Abstract
Objectives
To investigate the effects of 4 months of customised, home-based exergaming on physical function and pain after total knee replacement (TKR) compared with standard exercise protocol.
Methods
In this non-blinded randomised controlled trial, 52 individuals aged 60–75 years undergoing TKR were randomised into an exergaming (intervention group, IG) or a standard exercising group (control group, CG). Primary outcomes were physical function and pain measured before and after (2 months and 4 months) surgery using the Oxford Knee Score (OKS) and Timed Up and Go (TUG) test. Secondary outcomes included measures of the Visual Analogue Scale, 10m walking, short physical performance battery, isometric knee extension and flexion force, knee range of movement and satisfaction with the operated knee.
Results
Improvement in mobility measured by TUG was greater in the IG (n=21) at 2 (p=0.019) and 4 months (p=0.040) than in the CG (n=25). The TUG improved in the IG by −1.9 s (95% CI, −2.9 to −1.0), while it changed by −0.6 s (95% CI −1.4 to 0.3) in the CG. There were no differences between the groups in the OKS or secondary outcomes over 4 months. 100% of patients in the IG and 74% in the CG were satisfied with the operated knee.
Conclusion
In patients who have undergone TKR, training at home with customised exergames was more effective in mobility and early satisfaction and as effective as standard exercise in pain and other physical functions. In both groups, knee-related function and pain improvement can be considered clinically meaningful.
Trial registration number
Keywords: rehabilitation, exercise, osteoarthritis, knee surgery
WHAT IS ALREADY KNOWN ON THIS TOPIC
Gamified exercising, that is, exergaming, has proven to improve physical outcomes in older adults. However, little is known about its effects on physical function and pain in rehabilitation in aged surgical patients.
WHAT THIS STUDY ADDS
Home-based exergaming after total knee replacement (TKR) surgery was more effective on mobility than standard post-TKR exercise.
Patients who underwent gamified rehabilitation were more satisfied with the operated knee than those who did standard exercising.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Although exergaming was not superior to the standard protocol, it could be used in physical function and pain management in rehabilitation after TKR.
Introduction
Total knee replacement (TKR) is a surgical treatment for severe knee osteoarthritis (OA). To maximise the individual benefits of TKR surgery, it is important to offer rehabilitation protocols that have the potential to improve compliance in home-based rehabilitation and thus have a beneficial effect on the postoperative outcomes.1–3 One such novel protocol is rehabilitation using exergames.4
In physical rehabilitative exergames, therapeutic exercise is exploited using computer games controlled through the player’s bodily movements or reactions. Exergames may be tailored to the therapeutic exercises of a specific group of patients,5 taking into account the progression of the rehabilitation process.6 Moreover, exergames may be implemented at a person’s home with self-directed rehabilitation,7 for which there is a growing need in situations where the increased demand for rehabilitation is unmet due to, for example, long distances or restrictions imposed by the pandemic.
The effects of exergaming have been studied in older adults and have shown promising results in improving physical outcomes.4 8 9 However, few studies have evaluated the effectiveness of exergames in aged surgical patients, such as those with TKR.10–12 More research is needed to strengthen the evidence on the effect of exergames used as therapeutic exercises after TKR surgery, especially when performed self-directedly with customised exercises at home.13 14 Therefore, this randomised controlled trial (RCT) aimed to investigate whether home-based exercise with custom exergames for post-TKR rehabilitation is effective for physical function and pain reduction in older adults after TKR surgery compared with home exercise using a standard protocol.
Methods
Trial design
This study was a 4-month non-blinded, dual-centre RCT with parallel groups (allocation ratio 1:1) comparing unsupervised exergame-based home exercise (intervention group, IG) with unsupervised home exercise by standard protocol (control group, CG) after TKR surgery in older adults. The study was conducted using the same protocol in Finland’s Southwest and Central Finland Healthcare Districts.
Knee-related pain and physical function, including knee-related function, mobility, walking and lower extremity performance and strength, were assessed using several measurements. Measurements were performed before (baseline) and after (2-month and 4-month follow-up) TKR surgery in the exercise laboratory, according to the patients’ residential area. Baseline assessment was performed within 2 weeks before the day of surgery, and 2-month and 4-month follow-up assessments were performed within ±5 days from the time point calculated according to the day of surgery. Trained physical therapists completed assessments of individual participants.
During the year 2020, the COVID-19 pandemic caused unavoidable situations in the study; the number of elective surgeries decreased, hospitals and laboratories had lockdowns, and some felt that coming to follow-up assessments at the exercise laboratory could pose a high risk of developing the disease. Recruitment slowed and was suspended, thus causing a reduction in the number of potential patients for recruitment. Tests could not be performed on participants who did not attend the assessments in the exercise laboratory.15 The outcomes collected by pen and paper were gathered by mail from these participants.
