Abstract
Background and Objectives
This prospective study seeks to examine the utility of subjective cognitive decline (SCD) as a marker of future progression to dementia in a community-based cohort of non-Latinx White, non-Latinx Black, and Latinx individuals. Debate surrounds the utility of SCD, the subjective perception of decline in one's cognition before such impairment is evident in traditional neuropsychological assessments, as an early indicator of impending Alzheimer disease. Unfortunately, most studies examining SCD have been conducted in non-Latinx White samples and commonly exclude groups of individuals shown to be most vulnerable to dementia.
Methods
Participants were enrolled into this cohort study from the Washington Heights–Inwood Columbia Aging Project if they were cognitively unimpaired, had baseline measurement of SCD, and self-identified as non-Latinx White, non-Latinx Black, or Latinx. SCD was measured as a continuous sum of 10 items assessing cognitive complaints. Competing risk models tested the main effects of baseline SCD on progression to dementia. Models were adjusted for age, sex/gender, years of education, medical comorbidity burden, enrollment cohort, and baseline memory test performance with death jointly modelled as a function of race/ethnicity.
Results
A total of 4,043 (1,063 non-Latinx White, 1,267 non-Latinx Black, and 1,713 Latinx) participants were selected for this study with a mean age of 75 years, 67% women, and with a mean follow-up of 5 years. Higher baseline SCD was associated with increased rates of incident dementia over time in the full sample (hazard ratio [HR] 1.085, CI 1.047–1.125, p < 0.001) and within Latinx (HR 1.084, CI 1.039–1.130, p < 0.001) and non-Latinx Black individuals (HR 1.099, CI 1.012–1.194, p = 0.024).
Discussion
Overall results of this study support SCD as a prodromal marker of dementia in a multiracial community sample, and in Latinx and non-Latinx Black individuals in particular. Because models examining the risk of dementia were adjusted for baseline memory test performance, the results support the idea that SCD, a subjective reflection of one's own current cognitive functioning, contributes information above and beyond standard memory testing. Current findings highlight the importance of carefully evaluating any memory concerns raised by older adults during routine visits and underscore the potential utility of screening older adults for SCD.
Subjective cognitive decline (SCD) was defined over a decade ago as the subjective perception of decline in one's cognition before such impairment is evident in traditional neuropsychological assessments.1 In recent years, SCD has emerged as a potential and valuable marker for a prodromal stage of dementia.2 Multiple studies show that the presence of SCD can increase risk for future progression to dementia and cognitive decline.3 Furthermore, Alzheimer disease (AD) biomarkers, including β-amyloid and cortical atrophy in AD-related regions, are associated with SCD.4 However, the utility of SCD as an early marker of AD is debated.5 Moreover, as with much research in AD and other neurodegenerative diseases, most studies examining SCD have been conducted in non-Latinx White samples. The lack of representativeness in the SCD literature threatens the validity and generalizability of conclusions by commonly excluding groups of individuals shown to be most vulnerable to dementia.
Decades of work have established differences across ethnoracial groups in the diagnostic sensitivity,6,7 clinical manifestations,8 and anatomic correlates9,10 of cognitive impairment and clinical AD. These differences have been linked to a number of social determinants of health such as quality of education, socioeconomic status, and racial socialization.11 There is evidence that the predictive utility of SCD for dementia is affected both by task factors (i.e., the characteristics of the tools we use)12 and person factors (i.e., the characteristics of the individual being evaluated),13,14 effects which may vary across ethnoracial groups. Previous studies examining SCD in non-Latinx Black individuals have produced conflicting support for SCD as a possible dementia prodrome. Multiple studies in non-Latinx Black older adults have linked SCD to memory function or future decline15-20; however, the results from at least 4 studies have suggested that SCD is unrelated to actual memory function in this group.21-24 Negative findings could reflect the cross-sectional nature of these studies, a lack of sensitive cognitive outcome measures,24 or limitations in the SCD instrument.22,24 Moreover, potential inclusion of individuals with mild cognitive impairment (MCI)22-24 could reduce the association between SCD and memory, given the degradation in memory awareness that accompanies advancing disease. Indeed, studies have shown that as many as 60% of patients with MCI may have impaired awareness of memory loss25 and thus endorse fewer subjective memory problems than individuals in an earlier stage of disease (i.e., SCD) despite having worse memory performance. Nonetheless, in the study by Jackson et al.21 in which individuals with MCI were carefully excluded, and sensitive measures of cognition and SCD were used, SCD was related to memory in Whites but unrelated to memory in a group of non-Latinx Black individuals matched on age, sex, estimated verbal IQ, and socioeconomic status. The authors speculated that cultural, health, environmental, and lifestyle factors might account for qualitative differences in how each racial group endorsed cognitive concerns, which in turn lead to differences in relationships with objective memory. However, this study was also cross-sectional; longitudinal designs are needed to more definitively establish SCD as a potential risk factor.
