Abstract
Background and Objectives
Orthostatic hypotension (OH) increases dementia risk in patients with Parkinson disease (PD), although the underlying mechanisms and whether a similar association between OH and cognitive impairment exists in other synucleinopathies remain unknown. The aim is to evaluate the association between OH and dementia risk in patients with PD, and cognitive impairment risk in patients with multiple system atrophy (MSA), and to explore relevant clinical and neuropathologic factors to understand underlying pathogenic mechanisms.
Methods
This is a retrospective cohort study. Medical records throughout the entire disease course of consecutive patients with neuropathology-confirmed PD and MSA from the Queen Square Brain Bank were systematically reviewed. Time of onset and severity of OH-related symptoms were documented, and their association with other clinical and neuropathologic variables was evaluated. Dementia risk for patients with PD and cognitive impairment risk for patients with MSA were estimated using multivariable hazard regression.
Results
One hundred thirty-two patients with PD and 137 with MSA were included. Patients with MSA developed OH more frequently, earlier in the disease course and with more severe symptoms. Cumulative dementia prevalence was higher in patients with PD. Multivariable adjusted regression models showed that early OH, but not its symptom severity, increased dementia risk in patients with PD by 14% per year (hazard ratio [HR] = 0.86; 95% CI, 0.80–0.93) and cognitive impairment risk in patients with MSA by 41% per year (HR = 0.59; 95% CI, 0.42–0.83). Early OH was not associated with increased α-synuclein, β-amyloid, tau, Alzheimer, or cerebrovascular pathologies. No significant associations were found between severity of OH symptoms and other clinical or neuropathologic variables.
Discussion
Early OH, but not its symptom severity, increases the risk of cognitive impairment in patients with PD and MSA. OH is not associated with more extensive Lewy, β-amyloid, tau, Alzheimer, or cerebrovascular pathologies. It is likely that OH contributes to cognitive impairment in patients with PD and MSA by hypoxia-induced nonspecific neurodegeneration. Further research should evaluate whether improving brain perfusion by treating OH may modify the risk of dementia in these conditions.
Orthostatic hypotension (OH) is one of the most common manifestations of Parkinson disease (PD) and can present at early stages.1 A growing body of evidence suggests that there is an association between OH and increased dementia risk in patients with PD.2,3 Therefore, OH could be a potential modifiable risk factor for cognitive impairment in patients with PD.4 However, the pathogenic mechanisms linking both conditions are complex and remain poorly understood.3 This association is independent of other autonomic symptoms, and it is present even in asymptomatic OH,5 suggesting a causative link rather than shared neuroanatomical basis or a more diffuse neuropathologic involvement. OH has shown to cause impairment in cerebral blood supply, and the most accepted hypothesis is that chronic hypoxia secondary to repeated episodes of cerebral hypoperfusion would lead to increased vascular lesions and synergistic neurodegeneration resulting in cognitive impairment.2,3 OH correlates with severity of white matter hyperintensities on magnetic resonance, which are a presumed imaging marker of small vessel disease independently associated with cognitive impairment in patients with PD.6,7 Moreover, additional studies have shown that OH can be associated with imaging markers of neurodegeneration, such as anterior temporal and mediotemporal atrophies on MRI,8 and with increased CSF neurofilament light chain levels, a nonspecific marker of neuronal damage.9
OH is one of the diagnostic features of multiple system atrophy (MSA), and its associated symptoms are more prevalent and usually more severe than in patients with PD.10 OH has been associated with reduced survival in patients with pathologically proven MSA.11 Despite OH being more prevalent and symptomatic, dementia in patients with MSA is rarer and was initially regarded as a feature to reconsider diagnosis. Recent studies showed that <30% of patients with MSA confirmed neuropathologically showed cognitive impairment with a pattern of frontal executive dysfunction similar to patients with PD and PSP that can be present at early stages.12,13 Cognitive deficits in patients with MSA remain poorly characterized, and whether OH increases the risk of future dementia in patients with MSA as in PD remains to be evaluated.
Last, up to 50% of patients with PD or MSA and OH may have concomitant supine hypertension (SH), defined as the presence of blood pressure ≥140/90 mmHg measured after at least 5 minutes of rest in the supine position.14 Emerging evidence suggests that SH may contribute to cognitive impairment in neurodegenerative disorders, although data on patients with PD and MSA are scarce.15,16
Our hypothesis is that earlier and more severe OH will be associated with greater risk of dementia in patients with PD and MSA. Based on currently accepted pathophysiologic explanations, we hypothesized that those with earlier and more severe OH will show more severe neurodegenerative proteinopathies and/or cerebrovascular changes at neuropathologic examination. In this study, we assessed the impact of OH parameters (timing of onset and severity of symptoms) on progression to cognitive impairment, evaluated whether the association between OH and cognitive dysfunction differed by the presence of concomitant SH, and correlated these data with the neuropathologic findings in a large cohort of pathology-proven patients with PD and MSA. We explored similarities and differences in OH and cognition in these synucleinopathies to further understand the pathophysiologic pathways linking OH with dementia because this could lead to identification of new therapeutic targets for cognitive impairment in these diseases.
Methods
Participants and Study Design
This retrospective cohort study included consecutive pathology-confirmed patients with brain donation to the Queen Square Brain Bank in London, United Kingdom, between 2009 and 2019 for patients with PD and between 2002 and 2018 for patients with MSA. Patients were excluded if an additional diagnosis of a neuropathologic condition was found on postmortem examination or comorbidities known to affect autonomic function (e.g., diabetic neuropathy) were present.
