Abstract
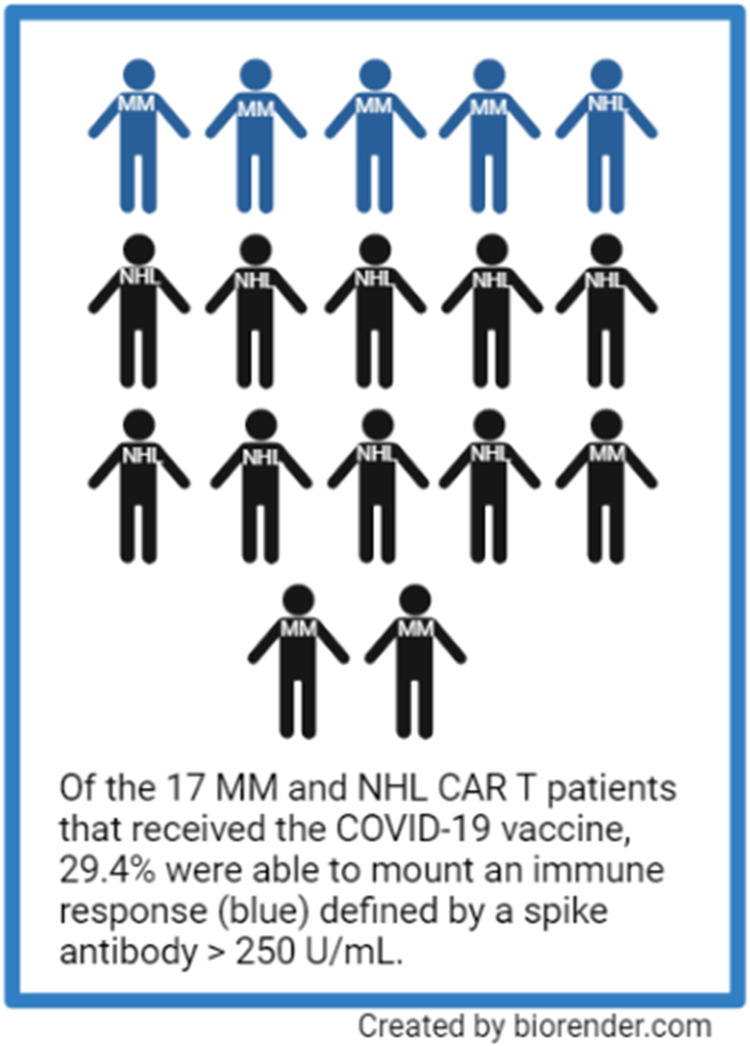
COVID-19 adversely affects individuals with cancer. Several studies have found that seroconversion rates among patients with hematologic malignancies are suboptimal when compared to patients without cancer. Patients with non-Hodgkin lymphoma (NHL) and multiple myeloma (MM) are immunocompromised due to impaired humoral and cellular immunity in addition to prescribed immunosuppressive therapy. Chimeric antigen receptor T-cell (CAR T) therapy is now widely used for NHL and MM, but little is known about seroconversion rates after COVID-19 vaccination among these populations. We evaluated SARS-CoV-2 spike-binding IgG antibody levels following COVID-19 vaccination among NHL and MM CAR T therapy recipients. Out of 104 CAR T infusions, 19 patients developed known COVID-19 infection post-CAR T. We tested 17 patients that received CAR T for antibody spike titers post COVID-19 vaccination, only 29 % (n = 5) were able to mount a clinically relevant antibody response (>250 IU/mL).
Keywords: COVID-19 vaccine, Cellular therapy, Dysproteinemia, Lymphoma, Immunosuppressed
Micro-Abstract
This study evaluated SARS-CoV-2 spike-binding IgG antibody levels following COVID-19 vaccination among non-Hodgkin lymphoma and multiple myeloma CAR T therapy recipients. In this retrospective study, we evaluated 104 patients that received CAR T therapy; of those 17 patients were evaluated for antibody spike titers following CAR T therapy. We found that only a minority of non-Hodgkin lymphoma and multiple myeloma patients were able to mount a clinically relevant (>250 IU/mL) antibody response.
