Abstract
Introduction
Nirmatrelvir/ritonavir (Paxlovid®) is currently one of the few therapeutic options for coronavirus disease 2019 (COVID-19) curative treatment in non-oxygen-requiring adult patients at-high risk of progressing to severe disease. This recently approved boosted antiviral therapy presents a significant risk of drug-drug interactions (DDI). As part of the enhanced surveillance program in France for COVID-19 drugs and vaccines, the French national pharmacovigilance database (BNPV [base nationale de pharmacovigilance]) was queried in order to better characterize the drug safety profile, with a special focus on DDI. The aim of the study was to describe the adverse drug reactions reported through the BNPV.
Method
All nirmatrelvir/ritonavir reports validated in the BNPV from the first authorization in France (January, 20th 2022) to December, 3rd 2022 (date of the query) were considered. An analysis of the scientific literature (PubMed®) and from the WHO pharmacovigilance database (Vigibase) was also performed.
Results
Over this period (11 months), 228 reports (40% of serious reports) were registered with a sex ratio of 1.9 female/1 male and a mean age of 66 years old. DDI reports account for more than 13% of reports (n = 30) and were mainly related to immunosuppressive drugs overexposure (n = 16). A total of 10/228 reports with fatal outcomes were reported in complex clinical settings. The main reported unexpected adverse drug reaction (ADRs) were high blood pressure (n = 7), confusion (n = 5), acute kidney injuries (AKI, n = 7) and various skin reactions (n = 22). Apart from situations of disease recurrence (not found in this analysis), data from Pubmed® and Vigibase also reported the above-mentioned events of interest.
Conclusion
Overall, this analysis shows that nirmatrelvir/ritonavir safety profile was conform to current summary of product characteristics (SmPC). The main concern was the risk of DDI. Therefore, SmPC and expert recommendations should be systematically consulted before initiation of this antiviral, which is particularly indicated in polypharmacy patients. A case-by-case multidisciplinary approach including a clinical pharmacologist is required in these complex situations. Blood pressure elevation, confusion, cutaneous reactions and AKIs were the main unexpected ADRs of interest to follow, but need to be confirmed with a qualitative approach over time and new reports.
Keywords: Nirmatrelvir/ritonavir, COVID-19, Pharmacovigilance, Drug drug interaction
Abbreviations
- ADR
adverse drug reaction
- AKI
acute kidney injury
- ANSM
Agence nationale de sécurité du médicament et des produits de santé
- BNPV
base nationale de pharmacovigilance (French Pharmacovigilance Database)
- COVID-19
coronavirus disease 2019
- DDI
drug-drug interaction
- EMA
European Medicines Agency
- FSPT
French Society of Pharmacology or Therapeutics
- INR
international normalized ratio
- IS
immunosuppressant
- MedDRA
Medical Dictionary for Regulatory Activities
- ND
not documented
- PCR
polymerase chain reaction
- SARS CoV-2
severe acute respiratory syndrome coronavirus 2
- SEM
standard error of the mean
- SmPC
summary of product characteristics
- TDM
therapeutic drug monitoring
- VKA
vitamin K antagonist
- WHO
World Health Organization
Introduction
Vaccination combined with “barrier” measures is the pillars of coronavirus disease 2019 (COVID-19) management. It allows preventing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and the onset of symptoms that may evolve into a serious form of the disease notably in the most vulnerable patients (i.e., elderly, immunosuppressed or patients with comorbidities) [1].
Nirmatrelvir/ritonavir obtained a marketing authorization in Europe on January 28, 2022 [2]. In France, it was initially available through within an early access program from January 20, 2022 to May 06, 2022. It is currently one of the few therapeutic options in the curative treatment of COVID-19 in adult patients who do not require oxygen supplementation and are at high risk of developing into a severe form of COVID-19 [3]. Indeed, the use of monoclonal antibodies is currently challenged due to their loss of activity against emerging strains of SARS-CoV-2 leading the European Medicine Agency (EMA) to recently warn that the monoclonal antibodies may not be effective in the current epidemic context [4].
Nirmatrelvir (previously known as PF-07321332 is a peptidomimetic inhibitor of the main protease of SARS-CoV-2 (Mpro), also known as 3C-like protease (3CLpro) or nsp5 protease. Mpro inhibition of SARS-CoV-2 makes the protein incapable of treating polyprotein precursors, leading to the prevention of viral replication [3]. Ritonavir inhibits the CYP3A-mediated metabolism of nirmatrelvir, resulting in increased plasma concentrations of nirmatrelvir [3].
Nirmatrelvir/ritonavir has a significant risk of drug-drug interactions (DDI) and medication error primarily due to ritonavir, an inhibitor of CYP3A used as an enzyme booster.