Clinical Assessment
A systematic review of all available medical records was retrospectively performed. All patients were regularly reviewed by experienced hospital specialists (neurologists and geriatricians) in the United Kingdom throughout their disease course and by primary care professionals according to clinical needs. Medical records included primary care medical notes, National Hospital for Neurology and Neurosurgery medical records, correspondence from hospital specialists, Queen Square Brain Bank reports, and self-assessment forms to the time of death. Clinical data were collated by neurologists with expertise in movement disorders (I.R.B., Y.M., and E.d.P.-F.), and all clinical data were obtained from routine clinical information. Patients without detailed clinical information sufficient to accurately document the main variables of the study throughout the disease were not included. OH was defined by a documented decrease >20 mm Hg in systolic or >10 mm Hg in diastolic blood pressure on standing or by the presence of orthostatic symptoms suggesting OH persistent over at least 6 months and after therapeutic measures to address non-neurogenic OH had been implemented based on the clinical impression of the treating physician. Patients with suspected non-neurogenic OH were not included in the study. Severity of OH symptoms was graded using a 4-point semiquantitative scale based on the impact and necessity of therapeutic intervention as previously described17: (0) absent; (1) mild severity/mild distress to patient/no therapeutic intervention required; (2) moderate severity/moderate distress/good symptomatic control with therapeutic intervention; and (3) severe intensity/severe distress/poor symptomatic control despite therapeutic interventions. SH was evaluated during the diagnosis of OH and graded as mild (140–159/90-99 mm Hg), moderate (160–179/100-109 mm Hg) or severe (≥180/110 mm Hg) as per consensus definition.14 Dementia was defined as cognitive dysfunction documented by a clinician or neuropsychological test severe enough to significantly affect tasks of daily living not attributable to motor impairment. When objective documented cognitive deficits were insufficient to interfere with functional independence, they were categorized as mild cognitive impairment.12,18 Subjective patient's or caregiver's cognitive complaints were excluded from these categories unless confirmed by a clinician or documented neuropsychological test. In patients with PD with a detailed cognitive examination, cognitive impairment was classified into “frontal executive” or “posterior cortical” according to the pattern of deficits.19 Similarly, patients with MSA and cognitive impairment were classified as fronto-subcortical or memory impairment predominant as previously described.20 Progression of cognitive deficits was evaluated using the time from diagnosis to dementia for patients with PD or to any type of cognitive impairment (including mild cognitive impairment and dementia) in patients with MSA because cases with incident dementia were expected to be low in this group. Response to initial levodopa treatment was measured using a 4-point semiquantitative scale (nil to mild; moderate; good; and excellent) derived from the UK Parkinson Brain Bank criteria response to levodopa therapy based on the clinical impression documented by the treating physician.11,21 Maximum levodopa equivalent daily dose in mg was estimated for patients with PD.22 Additional clinical variables were collected for the PD group. Patients were classified according to PD motor subtype into tremor predominant, akinetic rigid or postural instability, and gait difficulty. Severity of motor and cognitive symptoms during diagnosis was graded using the similar 4-point semiquantitative score used for OH symptoms based on symptom severity, functional impairment, and/or therapeutic need and response as previously published.17,23
Neuropathologic Assessment
Neuropathologic evaluation followed standard Queen Square Brain Bank protocols. Brain tissue was fixed in 10% formalin buffer for 3 weeks, and paraffin-embedded 8-µm sections were sampled from representative regions. Sections were stained with hematoxylin and eosin and a panel of immunohistochemistry antibodies for neurodegenerative diseases including α-synuclein, β-amyloid, 3-repeat tau, 4-repeat tau, and phosphorylated tau. Neuropathologic diagnosis of PD and MSA was performed according to current consensus criteria. Severity and distribution of Lewy pathology was evaluated using the Lewy body–type classification (brainstem; limbic; and diffuse neocortical) and Braak staging system.24,25 Patients with MSA were classified into 4 pathologic subtypes based on previously published criteria.26,27
Only data from the systematic neuropathologic assessment of patients with PD is presented in this study because the neuropathologic abnormalities underlying cognitive impairment in patients with MSA have been previously reported elsewhere in detail.20,28 For those with PD, β-amyloid deposition, neuritic plaques, and neurofibrillary tangles were also evaluated according to Thal phase, the Consortium to Establish a Registry for Alzheimer Disease scheme, and Braak and Braak stage, respectively.29-31 Alzheimer disease neuropathologic changes were assessed using the ABC scoring system proposed by the National Institute on Aging (absent, low, intermediate, and high).32 Assessment of cerebrovascular pathology following the Vascular Cognitive Impairment Neuropathology Guidelines33 including semiquantitative grading of multiple vascular changes (arteriosclerosis, arteriolosclerosis, macroinfarcts/microinfarcts and hemorrhages, and cortical, capillary and leptomeningeal cerebral amyloid angiopathy changes) in representative brain regions was available in a subgroup of patients.
Statistical Analysis
Comparisons between groups were performed using the χ2 test for categorical variables, independent t test for continuous variables, and Kruskal-Wallis test for ordinal variables, as appropriate. A linear regression model was used to analyze potential associations between OH parameters and the main explanatory variables. Multivariable Cox proportional hazards regression models were used to estimate the risk of dementia for patients with PD and the risk of cognitive impairment (including mild cognitive impairment and dementia) for patients with MSA. The decision to focus the regression analysis on patients with MSA and any type of cognitive impairment was made for statistical purposes (because dementia is a rare feature of MSA and the expected low number of cases with incident dementia in the MSA group was unlikely to have enough statistical power to confidently evaluate the association), based on the methodology used in previous research evaluating cognitive function in patients with MSA13,20,34-37 and the fact that dementia is an exclusion feature in the diagnostic criteria.38 Grouping patients with MSA with cognitive impairment was also felt to be more relevant for the identification of potential pathogenic mechanisms. Other potential confounders in the association, including age at diagnosis, sex, pattern of cognitive impairment, and PD clinical features, were included in the model as explanatory variables. Only those with a relevant association in the adjusted multivariate analysis were included in the final model. Adjusted hazard ratios (HRs) and 95% confidence intervals were calculated to estimate effect size. Patients with PD and MSA were divided according to the time of OH onset into 2 equal-sized groups by the median value (early OH and late OH) for visual representation purposes. Kaplan-Meier curves of dementia and survival were plotted and their visual inspection and plots of scaled Schoenfeld residuals against time were used to assess the proportional hazards assumption. Censoring was considered uninformative. Results were considered statistically significant at 2-tailed p < 0.05. Stata statistical software version 17 (StataCorp) was used to perform all statistical analyses.
Standard Protocol Approvals, Registrations, and Patient Consents
The brain donor program and protocol were approved by the London-Central Research Ethics Committee (18/LO/0721), all donors provided written informed consent, and brain tissue is stored for research under a license issued by the Human Tissue Authority (12198).
Data Availability
Data not provided in the article because of space limitations may be shared (anonymized) at the request of any qualified investigator, if in compliance with regulatory ethical approvals, for purposes of replicating procedures and results.