The study was prospectively registered at ClinicalTrials.gov (NCT03717727), and the study protocol has been described in detail elsewhere.16 Guidelines were followed in reporting.15 17–19
Participants
At the preoperative polyclinic visit, eligibility screening was performed for individuals aged 60–75 with knee OA (n=78) who were scheduled to undergo TKR surgery and were interested in participating in the study. The inclusion criteria were (1) first primary unilateral TKR, (2) mechanical axis of the limb in varus, (3) posterior stabilising or cruciate-retaining prosthesis and (4) normal vision with or without eyeglasses. Individuals were excluded if they had fractures, rheumatoid arthritis or other biomechanical disruptions in the affected lower limb within 1 year before surgery, a diagnosed memory disorder, cognitive impairment or a neurological condition. Before the TKR surgery, the researcher contacted patients by phone, ensured eligibility, provided a detailed description of the study and scheduled the time for the baseline assessment (n=52) in the exercise laboratory. Eligible individuals provided written informed consent before enrolment.
Randomisation and blinding
Patients were randomly allocated to either the IG or CG. Randomisation was performed using blocks of two and four in random order and stratified by the place of recruitment, gender and 10 s time limit in the timed Up and Go (TUG) test (fast/slow).20 21 Two persons unrelated to the study implemented the random allocation sequence and concealment: one generated a randomisation procedure, and the other concealed group allocation cards to consecutively marked opaque envelopes. Allocation to the groups occurred at the end of the baseline assessment. The research physical therapist assigned the participants to groups by selecting and opening a valid envelope. Participants allocated to the IG received gaming equipment, installation and exergaming instructions. The blinding of participants and outcome assessors was impossible because of the nature of the interventions and the collected exergame-related questionnaires.
Outcomes
Primary outcomes
Knee-related function and pain were assessed using the Oxford Knee Score (OKS) 12-item questionnaire.22 23 Each item is scored from 0 to 4, from the highest to the lowest severity of function and pain. The total score ranged from 0 to 48, with 48 indicating the best function and the least (or no) pain.
Mobility was measured using the TUG test.24 Time in seconds was measured while the participant raised from a chair, walked 3 m, turned, walked back to the chair and sat down. A shorter test time indicated better mobility.
Secondary outcomes
Knee pain was assessed using the pen-and-paper Visual Analogue Scale (VAS).25 Participants rated their average pain intensity over a week from 0 to 100, ranging from no knee pain to the worst possible knee pain.
Walking was measured using the 10 m walking test.26 The time in seconds was measured while the participant walked 10 m fast. The results were expressed as walking speed (m/s). A higher m/s value in the test indicated better walking performance.
Lower extremity performance was measured using the short physical performance battery (SPPB) test.27 The SPPB test includes three subtests measuring balance, mobility and lower extremity strength, each scored from 0 to 4, from poor to best performance. The total score ranged from 0 to 12, with 12 indicating the best lower extremity performance.
Muscle strength of the operated lower limb was measured using isometric knee extension and flexion force tests.28 A higher force value in Newton metres indicated better lower-extremity muscle strength.
The knee range of movement (ROM) of the operated lower limb was measured using a goniometer.29 A smaller degree of active knee extension and a higher degree of active knee flexion (ie, wider ROM) indicated a better joint range of motion.
Early satisfaction with the operated knee was assessed with the question: ‘How satisfied are you with your operated knee?’ Responses were scored from 1 to 4, from ‘very satisfied’ to ‘very dissatisfied’.
Interventions
All participants received their usual treatment after TKR. In addition, regardless of the assigned group, all participants received standard protocol home exercise instructions from a physical therapist during the hospital stay. Interventions in the IG and CG were initiated after discharge and lasted for 16 weeks. In structured diaries, the participants reported the duration of daily exergaming, standard protocol exercising, and other physical activity (PA). Self-reported PA minutes were calculated as metabolic equivalents of task hours per week according to the marked activity and self-evaluated intensity.30 In addition, gaming computers recorded the daily duration of the games. Participants’ adverse events spontaneously mentioned were recorded, and their possible causal connections to the interventions were assessed.
Home exercise by standard protocol
The CG protocol included 11–12 exercises. Progression of postoperative exercise over time was ensured by increasing the exercise time (from 2 to 5 times a day), the number of repetitions (from 3 to 15), and the number of sets (from 1 to 3).
Home-based exergame intervention
The IG protocol included 11 games. Progression of postoperative exergaming over time was ensured by changing the weekly number of games (from 4 to 5 exergames), duration (from 90 s to 360 s), number of repetitions (from 5 to 12), number of sets (from 1 to 3) and intensity (from slow to fast). Participants were instructed to complete the exergame programme assigned to the intervention week several times a day.
Interventions are presented in more detail in the protocol.16
Sample size
The calculation of sample size was based on the primary outcome OKS and was determined to be 100 participants to detect 5 point difference between groups at an alpha of 0.05, power of 80%, and anticipate a 10% drop-out rate during follow-up.31 32
Statistical methods
All available data in the full analysis set were analysed using Stata software (V.17.0; StataCorp). Participants assigned to the IG or CG received the allocated intervention. They had any assessments at the baseline, and 2-month or 4-month follow-ups were included in the intention-to-treat (ITT) analysis. Missing data resulting from drop-outs, technical or human errors in the data collection and interruptions to routine data collection were not imputed. Repeated measurements were obtained at different time points, including baseline and 2 and 4 months. Repeated measures of the changes in primary and secondary outcomes were compared between the IG and CG using mixed-effects models and an unstructured covariance structure (ie, the Kenward-Roger method for calculating df). The fixed effects included group, time, and group×time interactions. Mixed models allow the analysis of unbalanced datasets without imputation.
