint (1–3,28). This appears to be a consequence of increased effective circulating blood volume and not merely a sign of diastolic dysfunction as is commonly cited (2). The splanchnic nerves supply the abdominal compartment with sympathetic fibers and are thus the major regulator of splanchnic vascular tone (29,30). Activation of splanchnic nerves recruits blood volume into the central circulation, whereas SNB results in vasodilation and redistribution of blood back into the splanchnic vascular compartment (4,5,29).
Our investigation supports several key concepts in cardiovascular physiology. First, we provide supportive evidence of a high SBV in chronic HFrEF and even more so in acute HFrEF. Using invasive methods to determine SBV in patients without HF, Magder and De Varennes (31) quantified SBV in 5 patients without HF on cardiac bypass to be in average 1,290 ± 296 ml, which amounted to ~30% of predicted total blood volume. In a separate investigation by Mass et al. (32), SBV was measured in 15 intubated patients using a sequence of breath hold, volume infusion and withdrawal, and arm stop-flow maneuvers while measuring CO and arterial and venous pressures. The SBV was 1,265 ± 541 ml, and approximately 28.5% of the predicted TBV. However, due to their highly invasive nature, these methods are unlikely to be applied widely to investigate the role of the venous system in patients with HF. The method used in the present study is based on a validated cardiovascular simulation and readily allows for application for scientific investigations.
Second, our investigation confirms that the SNB reduces eSBV and that this is likely the principle mechanism of action responsible for reducing cardiac filling pressures at rest and during exercise. Notably, SNB also lowered SVR (but not pulmonary vascular resistance) in ambulatory patients which can contribute to the observed hemodynamic benefits of SNB. Pharmacological vasodilation can reduce the SBV in preclinical models of HF that have increased SBV. Splanchnic-induced venodilation not only results in a functional decrease of global eSBV but also a physical redistribution of blood from the pulmonary circulation to the splanchnic vascular bed (33). These effects were most pronounced with venodilators such as nitroglycerin (which increased UBV by ~30%) (28,34). Agents targeting primarily arterial tone have been shown to have the least effect in venous capacity and are less effective at reducing cardiac filling pressures (25,34–36). Combined arterial and venous dilators such as angiotensin-converting enzyme inhibitors have intermediary effects on SBV and reductions of cardiac filling pressures (increase in UBV by 11%) (25,34,37).
As emphasized above, estimates of SBV were obtained using a parameter optimization algorithm applied to a comprehensive cardiovascular model to fit each individual patient’s hemodynamic profile. This approach was adopted because direct measurement of SBV and UBV is not feasible in patients, especially during exercise. Even in the setting of preclinical studies with highly invasive instrumentation and extracorporeal reservoirs, it is typically possible to only measure changes of SBV, not absolute values of SBV, in response to physiological and pharmacological interventions (16,38–42). More recently, Uemura et al. (19) derived and validated a simple analytic formula: SBV = T · CO + Cs · CVP + Cp · PCWP. T is the characteristic vascular time constant determined by the distribution of resistance and compliance over the entire vascular system. Cs and Cp are total systemic and pulmonary vascular compliances, respectively. T/Cs and T/Cp give resistances to systemic and pulmonary venous returns. SBV/T defines maximum venous return. While simple to calculate, an important limitation of this approach, however, is that it does not account for nonlinearities of vascular pressure-volume curves at the high values of CVP and PCWP encountered in patients with HF, especially during exercise. As a consequence, this equation can result in unrealistically large values of eSBV. Nevertheless, this equation provides the theoretical foundation for a simple but powerful concept: there is a tight link between bilateral cardiac filling pressures, CO, and SBV. It is for this reason that it was important to confirm that the parameter fitting algorithm accurately predicted CVP, PCWP, and CO (Supplemental Figure 3). Although future studies will be required to provide further validation of this approach, it is recognized that at the current time there is no other approach to estimate SBV in patients.
STUDY LIMITATIONS.
Both Splanchnic HF-1 and HF-2 studies used short-term SNB, which limits the interpretation of potential effects of more permanent SNB on long-term SBV and hemodynamics. The studies are further limited by small patient numbers and open-label design. Yet, hemodynamic values were evaluated in a blinded fashion and results were consistent between patients and between the 2 SNB studies.
