Abstract
Background
Enterococci role in the microbiome remains controversial, and researches regarding enterococcal infection (EI) and its sequelae are limited. The gut microbiome has shown to play an important role in immunology and cancer. Recent data have suggested a relationship between the gut microbiome and breast cancer (BC).
Methods
Patients in a Health Insurance Portability and Accountability Act (HIPAA) compliant national database (2010 - 2020) were used for this retrospective study. International Classification of Disease (ICD) Ninth and Tenth Codes, Current Procedural Terminology (CPT), and National Drug Codes were used to identify BC diagnosis and EI. Patients were matched for age, sex, Charlson comorbidity index (CCI), antibiotic treatment, obesity, and region of residence. Statistical analyses were implemented to assess significance and estimate odds ratio (OR).
Results
EI was associated with a decreased incidence of BC (OR = 0.60, 95% confidence interval (CI): 0.57 - 0.63) and the difference was statistically significant (P < 2.2 × 10-16). Treatment for EI was controlled for in both EI and noninfected populations. Patients with a prior EI and treated with antibiotics were compared to patients with no history of EI and received antibiotics. Both populations subsequently developed BC. Results remained statistically significant (P < 2.2 × 10-16) with an OR of 0.57 (95% CI: 0.54 - 0.60). In addition to standard matching protocol, obesity was controlled for in both groups by exclusively containing obese patients, but one group with prior EI and the other without. In obese patients, a lower incidence of BC was shown in the infected group compared to the noninfected group. Results were statistically significant (P < 2.2 × 10-16) with an OR of 0.56 (95% CI: 0.53 - 0.58). Age of BC diagnosis with and without a prior EI was analyzed and demonstrated increased BC incidence with increasing age in both groups, but less in the EI group. Incidence of BC based on region was analyzed, which showed lower BC incidence across all regions in the EI group.
Conclusion
This study shows a statistically significant correlation between EI and decreased incidence of BC. Further exploration is needed to identify and understand not only the role of enterococcus in the microbiome, but also the protective mechanism(s) and impact of EI on BC development.
Keywords: Breast cancer, Microbiome, Enterococcus, Infection, Immunology
Introduction
Breast cancer (BC) is the most common cancer in women worldwide and is the second most common cause of death from cancer in women in the United States [1, 2]. BC is also the most research-funded cancer worldwide, receiving substantially higher grants than any other cancer [3]. The prevalence and impact of BC on patients and their families and friends are wide reaching, which in part helps explain its popularity in research. Recently, BC and the microbiome has been a trending focus of research [4-9]. Many bacteria in the gut microbiota possess genes capable of metabolizing estrogen, suggesting a relevant and important relationship to BC [4, 6, 8]. To further investigate and contribute to this area of research, the objective of this study was to assess the correlation between prior infection with enterococci and risk of BC development.
Enterococci are gram-positive, non-sporulating, facultative anaerobic cocci that form short and medium chains [10]. Enterococci are ubiquitous and can be easily isolated from a broad spectrum of hosts, soil, water, food, plants, and sewage [11]. In humans, enterococci commonly colonize and can be found on the skin, in the oral cavity, and in the large intestine [12]. Enterococci are robust bacteria capable of tolerating a wide range of growth conditions, and as facultative organisms, grow best under reduced or oxygenated conditions [13].
Enterococci do not produce toxins. Instead, their virulence derives from other features such as structure, durability, and antibiotic resistance [14]. Their surface components include a polysaccharide capsule, adhesins, pili, and an aggregation substance. Because enterococci can form biofilms, a favorable environment encourages adherence to catheters, dental prosthetics, and heart valves [12]. This characteristic further facilitates persistent infection. Notoriously, enterococci are intrinsically resistant to a multitude of antibiotics, such as cephalosporins, aminoglycosides, lincosamides, and streptogramins [15]. They are also capable of acquiring and transferring antibiotic resistance through conjugative transposons, or mobile gene elements, which contain genes encoding traits that confer resistance [11].