Results
One hundred thirty-two patients with PD and 137 patients with MSA were included in the analysis, and their demographic data and clinical features are summarized in Table 1. Patients with MSA had younger age at onset and faster disease progression with shorter survival. In patients with PD, 67 of 132 (51%) developed OH (confirmed with blood pressure measurements or cardiovascular autonomic test in 91%) while 107 of 137 (78%) patients with MSA had OH (confirmed with blood pressure measurements or cardiovascular autonomic test in 98%). Patients with MSA developed OH earlier in the disease course and with more severe symptoms than patients with PD (Table 1). Among those with OH, 22 (35.5%) patients with PD and 30 (29.4%) with MSA had concomitant SH during the diagnosis of OH. The cumulative prevalence for dementia in patients with PD was 57.6% (76 of 132) while only 10 patients with MSA of 137 (7.3%) developed frank dementia (χ2 = 39.043; p < 0.001) with 10 more additional patients with MSA with mild cognitive impairment. A frontal-subcortical cognitive syndrome was the predominant pattern of cognitive impairment in both conditions. Dementia was a late feature in patients with PD and occurred at earlier stages of disease in patients with MSA (Table 1). Neuropathologic assessment showed that patients with PD had advanced α-synuclein pathology with 115 of 132 (87.1%) cases with Braak stage 6 and 111 of 132 (84.1%) cases with diffuse neocortical Lewy pathology. Further details on β-amyloid, tau, and cerebrovascular pathologies are shown in Table 2.
Table 1.
Demographic and Clinical Characteristics
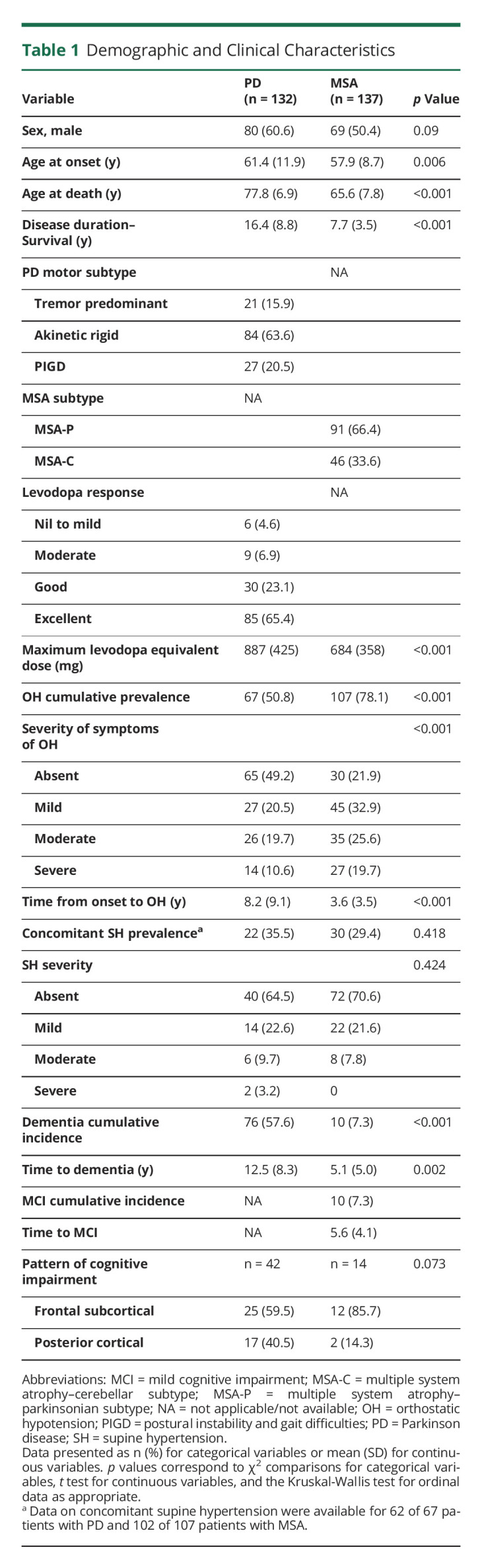
Table 2.
Neuropathologic Data of Cases With Parkinson Disease and Multiple System Atrophy
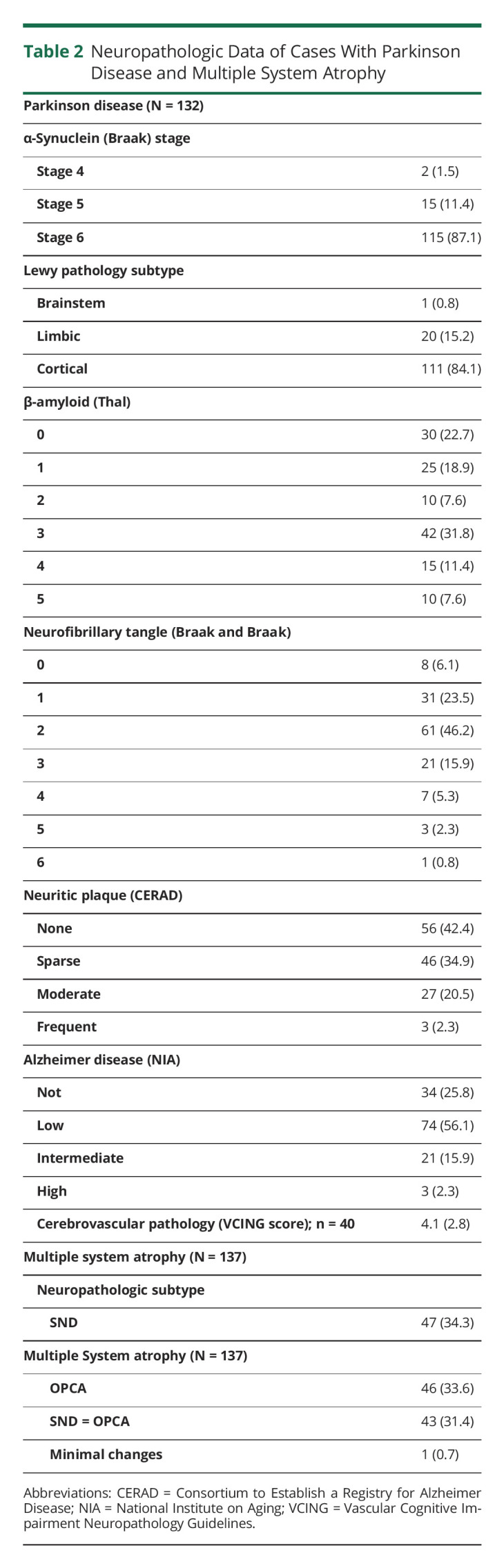
Association of Time of Onset of OH With Other Clinical and Pathologic Variables
Patients with PD and early OH showed a distinctive clinical phenotype, with older age at diagnosis, postural instability and gait difficulty motor subtype, poorer response to levodopa, and poorer cognitive performance at diagnosis, although there was no association with severity of motor symptoms (see Table 3 for effect size estimates). In patients with MSA, development of OH was associated with older age. The pattern of cognitive impairment in patients with PD with early OH tended to have a posterior cortical pattern, although this association did not reach statistical significance. Early OH in patients with PD was associated with limbic distribution of α-synuclein pathology (limbic subtype), although there were no other significant associations with Braak stages of α-synuclein deposition, β-amyloid, tau, or vascular pathologies (Table 3).
