Abstract
Introduction
Postoperative delirium (POD) is seen in approximately 15% of elderly patients and is related to poorer outcomes. In 2017, the Federal Joint Committee (Gemeinsamer Bundesausschuss) introduced a ‘quality contract’ (QC) as a new instrument to improve healthcare in Germany. One of the four areas for improvement of in-patient care is the ‘Prevention of POD in the care of elderly patients’ (QC-POD), as a means to reduce the risk of developing POD and its complications.
The Institute for Quality Assurance and Transparency in Health Care identified gaps in the in-patient care of elderly patients related to the prevention, screening and treatment of POD, as required by consensus-based and evidence-based delirium guidelines. This paper introduces the QC-POD protocol, which aims to implement these guidelines into the clinical routine. There is an urgent need for well-structured, standardised and interdisciplinary pathways that enable the reliable screening and treatment of POD. Along with effective preventive measures, these concepts have a considerable potential to improve the care of elderly patients.
Methods and analysis
The QC-POD study is a non-randomised, pre–post, monocentric, prospective trial with an interventional concept following a baseline control period. The QC-POD trial was initiated on 1 April 2020 between Charité-Universitätsmedizin Berlin and the German health insurance company BARMER and will end on 30 June 2023. Inclusion criteria: patients 70 years of age or older that are scheduled for a surgical procedure requiring anaesthesia and insurance with the QC partner (BARMER). Exclusion criteria included patients with a language barrier, moribund patients and those unwilling or unable to provide informed consent. The QC-POD protocol provides perioperative intervention at least two times per day, with delirium screening and non-pharmacological preventive measures.
Ethics and dissemination
This protocol was approved by the ethics committee of the Charité-Universitätsmedizin, Berlin, Germany (EA1/054/20). The results will be published in a peer-reviewed scientific journal and presented at national and international conferences.
Trial registration number
Keywords: PREVENTIVE MEDICINE, Delirium & cognitive disorders, Protocols & guidelines, QUALITATIVE RESEARCH
STRENGTHS AND LIMITATIONS OF THIS STUDY.
The quality contract (QC)-postoperative delirium (POD) study is a monocentric clinical trial, being conducted in a standard clinical setting.
The QC-POD protocol is inexpensive, with a balanced and interdisciplinary division of tasks through holistic, perioperative intervention protocols.
The effectiveness of the QC-POD protocol will depend on the adherence of clinicians and relatives to the intervention protocol, after being briefed on preventive strategies.
Delirium is a fluctuating disorder, and detection may occasionally be missed despite rigorous and validated assessment methods.
Pre–post design generates bias according to internal validity, such as seasonal influences, and does not allow blinding of study personnel, patients or relatives.
Introduction
The term delirium describes acute mental disorders with an organic cause, and is associated with an attention deficit disorder. The symptoms fluctuate and may be accompanied by other cognitive impairments (mental disorder and/or consciousness disorder).1 The incidence of acute postoperative delirium (POD) for different surgical patient groups ranges between 5% and 72%.2–7 Patients at high risk of developing POD have predisposing und precipitating risk factors,8 whereas with an increasing amount of predisposing risk factors, the organic brain systems of patients become increasingly unstable. This instability renders patients more susceptible to the development of POD, even if faced with minor precipitating factors. Elderly patients are an extremely vulnerable group, seen by higher rates of POD, as well as an increased risk of subsequently developing persistent postoperative neurocognitive disorders (NCDs), often associated with loss of functionally and care dependency.9 10 Failure to diagnose POD or treatment delays can directly increase the rates of complications and mortality.11 Additionally, POD is associated with a high level of distress for the affected patients and their relatives, an additional workload for the medical staff and significantly higher costs.12 13 Unfortunately, the significantly higher medical workload related to delirious patients is not adequately represented within the German system of Diagnosis-Related Groups, and the higher costs associated with their care are not covered in the current system. Delirious patients are often admitted to ICUs, where the standard workload scores in intensive care units (ICUs) (Therapeutic Intervention Scoring System (TISS)-10, TISS-28, Nine Equivalents of Nursing Manpower) also fail to reflect the increased daily effort of medical staff.14 Furthermore, delirium often remains undetected in the absence of structured screening strategies, so that patients and relatives receive no guidance on long-term consequences, such as postoperative NCDs. With an increasing life expectancy and medical progress, complex surgical procedures in older patients are increasingly common, and this trend is clearly reflected in patient care: in 2017, from 60.0 million operations and medical procedures performed as full in-patient cases in Germany, 31.4 million (52.3%) were performed on patients age 65 or older. In 2018, these numbers rose further to 32.2 million (52.5%).15 In summary, there is a lack of consistent and reliable screening strategies for POD that employ validated screening tools in in-patient care, especially in the normal wards. As a consequence, POD is regularly not recognized, or is associated with significant diagnosis and treatment delays. Furthermore, there is a lack of well-structured, protocol-based interventional measures to prevent POD, as well as follow-up concepts and continued outpatient care for affected patients. The current situation is unsatisfactory for all involved, and will get progressively worse as our society ages.
Impulse of ‘quality contract: prevention of pod in the care of elderly patients’ (POD): justification
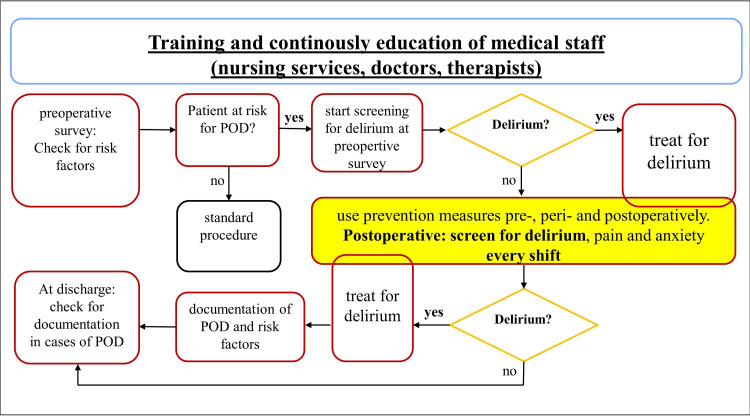
Non-pharmacological preventive measures for delirium are highly effective and have the highest degree of recommendations in the guidelines.16 17 Due to their preventive nature, these measures can only be effective if they are applied continuously and as complete as possible.18–22 However, in current practice, there is a lack of preventive measures and screening for delirium during in-patient care (online supplemental figure S1).23 Even if performed, there is no structured and transparent mapping tool for documentation, so that missing preventive measures maybe not noticed in a busy workflow environment. This represents a dangerous gap in the treatment concept for patients who are at high risk of developing POD.24 25 When applied thoroughly, the antidelirium bundle measures can improve the short-term and long-term outcome of elderly patients. An optimised treatment pathway for POD is shown in figure 1. In this flow chart, preventive measures are taken into account from the very first contact until discharge from the hospital.