CONCLUSIONS
In further analysis of 2 studies investigating the effects of SNB on hemodynamics in patients with decompensated and ambulatory HFrEF, we found that SNB reduced the eSBV in both clinical scenarios. The reduction in eSBV was maintained throughout exercise. Future investigation of SNB must investigate whether short-term effects on eSBV persist over a prolonged period, and whether a reduction in eSBV is sufficient to improve functional and clinical outcome in HF.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE:
Decompensated patients with HFrEF had a higher eSBV than chronic ambulatory patients. SNB reduced the eSBV up to 0.5 l of blood in both decompensated and chronic ambulatory patients with HFrEF.
TRANSLATIONAL OUTLOOK:
Whether the short-term effects of SNB on eSBV persist over a prolonged period remains to be investigated. Whether a reduction in eSBV is sufficient to improve functional and clinical outcome in HF is unknown.
FUNDING SUPPORT AND AUTHOR DISCLOSURES
This study was supported by American Heart Association (AHA) Grant, 17MCPRP33460225 and Translating Duke Health Award. Dr. Fudim has received grants from the American Heart Association (Grant 17MCPRP33460225), National Institutes of Health (NIH) T32 grant 5T32HL007101, Mario Family Award, Translating Duke Health Award; and has received consulting fees from AxonTherapies, Daxor, and Galvani. Dr. M.R. Patel has received research grants from HeartFlow, Bayer, Janssen, and the National Heart, Lung, and Blood Institute (NHLBI); and is on advisory boards for HeartFlow, Bayer, and Janssen. Dr. Borlaug has received research funding from National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI) (R01 HL128526, U01 HL125205), AstraZeneca, Corvia, Medtronic, Mesoblast, GlaxoSmithKline, and TENAX; and is on advisory boards/consults for Merck, Novartis, Lilly, and Novo Nordisk. Dr. DeVore has received research funding through his institution from the American Heart Association, Amgen, AstraZeneca, Bayer, Intra-Cellular Therapies, American Regent, Inc., the NHLBI, Novartis, and PCORI; has received consulting fees from Amgen, AstraZeneca, Bayer, CareDx, InnaMed, LivaNova, Mardil Medical, Novartis, Procyrion, scPharmaceuticals, Story Health, and Zoll; and has received nonfinancial support from Abbott for educational activities. Dr. Lopes has received research grants from Bristol-Myers Squibb and Pfizer; has received personal fees from Bristol-Myers Squibb, Pfizer, Boehringer Ingelheim, and Bayer AG; and has received grants from Amgen Inc., GlaxoSmithKline, Medtronic PLC, and Sanofi Aventis outside the submitted work. Dr. Mentz has received research support and honoraria from Abbott, American Regent, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim/Eli Lilly, Boston Scientific, Cytokinetics, Fast BioMedical, Gilead, Medtronic, Merck, Novartis, Roche, Sanofi, and Vifor. Dr. Felker has received research funding from NHLBI, AHA, Amgen, Cytokinetics, Merck; and has received consulting fees from Novartis, Amgen, Cytokinetics, Bristol-Myers Squibb, Innolife, Medtronic, EBR Systems, Cardionomic, SC Pharma, and Myokardia. Dr. Hernandez has received research funding from AstraZeneca, GlaxoSmithKline, Merck, and Novartis; and has received consulting fees from AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Merck, Novartis, and Pfizer. Dr. Burkhoff has received institutional grants from Abiomed, Ancora Heart, Tenax Therapeutics, and Fire 1; and has received consulting fees from PVLoops LLC and Axon Therapeutics. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- CO
cardiac output
- CVP
central venous pressure
- HF
heart failure
- HFrEF
heart failure with reduced ejection fraction
- PCWP
mean pulmonary capillary wedge pressure
- SBV
stressed blood volume
- SNB
splanchnic nerve block
- UBV
unstressed blood volume
Footnotes
APPENDIX
For supplemental figures, please see the online version of this paper.
