Abstract
Ovarian carcinoma (OC) is considered the deadliest gynecological malignancy. It is typically diagnosed in the advanced stages of the disease, with metastatic sites widely disseminated within the abdominal cavity. OC treatment is challenging due to the high rate of disease recurrence, which is further complicated by acquired chemoresistance caused by the reversion of the pathological variant. Therefore, more effective treatments are still being sought. Histologically, OC is classified into serous, mucinous, endometrioid, clear cell, and transitional cell carcinomas and malignant Brenner tumor. Recent clinicopathological and molecular biological studies demonstrated that these subtypes differ in histogenesis and anti-tumor agent sensitivity. In Japan, the incidence rates of the histological types of OC, namely, serous carcinoma, mucinous carcinoma, endometrioid carcinoma, and clear cell adenocarcinoma, are 39%, 12%, 16%, and 23%, respectively. Serous carcinoma is classified as high or low grade, with the former accounting for the overwhelming majority. In this study, the molecular pathological classification of OC has been described based on the characteristics of the two types of OC, types 1 and 2. Compared with Europe and the United States, Japan has a higher prevalence of type 1 OC and a lower prevalence of type 2 OC. The prevalence of each type of OC varies by race. It has been elucidated that the prevalence rate of each type of ovarian cancer in Asian countries is similar to that in Japan. Thus, OC is a heterogeneous disease. Furthermore, OC has been attributed to molecular biological mechanisms that vary among tissue subtypes. Therefore, it is necessary to conduct treatment based on accurate diagnoses of each tissue type and establish an optimal treatment strategy, and now is the transition period.
Keywords: Ovarian cancer, High-grade serous carcinoma, BRCA, Prevention, HBOC
Introduction
Ovarian carcinoma (OC) accounts for approximately 239,000 new cases and 152,000 deaths worldwide annually [1]. The highest rates were reported in Eastern and Central Europe (11.4 per 100,000 and 6.0 per 100,000, respectively). Although China has a low incidence of OC (4.1 per 100,000), its large population translated to an estimated 52,100 new cases and 22,500 related deaths in 2015 [2]. In the USA, 21,290 new cases and 14,180 related deaths were reported during the same year [3]. The advanced stage of OC is considered an important prognostic factor, and the prognosis of patients with stages III and IV is poor [3]. In addition, because the ovary is an internal organ of the pelvis, few subjective symptoms occur at an early stage even if a tumor develops. Due to the advanced-stage distribution of OC, approximately 40-50% of cases are classified as stage III or IV, which has a poor prognosis [1-5]. In short, the diagnosis of ovarian cancer is difficult because the ovaries are located deep in the body. In particular, high-grade serous ovarian cancer, classified as type 1, is a refractory malignant tumor that repeatedly recurs and metastasizes, although it is sensitive to the combination therapy of platinum agents and Taxol. Therefore, better treatment outcomes for advanced cases are important in the treatment of OC.
OC is a malignant tumor with a wide variety of histological types. Furthermore, the molecular mechanisms leading to its origin and carcinogenesis significantly differ for each histological type; thus, our understanding of OC is limited. The biological features, pathological characteristics, and clinical features of OC are strongly dependent on the tissue type. Because the biological properties of OC have not yet been elucidated, a treatment, particularly a radical treatment for advanced-stage cancer, has also not yet been established. Therefore, the development of new anti-tumor agent and identification of biomarkers (also called draggable variants) that enable early detection are warranted based on an understanding of the exact molecular biological and carcinogenic mechanisms of OC. In this study, we outline a new classification of OC and describe the mechanisms of carcinogenesis that have been elucidated to date.
Transition of the Classification of Ovarian Cancer
The ovaries have a number of anatomical and histological structures, and thus, a wider variety of tumors develop there than in other organs. Because ovarian tumors are classified by identifying the tissue from which the tumor originates, OC classification is very complex and diverse [6]. In the molecular pathological classification of ovarian tumors, the World Health Organization (WHO) classification emphasized that histogenesis is widely used. In Japan, the WHO classification and convertible tissue classification (ovarian tumor-handling regulations) are used. Based on the 2003 WHO classification, ovarian tumors were broadly divided into three groups, namely, superficial epithelial/stromal, sex cord stromal, and germ cell tumors [7], with incidence rates of 70%, 10%, and 20%, respectively. Recently, high-grade serous carcinoma (HGSC), which accounts for the majority of superficial epithelial/stromal tumors, was shown to be derived from fallopian tube epithelial cells [8]. Many molecular, pathological, and clinical findings have been accumulated on the development of ovarian tumors, and in 2020, the OC classification was revised as the 2020 WHO classification, which significantly changed the category of epithelial tumors. The conventional rules for handling ovarian tumors/cancer were revised to “Handling rules for ovarian tumors/cancer, fallopian tube cancer, and peritoneal cancer” in accordance with the 2020 WHO classification.
In the previous code, the definition of a superficial epithelial/stromal tumor was described as “a tumor originating from the ovarian superficial epithelium and composed of various proportions of interstitial components.” However, because some serous carcinomas (SCs) are derived from fallopian tube epithelial cells, the term “epithelial” only became “surface epithelial/interstitial,” and stromal tumors were classified as separate items. In the revised version of the code, SCs are classified as low or high grade [9], which are basically considered separate malignant tumors in terms of histogenesis (Table 1) [10-14]. Thus, over the last decade, OC classification has significantly changed. Based on the characteristics of both cancer cells and genetic abnormalities, Kurman et al [15, 16] proposed an oncogenic model in which OC was classified into types I and II (Table 1) [10-14].