Figure 1.
Optimised treatment pathway for postoperative delirium (POD), modified figure of the Institute for Quality Assurance and Transparency in Health Care (IQTIG). Optimisation of the inpatient treatment pathway includes training and continuous education of medical staff (nurses, physicians, therapists) on the topic of delirium, its preventive measures and therapy, screening for risk factors for postoperative delirium on hospital admission, screening on admission and immediate treatment of the cause of delirium in case of a positive delirium screening. Delirium prevention measures are listed as an integral part of treatment and form the core of the optimised treatment pathway. At discharge, for patients who have been affected by delirium, the duration and treatment of delirium is documented in the discharge document. The boxes outlined in red and blue represent new structures for preventing POD in current inpatient care practices and compensate for the deficits mentioned in online supplemental figure S1.
bmjopen-2022-066709supp001.pdf (104.1KB, pdf)
Objectives
POD has an organic cause, and quality contract (QC)-POD intervention aims to systematically assess and compensate for deficits that could trigger the development of POD. Screening documentation in the patient data management system (PDMS) with validated delirium, anxiety and pain scores at least twice a day objectively and systematically maps the patient’s course up to the third, respectively, fifth postoperative day. The QC-POD intervention includes all non-pharmacological preventive measures against POD, and employs validated screening instruments to ensure early diagnosis. At the first notice of POD, medical staff can systematically seek triggers among a list of possible differential diagnoses and provide early and targeted treatment. Therefore, the current protocol will actively employ measures to prevent the development of POD and limit its complications, shorten hospital stays and reduce treatment costs. The hypothesis is that a higher implementation rate of delirium screening (primary outcome) can be achieved within the period of the QC-POD study (three study years).
Study design
The proposed QC-POD study is designed as an open, non-randomised, monocentric, pre–post, prospective clinical trial with an intervention period of 33 months (intervention group), following a baseline period of 6 months (control group).
Methods: participants, interventions and outcomes
Study setting
The QC-POD study is performed in academic hospitals of Charité-Universitätsmedizin Berlin, Germany: Campus Virchow-Klinikum, Charité Campus Mitte, and Campus Benjamin Franklin.
Eligibility criteria
Enrolment requires written informed consent for data processing from patients or their legal representatives. In case of emergency surgery, written consent may be obtained at the earliest 24 hours after the surgical procedure. All required preventive measures of QC-POD protocol (online supplemental table S1–S4) are assessed immediately after hospital admission. The target population are patients of at least 70 years of age and insurance with the QC partner (BARMER), requiring surgery in general, local or combined anaesthesia. All types of operations and urgency levels (elective and emergency procedures) may be included. Exclusion criteria are moribund patients and patients with a significant language barrier (table 1). Currently, only patients who are insured by the QC partner BARMER may be included, although additional health insurers may participate in the QC-POD over the course of the contract period. This possibility has the potential to increase the number of participants significantly. A professional team of trained employees (Delirium-Expert-Support-Team (DES-Team)) will carry out the intervention. Within the intervention period (33 months), the DES team supports ward staff on the normal wards and offers needs-based training. At the end of the intervention period, the ward staff (nurses and physicians) perform the intervention in the intervention group according to the QC-POD protocol.
Table 1.
Inclusion and exclusion criteria of QC-POD
| Inclusion criteria | Exclusion criteria |
|
|
POD, postoperative delirium; QC, quality contract.
bmjopen-2022-066709supp002.pdf (161KB, pdf)
Study population and participant timeline
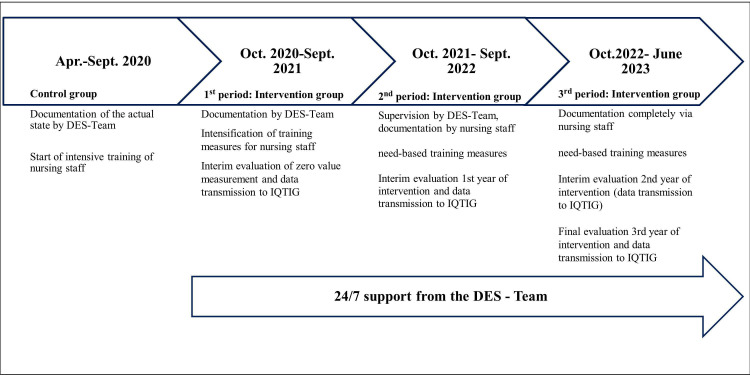
The study population is divided in a control group and an intervention group. Control group patients have been included between 1 April 2020 and 30 September 2020, and the routine data of at least n=500 participants will be collected. Intervention group patients will be included between 1 October 2020 and 30 June 2023, and they will receive the entire interventional bundle, including protocol-based preventive measures for POD (figure 2). For detailed information on each prevention measure based on the QC-POD protocol, please refer to online supplemental table S1–S4.
Figure 2.
Timeline of quality contract (QC): first patient in 1 April 2020, last patient in: 30 June 2023. DES-Team, Delirium-Expert-Support-Team (professional team of trained nurses and physicians, 24/7 service).
Intervention
Genesis of the QC-POD protocol
The guiding principle for the development of the QC-POD protocol are the existing evidence-based guidelines.16 17 26 All steps of this multistage process are explained below.
Step 1 (selection of suitable intervention parameters): the content of the European Society of Anesthesiology guideline for POD16 and the S3 guideline for analgesia, sedation and delirium management17 were reviewed. All evidence-based and consensus-based recommendations for the prevention of postoperative and ICU delirium mentioned in the guidelines were selected and processed in their individual aspects. In addition, the recommendations of the modified Hospital Elderly Life Programme (mHELP),27 28 a delirium prevention and treatment protocol for geriatric in-patient care of elderly patients were integrated.
Step 2 (preparation of the checklist): all selected preventive measures were placed into a structured chronological order that reflects the course of in-patient treatment during the hospital stay. Each individual area in in-patient care was considered with its specific modalities and mapped in a checklist format. The selected preventive measures for the individual areas (preoperative, perioperative and postoperative) were embedded in an interdisciplinary and interunit treatment concept for the care of hospitalised elderly patients. In terms of an optimised treatment pathway, the Institute for Quality Assurance and Transparency in Health Care (here referred to as IQTIG) recommends ensuring preventive measures throughout in-patient care. The optimised treatment pathway is completely implemented into the QC-POD protocol in all in-patient areas (preoperative, perioperative, postoperative) (figure 1).
Step 3 (planning the process steps): the checklist obtained from the completion of steps 1 and 2 was the basis for planning all further process steps. Additional personnel expenditure resulting from the implementation of all QC-POD protocol-based preventive strategies was calculated based on the internal clinical evaluations of previous clinical studies. The details for the implementation and documentation of each individual preventive measure were defined in terms of minutes per patient or minutes per case. In addition, the implementation of preventive measures and the documentation effort was distributed according to the workload of the different professional groups or relatives (nurse/doctor/therapist/relatives). Patient representatives were involved in the planning and implementation of the QC-POD protocol, providing valuable opinions and suggestions for educational concept for patients and relatives.