Regarding studies in Latinx older adults, evidence for SCD as a clinical precursor of AD is also inconsistent.20,26-30 Several studies have highlighted the association between SCD and lower cognitive performance.20,28,29 In a 2021 study by Nakhla et al.,26 SCD was associated with cognition and reduced entorhinal thickness and left hippocampal volume. In the latter study, the authors used a 12-item Likert scale to assess SCD as opposed to the 5-item dichotomous measure used in a previous study finding no link between SCD and cognition.27 Nonetheless, a recent longitudinal study found that SCD predicted later impairment on the telephone interview for cognitive status in non-Latinx Whites, but not Latinx or non-Latinx Black older adults.30 The authors speculated that differing trends in cognitive status perception across ethnoracial groups in addition to systemic inequities that may influence their decision to express their concerns may contribute to the varying predictive utility of SCD that they observed.
Taken together, most studies suggest that SCD is associated with cognitive function and/or progression to dementia across ethnoracial groups including non-Latinx White, non-Latinx Black, and Latinx older adults. However, several recent longitudinal studies challenge this idea, and no study has simultaneously examined the utility of SCD across all 3 ethnoracial groups for predicting conversion to dementia. To more rigorously examine the utility of SCD in diverse cohorts, this study investigates the predictive utility of SCD on progression to dementia in non-Latinx Black, Latinxs and non-Latinx, and non-Latinx White older adults.
Methods
Participants
Participants were selected for this study from the Washington Heights–Inwood Columbia Aging Project (WHICAP), a community-based, prospective cohort study of cognitive aging and dementia in Northern Manhattan, New York. WHICAP enrollees consist of a diverse sample of Medicare-eligible older adults who reside in the Washington/Hamilton Heights and Inwood area. Participants were enrolled in 3 primary waves: 1992 (n = 2,338), 1999 (n = 2,183), and 2009 (n = 2,128). WHICAP participants are followed at 18- to 24-month intervals; at each visit, they receive a variety of medical, neurologic, functional, and neuropsychological measures. Measures are given in either English or Spanish based on the participant's language preference.
For purposes of this study, participants were included if (1) their primary self-reported race/ethnicity was non-Latinx White, non-Latinx Black, or Latinx; (2) they were cognitively unimpaired at baseline (i.e., no dementia or MCI)31,32; and (3) they had subjective cognitive data at their baseline visit. This resulted in a sample of 4,043 individuals.
Measures
Demographic and Clinical Measures
Self-reported race/ethnicity was measured based on the 1990 US Census guidelines. Participants were first asked whether they were Latinx or Latino and then asked to classify themselves racially as non-Latinx White, non-Latinx Black, Asian, American Indian, Pacific Islander, or other. Sex/gender was assessed by asking participants if they were male or female; because it is unknown whether participants answered based on their sex at birth or the gender they identify with, we refer to this variable as “sex/gender.”33 Education was defined as the highest level of educational achievement and transformed into the corresponding number of years to obtain a level ranging from 0 to 20.
A total score of medical burden ranging from 0 to 14 was calculated based on participants' self-reported history of hypertension, diabetes, heart disease, stroke, chronic obstructive pulmonary disease, thyroid disease, liver disease, renal insufficiency, peptic ulcer disease, peripheral vascular disease, cancer, Parkinson disease, multiple sclerosis, and essential/familiar tremor.34 Time to death was coded as years from baseline to time of death.
Self-reported depressive symptoms were measured via the Center for Epidemiological Studies-Depression35 10-item questionnaire; this score ranges from 0 to 10 with higher scores indicating more depressive symptoms.
Subjective Cognitive Decline
SCD was defined as a continuous sum variable based on the number of cognitive complaints from 10 items as defined in eTable 1 (http://links.lww.com/WNL/C501). Items were drawn from existing questionnaires including the Blessed Dementia Scale, the Comprehensive and Referral Evaluation, and WHICAP-specific medical questionnaires.36-38
Neuropsychological Testing
As part of the parent study, participants underwent a full neuropsychological battery described previously39,40 that assessed cognitive domains of memory, language, speed/executive function, and visuospatial ability. In this study, memory performance was included as a covariate. Memory was assessed via the Selective Reminding Test's immediate, delayed, and recognition trials.41 Individual raw scores were converted to z scores and averaged based on a confirmatory factor analysis approach to obtain a composite memory score.40
Dementia Diagnosis
Participants received diagnoses of all-cause dementia via consensus case conference based on neurologic, neuropsychological, functional, medical, and psychiatric information gathered from self-reports from participants and/or informants and followed standard research criteria for the all-cause dementia.42
Statistical Methods
A one-way analysis of variances with Tukey post hoc tests and χ2 analyses were conducted to examine demographic and clinical differences across ethnoracial groups. A one-way analysis of covariance was also conducted to examine differences of SCD endorsement while adjusting for age, sex/gender, and education. Fine-Gray competing risk models43 tested main effects of baseline SCD on progression to dementia and the group-specific stratified effects of race/ethnicity and SCD. Death was jointly modeled during follow-up as a function of race/ethnicity. Models were adjusted for age, sex/gender, years of education, medical comorbidity burden, enrollment cohort, and baseline memory functioning. Owing to missing data on the depression scale, additional models adjusting for depressive symptoms were conducted in supplementary analyses.