REFERENCES
- 1.Burkhoff D, Tyberg JV. Why does pulmonary venous pressure rise after onset of LV dysfunction: a theoretical analysis. Am J Physiol 1993;265: H1819–28. [DOI] [PubMed] [Google Scholar]
- 2.Fallick C, Sobotka PA, Dunlap ME. Sympathetically mediated changes in capacitance: redistribution of the venous reservoir as a cause of decompensation. Circ Heart Fail 2011;4:669–75. [DOI] [PubMed] [Google Scholar]
- 3.Fudim M, Hernandez AF, Felker GM. Role of volume redistribution in the congestion of heart failure. J Am Heart Assoc 2017;6:e006817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bapna A, Adin C, Engelman ZJ, Fudim M. Increasing blood pressure by greater splanchnic nerve stimulation: a feasibility study. J Cardiovasc Transl Res 2019;13:509–18. [DOI] [PubMed] [Google Scholar]
- 5.Fudim M, Yalamuri S, Herbert JT, Liu PR, Patel MR, Sandler A. Raising the pressure: hemodynamic effects of splanchnic nerve stimulation. J Appl Physiol (1985) 2017;123:126–7. [DOI] [PubMed] [Google Scholar]
- 6.Bradley SE, Childs AW, Combes B, Cournand A, Wade OL, Wheeler HO. The effect of exercise on the splanchnic blood flow and splanchnic blood volume in normal man. Clin Sci 1956;15:457–63. [PubMed] [Google Scholar]
- 7.Flamm SD, Taki J, Moore R, et al. Redistribution of regional and organ blood volume and effect on cardiac function in relation to upright exercise intensity in healthy human subjects. Circulation 1990;81:1550–9. [DOI] [PubMed] [Google Scholar]
- 8.Fudim M, Boortz-Marx RL, Ganesh A, et al. Splanchnic nerve block for chronic heart failure. J Am Coll Cardiol HF 2020;8:742–52. [DOI] [PubMed] [Google Scholar]
- 9.Fudim M, Ganesh A, Green C, et al. Splanchnic nerve block for decompensated chronic heart failure: splanchnic-HF. Eur Heart J 2018;39: 4255–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fudim M, Jones WS, Boortz-Marx RL, et al. Splanchnic nerve block for acute heart failure. Circulation 2018;138:951–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doshi D, Burkhoff D. Cardiovascular simulation of heart failure pathophysiology and therapeutics. J Card Fail 2016;22:303–11. [DOI] [PubMed] [Google Scholar]
- 12.Lau VK, Sagawa K, Suga H. Instantaneous pressure-volume relationship of right atrium during isovolumic contraction in canine heart. Am J Physiol 1979;236:H672–9. [DOI] [PubMed] [Google Scholar]
- 13.Alexander JJ, Sunagawa K, Chang N, Sagawa K. Instantaneous pressure-volume relation of the ejecting canine left atrium. Circ Res 1987;61: 209–19. [DOI] [PubMed] [Google Scholar]
- 14.Maughan WL, Shoukas AA, Sagawa K, Weisfeldt ML. Instantaneous pressure-volume relationship of the canine right ventricle. Circ Res 1979;44:309–15. [DOI] [PubMed] [Google Scholar]
- 15.Suga H, Sagawa K. Instantaneous pressure-volume relationships and their ratio in the excised, supported canine left ventricle. Circ Res 1974;35:117–26. [DOI] [PubMed] [Google Scholar]
- 16.Shoukas AA, Brunner MC. Epinephrine and the carotid sinus baroreceptor reflex: influence on capacitive and resistive properties of the total systemic vascular bed of the dog. Circ Res 1980; 47:249–57. [DOI] [PubMed] [Google Scholar]
- 17.Sakamoto T, Kakino T, Sakamoto K, et al. Changes in vascular properties, not ventricular properties, predominantly contribute to baroreflex regulation of arterial pressure. Am J Physiol Heart Circ Physiol 2015;308:H49–58. [DOI] [PubMed] [Google Scholar]
- 18.Burkhoff D, Dickstein ML, Schleicher T. Harvi 2017. Available at: https://harvi.online. Accessed February 1, 2021.