Step 4 (consensus-building): in a multistage process within the hospital, all preventive measures summarised in the checklist were agreed on. The approved form of the checklist was transferred to a data dictionary.
Preoperative area (anaesthesia out-patient clinic).
Perioperative area (operating room).
Postoperative area (ICU/intermediate care (IMC)/postanesthesia care unit (PACU)/recovery room/normal ward).
Out-patient care area (anaesthesia out-patient clinic).
The entire QC-POD study protocol for interventional with preventive measures were implemented in the PDMS as a COPRA6-form. The preventive measures were added to the existing documentation templates in COPRA6-form, whereas a completely new documentation template was created for the postoperative documentation of the preventive measures. These innovations tools were made available to the medical staff in practical training courses, video tutorials and theoretical work instructions for self-study and blended learning concepts (e-learning, simulator-training and on the job training). Each area was included in a workflow and uniformly defined from the initial contact with patients until discharge. The documentation of the items was summarised in a work instruction for the medical staff.
Subject/rationale of this method protocol
As part of its role as the highest decision-making body within the German statutory healthcare system, the Federal Joint Committee (Gemeinsamer Bundesausschuss, G-BA) introduced the QC in May 2017, as a new instrument to improve healthcare in Germany. The G-BA defined four in-patient service areas (Leistungsbereiche) for which the new instrument of QC should be evaluated, and one of these areas was the ‘Prevention of POD in the care of elderly patients’. Evaluation of the QC has been assigned to the IQTIG, a scientifically independent institution responsible for evaluating quality of care in the German statutory healthcare system. IQTIG is the central institute for legally anchored quality assurance in the healthcare system in Germany, and it has created many indicators to address issues (P0–P6) with the current healthcare practice (online supplemental figure S1). All indicators add to the entire optimised overall treatment pathway (figure 1). Related to POD, these indicators reflect the evidence-based and consensus-based recommendations for preventive measures from the national, international and European guidelines for POD.16 17 26 The main approach to resolve issues related to POD would be the systematic closure of gaps in current practice by implementing a transparent interdisciplinary and holistic concept for non-pharmacological prevention of POD. The implementation of evidence-based and consensus-based medical measures are prerequisites to avoid POD in the care of elderly patients, as specified in the service area (Leistungsbereich) of QC-POD.
Intervention per QC-POD protocol in general
The intervention in QC-POD is a holistic approach consisting of well-defined, non-pharmacological preventive measures to avoid POD, early detection and early initiation of therapy for POD (figure 1 and online supplemental table S1, S3 and S4). All preventive measures are subject to the current evidence-based recommendations of national and European guidelines on POD.16 17 For each area that patients stay during their hospitalisation, specific preventive measures are summarised as an intervention and are implemented by trained professional staff at the bedside. Interdisciplinary exchange, integration of relatives into the treatment concept, training of relatives and staff, and documentation of preventive measures are important elements and clearly defined in the QC-POD protocol. If patients are affected by POD, there are well-structured algorithms for confirming the diagnosis and treating the cause(s). To explain the algorithm in detail is beyond the scope of this protocol. Therefore, the algorithm will be published in a further paper.
Patient and public involvement
In addition, the content for the QC-POD protocol was evaluated through patient and family interviews. The needs of patients and relatives were given priority in the protocol. These include the desire for rapid provision with aids (glasses, dentures, hearing aids) to compensate for sensory deficits or the desire of relatives to be in contact with the patient even in times of severe pandemic. In addition to the patient and public participation, an information flyer on the prevention of POD with important information for patients and relatives was prepared with patient representatives (QC-POD Flyer). The information flyer was additionally dubbed as an audio file to make the contents of the flyer accessible to patients with severe visual impairment (QC-POD Audio). A film for the visual presentation of the preventive measures was also produced in this cooperative effort and is freely available to the public (QC-POD Video).
Intervention preoperatively at baseline assessment
The European guidelines on POD suggested that baseline assessment includes cognitive, functional and mental function,16 so the QC-POD intervention begins with the first contact with patients in the anaesthesiology outpatient clinic. This intervention involves screening for predisposing and precipitating risk factors for POD,9 16 29 as well as employing a screening to detect delirium before surgery. Our intervention also includes the results of a minigeriatric assessment, including a modified Fried frailty assessment30 with questions on unintentional weight loss, fatigue, physical activity measured by metabolic equivalents, measurement of hand strength with a dynamometer and measurement of gait speed.30 31 In addition, the Timed Up and Go Test is performed at baseline, along with information regarding the patient’s history of falls.32 The social situation is assessed with a questionnaire created by Nikolaus et al (SOS-I)33; nicotine consumption with Heaviness of Smoking Index,34 and alcohol consumption with Alcohol use Disorders Identification Test-Consumption Items35 36 are also recorded with a specific questionnaire. Furthermore, the assessment of an existing polypharmacy and check for anticholinergic medication,37 38 the health questionnaire (Patient Health Questionnaires-8, PHQ-8) for signs of depression,39 and a mini-cognitive test (clock drawing test, three-word memory test) are included. Anxiety or stress is measured with the self-assessment tool Faces Anxiety Scale,40 41 and pain with the 0-10 visually Numeric Rating Scale (NRS-V).42
Intervention intraoperatively
The incidence of POD in the elderly can be reduced by monitoring depth of anaesthesia with a routine EEG monitoring device, with the aim to prevent burst suppression during surgical procedures.16 43 In the Charité Universitätsmedizin Berlin, such devices (Root with SedLine Brain Function Monitoring, MASIMO Corporation, California/ USA,) are routinely employed for monitoring depth of anaesthesia during surgery. Additionally, a multimodal pain concept for opioid-sparing strategy is in place, including the use of regional anaesthesia as an optional procedure, which may be combined with general anaesthesia if indicated (and in the absence of contraindications). Other intraoperative and perioperative preventive measures include avoiding anticholinergic medication, the use of patient blood management, limiting fasting periods (only in the necessary time window) and patient-oriented treatment (sensory aids, barriers to mobilisation, etc).
Intervention perioperatively and postoperatively
Screening for POD in the project is performed with validated screening instruments in the anaesthesiology outpatient clinic (Nu-DESC: Nursing Delirium Screening Scale), recovery room (Nu-DESC), normal ward (Nu-DESC, Delirium Observation Scale)44 45 and ICU/IMC (Confusion Assessment Method for ICU, Intensive Care Delirium Screening Checklist, Delirium Detection Score).46 47 In the intervention group, delirium screening takes place postoperatively at least once per shift in three shift service systems. However patients will not be awakened for screening during the night in order not to disrupt their circadian rhythm. In case of a positive delirium screening with Nu-DESC in the areas outside the ICU/IMC, delirium is verified by an attending trained physician according to the Diagnostic and Statistical Manual of Mental Disorders -fifth edition (DSM-5) criteria.48 The delirium screening will be repeated once per shift until day 5 postoperatively.