Table 3.
Association of Time of Onset of Orthostatic Hypotension With Other Variables in Cases With PD and MSA
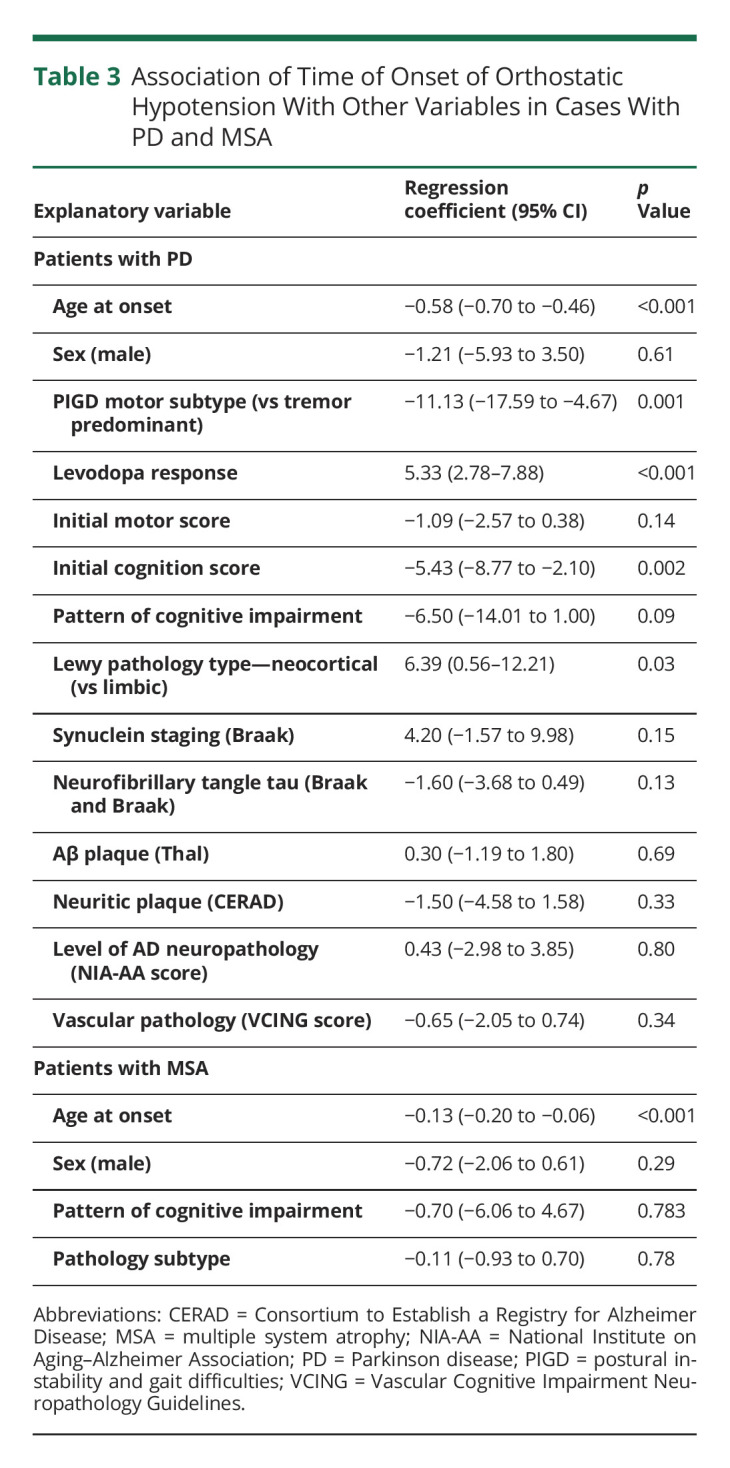
Association of Severity of OH Symptoms With Other Clinical and Pathologic Variables
Analysis of the severity of OH symptoms did not show any significant association with demographic, clinical, or pathologic variables in patients with PD or MSA (Table 4).
Table 4.
Association of Severity of Orthostatic Hypotension Symptoms With Other Variables in Cases With PD and MSA
OH and Risk of Cognitive Impairment and Dementia
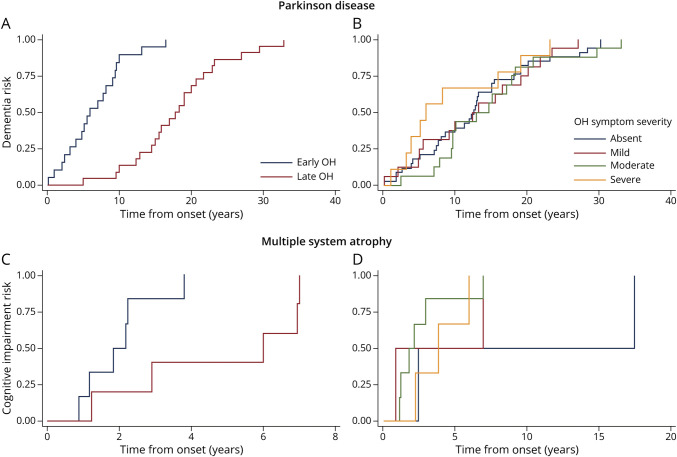
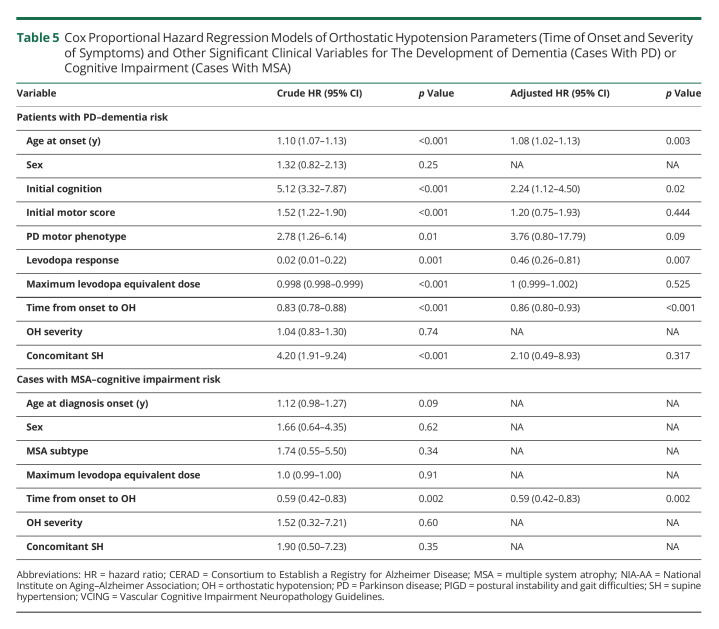
Earlier development of OH was associated with developing dementia in patients with PD (Figures 1 and 2). Development of OH increased the risk of dementia by 14% per year (HR = 0.86; 95% CI, 0.80–0.93) after adjusting for other potential confounding variables (Table 5). In the univariate analysis, severity of OH symptoms was not associated with increased dementia risk in patients with PD (Figure 2). The risk of severity of different proteinopathies and dementia was also evaluated. In the univariate analysis, severity of tau pathology and Alzheimer pathologies increased dementia risk in patients with PD, although only the latter remained statistically significant in the multivariate analysis (high Alzheimer neuropathology NIA score HR 9.58 (2.52–36.41); p = 0.001). The presence of concomitant SH was associated with an increased risk of dementia in patients with PD in the univariate analysis, although it did not remain significant after adjusting for other relevant factors in the multivariate analysis. In addition to early OH, older age, poor cognitive performance at diagnosis, and poor response to levodopa were other independent variables associated with dementia in PD in the multivariate model (Table 5).
