Abstract
Background and Objectives
A causal relationship between statin use and intracerebral hemorrhage (ICH) is uncertain. We hypothesized that an association between long-term statin exposure and ICH risk might vary for different ICH locations.
Methods
We conducted this analysis using linked Danish nationwide registries. Within the Southern Denmark Region (population 1.2 million), we identified all first-ever cases of ICH between 2009 and 2018 in persons aged ≥55 years. Patients with medical record–verified diagnoses were classified as having a lobar or nonlobar ICH and matched for age, sex, and calendar year to general population controls. We used a nationwide prescription registry to ascertain prior statin and other medication use that we classified for recency, duration, and intensity. Using conditional logistic regression adjusted for potential confounders, we calculated adjusted ORs (aORs) and corresponding 95% CIs for the risk of lobar and nonlobar ICH.
Results
We identified 989 patients with lobar ICH (52.2% women, mean age 76.3 years) who we matched to 39,500 controls and 1,175 patients with nonlobar ICH (46.5% women, mean age 75.1 years) who we matched to 46,755 controls. Current statin use was associated with a lower risk of lobar (aOR 0.83; 95% CI, 0.70–0.98) and nonlobar ICH (aOR 0.84; 95% CI, 0.72–0.98). Longer duration of statin use was also associated with a lower risk of lobar (<1 year: aOR 0.89; 95% CI, 0.69–1.14; ≥1 year to <5 years aOR 0.89; 95% CI 0.73–1.09; ≥5 years aOR 0.67; 95% CI, 0.51–0.87; p for trend 0.040) and nonlobar ICH (<1 year: aOR 1.00; 95% CI, 0.80–1.25; ≥1 year to <5 years aOR 0.88; 95% CI 0.73–1.06; ≥5 years aOR 0.62; 95% CI, 0.48–0.80; p for trend <0.001). Estimates stratified by statin intensity were similar to the main estimates for low-medium intensity therapy (lobar aOR 0.82; nonlobar aOR 0.84); the association with high-intensity therapy was neutral.
Discussion
We found that statin use was associated with a lower risk of ICH, particularly with longer treatment duration. This association did not vary by hematoma location.
Statins effectively reduce the occurrence of recurrent ischemic stroke and other cardiovascular events in high-risk populations.1 An individual patient data meta-analysis of statin trials2 and exploratory analyses of data from the Stroke Prevention by Aggressive Reductions in Cholesterol Levels trial3 and the Heart Protection Study4, however, raised concerns of an increased risk of ICH among statin users with a history of stroke. The results of subsequent observational studies, including 4 meta-analyses,5-8 focusing on patients both with9-13 and without14,15 prior stroke are inconsistent, although most found no increase in bleeding. Furthermore, more recent studies that assessed the effects of intensity of treatment,16 duration-response relationships,15,17 or both18 found a lower risk of ICH among statin users and that the risk was lower with longer duration and higher intensity of statin treatment.
There are few data assessing the association between statin use and the location of ICH.19-22 Parenchymal ICH can be lobar (∼35%) or deep (nonlobar, ∼35%) in part reflecting differing underlying pathologies. Cerebral amyloid angiopathy (CAA) is associated with lobar but not nonlobar ICH.23 Arteriolosclerosis, which is strongly associated with hypertension, is a common histologic finding in ICH regardless of hemorrhage location.24 In a population-based study, histologically verified CAA was present in 58% of patients with a lobar ICH, but the majority also had evidence of arteriolosclerosis, with only 13% having isolated CAA pathology.24 Whether differences in the underlying pathophysiology of lobar vs nonlobar ICH differentially affect the relationship between statin use and ICH risk has only been assessed in few population-based studies.16,22,25,26 These studies did not assess the relationship between the duration of statin use with ICH location.
We hypothesized that statin use is associated with a lower overall risk of ICH but that the magnitude of the association varies by lCH mechanism (i.e., the benefit of statin treatment is greater in reducing the risk of nonlobar ICH vs lobar ICH, reflecting a more pronounced statin effect on arteriosclerosis compared with amyloid angiopathy). To explore this hypothesis, we conducted a case-control study in which we classified hemorrhage location in all patients in a Danish region with incident ICH and compared their prior long-term use of statins with general population controls. Statin exposure for cases and controls was obtained from a prospective registry of dispensed prescriptions with full coverage of the source population for the prior 25 years.
Methods
Design
We conducted a population-based nested case-control study within the Region of Southern Denmark (RSD) based on nationwide Danish registry data.27-30 Data were linked across registries using the unique 10-digit personal identifier assigned to all residents at birth or on immigration.30
Setting and Data Sources
Health care is tax financed and free of personal charge to all residents of Denmark. Patients suspected of having a stroke are evaluated as medical emergencies with organized pathways including rapid access to neuroimaging and subsequent admission or transfer to stroke units for specialized care. The RSD (population 1.2 million), 1 of the 5 regions in the country, includes 5 stroke units and a single neurosurgical department at a university hospital and is representative of the Danish population regarding sociodemographic and health-related characteristics.31
Standard Protocol Approvals, Registrations, and Patient Consents
The study was approved by the RSD, and informed consent was waived. Data were pseudonymized.32
Data Availability
Danish law prohibits the authors from sharing or granting access to the data used for this study.
Source Population
The source population were all persons 55 years or older and residing in the RSD between 2009 and 2018. The Patient Registry data for 2019–2022 have only recently been released for research, which limited the inclusion of later data for this analysis. We chose the patient age range as it is one of the modified Boston criteria for CAA33 and because younger people are less likely to be treated with statins.
Cases
We identified cases ad hoc, as previously described.34 In brief, we used population-based sources to trace all individuals in the RSD in the study period with a first-ever diagnosis of spontaneous ICH (s-ICH; patients having a traumatic ICH or an ICH related to aneurysmal SAH, vascular malformations, or tumors were excluded). The s-ICH diagnosis was verified by physicians who also classified the location of the hemorrhage based on discharge summaries and brain imaging reports in accordance with a previously validated method.35 We assigned the index date as the date of the ICH, if established as part of the verification process and alternatively as the date of hospital admission. We linked the ad hoc collected s-ICH data with prospectively collected information on all residents of the RSD available from 4 nationwide data sources (i.e., the Danish Civil Registration System,30 the Danish National Patient Registry29 [Patient Registry], the Danish Stroke Registry27 [Stroke Registry], and the Danish National Prescription Registry28 [Prescription Registry]).