Every patient in the QC-POD study intervention group receives at least two visits per day from the DES-Team. During these visits, preventive measures are applied and documented by using the QC-POD protocol in the PDMS. Daily visits also include screening with validated instruments for delirium, anxiety and pain at least twice a day. Due to the structured, protocol-based documentation in the PDMS, deficits can be quickly noticed and rectified by the DES-Team. An example is a patient who has an unplanned intensive care unit stay following an operation, and arrives there without his/her aids (glasses, hearing aids, telephone, dentures). The early compensation of sensory deficits supports the reorientation and the circadian rhythm. Through a systematic query as to whether and which aids are needed, these can be immediately brought from the normal ward and given to the patient.
Intervention: follow-up 3 months after discharge
Postoperative follow-up care is offered to patients of the intervention group who were affected by POD during their in-patient stay. Patients affected by POD who consented to follow-up will be contacted 3 months after their discharge and interviewed via phone or video consultation. The intervention is a protocol-based interview according to an in-house standardised operating procedure (SOP) with validated questionnaires:
Video Montreal-Cognitive-Assessment (Video MoCA) (only for video consultation).
MoCA Blind Version.
Self-assessment of memory performance.
Multifactorial Memory Questionnaire.
Instrumental Activities of Daily Living Scale
PHQ-8.
Generalised Anxiety Disorder Scale-7.
Brief diagnostic interview for mental disorders (Diagnostisches Kurz-Interview bei psychischen Störungen).
The Informant Questionnaire on Cognitive Decline in the Elderly.
In addition, a paper-based questionnaire is handed out per mail for patients and for relatives to complete and return:
Insomnia Severity Index.
Perceived Stress Questionnaire-20.
Berlin Social Support Scales-17.
NRS-V.
Questions about hospitalisation after discharge and current medication.
The interview is conducted by trained personnel with psychological training. A questioning of the life partner or a person close to the patient is the subject of the SOP. For this purpose, patients and relatives are offered an appointment following discharge to discuss neurocognitive testing results, functional and mental states, as well as outpatient support and care options. The goal is to offer patients and relatives interdisciplinary support if cognitive impairment develops after delirium, objectively or as a matter of subjective concern.
Additional data collection
The baseline assessment of the QC-POD protocol routinely collects demographic information, a detailed medical history, surgical history, current medication and physical examination results.
Outcomes
The primary outcome of the project is the implementation rate of delirium screening according to the guideline recommendations.16 17 The implementation rate is measured as the number of patients who received delirium screening at least twice a day within the first three postoperative days, divided by the total number of participants in the respective period. Implementation rates of at least 60% for screening should be achieved, growing to at least 70% in the second year and at least 80% in the third year.
Secondary outcomes of the QC-POD project are implementation rate within the first five postoperative days, incidence rate of POD in the study population and total duration of POD in days. The intention to-treat principle will be followed for all analyses, and group differences will be presented with 95% CI. A complete list of secondary outcomes is available under the study registration (ClinicalTrials.gov Registry NCT04355195).
In order to assess the progress of the implementation, the G-BA defined general milestones for the project, which will be independently evaluated by the IQTIG.
Sample size
The control group will include at least n=500 patients for standard procedure and collection of routine data. The approximate number of patients eligible for the intervention group is estimated to be n=1700 per year. In both groups currently only patients who are insured by the QC partner BARMER will be assessed.
Recruitment
Eligible participants will be identified primarily by reviewing the operation schedule in the PDMS. Additionally, all patients ≥70 years old that take part in the QC-POD (control group and intervention group) will receive a risk factor analysis of their individual predisposing and precipitating risk factors for POD during the preoperative evaluation with the anaesthesiologist. The risk factors for POD, preoperative delirium screening, preoperative anxiety screening and pain screening, as well as the geriatric assessment are all collected in the digital COPRA6 form. All relevant items are visible in the routine layout of the standard preoperative evaluation interview forms (online supplemental table S2–S4). The enrolment of the patients is required exclusively for data processing and analysis. The intervention can, therefore, start independently from written consent. This occurs in emergencies, when written consent cannot be obtained immediately.
Methods: assignment of interventions (for controlled trials)
Assignment
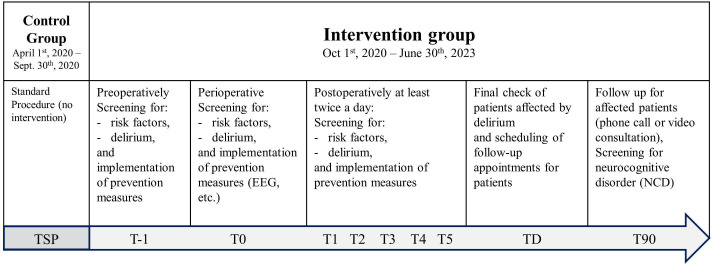
Participants will not be randomly assigned to either control or intervention group. Study physicians will assign participants in the first period of the QC-POD study to the control group (for standard procedure and collection of routine data), and in the following three periods participants will be automatically assigned to the intervention group (participant timeline, figure 3).
Figure 3.
Participant timeline. Control group patients will be included between 1st April 2020 and 30th September 2020, and will receive standard procedure (TSP). Intervention group patients will be included between 1st October 2020 and 30th June 2023, and will receive the entire interventional package, including protocol-based preventive measures for POD from T-1 to T90. T-1: day of hospital admission, T0: day of surgery, T1: first postoperative day, T2: second postoperative day, T3: third postoperative day, T4: fourth postoperative day, T5: fifth postoperative day, TD: day of discharge, T90: day of follow-up treatment three months after discharge. For more detail information about single prevention measures intervention period, we kindly refer to online supplemental table S1–S4. EEG, electroencephalography.
Implementation
For the implementation of the intervention, interdisciplinary conferences, education of patients, training of staff (physicians, nurses, therapists) and relatives will be key to improve POD sensitisation and awareness. Content of staff trainings are:
Intervention with preventive measures according to the QC-POD protocol.
Use of validated screening tools to screen for delirium, anxiety/stress and pain.
Procedure in case of positive delirium screening: algorithm for a practical intervention scheme.
Relatives integration as an elementary component of the intervention.
Patient and relatives education sessions are based on provided informational material on POD. Patients and relatives have the option to access analogue or QR code informational material as brochure, audio file or video clip. Additional monitoring and integration of relatives in the treatment pathway is accompanied by the on-site support team.
Blinding (masking)
No blinding mechanism has been established.