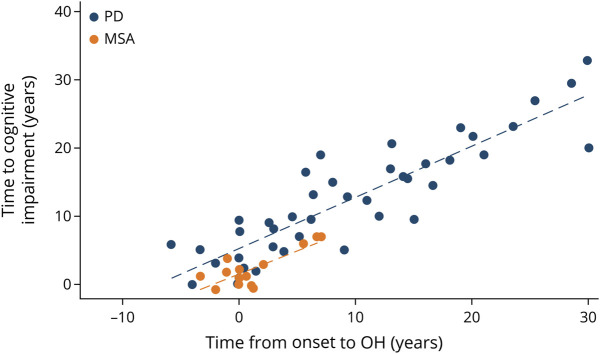
Figure 1. Scatterplot Showing the Association Between Time of Onset of Orthostatic Hypotension and Time to Development of Cognitive Impairment for Cases With MSA (Orange) and Dementia for PD Cases (Blue).
Figure 2. Kaplan-Meier of Cumulative Risk of Dementia in PD and Risk of Cognitive Impairment in MSA by Time of Onset of Orthostatic Hypotension (A) and (C) and Severity of Symptoms of Orthostatic Hypotension (B) and (D).
Table 5.
Cox Proportional Hazard Regression Models of Orthostatic Hypotension Parameters (Time of Onset and Severity of Symptoms) and Other Significant Clinical Variables for The Development of Dementia (Cases With PD) or Cognitive Impairment (Cases With MSA)
Similar results were found in patients with MSA. Early OH was associated with an increase of 41% risk of future cognitive impairment per year (HR = 0.59; 95% CI, 0.42–0.83), although severity of OH symptoms or concomitant SH did not show any significant change in cognitive impairment risk (Table 5 and Figures 1 and 2).
Discussion
This large retrospective clinicopathologic study demonstrates an association between early development of OH—but not severity of OH symptoms—and future dementia in PD and cognitive impairment in MSA despite clinical differences in OH and cognitive deficits between these conditions. The pattern of cognitive impairment associated with OH did not show distinctive features for these conditions, although detailed neuropsychometry was available only in a proportion of cases. Our clinical and neuropathologic data demonstrated that the association between OH and cognitive impairment is not due to shared neuroanatomical basis, more extensive specific neurodegenerative proteinopathies, or an increased burden of cerebrovascular disease. Repeated cerebral hypoperfusion secondary to OH may induce nonspecific neurodegeneration contributing to cognitive impairment.
Our results demonstrated that OH is associated with an increased risk of dementia of 14% per year in patients with PD and 41% risk of cognitive impairment per year in patients with MSA over the entire course of the disease. The increased risk is independent of the presence of concomitant SH and other factors associated with cognitive impairment. These results are consistent with previous prospective cohort studies showing a 3-fold increased risk of dementia in patients with PD and OH over a mean 4 years of follow-up.4,39 Data from literature evaluating this association in patients with MSA are scarce and derive mainly from relatively small series, with short follow-up periods and without neuropathologic confirmation. Results are less conclusive than for patients with PD, with some studies showing a positive association,34,36 whereas others did not find any correlation.35,37 Our study represents a large clinicopathologic series of patients with pathology-confirmed MSA with clinical information on OH and cognition throughout the entire disease course providing robust data demonstrating an increased risk of cognitive impairment in patients with MSA with OH. Although OH seems to independently increase the risk of cognitive impairment in both synucleinopathies, there are additional factors contributing to cognitive deterioration that could modify effect size of this association, explaining the paradoxical finding that patients with MSA have lower rates of cognitive impairment despite more frequent and earlier development of OH than patients with PD. The most obvious factors that may explain these pathophysiologic differences are disease duration and age at death. Assuming a cumulative damage from early development of OH, patients with MSA have shorter survival and therefore may not have sufficient exposure to OH-related chronic hypoxia to develop cognitive difficulties. Younger age at death means that they are less likely to have aging-related changes or additional proteinopathies contributing to neurodegenerative changes associated with cognitive impairment because deposition of β-amyloid is associated with age and is more common in patients with PD.40 Growing evidence suggest that differences in neuronal vulnerability and molecular characteristics of α-synuclein between these diseases may additionally contribute to the distinct phenotypes between synucleinopathies41,42 and, potentially, they could also have an influence on the development and effect of OH on cognitive function.
In both patients with PD and patients with MSA, time of onset of OH was the relevant factor in association with cognitive dysfunction, while the severity of OH symptoms did not increase the risk of cognitive impairment in either condition. These findings suggest that the deleterious effect of OH causes cumulative damage leading to cognitive deficits and dementia, even when asymptomatic or mildly symptomatic. Repeat bouts of OH compromising brain perfusion and inducing chronic hypoxia is the most likely pathogenic mechanism. Both ischemic and neurodegenerative changes secondary to chronic hypoxia have been demonstrated in animal models and human studies.2,3,43
In our study, 35.5% patients with PD and 29.4% patients with MSA had concomitant SH at the time of diagnosis of OH. The deleterious effects of OH on cognitive function were independent of the presence of hypertension, and concomitant SH was not associated with cognitive dysfunction in patients with PD or MSA in our cohort. Our results are in contrast with recent research showing that SH may contribute to the development of cognitive impairment in patients with PD by increasing the burden of white matter hyperintensities (a surrogate biomarker of brain damage associated with cognitive impairment).15 Both OH and SH may be different manifestations of the blood pressure dysregulation present in patients with PD or MSA and autonomic dysfunction. Based on emerging evidence from studies on the general population,44 the concept of blood pressure variability has attracted increasing interest as an independent additional factor contributing to cognitive impairment, and further research is warranted to evaluate the contribution of SH and blood pressure variability in patients with PD and MSA as potential modifiable factors.