Table 1. Classification, Origin, and Mechanism of Epithelial Ovarian Cancer.
| Tumor type | Serous |
Endometrioid | Clear cell | Mucinous | |
|---|---|---|---|---|---|
| High grade | Low grade | ||||
| Incidence | 34% | 5% | 16% | 23% | 12% |
| Tissue origin | Fallopian tube epithelium | Fallopian tube epithelium | Endometrial cells | Endometrial cells | Ovarian surface epithelium |
| Ovarian surface epithelium | Ovarian surface epithelium | Ovarian surface epithelium | Ovarian surface epithelium | Brenner tumor | |
| Precancerous lesion | STIC [10] | Borderline malignant tumor [11] | Atypical endometriosis [12] | Atypical endometriosis | Borderline malignant tumor [11] |
| Molecular biological abnormalities | p53 mutation | KRAS mutation | ERa high expression | HNF-1β high expression | KRAS mutation |
| BRCA1/2 mutation | BRAF mutation | PI3KCA mutation | PI3KCA mutation | HER2 high expression | |
| Chromosome instability | BRCA1/2 mutation | CTNNB1 mutation | PTEN mutation | ||
| ARID1A mutation | ARID1A mutation | ||||
| BRCA1/2 mutation | |||||
| Microsatellite instability | |||||
| Sensitivity to chemotherapy | High | Middle | High | Low | Low |
| HBOC [13, 14] | +++ | ++ | + | - | - |
| RRSO [13, 14] | Recommend | - | - | - | - |
| Type I/II | Type II | Type I | |||
| Incidence | Europe, USA > Asia | Europe, USA < Asia | |||
Borderline malignant tumors are abnormal cells that form in the tissue covering the ovary. They are not cancerous and are generally cured with surgery. Approximately 15 out of 100 ovarian tumors (15-20%) are borderline tumors [11]. They are also described as atypical proliferative tumors and were previously called tumors with low malignant potential [11]. They are different from ovarian cancer because they do not grow into the supportive tissue of the ovary (the stroma). They slowly grow and in a more controlled way than cancer cells. Borderline tumors generally affect women aged between 20 and 40 years. They are usually diagnosed at an early stage when the abnormal cells are still within the ovary. Ovarian atypical endometriosis is a premalignant lesion, and its potential to progress to endometriosis-associated ovarian cancer highlights its significance [12]. In BRCA1/2-associated hereditary breast and ovarian cancer (HBOC), female and male breast and ovarian cancers (including fallopian tube and primary peritoneal cancers) can occur [13]. To a lesser extent, the development of other cancers, such as prostate and pancreatic cancers, is allowed [13]. Melanoma primarily affects individuals with BRCA2 pathogenic variants. The risk of developing associated cancers depends on whether HBOC is caused by BRCA1/2 pathogenic variants. Pathogenic germline variants underlie up to 20% of ovarian carcinoma (OC) cases and are associated with varying degrees of OC risk [14]. For pathogenic mutations in high-penetrance genes, such as BRCA1/2, the role of risk-reducing bilateral salpingo-oophorectomy (RRSO) in cancer prevention is well established, thus improving the mortality rate [14]. STIC: serous tubal intraepithelial carcinoma; HNF-1β: hepatocyte nuclear factor-1 beta; HGSC: high-grade serous carcinoma; ER: estrogen receptor.
Type I OCs are classified into low-grade serous, low-grade endometrioid, mucinous, and clear cell carcinomas and malignant Brenner tumor. It is suggested that type I OCs behave indolently and appear to form part of a morphological and molecular continuum starting with cystadenoma/adenofibroma benign tumors that subsequently develop toward atypical proliferative or borderline tumors and then finally toward invasive tumors [16]. Tumors classified as type I include benign cystadenomas, borderline malignant tumors, or endometriosis as precancerous lesions, and tumors that develop cancer along the “adenoma-carcinoma sequence.” As a molecular biological feature, activating mutations, such as the abnormalities including the tumor suppressor genes (AT-rich interactive domain-containing protein 1A (ARID1A), beta-catenin-interacting protein 1 (CTNNB1), B-raf proto-oncogene (BRAF), human epidermal growth factor receptor 2 (HER2)/neu, Kirsten ras (KRAS) oncogene, phosphatidylinositol-4, 5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA), and phosphatase and tensin homolog (PTEN), as well as tumor protein 53 (TP53) mutations are observed at a high rate (Table 1) [10-14]. Tumors generally classified as type I exhibit relatively slow cell growth; furthermore, they are localized in the ovary and detected at an early stage at the time of diagnosis. However, because clear cell and mucinous carcinomas are difficult to detect at an early stage, their prognosis is currently worse than that of type I tumors [17].
