To the editor,
Autosomal dominant gain-of-function (GOF) mutations in the signal transducer and activator of transcription 1 (STAT1) cause heterogeneous manifestations including chronic mucocutaneous candidiasis (CMC), autoimmunity, vascular abnormalities, malignancies, and recurrent respiratory and skin infections (S1). STAT1 GOF patients show increased levels of total STAT1 and its phosphorylated (p) form pSTAT1 after cytokine stimulation. JAK inhibitors (JAKinibs) such as Ruxolitinib and less frequently used Baricitinib have shown to be effective in controlling disease manifestations [1].
Hidradenitis suppurativa (HS) is a chronic inflammatory skin disorder defined by typical lesions, distribution, chronicity, and recurrence [2]. Although tumor necrosis factor (TNF) and interleukin (IL)-1β are key mediators in HS development and progression, increased expression of proinflammatory cytokines such as type I–II Interferons and the Janus Kinase (JAK)-STAT pathway components have been observed in HS lesions. Anti-TNF alpha therapy is currently used in HS refractory to antibiotics and corticosteroids, but recently JAKinibs have been reported to show good clinical responses (CR) (S2, S3).
We describe a patient with STAT1 GOF and HS, a manifestation not yet described. Baricitinib was safe and effective in treating STAT1 GOF-typically associated symptoms as well as HS.
Case Report
A 35-year-old woman was diagnosed with Behçet’s disease aged 11 years. She was born to unrelated parents and her family history was unremarkable. She presented at 5 days of life with recurrent aphthosis and blepharitis, managed with antibiotics (Fig. 1A). During the first 11 years of life, the patient suffered from flare-ups that were treated with antibiotics or corticosteroids. She also presented unspecific arthralgia and CMC (mostly thrush and onychomycosis), the latter attributed to the use of antibiotics and corticosteroids. Blood analysis revealed positive antinuclear antibodies (1/1280), lupus anticoagulant, rheumatoid factor, and negative HLA B51 and B27. As she did not fulfil the international criteria for Behçet’s disease, (3/10 points) incomplete Behçet’s disease was diagnosed based on oral aphthosis and skin lesions and Colchicine plus Azathioprine treatment was initiated.
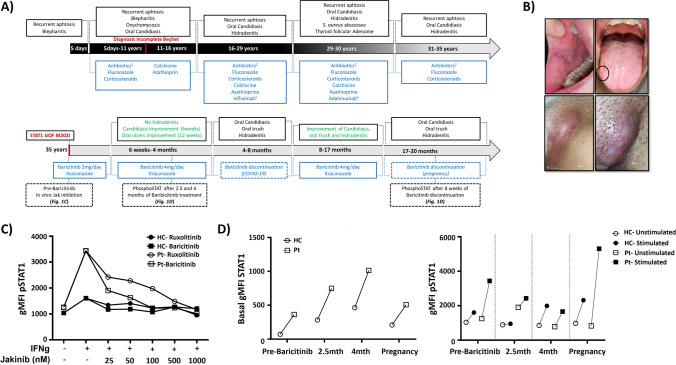
Fig. 1.
Summary of the clinical course of the patient and laboratory results. A Clinical symptoms and treatments prescribed. 1Antibiotics: metronidazole, amoxicillin/clavulanic acid, and minocycline; 2ciprofloxacin and cloxacillin; 3Infliximab 5 mg/kg every 8 weeks; 4Adalimumab 80 mg every 2 weeks. B Clinical features of the patient with oral thrush (top) and hidradenitis lesions (bottom). C IFN-γ-dependent STAT1 activation in patient and healthy control’s CD14 + monocytes in the presence of different Ruxolitinib or Baricitinib concentrations. D IFN-γ-dependent STAT1 activation in patient and healthy control’s CD14 + monocytes at different time points: before Baricitinib initiation, after 2.5 and 4 months, and during Baricitinib discontinuation because of pregnancy. Geometric mean fluorescence intensity (gMFI) of total STAT1 (left) and pSTAT1 (right). The same age and sex matched healthy control was used for all time points
At 16 years of age, she started to suffer from HS. Infliximab was started and continued during the following 13 years despite symptom persistence. At 29 years of age, Infliximab was replaced by Adalimumab. However, treatment was discontinued after 12 months as no benefit was observed and Staphylococcus aureus skin abscesses occurred. She underwent thyroid follicular adenoma surgery. The subsequent withdrawal of immunosuppressive treatment resulted in recurrence of CMC, aphthosis, and HS (Fig. 1B). Symptomatic treatment was reinitiated consisting in antibiotics, fluconazole, and corticosteroids.