Our findings have important clinical implications and raise questions about the current management of OH in patients with PD and MSA. Current treatment recommendations are guided by symptom severity and aimed at reducing the symptomatic burden and improving functional capacity. Owing to the chronicity of OH in patients with PD and MSA, cerebral blood flow regulatory mechanisms in these patients develop adaptive changes. Therefore, OH symptoms may not be an accurate indicator of cerebral hypoperfusion, and some authors propose the incorporation of vasodynamic parameters (standing mean blood pressure) in therapeutic decision-making.45 Regular blood pressure measurements were not available in all our patients, although previous studies suggested that the dementia risk associated with OH may correlate with vasodynamic and neurocirculatory abnormalities.46,47 Therefore, OH could be one of the very few potential modifiable factors contributing to cognitive impairment,4 although it is likely that any potential benefit obtained would require therapeutic interventions aimed at improving these circulatory abnormalities rather than guided by symptomatic severity.
Early OH was associated with more severe α-synuclein deposition in limbic structures in people with PD according to the Lewy-related pathology subtypes. Early and more severe Lewy pathology deposition in interconnected neuroanatomical structures of the limbic system involved in autonomic regulatory control such as the anterior cingulate, amygdala or insula, may explain the early development of OH in these patients. Our results go against the hypotheses that OH and dementia may be associated due to shared neuroanatomical basis (because no significant differences were found in cortical Lewy pathology) or secondary to a more diffuse neurodegeneration (because global Lewy pathology did not differ between subgroups). Neuropathologic analysis did not show differences in β-amyloid, tau deposition, or Alzheimer-type pathology, which are well-recognized factors contributing to cognitive impairment in patients with PD48 or increased global cerebrovascular pathology in a subgroup of patients (n = 40). Imaging studies have explored the association between OH and white matter hyperintensities as a marker of small vessel disease with inconsistent results. While some studies have shown that OH correlates with white matter hyperintensities on magnetic resonance imaging and cognitive outcomes in patients with PD,6,7,49 it is unclear whether these changes are related to concomitant SH.15 Of interest, a large multicenter study did not find an association between OH and white matter hyperintensities, although OH was associated with anterior temporal and mediotemporal atrophies independent from SH.8 Other clinical studies using CSF biomarkers have demonstrated that OH is associated with increased neurofilament light chain levels, a nonspecific marker of neuronal damage.9 We evaluated the cerebrovascular pathologic changes of OH in patients with PD, and our results showed that cerebral hypoperfusion secondary to OH did not translate into more severe cerebrovascular burden. Taken together, these data suggest that the deleterious effects of OH on cognition are not mediated by an increased burden of cerebrovascular disease and that repeated cerebral hypoperfusion may lead to chronic hypoxic changes activating molecular pathways leading to nonspecific neuronal damage and neurodegeneration.43
Last, although the effect of OH on cognition was independent from other confounders, early OH in patients with PD was associated with other clinical variables such as older age at diagnosis, suboptimal response to levodopa, and more severe postural and gait difficulties at diagnosis. These clinical features commonly present together and have become increasingly recognized as defining features of the so-called “diffuse or malignant” PD subtype, with more rapid disease progression and reduced survival.17
The main strengths of our study are the pathologic confirmation of the diagnosis, the big sample size, and the availability of detailed clinical information throughout the entire disease course. In addition, the detailed neuropathologic assessment allowed novel pathophysiologic insights to be made. Our results have a few limitations inherent to retrospective clinicopathologic studies. The assessment by different professionals without clear methodologic homogeneity may potentially account for some limitations in the accuracy of the recording and interpretation of the symptoms. To limit this potential bias, only cases with detailed clinical information and regular assessments throughout the disease course were included. The clinical assessment of OH in the study did not allow a confirmation of the neurogenic origin of OH in all patients, although we restricted participants to those with persistent OH >6 months and excluded those with suspected non-neurogenic OH. Moreover, clinical parameters evaluated in the study were selected based on their relevance in clinical practice and are more likely to be confidently documented in medical records. Our study mostly relied on the clinical judgment of the treating specialists for the diagnosis and symptom evaluation of OH. We acknowledge that the lack of regular blood pressure measurements during orthostatism and the potential underreporting of atypical or mild OH–associated symptoms without relevant effect on clinical practice may have delayed the detection of OH in some patients. The lack of use of validated quantitative scales for OH symptoms (such as the Orthostatic Hypotension Questionnaire50) and cognitive assessments prevented a more detailed evaluation of the potential pathogenic mechanisms. Last, although we reported the effect of concomitant SH on cognition in those with OH during diagnosis, our data did not allow the assessment of SH without OH or a longitudinal assessment of incident SH throughout the disease course. Further research is warranted to explore the impact of SH and blood pressure variability on cognitive function in patients with PD or MSA.
We found that early development of OH, but not severity of OH symptoms, is independently associated with an increased dementia risk in a large cohort of patients with pathology-confirmed PD and MSA. Systematic neuropathologic analysis did not show significant differences in the severity of α-synuclein deposition in Braak stages and β-amyloid, tau, or cerebrovascular pathologies, suggesting that the association between OH and cognitive impairment is not due to shared neuroanatomical basis, more extensive neuronal damage secondary to specific neurodegenerative proteinopathies, or an increased burden of cerebrovascular disease. Taking our results and evidence from previous clinical studies together, it is likely that repeated cerebral hypoperfusion events secondary to OH may induce nonspecific hypoxia-related neurodegeneration contributing to cognitive impairment. Further research is required to evaluate whether interventions aimed at improving circulatory abnormalities and brain hypoperfusion associated with OH are able to reduce dementia risk in PD and MSA.
Acknowledgment
Acknowledgments
The authors thank the patients and their families, without whose generous donation and support none of this research would have been possible.