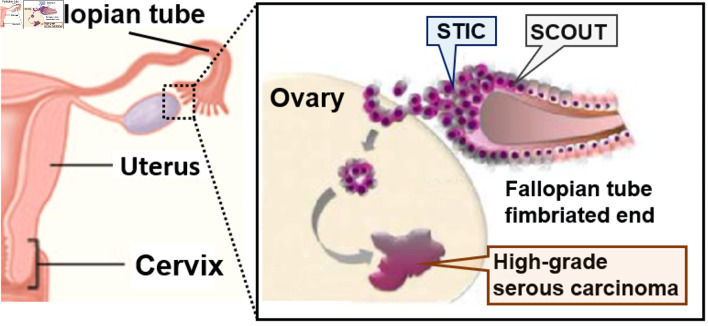
OCs classified as type II are the cause of death in most patients and include HGSC, high-grade endometrioid cancer, ovarian carcinosarcoma, and undifferentiated cancer. These cancers have strongly proliferating cells and a poor prognosis as they are detected in advanced stages. Tumors classified as type II are considered “de novo cancer” without precancerous lesions, and the origin of HGSC was shown to be secretory cell outgrowth, serous tubal intraepithelial neoplasia, and serous tubal intraepithelial carcinoma (STIC) (Fig. 1). Molecular biology is characterized by TP53 mutations in almost all cases, resulting in high rates of genomic and chromosomal instabilities during carcinogenesis. Several lines of evidence now indicate that these tumors may originate from the epithelium of the fimbrial portion of the fallopian tube and/or the ovarian surface epithelium [18-21]. Uterine serous carcinoma (USC) is an overlooked component of breast cancer susceptibility gene 1/2 (BRCA1/2)-associated hereditary breast and ovarian cancer (HBOC) syndrome. For patients diagnosed with USC, particularly those with a positive first-degree family history of HBOC syndrome, screening for germline BRCA1/2 pathogenic mutations needs to be considered [22]. Women with pathogenic variants in BRCA1/2 have a high lifetime risk of developing ovarian cancer [23-26]. To date, a reliable early detection method for ovarian cancer has not yet been established. Patients with advanced ovarian cancer have a poor prognosis. A meta-analysis of women with BRCA1/2 pathogenic variants revealed a 79% reduced risk of developing ovarian or fallopian tube cancer following risk-reducing salpingo-oophorectomy (RRSO) [27]. Therefore, RRSO has been performed to prevent the development of ovarian cancer in women with BRCA1/2 pathogenic variants.
Figure 1.
Fallopian tube hypothesis on the origin of high-grade serous carcinoma (HGSC). Fallopian tube epithelium cells of the fimbriated ends undergo initial neoplastic transformation and become serous tubal intraepithelial carcinoma (STIC). STIC cells are resistant to anoikis, which favors settlement and ovarian surface invasion. The ovarian microenvironment, rich in hormonal and inflammatory factors, drives the full neoplastic transformation to invasive high-grade serous carcinoma (HGSC). SCOUT: secretory cell out-growth.
Origin of Ovarian Cancer and Molecular Biological Abnormalities
In the development process of embryonic ovaries, researchers may be able to establish why OC has different tissue types. In the fetal period, the body cavity is covered by mesothelial cells, and certain tissues within it form the serosal epithelium, which becomes the ovarian superficial epithelium. Similarly, mesothelial cells give rise to the Mullerian tube (medium pararenal tube), from which the oviduct, uterus, and vagina develop. In other words, the concept (secondary Mullerian system) that the origin of the ovarian superficial epithelium and that of Mullerian duct-derived organs are the same has been proposed [28]. Thus, the origin of OC was considered a superficial epithelial inclusion cyst formed when the ovarian surface epithelium invades the ovarian stroma from the wound that occurred during ovulation. Encapsulated cyst cells that have undergone cellular stress accumulate gene mutations and eventually metamorphose into tissue types derived from the Mullerian tube, such as the fallopian tube epithelium, endometrium, and cervical glands, of the same developmental origin. Epithelial OC with various tissue types may develop. During pregnancy, SC is similar to the fallopian tube epithelium, mucinous carcinoma to the cervical mucosa, endometrioid carcinoma to the endometrial gland, and clear cell carcinoma to the endometrial gland. Thus, OC was considered to originate from the ovary itself. However, recent studies demonstrated that some OCs may originate from tissues other than the ovary, such as ovarian endometriosis or tubal fistulas that make up the fallopian tube. A paradigm-shifting theory that the onset of OC is secondary to the ovary has been proposed [16, 29].
Despite the advancements of debulking surgery with additional platinum-based chemotherapy, the outcome of OC remains poor, with a 5-year survival rate of less than 35%; thus, more effective treatments are still being investigated [30]. OC is histologically classified into serous, mucinous, endometrioid, clear cell, and transitional cell carcinomas and malignant Brenner tumor. Recent clinicopathological and molecular biological studies demonstrated that these subtypes differ in histogenesis and anticancer drug sensitivity. In Japan [30], the incidence rates of the histological types of OC, namely, SC, mucinous carcinoma, endometrioid carcinoma, and clear cell adenocarcinoma, are 39%, 12%, 16%, and 23%, respectively (Table 1) [10-14].
Serous carcinoma
Serous carcinoma is classified as high or low grade, with the former accounting for the overwhelming majority. The most common histological type of OC is HGSC, and many advanced OCs with clinicopathological features exist. HGSC reflects the classic clinical picture of OC that is highly sensitive to anticancer drugs. More than 90% of cases have TP53 mutations, of which approximately 50% are considered to originate from the fallopian tube [31]. On the other hand, low-grade SC is considered to occur in the background of serous borderline malignant tumors; however, there are not many advanced cases, and the proliferative activity and anticancer drug sensitivity are low. Many cases of low-grade SC have KRAS and BRAF somatic mutations [32, 33]. Although the estrogen receptor is positive, the effectiveness of hormonal therapy has not been confirmed in clinical studies [32, 33].
Clear cell carcinoma
Ovarian clear cell carcinoma accounts for about half of stage I cases and has the following characteristics: related to endometriosis, difficult dissemination, local growth, and resistance to chemotherapy. Therefore, ovarian clear cell carcinoma is different from SC. In advanced cases, ovarian clear cell carcinoma has a poorer prognosis than SC. Many cases are caused by endometriosis and have ARID1A and PIK3CA somatic mutations [34]. The importance of endometriosis as the origin of ovarian clear cell carcinoma has been reported by many specialist journals. A large population-based study in Scandinavia demonstrated that the incidence of OC caused by endometriosis was 2.01-fold higher in women in their 20s and 1.76-fold higher in those in their 30s than in healthy women [35]. Furthermore, no significant increases were observed in the incidence of OC in women in their 40s or older.