The majority of enterococcal infections (EIs) are caused by Enterococcus faecalis, and the remaining are caused by Enterococcus faecium. These two species comprise over 90% of the total EIs [10]. The former is more likely to display factors related to overt virulence, however, are also more likely to maintain sensitivity to at least one antibiotic [13]. The latter species, in contrast, distinctly lack overt pathogenicity but are effectively more resistant to even last resort antibiotics [15].
Enterococci are recognized as the second leading cause of nosocomial infections, the first being staphylococci. Common infections include bacteremia, urinary tract infections, surgical wound infections, and endocarditis [12, 13]. More rarely, they may also cause intra-abdominal infections and meningitis [12]. Over the last few decades, enterococci have gained notoriety not only due to their increasing presence in hospital-acquired infections, but also for their rapid rise in antibiotic resistance and difficult treatment course.
Although enterococci can cause a variety of severe infections and pose a major threat to modern medicine, these bacteria do not always contribute to infection. Enterococci are normal inhabitants of the gut and live in the gastrointestinal (GI) tract of virtually all animals, including humans, and are regarded as commensal organisms [11]. Over 50 species of enterococci that reside as commensal bacteria have been isolated from the GI tract of insects, birds, reptiles, and mammals [16]. In humans, enterococci are one of the earliest colonizers of the gut flora and are prominent members of the intestinal microbiome [14]. They have been shown to play an important and beneficial role in the gut microbiome. For example, enterococci have been extensively studied as a potential probiotic candidate for treatment and/or prevention in specific human and animal diseases [17].
Enterococci have also been implicated in the development of colorectal cancer (CRC), although the current literature is equivocal and no strong relationship between enterococcus and CRC has been established [18]. Many studies have supported a positive correlation of enterococci and CRC [19-21], while many others have disputed its significance [22, 23]. Enterococci and its role in various domains continue to prove controversial. The benefit versus harm juxtaposition of enterococci is evident in the current literature [18], which necessitates further research in our understanding of this bacterium.
Materials and Methods
Patient cohorts
This study was conducted using a Health Insurance Portability and Accountability Act (HIPAA) compliant national database provided by Holy Cross Health, Fort Lauderdale, Florida for academic research. The PearlDiver Mariner database, in conjunction with the Bellwether interface, was used to run queries, stratify data, and perform statistical analyses. PearlDiver contains over 41 billion HIPAA compliant patient records, which are acquired from analysis of private insurance claims from Humana, United Healthcare, and government claims from Medicare [24]. The Patient Population Database in the United States was retrospectively reviewed from January 2010 to December 2019 using International Classification of Disease Ninth and Tenth Codes (ICD-9, ICD-10), Current Procedural Terminology (CPT), and National Drug Codes were used for identifying EI and BC diagnosis. Two groups were first identified and isolated based on history of EI and no history of EI, which comprised of the experimental group and control group, respectively. Criteria for defining the type of EI were determined and identified using ICD-9 and ICD-10 codes: “Sepsis due to Enterococcus” and “Enterococcus as the cause of disease classified elsewhere”. These groups were then matched based on age, sex, and Charlson comorbidity index (CCI) to allow for the optimal comparison, while mitigating confounding variables on outcome measures. This initial match was further stratified to include antibiotic treatment exposure. This was done in an effort to control potential treatment effects on outcome measures and overall interpretation of the data. The inclusion criteria included patients who were active in the database for at least 8 years with a history of BC, and a history of exposure to similar antibiotic treatment regimen. The development of BC was the primary outcome measure of this study. Standard statistical methods were utilized to analyze the results and assess statistical significance. Chi-squared, logistic regression, and odds ratio (OR) were implemented to interpret and assess the significance of the data. Data from the initial query were subsequently stratified based on patient demographics and other risk factors in both patient groups. Figure 1 illustrates a flow chart of the two patient groups, including the number of total patients (n) in each respective group and how the groups were further stratified and compared.
Figure 1.