Based on slightly modified criteria used in a previous population-based study,36 we classified ICH location as nonlobar if the patient had a single infratentorial ICH (located in the brain stem or cerebellum), a single supratentorial deep ICH (located in the basal ganglia, internal or external capsule, or thalamus), or multiple ICHs in solely nonlobar locations (either supratentorial deep, or infratentorial); all other ICHs were classified as lobar. We subclassified nonlobar hemorrhages as deep and cerebellar (a location reported to be rarely associated with CAA37). We considered deep ICH as the group most likely being hypertension-related38 and due to small vessel disease (SVD). We included isolated intraventricular hemorrhages (IVHs), which can be related to SVD,39 in overall analyses of ICH but excluded them from location-specific analyses.
Controls
We sampled the Danish Civil Registration System30 to identify 40 controls without ICH from the source population for each case. Using risk-set sampling, we matched controls by birth year and sex to their index case and assigned an index date identical to the index date of their corresponding case. Cases were eligible to be selected as controls before their first-ever s-ICH. This design ensures that the OR is an unbiased estimate of the incidence rate ratio and enables the calculation of absolute rate differences. We retrieved nationwide data on controls from the same registry sources as for cases (see above).
Exposure to Statins
During the study period, simvastatin, lovastatin, pravastatin, fluvastatin, atorvastatin, and rosuvastatin were available in Denmark for clinical use (cerivastatin was withdrawn from the Danish market in 2001). Exposure to any of these statins for s-ICH cases and controls up to the index date was determined based on prescription registry data (codes in eTable 1, links.lww.com/WNL/C492).
Based on the most recent episode of statin use before the index date, and as in a previous study by our group,18 statin exposure was classified as follows: current use (prescription supply covered the index date or ended within 30 days of the index date); recent use (prescription supply ended 31–90 days before the index date); past use (prescription supply ended 91–365 days before the index date); and nonuse (no recorded prescription before the index date or most recent prescription ended more than 365 days before the index date). We based the length of a prescription on the number of tablets dispensed (assuming a posology of once a day). A statin treatment episode comprised the length of consecutive prescriptions allowing for gaps between prescriptions of 60 days or less. We assessed potential duration-response relationships in relation with ICH location. For this analysis, we classified the duration of statin current use into the following categories: <1, ≥1 to <5, and ≥5 years. The duration was based on the most recent episode of statin treatment before the index date. We focused on duration of the most recent episode of current use of statins as a previous Danish nationwide study we conducted found that ICH risk was only associated with the duration of current use of statins with null associations for past statin use.18
We classified the intensity of statin therapy based on the 2013 ACC/AHA guidelines40 as high intensity (atorvastatin 40–80 mg/d; rosuvastatin 20–40 mg/d; simvastatin 80 mg/d), moderate intensity (atorvastatin 10–20 mg/d; rosuvastatin 5–10 mg/d; simvastatin 20–40 mg/d; pravastatin 40–80 mg/d; lovastatin 40 mg/d), or low intensity (simvastatin 10 mg/d; pravastatin 10–20 mg/d; lovastatin 20 mg/d; fluvastatin 20–40 mg/d). Simvastatin 80 mg/d was not included in ACC/AHA guidelines due to a high risk of adverse effects40; however, we included this dose in the high-intensity category as there was some use of that intensity in our population. We used the maximum tablet strength prescribed during the most recent episode of statin treatment assuming a 1 tablet per day use for this classification, as information on the physician-ordered dose was not available in the Prescription Registry. Further details on the classification of exposure to statins and other medications are provided in the eMethods.
Supplementary Analyses
We also retrieved data on vital status from the Danish Civil Registry from which we identified patients with ICH who died within 30 days of the index date (fatal ICH). Patients with large hemorrhages for whom location could not be classified were not included in the main analyses but were assessed separately in a subanalysis.
To assess the effect of newly beginning a statin on ICH risk (i.e., the effect in new users41), we defined new users among cases and controls as those having no use of statins in 1995, the year the Prescription Registry became operational, and having a single episode of statin use recorded between 1996 and up to their index date (i.e., excluding those who started, stopped, and restarted statins during the period beginning in 1996).
We repeated the main analyses using cases only and comparing statin exposure for nonlobar vs lobar ICH. This analysis included the same confounders as the main analysis in addition to age, sex, and calendar time. This design was chosen to assess potential location-specific statin associations.
Brain CT Scan Substudy
We primarily used discharge records and brain scan reports to verify the diagnosis of s-ICH and to classify hematoma location. Although this method has the advantage of reflecting clinical routine, it could result in bias (e.g., radiologists were aware of clinical data, which could influence their evaluation of brain scans) and misclassification of hematoma location (e.g., either due to location not being mentioned in brain report or due to less accurate descriptions, possibly more so for large hematomas (e.g., large supratentorial ICH). Therefore, in a substudy, index CTs of 1,060 patients were evaluated for hematoma location (using CHARTS42), hematoma volume (using ABC/243), hematoma intraventricular extension (using Graeb score44), and evidence of SVD (i.e., presence of white matter lucencies [using van Swieten45] or lacunar infarct sequelae) masked to clinical data (eMethods, eFigure 1, links.lww.com/WNL/C492). We repeated the main analyses based on the data available from this substudy and using the above-outlined classifications for overall s-ICH and by hematoma location.
Covariates
We obtained data on potential confounders, proxies for confounders, and risk factors based on information retrieved from the Patient Registry, Stroke Registry, and Prescription Registry for both incident s-ICH cases and controls (eTable 1, eMethods, links.lww.com/WNL/C492).
Statistical Analysis
We performed descriptive analyses with categorical data presented using frequency counts and percentages and continuous data using mean and SD. We conducted nested case-control analyses using conditional logistic regression to calculate ORs as a measure of the risk of s-ICH (overall and by location) associated with use of statins (vs. nonuse), adjusted for the following covariates: history of chronic obstructive pulmonary disease (as a marker of smoking), disorders indicative of high alcohol consumption, hypertension, history of ischemic stroke, diabetes, chronic hepatic disease, chronic kidney disease, heart failure, ischemic heart disease, peripheral artery disease, cancer, and current use of drugs with antihypertensive effects (loop diuretics, nonloop diuretics, beta-blockers, calcium channel blockers, angiotensin-converting enzyme inhibitors, and angiotensin II receptor blockers), antiplatelet drugs, anticoagulants, nonsteroidal anti-inflammatory drugs, selective serotonin reuptake inhibitors, or proton pump inhibitors, hormone replacement therapy, or oral corticosteroid drugs. We chose covariates for inclusion in the model based on current knowledge regarding potential confounders and risk factors for ICH.
