Abstract
Background
Data on whether the graft CD3-positive (CD3+) T-cell dose in T-cell-replete human leukocyte antigen (HLA)-mismatched allogeneic hematopoietic peripheral blood stem cells transplantation (PBSCT) influences post-transplant outcomes are controversial.
Methods
Using King Hussein Cancer Center (KHCC) Blood and Marrow Transplantation (BMT) Registry database, 52 adult subjects, receiving the first T-cell-replete HLA-mismatched allogeneic hematopoietic PBSCT for acute leukemias or myelodysplastic syndrome, were identified, from January 2017 to December 2020. The cutoff value of graft CD3+ T-cell dose was identified using the receiver operating characteristic (ROC) formula and Youden’s analysis. Subjects were divided into two cohorts: cohort 1 with low CD3+ T-cell dose (n = 34) and cohort 2 with high CD3+ T-cell dose (n = 18). Correlative analyses were performed between CD3+ T-cell dose and the risk of graft-versus-host disease (GvHD), relapse, relapse-free survival (RFS), and overall survival (OS). P-values were two-sided and considered significant when P < 0.05.
Results
Subject covariates were displayed. Subject’s characteristics were comparable, except for higher nucleated cells and more female donors in the high CD3+ T-cell cohort. The 100-day cumulative incidence of acute GvHD (aGvHD) was 45±7% and 3-year cumulative incidence of chronic GvHD (cGvHD) was 28±6.7%. There was no statistically significant difference between the two cohorts in aGvHD (50% vs. 39%, P = 0.4) or cGvHD (29% vs. 22%, P = 0.7). The 2-year cumulative incidence of relapse (CIR) was 67.5±16.3% for low compared with 14.3±6.8% for high CD3+ T-cell cohort (P = 0.018). Fifteen subjects relapsed and 24 have died, 13 due to disease relapse. There was an improvement in 2-year RFS (94% vs. 83%; P = 0.0022) and 2-year OS (91% vs. 89%; P = 0.025) in low CD3+ T-cell cohort compared with high CD3+ T-cell cohort. Graft CD3+ T-cell dose is the only significant risk factor for relapse (P = 002), and OS (P = 0.030) in univariate analysis which was maintained in multivariate for relapse (P = 0.003), but not for OS (P = 0.050).
Conclusions
Our data suggest that high graft CD3+ T-cell dose is associated with lower risk of relapse, and might improve long-term survival, but has no influence on the risk of developing aGvHD or cGvHD.
Keywords: Graft CD3+ T-cell dose, GvHD, Relapse-free survival, Overall survival, PBSC, Allogeneic hematopoietic cell transplantation
Introduction
The outcome of allogeneic hematopoietic cell transplantation (allo-HCT) performed for advanced hematological malignancies relies on both disease-related and transplant-related factors, including disease status at the time of transplant, conditioning intensity, donor type, stem cells source and immunotherapy exploiting the graft-versus-tumor (GVT) effect, which is derived primarily from donor immune effector cells.
Haploidentical stem cell transplant is an alternative option for patients who do not have a human leukocyte antigen (HLA)-matched related or unrelated donor, but associated with higher incidence of graft-versus-host disease (GvHD), graft rejection, and delayed immune reconstitution. Graft composition has different effects on hematopoietic engraftment, immune recovery, GVT, GvHD and survival outcomes [1]. The interaction between the graft immune effector cells, including antigen-presenting cells, CD3+, CD4+, CD8+ cells, regulatory T cells (Tregs) and natural killer (NK) cells, is responsible for both GVT and GvHD. Among these cells, the most well-studied are CD3+ T cells. The donor CD3+ T cells are the main player in GVT process, increased dose of which results in increasing incidence of GvHD [2-6]. In vivo and ex vivo T-cell-depleted (TCD) grafts are associated with reduction of GvHD but at the expense of increased relapse and infections [6]. Peripheral blood stem cell (PBSC) grafts which carry 10 - 15 times higher quantity of CD3+ T cells also have higher risk of chronic GvHD (cGvHD) compared with bone marrow (BM) grafts [7, 8].
The impact of graft CD3+ T-cell dose on the clinical outcomes in different types of allo-HCT is still uncertain. Few single-center and registry studies assessed the role of graft CD3+ T-cell dose with respect to post-transplant outcomes [9-17]. These studies are variable in terms of selection criteria, types of donors, disease entity, and conditioning regimen. It has been reported that the incidence of aGvHD was higher in the graft with high counts of graft CD3+ T cells in HLA-matched allo-HCT [13], opposite to a published report showing high counts of graft CD3+ T-cell resulted in more intensive graft-versus-leukemia (GVL) without producing more severe GvHD and better overall survival (OS) in haploidentical BM combined with peripheral stem cells transplantation [14, 15]. Few small studies published reported on the impacts of graft CD3+ T-cell and CD8+ T-cell doses on the hematopoietic recovery, GvHD and survival outcomes in haploidentical allo-HCT using peripheral blood graft [16, 17].
Herein, we reported 52 subjects to assess the correlations between graft CD3+ T-cell dose and GvHD, disease relapse, relapse-free survival (RFS) and OS in T-cell-replete HLA-mismatched allogeneic peripheral blood hematopoietic cell transplantation.
