Background:
Ceftolozane/tazobactam, a cephalosporin-β-lactamase inhibitor combination, active against multidrug-resistant Gram-negative pathogens, is approved for treatment of adults with complicated urinary tract infections (cUTI). Safety and efficacy of ceftolozane/tazobactam in pediatric participants with cUTI, including pyelonephritis, were assessed.
Methods:
This phase 2 study (NCT03230838) compared ceftolozane/tazobactam with meropenem for treatment of cUTI in participants from birth to <18 years of age. The primary objective was safety and tolerability. Key secondary end points included clinical cure and per-participant microbiologic response rates at end of treatment (EOT) and test of cure (TOC) visits.
Results:
The microbiologic modified intent-to-treat (mMITT) population included 95 participants (ceftolozane/tazobactam, n = 71; meropenem, n = 24). The most common diagnosis and pathogen were pyelonephritis (ceftolozane/tazobactam, 84.5%; meropenem, 79.2%) and Escherichia coli (ceftolozane/tazobactam, 74.6%; meropenem, 87.5%); 5.7% (ceftolozane/tazobactam) and 4.8% (meropenem) of E. coli isolates were extended-spectrum β-lactamase-producers. Rates of adverse events were similar between treatment groups (any: ceftolozane/tazobactam, 59.0% vs. meropenem, 60.6%; drug-related: ceftolozane/tazobactam, 14.0% vs. meropenem, 15.2%; serious: ceftolozane/tazobactam, 3.0% vs. meropenem, 6.1%). Rates of clinical cure for ceftolozane/tazobactam and meropenem at EOT were 94.4% and 100% and at TOC were 88.7% and 95.8%, respectively. Rates of microbiologic eradication for ceftolozane/tazobactam and meropenem at EOT were 93.0% and 95.8%, and at TOC were 84.5% and 87.5%, respectively.
Conclusions:
Ceftolozane/tazobactam had a favorable safety profile in pediatric participants with cUTI; rates of clinical cure and microbiologic eradication were high and similar to meropenem. Ceftolozane/tazobactam is a safe and effective new treatment option for children with cUTI, especially due to antibacterial-resistant Gram-negative pathogens.
Keywords: cUTI, antibacterial, pyelonephritis, Escherichia coli
Multidrug-resistant (MDR) Gram-negative bacteria, including Enterobacterales and Pseudomonas aeruginosa, are well recognized as a global public health issue.1,2 Complicated urinary tract infections (cUTI), including pyelonephritis, are characterized by pyuria, presence of microbial pathogen(s) on culture of urine or blood, local and systemic signs and symptoms of infection [fever (>38°C), chills, malaise, flank pain, back pain, and/or costo-vertebral angle pain/tenderness], and occur in the presence of a functional or anatomical abnormality of the urinary tract or in the presence of catheterization.3 The most common causative pathogens for pediatric urinary tract infections are Escherichia coli (>80%), followed by Klebsiella pneumoniae, P. aeruginosa, Enterococcus spp.‚ and Proteus spp., with each isolated in approximately 4% of cases or less.4,5 Increasingly, neonatal and pediatric UTI are caused by antibacterial-resistant Gram-negative pathogens, with an estimated global pooled prevalence of extended-spectrum β-lactamase (ESBL)–producing Enterobacterales of 14% among pediatric UTI cases; rates are higher in patients with recurrent UTIs caused by uropathies such as vesicoureteral reflux in some parts of the world.6,7 Antibacterial resistance may be even more prevalent among P. aeruginosa isolates from patients with nosocomial UTI, with a global rate of nonsusceptibility to ceftazidime in adults and children between 20.3% and 50%.8
UTI are common in the pediatric population, with prevalence rates of 7.0% among febrile infants ≤24 months of age, and 7.8% among children <19 years of age with urinary symptoms and/or fever.9 Compared with children without uropathies, those with vesicoureteral reflux have higher 2-year rates of recurrent UTI (25.4% vs. 17.3%).10 Children with cUTI are at risk for complications including bacteremia, renal scarring‚ and chronic abdominal pain.11–13 There is significant unmet medical need for new antibacterial agents that are approved for use in adults to be available for the neonatal and pediatric populations to provide more options for cUTI treatment should local resistance rates indicate the need for alternative therapy.
Ceftolozane/tazobactam is a cephalosporin-β-lactamase inhibitor combination14 approved to treat cUTI, including pyelonephritis, complicated intra-abdominal infection (cIAI)‚ and nosocomial pneumonia in adults.15 In a large phase 3 study in adults (ASPECT-cUTI), ceftolozane/tazobactam had a favorable safety profile and was effective for the treatment of cUTI.16 Ceftolozane/tazobactam was recently approved for the treatment of cUTI in pediatric patients in the United States (birth to <18 years of age).15
There are limited data related to the use of ceftolozane/ tazobactam in children and adolescents, although a pharmacokinetic study indicated that exposures in pediatric participants are comparable with those observed in adults.17 This study assessed the safety and efficacy of ceftolozane/tazobactam compared with meropenem for the treatment of cUTI, including pyelonephritis, in neonatal and pediatric participants.