Our main analysis focused on the risk of ICH associated with long-term use (≥5 years) of statins. We performed analyses assessing the effects of duration of current use of statins, intensity of treatment stratified by treatment duration (i.e., <5 vs ≥ 5 years). We investigated the presence of potential effect measure modification of age (classified as 55–74, ≥75 years; in a separate analysis), sex, concurrent use of antithrombotic drugs, concurrent use of drugs with antihypertensive properties, and by history of ischemic stroke (to support comparability with a prior report that excluded patients with a history of any type of stroke in the control group22). Analyses of effect modifications by the indicated medications and history of ischemic stroke were performed using unconditional logistic regression (i.e., without matching and with inclusion of the original matching variables in the multivariable model), and differences in strength of association between subgroups were evaluated using the 2-sample Wald test. We also conducted main analyses stratified into fatal and nonfatal ICH using their respective matched controls. To ensure that our results were not subject to prevalent user bias, we also performed new user analyses.41 As above, we repeated the main analyses based on cases only (i.e., comparing nonlobar vs lobar [reference] and deep vs lobar [reference]). Dose-response trends in associations between drug use duration and ICH risk were tested using the duration in days as an explanatory variable in the regression. We calculated the E-values for the main results as a measure of the potential effect of unmeasured confounding.46 The E-value is the minimum strength of association that an unmeasured confounder would need to have with both the exposure and outcome to fully explain the observed association. With higher values, the observed association is less likely to be explained by unmeasured confounding.
For the brain CT substudy, we performed descriptive analyses of demographic, comorbidity, medication, and radiologic (e.g., hematoma volume and Graeb score) characteristics by hematoma location and statin exposure. We repeated the main analysis of the association of duration of current statin use with s-ICH by location in case-control (lobar vs nonlobar) and case-only analyses (lobar vs nonlobar and lobar vs deep, respectively), adjusting for confounding with the same covariates as for the main analysis.
Results
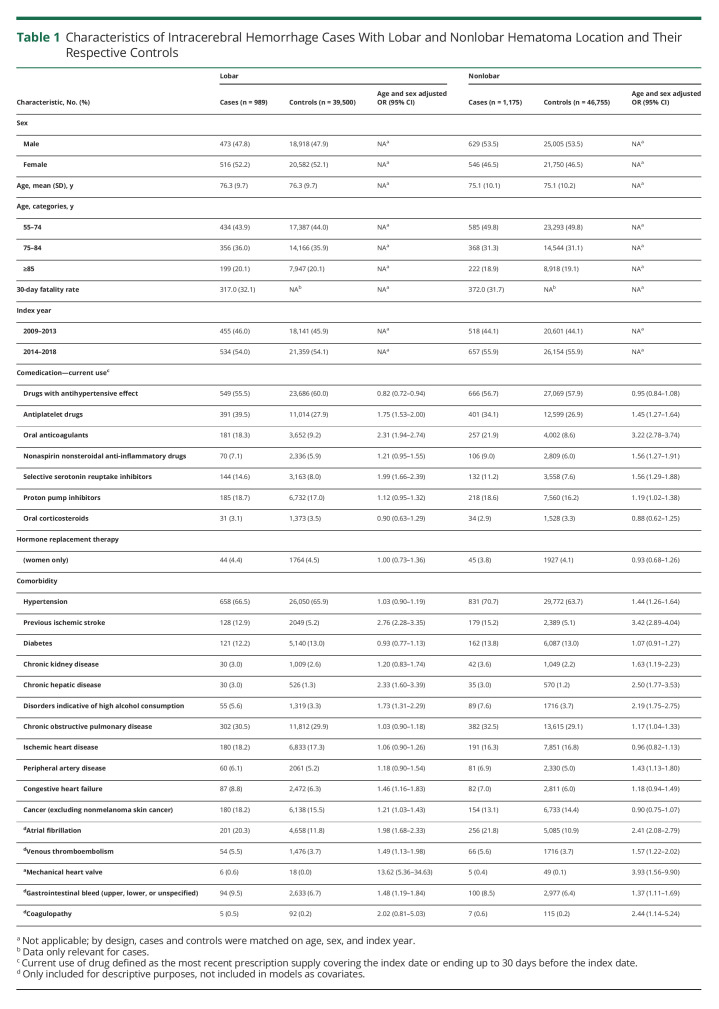
After cross-linking the Stroke Registry and Patient Registry, we identified 4,621 patients with codes compatible with ICH in the study period. We identified discharge summaries and brain scan reports for 4,430 (95.9%) patients, and based primarily on this information, we verified the diagnosis of s-ICH in 2,819 cases (eFigure 1, links.lww.com/WNL/C492). After exclusion of those younger than 55 years, our sample included 2,519 cases with s-ICH. Of these, 989 (39.3%) were classified as lobar ICH (52.2% women, mean age 76.3 years) and 1,175 (46.6%) as nonlobar ICH (46.5% women, mean age 75.1 years). A further 275 (10.9%) were large ICHs unclassifiable with respect to location, 46 (1.8%) had isolated IVH, and 34 (1.3%) were unclassifiable due to missing information. Compared with their controls, patients with lobar ICH more frequently had a history of previous ischemic stroke, chronic hepatic disease, congestive heart failure, atrial fibrillation, venous thromboembolism, mechanical heart valve, gastrointestinal bleed, coagulopathy, previous cancer, and disorders indicative of high alcohol intake (Table 1). These conditions (except congestive heart disease and previous cancer) were also more frequent in patients with a nonlobar ICH, who in addition had a higher prevalence of hypertension, peripheral arterial disease, and ischemic heart disease. Compared with their controls, lobar and nonlobar ICH cases had more frequently used anticoagulants, platelet inhibitors, and selective serotonin reuptake inhibitors. In addition, nonlobar ICH cases had more frequent preadmission use of nonsteroidal anti-inflammatory drugs and proton pump inhibitors (Table 1). History of previous disorders and preadmission drug use in nonlobar cases subclassified into deep and cerebellar were similar to nonlobar cases (eTable 2); however, there were stronger associations with preadmission use of oral anticoagulants among those with cerebellar ICH. This was also true for large unclassifiable ICH (eTable 2).
Table 1.