Materials and Methods
We used King Hussein Cancer Center Registry Bone Marrow Transplantation database to identify subjects > 18 years, who received first T-cell-replete HLA-mismatched allogeneic peripheral blood cell transplantation, from January 2017 to December 2020. Subjects with acute leukemia or myelodysplastic syndrome (MDS) were eligible for the study. We excluded ex vivo (TCD and CD34+-selected grafts) allo-HCT, second transplants, matched related, and matched or mismatched unrelated transplants. Donors with one or two alleles mismatched or 5/10 mismatched using high-resolution typing at HLA-A/B/C/DRB1/DQB1 were included.
All subjects received blood grafts. Haploidentical allo-HCT received fludarabine/total body irradiation (Flu/TBI) conditioning (fludarabine 30 mg/m2 actual weight body surface area (BSA)) on days (-6), (-5) and (-4) and TBI 200 cGy two fractions per day on days (-3), (-2), and (-1) from stem cell infusion day in myeloablative and classic Baltimore regimen of Flu/cyclophosphamide/TBI (FluCyTBI) conditioning (30 mg/m2 actual weight BSA) on days (-6), (-5), (-4), (-3) and (-2) and cyclophosphamide 14.5 mg/kg adjusted ideal body weight) on days (-6) and (-5) and TBI 200 one fraction per day on day (-1) from stem cell infusion in reduced intensity allo-HCT. Five mismatched related allotransplant recipients received different intensity conditioning including busulfan/cyclophosphamide (BU/CY), Flu/Bu4, and FluBu2. All subjects received cyclosporine and short course methotrexate or mycophenolate mofetil with post-transplant cyclophosphamide (PTcy) given on day +3 and day +5 after haploidentical allo-HCT. Subjects with one or two alleles mismatched, received 5 mg/kg of anti-thymocyte globulin (ATG). Acute GvHD was defined based on 1994 Consensus Conference recommendations on aGvHD grading held in Keystone in January 1994 and cGvHD was based on National Institutes of Health (NIH) consensus criteria [18, 19]. All donors were mobilized using 10 µg/kg/day myeloid hematopoietic growth factors (granulocyte colony-stimulating factors (G-CSF)) for four consecutive days before stem cells collection. The numbers of total nucleated, CD34+ and CD3+ cells in the graft were assessed before stem cell infusion. CD34+ and CD3+ cells were calculated by flow cytometer, and data were acquired and analyzed by an eight-color BD FACSCanto II flowcytometer.
All subjects’ demographics were retrospectively collected from the electronic charts, including age, gender, donor/recipient sex, date of diagnosis, disease subtype, hematopoietic stem cell comorbidity index (HSCT-CI) [20], time from diagnosis to allo-HCT, treatment regimens, conditioning regimen, GvHD prophylaxis, pre-transplant disease status, type of donor, GvHD, post-transplant disease status, CD3+ T-cell dose, CD34+ cell dose, donor/recipient cytomegalovirus (CMV) serostatus, donor/recipient ABO blood group match, and cause of death.
We plotted all subjects on the XY lines and using receiver operating characteristic (ROC) analysis and Youden’s index, we identified the cutoff value of graft CD3+ T-cell dose, where the risk of GvHD is the lowest [21, 22]. We divided all subjects into two cohorts (cohort 1: low CD3+ T-cell; cohort 2: high CD3+ T-cell) based on the cutoff value of CD3+ T-cell. Cumulative incidence of relapse (CIR), aGvHD, cGvHD and survival outcomes were compared between the two cohorts. This study was approved and informed consent was exempted by King Hussein Cancer Center (KHCC) Institutional Review Board (IRB), study number 20KHCC 191, compliant with the principles of the Declaration of Helsinki.
Statistics
Descriptive statistics were used to describe subjects’ demographics. Categorical variables were compared by using Chi-square test. The cutoff value of graft CD3+ T-cell was calculated based on ROC analysis, identifying the point on ROC curve where the sensitivity and specificity of the test are equal: the point on the curve with minimum distance from the left-upper corner of the unit square, and the point where the Youden’s index is maximum [21, 22]. OS was defined as date of transplant to death from any cause and surviving patients were censored at last encounter. RFS is defined, as date of transplant to either progression/relapse or death from any cause, and patients alive without evidence of disease relapse/progression were censored at last encounter. The 100-day cumulative incidence rates of aGvHD and cGvHD at 3 years were calculated based on a competing risk analysis. CIR was calculated from the date of initial diagnosis to the date of relapse. Survival curves were plotted using Kaplan-Meier curves and survival analyses were performed by a log-rank test. To identify the determinant outcomes, covariates were selected based on their association with post-transplant outcomes including GvHD, relapse and survival outcomes in previously published reports. Significant covariates in univariate analysis were analyzed using multivariate analysis. Multivariate Cox proportional hazard model was used for the survival analyses and expressed in hazard ratio (HR) along with a 95% confidence interval (CI). All tests were two-sided and P-value of < 0.05 was considered statistically significant. Statistical analysis was done using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
Subject- and disease-related covariates
Fifty-two subjects were included in the analysis. Subject covariates are displayed in Table 1. CD3+ T-cell cutoff value was identified (23.4 × 107). All subjects had good Karnofsky performance status (KPS) and 49 (94%) had low HSCT-CI. Forty-five (87%) of subjects were in histological complete remission (CR) at the time of transplant, six with active MDS (11.5%) and one received upfront sequential allo-HCT. Indication for allo-HCT was myeloid neoplasms (acute myeloid leukemia (AML)and MDS) in 37 subjects (71%) and acute lymphoblastic leukemia (ALL) in 15 (29%). Median recipient (R) and donor age was 30 years (range: 17 - 63 years) and 28 years (range: 8 - 59 years). Thirty-eight subjects were male (73%); 19 had gender mismatch (36.5%); six (female-male in six). All, but one D-R CMV serostatus was IgG negative to IgG positive. All subjects received G-CSF mobilized blood grafts. Myeloablative conditioning was used in 43 (83%), TBI-based was in 24 (46%) and nine (17%) received ATG. The baseline characteristics of two cohorts were comparable, except for higher nucleated cells (7.78 × 108/kg versus 6.76 × 108; P = 0.034), and more female donors into high versus low cohort (26% versus 67%), respectively. The median mononuclear and CD34+ cells infused were 7.2 × 106 (interquartile range (IQR): 5.9 - 8.3) and 7 × 106/kg (IQR: 5.2 - 9.1), respectively. The median dose of CD3+ T cells was 19.5 × 107/kg (IQR: 15.5 - 24.9).