In addition to these issues inherent to the risk of DDI, the most expected adverse effects of nirmatrelvir/ritonavir are diarrhea, headache, vomiting, nausea and dysgeusia (metallic/bitter taste in the mouth after each drug intake) [3]. All these adverse effects are common but generally resolve upon discontinuation.
As part of the enhanced surveillance in France for COVID-19 drugs and vaccines, we thus queried the national (French) pharmacovigilance database (called BNPV) in order to better characterize the clinical, paraclinical, and prognostic features of the safety profile of nirmatrelvir/ritonavir, with particular attention to DDI.
Method
Reports identification process
All reports validated in BNPV from the first authorization (January 20th, 2022) to December 3rd, 2022 (date of the query) were considered. Briefly, the BNPV gathers spontaneous reports of adverse drug reactions from French healthcare professionals or patients. Each report is assessed by clinical pharmacologists in the relevant regional pharmacovigilance center – according to the French method for the causality assessment of adverse drug reactions – before being recorded in the database. The French method for the causality assessment of adverse drug reactions (also named “imputability”) takes into account the following parameters [5], [6]:
-
•
intrinsic “imputability”, which ranges from I0 (no association between the reaction and a drug) to I6 (strong association between the events) and combines a chronological score (temporal link) and a semiological score (etiological link), each ranging from 0 to 3;
-
•
extrinsic score, based on previously published similar reports (bibliographic documentation), which ranges from B1 (adverse drug reaction not published) to B4 (expected adverse drug reaction listed in SmPC).
The BNPV is administered by the Agence nationale de sécurité du médicament et des produits de santé (ANSM), the French Health Agency. All pharmacovigilance reports registered anonymously are pseudonymized.
Inclusion criteria were as follows:
-
•
active ingredients: nirmatrelvir/ritonavir as suspect or interacting drug;
-
•
reaction: no restriction.
Exclusion criteria were: lack of documentation, including the absence of a narrative with a least clinical symptoms description.
Literature analysis
Literature review was conducted using the Medline databases for papers published in English and French up to October 13th, 2022. The keyword research was performed using combinations of the following terms (nirmatrelvir [All Fields] + Filters: English, French).
VigiBase
Extraction of data from the WHO Pharmacovigilance Database (VigiBase) was made as follows: “Nirmatrelvir;Ritonavir” (Active ingredient) as of January 01, 2023.
Other sources
The “Paxlovid® oral antiviral use study from February 4 to June 29, 2022 – a national study based on the French national health data systemdata” (EPIPHARE) published on September 13, 2022 was also included in this analysis [6].
Data availability policy
The use of confidential, electronically processed patient data was approved by the French national commission for data protection and liberties (Commission nationale de l’informatique et des libertés; reference number, 1735841).
Statistics
Quantitative variables were described using means and standard deviation or using median and interquartile range. Categorical variables were described by the number and proportion of subjects in each class.
Results
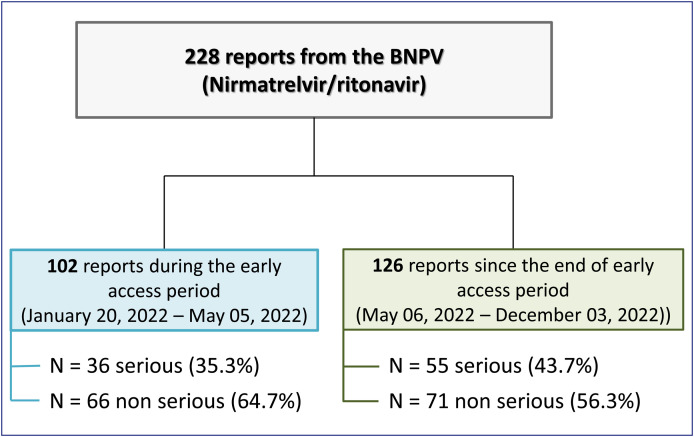
Since the launch of nirmatrelvir/ritonavir in France, a total of 228 reports (of which 91 serious reports including 10 deaths) (Table 1 ) were registered by regional pharmacovigilance centers in the BNPV at the date of extraction (December, 3rd 2022). Based on the treatment dates registered, 102 of these reports concern patients treated within the period of early access of nirmatrelvir/ritonavir (January, 20th 2022–May, 5th 2022) and 126 have been reported for patients treated since the end of this early access (Fig. 1 ).
Table 1.