Standard Protocol Approvals, Registrations, and Patient Consents
This study was approved by the institute review board at Columbia University as a human's subject protocol IRB-AAAO9804. Participants were consented before testing with a full written consent.
Data Availability
Data are available upon reasonable request to the WHICAP Publications Committee. Data requests should be submitted at cumc.co1.qualtrics.com/jfe/form/SV_6x5rRy14B6vpoqN.
Results
Descriptives
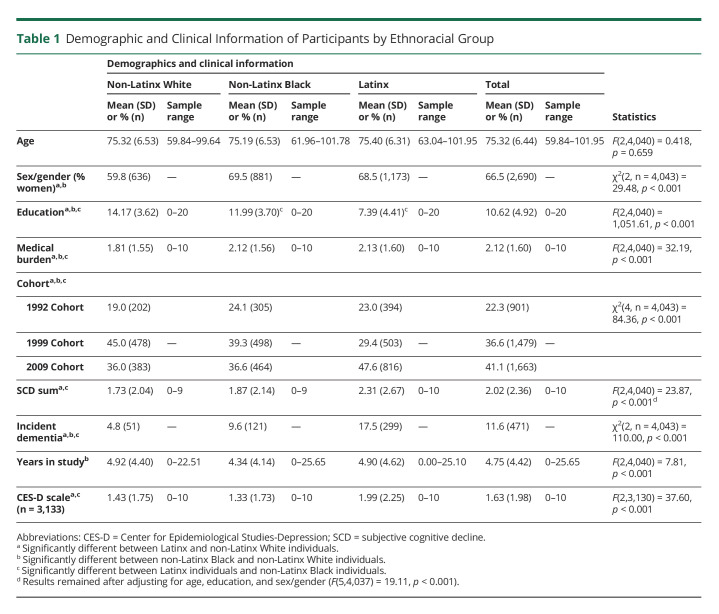
Table 1 summarizes participants' demographic and clinical information. As observed in Table 1, Latinx participants had lower education than non-Latinx Black participants; both Latinx and non-Latinx Black participants had lower educational attainment than non-Latinx White participants. The medical burden was higher in Latinx participants as compared to non-Latinx Black and non-Latinx White participants, with non-Latinx White individuals having less burden that non-Latinx Black and Latinx participants. Latinx participants also had higher endorsement of depressive symptoms than non-Latinx Black and non-Latinx Whites participants. No differences emerged in age. There was a larger proportion of women in Latinx and non-Latinx Black participants compared with non-Latinx White participants. The overall endorsement of SCD was different across groups such that Latinx participants reported more complaints than non-Latinx Whites and non-Latinx Black participants. This difference remained after adjusting for education, age, and sex/gender. No significant differences were observed between non-Latinx White and non-Latinx Black participants in overall SCD (p > 0.05). Finally, Latinx participants had higher incidence rates of dementia than non-Latinx Black and non-Latinx White participants; non-Latinx Black participants had a higher incidence compared with non-Latinx White participants.
Table 1.
Demographic and Clinical Information of Participants by Ethnoracial Group
SCD Predicting Progression to Dementia
Table 2 includes the estimates of the overall effect of SCD on incident dementia in the whole sample. Higher baseline SCD was related to greater likelihood of progression to dementia such that at any given time and additional 1 point on the 10-point SCD scale is associated with an 8.5% higher risk of having dementia, roughly comparable with the effect of 2 years of aging. Similarly, being a woman and being Latinx increased the risk of conversion to dementia. Higher memory performance and higher educational attainment were associated with a reduced in risk of dementia (Table 2 and Figure).
Table 2.