Results
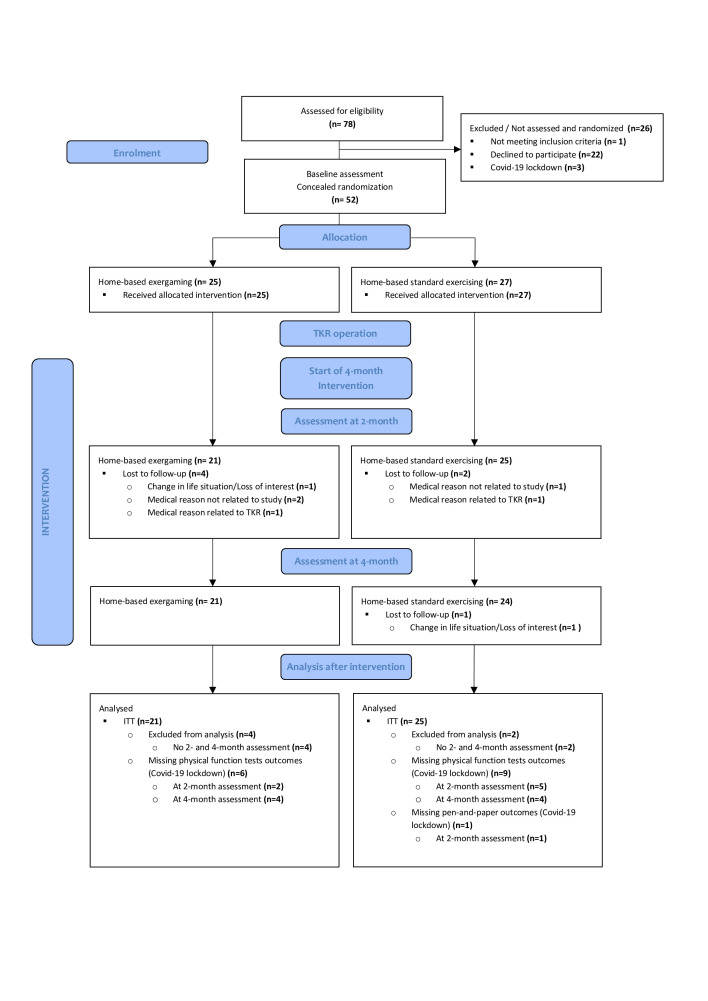
Recruitment started in November 2018 and ended in December 2020 at the scheduled closure date. Fifty-two eligible and voluntary TKR patients were randomly allocated to the IG (n=25) or CG (n=27) after baseline assessment. Both groups’ demographic and clinical characteristics were similar at the baseline (table 1). Figure 1 presents the flow and number of randomly assigned participants by the group throughout the study, together with losses after randomisation. The drop-out rate during the 4-month intervention period was 16.0% in the IG and 11.1% in the CG. Drop-outs related to TKR (n=2) were due to inflammation in the operated prosthesis. Forty-six participants (IG, n=21; CG, n=25) were included in the ITT analysis. No adverse events related to the intervention were observed.
Table 1.
Baseline characteristics of the patients scheduled to undergo a TKR surgery
| Variables | Intervention group (exergame) (n=25) |
Control group (standard exercise) (n=27) |
| Age, years, mean (SD) | 66.9 (3.1) | 66.4 (4.5) |
| Women, n (%) | 16 (64.0) | 17 (63.0) |
| Healthcare district, n (%) | ||
| South West Health Care District | 17 (68.0) | 20 (74.1) |
| Central Finland Health Care District | 8 (32.0) | 7 (25.9) |
| Height, mean (SD) | 167.4 (9.3) | 167.0 (7.9) |
| Weight, mean (SD) | 86.9 (16.0) | 84.4 (10.4) |
| BMI, mean (SD) | 31.0 (5.3) | 30.3 (3.4) |
| ICD-10, n (%)* | ||
| M17.0 | 8 (32.0) | 9 (33.3) |
| M17.1 | 17 (68.0) | 18 (66.7) |
| Model of the completed TKR, n (%) | ||
| Cruciate retaining | 24 (96.0) | 27 (100.0) |
| Posterior stabilising | 1 (4.0) | 0 (0.0) |
| Knee pain (VAS 0–100), mean (SD) | 54.8 (20.4) | 53.7 (20.9) |
| Self-reported comorbidity, n (%) | ||
| OA in joints other than the knee | 10 (40.0) | 9 (33.3) |
| Musculoskeletal disease other than OA | 2 (8.7)† | 3 (11.1) |
| Tibia fracture in the operated lower limb | 1 (4.0) | 0 (0.0) |
| Diabetes | 3 (12.0) | 4 (14.8) |
| Coronary artery disease | 1 (4.0) | 2 (7.4) |
| Hypertension | 11 (44.0) | 17 (63.0) |
| Respiratory disease | 1 (4.0) | 2 (7.4) |
| Life situation, n (%) | ||
| Working | 4 (16.0) | 8 (29.6) |
| Unemployed | 0 (0.0) | 1 (3.7) |
| Retired | 21 (84.0) | 18 (66.7) |
| Daily walking, km, n (%) | ||
| <0.5 | 1 (4.0) | 1 (3.7) |
| 0.5–0.9 | 9 (36.0) | 6 (22.2) |
| 1–3.9 | 15 (60.0) | 14 (51.9) |
| 4–5.9 | 0 (0.0) | 2 (7.4) |
| ≥6 | 0 (0.0) | 4 (14.8) |
| Level of physical activity (PA), n (%) | ||
| Hardly any PA | 3 (12.0) | 0 (0.0) |
| Light PA, 1–2 times a week | 5 (20.0) | 8 (29.6) |
| Light PA, >2 times a week | 6 (24.0) | 5 (18.5) |
| Moderate PA, 1–2 times a week | 4 (16.0) | 5 (18.5) |
| Moderate PA, >2 times a week | 7 (28.0) | 6 (22.2) |
| Active sports, >2 times a week | 0 (0.0) | 3 (11.1) |
| Competitive sports | 0 (0.0) | 0 (0.0) |
*M17.0 Bilateral primary osteoarthritis of knee, M17.1 Unilateral primary osteoarthritis of knee.