Table 1. Subjects Demographics for Both Cohorts (n = 52).
| Variable | Low CD3+ T-cell dose (34 (65.4%)) | High CD3+ T-cell dose (18 (34.6%)) | P-value |
|---|---|---|---|
| Recipient age, median (range) | 29 (22 - 39) | 32 (25 - 40) | 0.6 |
| Donor age, median (range) | 28 (24 - 34) | 28 (21 - 42) | > 0.9 |
| Recipient gender | |||
| Female | 8 (24%) | 6 (33%) | 0.5 |
| Male | 26 (76%) | 12 (67%) | |
| Donor gender | |||
| Female | 9 (26%) | 12 (67%) | 0.4 |
| Male | 25 (74%) | 6 (33%) | |
| Recipient/donor sex match | |||
| Female/female | 4 (12%) | 4 (22%) | 0.021 |
| Male/female | 3 (8.8%) | 3 (17%) | |
| Donor/recipient ABO match | |||
| D-R | 17 (50%) | 8 (44%) | 0.7 |
| Recipient CMV seropositive | |||
| Negative positive | 1 (3.0%) | 0 (0%) | 0.6 |
| Positive-positive | 0 (0%) | 1 (5.6%) | |
| Positive-positive | 32 (97%) | 17 (94%) | |
| Nucleated cells, median (range) | 6.76 (5.72 - 7.84) | 7.78 (6.97 - 9.92) | 0.034 |
| CD3+ T-cell dose, median (range) | 17 (13 - 19) | 28 (25 - 30) | < 0.001 |
| CD34+ T-cell dose, median (range) | 7.0 (5.40 - 9.50) | 6.32 (5.03 - 8.54) | 0.40 |
| Disease type | |||
| ALL | 10 (29%) | 5 (28%) | 0.6 |
| AML-MDS | 24 (71%) | 13 (72.5%) | |
| HSCT-CI | |||
| 0-1 | 33 (97%) | 16 (89%) | 0.3 |
| 3-4 | 1 (2.9%) | 1 (5.6%) | |
| Pre-transplant disease status | |||
| Complete remission | 19 (61%) | 9 (56%) | 0.7 |
| Conditioning regimen | |||
| Myeloablative | 30 (88%) | 13 (72%) | 0.2 |
| Reduced intensity | 3 (8.8%) | 4 (22%) | |
| Reduced toxicity | 0 (0%) | 1 (5.6%) | |
| TBI-based conditioning | 14 (41%) | 10 (56%) | 0.3 |
| ATG | 6 (18%) | 3 (17%) | > 0.9 |
| Donor type | |||
| Haploidentical | 29 (85%) | 14 (78%) | 0.7 |
| Mismatched related | 5 (15%) | 4 (22%) | |
| GvHD prophylaxis | |||
| CSA or TAC + PTcy | 29 (85%) | 14 (78%) | 0.4 |
| CSA or TAC/MTX | 3 (8.8%) | 1 (5.6%) | |
| Acute GvHD | 17 (50%) | 7 (39%) | 0.4 |
| Chronic GvHD | |||
| Total | 10 (29%) | 4 (22%) | 0.7 |
| NIH score 2-3 | 6 (60%) | 3 (75%) | > 0.9 |
R: recipient; D: donor; CMV: cytomegalovirus; AML: acute myeloid leukemia; MDS: myelodysplastic syndrome; ALL: acute lymphoblastic leukemia; HSCT-CI: hematopoietic stem cell comorbidity index; ATG: anti-thymocyte globulin; TBI: total body irradiation; GvHD: graft-versus-host disease; CSA: cyclosporine A; PTcy: post-transplant cyclophosphamide. TAC: Tacrolimus; MTX: methotrexate; NIH: National Institutes of Health.
GvHD
The 100-day cumulative incidence of aGvHD was 45±7% and 3-year cumulative incidence of cGvHD was 28±6.7% calculated based on competing risk analyses with deaths.
There is no statistically significant difference between low and high CD3+ T-cell dose in regard to aGvHD (50% vs. 39%, P = 0.4), grade ≥ 2 (53% vs. 43%; P = 0.4) and organ specific (all sites P > 0.3) or cGvHD (29% vs. 22%, P = 0.7) and moderate to severe cGvHD (70 vs. 75%; P = 0.9).
