Abstract
Immune checkpoint inhibitors (ICIs) are associated with a wide range of immune-related adverse events. As oncological indications for ICIs widen, their rare side effects become increasingly visible in clinical practice and impact therapy decisions.
Here, we report a rare case of early-onset, mild cytokine release syndrome (CRS) in a patient who received ICIs for a metastasized renal cell carcinoma, which led to treatment discontinuation.
We further provide a systematic review of the literature of CRS and related life-threatening side effects of ICI treatment, such as hemophagocytic lymphohistiocytosis (HLH). We searched Medline, Embase and the Web of Science Core Collection from inception to October 2021 for reports on CRS, cytokine storm, macrophage activation syndrome, HLH, and related hyperinflammatory disorders in patients with solid cancers receiving ICIs. We found n=1866 articles, which were assessed for eligibility independently by two examiners. Of those, n=49 articles reporting on n=189 individuals were eligible for review. We found that the median time from last infusion to the occurrence of CRS/HLH was approximately nine days, while the onset of symptoms varied from immediately after infusion to one month after treatment. Most patients were treated with either corticosteroids or the anti-interleukin 6 (IL-6) antibody tocilizumab, and although the majority of patients recovered, a few cases were fatal. Concomitant IL-6 and ICI treatment were reported as beneficial for both the antitumoral effect and for limiting side effects. Data from international pharmacovigilance databases underscored that ICI-related CRS and HLH are rare events, but we identified significant differences in reported frequencies, which might suggest substantial under-reporting.
The results from this first systematic review of CRS/HLH due to ICI therapy highlight that life-threatening systemic inflammatory complications of ICIs are rare and might be associated with fatal outcome in approximately 10% of patients. Limited data support the use of IL-6 inhibitors in combination with ICIs to augment the antitumoral effect and reduce hyperinflammation.
Keywords: Melanoma, Lung Neoplasms, Inflammation Mediators, Immunotherapy
Introduction
Immune checkpoint inhibitors (ICIs) have become important therapeutic options for various tumor types. ICIs are associated with specific toxicities, commonly referred to as immune-related adverse events (irAEs), which can lead to treatment interruption or discontinuation. International guidelines aid clinicians in the diagnosis and management of relatively common irAEs, such as skin rashes, colitis, thyroiditis, and pneumonitis.1 2 However, the increasing volume of patients treated with ICIs is starting to reveal less common side effects, including systemic hyperinflammatory syndromes. Nonspecific systemic inflammatory reactions to ICIs, such as self-limiting fever or skin rashes during or shortly after infusion2 need to be distinguished from severe, persistent, and potentially life-threatening conditions such as cytokine release syndrome (CRS) and hemophagocytic lymphohistiocytosis (HLH)/macrophage-activation syndrome (MAS).3 Due to their severity, these irAEs are of particular clinical importance and require a decision on continuation of ICI treatment, for which evidence is lacking. Because of their rarity, hyperinflammatory syndromes are incompletely captured in randomized clinical trials with ICIs4 5 and most international irAE guidelines lack specific recommendations for their management.1 2 Although a recent irAE guideline from the Society of Immunotherapy for Cancer discusses HLH as an irAE with potentially high lethality, no specific treatment recommendation could be made.6 Hence, real-world data and case reports of rare irAEs are needed to understand their frequency and severity, and to improve clinical management.
In cancer therapy, CRS is best understood in the context of chimeric antigen receptor (CAR) T cell therapies, where it occurs in a substantial proportion of patients at different levels of severity.7 CRS is believed to be mainly driven by T cell-derived interferon gamma (IFN-γ), which stimulates macrophages to produce various proinflammatory substances including interleukin 6 (IL-6) and tumor necrosis factor alpha (TNF-α).8 Therapeutically, IL-6 inhibition with specific anti-IL-6 receptor antibodies, such as tocilizumab, has proven highly effective against CRS, reflected by the US Federal Drug Agency’s approval of tocilizumab for CAR T cell-induced CRS.9 This is important because IL-6 inhibition could allow for the continuation of any treatment associated with mild CRS.10
HLH is an umbrella term for life-threatening hyperinflammatory conditions with supramaximal activation of the immune system. For the diagnosis of HLH, the HLH-2004 diagnostic criteria are frequently used, although they were developed for the pediatric population.3 The criteria include clinical features such as fever and splenomegaly, as well as laboratory findings such as cytopenias, hypertriglyceridemia, hyperferritinemia, evidence of hemophagocytosis, and the absence of natural killer (NK) cell activity.3 More recently, the HScore, which includes similar criteria to HLH-2004, was developed to estimate the probability for reactive HLH in adults with inflammatory syndromes.11 Genetic analyses from pediatric patients have revealed a wide variety of predisposing variants that presumably play a role for different immune cell types, suggesting that HLH-related conditions represent a complex disease continuum.12
Reports on hyperinflammatory syndromes due to ICI treatment have started to emerge during recent years, and have suggested that these are relatively rare, but potentially life-threatening events.13 Both HLH and CRS can be fatal by causing hypotension, capillary leak syndrome, and consequently organ dysfunction.8 Because of the potential risk of increasing the severity of CRS/HLH on repeated exposure to a particular trigger, suspicion of these hyperinflammatory syndromes in clinical practice most often leads to treatment cessation. Hence, a better understanding of this complex disease spectrum in the context of ICI treatment is needed to guide decision-making on treatment continuation and optimal management.
Here, we present a rare case of early-onset CRS after ICI treatment of a metastatic renal cell carcinoma. To set the stage for a more evidence-based approach to CRS, we provide a systematic review of the literature, in which we identify n=49 articles on n=189 patients with hyperinflammatory syndromes due to ICIs. The results reveal that most patients with CRS/HLH recovered and that a fatal outcome occurred in approximately 10% of all patients. In addition, the literature reveals that corticosteroids and IL-6 inhibition may provide effective therapies. Extrapolating from preclinical data, which we review in brief, we posit that rechallenging with ICIs after CRS/HLH should be considered at least in patients with mild hyperinflammatory ICI side effects who are expected to benefit from ICI treatment. Recent data suggest that ICIs in combination with IL-6 antagonists may boost the antitumoral effect, while simultaneously protecting from severe irAEs, which makes ICIs in combination with IL-6 inhibition an attractive option for rechallenge that warrants further research.
Methods
Case report
The clinical case of CRS was encountered by the authors in their clinical practice at Karolinska University Hospital, Stockholm, Sweden. Clinical data were gathered through review of the electronic patient journal. Data were visualized using GraphPad Prism V.9.4.0.
Systematic review
We performed a systematic search for published reports on CRS, HLH and related hyperinflammatory diseases in MEDLINE, Embase, and the Web of Science databases as of October 2021. The search strategies were designed in collaboration with the Karolinska Institute library and are included as online supplemental figure 1. Deduplication was performed as previously described.14
jitc-2022-005841supp001.pdf (185.7KB, pdf)
Additional manual searches were performed based on the lists of references in the eligible studies, and a reduced set of keywords in the MEDLINE database only, as of June 13, 2022. Inclusion criteria for abstract review were assessed independently by two reviewers (LLL and MG) and defined as follows: (1) report on any hyperinflammatory syndrome (CRS, HLH, MAS, or any indication of these or related systemic syndromes) on human patients with solid tumors, (2) reported use of any ICI at any time of the treatment, (3) case series or case reports, that is, no randomized controlled trial, unless explicitly stated that CRS/HLH/MAS or any other hyperinflammatory syndrome was reported, (4) articles in English, Swedish, Chinese or German. LLL and MG agreed on article inclusion by discussing the articles for which the initial decision on inclusion differed. For all eligible articles, the full-text was downloaded by LLL or MG. Data were extracted by either LLL, MS, or MG, and all authors convened to agree on final inclusion. As the majority of the included studies were case reports on rare events, no further criteria for study quality assessment were applied and the risk for bias was not assessed. The systematic review was not preregistered.
Results
Case report
A patient in her early 70s had presented to her primary care physician with increasing fatigue and right upper quadrant pain radiating to the spine. She reported involuntarily weight loss of 20 kg during the last year. She had a history of arterial hypertension, hyperlipidemia, and she had been a smoker for 35 years, but had quit more than 10 years previously. No history of allergic reactions was recorded. At the time of presentation, she was on treatment with metoprolol, amlodipine, enalapril, atorvastatin, and ketoprofen. Workup with a CT scan revealed a 12.5×8×15 cm multicystic renal tumor, lymph node metastases, at least two liver metastases, the largest of which was 5 cm in diameter, three pulmonary/pleural metastases, and a bone metastasis in the left acetabulum. A needle biopsy from the lesion in the right kidney showed clear cell renal cell carcinoma, grade 2 according to the International Society of Urologic Pathologists. She had a performance score of 2 according to the Eastern Cooperative Oncology Group (ECOG). According to the International metastatic RCC database consortium prognostic criteria,15 she had a poor prognosis due to anemia, elevated neutrophils and a short period from diagnosis to start of systemic treatment. For the pain, the patient was started on paracetamol and oxycodone, which she used sparingly, and she needed transfusions because of low hemoglobin levels.