Characteristics of Intracerebral Hemorrhage Cases With Lobar and Nonlobar Hematoma Location and Their Respective Controls
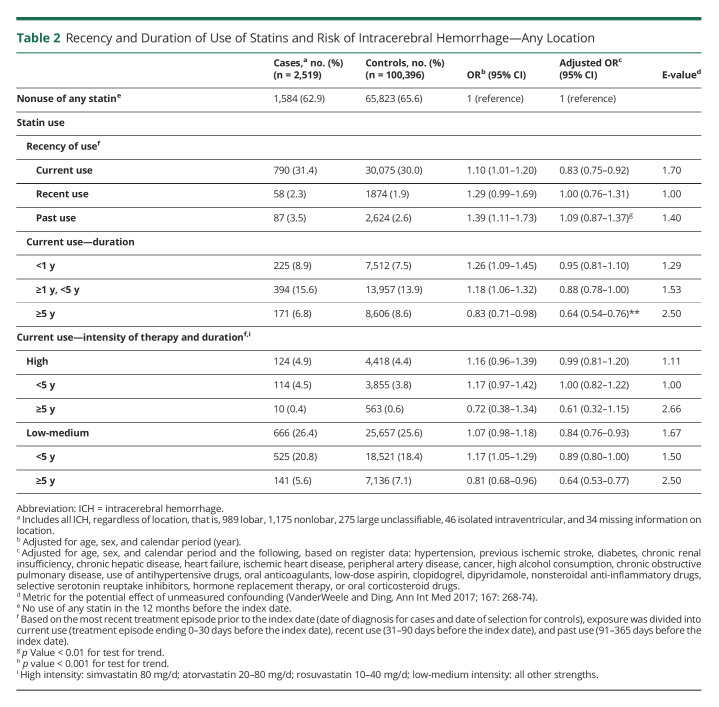
Table 2.
Recency and Duration of Use of Statins and Risk of Intracerebral Hemorrhage—Any Location
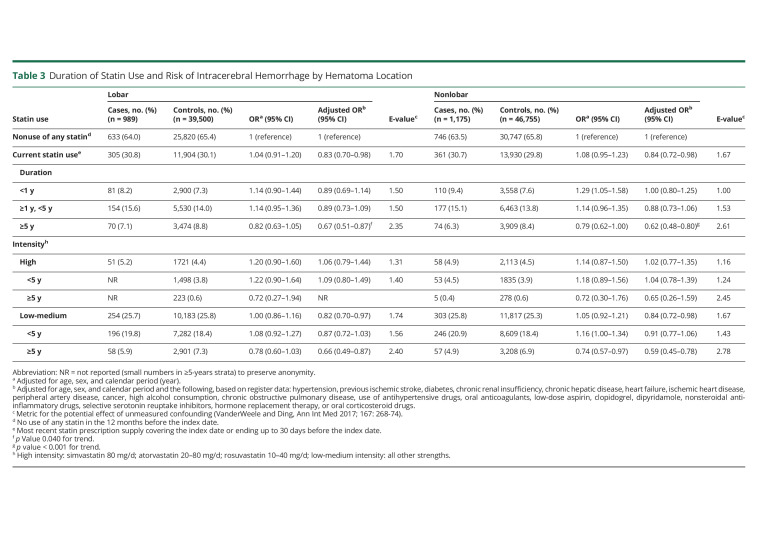
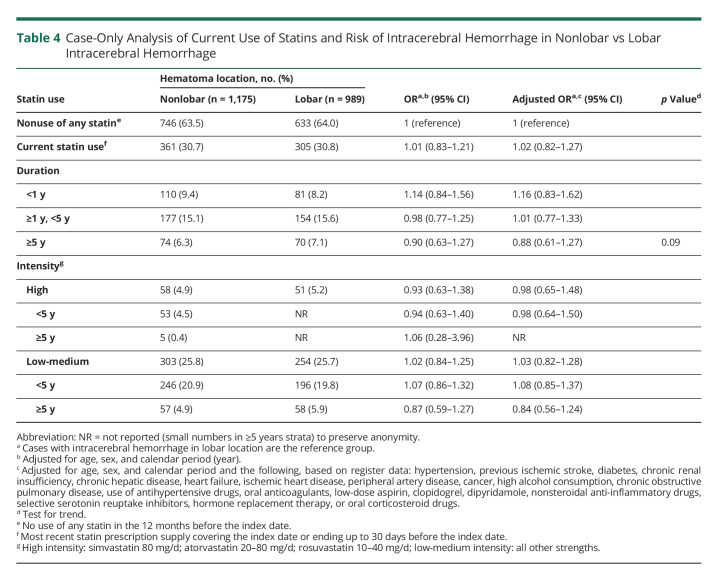
Current use of statins was associated with a lower risk of ICH overall (adjusted OR [aOR] 0.83; 95% CI, 0.75–0.92) (Table 2), a risk that was similar for lobar (aOR 0.83; 95% CI, 0.70–0.98) and nonlobar ICH (aOR 0.84; 95% CI, 0.72–0.98) (Table 3). A longer duration of statin use was also associated with a lower risk of ICH that was similar for lobar (aOR 0.89; 95% CI, 0.69–1.14 for <1 year to aOR 0.67; 95% CI, 0.51–0.87 for use ≥5 years; p for trend 0.040) and nonlobar ICH (aOR 1.00; 95% CI, 0.80–1.25 for <1 year to aOR 0.62; 95% CI 0.48–0.80 for ≥5 years; p for trend <0.001) (Table 3). This duration-response relationship was also present within strata of low-medium–intensity and high-intensity statin use, albeit based on small numbers (Table 3). Estimates stratified only by intensity of therapy were similar to the main estimates for low-medium intensity therapy (lobar aOR 0.82; nonlobar aOR 0.84), whereas the associations for high-intensity therapy were neutral (lobar aOR 1.06; nonlobar aOR 1.02) (Table 3). Similar results were found among new users (eTable 3, links.lww.com/WNL/C492) and analyses restricted to deep ICH cases and their controls (eTable 4). In analyses restricted to patients with ICH, there was no difference in ICH location based on the duration of statin use (Table 4). E-values (Tables 2 and 3) were almost consistently above 2.5 for long-term exposure (≥5 years) and lower than 2.5 for short-term (<5 years) or indefinite (e.g., current) exposure.
Table 3.
Duration of Statin Use and Risk of Intracerebral Hemorrhage by Hematoma Location
Table 4.