Disease relapse and transplant-related mortality (TRM)
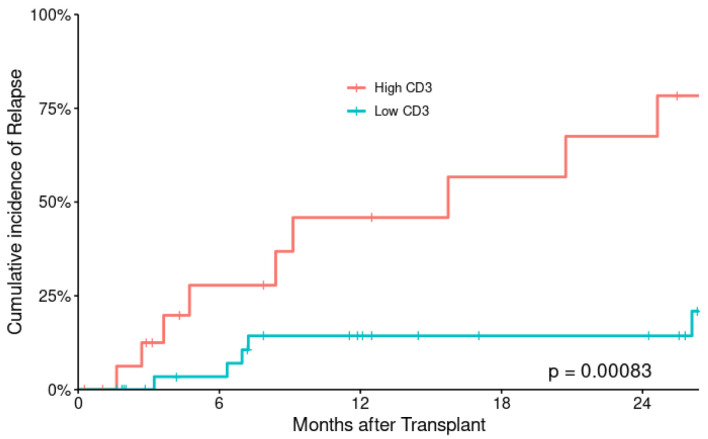
At last encounter, 15 subjects relapsed (29%); 24 subjects have died, and 28 were alive. The 2-year CIR was 31.3±7.9% for the entire patient’s population. There are more relapses in low CD3+ T-cell cohort with 2-year CIR 67.5±16.3% for low CD3+ T-cell cohort compared with 14.3±6.8% for high CD3+ T-cell cohort with statistically significant P value (P = 0.018) (Fig. 1). Disease relapse was the most common cause of death (n = 13), followed by sepsis (n = 8), pulmonary hemorrhage (n = 2) and one subject died of GvHD. The 2-year cumulative incidence of TRM is 21% (seven vs. three subjects in low and high CD3+ T-cell cohorts).
Figure 1.
Cumulative incidence of relapse according to CD3+ T-cell dose.
RFS and OS
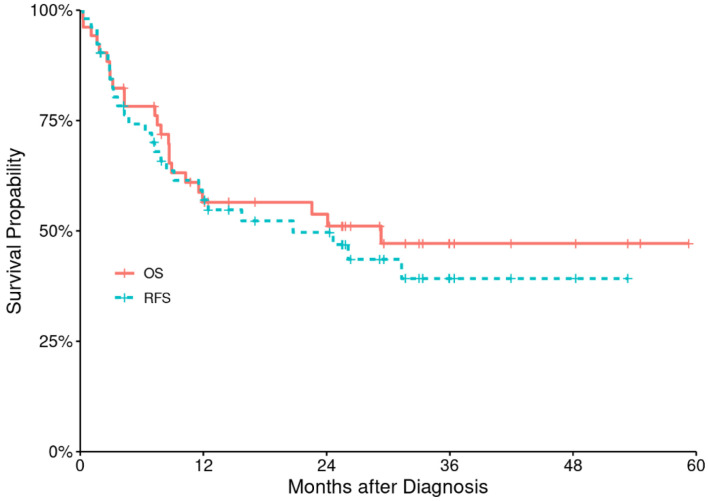
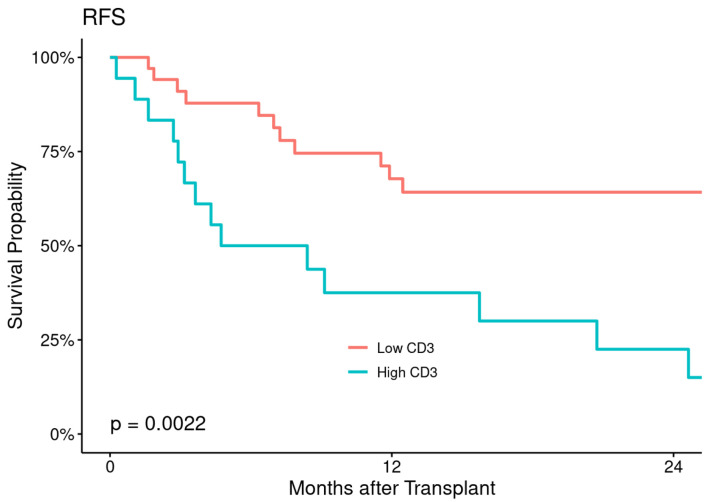
At a median follow-up of 26 months (range: 2 - 60 months), median RFS and OS were 11.7 months (range: 0.3 - 53 months) and 12 months (range: 0.3 - 59 months), respectively for the entire patient’s population, corresponding to 2-year RFS and OS of 49% (95% CI: 37-66%) and 54% (95% CI: 41-70%), respectively (Fig. 2). The estimated 2- and 5-year RFS were 94% (86-100%) in low CD3+ T-cell and 83% (68-100%) in high CD3+ T-cell cohort and 88% (77-99%) in low CD3+ T-cell and 50% (32-79%) in high T-cell cohort respectively with statistically significant P value (P = 0.0022) (Fig. 3).
Figure 2.
Relapse-free survival (RFS) and overall survival (OS) for the entire study population.
Figure 3.
Relapse-free survival (RFS) according to CD3+ T-cell dose.