Summary of reports recorded in the French pharmacovigilance database over the period from 01/20/2022 to 12/03/2022.
| Early access (from 01/20/2022 to 05/05/2022) | Post-early access (from 06/05/2022 to 12/03/2022) | Total | |
|---|---|---|---|
| Number of exposed patients (02/04/2022–06/29/2022)a | 12,179 subjects who received at least one issue of Paxlovid® from city pharmacies (of which nearly 43% during the early access period) | ||
| Number of reports from the BNPV | 102 | 126 | 228 |
| Number of serious reports (n, %) | 36 (35.3%) | 55 (43.7%) | 91 (39.9%) |
| Number of non-serious reports (n, %) | 66 (64.7%) | 71 (56.3%) | 137 (60.1%) |
| Number of deaths (n, %) | 7 (6.9%) | 3 (2.4%) | 10 (4.4%) |
| Sex | |||
| Female (n, %) | 68 (66.7%) | 81 (64.3%) | 149 (64.0%) |
| Male (n, %) | 34 (33.3%) | 45 (35.7%) | 79 (34.6%) |
| Mean age (years) | 66 | 66 | 66 |
BNPV: base nationale de pharmacovigilance (French Pharmacovigilance Database).
From EPIPHARE study [7].
Figure 1.
Study flow chart of nirmatrelvir/ritonavir reports recorded in the French pharmacovigilance database over the period from 01/20/2022 to 12/03/2022.
The sex ratio is on average 1.9 female/1 male, with a global mean age of 66 years old at the time to onset of the effects.
A total of 434 adverse reactions were reported over the period, of which 200 can be considered as expected (46.1%) in the light of the European summary of product characteristics (SmPC) (notably dysgeusia and gastro-intestinal disorders). In addition, 12 effects (2.8%) in relation to medication errors and at least 5 reports of treatment failure – which are not unexpected based on the final results of the pivotal phase 3 study – were reported.
Drug-drug interactions analysis
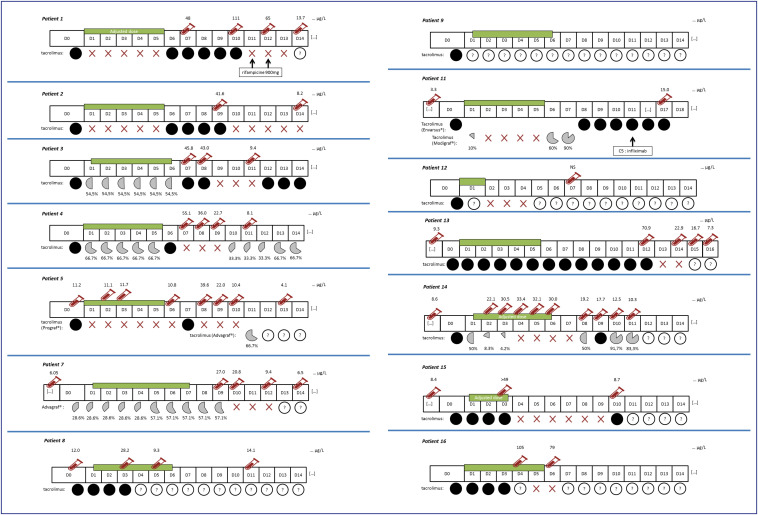
A total of 30 reports of DDI (among which 20 serious reports) was identified among the 228 selected reports (13.1%), mostly with an immunosuppressant (IS) overdose (i.e., tacrolimus n = 14 & ciclosporin n = 2). These reports are summarized in Table 2 . The Fig. 2 specifically details the individual reports of tacrolimus overdoses.
Table 2.
Summary of reports of overexposure in immunosuppressant due to a drug-drug interaction with nirmatrelvir/ritonavir (Paxlovid®) recorded in the French Pharmacovigilance database over the period from 01/20/2022 to 12/03/2022.