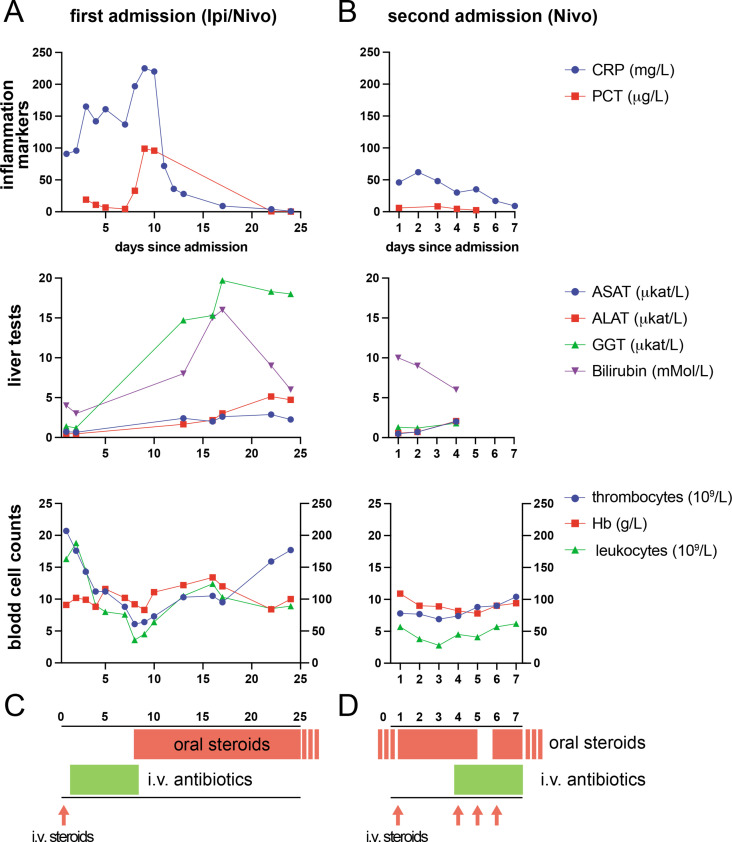
One month after diagnosis, the patient was started on palliative first-line therapy with the ICIs ipilimumab (1 mg/kg, total of 64 mg) and nivolumab (3 mg/kg, total of 200 mg) at the Karolinska University Hospital. She received her first course of this combination from 08:30 onwards and left the ambulatory treatment unit without signs of complications. On her way home, she began to feel sick, developed a fever of 39.5°C, and experienced chills and confusion, on which an ambulance was called. She was brought into the emergency department with suspected sepsis. On arrival, her heart rate was 110 /min, her blood pressure was 120/80 mm Hg, and her ECG showed sinus tachycardia without signs of ischemia. Her C-reactive protein (CRP) level on arrival was 102 mg/L (normal: <3 mg/L). In the emergency department, an adverse reaction to the ICI treatment was suspected, and the patient was given antihistamines and 100 mg of hydrocortisone intravenously. Because she continued to have fever and chills, the antibiotic piperacillin/tazobactam was added ex juvantibus. Repeated blood cultures were negative. The patient continuously received both antibiotic and corticosteroid treatment during her inpatient stay, following which decreasing inflammatory parameters were seen (figure 1A, C). The patient was hospitalized for a total of 22 days and was discharged with betamethasone 8 mg per os daily with a planned tapering over a period of almost 2 months. Five weeks after discharge, the patient presented to the outpatient clinic with an improved ECOG performance score of 1. A CT scan showed partial response. A clinical conference decision was made to continue with ICI treatment, but as a monotherapy with nivolumab to reduce the risk of inflammatory side effects. She received the second ICI treatment of 480 mg nivolumab 69 days after the first cycle with ipilimumab and nivolumab. Directly after completion of the infusion, she developed a fever of 38.6°C, sinus tachycardia of 130 beats/min and hypertension of 170/100 mm Hg. Another adverse reaction related to ICI was suspected and she was given hydrocortisone (200 mg intravenously) and paracetamol (1000 mg intravenously); in addition, she was already on treatment with betamethasone 0.5 mg per os daily after the first adverse reaction to ICI. The patient was admitted to the oncological inpatient ward for the second time (figure 1B, D). On admission, the patient’s CRP level was 46 mg/L (normal: <3 mg/L), her procalcitonin level was 5.9 µg/L (normal: >0.5 µg/L), and leukocyte counts were normal. The day after admission, the patient’s vital parameters were stable, serum IL-6 was at 51 ng/L (normal: <7.0 ng/L). Her CRP reached a maximum level of 62 mg/L the day after admission and her procalcitonin level rose to a maximum of 8.2 µg/L 2 days after the ICI infusion. Erythrocyte count, leucocyte count and platelets were all suppressed and reached their nadir 3–4 days post-ICI infusion (figure 1). As the patient had intermittent fever several days after the ICI treatment, piperacillin/tazobactam was administered intravenously for 4 days, and treatment was discontinued when the patient was discharged, as her inflammatory parameters had decreased, and no pathogens were detected in blood cultures.
Figure 1.
Laboratory tests after immune checkpoint inhibitor (ICI) treatment in a patient with metastatic renal cell carcinoma. (A) Routine blood tests for the indicated markers at the first admission after treatment with ipilimumab and nivolumab; legend as indicated in (C). Left y-axis in lower panel for leukocytes, right y-axis for thrombocytes and hemoglobin. (B) The same blood tests as in (A) at the occasion of the second admission after treatment with nivolumab. Day ‘1’ is the day of treatment, which was also the day of admission in both cases. (C and D) Graphic illustration of the timing of treatment on first (C) and second (D) admission. ALAT, alanine aminotransferase; ASAT, aspartate aminotransferase; CRP, C reactive protein; GGT, gamma-glutamyltransferase, Hb, hemoglobin; Ipi, ipilimumab, Nivo, nivolumabb; PCT, procalcitonin.
According to the American Society for Transplantation and Cellular Therapy criteria for grading CRS after CAR T cell therapy,16 the patient presented with grades 1–2 CRS on both occasions.
At the first follow-up visit after monotherapy with nivolumab, the patient had elevated transaminases, which was considered as ICI related toxicity, hepatitis grade 3. Because of these severe side effects after monotherapy with nivolumab, ICIs were permanently discontinued. Oral corticosteroids were tapered over several weeks and then discontinued without signs of a recurrent inflammatory flare. Several follow-up CTs showed initially partial response in several metastases and thereafter stable disease. However, a CT scan 10 months following ICI therapy cessation confirmed progressive disease and the patient was switched to a second-line tyrosine kinase inhibitor (TKI). The TKI was discontinued after four months of therapy due to severe treatment-related adverse events, including mucositis, fatigue, and diarrhea. The patient was started on third-line everolimus 18 months after diagnosis and remains free of irAEs.
In summary, this patient developed early-onset, mild CRS that was manageable in a standard oncology ward without the need for vasopressors or invasive ventilation.
Systematic review
To chart our current knowledge of hyperinflammatory syndromes, such as CRS and HLH, as rare side effects of treatment with ICIs, we conducted a systematic review of the literature housed in the major medical databases as of October 2021. In addition, we manually updated the list of eligible studies as of 1June 13, 2022. Online supplemental figure 2 presents the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting chart for the study.
jitc-2022-005841supp002.pdf (304.5KB, pdf)
We identified a total of n=49 articles, reporting on n=189 patients treated in the USA, the UK, Switzerland, Spain, Poland, Lebanon, Japan, Israel, Germany, France, China, Canada, Australia, and Singapore (table 1). Of these studies, n=45 reports were on n=<5 individual patients (comprizing a total of n=56 individuals), while n=4 studies reported case series or data from queries of pharmacovigilance databases. Table 2 summarizes the most important findings from the reports on individual patients. Definitions of CRS/HLH varied significantly between reports, and retrospective diagnostic assessment was not possible due to partially incomplete and heterogeneous clinical data. Therefore, we used the respective authors’ assessment of the hyperinflammatory condition as CRS, HLH, or related hyperinflammatory diseases for further analysis. The earliest published report was from 2016.13
Table 1.