Flowchart of infected vs. noninfected groups with and without antibiotic treatment. Left side: total patients in database (n = 53 × 106). Second column from left: evaluation of incidence of breast cancer in patients with prior enterococcus infection (dark blue) and without infection (light blue). Third column from left: additional stratification and matching were done based on patient comorbidities and age with and without infection (very dark blue and dark blue, n = 28,518), and with antibiotic treatment with and without infection (very light blue and light blue, n = 11,523).
Literature review
PubMed (2000 - present) and Google Scholar (2000 - present) were used for the purpose of a literature review. The literature reviewed aimed at inquiring data relating to any and all relationships between BC and EI. The review was guided using keywords in varying orders: “breast cancer”, “enterococcal infection”, “microbiome”, “carcinogenesis”, “immunology”, “immunomodulator”, and “bacterial infection”. The literature included findings relating to the microbiome and its impact on carcinogenesis and BC. Review of the literature also indicated a diverse, yet inconclusive role of enterococcus in the gut.
IRB approval
This study was exempt from IRB approval because all data were obtained from a database that provided deidentified patient information. The study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Results
Patients with a history of EI had significantly lower incidence of BC
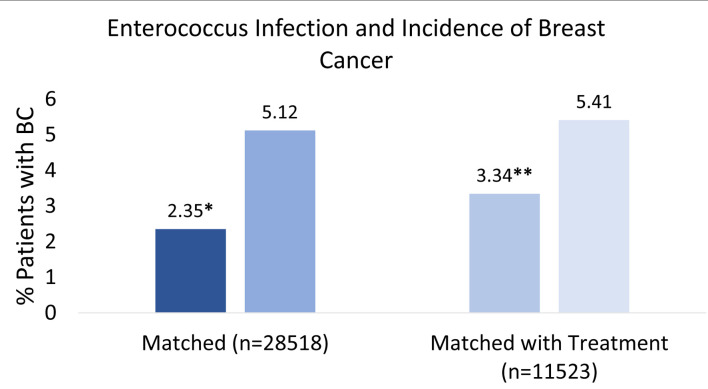
The initial matched query based on patient CCI score and age (Fig. 1) yielded 28,518 patients who had a positive history of EI (very dark blue square in third column from the left in Fig. 1) and 28,518 patients with no prior history of EI (dark blue square in third column from the left in Fig. 1). Six hundred seventy-one out of 28,518 (2.35%) patients with a prior EI (very dark blue square in far-right column in Fig. 1) and 1,459 out of 28,518 (5.12%) patients without EI (dark blue square in far-right column in Fig. 1) subsequently developed BC (left side of Fig. 2). The difference was statistically significant (P < 2.2 × 10-16). Logistic regression also indicated EI was associated with a decreased incidence of BC (OR = 0.60, 95% CI: 0.57 - 0.63).
Figure 2.
Percent breast cancer incidence after enterococcus infection. This figure illustrates the percentage of patients who developed breast cancer with or without a prior enterococcus infection. Left side: patients with enterococcus infection (very dark blue) and no infection (dark blue) were compared based on similar comorbidities and age before incidence of breast cancer (n = 28,518). Score *P < 2.2 × 10-16. Right side: patients with enterococcus infection with antibiotic treatment (light blue) and no infection with antibiotic treatment (very light blue) were controlled and similarly compared based on comorbidities and age before incidence of breast cancer (n = 11,523). Score **P < 2.2 × 10-16.
Association of the history of EI and lower incidence of BC remained significant among the patients who underwent antibiotics treatment
When treatment regimen was controlled, 11,523 patients with a positive history of EI but treated with antibiotics were matched with 11,523 patients with no prior history of EI but similarly treated with antibiotics. The query yielded 398 out of 11,523 (3.34%) patients with a prior EI and treated with antibiotics (light blue square in far-right column in Fig. 1), and 624 out of 11,523 (5.41%) patients with no history of EI (control) and received antibiotic treatment (very light blue square in far-right column in Fig. 1). Both populations subsequently developed BC. Both groups were compared, and results remained statistically significant (P < 2.2 × 10-16) with an OR of 0.57 (95% CI: 0.54 - 0.60). The overall findings and interpretation from the data are illustrated in Figure 2. OR with CIs among the two different matching criteria groups are shown in Figure 3.