Introduction/Background
Coronavirus disease 2019 (COVID-19) adversely affects individuals with cancer. Patients with cancer were initially excluded from COVID-19 vaccine clinical trials. Therefore, this population's response to COVID-19 vaccination is an essential area of research. Several studies have found significantly lower seroconversion rates in patients with hematologic malignancies compared to their solid tumor counterparts.1 , 2 In the CANVAX study, antibody responses were lower in cancer patients compared to controls, irrespective of vaccine type (P < .001); however mRNA-1273 was the most immunogenic and patients that received additional vaccine doses displayed increased antibody titers.3
Even with increased vaccine availability, COVID-19 continues to mainly threaten vulnerable populations. Among patients with hematologic malignancies, seroconversion rates also appear to be influenced by recent treatment and type of treatment.4, 5, 6, 7, 8 Patients with chronic lymphocytic leukemia who underwent treatment with anti-CD20 antibodies in the preceding 12 months have low to no seroconversion rates following the BNT162b2 mRNA COVID-19 vaccine.9 Likewise, patients with Waldenstrm macroglobulinemia have low humoral response after COVID-19 vaccination (either 2 doses of the BNT162b2 or 1 dose of the AZD1222).10 In addition, patients with multiple myeloma (MM) on active therapy with anti-CD38 regimens and BCMA-targeted therapy have significantly lower seroconversion when compared to patients with MM not on these active therapies.11
Less is known about vaccine immunogenicity among patients that have undergone treatment following chimeric antigen receptor T cell (CAR T) therapy. Two studies evaluated seroconversion rates following COVID-19 vaccination in a small number of CAR T therapy recipients and both showed low seroconversion rates (approximately 20%).12 , 13 A pooled analysis of SARS-CoV-2 vaccine responses of 236 CAR T recipients revealed a response rate of approximately 28%.14 This poor seroconversion rate may not be specific to COVID-19 vaccines but other vaccines as well.15
Patients with NHL and MM are immunocompromised due to impaired humoral and cellular immunity in addition to immunosuppressive therapy.11 CAR T therapy is now widely used for NHL and MM, but seroconversion rates are less known after COVID-19 vaccination among these populations. Current national guidelines recommend COVID-19 vaccination be offered to CAR T therapy recipients as early as 3 months postcellular infusion.16 This study aimed to assess seroconversion rates and mortality following COVID-19 vaccination after CAR T infusion.
Patients and Methods
This retrospective study was conducted at 3 Mayo Clinic sites (Arizona, FL, and MN) looking at NHL and MM patients receiving CAR T therapy from September 2016 to June 2021 (some of which received CAR T prior to the COVID 19 pandemic and vaccination approval on November 12, 2020). We evaluated severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike IgG antibody levels following COVID-19 vaccination among NHL and MM CAR T therapy recipients. Baseline characteristics, date of vaccination(s), number of vaccine doses, and documented COVID-19 infections were obtained from medical records. Laboratory values were obtained at approximately the same time that COVID-19 SARS-CoV-2 antibodies were obtained. All NHL and MM patients who received CAR T therapy at any point and were alive at the time that the first COVID-19 vaccine became commercially available in the United States (November 12, 2020) were eligible for inclusion for antibody response evaluation. This study was approved by the Institutional Review Board of Mayo Clinic.
Testing was performed using the Roche Elecsys anti-SARS-CoV-2 reagent assay (Roche Diagnostics, Mannheim, Germany), which has received Emergency Use Authorization by the U.S. Food and Drug Administration. The assay detects antibodies to the SARS-CoV-2 spike glycoprotein. Antibody levels > 0.8 U/mL were considered positive per our institution's laboratory guidelines. Other publications have defined clinical response according to spike antibody levels as follows: clinically relevant response (>250 IU/mL), partial response (50-250 IU/mL), and no response (<50 IU/mL).17 , 18 To align ourselves with recent publications, we also adopted similar cutoffs to render our final results.19 The date of last follow up for the entire cohort was September 2021. The 17 patients with evaluable COVID-19 CoV-2 spike IgG antibody levels were reassessed for survival status update in February of 2022.