Methods: data collection, management and analysis
Data collection and data management
COPRA is the standard PDMS documentation programme for all anaesthesiology and ICU units at the Charité Universitätsmedizin Berlin. Since 2015, premedication rounds, surgical protocols, postoperative recovery room stays and all aspects of treatment at the intensive care units, as well as in the pain clinic, have been fully and transparently documented in this program (COPRACOPRA6).
The COPRA6 program allows data exchange with the POD documentation form, so that scores (validated delirium screening scores, pain scores, anxiety score) are directly transferred into the POD documentation form and are available for all medical staff. In the surgical setting, and postoperatively in the recovery room or intensive care unit, all non-pharmacological preventive measures are already part of the standard procedure and are mapped and documented in the protocols of these areas (operation/intensive care unit/ recovery room). The COPRA6 form for the documentation of preventive measures during the postoperative phase on the normal ward is divided into five domains: (1) delirium screening, (2) pain/stress/anxiety screening, (3) stimulation of cognition and circadian rhythm, (4) nutrition and mobilisation and (5) indwelling catheters and external devices (online supplemental table S4). The domains can be accessed easily and independently using a sidebar. Each domain contains queries on non-pharmacological preventive measures, which are stored along with the date and time of the conducted visit. The checklist format is kept for the postoperative phase and is intended to ensure that staff can complete it quickly and easily during bedside visits. When patients are discharged or transferred from the hospital, the documentation in the postoperative COPRA6 application also ends with a final query on the presence of delirium. Patients affected by a POD will be offered a follow-up consultation to the out-patient anaesthesiology department 3 months after discharge.
Statistical methods
Data analysis
After study completion, the clinical impact of the QC-POD protocol implementation will be evaluated by using a pre-specified rate. The primary outcome (implementation rate) is measured by the number of patients who received delirium screening and preventive measures at least twice a day within the first three postoperative days, divided by all recruited patients in the respective study period. This rate comprises the number of days with respect to the first three postoperative days (or length of hospitalisation, if less than 3 days) in which the patients received at least two daily interventions. One intervention consists of delirium screening and preventive measures. Our aim is to achieve at least a 60% implementation rate in the first year of the intervention phase, at least 70% in the second and at least 80% in the third year.
The secondary outcomes are implementation rate for delirium screening and preventive measures at least twice a day within the first five postoperative days divided by all recruited patients in the respective study period, the reduction of POD incidence and a decrease in the duration of POD. To account for the problem of interval censoring and the oscillatory nature of POD, each positive delirium screening score after the establishment of the diagnosis will add 12 hours to the total POD duration for a given patient. Within this period, if the patient receives another positive screening score, only the difference in time between these two scores will be added to the total, plus another 12 hours. The same procedure is defined for negative screening, so that a delirium-free phase is assumed for 12 hours or in the period until the next negative screening. This form of analysis achieves a summation of hours for each study patient for the duration of POD. A sensitivity analysis will be performed using the last seen state as unchanged, until another assessment has been made.
Statistical evaluation of all other secondary outcomes will be defined depending on the distribution and the research question in the future papers. The statistical evaluation pertaining to these secondary outcomes and other research questions begins with a detailed exploratory analysis of the data in the form of determining statistical parameters such as mean value and variance (metric scaling), median and IQR (ordinal scaling) or frequencies with percentages (qualitative data), and is carried out by examining the distributions of the parameters added. Multivariable regression analysis will be done to better estimate the effect size of different covariates in relation to the outcomes. Effect estimates with the corresponding 95% CIs will be reported.
Data monitoring
Patient data from the entire QC-POD study protocol for baseline and intervention with preventive measures was implemented in the PDMS as a COPRA6 form. The preventive measures have been added to the existing documentation templates in COPRA6 form, with an innovative documentation template being created for the postoperative documentation of the preventive measures. Data quality control is ensured by on-site monitoring by the study team to ensure accuracy and to query implausible or missing data on a regular basis.
Discussion
The QC-POD protocol was developed to close the gaps in prevention of POD in in-patient care for the elderly through consistent application of existing guidelines.
Each preventive measure listed in national and international guidelines has been shown to have an effect on the incidence of POD in preliminary studies.16 17 26 In summary, preoperative (assessment of risk factors for delirium, avoidance of benzodiazepines and excessive preoperative fasting), intraoperative (EEG monitoring during general anaesthesia, multimodal analgesia) and postoperative (screening for delirium, pain and anxiety with validated tools, cognitive stimulation and training, delivering sensory aids, non-pharmacological support of circadian rhythm, involvement of relatives, enhanced recovery after surgery through early mobilisation, early enteral nutrition, early removal of indwelling catheters) interventions can potentially reduce delirium and NCDs in this surgical population. Since each of these measures is relevant to avoid POD, the effect will be more powerful and long-lasting if they are used permanently in a bundle, as shown in other settings, such as mHELP.49 Efforts to support preoperative neurocognitive function in an elderly surgical population might constitute a further preventive measure, which is not yet recommended by current guidelines nor routinely employed in preoperative care. Moreover, surgery in elderly patients is not always appropriately planned, especially in cases of urgent surgery, which does not allow enough time for preoperative physical or neurocognitive training. Especially in emergency situations requiring immediate surgical treatment, there is a high risk for POD with limited or no capacity for prehabilitation, preparation and mobilisation of resources in elderly patients.50
In the current hospital routine, there is a lack of standardised protocols to prevent POD in elderly surgical patients.51 Furthermore, regulation of responsibilities for monitoring preventive measures for POD are in general not yet supported by multiprofessional delirium-teams in in-patient care. This makes it difficult to guarantee the implementation of preventive measures. Especially in postoperative in-patient care and follow-up out-patient care, there is a lack of structured concepts that enable medical staff to act quickly and provide appropriate treatment. There are no comparable publications describing concepts of structured documentation and transparent mapping of all preoperative, intraoperative and postoperative preventive measures for POD in hospitalised elderly patients. However, the mHELP of Inouye et al.27 28 offers a concept for the prevention of delirium in these patients. It describes a comprehensive package with a free toolkit for the targeted implementation of a prevention strategy for delirium that focuses on a mobility programme. General goals in the treatment of geriatric patients for the prevention of POD are summarised in mHELP and are also used in our protocols. The lack of structured documentation templates for transparent mapping and survey of the preventive measures limits comparability with the concept of QC-POD. The present concept is not restricted to elective procedures, but includes all levels of urgency as well. This is important, as emergency surgical procedures often increase the risk of POD due to a generally increased predisposing and precipitating risk profile in this patient population. Every patient with a minimum age of 70 years receives a report of the predisposing and triggering risk factors present. Based on this assessment, the treatment team can quickly identify deficits that are present in patients, so that each step in the treatment concept can be quickly and easily adapted to the needs of the individual patient. The holistic and transparent mapping of preventive measures enables medical staff to identify deficits in the application of preventive measures at any time during in-patient treatment. Thus, identified gaps in the provision of care can be quickly rectified by medical staff. This concept, coupled with the described documentation framework, can improve care for patients ≥70 years of age, and allows staff to quickly detect acute events in affected patients and offer treatment at an early stage. At this point, it must be emphasised that there is no pharmacological intervention to cure delirium, and medication should only be considered to control symptoms of the condition. Non-pharmacological preventive strategies, on the other hand, have been shown to decrease the incidence of POD significantly. So far, they are the most powerful tool available against POD and its consequences. A standardised delirium prevention and treatment strategy, especially designed for elderly patients undergoing surgery, must no longer remain theoretical, but requires urgent implementation in routine practice.