MATERIALS AND METHODS
Study Design
This was a phase 2, randomized, double-blind study (NCT03230838; EudraCT 2016-004153-32; protocol MK-7625A-034) in pediatric participants with cUTI, including pyelonephritis, conducted at 28 study sites in 8 countries across Western Europe, Eastern Europe‚ and North America between April 2018 and December 2020. The study was conducted in accordance with the principles of Good Clinical Practice and the protocol was approved by the appropriate institutional review boards and regulatory agencies. All participants had a legally acceptable representative, and documented informed consent/assent was provided for the study. Blinding, randomization, and masking procedures are included in Methods, Supplemental Digital Content 1, http://links.lww.com/INF/E917.
Participants
Male and female participants from birth (defined as >32 weeks gestational age and ≥7 days postnatal) to <18 years of age were eligible. Eligible participants required intravenous (IV) antibacterial therapy for the treatment of cUTI, had a pretreatment baseline urine culture specimen obtained within 48 hours before the administration of the first dose of study treatment, and had pyuria. Participants were also required to have had clinical signs and/or symptoms of cUTI (pyelonephritis or cUTI with a urological abnormality); the clinical diagnosis was at the discretion of the investigator. Participants were excluded if they had a history of cUTI within the previous year caused by a pathogen known to be resistant to either IV study treatment, a concomitant infection that required nonstudy systemic antibacterial therapy (antibacterials with only Gram-positive activity were permitted), receipt of potentially therapeutic antibacterial therapy for >24 hours during the 48 hours preceding the first dose of study treatment (except in cases of participants receiving ≥48 hours of prior antibacterial therapy who were deemed to have failed treatment)‚ or had moderate or severe impairment of renal function (defined as an estimated creatinine clearance <50 mL/min/1.73 m2). Full inclusion and exclusion criteria are provided in Methods, Supplemental Digital Content 1, http://links.lww.com/INF/E917.
The microbiologic modified intent-to-treat (mMITT) population included all randomized participants who received any amount of study treatment and had ≥1 causative uropathogen from a study-qualifying baseline urine culture. The safety population consisted of all randomized participants who received any amount of study treatment.
Treatment
Randomized participants were stratified and dosed by age group. The selected doses were based on population pharmacokinetic modeling and simulations.18 For participants in the ceftolozane/tazobactam group, those 12 to <18 years of age were given 1.0 g ceftolozane and 0.5 g tazobactam (the dose indicated for adult patients with cUTI),15 and those from birth to <12 years of age were given 20 mg/kg ceftolozane and 10 mg/kg tazobactam (maximum of 1.0 g ceftolozane and 0.5 g tazobactam per dose). All participants in the meropenem group received 20 mg/kg (maximum of 1.0 g per dose), with higher dosing up to 30 mg/kg for participants who were 14 days to <3 months of age permitted at the investigator’s discretion. Each dose of ceftolozane/tazobactam or meropenem was administered as a 60-minute (±10 minutes) infusion and dosed every 8 hours (±1 hour) after the previous infusion.
Treatment duration was 7-14 days. After 3 days (9 doses) of IV therapy, optional open-label, standard-of-care, oral step-down therapy was permitted at the investigator’s discretion, with choice of therapy guided by culture and antibacterial susceptibility results, as well as local standard of care for treatment of cUTI. Recommended options for oral step-down therapy were β-lactam/β-lactamase inhibitor combinations, cephalosporins, fluoroquinolones, nitrofurantoin, trimethoprim‚ or trimethoprim/sulfamethoxazole.
Specimen Collection
A baseline urine sample for culture was obtained ≤48 hours before the start of administration of the first dose of study treatment. Urine specimens were obtained by suprapubic aspiration, clean urethral catheterization, indwelling urethral catheter‚ or midstream clean catch. Additional details of specimen collection and culture of urine specimens are included in Methods, Supplemental Digital Content 1, http://links.lww.com/INF/E917.
Assessments and End Points
Clinical and microbiologic assessments were performed at the end of treatment (EOT) visit, scheduled <24 hours after the last IV dose of therapy or <48 hours after the last dose of oral step-down therapy (if applicable), the test of cure (TOC) visit, occurring 5–9 days after the last dose of study treatment (IV or oral) and the end of IV therapy (EOIV) visit (<24 hours after the last of IV therapy).
The primary end points were rates of adverse events (AEs) and changes in laboratory values and vital signs through the last follow-up visit, which occurred 28-35 days after the last dose of study treatment (IV or oral). In all treated participants, AEs were evaluated from the first dose of study treatment to the last study evaluation. Key secondary end points included rates of clinical success and per-participant microbiologic eradication, defined as the proportion of participants who had a clinical response of cure and proportion of participants who had microbiologic eradication or presumed eradication at the EOT and TOC visits, respectively. Additional exploratory end points included per-pathogen microbiologic eradication, which was determined for each uropathogen isolated from the baseline study-qualifying culture, clinical response at the EOIV visit‚ and composite success, defined as a clinical response of success and per-participant microbiologic response of eradication. See additional details about end points in Methods, Supplemental Digital Content 1, http://links.lww.com/INF/E917.
Statistical Analysis
No formal hypothesis testing was performed. For the primary safety analysis, 95% CIs were derived for the between-treatment differences in the percentage of participants with events, and these analyses were performed using the unstratified Miettinen & Nurminen method.19 Changes in laboratory and vital sign values from baseline were summarized using descriptive statistics. For the secondary efficacy analyses, 2-sided 95% CIs based on the Miettinen & Nurminen method19 and stratified by age group were used to evaluate the treatment differences for clinical success and per-participant microbiologic eradication at the EOT and TOC visits. The sample size calculation is described in Methods, Supplemental Digital Content 1, http://links.lww.com/INF/E917.