Figure 3.
Odds ratio ((OR) with 95% confidence intervals (CIs). OR in enterococcus infected (dark blue) and enterococcus infected with antibiotic treatment (light blue) showed a decreased likelihood of developing breast cancer. OR in infected group (light blue) was 0.60 with a 95% CI of 0.57 - 0.63. OR in infected group with antibiotic treatment (dark blue) was 0.57 with a 95% CI of 0.54 - 0.60.
Association of the history of EI and lower incidence of BC remained significant among obese patients
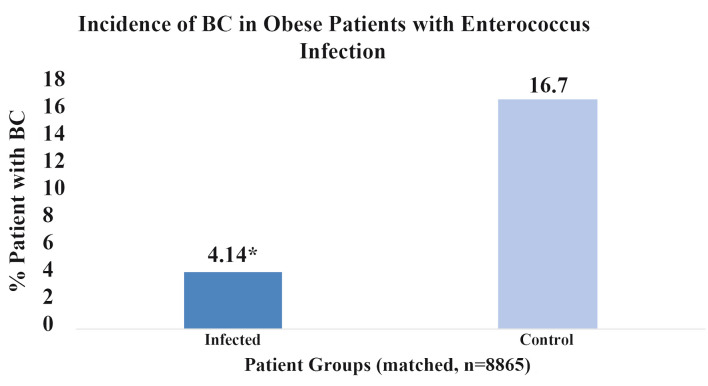
The impact of obesity on BC incidence following an EI was additionally analyzed and results are shown in Figure 4. When both patient groups were controlled for obesity in addition to the standard matching protocol, but one group had a prior EI and the other did not, a lower relative incidence of BC was shown in the EI group. Both groups comprised of 8,865 patients who satisfied criteria for obesity based on body mass index (BMI). Obesity was defined by patients with BMI’s ≥ 30. Results were statistically significant with a P-value of less than 2.2 × 10-16 and OR of 0.56 (95% CI: 0.53 - 0.58). Compared to the original matched group of patients with no prior EI, both with and without antibiotic treatment control, obese patients with no prior EI had higher incidences of BC. These results are expected due to the increased risk of BC in obese patients. However, the difference in incidence of BC in obese patients with a prior EI compared to no infection was greater than the difference in incidence in the original match when obesity was not controlled. These results suggest that the proposed protective effect of EI may outweigh the harm or risk of obesity when considering BC.
Figure 4.
Percent breast cancer incidence in obese patients with enterococcus infection. This figure illustrates the percentage of obese patients (n = 8,865) who developed breast cancer with a prior history enterococcal infection (dark blue) and obese patients without infection (light blue). Score *P < 2.2 × 10-16.
Association of the history of EI and lower incidence of BC was consistent across all age ranges
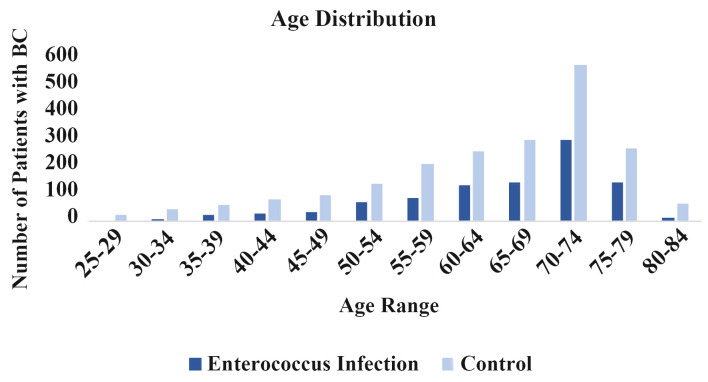
The incidence of BC following a prior EI was further analyzed based on age of BC diagnosis. Figure 5 shows the incidence of BC based on age of diagnosis. These findings are consistent with the overall understanding that BC incidence increases with age. Furthermore, the patients with the history of EI showed a lower incidence of BC across all age ranges.