Endometrioid carcinoma
Endometrioid carcinoma is often low grade and rarely advanced [36, 37]. It is associated with endometriosis and may have somatic mutations in PIK3CA in addition to ARID1A [36, 37]. Overexpression of hepatocyte nuclear factor-1 beta (HNF-1β) and somatic mutations in the ARID1A gene have also been observed in atypical endometriosis adjacent to the carcinoma [38, 39]. Also, the histogenesis of endometrioid carcinoma may be from endometriosis, which originates from HNF-1β-negative inclusion cyst cells.
Mucinous carcinoma
Because mucinous carcinoma often co-exists with intestinal mucinous borderline malignant tumors, it is regarded as a tumor originating from a benign mucinous tumor [40]. An internal cervical borderline malignant tumor rarely becomes cancerous. Mucinous carcinoma macroscopically forms multilocular cysts, and the tumor diameters are often greater than 10 cm. Regarding mucinous carcinoma, there are few advanced cases and bilateral tumors, and KRAS somatic mutations are frequently observed [41]. Primary transitional cell carcinoma of the ovary is classified as surface epithelial/interstitial tumor and is a relatively rare disease accounting for 0.38% of all OC cases [42]. In the primary transitional cell carcinoma of the ovary, imprint and ascites cytologies have been reported, but there has been no definite view of the cytology. It is suggested that the nature of transitional cell carcinoma is HGSC or poorly differentiated endometrioid carcinoma [43-45]. Thus, OC is a heterogeneous disease that occurs via different molecular biological mechanisms for different tissue subtypes [46]. Treatment must be administered with an accurate diagnosis for each tissue type, and an optimal treatment strategy needs to be developed.
Fallopian Tube Epithelium as the Origin of Serous Ovarian Cancer
OC is the fifth leading cause of cancer deaths in women. Generally, most patients are diagnosed at an advanced stage, with fewer than 30% surviving longer than 10 years. Some researchers have attributed the most common type of OC to the fallopian tube, a thin fibrous tube connecting the ovary to the uterus. Previous findings obtained from nine OC patients indicated that the genomic origin of many ovarian tumors was the fallopian tube [47, 48]. Previous studies provided insights into the origin of OC and the potential of novel interventions for the prevention and treatment of OC. Over the last few decades, no significant change has been observed in the treatment of OC. This may be because doctors and researchers are investigating the wrong focal tissue for OC. Therefore, if extensive research confirms that most OCs originate from the fallopian tube, the strategies employed to clinically manage OC in patients with a genetic risk of OC may significantly change in the future. In recent studies, clinical researchers at the Johns Hopkins University Kimmel Cancer Center and the Dana-Farber Cancer Center in Boston collected normal cells, OC, and distant metastases to elucidate the origin cells of OC. A small cancer tissue sample found in the fallopian tube was also collected. Small cancers in the fallopian tube included a single cell layer cancer called the “TP53 signature” and SC in situ of the fallopian tube, i.e., STIC lesion. Tissue samples were collected from five patients with high-grade serous OC. This type of cancer accounts for 75% of an estimated 22,000 cases of OC diagnosed each year in the United States.
Researchers removed serous fallopian tube carcinoma and normal tissue from patients with a pathological variant of the BRCA1/2 genes associated with OC and breast cancer and from four patients who underwent prophylactic resection of the ovary and fallopian tube due to a pelvic mass. Cells containing mutant TP53 genes that were previously shown to be associated with a number of cancer types have been highlighted by clinical researchers. They then used an infrared laser to remove the cancer cells highlighted by staining. For all known genes in all cancer tissues, whole-exome sequencing was performed, and a blueprint for the gene encoding the protein was created. Without this approach, the findings of genome sequencing will be buried in genetic information from normal cells, and detecting cancer-related DNA errors will be difficult. The Johns Hopkins and Dana-Farber teams subsequently searched for DNA sequence errors, such as regions that switch to another DNA or changes in a large region of a particular chromosome. The findings from genome-wide approach studies indicated that the region of chromosome 17, where the cancer-related TP53 gene is located, was completely deleted in tissues containing early serous intratubal carcinoma lesions obtained from nine patients [49]. Thus, the “misprint” of or a defect in the TP53 gene is an early stage of OC development. Furthermore, all nine patients had a deletion in part of the chromosome containing the BRCA1 or BRCA2 gene or both. These genes have been linked to hereditary and sporadic breast cancers and OC, i.e., HBOC syndrome. Four of the patients lacked chromosome 10, which contains another cancer-related gene called PTEN. The findings of these genomic studies indicated that some cancer cells prone to mutations were estimated. Origin cancer cells may have fewer germline and/or somatic mutations than successor cells. Therefore, a phylogenetic tree was created for OC in five women. The findings indicated that each serous OC was caused by a serous fallopian tube intraepithelial carcinoma lesion in the fallopian tube or a mistake in early lesions. Additional DNA misses were detected in cancer cells that remained in the ovaries, near the fallopian tubes, and in metastatic sites [50]. Overall, these studies demonstrated that early tumors of serous intratubal carcinoma in the fallopian tube already have important DNA changes necessary for OC development. These findings suggest that serous fallopian tube carcinoma migrated to the ovary and carcinoma progressed in the ovary [47, 48, 50] (Fig. 1).