| Patient number | Sex | Age group | Seriousness? (Y/N) | Drug (speciality) | IS current daily dose (mg) | Posology adaptation? (Y/N) | ADRs related to the DDI | Paxlovid® duration (days) | Overdosage discovery time since initiation (D) | Main clinical context |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 50–60 | Y | Tacrolimus (Advagraf®) | 12 mg | Yes | Acute renal insufficiency (+ hyperkalemia + metabolic acidosis) | 5 | 6 | Liver transplantation on alcoholic hepatitis Hypertension Psoriasis IgA mesangial deposition nephropathy Chronic kidney disease |
| 2 | F | 30–40 | N | Tacrolimus (Advagraf®) | ND | Yes | None | 5 | 8 | Lupus nephritis Kidney transplant |
| 3 | M | 60–70 | N | Tacrolimus (Envarsus®) | 2.75 mg | Yes | None | 5 | 7 | Organ transplant (no other details) |
| 4 | F | 50–60 | N | Tacrolimus (Advagraf®) | 3 mg | Y | Acute renal insufficiency + encephalopathy (tremor + +) | 4 | 7 | Organ transplant (no other details) |
| 5 | F | 40–50 | Y | Tacrolimus (Prograf®) | 9 mg | Y | None | 5 | 8 | Heporenal polykystosis with preemptive graft Hypertension Nephritic colic |
| 6 | F | 30–40 | Y | Ciclosporin (Neoral®)/prednison | 270 mg | Y | None (suspected hyperglycemia) | 5 | 3 | COVID-19 Organ transplant (no other details) Cystic fibrosis Renal insufficiency Diabetes |
| 7 | M | 50–60 | Y | Tacrolimus (Advagraf®) | 3.5 mg | Y | Acute renal insufficiency | 6 | 9 | Liver transplantation for primary sclerosing cholangitis Benign prostatic hyperplasia |
| 8 | F | 60–70 | N | Tacrolimus (Adoport®) | 5 mg | N | None | 5 | 3 | Organ transplant (no other details) |
| 9 | M | 40–50 | Y | Tacrolimus (ND) | 4 mg | ND | Acute renal insufficiency | 5 | 10 | Organ transplant (no other details) |
| 10 | M | 60–70 | Y | Ciclosporin (Neoral®) | 240 mg | ND | Encephalopathy (coma) | 3 | 5 | Organ transplant (no other details) |
| 11 | M | 30–40 | Y | Tacrolimus (Envarsus®) | 2 mg | Y | None | 5 | 10 | Autoimmune hepatitis Cirrhosis IgG4-related sclerosing disease Ulcerative colitis |
| 12 | F | 60–70 | Y | Tacrolimus (Advagraf®) | ND | ND | Vomiting Diarrhea Lethargy Weight loss |
1 | 6 | COVID-19 Liver transplantation |
| 13 | M | 50–60 | N | Tacrolimus (Advagraf®) | 8 mg | ND | None | 5 | 11 | Kidney transplant |
| 14 | M | 10–20 | Y | Tacrolimus (Prograf®) | 12 mg | Y | Acute renal insufficiency (aggraved) | 5 | 1 | Kidney transplant |
| 15 | F | 40–50 | Y | Tacrolimus (Advagraf®) | 9 mg | Y | Acute renal insufficiency | 2 | 2 | Kidney transplant |
| Pravastatine | 10 mg | Yes (but not applicated by the patient) | Rhabdomyolysis | – | – | |||||
| 16 | F | 30–40 | Y | Tacrolimus (ND) | 15 mg | Yes (but not applicated by the patient) | Acute renal insufficiency | 4 | 2 | Kidney transplant |
ADR: adverse drug reaction; COVID-19: coronavirus disease 2019; D: day; F: female; N: no; Y: yes.
Figure 2.
Individual history of tacrolimus overexposures due to a drug-drug interaction with nirmatrelvir/ritonavir (Paxlovid®) from the French Pharmacovigilance database over the period from January 20, 2022 to December 03, 2022.
For these reports of DDI with IS (n = 16 of which 11 serious), despite a dose adjustment performed in the majority of reports (n = 10, 62.5%), an overdose was diagnosed within an average of 6 days, the day after the theoretical cessation of antiviral treatment. However, for most of the tacrolimus reports (11 out of 14), recommendations for discontinuation or dosage adjustment and/or modality for immunosuppressant treatment restart has not been respected (Fig. 2). For two of the remaining reports (patients no 9 and no 12), no dosage information has been provided. For the last patient (no 11), recommendations have only been partly implemented with an initial dosing reduction to 10% of the initial dose on day 1 before tacrolimus discontinuation (as recommended) and then a restart at 60% and 90% of the initial dose on day 6 and 7 respectively, while 50% and 75% were recommended. Of note, the overexposure episode in that report (15 ng/mL on day 17) was moderate. These IS overexposures were asymptomatic in 7 reports (patients 2, 3, 5, 6, 8, 11 & 13). In the other situations, overdoses were identified following the finding of an acute renal failure (n = 9; patients 1, 4, 7, 9, 10, 12, 14, 15 & 16). Finally, outcome was constantly favorable either after nirmatrelvir/ritonavir discontinuation, or suspension/dose adjustment of the IS.
Other DDI situations in the remaining 14 reports are detailed in Table 3 . Six (6) concerned anticoagulants, 2 of which occurred as part of a switch of an oral anticoagulant (i.e., rivaroxaban) to heparin therapy in accordance with the current recommendations of the French Society of Pharmacology & Therapeutics (FSPT) [7], [8]. In one report, the non-recommendation of association with rivaroxaban was not taking to account, regarding the risk of DDI. Three other reports concerned INR modification under vitamin K antagonist (VKA), of which 2 report INR increase while the reverse would be expected due to the suspected mode of interaction (i.e., INR decrease related to induction of the VKA metabolism).
Table 3.