Figure 5.
Age distribution. Incidence of breast cancer is sorted by age in enterococcus infected (dark blue) and noninfected (light blue) groups.
Association of the history of EI and lower incidence of BC was consistent across all regions in the USA
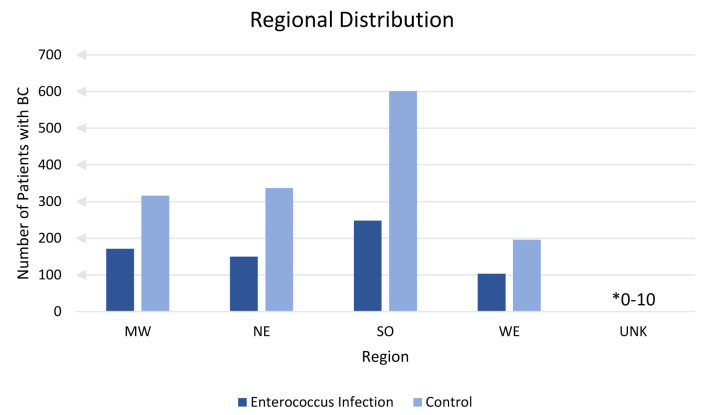
Incidence of BC with or without a previous EI was analyzed based on region of diagnosis and is shown in Figure 6. Analysis of region was confined to the USA. Patients residing in an unknown (unk) region comprised of less than 10 but greater than 0 cases of BC. A lower incidence of BC was found in all regions where patients had a prior EI compared to no history of EI.
Figure 6.
Regional distribution. This figure shows number of patients with breast cancer sorted by United States region with prior enterococcus (dark blue) and without prior enterococcus infection (light blue). Unknown (unk) is a label provided by the database for patient records with unidentified or no known region of residence.
Discussion
This study is the first to demonstrate a statistically significant correlation between a previous EI and a decreased incidence of BC among adults in the United States. This correlation remains significant when controlling for antibiotic treatment, obesity, age, and regions in the USA. This study also includes a large sample size with effective group matching to reduce potential confounding variables and bias.
When controlling for antibiotic use, the sample size decreased by approximately 17,000. This may be due to the differences in standard clinical practice when treating an EI compared to other bacterial infections. As stated in the introduction, EIs are notoriously durable against many different antibiotics, most of which are commonly used to treat other bacterial infections. Treatment of EIs often necessitates stronger antibiotics, which may explain why patients with no previous history of EI did not fulfill matching criteria with patients who had EIs. Essentially, roughly 17,000 patients with no history of EI were excluded from analysis because they did not receive identical antibiotic treatment as patients with EIs.
Current literature on the relationship between enterococci and cancer primarily emphasizes CRC, and despite the predominance, the role of enterococci in this light is unestablished and remains controversial [18]. While some published literature reports a harmful role of enterococci via enhancing cellular proliferation [19, 20, 25], other data have shown either no correlation or a favorable anti-proliferative affect [26]. In general, the majority of current data have been published regarding the impact of the microbiome and/or various bacteria on GI cancers [8-11, 16, 18, 25, 27]. Little research has focused on the microbiome and BC specifically, and even less on enterococci and BC. Although previous studies have shown anti-BC effects of enterococci on malignant BC cell lines [26], the data of this study are the first to indicate a relationship between EI and BC incidence in a prophylactic respect. In view of the broad body of literature on enterococci in relation to cancer, it is clear that enterococci possess a unique capacity. Enterococci ability to modulate cancer risk may be potentiated through infection only and not exclusively as commensals. Considering the controversial role of enterococci in the microbiome, the results from this study substantiate the body of literature on behalf of enterococci as an oncoprotective organism. Further, in the light of recent focus on anti-cancer immunity, we cannot help but speculate that EI may enhance the anti-cancer immunity that prevents BC carcinogenesis.