Studies included after systematic review
| Authors ref year country |
Type and stage |
No. of patients |
Age* and sex | Antibody | Main complication† |
ICI cycle | Time- to-symptoms (days) |
Main symptoms | Intervention | CRS/HLH outcome | ICI rechallenge | Tumor- specific outcome |
| Sharma et al13 2016 USA |
Lung cancer, ‘progressive’ | 12 | Unclear | Nivolumab | SIRS | ‘Fever, tachypnea, tachycardia, hypotension’ | Steroids, tocilizumab | Unclear | Unclear | Unclear | ||
| El Rassy et al.31 2017 Lebanon |
Lung squamous cell carcinoma, stage 4 | 1 | 60–70 M | Pem brolizumab |
CRS | 7 | 1 | ‘Persistent low-grade fever(…), diffuse swelling’ | Furosemide, steroids. | Recovery | Yes, up to at least 13 cycles. | Unclear |
| Shah et al.46 2017 USA |
Bladder, stage 4 | 1 | 70–80 M | Pem brolizumab |
HLH | 9 months of therapy | Unclear | Fever, tachycardia, rash, acute renal failure. | Etoposide, steroids | Unclear | Unclear | CR |
| Rotz et al.47 2017 USA |
Sarcoma, stage 4 | 1 | 20–30 F | Nivolumab | CRS | 2 | 4 | Fever, rash, encephalopathy, tachycardia, hypotension. | Steroids, broad-spectrum ABs, tocilizumab. | Recovery | No | Unclear |
| Urosevic-Maiwald et al48 2017 Switzerland |
Melanoma, stage 4 | 2 (1) | 40–50 F | Pem brolizumab |
SIRS | 28 | Fever, hypotension, tachycardia, anuria. | ABs, steroids | Recovery | No. | ||
| Urosevic-Maiwald et al48 | Melanoma, stage 4 | 2 (2) | 40–50 F | Pem brolizumab |
‘Hhyper sensitivity syndrome’ or DRESS |
8 | Face swelling, pruritic eruption, hypotension, tachycardia and fever | Steroids | Recovery | No | PD after around 7 months | |
| Takeshita et al49 2017 Japan |
Squamous lung cancer, stage 4 | 1 | 60–70 F | Nivolumab | CRS | 2 | 25 | Severe general fatigue and high-grade fever | Steroids | Recovery | No | Regression |
| Michot et al40 2018 France |
Melanoma, stage 4 | 1 | 50–60 F | Ipilimumab | HLH | 1 (8 pembrolizumab) | 8 weeks after the last dose of ipilimumab | ‘Fever with pancytopenia and disseminated intravascular coagulation’ | Steroids, etoposide | Death from brain hemorrhage at metastatic site. | No | Unclear |
| Sadaat et al50 2018 USA |
Melanoma, stage 4 | 1 | 50–60 M | Pem- brolizumab |
HLH | 6 | 31 | Fever. | Steroids | Recovery | No | CR for 1 year. |
| Hantel et al51 2018 USA |
Melanoma, stage 4 | 1 | 30–40 F | Ipilimumab/ nivolumab |
HLH | “first doses” | 21 | Tachycardia, hypotension. | Steroids. | Recovery | No | CR |
| Satzger et al52 2018 Germany |
Melanoma, stage 4 | 1 | 20–30 F | Ipilimumab/ nivolumab |
HLH | 4 | 7 | Fever. | Steroids, mycophenolate mofetil. | Recovery | No | CR |
| Shah and Melissa53 2018 USA |
Bladder cancer and thymoma, stage 4 | 1 | 70–80 M | Pem brolizumab |
HLH | Fever, fatigue | ||||||
| Sasaki et al54 2018 Japan |
Melanoma, stage 4 | 1 | 60–70 F | Pem brolizumab |
HLH | ‘1 month after last ICI’ | 13 | Fever, hep atosplenomegaly, erythema multiforme-like lesions. |
Steroids | Recovery | No. | PR |
| Laderian et al55 2019 USA |
Thymic carcinoma, stage 4 | 1 | 40–50 M | Pem brolizumab |
HLH | Several over the course of 1 year | Pancytopenia, hemophagocytosis. | Deceased | ‘Clinical benefit’ | |||
| Kogure et al32 2019 Japan |
Lung adeno- carcinoma, stage 3b |
1 | 60–70 M | Pem brolizumab |
CRS | 1 | 2 | Fever, tachycardia, hypotension. | Steroids | Recovery | Yes | Pseudoprogression, after 3 cycles of pembrolizumab PR. |
| Oda et al56 2019 Japan |
Gastric adeno- carcinoma, stage 4 |
1 | 40–50 M | Nivolumab | CRS | 1 | 8 | Fever, tachycardia, malaise. | Steroids, mycophenolate mofetil. | Death “from gastric cancer.” | No | PD |
| Noseda et al18 2019 France, Japan, Germany, Switzerland, Canada, USA |
diverse | 38 | M: 29 F: 9 | Ipilimumab, nivolumab, pem- brolizumab, atezolizumab, and combinations |
||||||||
| Lorenz et al57 2019 Germany |
Prostate, stage 4 | 1 | 60–70 M | Pem- brolizumab |
HLH | Fever, hep atosplenomegaly. |
Steroids, plasmapheresis, ciclosporin A, etoposide, tacrolimus. | Recovery | No | CR | ||
| Okawa et al58 2019 Japan |
Lung squamous cell carcinoma, stage 3b | 1 | 70–80 M | Pem- brolizumab |
HLH | 1 | 10 | Fatigue, fever, jaundice, splenomegaly. | Steroids, ABs. | Recovery | No | CR |
| Chin et al59 2019 Australia |
Melanoma, stage 4 | 1 | 60–70 F | Nivolumab | HLH | 30 | Fevers, lethargy, abdominal distention. Hep- atosplenomegaly. |
Steroids | Recovery | No | SD | |
| Honjo et al60 2019 Japan |
Pulmonary pleomorphic carcinoma, stage 3 | 1 | 50–60 F | Nivolumab | CRS | ‘After the last nivolumab administration’ | 14 | Asthenia, fever. Livedo reticularis with systemic purpura. | Steroids, thrombo modulin and my cophenolate mofetil, hemodi afiltration. |
Recovery | No | PR |
| Adashek and Feldman61 2019 USA |
Lung adeno- carcinoma, stage 4 |
1 | 70–80 M | Pem- brolizumab |
CRS | 3 then again after 4 | 1 | Fever, hypotension, mental status change the first time. Then hypotension, respiratory distress. | Tocilizumab | Recovery | Unclear | Unclear |
| Slota et al20 2019 USA |
Melanoma, stage 4 | 1 | 70–80 M | Nivolumab | CRS | 17 | Altered mental status, hypotension, tachycardia, fever, hypoxia. Grade 3 maculopapular rash. | ABs, steroids, tocilizumab | Recovery, then relapse 6 weeks later (deceased) | No | PR | |
| Takahashi et al62 2020 Japan |
Lung adeno- carcinoma, stage 4 |
1 | 70–80 M | Pem brolizumab |
HLH | 1 | 7 | Fever, diarrhea | Steroids, Abs. | Recovery | No | SD for 3 months |
| Ohira et al63 2020 Japan |
Renal cell carcinoma, stage 4 | 1 | 70–80 M | Nivolumab, ipilimumab | CRS | 2 | 2 | "Dermatomyositis (…)high fever, hypotension, respiratory failure, impaired consciousness@ |
Steroids, mycophenolate mofetil, plasma exchange. | Recovery | No | SD for 2 months |
| Normand et al64 2020 Switzerland |
Lung adeno- carcinoma, stage 4 |
1 | 70–80 M | Pem brolizumab |
CRS | 1 | 1 | Fever, renal impairment, confusion, dyspnea. | ABs, steroids. | Recovery | No | SD for 6 months |
| Gao et al65 2020 China |
Esophageal squamous cell carcinoma, locally advanced | 1 | 60–70 M | Sintilimab | CRS | 3 | Fever, diarrhea. | Methyl- prednisolone, tocilizumab, mycophenolate mofetil, immuno globulin. |
Recovery | Unclear | Unclear | |
| Özdemir et al66 2020 Switzerland |
Melanoma, stage 4 | 3 (1) | 40–50 M | Ipilimumab, nivolumab | HLH | 2 | Fever, nausea, extreme fatigue. | Steroids, tocilizumab, plasma. | Recovery | Unclear | CR | |
| Özdemir et al66 | Melanoma, stage 4 | 3 (2) | 30–40 M | Nivolumab | HLH | 5 | Splenomegaly, fever and extreme fatigue. | Tocilizumab, steroids, low dose heparin prophylaxis. | Recovery | Unclear | PR | |
| Özdemir et al66 | Melanoma, stage 4 | 3 (3) | 30–40 M | Ipilimumab, nivolumab | HLH | 3 | Hep atosplenomegaly, fever and fatigue. |
Tocilizumab, plasma, low dose heparin prophylaxis | Recovery | Unclear | PR | |
| Azari et al67 2020 UK |
Renal cell carcinoma, stage 4 | 1 | 50–60 M | Nivolumab, ipilimumab | HLH | 1 | 6 | ‘Myalgia, fevers, frontal headache, photophobia, blurry vision, and vomiting’. | Abs, methyl prednisolone and anakinra. |