Competing Risks Model of Baseline SCD as a Predictor of Dementia in Whole Sample
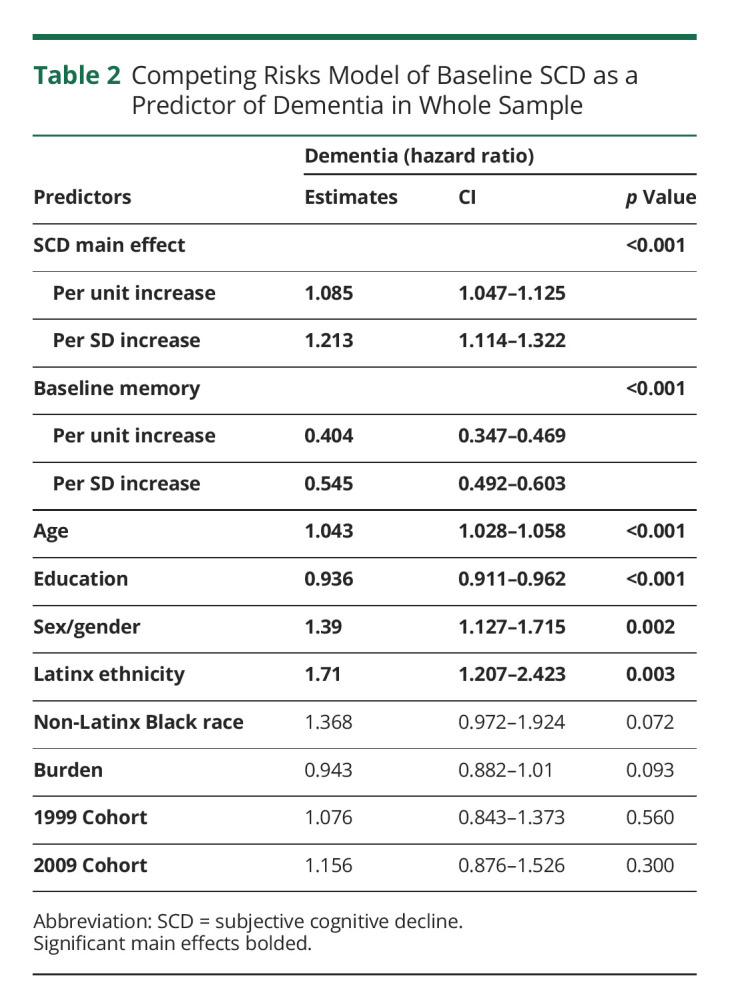
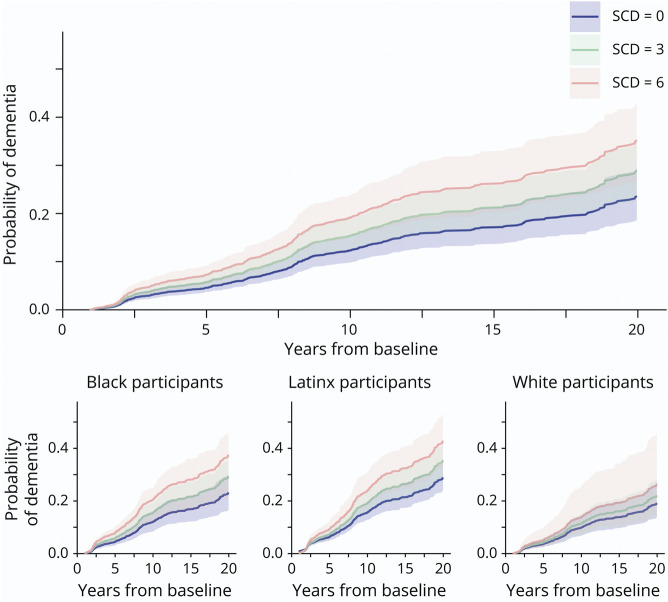
Figure. Dementia Risk by Baseline SCD in the Whole Sample and by Ethnoracial Group.
SCD = subjective cognitive decline.
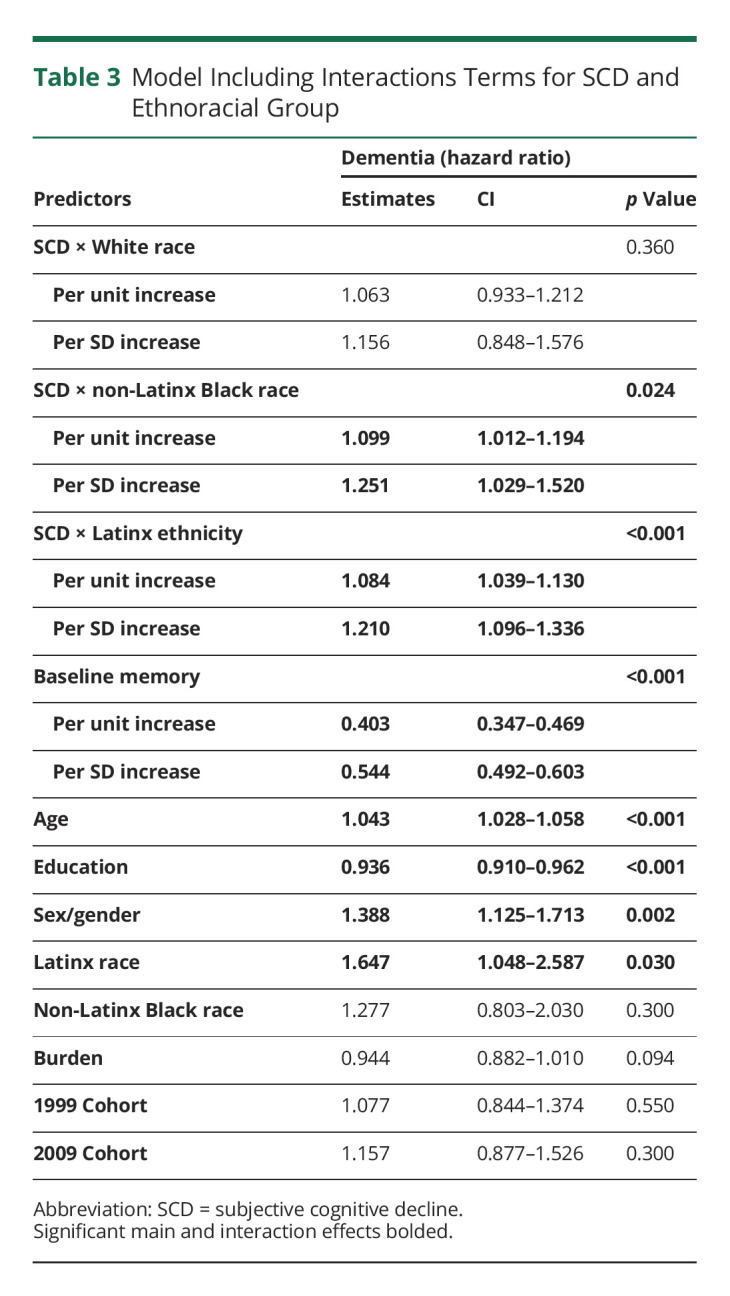
Table 3 includes the estimates of SCD by ethnoracial groups on incident dementia. SCD increased the risk of conversion to dementia in Latinx and non-Latinx Black participants but not in non-Latinx White participants, although the effect size and CI were comparable across groups (Figure). Finally, models included in Tables 2 and 3 were then adjusted for depressive symptoms included in eTables 2 and 3 (http://links.lww.com/WNL/C501). The overall effect of SCD on dementia remained significant in the whole sample and in Latinx participants. The effect within non-Latinx Black participants was similar but lost its significance at the margin (p = 0.077, eTable 3).
Table 3.
Model Including Interactions Terms for SCD and Ethnoracial Group
Discussion
This study examined whether SCD is a marker of dementia risk in a community-based multiethnic and multiracial sample. Latinx participants endorsed higher levels of SCD and also had the greatest likelihood of progressing to dementia compared with non-Latinx Black and non-Latinx White participants. Overall results of this study support SCD as a preclinical marker of dementia in the full sample, and in Latinx and non-Latinx Black individuals in particular. Although the association between baseline SCD and future progression to dementia was not statistically significant in non-Latinx White participants, the effect size and confidence intervals were comparable with the other racial and ethnic groups. Models examining the risk of dementia were also adjusted for baseline memory test performance supporting the idea that SCD, a subjective reflection of one's own current cognitive functioning, contributes information above and beyond a clinical neuropsychological assessment of memory. Models examining the risk of dementia were also adjusted for baseline memory test performance supporting the idea that SCD, a subjective reflection of one's own current cognitive functioning, contributes information above and beyond a clinical neuropsychological assessment of memory. Perhaps as would