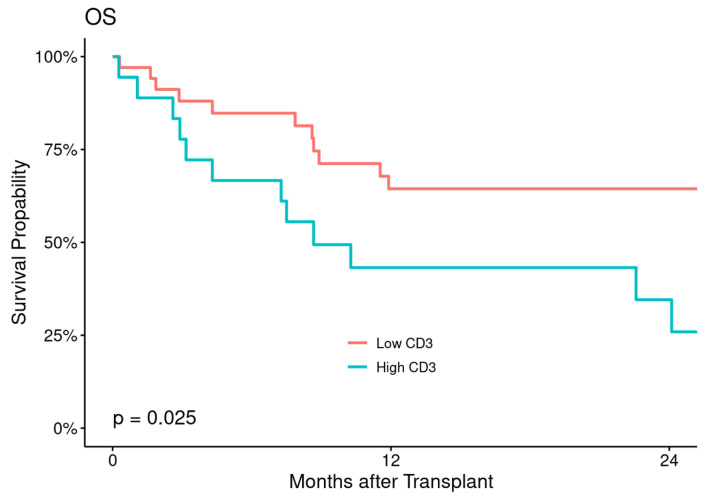
The estimated 2- and 5-year OS were 91% (82-100%) and 85% (73-98%) in low CD3+ T-cell cohort and 89% (76-100%) and 67% (48-92%) in high CD3+ T-cell cohort with statistically significant difference between the two cohorts (P = 0.025) (Fig. 4).
Figure 4.
Overall survival (OS) according to CD3+ T-cell dose.
Univariate and multivariate analyses for several factors including graft CD3+ T-cell dose (low versus high), recipient age (< 40 years versus ≥ 40 years), donor age, sex mismatch (female to male versus other), recipient CMV seropositive (positive versus negative), disease status at the time of transplant (CR versus active), conditioning regimen intensity (myeloablative versus reduced intensity), TBI based or not, type of transplant (haplo versus mismatched related) and the use of ATG in mismatched related donor (MMSD) transplant were analyzed. Graft CD3+ T-cell dose is the only significant risk factor for relapse (P = 002), and OS (P = 0.030) in univariate analysis which was maintained in multivariate analysis for relapse (P = 0.003), but lost its statistical significance for OS (P = 0.050). None of the other factors was significant in regard to aGvHD and cGvHD (all P > 0.2).
Discussion
T-cell-replete HLA-mismatched allogeneic transplantation using peripheral blood grafts is universally increasing. Blood grafts, which carry 10 - 15 times higher quantity of graft CD3+ T cells are associated with higher risk of aGvHD and more cGvHD compared with BM grafts [7, 8]. This might be translated into increased non-relapsed mortality (NRM) and compromising survival outcomes.
Saad et al reported on 2,376 subjects to determine whether the T-cell graft composition can impact GvHD and survival outcomes of subjects with acute leukemia or MDS receiving peripheral blood allogeneic transplantation using an HLA-matched sibling donor (MSD) or matched unrelated donor (MUD). The authors concluded, currently used cutoff value of CD3+ T-cell doses of (14 × 107 cells/kg in MSD; 15 × 107 cells/kg in MUD) peripheral blood grafts did not significantly influence the risk of aGvHD, cGvHD, or other post-transplant outcomes [13]. Another relatively old European Society for Blood and Marrow Transplantation (EBMT) report, included AML subjects in CR, receiving reduced intensity conditioning in MUD transplants, with different design, analyzed by IQR, thus allowing comparison of grafts with the lowest below and the highest above 347 × 106/kg CD3+ T-cell dose content in the grafts is an independent prognostic factor associated with higher probability of severe aGvHD grades II-IV and III-IV for CD3+ T-cell and no effect on cGvHD and other post-transplant outcomes [12]. The first study analyzed recipients of mixed MSD and matched MUD transplants and the second study analyzed matched MUD transplants exclusively, used different conditioning intensity and methodology(ies) in identifying the cutoff CD+ T-cell graft dose. Results of both studies were in opposite to our study results and affirmed that myeloablative conditioning was more likely to be associated with more severe GvHD, as an advanced donor age and sex-mismatched grafts, which is consistent with our study results in the former study, but discordant with the last one.
Similarly, using TCD haploidentical transplantation procedures with manipulation and quantification of graft compositions, especially CD3+ T-cell count will influence post-transplant outcomes [16].
Our data show no associations between graft CD3+ T-cell dose of peripheral blood grafts and the risk of aGvHD, or cGvHD in T-cell-replete mismatched HLA-mismatched allogeneic hematopoietic PBSCT, but strongly associated with disease relapse risk and no statistical significance in OS outcomes in multivariate analysis in our study cohort. Nonetheless, the subgroup analyses suggest certain correlations, which merit further exploration prospectively.
Results of our study are opposite to several previously published reports in MSD, MUD mentioned above and MMSD, haploidentical allotransplants confirming increased risk of aGvHD grade 2-4 [10-12]. Our data contrast the report published from China by Zhang et al, including 30 subjects with acute leukemia and MDS, who received haploidentical allo-HCTs, using peripheral blood grafts which showed high CD3+ T-cell dose above the median (299.7 × 106/kg) in peripheral blood stem cell graft increased the rate of aGvHD, but not cGvHD and decreased the OS with no effect on relapse risk [16]. The discrepancy in the results between the two studies may be attributed to differences in median CD3+ T-cell dose in peripheral blood grafts, as well as in the statistical methodology used to categorize the primary outcome variable. In this study, CD3+ T-cell dose was categorized by using the median value, whereas in our study, we used a cutoff value of CD3+ T-cell dose using Youden’s analysis and ROC formula where it delineates the lowest and the highest risk of developing GvHD. Moreover, all subjects in this study received ATG for GvHD prophylaxis, in combination with cyclosporine, short-term methotrexate and mycophenolate mofetil, whereas in our study, we used PTcy in combination with cyclosporine A (CSA) or tacrolimus and mycophenolate mofetil in all haploidentical transplants recipients and ATG was given for mismatched transplants in addition to CSA and short course methotrexate or mycophenolate mofetil.