Recovery | No | Unclear |
| Dupré et al33 2020 France |
Pulmonary sarcomatoid carcinoma, stage 4 | 5 (1) | 50–60 M | Pem brolizumab |
HLH | 1 | 7 | Fever, asthenia, dyspnea. | Steroids, broad-spectrum ABs | Recovery | Yes | PD |
| Dupré et al33 | Melanoma, stage 4 | 5 (2) | 30–40 F | Ipilimumab, nivolumab | HLH | 1 | 21 | Asthenia, splenomegaly | Steroids, etoposide, intravenous immuno globulins, tocilizumab. |
Recovery | Yes | SD |
| Dupré et al33 | Melanoma, stage 4 | 5 (3) | 50–60 F | Ipilimumab, pem brolizumab |
HLH | ‘After ipilimumab perfusion’ | 30 | Asthenia, fever | Steroids, etoposide | Deceased | No | PD |
| Cont. Dupré et al33 | Melanoma, stage 4 | 5 (4) | 60–70 M | Ipilimumab, nivolumab | HLH | ‘Five weeks and two cycles after the introduction of the combination’. | Unclear | Fever Splenomegaly | Steroids | Recovery | Yes | PD |
| Dupré et al33 | Melanoma, stage 4 | 5 (5) | 20–30 M | Ipilimumab, nivolumab | HLH | Fever, meningitis, colitis, hepatic cytolysis. | Steroids | Recovery | No | Unclear | ||
| Akagi et al68 2020 Japan |
Lung adeno- carcinoma, stage 3b |
1 | 70–80 M | Pem- brolizumab |
HLH | 1 | 27 | Joint swelling, hypertension, fever diffuse macular rash. | Steroids, recombinant throm- bomodulin, G-CSF, etoposide. |
Recovery | No | CR |
| Thummalapalli et al69 2020 USA |
Glioblastoma | 1 | 70–80 M | Nivolumab | HLH | 2 | 17 | Fever, altered mental status. | Steroids, Abs. | Deceased | No | Unclear |
| Mizuta et al70 2020 Japan |
Melanoma, stage 4 | 1 | 60–70 F | Ipilimumab, nivolumab | HLH | 2 | 1 | Fever, malaise, headache, grade 2 diarrhea. | NSAID, ABs, steroids | Unclear | Unclear | Unclear |
| Ceschi et al19 2020 WHO database, inc. reports from N. America, Europe, Australia, Japan. |
Melanoma, lung cancer, others | 58 cases, 42 patients with non-hemato logical malignancies. |
Med. age 55 y (incl. hemato logical cases). M:34 F: 21 |
Ipilimumab, nivolumab, pem brolizumab, cemiplimab, atezolizumab, avelumab a nd combinations. |
CRS | 10 cases after a single administration. | ‘A median of 4 weeks’ | ‘CRS, defined accordingly to the correspondent MedDRA PT ‘cytokine release syndrome’ (MedDRA version 21.1)’. | No reported | Two fatal cases, unknown outcome in 20 cases | Unclear | Unclear |
| Kalmuk et al34 2020 USA |
Oropharyngeal squamous cell carcinoma, stage 4 | 1 | 60–70 M | Pem- brolizumab |
HLH | 14 | 4 | Fever, malaise. | Antibiotics, steroids, etoposide | Recovery | Yes | SD for 8 months |
| Hu et al71 2020 China |
Colon cancer, stage 4 | 1 | 50–60 M | Sintilimab | cytokine storm | 2 | 1 | Fever, hypotension, dyspnea. | IV fluids, vasopressors, steroids, ABs, nintedanib. | Recovery | Unclear | Unclear |
| Nieves-Borrero et al72 2020 USA |
Small cell lung cancer, metastatic | 1 | 60–70 M | Atezolizumab | CRS | 1 | 3 | Hypotension, cardiac arrest. | Hemodialysis, renal replacement therapy. | Deceased | No | Unclear |
| Dudda et al73 2020 Germany |
Melanoma, stage 4 | 1 | Unclear Unclear | Nivolumab | HLH | 21 | Splenomegaly, fever | Broad-spectrum Abs. | Recovery | No | PR | |
| Amlani et al74 2020 Canada |
Melanoma, stage 4 | 1 | 50–60 M | Nivolumab | CRS | Fatigue, nausea, vomiting, diarrhea, fever, and a purpuric eruption. Shock. | Broad-spectrum ABs, steroids, tocilizumab | Recovery | No | Unclear | ||
| Yomota et al75 2021 Japan |
Lung adeno- carcinoma, stage 4 |
1 | 50–60 M | Atezolizumab | CRS | 1 | 7 | ‘High fever, rash, DIC, reduced level of consciousness, heart failure…’. | Steroids, tocilizumab from day 11. | Recovery | No | PR |
| Percik et al.76 2021 Israel |
Melanoma, stage 3b | 2 (1) | 50–60 M | Nivolumab, ipilimumab | Capillary-leak syndrome | 21 | Generalized edema | Discontinuation of ICI | Initial recovery; proximal muscle weakness and death 1 month later. | No | PR | |
| Percik et al76 | Duodenal adeno- carcinoma, stage 2 |
2 (2) | 70–80 F | Pem brolizumab and ‘an investigational CTLA-4 blocker.’ |
Capillary-leak syndrome | 1 | Fever, fatigue, bilateral leg swelling, weight gain. | Steroids | Recovery | Unclear | Unclear | |
| Del Bello et al77 2021 France |
Squamous cell carcinoma, unclear stage | 1 | 80–90 M | Cemiplimab | Cytokine storm. | 1 | 7 | ‘Septic shock’. | Steroids | Dialysis, kidney necrosis trans- plantectomy. complications after surgery leading to death. |
No | Unclear |
| Sackstein et al78 2021 USA |
Lung adeno carcinoma, stage 4 |
1 | 50–60 M | Pem brolizumab |
CRS | 3 | 19 | Fever, chills, hypotension, tachypnea, lethargy. | ABs, steroids, ivermectin, hemodialysis, tocilizumab | Recovery | Unclear | Unclear |
| Olivares-Hernández et al79 2021 Spain |
Choroidal melanoma, stage 4 | 1 | 70–80 F | Ipilimumab | HLH | 3 | Fever, splenomegaly | Steroids, tocilizumab. | Recovery | Unclear | PR | |
| Kurozumi et al80 2021 Japan |
Lung adeno- carcinoma, stage 4 |
2 (1) | 70–80 M | Pem brolizumab |
HLH | 1 | 10 | Fever | Steroids | Unclear | Unclear | Unclear |
| Kurozumi et al80 | Lung adeno carcinoma. Stage 3b |
2 (2) | 60–70 F | Pem brolizumab |
HLH | ‘After last dose of pembrolizumab’. | 30 | Cytopenia, elevated liver enzyme levels. | Steroids | Unclear | Unclear | Unclear |
| Masood et al81 2021 USA |
Renal cell carcinoma, stage 4 | 1 | 60–70 M | Ipilimumab, nivolumab | HLH | Generalized weakness. intermittent fevers, splenomegaly. | Steroids | Recovery | Unclear | Unclear | ||
| Pacholczak-Madej et al82 2021 Poland |
Melanoma, stage 4 | 1 | 50–60 F | Ipilimumab, nivolumab | HLH | 4 | Fever, general malaise, dyspnea, splenomegaly. | Steroids, FFP, mycophenolate mofetil, cyclophos- phamide, etoposide, ciclosporin. |
Recovery | No | PR | |
| Tiu et al83 2021 UK |
Lung carcinoma, stage 4 | 3 (1) | 50–60 F | Unclear | HLH | 1 | 11 | Fever, rigors. | Broad-spectrum ABs, steroids. | Recovery | Unclear | Unclear |
| Tiu et al83 | Breast cancer, stage 4 | 3 (2) | 40–50 F | Unclear | HLH | 1 | 11 | Fever, maculopapular rash, dyspnea, hypoxia. | ABs, steroids, tocilizumab. | Recovery | Unclear | Unclear |
| Tiu et al83 | Bladder cancer, stage 4 | 3 (3) | 60–70 M | Unclear | HLH | 1 | 10 | Fever, rigors. | ABs, steroids, tocilizumab, siltuximab, anakinra, plasma exchange, intravenous immunoglobulins. | Recovery | Unclear | Unclear |
| Tay et al17 2022 Singapore |
NSCLC, renal cell carcinoma, hepatocellular carcinoma, melanoma | 25 | Med. age 64 M: 18 F: 7 | Pem brolizumab, nivolumab, ipilimumab, anti-LAG3-antibody. |
CRS | Median of 11 days | All had fever of 38°C or higher. | Steroids, tocilizumab | three fatal | In 7 patients, no grade 3/4 events. | PR: 6, SD: 5, P D: 10 | |
| Zhang et al84 2022 China |
Lung adeno carcinoma, stage 4 |
1 | 60–70 F | Pem brolizumab |
CRS | 1 | Fever, nausea, vomiting, chest pain. | Broad-spectrum ABs, intravenous fluids, steroids. | Recovery | No | PR |
*Age given in ranges due to journal constraints.