Results
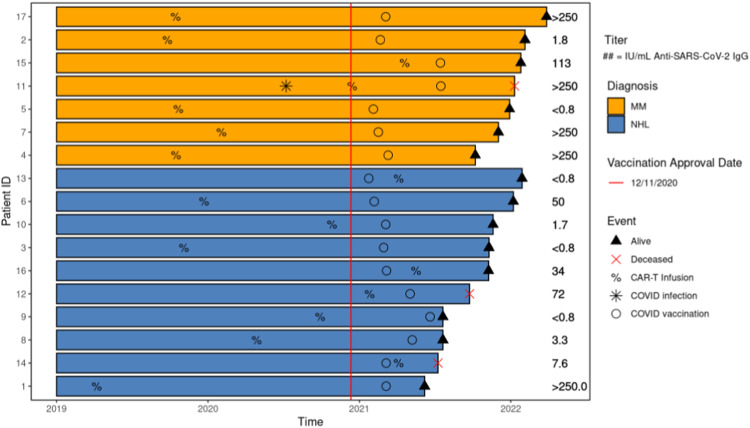
We evaluated 104 patients with MM and NHL that received CAR T infusions (Table 1 ). The median age of patients included in the study was 59.0 years (19-79) and most patients identified themselves as white (n = 86). Within our cohort, 76 patients were diagnosed with NHL and 26 had MM. Twenty-eight percent (n = 29) of individuals that received CAR T therapy were deceased and 72% (n = 75) were alive as of September 2021. Seven patients had COVID-19 infection pre-CAR T and all 7 were alive as of September 2021. Nineteen patients developed COVID-19 infection post-CAR T therapy (13 alive and 6 deceased; 3 patients were known to have received the COVID-19 vaccine; 1 patient had evaluable COVID-19 Co-V2 spike IgG antibody levels). The mortality of those that had COVID-19 infection post-CAR T therapy in our cohort was 31.5%. Of the 13 patients that survived COVID-19 infection, they received CAR T therapy a median of 337 days before COVID-19 infection (range = 54-1406); the 6 patients who died following COVID-19 infection (Table 2 ) received CAR T therapy a median of 164 days before COVID infection (range = 7-846). All 6 deceased patients did not receive COVID-19 vaccination pre-CAR T therapy (Table 2).
Table 1.
Patient Characteristics
| Patient Characteristics | |
|---|---|
|
Total, n Evaluable anti-SARS-Cov-2 antibodies |
104 17 |
|
Gender, male, n (%) |
75 (72) 13 (76) |
| Age, median (range) | 59.0 (19-79) 64 (35-77) |
|
Race/ethnicity White, n, (%) Hispanic or Latino, n (%) African American, n (%) Asian, n (%) Pacific Islander, n (%) American Indian, n (%) Other, n (%) Missing, n (%) |
86 (83) 13 (76) 8 (7.7) 4 (3.8) 1 (1.0) 1 (1.0) 1 (1.0) 2 (1.9) 1 (1.0) |
| BMI, median (range) | 26 (17.9-53.8) 28 (18.3 -34.7) |
|
Diagnosis NHL, n (%) MM, n (%) |
76 (73) 10 (59) 26 (25.0) 7 (41) |
|
Survival status Alive, n (%) Deceased, n (%) |
73 (70.) 31 (30) |
| COVID-19 pre-CAR T, n (%) | 7 (7) 2 (12) |
| Days from COVID-19 to CAR T, median (range) | 220 (30-265) (112-161) |
|
ASCT pre-CAR T NHL, n (%) MM, n (%) |
18 (17) 4 (40) 20 (19) 6 (86) |
| Days ASCT to CAR T median (range) | 605 (138-4409) 881 (272-2502) |
| Vaccinated pre-CAR T, n (%) | 4 (4) 3 (18) |
| COVID-19 post-CAR T, n (%) | 19 (18) $2 |
| Days from CAR T to COVID-19, median (range) | 220 (7-1406) |
| Days from CAR T to vaccine, median (range) | 170 (32-881) 95 (28-792) |
| Antibody info for vaccinated, n (%) | 17 (16) |
| % positive for antibody that had a vaccine, n (%) | 13 (76) |
| % negative for antibody that had a vaccine, n (%) | 4 (24) |
|
Cell and IgG Count WBC, median (range) ALC, median (range) CD4, median (range) IgG, median (range) |
3.6 × 104/µL (0.2-28.2) 5 × 104/µL (1.3-28.2) 0.64 × 104/µL (0.03-870) 0.73 × 104/µL (0.28-3.43) 110 × 106/µL (4-829) 137 × 104/µL (34-543) 517 g/L (<300-3611) 553 g/L (<300-3611) |
| Received IVIG post-CAR T, n (%) | 46 (44) 7 (41) |
|
Disease status post-CAR T Remission, n (%) Progression, n (%) Missing, n (%) |
58 (56) 11 (65) 43 (41) 5 (29) 3 (3) 1 |
| Received Rx post-CAR T, n (%) | 41 (39) 4 (24%) |
Characteristics of the 17 patients with evaluable anti-SARS-Cov-2 antibodies are represented in blue font. Survival status is based on the month/year of September 2021 for the cohort as a whole. Specific survival status for the 17 patients with evaluable anti-SARS-Cov-2 antibodies is discussed in the text as survival follow up was completed at a later date.