RESULTS
Study Participants
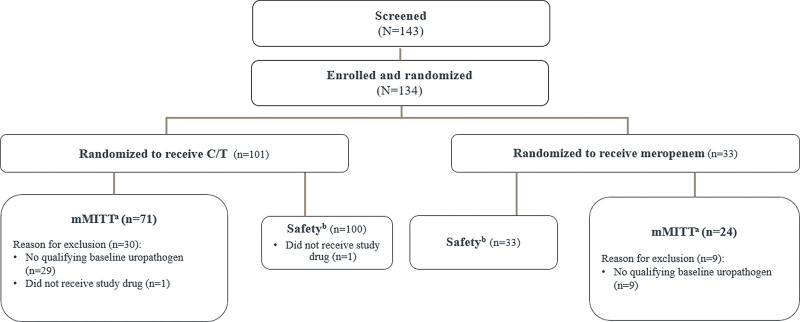
A total of 143 participants were screened and 134 were randomized. One randomized participant in the ceftolozane/tazobactam group was not treated because of a temperature excursion of the study treatment. The remaining 133 participants were randomized in a 3:1 ratio and treated with ceftolozane/tazobactam (n = 100) or meropenem (n = 33; Fig. 1). The mMITT population included 95 participants in the ceftolozane/tazobactam (n = 71) and meropenem (n = 24) groups. The most common reason for mMITT exclusion was lack of a qualifying baseline uropathogen (ceftolozane/ tazobactam, 28.7%; meropenem, 27.3%). One participant in the ceftolozane/tazobactam group had an AE of exacerbation of chronic kidney disease leading to discontinuation of study treatment. This AE met the protocol-defined discontinuation criterion excluding participants with creatinine clearance <50 mL/min and was not considered to be drug-related.
FIGURE 1.
Study disposition of all randomized participants. aRandomized participants who received any amount of study drug and have at least 1 acceptable causative uropathogen identified from a study-qualifying baseline urine culture. bAll randomized participants who received any amount of study treatment. C/T indicates ceftolozane/tazobactam; mMITT, microbiologic modified intent-to-treat.
The baseline characteristics of participants in each treatment group were comparable (Table 1). Pyelonephritis was the most common baseline diagnosis, occurring in 83.2% of participants overall. Among those participants with cUTI, the most common underlying problems were recurrent UTI (33.7%), congenital abnormalities of the urogenital tract (28.4%)‚ and anatomic abnormalities of the urogenital tract (27.4%). Other underlying problems were functional abnormalities of the urogenital tract (21.1%), obstructive uropathies (7.4%), recent bladder instrumentation (3.2%)‚ and temporary indwelling catheter (2.1%). More than one underlying problem per participant may have been present. Bacteremia was present in 3 participants in each treatment group (ceftolozane/tazobactam, 4.2%; meropenem, 12.5%). Urine samples were collected by catheter in 29 (40.8%) participants in the ceftolozane/tazobactam group and in 9 (37.5%) participants in the meropenem group, by midstream clean catch in 37 (52.1%) participants in the ceftolozane/tazobactam group and in 13 (54.2%) participants in the meropenem group and by suprapubic aspiration in 5 (7.0%) participants in the ceftolozane/tazobactam group and in 2 (8.3%) participants in the meropenem group.
TABLE 1.
Participant Demographics and Baseline Characteristics (mMITT Population)
| Characteristic | C/T (N = 71) | MEM (N = 24) |
|---|---|---|
| Female, n (%) | 40 (56.3) | 15 (62.5) |
| Age, n (%) | ||
| Birth to <3 mo* | 14 (19.7) | 6 (25.0) |
| 3 mo to <2 yr | 20 (28.2) | 6 (25.0) |
| 2 to <6 yr | 14 (19.7) | 6 (25.0) |
| 6 to <12 yr | 13 (18.3) | 4 (16.7) |
| 12 to <18 yr | 10 (14.1) | 2 (8.3) |
| White race, n (%)† | 70 (98.6) | 24 (100.0) |
| Median (range) weight, kg | 13.0 (2.6–75.3) | 10.4 (3.8–54.0) |
| Baseline diagnosis, n (%) | ||
| Pyelonephritis | 60 (84.5) | 19 (79.2) |
| cUTI with a urological abnormality | 11 (15.5) | 5 (20.8) |
| Bacteremia at baseline, n (%) | 3 (4.2) | 3 (12.5) |
| Urine sample collected via urinary catheter, n (%) | 29 (40.8) | 9 (37.5) |
| Baseline CrCl (mL/min/1.73 m2),‡ n (%) | ||
| CrCl ≥80 | 48 (67.6) | 16 (66.7) |
| CrCl ≥50 to <80 | 23 (32.4) | 7 (29.2) |
| CrCl ≥30 to <50 | 0 | 1 (4.2)§ |
| Failure of prior antibacterial therapy, n (%) | 3 (4.2) | 0 |
All enrolled participants in this age group were full-term neonates.
Race was determined by the investigator during the screening visit.
CrCl rates were calculated by the revised Schwartz equation at baseline.
One participant was enrolled with a baseline CrCl of 36.6 mL/min/1.73 m2; however, a follow-up CrCl measurement was >50 mL/min/1.73 m2, and the participant remained in the study.
C/T indicates ceftolozane/tazobactam; CrCl, creatinine clearance; MEM, meropenem, mMITT, microbiologic modified intent-to-treat.