Outlook
After the adaptation of the COPRA6 delirium documentation form has been completed, the visualisation of the status of preventive measures is the next programming milestone. The visualisation provides a simple and comprehensive overview of the status of preventive measures, for each individual domain, for the entire staff and for every patient. The intention is to sensitise medical personnel for the individual domains. In practical application, this form of visualisation helps to identify deficits in the implementation of preventive measures more efficiently. With this tool, medical personnel can intervene faster and in a more targeted manner.
The final system for the early detection of POD, after adequate development and evaluation, should be able to close the gap identified by the Federal Joint Committee in the prevention of POD in elderly patients. The closing of the gap will be achieved through the consistent application of already existing guidelines, assisted by a newly created and comprehensive digital documentation template to survey the application of the bundle measures in clinical practice. If a relevant improvement in performance can be demonstrated within the framework of this QC, the processes described here are to be made permanent and thus become the new standard of care in the surgical and interventional care of elderly patients in Germany.
Success of the QC-POD protocol will be demonstrated by improvements in the following areas related to the care of elderly patients:
Implementation rate for delirium screening (primary outcome).
Lower incidence rate of POD (secondary outcome).
Reduction in the duration of POD (secondary outcome).
Reduction in the length of hospitalisation (secondary outcome).
Reduction in treatment costs (secondary outcome).
Lower risk of rehospitalisation or need for long-term nursing care (secondary outcome).
QC-POD allows for a comprehensive evaluation of the effectiveness of both individual and combined preventive interventions for healthcare professionals and health economics, as well as an assessment of the suitability of the concept to become a standard of care. The QC-POD is a study that, for the first time, holistically and coherently encompasses all areas of in-patient care with preventive measures against POD. It compares the clinical and cost-effectiveness of non-pharmacological preventive measures, in terms of a quality improvement intervention, with the current standard care.
Ethics and dissemination
Together with the patient information sheets and consent forms, the protocol was approved by the ethics committee of the Charité-Universitätsmedizin, Berlin, Germany (EA1/054/20). The results will be published in a peer-reviewed scientific journal and presented at national and international conferences.
The protocol is built entirely on evidence-based and consensus-based recommendations for the prevention of postoperative and ICU delirium. These recommendations are mentioned in the current guidelines. By implementing such protocol, no additional risks to patients relative to standard of care are expected. Outcome data are routinely collected together with standard health data (the same applies for follow-up data). Consequently, there is no additional need for reporting or monitoring of adverse events.
Confidentiality
All sensitive patient data are stored in Charité’s current PDMS. For evaluation purposes, the data undergoes pseudonymisation and is then transferred to an internal security server. The storage location is data protection compliant. The data transfer to the QC partner takes place exclusively via password-protected data carriers: password and data medium are handed over in independent delivery systems. These data transfers occur at regular intervals. With each transfer, a pseudonym list of enrolled patient identifiers is generated. This list also contains defined treatment data, for the evaluation of the data by IQTIG.
IQTIG platform stores the data in PDF format. Access to this platform is only possible with a personal account, which is set by IQTIG on request. The Department of Anesthesiology and Operative Intensive Care Medicine (CCM, CVK), Augustenburger Platz 1, 13 353 Berlin and Chariteplatz 1, 10 117 Berlin, Germany, will have access to the final trial dataset. The QC partner (BARMER, Germany) has legally regulated access to the health insurance data.
Supplementary Material
Acknowledgments
Our great thanks go to our colleagues Mrs. Alissa Wolf, Mr. Friedrich Borchers, Mrs. Carolin Finger, Mr. Thomas Ocker, Mr. Michael Römer, Mrs. Maria Heinrich, Mrs. Anika Müller, Mr. Alawi Lütz, Mr. Björn Weiss, Mrs. Sinah Krüger, Mrs. Manuela Bergjan, Mrs. Christiane Franke, Mr. Adrian Rosada, Mrs. Müller-Werdan, Mr. Sascha Tafelski, Mr. Andreas Kopf, Mr. Thomas Fritzsche, and all other colleagues not mentioned by name who accompanied the study and proactively supported the processes through discussions, constructive suggestions, and interdisciplinary exchange. This approach has been essential to establish the interdisciplinary and holistic character in the study in terms of content and practice.
Footnotes
Contributors: CS conceptualised this study, has been heading the ESA guideline group and is the principal investigator and supervisor of the QC-POD. FY, EW and DH took over the clinical conceptual planning. J-DZ designed the documentation form for the normal ward and extended existing documentation masks by the content specifications. JKr and SKP supervised statistical analyses; JKi supported the content of the Feasibility Concept. KS supervised the preparation of the written informed consent forms and ethics application and provided constructive input on the manuscript. FY, DH, NS, EW, OARR, LH and LJ tested the documentation form during the test phase and provided constructive suggestions for practical implementation. FB supervised and supported the IT programming. FY and EW lead the hands-on training program at Berlin Simulation-Training-Center (BeST) for medical staff (nurses and doctors). FY, EW, OARR, NS and LJ lead education and integration of relatives. AHa and ED lead the standard education program for nurses at ICU and normal ward. RM is responsible for geriatric assessment and manages the teams in our department. TG and EF led the supervision and training of the DES-Teams. FL assigned staff positions to DES-Teams according to their responsibilities and supported team building with regular team meetings. LG and AEMH scheduled the follow-ups and operated them. LE is neuropsychologist, responsible for evaluating the follow-up examination, and for informing and advising patients. CS, MH and FY contributed the clinical aspects and backgrounds in the negotiations with UM, AHö, MR of BARMER health insurance together with the commercial center management and DS (staff unit Kassenverhandlungen of Charité-Universitätsmedizin Berlin), which led to the planning and financing of the contract contents. CS is principal investigator of the quality contract. FY is responsible for cross-campus project coordination and project management and conducts the interdisciplinary correspondence with the support of CS and ST. CS, FY, and MH are responsible for the implementation of the contract contents and the correspondence with IQTIG. FY wrote the manuscript (first authorship). All authors have critically read the final manuscript, contributed inputs and revisions, and approved the final manuscript.