Glossary
- HR
hazard ratio
- OH
orthostatic hypotension
- PD
Parkinson disease
- MSA
multiple system atrophy
- SH
supine hypertension
Appendix. Authors
Footnotes
Editorial, page 451
CME Course: NPub.org/cmelist
Study Funding
The authors report no targeted funding.
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Baschieri F, Sambati L, Guaraldi P, Barletta G, Cortelli P, Calandra-Buonaura G. Neurogenic orthostatic hypotension in early stage Parkinson's disease: new insights from the first 105 patients of the BoProPark study. Parkinsonism Relat Disord. 2021;93:12-18. doi: 10.1016/j.parkreldis.2021.11.002. [DOI] [PubMed] [Google Scholar]
- 2.McDonald C, Newton JL, Burn DJ. Orthostatic hypotension and cognitive impairment in Parkinson's disease: causation or association? Mov Disord. 2016;31(7):937-946. doi: 10.1002/mds.26632. [DOI] [PubMed] [Google Scholar]
- 3.Udow SJ, Robertson AD, MacIntosh BJ, et al. Under pressure': is there a link between orthostatic hypotension and cognitive impairment in alpha-synucleinopathies? J Neurol Neurosurg Psychiatry. 2016;87(12):1311-1321. doi: 10.1136/jnnp-2016-314123. [DOI] [PubMed] [Google Scholar]
- 4.Guo Y, Xu W, Liu FT, et al. Modifiable risk factors for cognitive impairment in Parkinson's disease: a systematic review and meta-analysis of prospective cohort studies. Mov Disord. 2019;34(6):876-883. doi: 10.1002/mds.27665. [DOI] [PubMed] [Google Scholar]
- 5.Merola A, Romagnolo A, Rosso M, et al. Orthostatic hypotension in Parkinson's disease: does it matter if asymptomatic? Parkinsonism Relat Disord. 2016;33:65-71. doi: 10.1016/j.parkreldis.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Ten Harmsen BL, van Rumund A, Aerts MB, et al. Clinical correlates of cerebral white matter abnormalities in patients with Parkinson's disease. Parkinsonism Relat Disord. 2018;49:28-33. doi: 10.1016/j.parkreldis.2017.12.029. [DOI] [PubMed] [Google Scholar]
- 7.Oh YS, Kim JS, Lee KS. Orthostatic and supine blood pressures are associated with white matter hyperintensities in Parkinson disease. J Mov Disord. 2013;6(2):23-27. doi: 10.14802/jmd.13006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pilotto A, Romagnolo A, Scalvini A, et al. Association of orthostatic hypotension with cerebral atrophy in patients with Lewy body disorders. Neurology. 2021;97(8):e814-e824. doi: 10.1212/wnl.0000000000012342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park DG, Kim JW, An YS, Chang J, Yoon JH. Plasma neurofilament light chain level and orthostatic hypotension in early Parkinson's disease. J Neural Transm. 2021;128(12):1853-1861. doi: 10.1007/s00702-021-02423-y. [DOI] [PubMed] [Google Scholar]
- 10.Ha AD, Brown CH, York MK, Jankovic J. The prevalence of symptomatic orthostatic hypotension in patients with Parkinson's disease and atypical parkinsonism. Parkinsonism Relat Disord. 2011;17(8):625-628. doi: 10.1016/j.parkreldis.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 11.O'Sullivan SS, Massey LA, Williams DR, et al. Clinical outcomes of progressive supranuclear palsy and multiple system atrophy. Brain. 2008;131(5):1362-1372. doi: 10.1093/brain/awn065. [DOI] [PubMed] [Google Scholar]
- 12.Stankovic I, Krismer F, Jesic A, et al. Cognitive impairment in multiple system atrophy: a position statement by the Neuropsychology Task Force of the MDS Multiple System Atrophy (MODIMSA) study group. Mov Disord. 2014;29(7):857-867. doi: 10.1002/mds.25880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown RG, Lacomblez L, Landwehrmeyer BG, et al. Cognitive impairment in patients with multiple system atrophy and progressive supranuclear palsy. Brain. 2010;133(8):2382-2393. doi: 10.1093/brain/awq158. [DOI] [PubMed] [Google Scholar]
- 14.Fanciulli A, Jordan J, Biaggioni I, et al. Consensus statement on the definition of neurogenic supine hypertension in cardiovascular autonomic failure by the American Autonomic Society (AAS) and the European Federation of Autonomic Societies (EFAS): endorsed by the European Academy of Neurology (EAN) and the European Society of Hypertension (ESH). Clin Auton Res. 2018;28(4):355-362. doi: 10.1007/s10286-018-0529-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palma JA, Redel-Traub G, Porciuncula A, et al. The impact of supine hypertension on target organ damage and survival in patients with synucleinopathies and neurogenic orthostatic hypotension. Parkinsonism Relat Disord. 2020;75:97-104. doi: 10.1016/j.parkreldis.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaufmann H, Palma JA. White matter hyperintensities in the synucleinopathies: orthostatic hypotension, supine hypertension, or both? Mov Disord Clin Pract. 2020;7(6):595-598. doi: 10.1002/mdc3.13000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Pablo-Fernandez E, Lees AJ, Holton JL, Warner TT. Prognosis and neuropathologic correlation of clinical subtypes of Parkinson disease. JAMA Neurol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Litvan I, Goldman JG, Troster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: movement disorder society task force guidelines. Mov Disord. 2012;27(3):349-356. doi: 10.1002/mds.24893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams-Gray CH, Evans JR, Goris A, et al. The distinct cognitive syndromes of Parkinson's disease: 5 year follow-up of the CamPaIGN cohort. Brain. 2009;132(11):2958-2969. doi: 10.1093/brain/awp245. [DOI] [PubMed] [Google Scholar]
- 20.Miki Y, Foti SC, Hansen D, et al. Hippocampal α-synuclein pathology correlates with memory impairment in multiple system atrophy. Brain. 2020;143(6):1798-1810. doi: 10.1093/brain/awaa126. [DOI] [PubMed] [Google Scholar]
- 21.Gibb WRG, Lees AJ. The significance of the Lewy body in the diagnosis of idiopathic Parkinson's disease. Neuropathol Appl Neurobiol. 1989;15(1):27-44. doi: 10.1111/j.1365-2990.1989.tb01147.x. [DOI] [PubMed] [Google Scholar]
- 22.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord. 2010;25(15):2649-2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- 23.De Pablo-Fernandez E, Tur C, Revesz T, Lees AJ, Holton JL, Warner TT. Association of autonomic dysfunction with disease progression and survival in Parkinson disease. JAMA Neurol. 2017;74(8):970-976. doi: 10.1001/jamaneurol.2017.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology. 2017;89(1):88-100. doi: 10.1212/WNL.0000000000004058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braak H, Tredici KD, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24(2):197-211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 26.Ozawa T, Paviour D, Quinn NP, et al. The spectrum of pathological involvement of the striatonigral and olivopontocerebellar systems in multiple system atrophy: clinicopathological correlations. Brain. 2004;127(12):2657-2671. doi: 10.1093/brain/awh303. [DOI] [PubMed] [Google Scholar]