be expected, objective memory as measured via a composite score representing immediate recall, delayed recall, and recognition memory on a rigorous list learning test exhibited a stronger effect than SCD on progression to dementia. The difference in the magnitude of the unstandardized effect partially reflects scaling differences in the 2 scores. When examining the standardized coefficients, the difference in the effect sizes is reduced. Nonetheless, memory function remains a stronger predictor of progression to dementia than SCD in this model. The deterioration of episodic memory in early AD is well known44; indeed, episodic memory testing is a core feature of MCI assessments.45 The additional contributory value of SCD for progression to dementia is important because SCD screening is far more practical than episodic memory testing in frontline settings. Moreover, the independent predictive value of SCD reinforces the idea that older adults' cognitive complaints might capture subtle weaknesses not yet detectable on clinical memory testing. Indeed, emerging studies show that SCD maps onto several sensitive cognitive tasks when clinical neuropsychological performance is within standard limits.12
The results from this study are in line with some18,26,28 but not all studies21,22,24,27,30 examining the association of SCD with dementia or cognitive functioning. Literature examining SCD in diverse populations is emerging, and there is no “gold standard” approach. Methodologies across studies vary widely, but some potential limitations of the studies with inconclusive results include exclusion of participants of Latinx descent; use of cognitive screeners as outcomes; and inclusion of patients with MCI, possibly including patients with disturbances of self-awareness (i.e., anosognosia). Moreover, most inconclusive studies were cross-sectional, a design which may exacerbate possible confounders such as differential thresholds for defining cognitive impairment, variable cognitive presentations, and cultural thresholds for reporting SCD,46 in addition to differences in psychological factors such as depression.47,48 This study addresses some of these concerns by using a longitudinal approach, simultaneously examining all 3 groups, and using a clinical consensus diagnosis to define incident dementia, a robust definition of future decline.