To determine the time for OC progression, clinical researchers used multiple statistical models, considering patient age at diagnosis and the total number of mutations in cancer cells. The findings of these studies indicated that serous intratubal carcinoma lesions progressed to OC within an average of 6.5 years in the analyzed patients [51]. When the serous intratubal carcinoma reached the ovaries, progression to metastatic disease was estimated to rapidly occur within an average of 2 years [51]. The recent findings are consistent with the data obtained in clinical practice. In many patients newly diagnosed with OC, the carcinoma cells have already spread within the pelvis. The clinical practice for OC management will remain unchanged until further studies verify that serous tubal carcinoma in situ is the origin of a new serous OC. If the findings of recent studies are validated, the loss of female hormones that increase the risk of heart and other diseases caused by ovariectomy may be avoided in some women. Because the development of metastatic disease from serous tubal carcinoma in situ takes 2 years, it is important to develop new screening strategies, such as liquid biopsy (testing with body fluids, such as blood), to detect serous OC early and easily [51]. In clinical practice, bilateral salpingectomy is performed to prevent the development of ovarian cancer in women with hereditary BRCA1 and BRCA2 pathogenic variants that cause serous cancer [14].
Difficulty of Preoperative Diagnosis at an Early Stage for HGSC
Clinical approaches elucidated two possible pathways of OC development, namely, adenoma-carcinoma sequence and de novo carcinogenesis [52]. Although half of OCs might suddenly develop from a normal-appearing ovary, previous clinical studies suggested that the remaining half develops secondarily from preexisting, benign-appearing cysts, endometriotic cysts, and STIC. Ovarian SC is an intra-ovarian metastasis of a primary malignant tumor, an STIC that does not clearly form a dominant tubal mass like other solid cancers. When STIC reaches the ovaries, progression to metastatic disease is estimated to rapidly occur within an average of 2 years [48, 51].
It has been reported that transvaginal and/or abdominal ultrasound examinations in conjunction with either color Doppler or power Doppler imaging increase the accuracy of diagnosis as malignant tumors exhibit characteristic changes in vascularity [53]. Furthermore, a three-dimensional ultrasound could be useful in the evaluation of benign and malignant ovarian lesions. However, STIC does not clearly form visualization mass; thus, STIC could not be easily detected at an early stage via transvaginal and/or abdominal ultrasound examination [54]. As a result, several patients with normal screening results were found to develop stage III HGSC; thus, it can be concluded that ultrasound screening may decrease the stage at detection but may not be effective in detecting HGSC cases at an early stage. Therefore, it should be kept in mind that SC or other histological types that appear to develop from normal-appearing ovary cannot be detected even by careful examination via transvaginal and/or abdominal ultrasound. This is a very serious point for gynecologist to explain to the patients who have developed OC, especially HGSC, at advanced stages during follow-up or clinical treatment.
Ethnicity Specifics in the Incidence of Ovarian Cancer
In the United States, approximately 21,000 individuals are diagnosed with OC and 14,000 die from the disease annually. According to the American Cancer Society, the diagnosis rate has slowly decreased over the past 20 years. Although OC may develop at any time in a woman’s life, it is rare among those younger than 40 years. According to the American Cancer Society in Atlanta, GA, 50% of all OCs occur in individuals aged 63 years and older. The diagnosis of and death from OC vary depending on race and ethnicity. Between 1999 and 2014, Caucasians were more likely to be diagnosed with or die from OC than those from any other ethnic groups, followed by black individuals, Asian Americans, Hispanics, Pacific Islanders, and then American Indian or Alaska Natives. OC is rare in women younger than 40 years. The latest data from the National Cancer Institute indicated that the percentage of new cases was 4% in those between the ages of 20 and 34 years. The percentage of OC-related deaths in the same age group was less than 1%. However, certain germline pathogenic variants, such as BRCA1 and BRCA2, significantly increase the risk of OC and breast cancer [55, 56]. These germline mutations may be inherited from a mother or a father. Women with an Ashkenazi Jewish or Eastern European genetic background also have a higher risk of these germline mutations (Fig. 1). The prevalence of mutations varies among ethnic groups and may be influenced by founder mutations.
Penetrance may be influenced by pathogenic variant-specific phenotypes and the potential modifying effects of genetic and environmental background in kindreds. The estimates of both germline mutation prevalence and mutation penetrance rates are inconsistent and occasionally controversial; thus, a clearer understanding is crucial for providing accurate risk information to each patient. The ability to identify germline BRCA1/2 pathogenic variants was found to be important in the gynecological management of HGSC. Based on implications for clinical care and advances in cancer prevention, the identification of racial differences in genetic or lifestyle factors, which may modify the risk of cancer due to germline BRCA1/2 pathogenic variants, is of high priority for future research. Clinicians who are interested in providing personalized cancer risk counseling for patients need to understand the potential modifying factors that are particular to a patient’s ethnicity, race, family history, and environmental influences.
Conclusions
Throughout many years of basic and clinical research on OC, as its carcinogenic mechanism and origin gradually become clear, a new classification of ovarian tumors will be identified based on new findings. However, the precise carcinogenic mechanisms that correspond to different OC histotypes are still unclear; therefore, the therapeutic outcome, especially in HGSC, remains unsatisfactory. An effective method for the early detection of HGSC has not yet been established. Therefore, RRSO for women with BRCA1/2 pathogenic variants is the only preventive measure against HGSC development. In the future, to establish a novel treatment for OC, in line with knowledge on the origin and mechanism of OC obtained from new pathological and molecular biological studies, it will be necessary to make an accurate and detailed classification of OC. Furthermore, appropriate clinical treatment for each tissue type and patient background is expected to ultimately contribute to the improvement of OC prognosis.
Acknowledgments
The authors thank Dr. Kaoru Abiko for critical reading of the manuscript and valuable intellectual discussions.