Summary of reports of drug-drug interaction (excluding immunosuppressant) with nirmatrelvir/ritonavir (Paxlovid®) recorded in the French Pharmacovigilance database over the period from 01/20/2022 to 12/03/2022.
| Sex | Age group (years) | Seriousness (Y/N) | Drug | DDI description | Dose adjustment before Paxlovid® initiation? | ADRs related to the DDI | Time of occurrence from the beginning of Paxlovid® (D) | Comment |
|---|---|---|---|---|---|---|---|---|
| F | 80–90 | Y | Digoxine | Proven plasma overdose of digoxin (via probable inhibition of P-gP by ritonavir) | None | None | 5 | Evolution: favorable after stopping Paxlovid® and suspension of digoxin |
| M | 90–100 | Y | Apalutamide | Probable elevated plasma concentrations of apalutamide (NB: risk of reduction of nirmatrelvir by apalutamide) | None | Prolonged QT interval (506 ms versus 475 ms) Movement disorder (without précision) |
5 | Contraindicated combination Evolution: death (on possible cerebral hemorrhage) at D5 of the discovery of the effect |
| F | 70–80 | N | Verapamil | Probable increase in plasma verapamil concentrations | None | Bradycardia | 1 | Not recommended combination |
| M | 70–80 | Y | Atenolol (switch from bisoprolol) amlodipine (switch from lercanidipine) | Not expected with β-blockers or amlodipine | None | Bradycardia Hypotension Palpitations |
2 | Pharmacodynamic interaction between antihypertensives due to slowing down lercanidipin removal? + Problem with dose equivalency during therapeutic switchs? |
| F | 80–90 | N | Rifampicin/clindamycin | Double DDI (on CYP3A4): – between clindamycin and rifampicin (risk of ineffectiveness of clindamycin and nirmatrelvir by induction) – between ritonavir and clindamycin (likely elevated plasma concentrations of clindamycin by inhibition) |
None | Hepatic cytolysis | 7 | Contraindicated combination (rifampicine & Paxlovid®) Unexpected adverse effect (i.e., hepatic cytolysis) in this context |
| F | 50–60 | Y | Dapsone | Probable increase in formation of methemogloucizing metabolite of dapsone (by enzymatic induction of CYP2C9 and 2C19) | None | Methaemoglobinaemia (6% vs. 0.3%) | 3 | Evolution: favorable after stopping Paxlovid® and suspension of dapsone |
| M | 50–60 | Y | Fentanyl (+ mirtazapine & sertraline) | Probable increase in plasma concentrations of fentanyl, mirtazapine and sertraline | None | Bradycardia | 2 | Association with fentanyl not recommended in FSPT recommendations + Association contraindicated not taken into account with simvastatin |
| F | 70–80 | N | Atorvastatine | Probable increase in plasma concentrations of atorvastatin | ND | Muscle cramps | 3 | Association not recommended |
| M | 70–80 | N | Atorvastatine/amiodarone | Probable increase in plasma concentrations of atorvastatin and amiodarone | None | Acute hepatitis Rhabdomyolysis |
2 | Association not recommended with atorvastatin Contraindicated combination with amiodarone |
| Fluindione | Not expected (via enzyme induction?) | INR increase | Increase in INR at distance from stopping Paxlovid® = paradoxical with respect to expected DDI | |||||
| M | 70–80 | N | Fluindione | Not expected (via enzyme induction?) | None | INR reduction | 11 | – |
| F | 70–80 | N | Acenocoumarol | Possible decrease in plasma VKA concentrations (via enzyme induction) | None | INR reduction and then increase | 18 | Increase in INR at distance from stopping Paxlovid® = paradoxical with respect to expected DDI Hypothesis of a rebound effect due to the lifting of enzymatic induction = unlikely (no change in AVK dosage |
| M | 70–80 | Y | Rivaroxaban | Plasma overdose proven in rivaroxaban | None | Melena | 4 | Not recommended combination Evolution: favorable after stopping Paxlovid® and suspension of oral anticoagulant |
| M | 70–80 | Y | Enoxaparin | Not expected with heparins (pharmacodynamic interaction between anticoagulants due to slower elimination of rivaroxaban?) | Yes (relay of oral anticoagulant by heparin) | Muscle hematoma Acute post-hemorrhagic anemia |
9 | Incidental discovery of bleeding away from stopping Paxlovid® (already present before Paxlovid®?) |
| M | 80–90 | Y | Calcium heparin | Muscle hematoma | 9 | Off label use (Oxygen Patient Requiring Prior to Paxlovid®) Incidental discovery of bleeding away from stopping Paxlovid® (already present before Paxlovid®?) |
ADR: adverse drug reaction; D: day; DDI: drug-drug interaction; F: female; FSPT: French Society of Pharmacology and Therapeutics; INR: international normalized ratio; M: male; Y: yes.
Among the other reports, one concern methemoglobinemia related to the formation of dapsone hydroxylamine (metabolite of dapsone) due to dapsone and ritonavir association through CYP2C9 induction [9], [10].
Finally, two reports of bradycardia occurred after association with anti-hypertensive drugs.