†n=23.
BMI, body mass index; ICD-10, International Classification of Diseases 10th Revision; OA, osteoarthritis; VAS, Visual Analogue Scale.
Figure 1.
Flow diagram. ITT, intention to treat; TKR, total knee replacement.
Adherence
There were no differences in the mean time spent for exergaming or standard protocol exercise in weeks 1–8 and 9–16, either in the IG or CG (figure 2, table 2). The IG had more PA during weeks 9–16 than during weeks 1–8 (table 2). Based on the gaming computers, two participants did not exergame. Several patients did not continue exergaming until the end of the study protocol (exergamed for less than 2 months (n=3), 3 months (n=2), or 4 months (n=3)).
Figure 2.

Mean minutes of exergaming from gaming computers during the 4-month intervention (n=21).
Table 2.
Self-reported standard protocol exercising, exergaming and PA from baseline to 2 months and from 2 months to the end of the intervention in the control and intervention groups
| Weeks | Control group (n=25) |
Intervention group (n=21) |
|||||||
| Standard exercise | PA | Exergaming | Standard exercise | PA | |||||
| Hours | Hours/week | MET hours | Hours | Hours/week | Hours | Hours/week | Total hours | MET hours | |
| 1–8 | 19.0 (16.9) | 2.4 (2.1) | 122.0 (164.7) | 19.9 (23.6) | 2.5 (3.0) | 5.7 (6.1) | 0.7 (0.8) | 25.6 (23.6) | 117.5 (95.6) |
| 9–16 | 17.4 (21.0) | 2.2 (2.6) | 180.8 (146.1) | 15.6 (17.1) | 2.0 (2.1) | 4.6 (4.7) | 0.6 (0.6) | 20.2 (18.8) | 179.4 (85.0) |
Values are mean (SD).
MET, metabolic equivalent of task; PA, physical activity.
Baseline assessments were performed on average 7.5 days (SD 3.7) before surgery, and follow-up assessments on average −0.2 days (SD 5.3) at 2 month, and 2.4 days (SD 7.2) at 4 months time point. Exceeding the target time limits was due to participants’ schedules (n=15) or the COVID-19 lockdown (n=4).
Outcomes
The TUG improved more in the IG than the CG over the 2 months (p=0.019) and the 4 months (p=0.040) time. Overall, during the 4-month intervention, in the IG, the mean TUG improved by −1.9 s (95% CI −2.9 to −1.0), and in the CG, it changed by −0.6 s (95% CI −1.4 to 0.3). There were no statistical differences between the groups in the OKS, but the score changed over the 4-month intervention in the IG by 12.1 points (95% CI 9.1 to 15.1) and in the CG by 9.8 points (95% CI 7.1 to 12.6). There were no statistical differences between the groups either in the secondary outcomes. Table 3, figure 3 and online supplemental appendix present the primary and secondary outcome changes from baseline in the IG and CG.
Table 3.