At this stage, a gene panel covering CMC-related genes identified a previously described heterozygous variant in STAT1 (c.606G > A, p.Met202IIe) (S1). Immunological work-up (Supplementary material) revealed increased STAT1 and pSTAT1 levels after cytokine stimulation (Fig. 1C and D). Ex vivo treatment (50 nM) with Baricitinib or Ruxolitinib normalized the patient’s pSTAT1 level (Fig. 1C). Oral Baricitinib treatment 2 mg daily was initiated in combination with Itraconazole. Due to symptom persistence, Baricitinib was increased to 4 mg daily. A decrease in the frequency of CMC (within 6 weeks) as well as oral ulcers (within 12 weeks) was observed and after 6 weeks of therapy, no further HS episodes were noted. In concordance with the clinical response (CR), functional assays also showed a pSTAT1 decrease. Whilst total STAT1 levels remained raised throughout the complete follow-up, pSTAT1 levels normalized after 4 months of treatment (Fig. 1D). Baricitinib treatment was interrupted due to administration of the 1st (for 36 days) and 2nd dose of COVID-19 vaccine (for 7 days), cystitis (for 26 days), and mild respiratory SARS-CoV2 infection (for 10 days). Baricitinib discontinuation resulted in reappearance of oral candidiasis and HS within 2 to 4 weeks. JAKinib re-initiation resulted in complete CR within 6–12 weeks with no further recurrence of HS and oral candidiasis. During this period, oral aphthosis was reduced in frequency and duration but has not completely resolved. No adverse events or side effects were noted during the follow-up apart from an upper respiratory tract infection which was treated with oral antibiotics. Recently, Baricitinib was stopped due to pregnancy resulting again in a marked increment in pSTAT1 levels and reoccurrence of symptoms (Fig. 1A and D).
Discussion
We describe an adult patient with a pathogenic STAT1 GOF variant presenting with recurrent oral ulcers, and CMC since childhood. She was initially diagnosed with incomplete Behçet’s disease but later developed HS. Importantly, several lines of standard immunosuppressive therapies did not control her symptoms.
She was eventually diagnosed with STAT1 GOF. Her first symptoms appeared in the 1990s, and CMC, a typical manifestation of STAT1 GOF patients, was then attributed to immunosuppressive treatment explaining the diagnostic delay.
Patients with Behçet’s disease carrying STAT1 GOF mutation have been previously reported. We therefore suggest that in patients with incomplete Behçet’s disease and mucocutaneous candidiasis, monogenic diseases such as STAT1 GOF should be considered despite concomitant immunosuppression. In our case, once STAT1 GOF was diagnosed, JAKinibs were considered. Most STAT1 GOF cases have been treated with Ruxolitinib; however, Baricitinib is increasingly used in rheumatology patients and is known to have less drug interactions, especially in combination with antifungals. In addition, the cost for the adult standard dosing of Ruxolitinib (20 mg daily) in our center is 2.5 times higher than Baricitinib (4 mg daily) treatment. This in addition to our results of ex vivo experiments (Fig. 1C) encouraged the use of Baricitinib. With standard dosing (2–4 mg), plasma concentrations between 50 and 100 nM can be achieved which have been shown to be effective in vitro (Fig. 1C) [3]. In our patient, complete CR was observed for CMC and HS after 6 weeks, and oral ulcers improvement was observed after 12 weeks, confirming previous published observations [1].
Whilst Baricitinib has not been used so far for the systemic treatment of HS, a recent phase II clinical trial reported improvement in quality of life and skin pain by week 1 using the JAKinib INCB054707 (S2). A retrospective study also observed positive CR for the JAKinib Upadacitinib by week 4 in 15/20 patients with HS (S3). In our case, CR was observed for HS with complete resolution of the lesions by week 6 and concurring with a decrease in pSTAT1 levels in monocytes (Fig. 1D). Interestingly, increased expression of the JAK-STAT pathway has been reported in HS lesions [4]. This may suggest a JAK-STAT-mediated physiopathological mechanism and reinforces the potential of JAKinibs in the management of HS.
Of note, Baricitinib treatment was discontinued several times and this resulted in rapid worsening of disease-related symptoms. We and others reported that JAKinib discontinuation might not be always necessary in STAT1 GOF with concomitant viral infections as they remain able to develop virus-specific immune responses after vaccination and are capable to control viral infections ([5], S4). As animal models have shown teratogenic effects of Baricitinib, the drug was stopped during pregnancy but this was followed by the appearance of HS lesions affecting new areas.