BC is often a hormonally driven malignancy with estrogen playing an essential role [28, 29]. The gut microbiota has been shown to modulate serum estrogen levels and affect response to endocrine therapy [4-7, 30, 31]. Numerous bacterial genes have been identified that produce estrogen-metabolizing enzymes [8]. Research has found that the microbiome of patients with BC differs from that of healthy patients [4, 6], which suggests the microbiome and its influence on estrogen balance may be a relevant focus point for research in patients with BC specifically. An EI may alter the gut flora, either exclusively or by downstream adaptations of other microbiota, in a manner that favors decreased estrogen levels consistent with a lower risk of BC development.
Like estrogen, obesity is a significant and relevant risk factor for BC. Obesity is associated with an increased risk for developing BC [32]. This is, in part, due to an increased estrone production and conversion in adipose tissue [32, 33]. The results from Figure 4, which depicts obesity as a controlled variable, encourage further discussion regarding the interrelation between estrogen modulation, EI, the microbiome, and BC evolution. The decreased risk of developing BC in obese patients with a prior EI may be greater than the risk of obesity alone in BC development.
The exact mechanism behind the findings of this study is not well understood and more research is needed to elucidate and support the potential protective impact of EI on BC development. While the PearlDiver database offers an immense number of patients, retrospective studies sustain inherent limitations, and this study is no exception. Selection bias, particularly selection of control groups, poses one limitation to this study. This is also a single cohort study, and the results are not validated. The results of this study heavily depend on accurate reporting, charting and recordkeeping by both healthcare professionals and insurance companies.
Although the role of EI in changing BC risk is not known, the results of this study do not support treatment as a reason for risk reduction. The precise mechanism is presumably complex, but likely involves a multi-leveled relationship involving the microbiome and its influence on endocrinology, the sequelae of bacterial infection and subsequent adaptive immunity, and oncogenesis. Despite the poorly understood and disputed significance of enterococci, therapeutics aimed at exploiting a safer, but similar, alternative to an EI may be a reasonable avenue in the future for prevention of BC.
Conclusion
This study shows a statistically significant correlation between history of EI and a decreased incidence of BC. Further investigation is needed to identify and understand the role of enterococcus in the microbiome and carcinogenesis of BC.
Acknowledgments
The authors acknowledge the support of Holy Cross Hospital and Nova Southeastern University Dr. Kiran C. Patel College of Allopathic Medicine. Kazuaki Takabe was supported by US National Institutes of Health: R37CA248018, R01CA250412, R01CA251545, R01EB029596, as well as US Department of Defense BCRP grants: W81XWH-19-1-0674 and W81XWH-19-1-0111.
Financial Disclosure
Grant support by Broward Community Foundation.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was exempted as this is a database study.
Author Contributions
Matthew Cardeiro is the first author and is responsible for data analysis, figures, literature review, and manuscript writing. Lexi Frankel is responsible for database extraction and analysis, manuscript writing and editing. Amalia Ardeljan is responsible for database extraction and analysis. Enoch Kim is responsible for supplemental editing. Authors Omar Rashid, corresponding author, and Kazuaki Takabe are responsible for manuscript writing, editing, and data analysis.
Data Availability
The data supporting the findings of this study are extracted from PearlDiver national database and available if needed.
Abbreviations
- BC
breast cancer
- CRC
colorectal cancer
- HIPAA
Health Insurance Portability and Accountability Act
- ICD
International Classification of Disease
- CPT
Current Procedural Terminology
- CCI
Charlson comorbidity index
- OR
odds ratio
- CI
confidence interval
- GI
gastrointestinal
- BMI
body mass index
- US
United States
References
- 1.Ghoncheh M, Pournamdar Z, Salehiniya H. Incidence and mortality and epidemiology of breast cancer in the world. Asian Pac J Cancer Prev. 2016;17(S3):43–46. doi: 10.7314/APJCP.2016.17.S3.43. [DOI] [PubMed] [Google Scholar]
- 2. Breast cancer - statistics. Cancer.Net. Published June 25, 2012. Accessed February 12, 2022. https://www.cancer.net/cancer-types/breast-cancer/statistics.