Abbreviations: ALC = absolute reticulocyte count; BMI = body mass index; CAR T: chimeric antigen receptor T-cells; IgG = immunoglobulin G; MM = multiple myeloma; N = number of patients; NHL = non-Hodgkin lymphoma; Rx = treatment; WBC = white blood cell count.
Table 2.
Characteristics of 6 Patients Deceased Post-CAR T Following COVID-19 Infection
| Patient | Age | Gender | Race | Malignancy | Vaccination Status | Days From CAR to COVID-19 |
|---|---|---|---|---|---|---|
| 1 | 73 | M | Hispanic | NHL | Unvaccinated | 7 |
| 2 | 67 | M | White | NHL | Unvaccinated | 208 |
| 3 | 62 | M | White | NHL | Unvaccinated | 101 |
| 4 | 55 | M | White | NHL | Unvaccinated | 846 |
| 5 | 63 | M | White | NHL | Unvaccinated | 120 |
| 6 | 63 | M | White | NHL | Vaccinated | 220 |
Attribution was not made by the investigator whether COVID-19 was the reason for death instead of a contributing factor.
Abbreviations: F = female; M = male; MM = multiple myeloma NHL = non-Hodgkin lymphoma.
Seventeen CAR T therapy recipients that received 2 doses of the mRNA-1273 or BNT162b2 vaccine or 1 dose for the Ad26.COV2.S vaccine were tested for spike antibody titers post COVID-19 vaccination(s). Baseline characteristics of these 17 patients appeared to be similar to the cohort as a whole with major differences likely secondary to sample size (blue font in Table 1). Among these 17 patients, 7 had MM, and 10 had NHL. Seventy-seven percent (n = 13) had vaccine immunogenicity according to our initial antibody spike values >0.8 U/mL ( Figure 2 ). We found this rate of vaccine immunogenicity to be higher than previously reported immunogenicity after CAR T therapy using a similar cutoff value (57% vaccine immunogenicity, n = 14).20 Using a positivity cutoff of 50 IU/mL (at least partial response), vaccination immunogenicity in our samples was 47% (n = 8). Using a positivity cutoff of 250 IU/mL (clinically relevant response), vaccination immunogenicity in our samples was 29% (n = 5). The median time from vaccination to antibody testing was 95 days (6-792). Six patients received the mRNA-1273 vaccine, 6 received the BNT162b2 vaccine, 2 received the Ad26.COV2.S vaccine, and 2 were unknown. One patient acquired COVID-19 infection, but this was prior to CAR T infusion. Of patients that received the vaccine, 14 (82%) were alive at the time of updated survival analysis. At the time of data collection tixagevimab/cilgavimab, pre-exposure prophylaxis for COVID-19 infection, was not available, therefore no patients in this cohort received it.
Figure 1.
Chronologic description of mortality post-CAR T. It was generated using the swimplot package 1.2.0. in R 4.0.3.
Figure 2.
COVID-19 vaccination immunogenicity in patients post-CAR T.
Conclusion
The frequency of vaccination immunogenicity in patients who underwent CAR T therapy among patients with NHL and MM was dependent on positivity cutoff values. Indeed, many studies use differing assays and cutoff values to define seroconversion rates.4, 5, 6, 7, 8 , 12, 13, 14 Approximately one-third of patients that had evaluable antibody spike values following COVID-19 vaccination and CAR T therapy were able to mount an immune response using the strictest criteria for vaccination immunogenicity in our relatively small sample. These findings emphasize that cutoff values need to be better defined. In addition, our results are limited by our small sample size, the retrospective/observational nature of our design, the fact that we did not assess cellular response, and the development of novel COVID-19 variants over time. We also did not evaluate immune response following additional vaccine administration (boosters) which have recently been reported in patients with hematologic malignancies receiving disease-directed therapies.21 , 22 These studies report that among those that did not seroconvert after their primary vaccination, 56%- 59% seroconverted after receiving a booster. It should also be noted that the term “immunogenicity” used in this text applies to only to measurable COVID-19 CoV-2 spike IgG antibody levels, but the authors acknowledge that immunogenicity is complex and includes factors that are not measured or reported in this study.