Pathogens at Baseline
Nearly all infections were monomicrobial (ceftolozane/ tazobactam, 98.6%; meropenem, 95.8%). E. coli was the most common qualifying baseline uropathogen in the mMITT population [ceftolozane/tazobactam, 53 (74.6%); meropenem, 21 (87.5%)], followed by K. pneumoniae [ceftolozane/tazobactam, 6 (8.5%); meropenem, 1 (4.2%)], and P. aeruginosa [ceftolozane/tazobactam, 5 (7.0%); meropenem, 2 (8.3%)]. Susceptibility rates among aerobic Gram-negative pathogens to both drugs were high [ceftolozane/tazobactam, 91/92 (98.9%); meropenem, 91/94 (96.8%)]. Among the E. coli isolates (n = 74), 5.4% were ESBL producers (ceftolozane/tazobactam, 5.7%; meropenem, 4.8%); all were susceptible to ceftolozane/tazobactam. A single carbapenemase-producing pathogen was isolated, a blaOXA-48-carrying K. pneumoniae isolate. No K. pneumoniae isolates were ESBL producers; 100% (7/7) were susceptible to ceftolozane/tazobactam. None of the 7 P. aeruginosa isolates overexpressed AmpC at baseline, and all were susceptible to ceftolozane/tazobactam. Antimicrobial activity of ceftolozane/tazobactam and meropenem against key pathogens is shown in the Table, Supplemental Digital Content 2, http://links.lww.com/INF/E918.
Safety
The mean (SD) duration of IV treatment was 6.1 (2.7) days and 5.7 (2.2) days in the ceftolozane/tazobactam and meropenem groups, respectively. Overall mean (SD) treatment duration (both IV and oral step-down) in the mMITT population was comparable in both treatment groups [ceftolozane/tazobactam, 10.2 (2.7) days; meropenem, 10.7 (2.1) days]. A total of 50 (70.4%) and 20 (83.3%) participants transitioned to optional oral step-down therapy in the ceftolozane/tazobactam and meropenem groups, for a mean (SD) duration of 5.8 (1.72) days and 6.0 (1.5) days, respectively. The most common oral step-down treatments (>10% in either treatment group) were cefixime, amoxicillin/clavulanate potassium‚ and cefuroxime; choice of oral step-down therapy was determined at the investigator’s discretion.
The overall incidences of AEs (all and drug-related), serious AEs (SAEs) and AEs leading to discontinuation were comparable between the ceftolozane/tazobactam and meropenem groups (Table 2). There were no AEs leading to death, drug-related SAEs‚ or discontinuations due to drug-related AEs or SAEs. Three participants (3.0%) in the ceftolozane/tazobactam group and 2 participants (6.1%) in the meropenem arm experienced SAEs, all of which were unrelated to study drug. The most commonly reported AEs and most commonly reported drug-related AEs are listed in Table 2. Overall, 5 participants in the ceftolozane/ tazobactam group had an AE of neutropenia (which was considered drug-related in 3 participants), and 2 additional participants in the ceftolozane/tazobactam group had an AE of decreased neutrophil count, 1 of which was considered drug-related. Of the 5 neutropenia cases, there was 1 mild and 2 each of moderate and severe, with durations ranging from 2.1 weeks to 1.5 months (1 participant was lost to follow-up after day 3 following withdrawal of parental consent). Both cases of decreased neutrophil count were classified as moderate, with one case having a duration of 1.1 months and the other lost to follow-up due to issues with IV access. Classification of these events was based on the investigator’s clinical judgement. Neutropenia or decreased neutrophil counts were not seen in the meropenem group. No clinically meaningful differences in mean change from baseline in neutrophil-related laboratory parameters were observed between the treatment groups. Five of the 7 participants were reported to have recovered. The 2 remaining participants were lost to follow-up; 1 participant withdrew on day 3 of the study because of loss of parental consent due to concern over pain associated with pharmacokinetic sampling, and 1 participant withdrew because of loss of parental consent due to IV access issues. There was no follow-up on either of these participants.
TABLE 2.
Adverse Events (All Treated Participants Population)
| Participants With, n (%) | C/T (N = 100) | MEM (N = 33) | Difference*† (95% CI) |
|---|---|---|---|
| ≥1 AE | 59 (59.0) | 20 (60.6) | −1.6 (−19.7 to 17.9) |
| Drug-related‡ AEs | 14 (14.0) | 5 (15.2) | −1.2 (−18.0 to 10.9) |
| Serious AEs§ | 3 (3.0) | 2 (6.1) | −3.1 (−16.9 to 3.9) |
| Serious drug-related‡ AEs | 0 | 0 | – |
| Death | 0 | 0 | – |
| Discontinuation due to AE | 1 (1.0) | 0 | 1.0 (−9.5 to 5.5) |
| Discontinuation due to drug-related‡ AE | 0 | 0 | – |
| Discontinuation due to serious AE | 0 | 0 | – |
| Most commonly reported AEs¶ | |||
| Thrombocytosis | 7 (7.0) | 3 (9.1) | – |
| Diarrhea | 7 (7.0) | 3 (9.1) | – |
| Urinary tract infection | 1 (1.0) | 3 (9.1) | – |
| Most commonly reported drug-related AEs‖ | |||
| Diarrhea | 3 (3.0) | 3 (9.1) | – |
| Eosinophilia | 0 | 1 (3.0) | – |
| Frequent bowel movements | 0 | 1 (3.0) | – |
| Increased appetite | 3 (3.0) | 0 | – |
| Increased aspartate aminotransferase | 2 (2.0) | 1 (3.0) | – |
| Neutropenia | 3 (3.0) | 0 | – |
| Phlebitis | 0 | 1 (3.0) | – |
Difference in C/T minus MEM.