Funding: The G-BA has enabled the implementation of the quality contracts through its resolution and the definition of the legal basis (§ 110a SGB V (Sozialgesetzbuch V)). This legal basis allows hospitals and health insurers to contract for funding of QC. BARMER reimburse the hospital for a part of the costs of conducting the clinical trial.
Disclaimer: This funding source had no role in the design of this study and will not have any role during its execution, analyses, interpretation of the data, or decision to submit results.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; peer reviewed for ethical and funding approval prior to submission.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Battle DE. Diagnostic and statistical manual of mental disorders (DSM). Codas 2013;25:191–2. 10.1590/s2317-17822013000200017 [DOI] [PubMed] [Google Scholar]
- 2.Androsova G, Krause R, Winterer G, et al. Biomarkers of postoperative delirium and cognitive dysfunction. Front Aging Neurosci 2015;7:112. 10.3389/fnagi.2015.00112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bin Abd Razak HR, Yung WYA. Postoperative delirium in patients undergoing total joint arthroplasty: a systematic review. J Arthroplasty 2015;30:1414–7. 10.1016/j.arth.2015.03.012 [DOI] [PubMed] [Google Scholar]
- 4.Kalisvaart KJ, de Jonghe JFM, Bogaards MJ, et al. Haloperidol prophylaxis for elderly hip-surgery patients at risk for delirium: a randomized placebo-controlled study. J Am Geriatr Soc 2005;53:1658–66. 10.1111/j.1532-5415.2005.53503.x [DOI] [PubMed] [Google Scholar]
- 5.Marcantonio ER, Flacker JM, Wright RJ, et al. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc 2001;49:516–22. 10.1046/j.1532-5415.2001.49108.x [DOI] [PubMed] [Google Scholar]
- 6.Saczynski JS, Marcantonio ER, Quach L, et al. Cognitive trajectories after postoperative delirium. N Engl J Med 2012;367:30–9. 10.1056/NEJMoa1112923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sockalingam S, Parekh N, Bogoch II, et al. Delirium in the postoperative cardiac patient: a review. J Card Surg 2005;20:560–7. 10.1111/j.1540-8191.2005.00134.x [DOI] [PubMed] [Google Scholar]
- 8.Inouye SK. Prevention of delirium in hospitalized older patients: risk factors and targeted intervention strategies. Ann Med 2000;32:257–63. 10.3109/07853890009011770 [DOI] [PubMed] [Google Scholar]
- 9.Evered L, Silbert B, Knopman DS, et al. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-2018. Br J Anaesth 2018;121:1005–12. 10.1016/j.bja.2017.11.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inouye SK. Delirium in hospitalized older patients. Clin Geriatr Med 1998;14:745–64. 10.1016/S0749-0690(18)30089-2 [DOI] [PubMed] [Google Scholar]
- 11.Pisani MA, Kong SYJ, Kasl SV, et al. Days of delirium are associated with 1-year mortality in an older intensive care unit population. Am J Respir Crit Care Med 2009;180:1092–7. 10.1164/rccm.200904-0537OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsieh SJ, Otusanya O, Gershengorn HB, et al. Staged implementation of awakening and breathing, coordination, delirium monitoring and management, and early mobilization bundle improves patient outcomes and reduces hospital costs. Crit Care Med 2019;47:885–93. 10.1097/CCM.0000000000003765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.González Tugas M, Uslar Nawrath W, Villarroel del Pino L, et al. Hospital costs associated with delirium in older medical patients. Rev Esp Geriatr Gerontol 2012;47:23–6. 10.1016/j.regg.2011.03.005 [DOI] [PubMed] [Google Scholar]
- 14.Guenther U, Koegl F, Theuerkauf N, et al. Nursing workload indices TISS-10, TISS-28, and NEMS: higher workload with agitation and delirium is not reflected. Med Klin Intensivmed Notfmed 2016;111:57–64. 10.1007/s00063-015-0056-5 [DOI] [PubMed] [Google Scholar]
- 15.DESTATIS . Operationen und prozeduren der vollstationären patientinnen und patienten in krankenhäusern (4-steller) - 2018. 2019. Available: https://www.destatis.de/DE/Themen/Gesellschaft-Umwelt/Gesundheit/Krankenhaeuser/Publikationen/Downloads-Krankenhaeuser/operationen-prozeduren-5231401187014.html
- 16.Aldecoa C, Bettelli G, Bilotta F, et al. European society of anaesthesiology evidence-based and consensus-based guideline on postoperative delirium. Eur J Anaesthesiol 2017;34:192–214. 10.1097/EJA.0000000000000594 [DOI] [PubMed] [Google Scholar]
- 17.Baron Ralf BA, Rolf B, Stephan B, et al. S3-leitlinie analgesie, sedierung und delirmanagement in der intensivmedizin (DAS-leitlinie 2015. 2015. Available: http://www.awmf.org/uploads/tx_szleitlinien/001-012l_S3_Analgesie_Sedierung_Deliermanagement_Intensivmedizin_2015-08.pdf [DOI] [PubMed]
- 18.Balas MC, Vasilevskis EE, Olsen KM, et al. Effectiveness and safety of the awakening and breathing coordination, delirium monitoring/management, and early exercise/mobility bundle. Crit Care Med 2014;42:1024–36. 10.1097/CCM.0000000000000129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bounds M, Kram S, Speroni KG, et al. Effect of ABCDE bundle implementation on prevalence of delirium in intensive care unit patients. Am J Crit Care 2016;25:535–44. 10.4037/ajcc2016209 [DOI] [PubMed] [Google Scholar]
- 20.Bryczkowski SB, Lopreiato MC, Yonclas PP, et al. Delirium prevention program in the surgical intensive care unit improved the outcomes of older adults. J Surg Res 2014;190:280–8. 10.1016/j.jss.2014.02.044 [DOI] [PubMed] [Google Scholar]
- 21.Eghbali-Babadi M, Shokrollahi N, Mehrabi T. Effect of family-patient communication on the incidence of delirium in hospitalized patients in cardiovascular surgery ICU. Iran J Nurs Midwifery Res 2017;22:327–31. 10.4103/1735-9066.212985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sullinger D, Gilmer A, Jurado L, et al. Development, implementation, and outcomes of a delirium protocol in the surgical trauma intensive care unit. Ann Pharmacother 2017;51:5–12. 10.1177/1060028016668627 [DOI] [PubMed] [Google Scholar]
- 23.IQTIG . Qualitätsverträge nach § 110a SGB V. Evaluationskonzept zur untersuchung der entwicklung der versorgungsqualität gemäß § 136b abs. 8 SGB V. Abschlussbericht. Institut für Qualitätssicherung und Transparenz im Gesundheitswesen; 2017. [Google Scholar]