In summary, the diagnosis of STAT1 GOF in patients with Behçet’s or Behçet’s-like disease and mucocutaneous candidiasis and/or inappropriate therapy responses should be considered and investigated. Similarly, HS may be a rare, STAT1 GOF–associated disease manifestation. Baricitinib treatment has been described in four STAT1 GOF cases only. We report the benefit of this therapy in a patient refractory to various immunosuppressors and highlight the transitory effect of JAK inhibitors and reoccurrence of symptoms when interrupted as well as its efficacy when reinitiated. Interestingly, withdrawal symptoms were not observed in our case. As more and more patients receive Jakinib therapy, questions regarding its safety during infectious episodes and physiologic events such as growth and pregnancy need further evaluation. Prospective studies investigating long-term JAKinib effects are needed. In this regard, an international working group [under the umbrella of ESID (European Society for Immunodeficiencies), IEWP (Inborn Error Working Party), and ERN-RITA (European reference network- Rare Immunodeficiency, Autoinflammatory and Autoimmune Diseases)] is currently elaborating consensus guidelines aiming to propose homogenous criteria for JAKinib treatment indication, dosing and monitoring.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank the patient and the healthy controls participating in this study.
Inborn Errors of Immunity (IEI), Dermatology and Rheumatology Research group:
Paloma Guisado Hernández1, Mercedes Rodríguez Serna3, Begoña Escutia Muñoz3, José A Román Ivorra2, Beatriz de Felipe Carrillo1, Elena Grau García2, José Cervera Zamora4, Teresa Jaijo Sanchís4
1Paediatric Infectious Diseases, Rheumatology and Immunology Unit, Hospital Universitario Virgen del Rocío, Instituto de Biomedicina de Sevilla, IBiS/ Universidad de Sevilla/CSIC, Red de Investigación Translacional en Infectología Pediátrica RITIP, Seville, Spain
2Servicio de Reumatología, Hospital Universitari i Politècnic La Fe, Valencia, Spain
3Servicio de Dermatología, Hospital Universitari i Politècnic La Fe, Valencia, Spain
4Unidad de Genética, Hospital Universitari i Politècnic La Fe, Valencia, Spain
Funding
This work was supported by Consejería de Salud de la Junta de Andalucía (SA0051/2020 to O.N.), Agencia de Innovación y Desarrollo de Andalucía (PI-0184–2018 to P.O.), Instituto de Salud Carlos III, Madrid, Spain [Sara Borrell, CD20/00124 to P.B.L, Juan Rodés JR18/00042 to P.O, FIS PI19/01471], the Job Research Foundation (NY, USA), and private donation from Zabrowski family.
Declarations
Ethics Approval
This study was performed in line with the principles of the Ethics Committee of the Hospitales Universitarios Virgen Macarena and Virgen del Rocío (0243-N-19).
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Olaf Neth, Email: oneth-ibis@us.es.
IEI, Dermatology and Rheumatology Research group:
Paloma Guisado Hernández, Mercedes Rodríguez Serna, Begoña Escutia Muñoz, José A Román Ivorra, Beatriz de Felipe Carrillo, Elena Grau García, José Cervera Zamora, and Teresa Jaijo Sanchís
References
- 1.Deya-Martinez A, Riviere JG, Roxo-Junior P, Ramakers J, Bloomfield M, Guisado Hernandez P, et al. Impact of JAK inhibitors in pediatric patients with STAT1 gain of function (GOF) mutations-10 children and review of the literature. J Clin Immunol. 2022;42(5):1071–1082. doi: 10.1007/s10875-022-01257-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sabat R, Jemec GBE, Matusiak L, Kimball AB, Prens E, Wolk K. Hidradenitis suppurativa. Nat Rev Dis Primers. 2020;6(1):18. doi: 10.1038/s41572-020-0149-1. [DOI] [PubMed] [Google Scholar]
- 3.Shi JG, Chen X, Lee F, Emm T, Scherle PA, Lo Y, et al. The pharmacokinetics, pharmacodynamics, and safety of baricitinib, an oral JAK 1/2 inhibitor, in healthy volunteers. J Clin Pharmacol. 2014;54(12):1354–1361. doi: 10.1002/jcph.354. [DOI] [PubMed] [Google Scholar]
- 4.Rumberger BE, Boarder EL, Owens SL, Howell MD. Transcriptomic analysis of hidradenitis suppurativa skin suggests roles for multiple inflammatory pathways in disease pathogenesis. Inflamm Res. 2020;69(10):967–973. doi: 10.1007/s00011-020-01381-7. [DOI] [PubMed] [Google Scholar]
- 5.Guisado Hernandez P, Blanco Lobo P, Villaoslada I, de Felipe B, Lucena JM, Martin Gutierrez G, et al. SARS-CoV-2 infection in a pediatrics STAT1 GOF patient under Ruxolitinib therapy-a matter of balance? J Clin Immunol. 2021;41(7):1502–1506. doi: 10.1007/s10875-021-01081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.