A major limitation of our study is that almost half of our patients (n = 46) received IVIG post CAR T therapy (11 patients were tested for spike antibody titers post COVID-19 vaccination; 4 of which had a detectable antibody spike value at any level), potentially resulting in cross-reactive antibody results as pooled immunoglobulin preparations may contain COVID-19 antibodies from vaccinated healthy donors. It is not known if these patients were receiving active IVIG therapy at the time of antibody assessment. Seropositivity in the United States at the time of this study assessment was reported at 43.3%; accordingly the timing of when studies were performed to quantify antibody response may influence the results.23 A recent study evaluating response to COVID-19 vaccination post anti-CD20 therapy in patients with NHL showed that longer time from last exposure to anti-CD20 therapy predicted higher response rates and elevated COVID-19 antibody titer.4 The ideal time from CAR T therapy to COVID-19 for optimal seropositivity remains to be answered. Interestingly we found that patients in our small sample that died after COVID-19 infection following CAR T therapy did not receive the vaccine prior to CAR T therapy. It should also be noted that whether COVID-19 directly attributed to death among these patients was not made by the investigators. We acknowledge that caution should be taken when interpreting survival in our retrospective review; the relationship of CAR T therapy to the to the pandemic was not fixed, thus some patients who received CAR T therapy years before the pandemic had more opportunity for immune recovery. Another limitation would be time bias since the mortality data on patients who received CAR T more recently (post vaccination approvals) will not be as mature.
Even though patients that receive CAR T have impaired humoral response, COVID-19 vaccination may still benefit this population and perhaps boosters further improve immune reconstitution.24 , 25 It should also be noted that because there were no standardized recommendations regarding screening patients for COVID-19 and different investigators had individualized practice, patients chosen for SARS-CoV-2 antibody testing may have introduced selection bias. To better understand the characteristics of the immunologic response against SARS-CoV-2 in patients post-CAR T therapy, more extensive multicenter studies exploring both humoral and cellular immunity will be needed.
Clinical Practice Points
-
•
Approximately one-third of patients with NHL and multiple myeloma following COVID-19 vaccination and CAR T therapy were able to mount an immune response using the strictest criteria for vaccination immunogenicity in our relatively small sample.
-
•
Even though patients that receive CAR T have impaired humoral response, COVID-19 vaccination may still benefit this population and perhaps boosters should follow immune reconstitution.
Ethics Approval and Consent to Participate
This study was approved by the Institutional Review Board of Mayo Clinic.
Consent for Publication
Not applicable.
Availability of Data and Materials
The datasets analyzed during the current study are not publicly available.
Authors’ Contributions
JEWN, MI, ACR, PV, HSM, and JM contributed to conception and design of the study and acquisition, analysis, or interpretation of data; JEWN, MI, ACR, and JM drafted the manuscript; JEWN, BJG, MJM, and RM performed statistical analysis; all authors helped in critical revision of the manuscript for important intellectual content. All authors read and approved the final manuscript.