- 3. Current grants by cancer type. Cancer.org. Accessed February 12, 2022. https://www.cancer.org/research/currently-funded-cancer-research/grants-by-cancer-type.html.
- 4.Bodai BI, Nakata TE. Breast cancer: lifestyle, the human gut microbiota/microbiome, and survivorship. Perm J. 2020;24:19.129. doi: 10.7812/TPP/19.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang N, Sun T, Xu J. Tumor-related microbiome in the breast microenvironment and breast cancer. J Cancer. 2021;12(16):4841–4848. doi: 10.7150/jca.58986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandez MF, Reina-Perez I, Astorga JM, Rodriguez-Carrillo A, Plaza-Diaz J, Fontana L. Breast cancer and its relationship with the microbiota. Int J Environ Res Public Health. 2018;15(8):1747. doi: 10.3390/ijerph15081747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eslami SZ, Majidzadeh AK, Halvaei S, Babapirali F, Esmaeili R. Microbiome and breast cancer: new role for an ancient population. Front Oncol. 2020;10:120. doi: 10.3389/fonc.2020.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Helmink BA, Khan MAW, Hermann A, Gopalakrishnan V, Wargo JA. The microbiome, cancer, and cancer therapy. Nat Med. 2019;25(3):377–388. doi: 10.1038/s41591-019-0377-7. [DOI] [PubMed] [Google Scholar]
- 9.Zitvogel L, Galluzzi L, Viaud S, Vetizou M, Daillere R, Merad M, Kroemer G. Cancer and the gut microbiota: an unexpected link. Sci Transl Med. 2015;7(271):271ps271. doi: 10.1126/scitranslmed.3010473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Tyne D, Martin MJ, Gilmore MS. Structure, function, and biology of the Enterococcus faecalis cytolysin. Toxins (Basel) 2013;5(5):895–911. doi: 10.3390/toxins5050895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fiore E, Van Tyne D, Gilmore MS. Pathogenicity of Enterococci. Microbiol Spectr. 2019;7(4):7.4.9. doi: 10.1128/microbiolspec.GPP3-0053-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Said MS, Tirthani E, Lesho E. Enterococcus infections. StatPearls; Treasure Island (FL): 2022. [PubMed] [Google Scholar]
- 13.Huycke MM, Sahm DF, Gilmore MS. Multiple-drug resistant enterococci: the nature of the problem and an agenda for the future. Emerg Infect Dis. 1998;4(2):239–249. doi: 10.3201/eid0402.980211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levitus M, Rewane A, Perera TB. Vancomycin-resistant Enterococci. StatPearls; Treasure Island (FL): 2022. [PubMed] [Google Scholar]
- 15.Manero A, Blanch AR. Identification of Enterococcus spp. with a biochemical key. Appl Environ Microbiol. 1999;65(10):4425–4430. doi: 10.1128/AEM.65.10.4425-4430.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dubin K, Pamer EG. Enterococci and their interactions with the intestinal microbiome. Microbiol Spectr. 2014;5(6):5.6.01. doi: 10.1128/microbiolspec.BAD-0014-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanchi H, Mottawea W, Sebei K, Hammami R. The Genus Enterococcus: Between Probiotic Potential and Safety Concerns-An Update. Front Microbiol. 2018;9:1791. doi: 10.3389/fmicb.2018.01791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Almeida CV, Taddei A, Amedei A. The controversial role of Enterococcus faecalis in colorectal cancer. Therap Adv Gastroenterol. 2018;11:1756284818783606. doi: 10.1177/1756284818783606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boonanantanasarn K, Gill AL, Yap Y, Jayaprakash V, Sullivan MA, Gill SR. Enterococcus faecalis enhances cell proliferation through hydrogen peroxide-mediated epidermal growth factor receptor activation. Infect Immun. 