Analysis of reports with fatal outcomes
A total of 10 fatal outcomes were reported over the period, involving 7 women and 3 men, with an average age of 86 years (Table 4 ). Of these 10 reports, 8 involved patients treated in the early access period. No expected DDI with nirmatrelvir/ritonavir has been identified as the direct or indirect cause of these deaths.
Table 4.
Summary of reports of deaths reported with nirmatrelvir/ritonavir (Paxlovid®) recorded in the French pharmacovigilance database over the period from 01/20/2022 to 12/03/2022.
| Sex (F/M)/age group (years) | Suspect drug | Reported ADRs | Reported cause of death | DDI? | Comment |
|---|---|---|---|---|---|
| F/70–80 | Comirnaty® | Vaccination failure | ARDS --> undetermined etiological shock on COVID19 variant Omicron BA.2 | No evidence of cessation of lercanidipine (risk of hypotension due to elevated plasma concentrations) | – |
| Paxlovid® | Drug inefficiency | ||||
| F/70–80 | Paxlovid® | Cardiorespiratory arrest | Unknown | No information concerning the cessation or continuation of certain drugs of the usual treatment (in particular lercanidipine and atorvastatin) | 93% oxygen saturation prior to Paxlovid® + multiple CV risk factors (hypertension, type 2 diabetes, obesity, high cholesterol, sleep apnea syndrome, chronic kidney failure, etc.) |
| F/80–90 | Paxlovid® | Pseudomonas pneumonia | Multi-organ failure due to COVID-19 infection and Pseudomonas aeriginosa superinfection | Undocumented | – |
| Drug inefficiency | |||||
| F/80–90 | Paxlovid® | Ischemic stroke at D6 from initiation of Paxlovid® | Ischemic stroke | Undocumented | Lack of SARS-CoV-2 vaccination |
| M/80–90 | Paxlovid® | Sudden death at D1 of Paxlovid® and D2 of nadolol | Sudden multifactor death | No | Context of liver decompensation on alcoholic cirrhosis, myeloproliferative syndrome being explored and multiple CV risk factors (hypertension, type 2 diabetes, CAAF, etc.) |
| Nadolol | |||||
| M/90–100 | Paxlovid® | Melena | Deglobulization + palliative management | No | Context of prostatic abscess treated with antibiotic therapy |
| Enoxaparine | |||||
| M/90–100 | Paxlovid® | Ischemic stroke at D17 from initiation of Paxlovid® | Ischemic stroke | Undocumented | – |
| F/80–90 | Paxlovid® | Drug inefficiency | Unknown | No | At D1: apixaban relai for enoxaparin with respect to DDI risk |
| Enoxaparine | |||||
| F/90–100 | Paxlovid® | Complicated abdominal hematoma from secondary hemorrhagic shock | Multivisceral failure following shock | No | At D1: rivaroxaban relai for enoxaparin with respect to DDI risk |
| Enoxaparine | |||||
| F/80–90 | Paxlovid® | Cardiogenic shock on myocardial ischemia | COVID-19 nosocomial infection complicated from myocardial infarction with cardio-respiratory failure | No | Difficult home support given severe cognitive impairment |
ADR: adverse drug reaction; ARDS: acute respiratory distress syndrome; CAAF: complete arrhythmia by atrial fibrillation; COVID-19: coronavirus disease 2019; CV: cardiovascular; D: day; DDI: drug-drug interaction; F: female; M: male; N: no; SARS-CoV2: severe acute respiratory syndrome coronavirus 2; Y: yes.
Of these 10 deaths, 2 occurred in a context of stroke. The first report concerned an octagenarian woman (not vaccinated against COVID-19) – with multiple cardiovascular risks including a history of stroke – who had an ischemic stroke 6 days after nirmatrelvir/ritonavir initiation (lack of information about the patient's usual treatment). The second report concerned a man in his nineties with several comorbidities (high blood pressure, ischemic stroke, dementia, sleep apnea syndrome) who died after an ischemic stroke 17 days after the end of nirmatrelvir/ritonavir treatment.
Three other deaths occurred as a result of COVID-19 disease which may reflect a lack of efficacy of nirmatrelvir/ritonavir.
Finally, 1 death occurred in an octagenarian woman after a hemorrhagic shock during heparin treatment as a part of switch of rivaroxaban to prevent the risk of DDI (Table 3).
Medication errors
Over the period, 7 reports of medication error (of which 5 serious) have been reported over the study period. In 4 reports, no compliance to recommendations resulting to DDI is reported. These reports have already been presented in the above section on DDI. Other situations are related to:
-
•
nirmatrelvir/ritonavir under-dosing without effect;
-
•
lack of nirmatrelvir/ritonavir dose adjustment to renal function;
-
•
nirmatrelvir intake without ritonavir due to computerized prescription misunderstanding.