Disclosure
JM receives consulting fees from Pharmacyclics/Abbvie, Bayer, Gilead/Kite Pharma, Pfizer, Janssen, Juno/Celgene, BMS, Kyowa, Alexion, Fosunkite, Innovent, Seattle Genetics, Debiopharm, Karyopharm, Genmab, ADC Therapeutics, Epizyme, Beigene, Servier, Novartis, Morphosys/Incyte, Secura Bio, TG Therapeutics, MEI, Lilly/Loxo; research funding from Bayer, Gilead/Kite Pharma, Celgene, Merck, Portola, Incyte, Genentech, Pharmacyclics, Seattle Genetics, Janssen, Millennium; Honoraria from Targeted Oncology, OncView, Curio, Kyowa, Physicians' Education Resource, and Seattle Genetics. Speaker's bureau from Gilead/Kite Pharma, Kyowa, Bayer, Pharmacyclics/Janssen, Seattle Genetics, Acrotech/Aurobindo, Beigene, Verastem, AstraZeneca, Celgene/BMS, Genentech/Roche. PV receives funds from DSMB from AbbVie, Vanda, Algernon; research from Cidara, Scynexis and fees paid to Mayo Clinic. PLB receives consulting and honoraria funding from Pfizer, Novartis, Janssen, Clgene, GSK, Genetech, and Oncopeptides. YW has financial research funding from InnoCare, Novartis, Genentech, MorphoSys and Membership on an entity's Board of Directors or advisory committees and research Funding with Incyte, LOXO Oncology, Eli Lilly, and TG Therapeutics. RF is a consultant for Amgen, BMS, Celgene, Takeda, Bayer, Janssen, Novartis, Pharcyclics, Sanofi, Merck, Juno, Kite, Aduro, OncoTracker, GSK, AbbVie, Patents and Royalties from Patent: Prognostication of myeloma via FISH and Membership on an entity's Board of Directors or advisory committees from OncoTracker, Adaptive Biotechnologies, and Caris Life Sciences. JMP receives research funding from PharmaEssentia, Incyte and consultancy and research funding from Protagonist, CTI BioPharma, and Sierra Oncology. DD receives consultancy fees from Alexion, Apellis, GSK, Sanofi, Janssen and research funding from Novartis. SK receives consultancy and research funding from BMS, Abbvie, Amgen, Takeda, Janssen, KITE, Astra-Zeneca, Roche-Genentech; consultancy fees from Beigene, Oncopeptides, and Bluebird Bio, and research funding from Tenebio, Carsgen, Merck, and Novartis. JP receives research funding from Karyopharm. SMA receives research funding from Bristol Myers Squibb, ADC Therapeutics, Seattle Genetics, Regeneron, Affimed, AI Therapeutics, Pfizer, Trillium, and Takeda. YL receives consultancy fees from Novartis, Juno, Legend, Sorrento, Gamida Cell; research funding from Merck and Takeda and consultancy and research funding from Kite, Janssen, Celgene, and Bluebird Bio. HSM receives research funding from CRISPR therapeutics.
Acknowledgements
We would like to thank the patients, families, and caregivers that have endured cancer through the COVID-19 pandemic.
References
- 1.Thakkar A., Pradhan K., Jindal S., et al. Patterns of seroconversion for SARS-CoV-2 IgG in patients with malignant disease and association with anticancer therapy. Nature Cancer. 2021;2(4):392–399. doi: 10.1038/s43018-021-00191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Addeo A., Shah P.K., Bordry N., et al. Immunogenicity of SARS-CoV-2 messenger RNA vaccines in patients with cancer. Cancer Cell. 2021;39(8):1091–1098e2. doi: 10.1016/j.ccell.2021.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naranbhai V., Pernat C.A., Gavralidis A., et al. Immunogenicity and reactogenicity of SARS-CoV-2 vaccines in patients with cancer: the CANVAX cohort study. J Clin Oncol. 2022;40(1):12–23. doi: 10.1200/JCO.21.01891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perry C., Luttwak E., Balaban R., et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with B-cell non-Hodgkin lymphoma. Blood Adv. 2021;5(16):3053–3061. doi: 10.1182/bloodadvances.2021005094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Redjoul R., Le Bouter A., Beckerich F., Fourati S., Maury S. Antibody response after second BNT162b2 dose in allogeneic HSCT recipients. Lancet. 2021;398(10297):298–299. doi: 10.1016/S0140-6736(21)01594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maillard A., Redjoul R., Klemencie M., et al. Antibody response after 2 and 3 doses of SARS-CoV-2 mRNA vaccine in allogeneic hematopoietic cell transplant recipients. Blood. 2022;139(1):134–137. doi: 10.1182/blood.2021014232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Redjoul R., Le Bouter A., Parinet V., Fourati S., Maury S. Antibody response after third BNT162b2 dose in recipients of allogeneic HSCT. Lancet Haematol. 2021;8(10):e681–e683. doi: 10.1016/S2352-3026(21)00274-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henriquez S., Zerbit J., Bruel T., et al. Anti-CD38 therapy impairs SARS-CoV-2 vaccine response against alpha and delta variants in patients with multiple myeloma. Blood. 