- 27.Ling H, Asi YT, Petrovic IN, et al. Minimal change multiple system atrophy: an aggressive variant? Mov Disord. 2015;30(7):960-967. doi: 10.1002/mds.26220. [DOI] [PubMed] [Google Scholar]
- 28.Asi YT, Ling H, Ahmed Z, Lees AJ, Revesz T, Holton JL. Neuropathological features of multiple system atrophy with cognitive impairment. Mov Disord. 2014;29(7):884-888. doi: 10.1002/mds.25887. [DOI] [PubMed] [Google Scholar]
- 29.Thal DR, Rub U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58(12):1791-1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 30.Mirra SS, Heyman A, McKeel D, et al. The Consortium to establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41(4):479-486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 31.Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112(4):389-404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol. 2012;123:1-11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skrobot OA, Attems J, Esiri M, et al. Vascular cognitive impairment neuropathology guidelines (VCING): the contribution of cerebrovascular pathology to cognitive impairment. Brain. 2016;139(11):2957-2969. doi: 10.1093/brain/aww214. [DOI] [PubMed] [Google Scholar]
- 34.Cuoco S, Carotenuto I, Cappiello A, et al. Relationship between orthostatic hypotension and cognitive functions in multiple system atrophy: a longitudinal study. Front Neurol. 2021;12:711358. doi: 10.3389/fneur.2021.711358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barcelos LB, Saad F, Giacominelli C, et al. Neuropsychological and clinical heterogeneity of cognitive impairment in patients with multiple system atrophy. Clin Neurol Neurosurg. 2018;164:121-126. doi: 10.1016/j.clineuro.2017.10.039. [DOI] [PubMed] [Google Scholar]
- 36.Ueda M, Nakamura T, Suzuki M, et al. Association of orthostatic blood pressure with the symptoms of orthostatic hypotension and cognitive impairment in patients with multiple system atrophy. J Clin Neurosci. 2020;75:40-44. doi: 10.1016/j.jocn.2020.03.040. [DOI] [PubMed] [Google Scholar]
- 37.Kawamura K, Shimohata T, Nakayama H, Tomita M, Ozawa T, Nishizawa M. Factors influencing the cognitive function in patients with multiple system atrophy. Mov Disord. 2010;25(16):2891-2892. doi: 10.1002/mds.23260. [DOI] [PubMed] [Google Scholar]
- 38.Wenning GK, Stankovic I, Vignatelli L, et al. The movement disorder society criteria for the diagnosis of multiple system atrophy. Mov Disord. 2022;37(6):1131-1148. doi: 10.1002/mds.29005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pilotto A, Romagnolo A, Tuazon JA, et al. Orthostatic hypotension and REM sleep behaviour disorder: impact on clinical outcomes in alpha-synucleinopathies. J Neurol Neurosurg Psychiatry. 2019;90(11):1257-1263. doi: 10.1136/jnnp-2019-320846. [DOI] [PubMed] [Google Scholar]
- 40.Robinson JL, Lee EB, Xie SX, et al. Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain. 2018;141(7):2181-2193. doi: 10.1093/brain/awy146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van der Perren A, Gelders G, Fenyi A, et al. The structural differences between patient-derived α-synuclein strains dictate characteristics of Parkinson's disease, multiple system atrophy and dementia with Lewy bodies. Acta Neuropathol. 2020;139(6):977-1000. doi: 10.1007/s00401-020-02157-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanudjojo B, Shaikh SS, Fenyi A, et al. Phenotypic manifestation of α-synuclein strains derived from Parkinson's disease and multiple system atrophy in human dopaminergic neurons. Nat Commun. 2021;12(1):3817. doi: 10.1038/s41467-021-23682-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heras-Garvin A, Danninger C, Eschlböck S, Holton JL, Wenning GK, Stefanova N. Signs of chronic hypoxia suggest a novel pathophysiological event in α-synucleinopathies. Mov Disord. 2020;35(12):2333-2338. doi: 10.1002/mds.28229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma Y, Tully PJ, Hofman A, Tzourio C. Blood pressure variability and dementia: a state-of-the-art review. Am J Hypertens. 2020;33(12):1059-1066. doi: 10.1093/ajh/hpaa119. [DOI] [PubMed] [Google Scholar]
- 45.Palma JA, Gomez-Esteban JC, Norcliffe-Kaufmann L, et al. Orthostatic hypotension in Parkinson disease: how much you fall or how low you go? Mov Disord. 2015;30(5):639-645. doi: 10.1002/mds.26079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anang JBM, Gagnon JF, Bertrand JA, et al. Predictors of dementia in Parkinson disease: a prospective cohort study. Neurology. 2014;83(14):1253-1260. doi: 10.1212/wnl.0000000000000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim JS, Oh YS, Lee KS, Kim YI, Yang DW, Goldstein DS. Association of cognitive dysfunction with neurocirculatory abnormalities in early Parkinson disease. Neurology. 2012;79(13):1323-1331. doi: 10.1212/wnl.0b013e31826c1acd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Compta Y, Parkkinen L, O'Sullivan SS, et al. Lewy- and Alzheimer-type pathologies in Parkinson's disease dementia: which is more important? Brain. 2011;134(5):1493-1505. doi: 10.1093/brain/awr031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dadar M, Fereshtehnejad SM, Zeighami Y, Dagher A, Postuma RB, Collins DL. White matter hyperintensities mediate impact of dysautonomia on cognition in Parkinson's disease. Mov Disord Clin Pract. 2020;7(6):639-647. doi: 10.1002/mdc3.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaufmann H, Malamut R, Norcliffe-Kaufmann L, Rosa K, Freeman R. The orthostatic hypotension questionnaire (OHQ): validation of a novel symptom assessment scale. Clin Auton Res. 2012;22(2):79-90. doi: 10.1007/s10286-011-0146-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data not provided in the article because of space limitations may be shared (anonymized) at the request of any qualified investigator, if in compliance with regulatory ethical approvals, for purposes of replicating procedures and results.