†As assessed by the authors of the respective study.
AB, antibiotics; CR, complete response; CRS, cytokine-release syndrome; EBV, Epstein-Barr virus; F, female; HLH, hemophagocytic lymphohistiocytosis; ICI, immune checkpoint inhibitor; M, male; PR, partial response; SD, stable disease; SIRS, systemic inflammatory response syndrome.
Table 2.
Summary of the studies reporting on individual patients
| Tumor-specific outcome (per individual patient) | |
| Complete response | 8 (14%) |
| Partial response | 13 (23%) |
| Stable disease | 6 (11%) |
| Progressive disease | 4 (7%) |
| Unspecified | 25 (45%) |
| Type of hyperinflammatory syndrome | |
| CRS | 16 (28%) |
| HLH | 34 (61%) |
| other | 6 (11%) |
| Type of ICI | |
| Pembrolizumab | 21 (38%) |
| Nivolumab | 11 (20%) |
| Ipilimumab | 2 (4%) |
| Atezolizumab | 2 (4%) |
| Ipilimumab+nivolumab | 13 (23%) |
| Cemiplimab | 1 (2%) |
| Ipilimumab+pembrolizumab | 1 (2%) |
| Sintilimab | 2 (4%) |
| Unspecified | 3 (5%) |
| CRS/HLH treatment with IL-6 blockade | |
| Yes | 14 (25%) |
| No | 42 (75%) |
| Rechallenge with ICIs | |
| Yes | 6 (11%) |
| No | 31 (55%) |
| Unspecified | 19 (34%) |
| CRS/HLH outcome | |
| Recovery | 43 (77%) |
| Deceased, related to CRS/HLH | 6 (11%) |
| Deceased, other reasons | 2 (4%) |
| Unspecified | 5 (9%) |
| Time to CRS/HLH onset | |
| Days after last administration* | 9 (3,75-21) |
| Cycles before symptoms onset* | 2(1-3) |
| Type of underlying malignancy | |
| Bladder cancer | 3 (5%) |
| Lung cancer | 15 (27%) |
| Malignant melanoma | 21 (38%) |
| Renal cell carcinoma | 3 (5%) |
| Other | 14 (25%) |
*Numbers given as mean with IQR
CRS, cytokine release syndrome; HLH, hemophagocytic lymphohistiocytosis; ICI, immune-checkpoint inhibitor.
The ICIs used included pembrolizumab (n=21 studies) and nivolumab (n=11 studies) as single agents, combined treatment with ipilimumab and nivolumab (n=13), and less frequently (n=2 studies each), ipilimumab, sintilimab or atezolizumab monotherapy, and cemiplimab (n=1). Often, ICIs were combined either with chemotherapeutical agents or other anticancer drugs, such as TKIs (ie, cabozantinib or dabrafenib/trametinib), or drugs that were used prior to development of the hyperinflammatory syndrome(s).
In the individual reports, n=16 patients were diagnosed with CRS, n=34 with HLH, and n=6 were described as having related hyperinflammatory conditions such as capillary leak syndrome with high fever, or systemic inflammatory response syndrome. The most frequently reported underlying malignancies in individual case reports were malignant melanoma (n=21 individual reports) and lung cancer (n=15 individual reports). The average age of the patients in the individual reports was 59 years. In individual reports that included the patients’ sex, n=35 patients were males, and n=20 patients were females. Higher frequencies of male patients were also reported in the largest case series from two centers in Singapore17 (n=18 males, n=7 females), and in a WHO pharmacovigilance database queried for HLH18 (n=29 males, n=9 females) and CRS, respectively19 (n=34 males, n=21 females). Most studies (n=46 studies out of 49) comprised data on patients with metastatic disease (table 1). Time from ICI infusion to onset of symptoms varied from hours to 1 month, with a median of n=9 days. The number of cycles of ICIs received prior to CRS diagnosis varied between n=1 and n=17, with a majority of cases developing CRS after more than one ICI administration (n=16 patients after one cycle of ICI, n=31 patients after more one than one cycle of ICI). In total, n=42 studies on n=56 individual patients (75%) reported recovery from ICI-induced irAEs, while n=6 cases (out of 56 [11%]) were fatal. Of those, n=3 cases were reported to have HLH, and n=3 had CRS, as per the authors’ assessment. Overall, n=42 patients from individual reports were reported to have recovered from their hyperinflammatory complications. Reports from pharmacovigilance data were incomplete for outcome data; a study including n=25 patients from Singapore reported n=3 fatal cases (0.12 %).
Treatments for CRS/HLH varied significantly and included different types of corticosteroids in almost all cases, and tocilizumab in n=14 patients (25% of all individual cases). None of the patients treated with tocilizumab had a fatal outcome after the reported CRS/HLH episode but one patient relapsed with CRS six weeks after the first episode and passed away despite tocilizumab treatment, although no further information on the second episode was provided.20 In contrast, fatal outcome was reported for n=6 patients in the remaining n=42 individual patients where tocilizumab was not mentioned as treatment (corresponding to 14%). Various other drugs were used in some patients, including etoposide in combination with dexamethasone, an established HLH treatment,21 22 as well as intravenous immunoglobulins, plasmapheresis, mycophenolate mofetil, and tacrolimus. Often, these were combined, at least initially, with antibiotic therapy because of suspected sepsis (table 1).
Discussion
Wider indications have continuously led to higher numbers of patients being treated with ICIs.23 Hence, the clinical need to understand rare side effects has increased, and higher patient numbers allow more informed clinical decisions. CRS and HLH are potentially life-threatening side effects of treatment with ICIs; however, they are relatively rare and therefore are challenging to study. In this systematic review, we provide a comprehensive picture of the reported cases in the literature to date. Our results underscore the notion that hyperinflammatory syndromes are rare, often treatable, and that fatal outcome occurs in a minority of the cases (table 2). It is interesting to note the predominance of male patients in case reports and case series, as well as in pharmacovigilance databases. This might partly be driven by a higher proportion of men among patients with lung cancer,24 which represent the second largest tumor group in the identified studies, and partly by sex differences in the immune response.25 Interestingly, pharmacovigilance data suggest that the outcome of CRS is more favorable in females than in males,19 warranting further research into sex differences in response to treatment and in side effect profiles of ICIs.
A diagnostic challenge that occurs with rapid onset of fever following ICI administration is to differentiate between infusion-related reactions and CRS/HLH. In the case presented here, the symptoms developed early after the start of treatment; the persistence of the hyperinflammatory state for days, together with elevated IL-6 suggest CRS rather than a hypersensitivity reaction as the cause, although there is considerable overlap in the symptoms and the cellular mechanisms of CRS and hypersensitivity reactions.26
As illustrated by our case report, a common clinical dilemma in patients receiving ICIs is whether to continue treatment despite severe irAEs. Key factors in this decision-making process are the risk of recurrence of a given irAE, the anticipated severity in case of recurrence, as well as its treatability. A recent study has suggested that IL-6 blockade given in parallel with ICIs ameliorates irAEs, while enhancing the antitumoral effect of ICIs.27 Earlier experimental data had already hinted at the potential benefit of adding IL-6 inhibitors to ICIs in mouse models of pancreatic cancer28 (which is largely resistant to ICI therapy), as well as hepatocellular carcinoma.29 Together, these data suggest that IL-6 blockade does not abrogate, but rather enhances, the activation of a beneficial antitumoral immune response, providing a potential oncological rationale for combining IL-6 inhibition and ICIs. Results from CAR-T cell therapy in hematological malignancies support the lack of antagonistic effects of IL-6 antagonists in combination with ICIs; for example, in refractory large B-cell lymphoma, response rates to CAR T cell therapy were independent of the use of concomitant tocilizumab to treat CRS.30
Our review of the literature revealed no fatal case among 14 patients who received the IL-6 inhibitor, tocilizumab, for CRS/HLH treatment, while the fatality rate was 14% in the patients for which anti-IL-6 treatment was not reported. One patient who had received tocilizumab passed away after a second CRS episode, but further details on the suspected CRS trigger and clinical course were not provided.20 Since the groups of patients are gathered from case reports and hence are not comparable, we cannot conclude that IL-6 inhibition is beneficial. Nevertheless, given the evidence that combined anti-IL-6/ICI treatment enhances the antitumoral effect, an important question is whether a rechallenge after mild CRS/HLH that responded well to anti-IL-6 treatment or corticosteroids should be considered. As summarized in table 2, only a few case reports exist on ICI rechallenge after CRS or HLH, none of which reports fatal outcomes after rechallenge.31–34 In the largest case series published containing n=25 cases, n=7 were cases after rechallenge with ICIs; none of these had grade 3 or 4 CRS,17 which lends some support for continuous ICI treatment despite CRS and could be appropriate in selected cases, as the risk of aggravated side effects when re-exposing patients to ICIs could be low. Furthermore, a meta-analysis of patients with non-small cell lung cancer rechallenged with ICIs suggests that certain patients with disease progression during ICI discontinuation might benefit from ICI rechallenge.35 Whether these data are applicable to ICI-related CRS remains to be seen.