Case-Only Analysis of Current Use of Statins and Risk of Intracerebral Hemorrhage in Nonlobar vs Lobar Intracerebral Hemorrhage
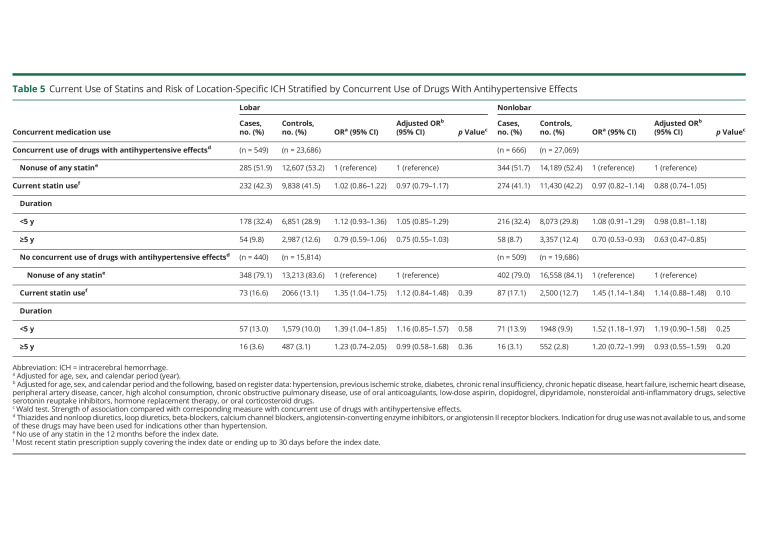
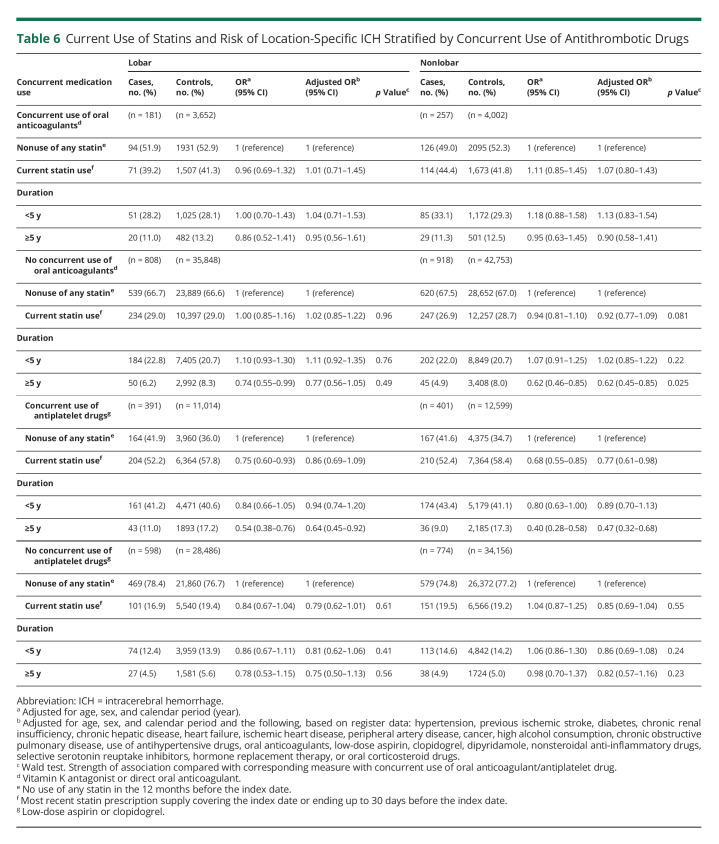
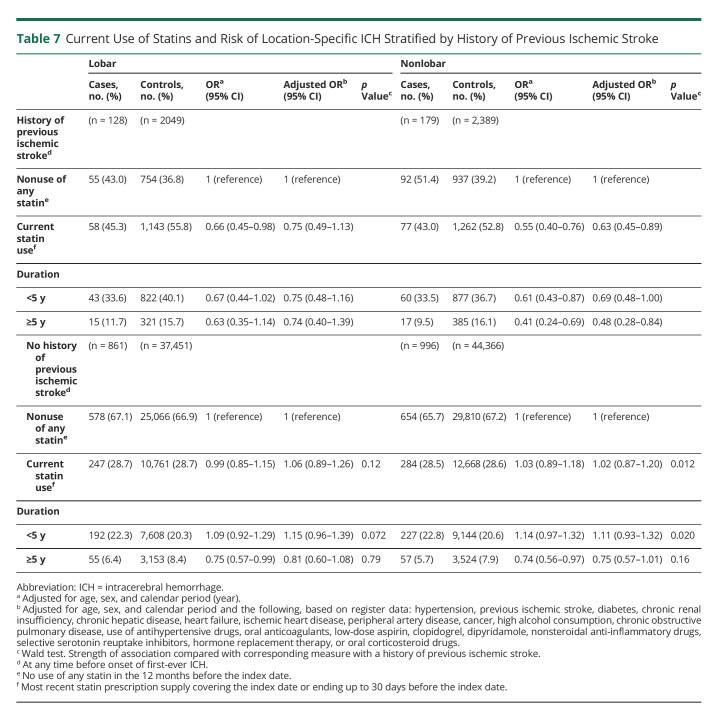
The duration-response relationship was present within subgroups defined by sex, age, and 30-day fatality of cases (eTables 5–7, links.lww.com/WNL/C492); however, in subgroups defined by concurrent use of other medications, the statin treatment duration-response relationship was only found in those treated with antihypertensive drugs (Table 5) and those not treated with oral anticoagulants (Table 6), whereas the relationship with antiplatelet drug use was less clear (Table 6). The duration-response relationship was present within subgroups defined by history of prior ischemic stroke (Table 7).
Table 5.
Current Use of Statins and Risk of Location-Specific ICH Stratified by Concurrent Use of Drugs With Antihypertensive Effects
Table 6.
Current Use of Statins and Risk of Location-Specific ICH Stratified by Concurrent Use of Antithrombotic Drugs
Table 7.
Current Use of Statins and Risk of Location-Specific ICH Stratified by History of Previous Ischemic Stroke
Brain CT Substudy Results
We included 467 lobar and 572 nonlobar ICH cases with characteristics similar to those included in the main study in the brain CT substudy (eTable 8, links.lww.com/WNL/C492). As expected,47,48 compared with lobar ICH, hematoma volumes were larger and intraventricular expansion more frequent in cases with nonlobar ICH; lacunar infarct sequelae were also more frequent in lobar cases. Total and LDL cholesterol values were similar in lobar and nonlobar cases as were renal function estimates (eGFR) (eTable 8). We found duration-response relationships similar to those found in the entire dataset in case-control (eTable 9) and case-only (eTables 10–11) substudy analyses.
Discussion
In this case-control study with 989 patients with lobar ICH and 1,175 patients with nonlobar ICH, we found that statin treatment was associated with a lower risk of both lobar and nonlobar ICH. Longer duration of treatment was also associated with lower ICH risk that was similar for lobar and nonlobar locations.