Mussetti et al also reported on 234 subjects receiving haploidentical BM and peripheral blood grafts transplants, and showed graft CD3+ T-cell content was associated with an increased incidence of all-grade cGvHD and the use of peripheral blood grafts was associated with an increased incidence of grade 2-4 aGvHD. This is in opposite to our study results in regard to GvHD [17].
An important observation in our study is that donor characteristics did not influence any of the study outcomes. These results are in contrast with HLA-identical donors and HLA-matched unrelated donors transplants, where donor age and gender correlated with outcomes [13, 23, 24]. Dezern et al in his study, reported, increasing donor age by decade was associated with poorer OS, worse progression-free survival and a higher risk for grade 2 to 4 and grade 3 to 4 aGvHD, but not for cGvHD in haploidentical allo-HCTs using reduced intensity conditioning [25]. The use of in vivo T-cell repletion with PTcy in most of our study participants may effectively abrogate alloreactivity, thus neutralizing differences related to donor characteristics. PTcy is known to neutralize HLA-mismatch disparities in the haploidentical transplant setting by blocking the proliferating fraction of donor CD3+ T-cell, thus reducing the risk of GvHD, and probably differences between donor’s characteristics [26, 27]. However, it is also possible that our study was underpowered to detect a significant influence of donor characteristics on outcomes. Lack of donor-recipient (D-R) combined age score variable analyzed in the uni- and multivariate analyses might have an effect on better assessment for aGvHD and cGvHD in our cohorts [28].
Although, there is a strong association of graft CD3+ T-cell dose with relapse risk (P = 0.02) which was confirmed in multivariate analysis and OS (P = 0.030) in univariate analysis, that lost it is statistical significance in multivariate analysis. So, our data are not strongly associated with survival outcomes, since the association was failed to be confirmed in multivariate analysis. This can be explained by the absence of other variables in the univariate and multivariate analyses like disease risk index and the inclusion of subjects with active MDS at the time of transplant. Other factors including conditioning intensity, TBI-based conditioning, type of donor, recipient and donor age, D-R sex and ABO mismatch were not associated with either GvHD or survival outcomes.
From clinical perspective, the use of in vivo T-cell depletion using PTcy in haploidentical and ATG in mismatched related transplants in recipients of peripheral blood grafts in our study cohort may result in ameliorating the risk of GvHD in high CD3+ T-cell cohort especially cGvHD.
Our study has important limitations including few heterogeneous subjects and disease- and transplant-related covariates, different conditioning intensities, the lack of inclusion of CD34 cell dose, nucleated cell dose in uni-multivariate analyses, that might affect the risk of GvHD and survival outcomes, lack of T-cell phenotypic subsets: CD4+, CD8+, or CD4+/CD8+ ratio for better assessment of the risk of GvHD and correlation with donor and recipient combined age score.
In conclusion, our data suggest, high CD3+ T-cell dose in peripheral blood grafts is associated with lower risk of relapse and might improve long-term survival outcomes, but has no influence on the risk of developing aGvHD or cGvHD in T-cell-replete mismatched peripheral blood hematopoietic cell transplantation. Our results need validation in the context of prospective study to determine whether the impact of CD3+ T-cell dose and the T-cell subsets (CD4+, CD8+, Tregs, and naive T cells) of the peripheral blood allografts have a meaningful influence on transplantation outcomes especially in haploidentical allo-HCTs, using PTcy.
Acknowledgments
We acknowledge patients and their families, and clinical care providers.
Financial Disclosure
No funding pertaining to this study was received.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
Informed consent has been granted an exemption by Institutional Review Board committee (IRB), King Hussein Cancer Center.
Author Contributions
Khalid Halahleh: designed and performed the study, and wrote the first draft and final manuscript; Rawan Mustafa: helped in study concept and design of the study and acquisition of data, writing the abstract; Dalia Al Rimawi helped in data analysis and interpretation of results; Dania Sarhan: acquisition of data and results interpretation; Hadeel Abdelkhaleq: data acquisition and data clearance, Isra Muradi: acquisition of data and interpretation of results; Iyad Sultan: data analysis, interpretation of results. All authors approved the final typescript and agreed to submit for publication.
Data Availability
Data are available at https://doi.org/10.1016/S2152-2650(22)01646-9; https://doi.org/10.3892/mco.2020.2027; https://doi.org/10.1016/j.bbmt.2019.05.007.