2012;80(10):3545–3558. doi: 10.1128/IAI.00479-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karpinski TM, Ozarowski M, Stasiewicz M. Carcinogenic microbiota and its role in colorectal cancer development. Semin Cancer Biol. 2022;86(Pt 3):420–430. doi: 10.1016/j.semcancer.2022.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Khodaverdi N, Zeighami H, Jalilvand A, Haghi F, Hesami N. High frequency of enterotoxigenic Bacteroides fragilis and Enterococcus faecalis in the paraffin-embedded tissues of Iranian colorectal cancer patients. BMC Cancer. 2021;21(1):1353. doi: 10.1186/s12885-021-09110-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan Z, Siddiqui N, Saif MW. Enterococcus faecalis infective endocarditis and colorectal carcinoma: case of new association gaining ground. Gastroenterology Res. 2018;11(3):238–240. doi: 10.14740/gr996w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Viljoen KS, Dakshinamurthy A, Goldberg P, Blackburn JM. Quantitative profiling of colorectal cancer-associated bacteria reveals associations between fusobacterium spp., enterotoxigenic Bacteroides fragilis (ETBF) and clinicopathological features of colorectal cancer. PLoS One. 2015;10(3):e0119462. doi: 10.1371/journal.pone.0119462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. About PearlDiver services. PearlDiver. Published January 19, 2022. Accessed May 27, 2022. https://pearldiverinc.com/about-us/
- 25.Turk S, Turk C, Temirci ES. et al. Assessing the genetic impact of Enterococcus faecalis infection on gastric cell line MKN74. Ann Microbiol. 2021;71(1):8. doi: 10.1186/s13213-020-01615-3. [DOI] [Google Scholar]
- 26.Hassan Z, Mustafa S, Rahim RA, Isa NM. Anti-breast cancer effects of live, heat-killed and cytoplasmic fractions of Enterococcus faecalis and Staphylococcus hominis isolated from human breast milk. In Vitro Cell Dev Biol Anim. 2016;52(3):337–348. doi: 10.1007/s11626-015-9978-8. [DOI] [PubMed] [Google Scholar]
- 27.Wu F, Yang L, Hao Y, Zhou B, Hu J, Yang Y, Bedi S. et al. Oral and gastric microbiome in relation to gastric intestinal metaplasia. Int J Cancer. 2022;150(6):928–940. doi: 10.1002/ijc.33848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yue W, Wang JP, Li Y, Fan P, Liu G, Zhang N, Conaway M. et al. Effects of estrogen on breast cancer development: Role of estrogen receptor independent mechanisms. Int J Cancer. 2010;127(8):1748–1757. doi: 10.1002/ijc.25207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Russo J, Russo IH. The role of estrogen in the initiation of breast cancer. J Steroid Biochem Mol Biol. 2006;102(1-5):89–96. doi: 10.1016/j.jsbmb.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neuman H, Debelius JW, Knight R, Koren O. Microbial endocrinology: the interplay between the microbiota and the endocrine system. FEMS Microbiol Rev. 2015;39(4):509–521. doi: 10.1093/femsre/fuu010. [DOI] [PubMed] [Google Scholar]
- 31.Baker JM, Al-Nakkash L, Herbst-Kralovetz MM. Estrogen-gut microbiome axis: Physiological and clinical implications. Maturitas. 2017;103:45–53. doi: 10.1016/j.maturitas.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 32.Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, Friedman ER, Slingerland JM. Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention. CA Cancer J Clin. 2017;67(5):378–397. doi: 10.3322/caac.21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cleary MP, Grossmann ME. Minireview: Obesity and breast cancer: the estrogen connection. Endocrinology. 2009;150(6):2537–2542. doi: 10.1210/en.2009-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of this study are extracted from PearlDiver national database and available if needed.