Our findings regarding a lower risk of ICH in association with statin use and long-term therapy with statins are consistent with results of recent observational studies.15,17,18 Our findings, however, did not support our hypothesis that there would be a greater benefit in reducing the risk of nonlobar ICH vs lobar ICH, as we found that the strength of the association between statin use and lower risk of lobar ICH was similar to that found for nonlobar ICH. Of note, similarity in the location-specific risks of lobar and nonlobar ICH was also found in a subanalysis using a case-only approach and was robust to use of an unbiased method of ICH location ascertainment (i.e., substudy with review of original brain scans according to CHARTS42 masked to clinical data). Our results on statin use and similar location-specific ICH risks could be explained by isolated CAA being uncommon in lobar ICH in unselected samples (i.e., ∼13% according to a population-based study with histologic confirmation24). Although lobar ICH is often (∼58%) associated with underlying CAA, this amyloid deposition disorder most frequently coexists with arteriolosclerosis,24 a condition that benefits from statin treatment. Our findings are consistent with those reported in a cohort study from Israel and a large case-control study from Italy.16,26 A multicenter study from the United States, however, reported a lower risk of nonlobar (OR 0.43; 95% CI, 0.29–0.63) compared with lobar ICH (OR 0.73; 95% CI, 0.45–1.19) with statin use.25 A nested case-control analysis of data from the Framingham Heart Study found a higher risk of lobar (OR 4.07; 95% CI, 1.16–14.21) but not nonlobar ICH (OR 0.99; 95% CI, 0.29–3.38) with statin use; these estimates, however, were based on a small sample (e.g., only 21 nonlobar cases [7 statin exposed] and 20 lobar cases [5 exposed]).22 Although methodological differences between studies limit direct comparisons, we found that the larger studies, including our own, reflect little or no variability in the association between statin use and the location of the hematoma in patients with an ICH.
Our findings indicate a possible effect modification of the association between statin use and risk of ICH in subgroups defined by concurrent use of antihypertensive medications or oral anticoagulants. We found that lower ICH risk was restricted to patients receiving both statins and concurrent antihypertensive drugs. Conversely, in analyses of oral anticoagulant comedications, only patients who were not concurrently on anticoagulants had a lower risk of ICH in association with statin use. These results could represent true effects (e.g., that a causal yet weak beneficial effect of statins is moderated by stronger effects of, e.g., untreated hypertension, or the use of anticoagulants). At least for concurrent use of antihypertensive drugs, however, spurious effects need to be considered such as healthy adherer bias,49 which may be present in statin-treated patients overall and possibly more so in those adhering to more than 1 chronic medication regimen. Also, lack of concurrent treatment with antihypertensives could be a marker for physicians who provide less optimal preventive treatment to their patients. The observational nature of our study does not allow us to determine to extent to which associations are causal.
Strengths of our study include the inclusion of all verified cases of spontaneous ICH in a defined population in 2009–2018.34,35 Our unselected cohort reflects current trends in preventive medication use and stroke diagnostic evaluation. Our use of registries, which hold data prospectively collected over decades, eliminated recall bias. By using the Civil Registration System, we could identify all residents of Southern Denmark in the study period and thereby select true general population controls.
Our study also has limitations. In a previous nationwide study with no information on hematoma location, we found that longer duration of statin treatment was associated with a lower risk of ICH within strata of therapeutic intensity, but therapeutic intensity per se (i.e., unstratified by duration of statin use) was not associated with a lower risk of ICH.18 Our study is underpowered for assessing associations of intensity of statin treatment and ICH risk, but our point estimates are indicative of effects similar to those found in the nationwide study. In the main analyses, we verified diagnoses based on partial medical records (i.e., discharge summaries and brain scan reports), which could raise concerns regarding diagnostic accuracy. The substudy with masked reevaluation of index brain scans using CHARTS,42 however, provided results similar to our main analyses, which should alleviate concerns on our method of classifying hematoma location in the main analysis. We lacked data on education and income or other markers of socioeconomic status (SES); however, in Denmark, health care is provided free of individual charge and drug costs are covered to a variable degree (depending on a patient's total health expenditure the previous year), factors that reduce the effect of SES. Furthermore, in previous analyses, addition of information on education and income to a model similar to the one used in this study had very little effect on ICH risk estimates.50 We lacked data on certain potentially important confounders (e.g., blood pressure measurements), had only partial data on others (e.g., cholesterol levels only available for cases in substudy), and needed to use proxies for smoking and alcohol. As our E-value analyses suggest, unmeasured confounding could explain some of our findings but would have to be fairly strong to account for the longer (≥5 years) statin treatment duration results. The available data did not include a measure of cognitive impairment, which can be caused by CAA, and we were unable to assess whether this might be an effect modifier for the association of statin use with ICH location. Finally, our results may not be generalizable to other populations, as the Danish population is of mainly European ancestry. Despite these caveats, we conclude that our results indicate that statin use and prolonged statin use are associated with a lower risk of spontaneous ICH that is not location specific.
Glossary
- aOR
adjusted OR
- CAA
cerebral amyloid angiopathy
- ICH
intracerebral hemorrhage
- IVH
intraventricular hemorrhage
- RSD
Region of Southern Denmark
- SVD
small vessel disease
Appendix. Authors
Footnotes
CME Course: NPub.org/cmelist
Study Funding
The project received funding from the Novo Nordisk Foundation (grant no. NNF20OC0064637; D.G.). The funding organization had no role in the design of the study or the collection, analysis, and the interpretation of the data or the interpretation of data and manuscript writing.
Disclosure
N.J. Boe, S.M. Hald, M.M. Jensen, J.A. Bojsen, M.T. Elhakim, S. Florrison, F.S.G. Harbo, O. Graumann, S. Möller, A. Clausen, and A. Saleh report no conflicts of interest. L.A. García Rodríguez works for CEIFE, which received research grants from Bayer AG for other research projects, and has also served as an advisory board member for Bayer AG. J. Hallas participated in projects outside the submitted work that were funded by AstraZeneca and Pfizer with money paid to his employer (no personal compensation). R. Al-Shahi Salman is an unpaid member of the Trial Steering Committee for the Statins In Intracerebral Hemorrhage (SATURN) trial (NCT03936361). L.B. Goldstein was a member of the Steering Committee for the Pfizer-supported SPARCL trial. D. Gaist received a speaker honorarium from Bristol-Myers Squibb and Pfizer unrelated to this work. Go to Neurology.org/N for full disclosures.