Based on Miettinen & Nurminen method.
Determined by the investigator to be related to the study drug.
Serious AEs in the C/T group were pyelonephritis (2 participants) and upper respiratory tract infection (1 participant); in the MEM group, the serious AEs were hypertension and pyrexia (1 participant each).
Incidence ≥7% in ≥1 treatment arm.
Incidence ≥3% in ≥1 treatment arm.
AE indicates adverse event; CI, confidence interval; C/T, ceftolozane/tazobactam; MEM, meropenem.
Efficacy
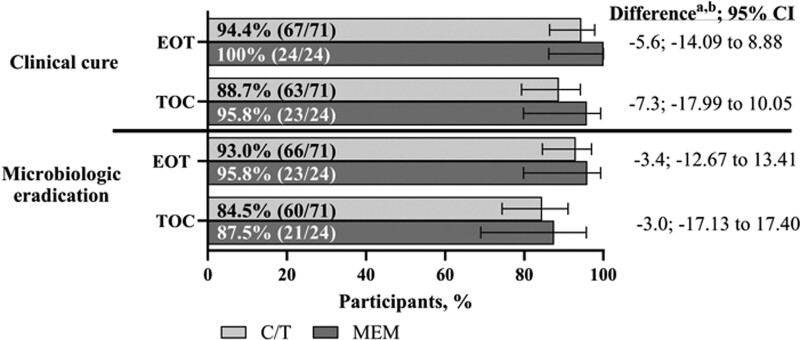
Clinical cure rates in the mMITT population were 94.4% and 100% for the ceftolozane/tazobactam and meropenem treatment groups, respectively, at the EOT visit, and 88.7% (ceftolozane/tazobactam) and 95.8% (meropenem) at the TOC visit (Fig. 2). Likewise, microbiologic eradication rates were 93.0% (ceftolozane/tazobactam) and 95.8% (meropenem) at the EOT visit and 84.5% (ceftolozane/tazobactam) and 87.5% (meropenem) at the TOC visit. (Fig. 2). Of the clinical failures at the EOT visit in the ceftolozane/tazobactam group, 2 (2.8%) were observed failures and 2 (2.8%) were classified as failures owing to having missing clinical response data (indeterminate response, thus imputed as a failure). Of the clinical failures at TOC, 1 (4.2%) in the meropenem group was an observed failure; within the ceftolozane/tazobactam group, 4 (5.6%) were observed failures, 1 (1.4%) was observed indeterminate and 3 (4.2%) were classified as clinical cure, partial improvement‚ or indeterminate at the previous visit but had missing clinical response data at the TOC visit.
FIGURE 2.
Clinical and microbiologic response at EOT and TOC (mMITT population). aDifference in C/T minus MEM. bThe percent difference was based on the Miettinen & Nurminen method stratified by age group with Cochran-Mantel-Haenszel weights. If there was a zero count in any class of the stratum, the groups with the lower count were pooled with the near age group stratum in the model. CI indicates confidence interval; C/T‚ ceftolozane/tazobactam; MEM, meropenem; mMITT, microbiologic modified intent-to-treat population; TOC, test of cure.
These results were generally consistent for the per-pathogen microbiologic response (Table 3), composite response (see Table, Supplemental Digital Content 3, http://links.lww.com/INF/E919), and all end points at the EOIV visit (see Table, Supplemental Digital Content 4, http://links.lww.com/INF/E920). Clinical cure rates were 100% in both treatment groups for the 4 total participants with ESBL-producing E. coli at baseline (ceftolozane/tazobactam, n = 3; meropenem, n = 1).
TABLE 3.
Per-pathogen Microbiologic Response at EOT and TOC (mMITT Population)
| EOT | TOC | |||
|---|---|---|---|---|
| Eradication by Pathogen, n/N1 (%) | C/T (N = 71) | MEM (N = 24) | C/T (N = 71) | MEM (N = 24) |
| All Enterobacterales | 62/66 (93.9) | 21/22 (95.5) | 58/66 (87.9) | 19/22 (86.4) |
| Escherichia coli | 50/53 (94.3) | 20/21 (95.2) | 45/53 (84.9) | 18/21 (85.7) |
| Klebsiella pneumoniae | 5/6 (83.3) | 1/1 (100) | 6/6 (100) | 1/1 (100) |
| Pseudomonas aeruginosa | 4/5 (80.0) | 2/2 (100) | 3/5 (60) | 2/2 (100) |
C/T indicates ceftolozane/tazobactam; EOT, end of treatment; MEM, meropenem; N1, number of participants in mMITT population for each specific pathogen; TOC, test of cure.