Abbreviations
- allo-HCT
allogeneic hematopoietic cell transplantation
- MRD
matched related donor
- MMRD
mismatched related donor
- MUD
matched unrelated donor
- MSD
matched sibling donor
- HLA
human leukocyte antigen
- GvHD
graft-versus-host disease
- aGvHD
acute graft-versus-host disease
- cGvHD
chronic graft-versus-host disease
- GVT
graft-versus-tumor
- DVL
graft-versus-leukemia
- CD3+ T cells
CD3-positive T cells
- CD34+
CD34-positive
- Tregs
regulatory T cells
- NK
natural killer
- PBSCs
peripheral blood stem cells
- BM
bone marrow
- RFS
relapse-free survival
- OS
overall survival
- CIR
cumulative incidence of relapse
- KHCC
King Hussein Cancer Center
- TCD
T-cell-depleted
- BSA
body surface area
- Flu/TBI
fludarabine/total body irradiation
- FluCyTBI
fludarabine/cyclophosphamide/total body irradiation
- BU/CY
busulfan/cyclophosphamide
- Flu/Bu
fludarabine/busulfan
- CSA
cyclosporine A
- PTcy
post-transplant cyclophosphamide
- ATG
anti-thymocyte globulin
- G-CSF
granulocyte colony-stimulating factor
- HSCT-CI
hematopoietic stem cell comorbidity index
- CMV
cytomegalovirus
- ABO
ABO blood group
- ROC
receiver operating characteristic
- IRB
Institutional Review Board
- NIH
National Institutes of Health
- KPS
Karnofsky performance status
- CR
complete remission
- AML
acute myeloid leukemia
- MDS
myelodysplastic syndrome
- ALL
acute lymphoblastic leukemia
- R
recipient
- D
donor
- M
male
- F
female
- NRM
non-relapsed mortality
- TRM
transplant-related mortality
- EBMT
European Society for Blood and Marrow Transplantation
References
- 1.Gu G, Yang JZ, Sun LX. Correlation of graft immune composition with outcomes after allogeneic stem cell transplantation: Moving towards a perfect transplant. Cell Immunol. 2018;323:1–8. doi: 10.1016/j.cellimm.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Kolb HJ, Schattenberg A, Goldman JM, Hertenstein B, Jacobsen N, Arcese W, Ljungman P. et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood. 1995;86(5):2041–2050. doi: 10.1182/blood.V86.5.2041.bloodjournal8652041. [DOI] [PubMed] [Google Scholar]
- 3.Boyiadzis M, Arora M, Klein JP, Hassebroek A, Hemmer M, Urbano-Ispizua A, Antin JH. et al. Impact of chronic graft-versus-host disease on late relapse and survival on 7,489 patients after myeloablative allogeneic hematopoietic cell transplantation for leukemia. Clin Cancer Res. 2015;21(9):2020–2028. doi: 10.1158/1078-0432.CCR-14-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Storb R, Gyurkocza B, Storer BE, Sorror ML, Blume K, Niederwieser D, Chauncey TR. et al. Graft-versus-host disease and graft-versus-tumor effects after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2013;31(12):1530–1538. doi: 10.1200/JCO.2012.45.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coghill JM, Sarantopoulos S, Moran TP, Murphy WJ, Blazar BR, Serody JS. Effector CD4+ T cells, the cytokines they generate, and GVHD: something old and something new. Blood. 2011;117(12):3268–3276. doi: 10.1182/blood-2010-12-290403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soiffer RJ, Lerademacher J, Ho V, Kan F, Artz A, Champlin RE, Devine S. et al. Impact of immune modulation with anti-T-cell antibodies on the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Blood. 2011;117(25):6963–6970. doi: 10.1182/blood-2011-01-332007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anasetti C, Logan BR, Lee SJ, Waller EK, Weisdorf DJ, Wingard JR, Cutler CS. et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med. 2012;367(16):1487–1496. doi: 10.1056/NEJMoa1203517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bensinger WI, Martin PJ, Storer B, Clift R, Forman SJ, Negrin R, Kashyap A. et al. Transplantation of bone marrow as compared with peripheral-blood cells from HLA-identical relatives in patients with hematologic cancers. N Engl J Med. 2001;344(3):175–181. doi: 10.1056/NEJM200101183440303. [DOI] [PubMed] [Google Scholar]
- 9.Reshef R, Huffman AP, Gao A, Luskin MR, Frey NV, Gill SI, Hexner EO. et al. High Graft CD8 Cell Dose Predicts Improved Survival and Enables Better Donor Selection in Allogeneic Stem-Cell Transplantation With Reduced-Intensity Conditioning. J Clin Oncol. 2015;33(21):2392–2398. doi: 10.1200/JCO.2014.60.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pastore D, Delia M, Mestice A, Carluccio P, Perrone T, Gaudio F, Curci P. et al. CD3+/Tregs ratio in donor grafts is linked to acute graft-versus-host disease and immunologic recovery after allogeneic peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2012;18(6):887–893. doi: 10.1016/j.bbmt.2011.10.039. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura R, Bahceci E, Read EJ, Leitman SF, Carter CS, Childs R, Dunbar CE. et al. Transplant dose of CD34(+) and CD3(+) cells predicts outcome in patients with haematological malignancies undergoing T cell-depleted peripheral blood stem cell transplants with delayed donor lymphocyte add-back. Br J Haematol. 2001;115(1):95–104. doi: 10.1046/j.1365-2141.2001.02983.x. [DOI] [PubMed] [Google Scholar]