In the case of the patient presented in the current report, the second occasion of CRS was milder than the first, although a switch from combined ipilimumab/nivolumab to nivolumab alone might have contributed to the milder clinical course on the second occasion. In addition, the patient was treated with oral betamethasone on rechallenge with nivolumab, which could have prevented severe CRS, although some authors have contested the preventive effect of steroids, at least when using bispecific antibodies.36 As a higher tumor burden has been shown to correlate with increased inflammation parameters and cytokine levels,37 it can be hypothesized that pretreatment tumor burden is associated with the risk of developing irAEs. Indeed, studies of CAR T cell treatment for B cell acute lymphoblastic leukemia have shown a significant correlation between severe CRS and a high tumor burden, which might lead to amelioration of CRS in case of treatment effects of previous courses38 39.
While we mostly found case reports, two studies reported pharmacovigilance data from the global WHO database, and two reports queried the Registry of Severe Adverse Reactions to Immunomodulatory Antibodies used in Oncology (REISAMIC), a database for ICI-related irAEs in France.33 40 The first report on REISAMIC data included n=16 patients with ‘fever reaction’ to ICI treatment, and based on their analysis of these cases, the authors concluded that this irAE can ‘usually be controlled with a short course of corticosteroids’. The second report also queried two other French databases specifically for HLH cases, and identified n=5 patients with HLH, one of which was fatal. In n=3 of the five cases, rechallenge of ICI was reported, in n=2 cases without recurrence of fever of HLH.33 One of the HLH cases was identified in REISAMIC among n=745 patients included ‘at a single center between 2014 and 2019’, suggesting that HLH indeed is a rare event. The rarity of HLH is also supported by the analysis of WHO pharmacovigilance data: 49,883 ICI-related adverse events were retrieved from the WHO database VigiBase on a search conducted in September 2018, n=38 of whom corresponded to HLH, and n=34 were directly linked to ICI treatment, usually developing more than 6 weeks after ICI treatment. Interestingly, the rate of other irAEs was below 20%.18 The same group of authors queried VigiBase for CRS as of January 2020. They found n=58 reports likely corresponding to CRS among a total of 80 700 reports on ICI-related adverse events, of which n=43 were definitely related to ICIs, and which occurred a median of approximately 4 weeks after initiation of ICI treatment.19 Two of those cases were fatal. Finally, a recent study presented a case series collected at two hospitals in Singapore between February 2014 and January 2021. They found that n=25 out of a total of n=539 patients that had received ICI developed CRS, which is a considerably higher frequency than suggested by the pharmacovigilance data, and the reason for this difference is unclear. In this cohort, n=7 patients with low-grade CRS were rechallenged with ICIs and did not relapse. A total of n=3 cases had fatal CRS despite tocilizumab treatment.17 The authors also suggested that time-to-fever-onset, low platelet count, and high urea levels at CRS presentation might serve as indicators of a severe course.17
Several studies have reported an association between irAEs and improved treatment outcome,41 42 although the type of irAE might predict treatment outcome, since specific irAEs, such as pneumonitits, might not predict ICI efficacy (reviewed in ref.41). We find that n=27 individual patients with hyperinflammatory syndromes had clinical benefit (CR in n=8 patients, PR in n=13 patients, SD in n=6 patients) from ICI treatment, while only n=4 patients had PD. Although follow-up time frames and assessment methods will vary substantially between studies and were inconsistently reported, it is tempting to speculate that CRS, like other irAEs, might be associated with improved treatment outcomes. A potential pitfall in this interpretation is that many of the studies assessed did not provide information about patient treatment outcome, potentially providing a bias toward the patients with beneficial treatment outcome.
It is still unclear whether ICI rechallenge after irAEs might be oncologically favorable. In accordance with current guidelines, most patients with grade 3 or 4 irAEs will discontinue treatment with ICI permanently. The rarity of cases, resulting in cohorts of limited size, poses a challenge when addressing this question. While recent studies have shown that rechallenge with ICI after irAE does not significantly improve overall survival,43 others have concluded that irAEs on rechallenge display milder toxicities, and suggested that rechallenge might be safe for most patients, depending on the type of irAE.44 45
In summary, the published data suggest that CRS and HLH are infrequent, potentially severe, but frequently treatable side effects of ICIs, and that rechallenge could be considered in selected cases, with IL-6 inhibition as an attractive preventive and therapeutic option.
Acknowledgments
The authors are grateful to Narcisa Hannerz and Sabina Gillsund of the Karolinska Institute’s Library, for expert help in conducting the systematic literature search. We are grateful to Marie Meeths for advice on the manuscript.
Footnotes
Twitter: @marcus_skribek, @cancer_invasion
Contributors: LLL and MG conceived the study and performed the literature search together with the librarians of Karolinska Institute’s library. LLL, MS, and MG decided on study inclusion and collected the data for the systematic review. LLL, MG, and UH collected and interpreted the data from the clinical case. MG wrote the manuscript, LLL, MS, and UH edited the manuscript.
Funding: This study received no dedicated funding. MG is supported by The Swedish Research Council (grant number 2018-02023), LLL is supported by The Swedish Society of Medicine and Swedish Society for Medical Research (grant number P17-0134).
Competing interests: None declared.
Provenance and peer review: Commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Haanen J, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv119–42. 10.1093/annonc/mdx225 [DOI] [PubMed] [Google Scholar]
- 2.Schneider BJ, Naidoo J, Santomasso BD, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clin Oncol 2021;39:4073–126. 10.1200/JCO.21.01440 [DOI] [PubMed] [Google Scholar]
- 3.La Rosée P, Horne A, Hines M, et al. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood 2019;133:2465–77. 10.1182/blood.2018894618 [DOI] [PubMed] [Google Scholar]
- 4.Onakpoya IJ. Rare adverse events in clinical trials: understanding the rule of three. BMJ Evid Based Med 2018;23:6. 10.1136/ebmed-2017-110885 [DOI] [PubMed] [Google Scholar]
- 5.Zhang S, Zhou Z, Wang L, et al. Rheumatic immune-related adverse events associated with immune checkpoint inhibitors compared with placebo in oncologic patients: a systemic review and meta-analysis. Ther Adv Chronic Dis 2021;12:2040622320976996. 10.1177/2040622320976996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brahmer JR, Abu-Sbeih H, Ascierto PA, et al. Society for immunotherapy of cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer 2021;9:e002435. 10.1136/jitc-2021-002435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frey N, Porter D. Cytokine release syndrome with chimeric antigen receptor T cell therapy. Biol Blood Marrow Transplant 2019;25:e123–7. 10.1016/j.bbmt.2018.12.756 [DOI] [PubMed] [Google Scholar]
- 8.Morris EC, Neelapu SS, Giavridis T, et al. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nat Rev Immunol 2022;22:85–96. 10.1038/s41577-021-00547-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le RQ, Li L, Yuan W, et al. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncologist 2018;23:943–7. 10.1634/theoncologist.2018-0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Si S, Teachey DT. Spotlight on tocilizumab in the treatment of CAR-T-cell-induced cytokine release syndrome: clinical evidence to date. Ther Clin Risk Manag 2020;16:705–14. 10.2147/TCRM.S223468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fardet L, Galicier L, Lambotte O, et al. Development and validation of the hscore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol 2014;66:2613–20. 10.1002/art.38690 [DOI] [PubMed] [Google Scholar]
- 12.Meeths M, Bryceson YT. Genetics and pathophysiology of haemophagocytic lymphohistiocytosis. Acta Paediatr 2021;110:2903–11. 10.1111/apa.16013 [DOI] [PubMed] [Google Scholar]
- 13.Sharma N, Stroud CRG, Walker PR, et al. Systemic inflammatory response syndrome (SIRS) with immune checkpoint inhibitors. JCO 2016;34:3061. 10.1200/JCO.2016.34.15_suppl.3061 [DOI] [Google Scholar]
- 14.Bramer WM, Giustini D, de Jonge GB, et al. De-duplication of database search results for systematic reviews in endnote. J Med Libr Assoc 2016;104:240–3. 10.3163/1536-5050.104.3.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heng DYC, Xie W, Regan MM, et al. External validation and comparison with other models of the international metastatic renal-cell carcinoma database consortium prognostic model: a population-based study. Lancet Oncol 2013;14:141–8. 10.1016/S1470-2045(12)70559-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant 2019;25:625–38. 10.1016/j.bbmt.2018.12.758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tay SH, Toh MMX, Thian YL, et al. Cytokine release syndrome in cancer patients receiving immune checkpoint inhibitors: a case series of 25 patients and review of the literature. Front Immunol 2022;13:807050. 10.3389/fimmu.2022.807050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noseda R, Bertoli R, Müller L, et al. Haemophagocytic lymphohistiocytosis in patients treated with immune checkpoint inhibitors: analysis of who global database of individual case safety reports. J Immunother Cancer 2019;7:117. 10.1186/s40425-019-0598-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ceschi A, Noseda R, Palin K, et al. Immune checkpoint inhibitor-related cytokine release syndrome: analysis of WHO global pharmacovigilance database. Front Pharmacol 2020;11:557. 10.3389/fphar.2020.00557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slota A, Khan R, Rahman A, et al. Cytokine release syndrome as a rare complication of nivolumab: a case report. Blood 2019;134:5630. 10.1182/blood-2019-127586 [DOI] [Google Scholar]
- 21.Henter J-I, Horne A, Aricó M, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer 2007;48:124–31. 10.1002/pbc.21039 [DOI] [PubMed] [Google Scholar]
- 22.Henter J-I, Samuelsson-Horne A, Aricò M, et al. Treatment of hemophagocytic lymphohistiocytosis with HLH-94 immunochemotherapy and bone marrow transplantation. Blood 2002;100:2367–73. 10.1182/blood-2002-01-0172 [DOI] [PubMed] [Google Scholar]
- 23.Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol 2021;16:223–49. 10.1146/annurev-pathol-042020-042741 [DOI] [PubMed] [Google Scholar]
- 24.Chen X, Mo S, Yi B. The spatiotemporal dynamics of lung cancer: 30-year trends of epidemiology across 204 countries and territories. BMC Public Health 2022;22:987. 10.1186/s12889-022-13281-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nature Reviews Immunology . Sex differences in immune responses. Available: https://www.nature.com/articles/nri.2016.90 [Accessed 28 Jun 2022].