Drug failure/worsening of the disease
Over the study period, 5 reports (all serious, including 2 deaths) describe a clinical presentation related to lack of efficacy or COVID-19 worsening. To these reports, at least 3 other could be related to nirmatrelvir/ritonavir inefficiency, with a COVID-19 symptoms worsening (mainly digestive). No expected DDI was retrieved to explain these inefficiencies.
Unexpected events of interest (analysis based on frequency and/or seriousness)
Apart from the above-detailed reports, the most frequently reported unexpected events were various cutaneous (n = 22), high blood pressure (n = 7), acute renal insufficiencies (n = 7) and confusion (n = 5).
Transient elevated blood pressure
A total of 7 reports (2 serious) of elevated blood pressure (without other suspect drug) were reported over the period (Table 5 ). These reports mainly concern women (n = 5) with an average age of 68 years, four of them without medical history of high blood pressure. The time to onset from the initiation of nirmatrelvir/ritonavir was variable (from D0 to D4). When the information was available (n = 6/7), outcome was favorable after discontinuation of antiviral treatment and in most reports without symptomatic treatment (n = 4/7). Finally, no expected DDI were identified to explain these transient blood pressure elevations, including the patient who received carfilzomib the day before nirmatrelvir/ritonavir initiation. Indeed, carfilzomib is extensively metabolized by the liver without CYP450 system involvement and probably a weak role for P-gp [11].
Table 5.
Summary of reports of blood pressure elevation reported with nirmatrelvir/ritonavir (Paxlovid®) recorded in the French pharmacovigilance database over the period from 01/20/2022 to 12/03/2022.
| Sex | Age group (years) | Seriousness (Y/N) | Paxlovid® duration (days) | Time to onset from initiation (days) | Tensional values (vs. usual values) | Possible expected DDI involved? | Outcome | Known hypertension (Y/N) | Comment |
|---|---|---|---|---|---|---|---|---|---|
| F | 50–60 | N | 2 | 8 | ND (ND) | ND | Recovering | N | Other reported effects: “nausea, diarrhea, malaise & thoracic oppression” |
| M | 70–80 | Y | 1 | 2 | ND (ND) | No | Recovered on symptomatic treatment | N | Discovery of thrombotic microangiopathy and decreased LVEF + carfilzomib chemotherapy context |
| F | 60–70 | N | 4 | 1 | 150 mmHg (vs. 110 mmHg) | No | Recovered after Paxlovid® withdrawal without symptomatic treatment | N | Other reported effects: “dysgeusia, xerrostomy and myalgia” |
| F | 70–80 | N | 5 | 4 | 180/80 mmHg (vs. normal or low pressure) | No | Recovered after Paxlovid® withdrawal without symptomatic treatment | N | Other effects described in the narrative: “oppression and chest pain” (pulmonary embolism discarded at angioscanner) |
| F | 40–50 | N | 3 | 2 | 140/80 mmHg + 150/95 mmHg (120/80 mmHg) | No | Unknown | N | Other reported effects: “Diarrhea, tremor and dysgeusia” |
| F | 80–90 | Y | 4 | 4 | ND (ND) | No | Recovered on symptomatic treatment | Y | Discovery of Type B Aortic Dissection during Cardiovascular Scans |
| M | 70–80 | N | 5 | 2 | 180/100 mmHg + 140/80 mmHg (vs. normal pressure under therapy) | No | Recovering | Y | Dose of Paxlovid® adapted to renal function |
CV: cardiovascular; D: day; DDI: drug-drug interaction; F: female; LVEF: left ventricular ejection fraction; M: male; N: no; ND: not documented; Y: yes.
Acute renal failure (excluding those resulting from DDI)
Except reports of AKI related to DDI between nirmatrelvir/ritonavir and immunosuppressants (n = 6), 7 other reports of AKI (all serious) occurred in 6 females and 1 male, with an average age of 82 years old, in various clinical circumstances (rhabdomyolysis, dehydration on profuse diarrhea and/or vomiting, underlying nephropathy, etc).
Cutaneous effects
A serious report of Stevens-Johnson syndrome (SJS) was reported in a male in his fifties, 3 days after nirmatrelvir/ritonavir end. This report with an unknown evolution was poorly documented without skin biopsy and was clinically atypical because of tense blisters. Moreover, the cutaneous eruption occurred in a context of infectious endocarditis treated by a multiple antibiotic therapy. The causality assessment identified at least two more suspect drugs than nirmatrelvir/ritonavir using ALDEN score [12] (vancomycin: ALDEN score + 3, amoxicillin/clavulanic acid: ALDEN score + 2, nirmatrelvir/ritonavir: ALDEN score 0, etc).