Financial Disclosure
This work was supported by the following agencies: Japan Society for the Promotion of Science (JSPS) for TH (grant no. 19K09840), START-program of Japan Science and Technology Agency (JST) for TH (grant no. STSC20001), National Hospital Organization multicenter clinical study for TH (grant no. 2019-Cancer in general-02), and Japan Agency for Medical Research and Development (AMED) (grant no. 22ym0126802j0001).
Conflict of Interest
The authors declare that they do not have any conflict of interest in undertaking this review. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author Contributions
All authors had full access to the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. Conceptualization: TH and IK. Writing-original draft: TH and IK. Writing-review and editing: IK. Visualization: TH and IK. Supervision: TH and IK. Funding acquisition: TH and IK.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
- OC
ovarian carcinoma
- WHO
World Health Organization
- HGSC
high-grade serous carcinoma
- ARID1A
AT-rich interactive domain-containing protein 1A
- CTNNB1
beta-catenin-interacting protein 1
- BRAF
B-raf protooncogene
- HER2
human epidermal growth factor receptor 2
- KRAS
Kirsten ras
- PIK3CA
phosphatidylinositol-4, 5-bisphosphate 3-kinase catalytic subunit alpha
- PTEN
phosphatase and tensin homolog
- TP53
tumor protein 53
- SCOUT
secretory cell out-growth
- STIN
serous tubal intraepithelial neoplasia
- STIC
serous tubal intraepithelial carcinoma
- USC
uterine serous carcinoma
- BRCA1/2
breast cancer susceptibility gene 1/2
- HBOC
hereditary breast and ovarian cancer
- RRSO
risk-reducing salpingo-oophorectomy
- HNF-1β
hepatocyte nuclear factor-1 beta
- NCI
National Cancer Institute
References
- 1.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Lyon, France: International Agency for Research on Cancer. 2013 [2016-09-09]. http://globocan.iarc.fr.
- 2.Yang R, Su YD, Ma R, Li Y. Clinical epidemiology of peritoneal metastases in China: The construction of professional peritoneal metastases treatment centers based on the prevalence rate. Eur J Surg Oncol. 2023;49(1):173–178. doi: 10.1016/j.ejso.2022.08.023. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 4.Kehoe S, Bhatla N. FIGO Cancer Report 2021. Int J Gynaecol Obstet. 2021;155(Suppl 1):5–6. doi: 10.1002/ijgo.13882. [DOI] [PubMed] [Google Scholar]
- 5.Koshiyama M, Matsumura N, Konishi I. Subtypes of ovarian cancer and ovarian cancer screening. Diagnostics (Basel) 2017;7(1):12. doi: 10.3390/diagnostics7010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 7.Cress RD, Chen YS, Morris CR, Petersen M, Leiserowitz GS. Characteristics of long-term survivors of epithelial ovarian cancer. Obstet Gynecol. 2015;126(3):491–497. doi: 10.1097/AOG.0000000000000981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee Y, Miron A, Drapkin R, Nucci MR, Medeiros F, Saleemuddin A, Garber J. et al. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J Pathol. 2007;211(1):26–35. doi: 10.1002/path.2091. [DOI] [PubMed] [Google Scholar]
- 9.Koshiyama M, Matsumura N, Konishi I. Recent concepts of ovarian carcinogenesis: type I and type II. Biomed Res Int. 2014;2014:934261. doi: 10.1155/2014/934261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurman RJ. Origin and molecular pathogenesis of ovarian high-grade serous carcinoma. Ann Oncol. 2013;24(Suppl 10):x16–21. doi: 10.1093/annonc/mdt463. [DOI] [PubMed] [Google Scholar]
- 11.Kurman RJ, Shih Ie M. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer—shifting the paradigm. Hum Pathol. 2011;42(7):918–931. doi: 10.1016/j.humpath.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cobb LP, Gaillard S, Wang Y, Shih Ie M, Secord AA. Adenocarcinoma of Mullerian origin: review of pathogenesis, molecular biology, and emerging treatment paradigms. Gynecol Oncol Res Pract. 2015;2:1. doi: 10.1186/s40661-015-0008-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bogaerts JMA, Steenbeek MP, van Bommel MHD, Bulten J, van der Laak J, de Hullu JA, Simons M. Recommendations for diagnosing STIC: a systematic review and meta-analysis. Virchows Arch. 2022;480(4):725–737. doi: 10.1007/s00428-021-03244-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laban M, Chen X, Guo B. Seromucinous and mucinous borderline ovarian tumors: we need to know more. Reprod Sci. 2022 doi: 10.1007/s43032-022-01143-2. [DOI] [PubMed] [Google Scholar]
- 15.Kim JM, Hong DG. Is ovarian cystectomy for atypical ovarian endometrioma safe? A single center study. Medicine (Baltimore) 2022;101(35):e30105. doi: 10.1097/MD.0000000000030272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petrucelli N, Daly MB, Pal T. In: BRCA1- and BRCA2-associated hereditary breast and ovarian cancer. Adam MP, Everman DB, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, et al., editors. GeneReviews((R)); Seattle (WA): 1993. [PubMed] [Google Scholar]
- 17.Hartmann LC, Lindor NM. The Role of Risk-Reducing Surgery in Hereditary Breast and Ovarian Cancer. N Engl J Med. 2016;374(5):454–468. doi: 10.1056/NEJMra1503523. [DOI] [PubMed] [Google Scholar]