- 26.Isabwe GAC, Garcia Neuer M, de Las Vecillas Sanchez L, et al. Hypersensitivity reactions to therapeutic monoclonal antibodies: phenotypes and endotypes. J Allergy Clin Immunol 2018;142:159–70. 10.1016/j.jaci.2018.02.018 [DOI] [PubMed] [Google Scholar]
- 27.Hailemichael Y, Johnson DH, Abdel-Wahab N, et al. Interleukin-6 blockade abrogates immunotherapy toxicity and promotes tumor immunity. Cancer Cell 2022;40:509–23. 10.1016/j.ccell.2022.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mace TA, Shakya R, Pitarresi JR, et al. Il-6 and PD-L1 antibody blockade combination therapy reduces tumour progression in murine models of pancreatic cancer. Gut 2018;67:320–32. 10.1136/gutjnl-2016-311585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu H, Shen J, Lu K. Il-6 and PD-L1 blockade combination inhibits hepatocellular carcinoma cancer development in mouse model. Biochem Biophys Res Commun 2017;486:239–44. 10.1016/j.bbrc.2017.02.128 [DOI] [PubMed] [Google Scholar]
- 30.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med 2017;377:2531–44. 10.1056/NEJMoa1707447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rassy EE, Assi T, Rizkallah J, et al. Diffuse edema suggestive of cytokine release syndrome in a metastatic lung carcinoma patient treated with pembrolizumab. Immunotherapy 2017;9:309–11. 10.2217/imt-2016-0134 [DOI] [PubMed] [Google Scholar]
- 32.Kogure Y, Ishii Y, Oki M. Cytokine release syndrome with pseudoprogression in a patient with advanced non-small cell lung cancer treated with pembrolizumab. J Thorac Oncol 2019;14:e55–7. 10.1016/j.jtho.2018.11.025 [DOI] [PubMed] [Google Scholar]
- 33.Dupré A, Michot J-M, Schoeffler A, et al. Haemophagocytic lymphohistiocytosis associated with immune checkpoint inhibitors: a descriptive case study and literature review. Br J Haematol 2020;189:985–92. 10.1111/bjh.16630 [DOI] [PubMed] [Google Scholar]
- 34.Kalmuk J, Puchalla J, Feng G, et al. Pembrolizumab-induced hemophagocytic lymphohistiocytosis: an immunotherapeutic challenge. Cancers Head Neck 2020;5:3. 10.1186/s41199-020-0050-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu S, Shukuya T, Tamura J, et al. Heterogeneous outcomes of immune checkpoint inhibitor rechallenge in patients with NSCLC: a systematic review and meta-analysis. JTO Clin Res Rep 2022;3:100309. 10.1016/j.jtocrr.2022.100309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kauer J, Hörner S, Osburg L, et al. Tocilizumab, but not dexamethasone, prevents CRS without affecting antitumor activity of bispecific antibodies. J Immunother Cancer 2020;8:e000621. 10.1136/jitc-2020-000621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 2014;6:224ra25. 10.1126/scitranslmed.3008226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hay KA. Cytokine release syndrome and neurotoxicity after CD19 chimeric antigen receptor-modified (CAR-) T cell therapy. Br J Haematol 2018;183:364–74. 10.1111/bjh.15644 [DOI] [PubMed] [Google Scholar]
- 39.Li M, Xue S-L, Tang X, et al. The differential effects of tumor burdens on predicting the net benefits of sscart-19 cell treatment on r/r B-ALL patients. Sci Rep 2022;12:378. 10.1038/s41598-021-04296-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Michot J-M, Pruvost R, Mateus C, et al. Fever reaction and haemophagocytic syndrome induced by immune checkpoint inhibitors. Ann Oncol 2018;29:518–20. 10.1093/annonc/mdx701 [DOI] [PubMed] [Google Scholar]
- 41.Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer 2019;7:306. 10.1186/s40425-019-0805-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hussaini S, Chehade R, Boldt RG, et al. Association between immune-related side effects and efficacy and benefit of immune checkpoint inhibitors-a systematic review and meta-analysis. Cancer Treat Rev 2021;92:102134. 10.1016/j.ctrv.2020.102134 [DOI] [PubMed] [Google Scholar]
- 43.Albandar HJ, Fuqua J, Albandar JM, et al. Immune-related adverse events (irAE) in cancer immune checkpoint inhibitors (ICI) and survival outcomes correlation: to rechallenge or not? Cancers (Basel) 2021;13:989. 10.3390/cancers13050989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Allouchery M, Lombard T, Martin M, et al. Safety of immune checkpoint inhibitor rechallenge after discontinuation for grade ≥2 immune-related adverse events in patients with cancer. J Immunother Cancer 2020;8:e001622. 10.1136/jitc-2020-001622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dolladille C, Ederhy S, Sassier M, et al. Immune checkpoint inhibitor rechallenge after immune-related adverse events in patients with cancer. JAMA Oncol 2020;6:865–71. 10.1001/jamaoncol.2020.0726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shah D, Shrestha R, Ramlal R, et al. Pembrolizumab associated hemophagocytic lymphohistiocytosis. Ann Oncol 2017;28:1403. 10.1093/annonc/mdx113 [DOI] [PubMed] [Google Scholar]
- 47.Rotz SJ, Leino D, Szabo S, et al. Severe cytokine release syndrome in a patient receiving PD-1-directed therapy. Pediatr Blood Cancer 2017;64:12. 10.1002/pbc.26642 [DOI] [PubMed] [Google Scholar]
- 48.Urosevic-Maiwald M, Mangana J, Dummer R. Systemic inflammatory reaction syndrome during combined kinase inhibitor therapy following anti-PD-1 therapy for melanoma. Ann Oncol 2017;28:1673–5. 10.1093/annonc/mdx187 [DOI] [PubMed] [Google Scholar]
- 49.Takeshita M, Anai S, Mishima S, et al. Coincidence of immunotherapy-associated hemophagocytic syndrome and rapid tumor regression. Ann Oncol 2017;28:186–9. 10.1093/annonc/mdw537 [DOI] [PubMed] [Google Scholar]
- 50.Sadaat M, Jang S. Hemophagocytic lymphohistiocytosis with immunotherapy: brief review and case report. J Immunother Cancer 2018;6:49. 10.1186/s40425-018-0365-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hantel A, Gabster B, Cheng JX, et al. Severe hemophagocytic lymphohistiocytosis in a melanoma patient treated with ipilimumab + nivolumab. J Immunother Cancer 2018;6:73. 10.1186/s40425-018-0384-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Satzger I, Ivanyi P, Länger F, et al. Treatment-related hemophagocytic lymphohistiocytosissecondary to checkpoint inhibition with nivolumab plus ipilimumab. Eur J Cancer 2018;93:150–3. 10.1016/j.ejca.2018.01.063 [DOI] [PubMed] [Google Scholar]
- 53.Shah K, Melissa K. 168 A case of epstein-barr virus-related hemophagocytic lymphohistiocytosis in association with pembrolizumab therapy - proquest. AJCP 2018;149:S71–2. 10.1093/ajcp/aqx121.167 [DOI] [Google Scholar]
- 54.Sasaki K, Uehara J, Iinuma S, et al. Hemophagocytic lymphohistiocytosis associated with dabrafenib and trametinib combination therapy following pembrolizumab administration for advanced melanoma. Ann Oncol 2018;29:1602–3. 10.1093/annonc/mdy175 [DOI] [PubMed] [Google Scholar]
- 55.Laderian B, Koehn K, Holman C, et al. Association of hemophagocytic lymphohistiocytosis and programmed death 1 checkpoint inhibitors. J Thorac Oncol 2019;14:e77–8. 10.1016/j.jtho.2018.11.035 [DOI] [PubMed] [Google Scholar]
- 56.Oda H, Ishihara M, Miyahara Y, et al. First case of cytokine release syndrome after nivolumab for gastric cancer. Case Rep Oncol 2019;12:147–56. 10.1159/000496933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lorenz G, Schul L, Bachmann Q, et al. Hemophagocytic lymphohistiocytosis secondary to pembrolizumab treatment with insufficient response to high-dose steroids. Rheumatology (Oxford) 2019;58:1106–9. 10.1093/rheumatology/key447 [DOI] [PubMed] [Google Scholar]