DISCUSSION
This study and the companion pediatric study of cIAI20 (NCT03217136) are the first randomized, active-controlled clinical studies to evaluate the safety and efficacy of ceftolozane/tazobactam treatment in the pediatric population. In this phase 2 study, ceftolozane/tazobactam had a favorable safety profile in children from birth (full-term neonates) to <18 years of age, comparable to that of meropenem for the treatment of cUTI. Furthermore, the safety profile of ceftolozane/tazobactam was consistent with that observed in the cUTI study in adults.16 Seven participants in the ceftolozane/tazobactam group had neutrophil-related AEs, but none of the events were considered serious and none led to discontinuation of study drug. Both participants with neutrophil-related AEs who discontinued from the study did so for reasons unrelated to the AE. There were no neutrophil-related AEs in the companion pediatric study mentioned above.20 Overall, no new safety concerns were identified for ceftolozane/tazobactam in this study or in the companion pediatric study of cIAI infection, and the results align with previously reported results about the safety of ceftolozane/tazobactam in adults.16 Based on these studies, ceftolozane/ tazobactam was recently approved in the United States for the treatment of both cUTI and cIAI in pediatric patients (birth to less than 18 years of age).15
Rates of clinical cure and microbiologic eradication were high at EOT and TOC for both treatment groups, and the results for exploratory efficacy endpoints were also comparable between treatment groups. While this study was not powered for comparison of between-group efficacy outcomes, the high clinical and microbiologic response rates observed were consistent with studies of ceftolozane/tazobactam in adults with cUTI.16,21,22
In the current study, E. coli was the most common pathogen identified in the mMITT population, followed by K. pneumoniae and P. aeruginosa. This is consistent with other studies in children with cUTI,23–25 suggesting that this participant population and the causative pathogens were representative of a typical pediatric population with cUTI. MDR pathogens were not common in this global study; 5.7% (ceftolozane/tazobactam) and 4.8% (meropenem) of E. coli isolates were ESBL producers. These isolates were from participants in Hungary and Turkey. Clinical cure occurred in all cases in both treatment groups for participants with ESBL-producing E. coli. This is similar to clinical response rates observed in the phase 3 clinical study of ceftolozane/tazobactam in adults with cUTI, in which clinical success was seen in 90.2% of participants with ESBL-producing Enterobacterales, including E. coli and K. pneumoniae.26 Surveillance study data also demonstrate that ceftolozane/tazobactam activity remains high against Gram-negative bacteria, including ESBL-producing strains,27–29 but MDR P. aeruginosa is becoming more common, where resistance to ceftazidime/avibactam has been reported to range from 2.9%30 to 18.0%,31 underscoring the need to have additional antibacterial options available to treat serious pediatric infections.
A limitation of this study is its small sample size, which is typical for pediatric studies. Its strength, however, is that it includes not only adolescents and children, but also 31 participants between 3 months and 2 years of age, and 22 participants from birth to 3 months of age, making the results generalizable to a broad range of pediatric age groups, including neonates. In addition, integrating the safety data from this study with the phase 2 study in pediatric participants with cIAI20 allowed for a more thorough assessment of the safety of ceftolozane/tazobactam in the pediatric population. A subanalysis of safety and efficacy in neonates and young infants <3 months of age will be published separately.
In this study, ceftolozane/tazobactam had a favorable safety profile that was comparable to meropenem and to the previously reported safety profile for ceftolozane/tazobactam in adults with cUTI. Furthermore, ceftolozane/tazobactam achieved high clinical cure and microbiologic eradication rates. Thus, ceftolozane/tazobactam is a safe and effective new treatment option active against antimicrobial-resistant Gram-negative bacteria for children, including neonates and young infants with cUTI.
ACKNOWLEDGMENTS
We thank the study participants and the investigators who made this study possible, particularly, as they dealt with the challenges imparted by the COVID-19 pandemic. Investigators included Emmanuel Roilides (Thessaloniki, Greece), Athanasios Michos (Athens, Greece), George A. Syrogiannopoulos (Larissa, Greece), Maria Tsolia (Athens, Greece), Vassiliki Papaevangelou (Athens, Greece), Laszlo Szabo (Budapest, Hungary), Tamas Szabo (Debrecen, Hungary), Csaba Bereczki (Szeged, Hungary), Ferenc Dicso (Bereg, Hungary), Tamas Decsi (Pecs, Hungary), Attila Szabo (Budapest, Hungary), Mercedes Macias Parra (Mexico City, Mexico), Martin Guerrero Becerra (Guadalajara, Mexico), Sarbelio Moreno Espinosa (Mexico City, Mexico), Cesar Adrian Martinez Longoria (Monterrey, Mexico), Irena Daniluk-Matras (Bydgoszcz, Poland), Marcin Tkaczyk (Lodz, Poland), Malgorzata Pawlowska (Bydgoszcz, Poland), Przemyslaw Janik (Torun, Poland), Beata Jurkiewicz (Lomianki, Poland), Dorota Drozdz (Krakow, Poland), Oana Falup-Pecurariu (Brasov, Romania), Ruxandra Beatris Neagu-Draghicenoiu (Bucuresti, Romania), Mihaela Balgradean (Bucharest, Romania), Dan Ioan Delean (Cluj-Napoca, Romania), Antonina Petrovna Zouzova (Smolensk, Russia), Sergey Viktorovich Minaev (Stavropol, Russia), Maria N. Kostyleva (Moscow, Russia), Anna Nikolaevna Galustyan (Saint Petersburg, Russia), Peter Nourse (Cape Town, South Africa), Rajendra Bhimma (Durban, South Africa), Jan Hendrik Reynor Becker (Pretoria, South Africa), Derya Alabaz (Adana, Turkey), Ates Kara (Ankara, Turkey), Ener Cagri Dinleyici (Eskisehir, Turkey), Nazan Dalgic (Istanbul, Turkey), Nataliia Dementieva (Dnipro, Ukraine), Ihor Ksonz (Poltava, Ukraine), Tetyana V. Lytvynova, (Kryvyy Rig, Ukraine), Natalia Karpenko (Kyiv, Ukraine), Igor Antonyan (Kharkiv, Ukraine), Oleksandr Fofanov (Ivano-Frankivsk, Ukraine), Nataliia Makieieva (Kharkiv, Ukraine), Negar Ashouri, (Orange, CA, USA), John S. Bradley (San Diego, CA, USA), Jason G. Newland (St. Louis, MO, USA), Michael N. Neely (Los Angeles, CA, USA), Leonard B. Weiner (Syracuse, NY, USA), Michael Bolton (Baton Rouge, LA, USA), Sowdhamini Wallace (Houston, TX, USA), William J. Muller (Chicago, IL, USA), and Jen-Jar Lin (Winston-Salem, NC, USA). Medical writing and/or editorial assistance was provided by Emily Burke, PhD, of The Lockwood Group, Stamford, CT, USA. This assistance was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. A portion of this study was conducted during the COVID-19 pandemic, and all standard operating procedures for study conduct, monitoring, and oversight during the pandemic were maintained, and a risk-based approach to assess and mitigate its impact on study conduct was employed.