- 18.Linz VC, Lowe A, van der Ven J, Hasenburg A, Battista MJ. Incidence of pelvic high-grade serous carcinoma after isolated STIC diagnosis: A systematic review of the literature. Front Oncol. 2022;12:951292. doi: 10.3389/fonc.2022.951292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsu CF, Seenan V, Wang LY, Chu TY. Ovulation Enhances Intraperitoneal and Ovarian Seedings of High-Grade Serous Carcinoma Cells Originating from the Fallopian Tube: Confirmation in a Bursa-Free Mouse Xenograft Model. Int J Mol Sci. 2022;23(11):6211. doi: 10.3390/ijms23116211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sisovska I, Minar L, Felsinger M, Anton M, Bednarikova M, Hausnerova J, Jandakova E. et al. [Current FIGO staging classification for cancer of ovary, fallopian tube and peritoneum] Ceska Gynekol. 2017;82(3):230–236. [PubMed] [Google Scholar]
- 21.Przybycin CG, Kurman RJ, Ronnett BM, Shih Ie M, Vang R. Are all pelvic (nonuterine) serous carcinomas of tubal origin? Am J Surg Pathol. 2010;34(10):1407–1416. doi: 10.1097/PAS.0b013e3181ef7b16. [DOI] [PubMed] [Google Scholar]
- 22.de Jonge MM, Mooyaart AL, Vreeswijk MP, de Kroon CD, van Wezel T, van Asperen CJ, Smit VT. et al. Linking uterine serous carcinoma to BRCA1/2-associated cancer syndrome: A meta-analysis and case report. Eur J Cancer. 2017;72:215–225. doi: 10.1016/j.ejca.2016.11.028. [DOI] [PubMed] [Google Scholar]
- 23.Kauff ND, Satagopan JM, Robson ME, Scheuer L, Hensley M, Hudis CA, Ellis NA. et al. Risk-reducing salpingo-oophorectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med. 2002;346(21):1609–1615. doi: 10.1056/NEJMoa020119. [DOI] [PubMed] [Google Scholar]
- 24.Rebbeck TR, Lynch HT, Neuhausen SL, Narod SA, Van't Veer L, Garber JE, Evans G. et al. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med. 2002;346(21):1616–1622. doi: 10.1056/NEJMoa012158. [DOI] [PubMed] [Google Scholar]
- 25.Finch A, Beiner M, Lubinski J, Lynch HT, Moller P, Rosen B, Murphy J. et al. Salpingo-oophorectomy and the risk of ovarian, fallopian tube, and peritoneal cancers in women with a BRCA1 or BRCA2 Mutation. JAMA. 2006;296(2):185–192. doi: 10.1001/jama.296.2.185. [DOI] [PubMed] [Google Scholar]
- 26.Domchek SM, Friebel TM, Neuhausen SL, Wagner T, Evans G, Isaacs C, Garber JE. et al. Mortality after bilateral salpingo-oophorectomy in BRCA1 and BRCA2 mutation carriers: a prospective cohort study. Lancet Oncol. 2006;7(3):223–229. doi: 10.1016/S1470-2045(06)70585-X. [DOI] [PubMed] [Google Scholar]
- 27.Rebbeck TR, Kauff ND, Domchek SM. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst. 2009;101(2):80–87. doi: 10.1093/jnci/djn442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lauchlan SC. The secondary mullerian system revisited. Int J Gynecol Pathol. 1994;13(1):73–79. doi: 10.1097/00004347-199401000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Kurman RJ, Shih Ie M. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34(3):433–443. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gurung A, Hung T, Morin J, Gilks CB. Molecular abnormalities in ovarian carcinoma: clinical, morphological and therapeutic correlates. Histopathology. 2013;62(1):59–70. doi: 10.1111/his.12033. [DOI] [PubMed] [Google Scholar]
- 31.Ghezelayagh TS, Pennington KP, Norquist BM, Khasnavis N, Radke MR, Kilgore MR, Garcia RL. et al. Characterizing TP53 mutations in ovarian carcinomas with and without concurrent BRCA1 or BRCA2 mutations. Gynecol Oncol. 2021;160(3):786–792. doi: 10.1016/j.ygyno.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gershenson DM, Sun CC, Iyer RB, Malpica AL, Kavanagh JJ, Bodurka DC, Schmeler K. et al. Hormonal therapy for recurrent low-grade serous carcinoma of the ovary or peritoneum. Gynecol Oncol. 2012;125(3):661–666. doi: 10.1016/j.ygyno.2012.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaldawy A, Segev Y, Lavie O, Auslender R, Sopik V, Narod SA. Low-grade serous ovarian cancer: A review. Gynecol Oncol. 2016;143(2):433–438. doi: 10.1016/j.ygyno.2016.08.320. [DOI] [PubMed] [Google Scholar]
- 34.Su YF, Tsai EM, Chen CC, Wu CC, Er TK. Targeted sequencing of a specific gene panel detects a high frequency of ARID1A and PIK3CA mutations in ovarian clear cell carcinoma. Clin Chim Acta. 2019;494:1–7. doi: 10.1016/j.cca.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Melin A, Sparen P, Persson I, Bergqvist A. Endometriosis and the risk of cancer with special emphasis on ovarian cancer. Hum Reprod. 2006;21(5):1237–1242. doi: 10.1093/humrep/dei462. [DOI] [PubMed] [Google Scholar]
- 36.Taha AAA, Koshiyama M, Matsumura N, Abiko K, Yamaguchi K, Hamanishi J, Baba T. et al. The effect of the type of dietary protein on the development of ovarian cancer. Oncotarget. 2018;9(35):23987–23999. doi: 10.18632/oncotarget.25253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Llobet D, Pallares J, Yeramian A, Santacana M, Eritja N, Velasco A, Dolcet X. et al. Molecular pathology of endometrial carcinoma: practical aspects from the diagnostic and therapeutic viewpoints. J Clin Pathol. 2009;62(9):777–785. doi: 10.1136/jcp.2008.056101. [DOI] [PubMed] [Google Scholar]