- 58.Okawa S, Kayatani H, Fujiwara K, et al. Pembrolizumab-induced autoimmune hemolytic anemia and hemophagocytic lymphohistiocytosis in non-small cell lung cancer. Intern Med 2019;58:699–702. 10.2169/internalmedicine.1001-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chin CK, Hall S, Green C, et al. Secondary haemophagocytic lymphohistiocytosis due to checkpoint inhibitor therapy. Eur J Cancer 2019;115:84–7. 10.1016/j.ejca.2019.04.026 [DOI] [PubMed] [Google Scholar]
- 60.Honjo O, Kubo T, Sugaya F, et al. Severe cytokine release syndrome resulting in purpura fulminans despite successful response to nivolumab therapy in a patient with pleomorphic carcinoma of the lung: a case report. J Immunother Cancer 2019;7:97. 10.1186/s40425-019-0582-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Adashek ML, Feldman M. Cytokine release syndrome resulting from anti-programmed death-1 antibody: raising awareness among community oncologists. J Oncol Pract 2019;15:502–4. 10.1200/JOP.19.00160 [DOI] [PubMed] [Google Scholar]
- 62.Takahashi H, Koiwa T, Fujita A, et al. A case of pembrolizumab-induced hemophagocytic lymphohistiocytosis successfully treated with pulse glucocorticoid therapy. Respir Med Case Rep 2020;30:101097. 10.1016/j.rmcr.2020.101097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ohira J, Kawamoto M, Sugino Y, et al. A case report of fulminant cytokine release syndrome complicated by dermatomyositis after the combination therapy with immune checkpoint inhibitors. Medicine (Baltimore) 2020;99:e19741. 10.1097/MD.0000000000019741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Normand CV, Zender HO, Staehli DM, et al. Acute cytokine release syndrome after a first dose of pembrolizumab as second-line treatment for metastatic, programmed death-ligand 1-positive, non-small-cell lung cancer. J Oncol Pharm Pract 2021;27:1528–33. 10.1177/1078155220980813 [DOI] [PubMed] [Google Scholar]
- 65.Gao C, Xu J, Han C, et al. An esophageal cancer case of cytokine release syndrome with multiple-organ injury induced by an anti-PD-1 drug: a case report. Ann Palliat Med 2020;9. 10.21037/apm-20-1310 [DOI] [PubMed] [Google Scholar]
- 66.Özdemir BC, Latifyan S, Perreau M, et al. Cytokine-directed therapy with tocilizumab for immune checkpoint inhibitor-related hemophagocytic lymphohistiocytosis. Ann Oncol 2020;31:1775–8. 10.1016/j.annonc.2020.08.2101 [DOI] [PubMed] [Google Scholar]
- 67.Azari AE, Stratton R, Singh A. First case of hemophagocytic lymphohistiocytosis secondary to cabozantinib with checkpoint inhibitors. Rheumatology (Oxford) 2021;60:e167–8. 10.1093/rheumatology/keaa750 [DOI] [PubMed] [Google Scholar]
- 68.Akagi Y, Awano N, Inomata M, et al. Hemophagocytic lymphohistiocytosis in a patient with rheumatoid arthritis on pembrolizumab for lung adenocarcinoma. Intern Med 2020;59:1075–80. 10.2169/internalmedicine.3889-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thummalapalli R, Heumann T, Stein J, et al. Hemophagocytic lymphohistiocytosis secondary to PD-1 and IDO inhibition in a patient with refractory glioblastoma. Case Rep Oncol 2020;13:508–14. 10.1159/000507281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mizuta H, Nakano E, Takahashi A, et al. Hemophagocytic lymphohistiocytosis with advanced malignant melanoma accompanied by ipilimumab and nivolumab: a case report and literature review. Dermatol Ther 2020;33:e13321. 10.1111/dth.13321 [DOI] [PubMed] [Google Scholar]
- 71.Hu J, Li Y, Chen X, et al. Pulmonary fibrosis and cytokine release syndrome after hyperactivation with sintilimab. J Clin Pharm Ther 2020;45:1474–7. 10.1111/jcpt.13217 [DOI] [PubMed] [Google Scholar]
- 72.Nieves-Borrero K, Adler S, Pow-anpongkul P, et al. 265 ATEZOLIZUMAB associated with tumor lysis syndrome, cytokine release syndrome, and autoimmune toxicity. Am J Kidney Dis 2020;75:613. 10.1053/j.ajkd.2020.02.267 [DOI] [Google Scholar]
- 73.Dudda M, Mann C, Heinz J, et al. Hemophagocytic lymphohistiocytosis of a melanoma patient under BRAF/MEK-inhibitor therapy following anti-PD1 inhibitor treatment: a case report and review to the literature. Melanoma Res 2021;31:81–4. 10.1097/CMR.0000000000000703 [DOI] [PubMed] [Google Scholar]
- 74.Amlani A, Barber C, Fifi-Mah A, et al. Successful treatment of cytokine release syndrome with IL-6 blockade in a patient transitioning from immune-checkpoint to MEK/BRAF inhibition: a case report and review of literature. Oncologist 2020;25:e1120–3. 10.1634/theoncologist.2020-0194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yomota M, Mirokuji K, Sakaguchi M, et al. Cytokine release syndrome induced by immune-checkpoint inhibitor therapy for non-small-cell lung cancer. Intern Med 2021;60:3459–62. 10.2169/internalmedicine.5922-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Percik R, Nethanel A, Liel Y. Capillary-leak syndrome: an unrecognized early immune adverse effect of checkpoint-inhibitors treatment. Immunotherapy 2021;13:653–9. 10.2217/imt-2020-0332 [DOI] [PubMed] [Google Scholar]
- 77.Del Bello A, Zakaroff AG, Meyer N, et al. Cytokine storm induced by a PD1 inhibitor in a renal transplant patient. Am J Transplant 2021;21:2616–8. 10.1111/ajt.16589 [DOI] [PubMed] [Google Scholar]
- 78.Sackstein P, Zaemes J, Kim C. Pembrolizumab-induced cytokine release syndrome in a patient with metastatic lung adenocarcinoma: a case report. J Immunother Cancer 2021;9:e002855. 10.1136/jitc-2021-002855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Olivares-Hernández A, Figuero-Pérez L, Amores Martín MA, et al. Response to treatment with an anti-interleukin-6 receptor antibody (tocilizumab) in a patient with hemophagocytic syndrome secondary to immune checkpoint inhibitors. Case Rep Oncol Med 2021;2021:6631859. 10.1155/2021/6631859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kurozumi A, Takahashi H, Watanabe T, et al. Two cases of lung cancer with hemophagocytic lymphohistiocytosis caused by immune checkpoint inhibitors. Thorac Cancer 2021;12:1625–8. 10.1111/1759-7714.13954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Masood A, Wahab A, Clifford T, et al. Secondary hemophagocytic lymphohistiocytosis due to nivolumab/ipilimumab in a renal cell cancer patient—a case report. Clinical Case Reports 2021;9:e05184. 10.1002/ccr3.5184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pacholczak-Madej R, Grela-Wojewoda A, Lompart J, et al. Effective treatment of a melanoma patient with hemophagocytic lymphohistiocytosis after nivolumab and ipilimumab combined immunotherapy. Prague Med Rep 2022;123:35–42. 10.14712/23362936.2022.4 [DOI] [PubMed] [Google Scholar]
- 83.Tiu C, Shinde R, Pal A, et al. A wolf in sheep’s clothing: systemic immune activation post immunotherapy. J Immunother Precis Oncol 2021;4:189–95. 10.36401/JIPO-21-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang M, Cheng Y, Hu Y, et al. Cytokine release syndrome and successful response to pembrolizumab therapy in a patient with EGFR-mutated non-small-cell lung cancer: a case report. Thorac Cancer 2022;13:1419–22. 10.1111/1759-7714.14390 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2022-005841supp001.pdf (185.7KB, pdf)
jitc-2022-005841supp002.pdf (304.5KB, pdf)