References
- 1.Kernan WN, Ovbiagele B, Black HR, et al. . Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160-2236. doi: 10.1161/STR.0000000000000024 [DOI] [PubMed] [Google Scholar]
- 2.Cholesterol Treatment Trialists (CTT) Collaboration. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174 000 participants in 27 randomised trials. Lancet. 2015;385(9976):1397-1405. doi: 10.1016/S0140-6736(14)61368-4 [DOI] [PubMed] [Google Scholar]
- 3.Goldstein LB, Amarenco P, Szarek M, et al. . Hemorrhagic stroke in the stroke prevention by aggressive reduction in cholesterol levels study. Neurology. 2008;70(24 Pt 2):2364-2370. doi: 10.1212/01.wnl.0000296277.63350.77 [DOI] [PubMed] [Google Scholar]
- 4.Collins R, Armitage J, Parish S, Sleight P, Peto R; Heart Protection Study Collaborative Group. Effects of cholesterol-lowering with simvastatin on stroke and other major vascular events in 20536 people with cerebrovascular disease or other high-risk conditions. Lancet Lond Engl. 2004;363(9411):757-767. doi: 10.1016/S0140-6736(04)15690-0 [DOI] [PubMed] [Google Scholar]
- 5.Hackam DG, Woodward M, Newby LK, et al. . Statins and intracerebral hemorrhage: collaborative systematic review and meta-analysis. Circulation. 2011;124(20):2233-2242. doi: 10.1161/CIRCULATIONAHA.111.055269 [DOI] [PubMed] [Google Scholar]
- 6.McKinney JS, Kostis WJ. Statin therapy and the risk of intracerebral hemorrhage: a meta-analysis of 31 randomized controlled trials. Stroke. 2012;43(8):2149-2156. doi: 10.1161/STROKEAHA.112.655894 [DOI] [PubMed] [Google Scholar]
- 7.Ziff OJ, Banerjee G, Ambler G, Werring DJ. Statins and the risk of intracerebral haemorrhage in patients with stroke: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2019;90(1):75-83. doi: 10.1136/jnnp-2018-318483 [DOI] [PubMed] [Google Scholar]
- 8.Sanz-Cuesta BE, Saver JL. Lipid-Lowering therapy and hemorrhagic stroke risk: comparative meta-analysis of statins and PCSK9 inhibitors. Stroke. 2021;52(10):3142-3150. doi: 10.1161/STROKEAHA.121.034576 [DOI] [PubMed] [Google Scholar]
- 9.FitzMaurice E, Wendell L, Snider R, et al. . Effect of statins on intracerebral hemorrhage outcome and recurrence. Stroke. 2008;39(7):2151-2154. doi: 10.1161/STROKEAHA.107.508861 [DOI] [PubMed] [Google Scholar]
- 10.Hackam DG, Austin PC, Huang A, et al. . Statins and intracerebral hemorrhage: a retrospective cohort study. Arch Neurol. 2012;69(1):39-45. doi: 10.1001/archneurol.2011.228 [DOI] [PubMed] [Google Scholar]
- 11.Chen PS, Cheng CL, Chang YC, Kao Yang YH, Yeh PS, Li YH. Early statin therapy in patients with acute intracerebral hemorrhage without prior statin use. Eur J Neurol. 2015;22(5):773-780. doi: 10.1111/ene.12649 [DOI] [PubMed] [Google Scholar]
- 12.Gaist D, Goldstein LB, Cea Soriano L, García Rodríguez LA. Statins and the risk of intracerebral hemorrhage in patients with previous ischemic stroke or transient ischemic attack. Stroke. 2017;48(12):3245-3251. doi: 10.1161/STROKEAHA.117.019141 [DOI] [PubMed] [Google Scholar]
- 13.Ribe AR, Vestergaard CH, Vestergaard M, et al. . Statins and risk of intracerebral hemorrhage in individuals with a history of stroke. Stroke. 2020;51(4):1111-1119. doi: 10.1161/STROKEAHA.119.027301 [DOI] [PubMed] [Google Scholar]
- 14.Åsberg S, Eriksson M. Statin therapy and the risk of intracerebral haemorrhage: a nationwide observational study. Int J Stroke Off J Int Stroke Soc. 2015;10(Suppl A100):46-49. doi: 10.1111/ijs.12539 [DOI] [PubMed] [Google Scholar]
- 15.Ribe AR, Vestergaard CH, Vestergaard M, et al. . Statins and risk of intracerebral haemorrhage in a stroke-free population: a nationwide Danish propensity score matched cohort study. EClinicalMedicine. 2019;8:78-84. doi: 10.1016/j.eclinm.2019.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saliba W, Rennert HS, Barnett-Griness O, et al. . Association of statin use with spontaneous intracerebral hemorrhage: a cohort study. Neurology. 2018;91(5):e400-e409. doi: 10.1212/WNL.0000000000005907 [DOI] [PubMed] [Google Scholar]
- 17.Jung M, Lee S. Effects of statin therapy on the risk of intracerebral hemorrhage in Korean patients with hyperlipidemia. Pharmacotherapy. 2019;39(2):129-139. doi: 10.1002/phar.2211 [DOI] [PubMed] [Google Scholar]
- 18.Rudolph DA, Hald SM, García Rodríguez LA, et al. . Association of long-term statin use with the risk of intracerebral hemorrhage: a Danish nationwide case-control study. Neurology. 2022;99(7):e711-e719. doi: 10.1212/WNL.0000000000200713 [DOI] [PubMed] [Google Scholar]
- 19.Woo D, Sauerbeck LR, Kissela BM, et al. . Genetic and environmental risk factors for intracerebral hemorrhage: preliminary results of a population-based study. Stroke. 2002;33(5):1190-1195. [DOI] [PubMed] [Google Scholar]
- 20.Haussen DC, Henninger N, Kumar S, Selim M. Statin use and microbleeds in patients with spontaneous intracerebral hemorrhage. Stroke. 2012;43(10):2677-2681. doi: 10.1161/STROKEAHA.112.657486 [DOI] [PubMed] [Google Scholar]
- 21.Romero JR, Preis SR, Beiser A, et al. . Risk factors, stroke prevention treatments, and prevalence of cerebral microbleeds in the Framingham Heart Study. Stroke. 2014;45(5):1492-1494. doi: 10.1161/STROKEAHA.114.004130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lioutas VA, Beiser AS, Aparicio HJ, et al. . Assessment of incidence and risk factors of intracerebral hemorrhage among participants in the Framingham heart study between 1948 and 2016. JAMA Neurol. 2020;77(10):1252-1260. doi: 10.1001/jamaneurol.2020.1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samarasekera N, Smith C, Al-Shahi Salman R. The association between cerebral amyloid angiopathy and intracerebral haemorrhage: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2012;83(3):275-281. doi: 10.1136/jnnp-2011-300371 [DOI] [PubMed] [Google Scholar]