- 12.Czerw T, Labopin M, Schmid C, Cornelissen JJ, Chevallier P, Blaise D, Kuball J. et al. High CD3+ and CD34+ peripheral blood stem cell grafts content is associated with increased risk of graft-versus-host disease without beneficial effect on disease control after reduced-intensity conditioning allogeneic transplantation from matched unrelated donors for acute myeloid leukemia - an analysis from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Oncotarget. 2016;7(19):27255–27266. doi: 10.18632/oncotarget.8463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saad A, Lamb L, Wang T, Hemmer MT, Spellman S, Couriel D, Alousi A. et al. Impact of T cell dose on outcome of T cell-replete HLA-matched allogeneic peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2019;25(9):1875–1883. doi: 10.1016/j.bbmt.2019.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalwak K, Porwolik J, Mielcarek M, Gorczynska E, Owoc-Lempach J, Ussowicz M, Dyla A. et al. Higher CD34(+) and CD3(+) cell doses in the graft promote long-term survival, and have no impact on the incidence of severe acute or chronic graft-versus-host disease after in vivo T cell-depleted unrelated donor hematopoietic stem cell transplantation in children. Biol Blood Marrow Transplant. 2010;16(10):1388–1401. doi: 10.1016/j.bbmt.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Urbano-Ispizua A, Rozman C, Pimentel P, Solano C, de la Rubia J, Brunet S, Perez-Oteiza J. et al. The number of donor CD3(+) cells is the most important factor for graft failure after allogeneic transplantation of CD34(+) selected cells from peripheral blood from HLA-identical siblings. Blood. 2001;97(2):383–387. doi: 10.1182/blood.V97.2.383. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Guo C, Sun C, Chen Y, Zhu H, Xi J, Zhang M. et al. High proportions of CD3(+) T cells in grafts delayed lymphocyte recovery and reduced overall survival in haploidentical peripheral blood stem cell transplantation. Mol Clin Oncol. 2020;12(6):574–580. doi: 10.3892/mco.2020.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mussetti A, De Philippis C, Carniti C, Bastos-Oreiro M, Gayoso J, Cieri N, Pennisi M. et al. CD3+ graft cell count influence on chronic GVHD in haploidentical allogeneic transplantation using post-transplant cyclophosphamide. Bone Marrow Transplant. 2018;53(12):1522–1531. doi: 10.1038/s41409-018-0183-8. [DOI] [PubMed] [Google Scholar]
- 18.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, Thomas ED. 1994 Consensus Conference on acute GVHD grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 19.Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, Palmer J. et al. National Institutes of Health Consensus Development Project on Criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21(3):389–401.e381. doi: 10.1016/j.bbmt.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, Storer B. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Han X, Shao Y. The ROC of Cox proportional hazards cure models with application in cancer studies. Lifetime Data Anal. 2021;27(2):195–215. doi: 10.1007/s10985-021-09516-6. [DOI] [PubMed] [Google Scholar]
- 22.Habibzadeh F, Habibzadeh P, Yadollahie M. On determining the most appropriate test cut-off value: the case of tests with continuous results. Biochem Med (Zagreb) 2016;26(3):297–307. doi: 10.11613/BM.2016.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loren AW, Bunin GR, Boudreau C, Champlin RE, Cnaan A, Horowitz MM, Loberiza FR. et al. Impact of donor and recipient sex and parity on outcomes of HLA-identical sibling allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2006;12(7):758–769. doi: 10.1016/j.bbmt.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 24.Kollman C, Howe CW, Anasetti C, Antin JH, Davies SM, Filipovich AH, Hegland J. et al. Donor characteristics as risk factors in recipients after transplantation of bone marrow from unrelated donors: the effect of donor age. Blood. 2001;98(7):2043–2051. doi: 10.1182/blood.V98.7.2043. [DOI] [PubMed] [Google Scholar]
- 25.DeZern AE, Franklin C, Tsai HL, Imus PH, Cooke KR, Varadhan R, Jones RJ. Relationship of donor age and relationship to outcomes of haploidentical transplantation with posttransplant cyclophosphamide. Blood Adv. 2021;5(5):1360–1368. doi: 10.1182/bloodadvances.2020003922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kasamon YL, Luznik L, Leffell MS, Kowalski J, Tsai HL, Bolanos-Meade J, Morris LE. et al. Nonmyeloablative HLA-haploidentical bone marrow transplantation with high-dose posttransplantation cyclophosphamide: effect of HLA disparity on outcome. Biol Blood Marrow Transplant. 2010;16(4):482–489. doi: 10.1016/j.bbmt.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCurdy SR, Zhang MJ, St Martin A, Al Malki MM, Bashey A, Gaballa S, Keesler DA. et al. Effect of donor characteristics on haploidentical transplantation with posttransplantation cyclophosphamide. Blood Adv. 2018;2(3):299–307. doi: 10.1182/bloodadvances.2017014829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bacigalupo A, Maria Raiola A, Dominietto A, Di Grazia C, Gualandi F, Lint MTV, Chiusolo P. et al. Graft versus host disease in unmanipulated haploidentical marrow transplantation with a modified post-transplant cyclophosphamide (PT-CY) regimen: an update on 425 patients. Bone Marrow Transplant. 2019;53(Suppl 2):708–712. doi: 10.1038/s41409-019-0594-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available at https://doi.org/10.1016/S2152-2650(22)01646-9; https://doi.org/10.3892/mco.2020.2027; https://doi.org/10.1016/j.bbmt.2019.05.007.