Socioeconomic disparities are a critical consideration in understanding mechanisms that may underlie potential differences in SCD and/or its association with cognition across ethnoracial groups. In this study, the Latinx group had lower education and higher SCD than non-Latinx White or Black older adults. When adjusting for education, which may partially address differences in SES, SCD remained higher in the Latinx group. Nonetheless, there is no clear moderating effect of race on the association between SCD and dementia. In other words, SCD seems to put all ethnoracial groups at a similar risk for dementia. It is certainly possible that differences in SES could have main effects on both SCD and dementia, as well as a moderating effect on the association between these 2 variables. Future studies will need to address this question directly.
From a public health standpoint, current findings highlight the importance of carefully evaluating any memory concerns raised by older adults during routine, primary care visits and underscore the potential utility of screening older adults for SCD. Delayed diagnoses of cognitive impairment in older adults are multifactorial, in part reflecting the belief that memory loss is a normal part of aging.49 SCD screening is highly practical: it is fast, easy, noninvasive, inexpensive, and adaptable to any setting. Because plasma-based biomarkers of AD become increasingly accessible and widespread practice across primary and specialized medical care facilities, it will be critical to interpret values in conjunction with cognitive symptoms.50
This study also has several limitations. First, the different ethnoracial groups were treated as monolithic groups when in fact each group is very heterogenous, with individuals representing many different backgrounds. It is thus important for future research to examine these topics with greater attention to this diversity. Second, although this study adjusted for depressive symptoms in supplementary models, missing data on the depression scale reduced the number of participants included in these models, limiting the comparison of results. Third, the SCD measurement was primarily focused on memory complaints and did not comprehensively cover cognitive domains. Furthermore, this study did not consider language of testing and cultural factors, which, as indicated above, could act as potential confounders. Future studies should examine the effects of language, cultural factors, and psychological factors on SCD as a function of race and ethnicity to fully optimize this measurement's utility. Finally, there is limited understanding of the pathology that drives SCD and the extent to which such pathology differs across ethnoracial groups. Efforts are needed to elucidate the potential neural pathways through which SCD manifests across these groups.
Glossary
- AD
Alzheimer disease
- MCI
mild cognitive impairment
- SCD
subjective cognitive decline
- WHICAP
Washington Heights–Inwood Columbia Aging Project
Appendix. Authors
Study Funding
This work was supported by NIH, National Institute on Aging grants P01-AG007232 and R01AG054525-01A1.
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Reisberg B, Prichep L, Mosconi L, et al. The pre-mild cognitive impairment, subjective cognitive impairment stage of Alzheimer's disease. Alzheimers Dement. 2008;4(1 suppl 1):S98-S108. doi: 10.1016/j.jalz.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 2.Jessen F, Amariglio RE, Boxtel M, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement. 2014;10(6):844-852. doi: 10.1016/j.jalz.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jonker C, Geerlings MI, Schmand B. Are memory complaints predictive for dementia? A review of clinical and population-based studies. Int J Geriatr Psychiatry. 2000;15(11):983-991. doi:. [DOI] [PubMed] [Google Scholar]
- 4.Amariglio RE, Mormino EC, Pietras AC, et al. Subjective cognitive concerns, amyloid-beta, and neurodegeneration in clinically normal elderly. Neurology. 2015;85(1):56-62. doi: 10.1212/wnl.0000000000001712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balash Y, Mordechovich M, Shabtai H, Giladi N, Gurevich T, Korczyn AD. Subjective memory complaints in elders: depression, anxiety, or cognitive decline? Acta Neurol Scand. 2013;127(5):344-350. doi: 10.1111/ane.12038. [DOI] [PubMed] [Google Scholar]
- 6.Weuve J, Barnes LL, Mendes de Leon CF, et al. Cognitive aging in Black and White Americans: cognition, cognitive decline, and incidence of Alzheimer disease dementia. Epidemiology. 2018;29(1):151-159. doi: 10.1097/ede.0000000000000747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnes LL, Wilson RS, Li Y, Gilley DW, Bennett DA, Evans DA. Change in cognitive function in Alzheimer's disease in African-American and white persons. Neuroepidemiology. 2006;26(1):16-22. doi: 10.1159/000089231. [DOI] [PubMed] [Google Scholar]
- 8.Cooper C, Tandy AR, Balamurali TB, Livingston G. A systematic review and meta-analysis of ethnic differences in use of dementia treatment, care, and research. Am J Geriatr Psychiatry. 2010;18(3):193-203. doi: 10.1097/jgp.0b013e3181bf9caf. [DOI] [PubMed] [Google Scholar]