Supplementary Material
Footnotes
Funding for this research was provided by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ.
M.G.J., J.L., F.-H.S., J.A.H., M.B., C.D.A., E.G.R. and C.J.B. are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ (MSD), who may own stock and/or hold stock options in the Merck & Co., Inc., Rahway, NJ. M.W.P. was an employee of MSD at the time of study conduct. E.R. reports grant funding and consulting fees from MSD to his institution. E.R., N.A., and J.S.B. report funding to conduct the study from MSD to their institutions.
All authors are responsible for the work described in this paper and meet ICMJE authorship criteria. All authors were involved in at least one of the following: conception, design of work or acquisition, analysis, interpretation of data, drafting the manuscript and/or revising/reviewing the manuscript for important intellectual content. All authors provided final approval of the version to be published. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com).
Contributor Information
Emmanuel Roilides, Email: roilides@auth.gr.
Negar Ashouri, Email: nashouri@choc.org.
John S. Bradley, Email: jsbradley@health.ucsd.edu.
Julia Lonchar, Email: julia.lonchar@merck.com.
Feng-Hsiu Su, Email: feng-hsiu.su@merck.com.
Jennifer A. Huntington, Email: Jennifer.Huntington@merck.com.
Myra W. Popejoy, Email: myra.popejoy@gmail.com.
Mekki Bensaci, Email: mekki.bensaci@merck.com.
Carisa De Anda, Email: carisa.de.anda@merck.com.
Elizabeth G. Rhee, Email: elizabeth.rhee@merck.com.
Christopher J. Bruno, Email: christopher.bruno@merck.com.
REFERENCES
- 1.World Health Organization. WHO publishes list of bacteria for which new antibiotics are urgently needed. Available at: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed. Accessed February 28, 2022.
- 2.Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399:629–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.FDA. Complicated urinary tract infections: developing drugs for treatment. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/complicated-urinary-tract-infections-developing-drugs-treatment. Accessed January 11, 2023.. [Google Scholar]
- 4.Koçak M, Büyükkaragöz B, Çelebi Tayfur A, et al. Causative pathogens and antibiotic resistance in children hospitalized for urinary tract infection. Pediatr Int. 2016;58:467–471. [DOI] [PubMed] [Google Scholar]
- 5.Lutter SA, Currie ML, Mitz LB, et al. Antibiotic resistance patterns in children hospitalized for urinary tract infections. Arch Pediatr Adolesc Med. 2005;159:924–928. [DOI] [PubMed] [Google Scholar]
- 6.Flokas ME, Detsis M, Alevizakos M, et al. Prevalence of ESBL-producing Enterobacteriaceae in paediatric urinary tract infections: a systematic review and meta-analysis. J Infect. 2016;73:547–557. [DOI] [PubMed] [Google Scholar]
- 7.Parente G, Gargano T, Pavia S, et al. Pyelonephritis in pediatric uropathic patients: differences from community-acquired ones and therapeutic protocol considerations. A 10-year single-center retrospective study. Children (Basel). 2021;8: 436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenthal VD, Duszynska W, Ider BE, et al. International Nosocomial Infection Control Consortium (INICC) report, data summary of 45 countries for 2013-2018, Adult and Pediatric Units, Device-associated Module. Am J Infect Control. 2021; 9:1267–1274. [DOI] [PubMed] [Google Scholar]
- 9.Shaikh N, Morone NE, Bost JE, et al. Prevalence of urinary tract infection in childhood: a meta-analysis. Pediatr Infect Dis J. 2008;27:302–308. [DOI] [PubMed] [Google Scholar]
- 10.Keren R, Shaikh N, Pohl H, et al. Risk factors for recurrent urinary tract infection and renal scarring. Pediatrics. 2015;136:e13–e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosen JM, Kriegermeier A, Adams PN, et al. Urinary tract infection in infancy is a risk factor for chronic abdominal pain in childhood. J Pediatr Gastroenterol Nutr. 2015;60:214–216. [DOI] [PubMed] [Google Scholar]
- 12.Megged O. Bacteremic vs nonbacteremic urinary tract infection in children. Am J Emerg Med. 2017;35:36–38. [DOI] [PubMed] [Google Scholar]
- 13.Shaikh N, Ewing AL, Bhatnagar S, et al. Risk of renal scarring in children with a first urinary tract infection: a systematic review. Pediatrics. 2010;126:1084–1091. [DOI] [PubMed] [Google Scholar]
- 14.van Duin D, Bonomo RA. Ceftazidime/avibactam and ceftolozane/tazobactam: second-generation β-lactam/β-lactamase inhibitor combinations. Clin Infect Dis. 2016;63:234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zerbaxa® (ceftolozane and tazobactam). US Prescribing information. Whitehouse Station, NJ, USA: Merck Sharpe & Dohme Corp. 2022. [Google Scholar]