2022;139(6):942–946. doi: 10.1182/blood.2021013714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herishanu Y., Avivi I., Aharon A., et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137(23):3165–3173. doi: 10.1182/blood.2021011568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gavriatopoulou M., Terpos E., Ntanasis-Stathopoulos I., et al. Poor neutralizing antibody responses in 106 patients with WM after vaccination against SARS-CoV-2: a prospective study. Blood Adv. 2021;5(21):4398–4405. doi: 10.1182/bloodadvances.2021005444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Oekelen O., Gleason C.R., Agte S., et al. Highly variable SARS-CoV-2 spike antibody responses to two doses of COVID-19 RNA vaccination in patients with multiple myeloma. Cancer cell. 2021;39(8):1028–1030. doi: 10.1016/j.ccell.2021.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhakal B., Abedin S., Fenske T., et al. Response to SARS-CoV-2 vaccination in patients after hematopoietic cell transplantation and CAR T-cell therapy. Blood. 2021;138(14):1278–1281. doi: 10.1182/blood.2021012769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sesques P., Bachy E., Ferrant E., et al. Immune response to three doses of mRNA SARS-CoV-2 vaccines in CD19-targeted chimeric antigen receptor T cell immunotherapy recipients. Cancer Cell. 2022;40(3):236–237. doi: 10.1016/j.ccell.2022.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uyemura B.S., Abbas Abid M., Suelzer E. Bilal Abid M. Efficacy of SARS-CoV-2 primary and booster vaccine doses in CAR-T recipients—targeting the target antigen. Bone Marrow Transplant. 2022;57(11):1727–1731. doi: 10.1038/s41409-022-01795-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vijenthira A., Gong I.Y., Fox T.A., et al. Outcomes of patients with hematologic malignancies and COVID-19: a systematic review and meta-analysis of 3377 patients. Blood. 2020;136(25):2881–2892. doi: 10.1182/blood.2020008824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. "Use of COVID-19 Vaccines in the United States." Last Reviewed November 14, 2022. https://www.cdc.gov/vannines/covid-19/clinical-considerations/covid-19-vaccines-us.html#table-03. Accessed December 12, 2022.
- 17.Stampfer S.D., Goldwater M., Jew S., et al. Response to mRNA vaccination for COVID-19 among patients with multiple myeloma. Leukemia. 2021;35(12):3534–3541. doi: 10.1038/s41375-021-01354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parry H., McIlroy G., Ali M., et al. Antibody responses after first and second Covid-19 vaccination in patients with chronic lymphocytic leukaemia. Blood Cancer J. 2021;11(7):136. doi: 10.1038/s41408-021-00528-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilbert P.B., Montefiori D.C., McDermott A.B., et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science. 2022;375(6576):43–50. doi: 10.1126/science.abm3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ram R., Hagin D., Kikozashvilli N., et al. Safety and Immunogenicity of the BNT162b2 mRNA COVID-19 vaccine in patients after allogeneic HCT or CD19-based CART therapy-a single-center prospective cohort study. Transplant Cell Ther. 2021;27(9):788–794. doi: 10.1016/j.jtct.2021.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ollila T.A., Masel R.H., Reagan J.L, et al. Seroconversion and outcomes after initial and booster COVID-19 vaccination in adults with hematologic malignancies. Cancer. 2022;128(18):3319–3329. doi: 10.1002/cncr.34354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abid M.B., Rubin M., Ledeboer N., et al. Efficacy of a third SARS-CoV-2 mRNA vaccine dose among hematopoietic cell transplantation, CAR T cell, and BiTE recipients. Cancer Cell. 2022;40(4):340–342. doi: 10.1016/j.ccell.2022.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. "COVID data tracker." https://covid.cdc.gov/covid-data-tracker/#datatracker-home. Accessed April, 2022.
- 24.Wang Y., Li C., Xia J., et al. Humoral immune reconstitution after anti-BCMA CAR T-cell therapy in relapsed/refractory multiple myeloma. Blood Adv. 2021;5(23):5290–5299. doi: 10.1182/bloodadvances.2021004603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baird J.H., Epstein D.J., Tamaresis J.S., et al. Immune reconstitution and infectious complications following axicabtagene ciloleucel therapy for large B-cell lymphoma. Blood Adv. 2021;5(1):143–155. doi: 10.1182/bloodadvances.2020002732. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are not publicly available.