- 38.Nemejcova K, Ticha I, Kleiblova P, Bartu M, Cibula D, Jirsova K, Dundr P. Expression, Epigenetic and Genetic Changes of HNF1B in Endometrial Lesions. Pathol Oncol Res. 2016;22(3):523–530. doi: 10.1007/s12253-015-0037-2. [DOI] [PubMed] [Google Scholar]
- 39.Wiegand KC, Shah SP, Al-Agha OM, Zhao Y, Tse K, Zeng T, Senz J. et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med. 2010;363(16):1532–1543. doi: 10.1056/NEJMoa1008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheasley D, Wakefield MJ, Ryland GL, Allan PE, Alsop K, Amarasinghe KC, Ananda S. et al. The molecular origin and taxonomy of mucinous ovarian carcinoma. Nat Commun. 2019;10(1):3935. doi: 10.1038/s41467-019-11862-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Panyavaranant P, Teerapakpinyo C, Pohthipornthawat N, Oranratanaphan S, Shuangshoti S, Triratanachat S. RAS mutation in mucinous carcinoma of the ovary. Asian Pac J Cancer Prev. 2019;20(4):1127–1132. doi: 10.31557/APJCP.2019.20.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ordonez NG. Transitional cell carcinomas of the ovary and bladder are immunophenotypically different. Histopathology. 2000;36(5):433–438. doi: 10.1046/j.1365-2559.2000.00844.x. [DOI] [PubMed] [Google Scholar]
- 43.Magrill J, Karnezis AN, Tessier-Cloutier B, Talhouk A, Kommoss S, Cochrane D, Chow C. et al. Tubo-ovarian transitional cell carcinoma and high-grade serous carcinoma show subtly different immunohistochemistry profiles. Int J Gynecol Pathol. 2019;38(6):552–561. doi: 10.1097/PGP.0000000000000538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marwah N, Mathur SK, Marwah S, Singh S, Karwasra RK, Arora B. Malignant Brenner tumour—a case report. Indian J Pathol Microbiol. 2005;48(2):251–252. [PubMed] [Google Scholar]
- 45.Green GE, Mortele KJ, Glickman JN, Benson CB. Brenner tumors of the ovary: sonographic and computed tomographic imaging features. J Ultrasound Med. 2006;25(10):1245–1251. doi: 10.7863/jum.2006.25.10.1245. quiz 1252-1244. [DOI] [PubMed] [Google Scholar]
- 46.Quan J, Jin L, Hu J, He T, Pan X, Ding Y, Peng J. et al. Brenner tumor of the testis: A case report and review of the literature. Mol Clin Oncol. 2017;6(1):119–121. doi: 10.3892/mco.2016.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Labidi-Galy SI, Papp E, Hallberg D, Niknafs N, Adleff V, Noe M, Bhattacharya R. et al. High grade serous ovarian carcinomas originate in the fallopian tube. Nat Commun. 2017;8(1):1093. doi: 10.1038/s41467-017-00962-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Konishi I, Abiko K, Hayashi T, Yamanoi K, Murakami R, Yamaguchi K, Hamanishi J. et al. Peritoneal dissemination of high-grade serous ovarian cancer: pivotal roles of chromosomal instability and epigenetic dynamics. J Gynecol Oncol. 2022;33(5):e83. doi: 10.3802/jgo.2022.33.e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quinn MC, Wojnarowicz PM, Pickett A, Provencher DM, Mes-Masson AM, Davis EC, Tonin PN. FKBP10/FKBP65 expression in high-grade ovarian serous carcinoma and its association with patient outcome. Int J Oncol. 2013;42(3):912–920. doi: 10.3892/ijo.2013.1797. [DOI] [PubMed] [Google Scholar]
- 50.McCluggage WG. Progress in the pathological arena of gynecological cancers. Int J Gynaecol Obstet. 2021;155(Suppl 1):107–114. doi: 10.1002/ijgo.13871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McGee J, Panabaker K, Leonard S, Ainsworth P, Elit L, Shariff SZ. Genetics consultation rates following a diagnosis of high-grade serous ovarian carcinoma in the Canadian province of Ontario. Int J Gynecol Cancer. 2017;27(3):437–443. doi: 10.1097/IGC.0000000000000907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nishio N, Kido A, Kataoka M, Kuwahara R, Nakao K, Kurata Y, Matsumura N. et al. Longitudinal changes in magnetic resonance imaging of malignant and borderline tumors associated with ovarian endometriotic cyst comparing with endometriotic cysts without arising malignancy. Eur J Radiol. 2018;105:175–181. doi: 10.1016/j.ejrad.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 53.Guerriero S, Alcazar JL, Ajossa S, Lai MP, Errasti T, Mallarini G, Melis GB. Comparison of conventional color Doppler imaging and power doppler imaging for the diagnosis of ovarian cancer: results of a European study. Gynecol Oncol. 2001;83(2):299–304. doi: 10.1006/gyno.2001.6377. [DOI] [PubMed] [Google Scholar]
- 54.Dubeau L, Drapkin R. Coming into focus: the nonovarian origins of ovarian cancer. Ann Oncol. 2013;24(Suppl 8):viii28–viii35. doi: 10.1093/annonc/mdt308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Madariaga A, Lheureux S, Oza AM. Tailoring ovarian cancer treatment: implications of BRCA1/2 mutations. Cancers (Basel) 2019;11(3):416. doi: 10.3390/cancers11030416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fackenthal JD, Olopade OI. Breast cancer risk associated with BRCA1 and BRCA2 in diverse populations. Nat Rev Cancer. 2007;7(12):937–948. doi: 10.1038/nrc2054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors declare that data supporting the findings of this study are available within the article.