Results for primary and secondary outcomes for the control and intervention groups
| Baseline | Change from baseline to 4 months | ||||
| Control group (n=25) | Intervention group (n=21) | Control group (n=25) |
Intervention group (n=21) | P value | |
| Mean (SD) | Mean (SD) | Mean (95% CI) | Mean (95% CI) | ||
| OKS | 26.9 (6.5) | 26.7 (6.7) | 9.8 (7.1 to 12.6) | 12.1 (9.1 to 15.1) | 0.27 |
| TUG | 8.3 (1.7) | 9.4 (3.6) | −0.6 (−1.4 to 0.3) | −1.9 (−2.9 to −1.0) | 0.04 |
| Pain | 54.2 (21.6) | 57.1 (18.3) | −26.7 (−36.4 to −17.0) | −36.3 (−46.7 to −25.8) | 0.18 |
| 10-MWT | 1.6 (0.3) | 1.6 (0.4) | 0.1 (−0.0 to 0.2) | 0.2 (0.1 to 0.3) | 0.06 |
| SPPB | |||||
| Total | 9.6 (1.5) | 9.5 (1.5) | 0.8 (0.1 to 1.4) | 1.1 (0.4 to 1.7) | 0.51 |
| Balance | 3.8 (0.5) | 3.8 (0.5) | 0.1 (−0.1 to 0.4) | −0.2 (−0.4 to 0.1) | 0.11 |
| Mobility | 3.9 (0.3) | 3.8 (0.8) | 0.1 (−0.1 to 0.3) | 0.2 (−0.0 to 0.4) | 0.64 |
| LE strength | 2.0 (1.1) | 2.0 (1.0) | 0.5 (0.1 to 1.0) | 1.1 (0.6 to 1.6) | 0.12 |
| Muscle force | |||||
| Extension | 1.1 (0.4) | 1.2 (0.5) | −0.1 (−0.2 to 0.0) | −0.1 (−0.2 to 0.0) | 0.85 |
| Flexion | 0.6 (0.2) | 0.6 (0.3) | 0.1 (−0.0 to 0.1) | 0.1 (−0.0 to 0.3) | 0.88 |
| ROM | |||||
| Extension | 6.6 (4.1) | 7.3 (7.7) | 0.1 (−2.7 to 2.9) | −0.5 (−3.5 to 2.5) | 0.76 |
| Flexion | 107.0 (13.0) | 107.0 (18.0) | −7.0 (−13.0 to -2.0) | −1.0 (−8.0 to 5.0) | 0.17 |
Group mean and SD values at baseline, mean and 95% CI values indicating the within-group change from baseline to the end of the 4-month intervention period, and p values indicating the intergroup change in 4-month intervention.
LE, lower extremity; Muscle force, isometric muscle force of the operated lower limb (Nm/weight); 10-MWT, 10 m Walking Test; OKS, Oxford Knee Score; ROM, range of motion; SPPB, Short Physical Performance Battery; TUG, Timed Up and Go.
Figure 3.
Mean changes in the Oxford Knee Score (A), Timed Up and Go (B) and knee pain intensity (C) for control (n=25) and intervention (n=21) groups from the baseline to the mid-intervention at 2 months and the end of intervention at 4 months.
bmjsem-2022-001416supp001.pdf (28.4KB, pdf)
After the intervention period in the IG (n=21), participants were either satisfied (52.4%) or very satisfied (47.6%) with their knees that had undergone an operation. In the CG (n=25), participants were very unsatisfied (8.7%), unsatisfied (17.4%), satisfied (39.1%), or very satisfied (34.8%) with their operations.
Discussion
Older adults who underwent TKR surgery participated in the 4-month home-based intervention using customised exergames, which improved their mobility more than those who exercised by the standard home exercise protocol. In addition, early satisfaction seemed to be more frequent in the IG. In both groups, there were positive changes in knee-related pain and physical function, including knee-related function, walking and lower extremity performance and strength over the 4 months; however, there were no statistically significant differences between the groups. This study’s results align with earlier studies investigating the use of exergames in enhancing physical function and pain in post-TKR rehabilitation.33 34
When observing changes in mobility using the TUG, improvement was greater in the IG than in the CG, both in the middle and at the end of the intervention. For example, a similar difference between guided high-intensity and low-intensity training after TKR has not been observed.35 The difference between IG and CG may be due, for example, to how the exergames may steer the training in a more progressive and goal-oriented direction than the instructions given for standard exercise. Moreover, it should be noted that the TUG did not change in the CG in the 2-month and 4-month follow-up points.
There were no intergroup changes when assessing changes in knee-related function and pain using the OKS. Positive changes were observed in both groups, indicating normal healing after TKR. When evaluating 95% CI, it can be speculated that when increasing the number of participants, CIs would narrow; thus, there may be significant differences in change between the groups.
When evaluating the clinical relevance of changes in knee-related function and pain using the OKS, the mean change in both groups was more than the estimate of the minimal clinically important change (MCIC) in the OKS in patients with TKR.31 In addition, a change in the OKS of 11 points or more 6 months after TKR has been related to satisfaction with the surgery.36 In the IG, this limit was already exceeded at 4 months, and 100% of the participants were either satisfied or very satisfied with the operated knee. In the CG, the percentage was 74%. The TUG MCIC has not been validated in patients with TKR. However, at 4 months, the mean change in the IG was similar to the TUG MCIC in lumbar surgery patients37 and thus may also support the observed early satisfaction. Moreover, gamification may affect patients’ expectations and experience of TKR and, thus, overall satisfaction.38
At 4 months, there were no significant intergroup changes in physical function or pain in the secondary outcomes. However, the pain intensity and lower extremity performance in both groups and walking in the IG changed slightly positively. When observing the results of the muscle force tests and knee ROM measures, there were no within-group changes in knee extension or knee flexion muscle force or knee extension ROM. The only negative change was observed in the knee flexion ROM in the CG, while in the IG, there was neither a negative nor positive change. Bade et al,39 who assessed patients with TKR following a standard rehabilitation programme, observed similar within-group results at 3 and 6 months after surgery; the knee flexion ROM remained below the baseline level.