- 16.Wagenlehner FM, Umeh O, Steenbergen J, et al. Ceftolozane-tazobactam compared with levofloxacin in the treatment of complicated urinary-tract infections, including pyelonephritis: a randomised, double-blind, phase 3 trial (ASPECT-cUTI). Lancet. 2015;385:1949–1956. [DOI] [PubMed] [Google Scholar]
- 17.Bradley JS, Ang JY, Arrieta AC, et al. Pharmacokinetics and safety of single intravenous doses of ceftolozane/tazobactam in children with proven or suspected gram-negative infection. Pediatr Infect Dis J. 2018;37:1130–1136. [DOI] [PubMed] [Google Scholar]
- 18.Larson KB, Patel YT, Willavize S, et al. Ceftolozane-tazobactam population pharmacokinetics and dose selection for further clinical evaluation in pediatric patients with complicated urinary tract or complicated intra-abdominal infections. Antimicrob Agents Chemother. 2019;63:e02578–e02518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miettinen O, Nurminen M. Comparative analysis of two rates. Stat Med. 1985;4:213–226. [DOI] [PubMed] [Google Scholar]
- 20.Jackson CCA, Newland J, Dementieva N, et al. Safety and efficacy of ceftolozane/tazobactam plus metronidazole versus meropenem in pediatric participants with complicated intra-abdominal infection: a phase 2, randomized clinical trial. Open Forum Infect. Dis. 2021;8:S668–S669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Popejoy MW, Paterson DL, Cloutier D, et al. Efficacy of ceftolozane/tazobactam against urinary tract and intra-abdominal infections caused by ESBL-producing Escherichia coli and Klebsiella pneumoniae: a pooled analysis of Phase 3 clinical trials. J Antimicrob Chemother. 2017;72:268–272. [DOI] [PubMed] [Google Scholar]
- 22.Huntington JA, Sakoulas G, Umeh O, et al. Efficacy of ceftolozane/tazobactam versus levofloxacin in the treatment of complicated urinary tract infections (cUTIs) caused by levofloxacin-resistant pathogens: results from the ASPECT-cUTI trial. J Antimicrob Chemother. 2016;71:2014–2021. [DOI] [PubMed] [Google Scholar]
- 23.Mirsoleymani SR, Salimi M, Shareghi Brojeni M, et al. Bacterial pathogens and antimicrobial resistance patterns in pediatric urinary tract infections: a four-year surveillance study (2009-2012). Int J Pediatr. 2014;2014:126142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bradley JS, Roilides E, Broadhurst H, et al. Safety and efficacy of ceftazidime-avibactam in the treatment of children ≥3 months to <18 years with complicated urinary tract infection: results from a phase 2 randomized, controlled trial. Pediatr Infect Dis J. 2019;38:920–928. [DOI] [PubMed] [Google Scholar]
- 25.Hanna-Wakim RH, Ghanem ST, El Helou MW, et al. Epidemiology and characteristics of urinary tract infections in children and adolescents. Front Cell Infect Microbiol. 2015;5:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bassetti M, Vena A, Giacobbe DR, et al. Ceftolozane/tazobactam for treatment of severe ESBL-producing Enterobacterales infections: a multicenter nationwide clinical experience (CEFTABUSE II Study). Open Forum Infect Dis. 2020;7:ofaa139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wi YM, Greenwood-Quaintance KE, Schuetz AN, et al. Activity of ceftolozane-tazobactam against carbapenem-resistant, non-carbapenemase-producing Pseudomonas aeruginosa and associated resistance mechanisms. Antimicrob Agents Chemother. 2018;62:e01970–e01917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lob SH, Hoban DJ, Young K, et al. Activity of ceftolozane-tazobactam and comparators against Pseudomonas aeruginosa from patients in different risk strata - SMART United States 2016-2017. J Glob Antimicrob Resist. 2020;20:209–213. [DOI] [PubMed] [Google Scholar]
- 29.Sader HS, Carvalhaes CG, Duncan LR, et al. Susceptibility trends of ceftolozane/tazobactam and comparators when tested against European Gram-negative bacterial surveillance isolates collected during 2012-18. J Antimicrob Chemother. 2020;75:2907–2913. [DOI] [PubMed] [Google Scholar]
- 30.Sader HS, Castanheira M, Shortridge D, et al. Antimicrobial activity of ceftazidime-avibactam tested against multidrug-resistant Enterobacteriaceae and Pseudomonas aeruginosa isolates from U.S. medical centers, 2013 to 2016. Antimicrob Agents Chemother. 2017;61:e01045–17(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gonzalez MD, McMullen AR, Wallace MA, et al. Susceptibility of ceftolozane-tazobactam and ceftazidime-avibactam against a collection of β-lactam-resistant gram-negative bacteria. Ann Lab Med. 2017;37:174–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.