- 24.Kratz T. Delirium with dementia. Z Gerontol Geriatr 2007;40:96–103. 10.1007/s00391-007-0435-5 [DOI] [PubMed] [Google Scholar]
- 25.Kratz T, Heinrich M, Schlauß E, et al. Preventing postoperative delirium. Dtsch Arztebl Int 2015;112:289–96. 10.3238/arztebl.2015.0289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med 2018;46:e825–73. 10.1097/CCM.0000000000003299 [DOI] [PubMed] [Google Scholar]
- 27.Inouye SK, Baker DI, Fugal P, et al. Dissemination of the hospital elder life program: implementation, adaptation, and successes. J Am Geriatr Soc 2006;54:1492–9. 10.1111/j.1532-5415.2006.00869.x [DOI] [PubMed] [Google Scholar]
- 28.Inouye SK, Bogardus ST, Baker DI, et al. The hospital elder life program: a model of care to prevent cognitive and functional decline in older hospitalized patients. Hospital elder life program. J Am Geriatr Soc 2000;48:1697–706. 10.1111/j.1532-5415.2000.tb03885.x [DOI] [PubMed] [Google Scholar]
- 29.Evered L, Silbert B, Knopman DS, et al. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-2018. J Alzheimers Dis 2018;66:1–10. 10.3233/JAD-189004 [DOI] [PubMed] [Google Scholar]
- 30.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–56. 10.1093/gerona/56.3.m146 [DOI] [PubMed] [Google Scholar]
- 31.Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci 2007;62:738–43. 10.1093/gerona/62.7.738 [DOI] [PubMed] [Google Scholar]
- 32.Podsiadlo D, Richardson S. The timed “up & go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991;39:142–8. 10.1111/j.1532-5415.1991.tb01616.x [DOI] [PubMed] [Google Scholar]
- 33.Nikolaus T, Specht-Leible N, Bach M, et al. Social aspects in diagnosis and therapy of very elderly patients. initial experiences with a newly developed questionnaire within the scope of geriatric assessment. Z Gerontol 1994;27:240–5. [PubMed] [Google Scholar]
- 34.Heatherton TF, Kozlowski LT, Frecker RC, et al. Measuring the heaviness of smoking: using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. Br J Addict 1989;84:791–9. 10.1111/j.1360-0443.1989.tb03059.x [DOI] [PubMed] [Google Scholar]
- 35.Bush K, Kivlahan DR, McDonell MB, et al. The audit alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. ambulatory care quality improvement project (ACQUIP). alcohol use disorders identification test. Arch Intern Med 1998;158:1789–95. 10.1001/archinte.158.16.1789 [DOI] [PubMed] [Google Scholar]
- 36.Nordqvist C, Johansson K, Bendtsen P. Routine screening for risky alcohol consumption at an emergency department using the AUDIT-C questionnaire. Drug Alcohol Depend 2004;74:71–5. 10.1016/j.drugalcdep.2003.11.010 [DOI] [PubMed] [Google Scholar]
- 37.Masnoon N, Shakib S, Kalisch-Ellett L, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr 2017;17:230. 10.1186/s12877-017-0621-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carnahan RM, Lund BC, Perry PJ, et al. The anticholinergic drug scale as a measure of drug-related anticholinergic burden: associations with serum anticholinergic activity. J Clin Pharmacol 2006;46:1481–6. 10.1177/0091270006292126 [DOI] [PubMed] [Google Scholar]
- 39.Kroenke K, Strine TW, Spitzer RL, et al. The PHQ-8 as a measure of current depression in the general population. J Affect Disord 2009;114:163–73. 10.1016/j.jad.2008.06.026 [DOI] [PubMed] [Google Scholar]
- 40.Chlan LL. Relationship between two anxiety instruments in patients receiving mechanical ventilatory support. J Adv Nurs 2004;48:493–9. 10.1111/j.1365-2648.2004.03231.x [DOI] [PubMed] [Google Scholar]
- 41.Chlan LL, Weinert CR, Heiderscheit A, et al. Effects of patient-directed music intervention on anxiety and sedative exposure in critically ill patients receiving mechanical ventilatory support: a randomized clinical trial. JAMA 2013;309:2335–44. 10.1001/jama.2013.5670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chanques G, Viel E, Constantin J-M, et al. The measurement of pain in intensive care unit: comparison of 5 self-report intensity scales. Pain 2010;151:711–21. 10.1016/j.pain.2010.08.039 [DOI] [PubMed] [Google Scholar]
- 43.Radtke FM, Franck M, Lendner J, et al. Monitoring depth of anaesthesia in a randomized trial decreases the rate of postoperative delirium but not postoperative cognitive dysfunction. Br J Anaesth 2013;110 Suppl 1:i98–105. 10.1093/bja/aet055 [DOI] [PubMed] [Google Scholar]
- 44.Luetz A, Heymann A, Radtke FM, et al. Different assessment tools for intensive care unit delirium: which score to use? Crit Care Med 2010;38:409–18. 10.1097/CCM.0b013e3181cabb42 [DOI] [PubMed] [Google Scholar]
- 45.Gaudreau J-D, Gagnon P, Harel F, et al. Fast, systematic, and continuous delirium assessment in hospitalized patients: the nursing delirium screening scale. J Pain Symptom Manage 2005;29:368–75. 10.1016/j.jpainsymman.2004.07.009 [DOI] [PubMed] [Google Scholar]
- 46.Ely EW, Truman B, Shintani A, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond agitation-sedation scale (RASS). JAMA 2003;289:2983–91. 10.1001/jama.289.22.2983 [DOI] [PubMed] [Google Scholar]
- 47.Bergeron N, Dubois MJ, Dumont M, et al. Intensive care delirium screening checklist: evaluation of a new screening tool. Intensive Care Med 2001;27:859–64. 10.1007/s001340100909 [DOI] [PubMed] [Google Scholar]
- 48.Neufeld KJ, Leoutsakos JS, Sieber FE, et al. Evaluation of two delirium screening tools for detecting post-operative delirium in the elderly. Br J Anaesth 2013;111:612–8. 10.1093/bja/aet167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen CC-H, Lin M-T, Tien Y-W, et al. Modified hospital elder life program: effects on abdominal surgery patients. J Am Coll Surg 2011;213:245–52. 10.1016/j.jamcollsurg.2011.05.004 [DOI] [PubMed] [Google Scholar]
- 50.O’Gara B, Marcantonio ER, Pascual-Leone A, et al. Prevention of early postoperative decline (peapod): protocol for a randomized, controlled feasibility trial. Trials 2018;19:676. 10.1186/s13063-018-3063-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berger M, Schenning KJ, Brown CH, et al. Best practices for postoperative brain health: recommendations from the fifth international perioperative neurotoxicity working group. Anesth Analg 2018;127:1406–13. 10.1213/ANE.0000000000003841 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-066709supp001.pdf (104.1KB, pdf)
bmjopen-2022-066709supp002.pdf (161KB, pdf)