The participants’ self-reported adherence to home exercise during the intervention was similar between the groups. However, the volume of exergaming and standard exercise varied widely between the participants over the intervention period. This may reflect the positive changes in physical function and pain achieved within 2 months, which may lower interest or motivation for rehabilitation. Moreover, variation may reflect interest or loss of training through novel games or individuals’ choices to exercise in the preferred way.6 In addition, this may be due to an increase in other self-reported PA,40 observed in the IG and is a positive change compared with the finding that PA may remain low during the first months after TKR surgery.41 42
The strength of this study is the accurate design and implementation of a dual-centre RCT.16 The randomisation was successful, and outcome variables selected in collaboration with researchers, orthopaedists and physiotherapists were validated and commonly used to measure the physical function and pain of patients with TKR.22 26 43–47 Self-employed therapeutic exercise implemented at participants’ homes ensured that despite the limitations caused by the COVID-19 pandemic in 2020, participants could continue therapeutic exercise in the assigned group without compromising their rehabilitation.
Study limitations
This study has several limitations. First, the number of participants was low, and half of the planned sample size was achieved in 2020 due to COVID-19. Second, assessors were not blinded after the baseline assessment because of the nature of the interventions and game-specific questionnaires collected from the exergame group. This may pose a risk of bias to the physical function follow-up assessments performed by a research physical therapist. Finally, due to COVID-19, some of the outcomes gathered by physical tests were not measured in this study. Because of the small sample size, the study results of physical function are indicative and will limit the generalisation and interpretation of the results. Future studies should aim to conduct similar studies among a larger cohort of participants.
Conclusions
In patients with TKR, training at home with customised exergames was more effective in mobility and early satisfaction and as effective as standard exercise compared with pain and other physical functions. In both groups, knee-related function and pain improvement can be considered clinically meaningful. Although exergaming was not superior to the standard protocol, it could be used in unsupervised home-based rehabilitation of physical function and pain after TKR.
Acknowledgments
The authors thank the funders, professionals in the Turku University Hospital, and Central Finland Central Hospital who were involved in the recruitment, and patients with TKR who participated in the study.
Footnotes
Contributors: All authors substantially contributed to the conception and design of the work. MJ, NK, JP, KP, HK, MK, KM and EA participated in the recruitment of participants. MJ, NK and EA collected the data. MJ, HK, AH and EA analysed and interpreted the data. MJ drafted the manuscript. All authors commented on the drafted manuscript and critically revised it. All authors approved the final version of the manuscript. AH is responsible for the overall content as guarantor.
Funding: This research was supported by the Päivikki and Sakari Sohlberg Foundation (personal grant to M. Janhunen), the Business Finland (grant numbers: 5794/31/2016, 5941/31/2016, 6057/31/2016) and Finnish partner companies: SE Innovations Oy (Senior Some Oy), Suunto Oy, Physiotools Oy, GoodLife Technology Oy, Lingsoft Oy, eSeteli Palveluverkko Oy, PN Turku Oy, Ade Animations Design & Effects Oy, Adesante Oy, 4FeetUnder, Intechso and Realmax Oy.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information. Metadata has been published in the Jyväskylä University Digital Repository (http://dx.doi.org/10.17011/jyx/dataset/85350).
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
Ethics approval
This study involves human participants and was approved by Ethics Committee of the South West Finland Health Care District (register ETMK Dnro 66/1801/2018, 19 June 2018). Participants gave informed consent to participate in the study before taking part.
References
- 1.Dávila Castrodad IM, Recai TM, Abraham MM, et al. Rehabilitation protocols following total knee arthroplasty: a review of study designs and outcome measures. Ann Transl Med 2019;7(Suppl 7):S255. 10.21037/atm.2019.08.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mistry JB, Elmallah RDK, Bhave A, et al. Rehabilitative guidelines after total knee arthroplasty: a review. J Knee Surg 2016;29:201–17. 10.1055/s-0036-1579670 [DOI] [PubMed] [Google Scholar]
- 3.Buhagiar MA, Naylor JM, Harris IA, et al. Assessment of outcomes of inpatient or clinic-based vs home-based rehabilitation after total knee arthroplasty: a systematic review and meta-analysis. JAMA Netw Open 2019;2:e192810. 10.1001/jamanetworkopen.2019.2810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor LM, Kerse N, Frakking T, et al. Active video games for improving physical performance measures in older people: a meta-analysis. J Geriatr Phys Ther 2018;41:108–23. 10.1519/JPT.0000000000000078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pirovano M, Surer E, Mainetti R, et al. Exergaming and rehabilitation: a methodology for the design of effective and safe therapeutic exergames. Entertainment Computing 2016;14:55–65. 10.1016/j.entcom.2015.10.002 [DOI] [Google Scholar]