- 19.Uemura K, Sugimachi M, Kawada T, et al. A novel framework of circulatory equilibrium. Am J Physiol Heart Circ Physiol 2004;286:H2376–85. [DOI] [PubMed] [Google Scholar]
- 20.Rapaport E, Weisbart MH, Levine M. The splanchnic blood volume in congestive heart failure. Circulation 1958;18:581–7. [DOI] [PubMed] [Google Scholar]
- 21.Funakoshi K, Hosokawa K, Kishi T, Ide T, Sunagawa K. Striking volume intolerance is induced by mimicking arterial baroreflex failure in normal left ventricular function. J Card Fail 2014; 20:53–9. [DOI] [PubMed] [Google Scholar]
- 22.Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail 2010;3:588–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borlaug BA. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction. Circ J 2014;78:20–32. [DOI] [PubMed] [Google Scholar]
- 24.Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2017;70:776–803. [DOI] [PubMed] [Google Scholar]
- 25.Wang SY, Manyari DE, Scott-Douglas N, Smiseth OA, Smith ER, Tyberg JV. Splanchnic venous pressure-volume relation during experimental acute ischemic heart failure. Differential effects of hydralazine, enalaprilat, and nitroglycerin. Circulation 1995;91: 1205–12. [DOI] [PubMed] [Google Scholar]
- 26.Wang SY, Manyari DE, Tyberg JV. Cardiac vagal reflex modulates intestinal vascular capacitance and ventricular preload in anesthetized dogs with acute myocardial infarction. Circulation 1996; 94:529–33. [DOI] [PubMed] [Google Scholar]
- 27.Maor E, Grossman Y, Balmor RG, et al. Exercise haemodynamics may unmask the diagnosis of diastolic dysfunction among patients with pulmonary hypertension. European journal of heart failure 2015;17:151–8. [DOI] [PubMed] [Google Scholar]
- 28.Borlaug BA, Reddy YNV. The role of the pericardium in heart failure: implications for pathophysiology and treatment. J Am Coll Cardiol HF 2019;7:574–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barnes RJ, Bower EA, Rink TJ. Haemodynamic responses to stimulation of the splanchnic and cardiac sympathetic nerves in the anaesthetized cat. J Physiol 1986;378:417–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greenway CV. Blockade of reflex venous capacitance responses in liver and spleen by hexamethonium, atropine, and surgical section. Can J Physiol Pharmacol 1991;69:1284–7. [DOI] [PubMed] [Google Scholar]
- 31.Magder S, De Varennes B. Clinical death and the measurement of stressed vascular volume. Crit Care Med 1998;26:1061–4. [DOI] [PubMed] [Google Scholar]
- 32.Maas JJ, Pinsky MR, Aarts LP, Jansen JR. Bedside assessment of total systemic vascular compliance, stressed volume, and cardiac function curves in intensive care unit patients. Anesth Analg 2012;115:880–7. [DOI] [PubMed] [Google Scholar]
- 33.Smiseth OA, Manyari DE, Scott-Douglas NW, et al. The effect of nitroglycerin on pulmonary vascular capacitance in dogs. Am Heart J 1991;121: 1454–9. [DOI] [PubMed] [Google Scholar]
- 34.Tyberg JV, Wang SY, Scott-Douglas NW, et al. The veins and ventricular preload. In: Maruyama Y, Hori M, Janicki JS, editors. Cardiac-Vascular Remodeling and Functional Interaction Tokyo, Japan: Springer Japan; 1997:257–66. [Google Scholar]
- 35.Raya TE, Gay RG, Aguirre M, Goldman S. Importance of venodilatation in prevention of left ventricular dilatation after chronic large myocardial infarction in rats: a comparison of captopril and hydralazine. Circ Res 1989;64:330–7. [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto J, Trippodo NC, Ishise S, Frohlich ED. Total vascular pressure-volume relationship in the conscious rat. Am J Physiol 1980; 238:H823–8. [DOI] [PubMed] [Google Scholar]
- 37.Risoe C, Hall C, Smiseth OA. Effect of enalaprilat on splanchnic vascular capacitance during acute ischemic heart failure in dogs. Am J Physiol 1994;266:H2182–9. [DOI] [PubMed] [Google Scholar]
- 38.Brunner MJ, Shoukas AA, MacAnespie CL. The effect of the carotid sinus baroreceptor reflex on blood flow and volume redistribution in the total systemic vascular bed of the dog. Circ Res 1981; 48:274–85. [DOI] [PubMed] [Google Scholar]
- 39.Rothe CF. Reflex control of veins and vascular capacitance. Physiol Rev 1983;63:1281–342. [DOI] [PubMed] [Google Scholar]
- 40.Rothe CF. Physiology of venous return. An unappreciated boost to the heart. Arch Intern Med 1986;146:977–82. [PubMed] [Google Scholar]
- 41.Rothe CF. Mean circulatory filling pressure: its meaning and measurement. J Appl Physiol (1985) 1993;74:499–509. [DOI] [PubMed] [Google Scholar]
- 42.Shoukas AA, Sagawa K. Control of total systemic vascular capacity by the carotid sinus baroreceptor reflex. Circ Res 1973;33:22–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