- 9.Zahodne LB, Manly JJ, Narkhede A, et al. Structural MRI predictors of late-life cognition differ across African Americans, Hispanics, and Whites. Curr Alzheimer Res. 2015;12(7):632-639. doi: 10.2174/1567205012666150530203214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnes LL, Leurgans S, Aggarwal NT, et al. Mixed pathology is more likely in black than white decedents with Alzheimer dementia. Neurology. 2015;85(6):528-534. doi: 10.1212/wnl.0000000000001834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manly JJ. Deconstructing race and ethnicity: implications for measurement of health outcomes. Med Care. 2006;44(11 suppl 3):S10-S16. doi: 10.1097/01.mlr.0000245427.22788.be. [DOI] [PubMed] [Google Scholar]
- 12.Chapman S, Sunderaraman P, Joyce JL, et al. Optimizing subjective cognitive decline to detect early cognitive dysfunction. J Alzheimers Dis. 2021;80(3):1185-1196. doi: 10.3233/jad-201322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cosentino S, Devanand D, Gurland B. A link between subjective perceptions of memory and physical function: implications for subjective cognitive decline. J Alzheimers Dis. 2018;61(4):1387-1398. doi: 10.3233/jad-170495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chapman S, Joyce JL, Barker MS, et al. Subjective cognitive decline is more accurate when metamemory is better. Front Aging Neurosci. 2022;14:787552. doi: 10.3389/fnagi.2022.787552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abner EL, Kryscio RJ, Caban-Holt AM, Schmitt FA. Baseline subjective memory complaints associate with increased risk of incident dementia: the PREADVISE trial. J Prev Alzheimers Dis. 2015;2(1):11-16. doi: 10.14283/jpad.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.John SE, Evans SA, Hanfelt J, Loring DW, Goldstein FC. Subjective memory complaints in white and African American participants. J Geriatr Psychiatry Neurol. 2020;33(3):135-143. doi: 10.1177/0891988719868305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arvanitakis Z, Leurgans SE, Fleischman DA, et al. Memory complaints, dementia, and neuropathology in older blacks and whites. Ann Neurol. 2018;83(4):718-729. doi: 10.1002/ana.25189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boggess MB, Barber JM, Jicha GA, Caban-Holt A. Subjective memory complaints are an important surrogate for objective cognitive performance in African Americans. Alzheimer Dis Assoc Disord. 2020;34(1):79-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parisi JM, Sharifian N, Rebok GW, Aiken-Morgan AT, Gross AL, Zahodne LB. Subjective memory, objective memory, and race over a 10-year period: findings from the ACTIVE study. Psychol Aging. 2021;36(5):572-583. doi: 10.1037/pag0000622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corlier FW, Shaw C, Hayes-Larson E, et al. Association between cognitive test performance and subjective cognitive decline in a diverse cohort of older adults: findings from the KHANDLE study. Alzheimer Dis Assoc Disord. 2020;34(3):198-205. doi: 10.1097/wad.0000000000000383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson JD, Rentz DM, Aghjayan SL, et al. Subjective cognitive concerns are associated with objective memory performance in Caucasian but not African-American persons. Age Ageing. 2017;46(6):988-993. doi: 10.1093/ageing/afx077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sims RC, Whitfield KE, Ayotte BJ, Gamaldo AA, Edwards CL, Allaire JC. Subjective memory in older African Americans. Exp Aging Res. 2011;37(2):220-240. doi: 10.1080/0361073x.2011.555640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sperling SA, Tsang S, Williams IC, Park MH, Helenius IM, Manning CA. Subjective memory change, mood, and cerebrovascular risk factors in older African Americans. J Geriatr Psychiatry Neurol. 2017;30(6):324-330. doi: 10.1177/0891988717732153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blazer DG, Hays JC, Fillenbaum GG, Gold DT. Memory complaint as a predictor of cognitive decline: a comparison of African American and White elders. J Aging Health. 1997;9(2):171-184. doi: 10.1177/089826439700900202. [DOI] [PubMed] [Google Scholar]
- 25.Vogel A, Stokholm J, Gade A, Andersen BB, Hejl AM, Waldemar G. Awareness of deficits in mild cognitive impairment and Alzheimer's disease: do MCI patients have impaired insight? Dement Geriatr Cogn Disord. 2004;17(3):181-187. doi: 10.1159/000076354. [DOI] [PubMed] [Google Scholar]