- 6.Skjæret N, Nawaz A, Morat T, et al. Exercise and rehabilitation delivered through exergames in older adults: an integrative review of technologies, safety and efficacy. Int J Med Inform 2016;85:1–16. 10.1016/j.ijmedinf.2015.10.008 [DOI] [PubMed] [Google Scholar]
- 7.Howes SC, Charles DK, Marley J, et al. Gaming for health: systematic review and meta-analysis of the physical and cognitive effects of active computer gaming in older adults. Phys Ther 2017;97:1122–37. 10.1093/ptj/pzx088 [DOI] [PubMed] [Google Scholar]
- 8.Gonçalves A, Muñoz J, Cameirão MS, et al. The benefits of custom exergames for fitness, balance, and health-related quality of life: a randomized controlled trial with community-dwelling older adults. Games Health J 2021;10:245–53. 10.1089/g4h.2020.0092 [DOI] [PubMed] [Google Scholar]
- 9.Corregidor-Sánchez AI, Segura-Fragoso A, Rodríguez-Hernández M, et al. Effectiveness of virtual reality technology on functional mobility of older adults: systematic review and meta-analysis. Age Ageing 2021;50:370–9. 10.1093/ageing/afaa197 [DOI] [PubMed] [Google Scholar]
- 10.Gianola S, Stucovitz E, Castellini G, et al. Effects of early virtual reality-based rehabilitation in patients with total knee arthroplasty: a randomized controlled trial. Medicine (Baltimore) 2020;99:e19136. 10.1097/MD.0000000000019136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoon S, Son H. EFFECTS of full immersion virtual reality training on balance and knee function in total knee replacement patients: A randomized controlled study. J Mech Med Biol 2020;20:2040007. 10.1142/S0219519420400072 [DOI] [Google Scholar]
- 12.Fung V, Ho A, Shaffer J, et al. Use of nintendo wii fit. Physiotherapy 2012;98:183–8. 10.1016/j.physio.2012.04.001 [DOI] [PubMed] [Google Scholar]
- 13.Blasco J, Igual-Camacho C, Blasco M, et al. The efficacy of virtual reality tools for total knee replacement rehabilitation: A systematic review. Physiother Theory Pract 2021;37:682–92. 10.1080/09593985.2019.1641865 [DOI] [PubMed] [Google Scholar]
- 14.Janhunen M, Karner V, Katajapuu N, et al. Effectiveness of exergame intervention on walking in older adults: A systematic review and meta-analysis of randomized controlled trials. Phys Ther 2021;101. 10.1093/ptj/pzab152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orkin AM, Gill PJ, Ghersi D, et al. Guidelines for reporting trial protocols and completed trials modified due to the COVID-19 pandemic and other extenuating circumstances: the CONSERVE 2021 statement. JAMA 2021;326:257. 10.1001/jama.2021.9941 [DOI] [PubMed] [Google Scholar]
- 16.Aartolahti E, Janhunen M, Katajapuu N, et al. Effectiveness of gamification in knee replacement rehabilitation: protocol for a randomized controlled trial with a qualitative approach. JMIR Res Protoc 2022;11:e38434. 10.2196/38434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (tidier) checklist and guide. BMJ 2014;348:g1687. 10.1136/bmj.g1687 [DOI] [PubMed] [Google Scholar]
- 18.Hopewell S, Clarke M, Moher D, et al. CONSORT for reporting randomized controlled trials in journal and conference abstracts: explanation and elaboration. PLoS Med 2008;5. 10.1371/journal.pmed.0050020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moher D, Hopewell S, Schulz KF, et al. Consort 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c869. 10.1136/bmj.c869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bade MJ, Wolfe P, Zeni JA, et al. Predicting poor physical performance after total knee arthroplasty. J Orthop Res 2012;30:1805–10. 10.1002/jor.22140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mizner RL, Petterson SC, Clements KE, et al. Measuring functional improvement after total knee arthroplasty requires both Performance-based and patient-report assessments: a longitudinal analysis of outcomes. J Arthroplasty 2011;26:728–37. 10.1016/j.arth.2010.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dawson J, Fitzpatrick R, Murray D, et al. Questionnaire on the perceptions of patients about total knee replacement. J Bone Joint Surg Br 1998;80:63–9. 10.1302/0301-620x.80b1.7859 [DOI] [PubMed] [Google Scholar]
- 23.Reito A, Järvistö A, Jämsen E, et al. Translation and validation of the 12-Item Oxford knee score for use in Finland. BMC Musculoskelet Disord 2017;18:74. 10.1186/s12891-017-1405-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Podsiadlo D, Richardson S. The timed “ up & go ”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991;39:142–8. 10.1111/j.1532-5415.1991.tb01616.x [DOI] [PubMed] [Google Scholar]
- 25.Thong ISK, Jensen MP, Miró J, et al. The validity of pain intensity measures: what do the NRS, vas, VRS, and FPS-R measure? Scand J Pain 2018;18:99–107. 10.1515/sjpain-2018-0012 [DOI] [PubMed] [Google Scholar]
- 26.Unver B, Baris RH, Yuksel E, et al. Reliability of 4-meter and 10-meter walk tests after lower extremity surgery. Disabil Rehabil 2017;39:2572–6. 10.1080/09638288.2016.1236153 [DOI] [PubMed] [Google Scholar]
- 27.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994;49:M85–94. 10.1093/geronj/49.2.m85 [DOI] [PubMed] [Google Scholar]
- 28.Maffiuletti NA, Bizzini M, Desbrosses K, et al. Reliability of knee extension and flexion measurements using the con-trex isokinetic dynamometer. Clin Physiol Funct Imaging 2007;27:346–53. 10.1111/j.1475-097X.2007.00758.x [DOI] [PubMed] [Google Scholar]