- 24.Rodrigues MA, Samarasekera N, Lerpiniere C, et al. . The Edinburgh CT and genetic diagnostic criteria for lobar intracerebral haemorrhage associated with cerebral amyloid angiopathy: model development and diagnostic test accuracy study. Lancet Neurol. 2018;17(3):232-240. doi: 10.1016/S1474-4422(18)30006-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martini SR, Flaherty ML, Brown WM, et al. . Risk factors for intracerebral hemorrhage differ according to hemorrhage location. Neurology. 2012;79(23):2275-2282. doi: 10.1212/WNL.0b013e318276896f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pezzini A, Grassi M, Iacoviello L, et al. . Serum cholesterol levels, HMG-CoA reductase inhibitors and the risk of intracerebral haemorrhage. The Multicenter Study on Cerebral Haemorrhage in Italy (MUCH-Italy). J Neurol Neurosurg Psychiatry. 2016;87(9):924-929. doi: 10.1136/jnnp-2015-312736 [DOI] [PubMed] [Google Scholar]
- 27.Johnsen SP, Ingeman A, Hundborg HH, Schaarup SZ, Gyllenborg J. The Danish stroke registry. Clin Epidemiol. 2016;8:697-702. doi: 10.2147/CLEP.S103662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pottegård A, Schmidt SAJ, Wallach-Kildemoes H, Sørensen HT, Hallas J, Schmidt M. Data resource profile: the Danish national prescription registry. Int J Epidemiol. 2017;46(3):798-798f. doi: 10.1093/ije/dyw213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmidt M, Schmidt SAJ, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449-490. doi: 10.2147/CLEP.S91125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidt M, Pedersen L, Sørensen HT. The Danish Civil registration System as a tool in epidemiology. Eur J Epidemiol. 2014;29(8):541-549. doi: 10.1007/s10654-014-9930-3 [DOI] [PubMed] [Google Scholar]
- 31.Henriksen DP, Rasmussen L, Hansen MR, Hallas J, Pottegård A. Comparison of the five Danish regions regarding demographic characteristics, healthcare utilization, and medication use—a descriptive cross-sectional study. PLoS One. 2015;10(10):e0140197. doi: 10.1371/journal.pone.0140197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thygesen LC, Daasnes C, Thaulow I, Brønnum-Hansen H. Introduction to Danish (nationwide) registers on health and social issues: structure, access, legislation, and archiving. Scand J Public Health. 2011;39(7 Suppl):12-16. [DOI] [PubMed] [Google Scholar]
- 33.Linn J, Halpin A, Demaerel P, et al. . Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology. 2010;74(17):1346-1350. doi: 10.1212/WNL.0b013e3181dad605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hald S, Sloth C, Agger M, et al. . The validity of intracerebral hemorrhage diagnoses in the Danish Patient Registry and the Danish Stroke Registry. Clin Epidemiol. 2020;12:1313-1326. doi: 10.2147/CLEP.S267583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hald SM, Kring Sloth C, Hey SM, et al. . Intracerebral hemorrhage: positive predictive value of diagnosis codes in two nationwide Danish registries. Clin Epidemiol. 2018;10:941-948. doi: 10.2147/CLEP.S167576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Samarasekera N, Fonville A, Lerpiniere C, et al. . Influence of intracerebral hemorrhage location on incidence, characteristics, and outcome: population-based study. Stroke. 2015;46(2):361-368. doi: 10.1161/STROKEAHA.114.007953 [DOI] [PubMed] [Google Scholar]
- 37.Pasi M, Pongpitakmetha T, Charidimou A, et al. . Cerebellar microbleed distribution patterns and cerebral amyloid angiopathy. Stroke. 2019;50(7):1727-1733. doi: 10.1161/STROKEAHA.119.024843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kremer PHC, Jolink WMT, Kappelle LJ, Algra A, Klijn CJM, SMART and ESPRIT study groups. Risk factors for lobar and non-lobar intracerebral hemorrhage in patients with vascular disease. PLoS One. 2015;10(11):e0142338. doi: 10.1371/journal.pone.0142338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Das AS, Regenhardt RW, Gokcal E, et al. . Idiopathic primary intraventricular hemorrhage and cerebral small vessel disease. Int J Stroke. 2022;17(6):645-653. doi: 10.1177/17474930211043957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stone NJ, Robinson JG, Lichtenstein AH, et al. . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2889-2934. doi: 10.1016/j.jacc.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 41.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158(9):915-920. doi: 10.1093/aje/kwg231 [DOI] [PubMed] [Google Scholar]
- 42.Charidimou A, Schmitt A, Wilson D, et al. . The cerebral haemorrhage anatomical RaTing inStrument (CHARTS): development and assessment of reliability. J Neurol Sci. 2017;372:178-183. doi: 10.1016/j.jns.2016.11.021 [DOI] [PubMed] [Google Scholar]
- 43.Kothari RU, Brott T, Broderick JP, et al. . The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27(8):1304-1305. doi: 10.1161/01.str.27.8.1304 [DOI] [PubMed] [Google Scholar]
- 44.Graeb DA, Robertson WD, Lapointe JS, Nugent RA, Harrison PB. Computed tomographic diagnosis of intraventricular hemorrhage. Etiology and prognosis. Radiology. 1982;143(1):91-96. doi: 10.1148/radiology.143.1.6977795 [DOI] [PubMed] [Google Scholar]
- 45.van Swieten JC, Hijdra A, Koudstaal PJ, van Gijn J. Grading white matter lesions on CT and MRI: a simple scale. J Neurol Neurosurg Psychiatry. 1990;53(12):1080-1083. doi: 10.1136/jnnp.53.12.1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167(4):268-274. doi: 10.7326/M16-2607 [DOI] [PubMed] [Google Scholar]
- 47.Roh D, Sun CH, Murthy S, et al. . Hematoma expansion differences in lobar and deep primary intracerebral hemorrhage. Neurocrit Care. 2019;31(1):40-45. doi: 10.1007/s12028-018-00668-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Witsch J, Bruce E, Meyers E, et al. . Intraventricular hemorrhage expansion in patients with spontaneous intracerebral hemorrhage. Neurology. 2015;84(10):989-994. doi: 10.1212/WNL.0000000000001344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patrick AR, Shrank WH, Glynn RJ, et al. . The association between statin use and outcomes potentially attributable to an unhealthy lifestyle in older adults. Value Health. 2011;14(4):513-520. doi: 10.1016/j.jval.2010.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hald SM, Möller S, García Rodríguez LA, et al. . Trends in incidence of intracerebral hemorrhage and association with antithrombotic drug use in Denmark, 2005-2018. JAMA Netw Open. 2021;4(5):e218380. doi: 10.1001/jamanetworkopen.2021.8380 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Danish law prohibits the authors from sharing or granting access to the data used for this study.