- 26.Nakhla MZ, Cohen L, Salmon DP, et al. Self-reported subjective cognitive decline is associated with global cognition in a community sample of Latinos/as/x living in the United States. J Clin Exp Neuropsychol. 2021;43(7):663-676. doi: 10.1080/13803395.2021.1989381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zlatar ZZ, Muniz M, Galasko D, Salmon DP. Subjective cognitive decline correlates with depression symptoms and not with concurrent objective cognition in a clinic-based sample of older adults. J Gerontol B Psychol Sci Soc Sci. 2018;73(7):1198-1202. doi: 10.1093/geronb/gbw207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zlatar ZZ, Tarraf W, González KA, et al. Subjective cognitive decline and objective cognition among diverse U.S. Hispanics/Latinos: results from the Study of Latinos-Investigation of Neurocognitive Aging (SOL-INCA). Alzheimers Dement. 2022;18(1):43-52. doi: 10.1002/alz.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodríguez D, Ayers E, Weiss EF, Verghese J. Cross-cultural comparisons of subjective cognitive complaints in a diverse primary care population. J Alzheimers Dis. 2021;81(2):545-555. doi: 10.3233/jad-201399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferraro KF, Sauerteig-Rolston MR, Barnes LL, Friedman E, Sands LP, Thomas PA. Subjective memory decline predicts incident cognitive impairment among white-but not black or Hispanic-older adults. Gerontologist. 2022; gnac086. doi: 10.1093/geront/gnac086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manly JJ, Tang M-X, Schupf N, Stern Y, Vonsattel J-PG, Mayeux R. Frequency and course of mild cognitive impairment in a multiethnic community. Ann Neurol. 2008;63(4):494-506. doi: 10.1002/ana.21326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):263-269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Avila JF, Vonk JMJ, Verney SP, et al. Sex/gender differences in cognitive trajectories vary as a function of race/ethnicity. Alzheimers Dement. 2019;15(12):1516-1523. doi: 10.1016/j.jalz.2019.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zahodne LB, Manly JJ, MacKay-Brandt A, Stern Y. Cognitive declines precede and predict functional declines in aging and Alzheimer's disease. PLoS One. 2013;8(9):e73645. doi: 10.1371/journal.pone.0073645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385-401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- 36.Golden RR, Teresi JA, Gurland BJ. Development of indicator scales for the Comprehensive Assessment and Referral Evaluation (CARE) interview schedule. J Gerontol. 1984;39(2):138-146. doi: 10.1093/geronj/39.2.138. [DOI] [PubMed] [Google Scholar]
- 37.Gurland B, Kuriansky J, Sharpe L, Simon R, Stiller P, Birkett P. The Comprehensive Assessment and Referral Evaluation (CARE): rationale, development and reliability. Int J Aging Hum Dev. 1978;8(1):9-42. doi: 10.2190/cl3j-0e20-97xx-mv5l. [DOI] [PubMed] [Google Scholar]
- 38.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114(512):797-811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 39.Stern Y, Andrews H, Pittman J, et al. Diagnosis of dementia in a heterogeneous population. Development of a neuropsychological paradigm-based diagnosis of dementia and quantified correction for the effects of education. Arch Neurol. 1992;49(5):453-460. doi: 10.1001/archneur.1992.00530290035009. [DOI] [PubMed] [Google Scholar]
- 40.Siedlecki KL, Manly JJ, Brickman AM, Schupf N, Tang M-X, Stern Y. Do neuropsychological tests have the same meaning in Spanish speakers as they do in English speakers? Neuropsychology. 2010;24(3):402-411. doi: 10.1037/a0017515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buschke H, Fuld PA. Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology. 1974;24(11):1019-1025. doi: 10.1212/wnl.24.11.1019. [DOI] [PubMed] [Google Scholar]
- 42.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Vol. 3. American Psychiatric Association, 1980. [Google Scholar]
- 43.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496-509. doi: 10.1080/01621459.1999.10474144. [DOI] [Google Scholar]
- 44.Dubois B, Feldman HH, Jacova C, et al. Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6(8):734-746. doi: 10.1016/s1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- 45.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):270-279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Teng EL. Cultural and educational factors in the diagnosis of dementia. Alzheimer Dis Assoc Disord. 2002;16(suppl 2):S77-S79. doi: 10.1097/00002093-200200002-00007. [DOI] [PubMed] [Google Scholar]
- 47.Morris EP, Byrd D, Summers AC, et al. Depressive symptoms differentially predict neurocognition in latinx and non-Hispanic white people living with HIV. J Int Neuropsychol Soc. 2021;27(3):249-260. doi: 10.1017/s1355617720000855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kohrt BA, Rasmussen A, Kaiser BN, et al. Cultural concepts of distress and psychiatric disorders: literature review and research recommendations for global mental health epidemiology. Int J Epidemiol. 2014;43(2):365-406. doi: 10.1093/ije/dyt227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liss JL, Seleri Assunção S, Cummings J, et al. Practical recommendations for timely, accurate diagnosis of symptomatic Alzheimer's disease (MCI and dementia) in primary care: a review and synthesis. J Intern Med. 2021;290(2):310-334. doi: 10.1111/joim.13244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hansson O, Edelmayer RM, Boxer AL, et al. The Alzheimer's Association appropriate use recommendations for blood biomarkers in Alzheimer's disease. Alzheimers Dement. 2022;1-18. doi: 10.1002/alz.12756. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request to the WHICAP Publications Committee. Data requests should be submitted at cumc.co1.qualtrics.com/jfe/form/SV_6x5rRy14B6vpoqN.