- 29.Gogia PP, Braatz JH, Rose SJ, et al. Reliability and validity of goniometric measurements at the knee. Phys Ther 1987;67:192–5. 10.1093/ptj/67.2.192 [DOI] [PubMed] [Google Scholar]
- 30.Ainsworth BE, Haskell WL, Herrmann SD, et al. 2011 compendium of physical activities: a second update of codes and Met values. Med Sci Sports Exerc 2011;43:1575–81. 10.1249/MSS.0b013e31821ece12 [DOI] [PubMed] [Google Scholar]
- 31.Beard DJ, Harris K, Dawson J, et al. Meaningful changes for the oxford hip and knee scores after joint replacement surgery. J Clin Epidemiol 2015;68:73–9. 10.1016/j.jclinepi.2014.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Judge A, Arden NK, Cooper C, et al. Predictors of outcomes of total knee replacement surgery. Rheumatology (Oxford) 2012;51:1804–13. 10.1093/rheumatology/kes075 [DOI] [PubMed] [Google Scholar]
- 33.Gazendam A, Zhu M, Chang Y, et al. Virtual reality rehabilitation following total knee arthroplasty: a systematic review and meta-analysis of randomized controlled trials. Knee Surg Sports Traumatol Arthrosc 2022;30:2548–55. 10.1007/s00167-022-06910-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peng L, Zeng Y, Wu Y, et al. Virtual reality-based rehabilitation in patients following total knee arthroplasty: a systematic review and meta-analysis of randomized controlled trials. Chin Med J (Engl) 2021;135:153–63. 10.1097/CM9.0000000000001847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bade MJ, Struessel T, Dayton M, et al. Early high-intensity versus low-intensity rehabilitation after total knee arthroplasty: a randomized controlled trial. Arthritis Care Res (Hoboken) 2017;69:1360–8. 10.1002/acr.23139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Judge A, Arden NK, Kiran A, et al. Interpretation of patient-reported outcomes for hip and knee replacement surgery: identification of thresholds associated with satisfaction with surgery. J Bone Joint Surg Br 2012;94:412–8. 10.1302/0301-620X.94B3.27425 [DOI] [PubMed] [Google Scholar]
- 37.Maldaner N, Sosnova M, Ziga M, et al. External validation of the minimum clinically important difference in the timed-up-and-go (TUG) test after surgery for lumbar degenerative disc disease. Spine 2021. 10.1097/BRS.0000000000004128 [DOI] [PubMed] [Google Scholar]
- 38.Hamilton DF, Lane JV, Gaston P, et al. What determines patient satisfaction with surgery? A prospective cohort study of 4709 patients following total joint replacement. BMJ Open 2013;3:e002525. 10.1136/bmjopen-2012-002525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bade MJ, Kohrt WM, Stevens-Lapsley JE. Outcomes before and after total knee arthroplasty compared to healthy adults. J Orthop Sports Phys Ther 2010;40:559–67. 10.2519/jospt.2010.3317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsunaga-Myoji Y, Fujita K, Ide S, et al. Changes in actual daily physical activity and patient-reported outcomes up to 2 years after total knee arthroplasty with arthritis. Geriatr Nur (Lond) 2020;41:949–55. 10.1016/j.gerinurse.2020.07.006 [DOI] [PubMed] [Google Scholar]
- 41.Mills K, Falchi B, Duckett C, et al. Minimal change in physical activity after lower limb joint arthroplasty, but the outcome measure may be contributing to the problem: a systematic review and meta-analysis. Physiotherapy 2019;105:35–45. 10.1016/j.physio.2018.04.003 [DOI] [PubMed] [Google Scholar]
- 42.Sašek M, Kozinc Ž, Löfler S, et al. Objectively measured physical activity, sedentary behavior and functional performance before and after lower limb joint arthroplasty: a systematic review with meta-analysis. J Clin Med 2021;10:24.:5885. 10.3390/jcm10245885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poitras S, Wood KS, Savard J, et al. Assessing functional recovery shortly after knee or hip arthroplasty: a comparison of the Clinimetric properties of four tools. BMC Musculoskelet Disord 2016;17:478. 10.1186/s12891-016-1338-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lienhard K, Lauermann SP, Schneider D, et al. Validity and reliability of isometric, isokinetic and isoinertial modalities for the assessment of quadriceps muscle strength in patients with total knee arthroplasty. J Electromyogr Kinesiol 2013;23:1283–8. 10.1016/j.jelekin.2013.09.004 [DOI] [PubMed] [Google Scholar]
- 45.Wylde V, Bruce J, Beswick A, et al. Assessment of chronic postsurgical pain after knee replacement: a systematic review. Arthritis Care Res (Hoboken) 2013;65:1795–803. 10.1002/acr.22050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Medina-Mirapeix F, Vivo-Fernández I, López-Cañizares J, et al. Five times sit-to-stand test in subjects with total knee replacement: reliability and relationship with functional mobility tests. Gait Posture 2018;59:258–60. 10.1016/j.gaitpost.2017.10.028 [DOI] [PubMed] [Google Scholar]
- 47.Brosseau L, Balmer S, Tousignant M, et al. Intra- and intertester reliability and criterion validity of the parallelogram and universal goniometers for measuring maximum active knee flexion and extension of patients with knee restrictions. Arch Phys Med Rehabil 2001;82:396–402. 10.1053/apmr.2001.19250 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjsem-2022-001416supp001.pdf (28.4KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information. Metadata has been published in the Jyväskylä University Digital Repository (http://dx.doi.org/10.17011/jyx/dataset/85350).