Twenty-one other reports of cutaneous effects were reported including six serious reports during the period, with an average delay of 4.2 days from initiation of nirmatrelvir/ritonavir. Among these reports, at least 5 reports of urticaria/angioedema were reported with available data (clinical presentation, time of occurrence, evolution) not suggestive of an immediate immuno-allergic mechanism (Ig-E mediated). The other reports of cutaneous effects are more difficult to characterize. Lastly, in all these reports, COVID-19 itself may have played a role in the eruption.
Confusion
Over the period, 5 reports of acute confusion were reported. Among the serious reports (n = 4/5), one occurred in a male in his fifties with multiple comorbidities (hypertension, lymphoma in remission) who experienced confusion at D2 from the administration of nirmatrelvir/ritonavir, requiring hospitalization. No infectious cause was found (blood cultures, urine culture, lumbar puncture, or at D4 from the administration of antiviral treatment also the day of withdrawal) with a favorable outcome after 3 days of probabilistic antibiotic therapy.
Another report concerned a nonagenarian man who presented an acute confusion with dysarthria at D3 of nirmatrelvir/ritonavir administration without any other identified cause (including stroke). The evolution was spontaneously favorable within one week after antiviral withdrawal.
Another report of acute confusion occurred in a male – over 80-years-old – approximately one hour after the first intake of nirmatrelvir/ritonavir with a spontaneous resolution after withdrawal cessation of treatment (by the patient himself).
The fourth report of acute confusion occurred in a nonagenarian woman the day after nirmatrelvir/ritonavir initiation.
Finally, an additional report of acute confusion occurred at D1 of nirmatrelvir/ritonavir and was already described in the previous section of medication errors.
When data was available, the analysis of drug combinations doesn’t identify any possible DDI as a cause of these confusions.
Vigibase analysis
Research in VigiLyze (date of request: 01/01/2023) found 26,907 deduplicated reports. The countries who reported most of the ARDs are the United States of America (n = 22,450, 83.4%), followed by the United Kingdom (n = 1224, 4.5%), Malaysia (n = 796, 3.0%), Italy (n = 669, 2.5%), Germany (n = 599, 2.2%) and France (n = 426, 1.6%). The percentage of serious reports was 12.6% (n = 3397 reports), of which 99 reports had a fatal outcome (0.4% of the total).
Regarding demographic characteristics, the sex ratio was 1.8 female to 1 male with a mean age of 58-years-old.
A total of 68,309 effects were reported – 2.5 effects per report on average – and there was an average of 1.1 suspected drugs per report. The main co-suspected drugs during nirmatrelvir and ritonavir treatment, were tacrolimus (n = 111), simvastatin (n = 97), atorvastatin (n = 51), apixaban (n = 48), rosuvastatin (n = 30), amlodipine (n = 29).
At least 294 reports (for 395 interacting drugs, excluding nirmatrelvir/ritonavir) of DDI were identified among the 26,907 reports identified. The main drugs involved were immunosuppressants for 41.5% (i.e., tacrolimus mainly, n = 158 reports), statins for 7.1% (atorvastatin: n = 16 reports, simvastatin: n = 7 reports and rosuvastatin: n = 5 reports) and oral anticoagulants for 8.1% (VKA: n = 11 reports, apixaban: n = 16 and rivaroxaban: n = 5 reports).
The most frequently reported adverse reactions concerned expected effects (i.e., dysgeusia, diarrhea, headache, nausea/vomiting) and could be:
-
•
probably related to underlying infectious syndrome;
-
•
therapeutic inefficiency;
-
•
medication error;
-
•
DDI.
Among the other reported ADRs, the most notable ones (on a clinical and frequency basis) concerned phenomena of “recurrence/rebound” of COVID-19 (N > 10,130 effects), elevation of blood pressure and hypertension (N > 370 effects) as well as skin reactions (N > 880 effects).
Finally, the disproportionality analysis based on WHO indices (i.e., IC0.25 & ROR) provided no additional information but confirmed the above-mentioned data.
Literature
On October, 13th 2022, PubMed® research retrieved at least 261 publications related to nirmatrelvir. Almost all of this literature reported almost no reports of adverse effects related to the use of this antiviral, apart from several reports or series of “rebound” or “recurrence” phenomena, pharmacovigilance data from the few published clinical studies and recommendations for management of drug interaction risk.
Concerning DDI, two articles described the occurrence of tacrolimus overdose in combination with nirmatrelvir/ritonavir in a context of kidney transplant. One involved a 14-years-old renal transplanted adolescent with a tacrolimus overdose following the introduction of nirmatrelvir/ritonavir without immunosuppressant dose adjustment [13]. The second report concerned a 34-years-old man not vaccinated against SARS-CoV-2, who experienced a tacrolimus overexposure (again without dose adjustment of the immunosuppressive drug as recommended in appropriate guidelines) at D2 of the initiation of an adjusted dose of nirmatrelvir/ritonavir [14].
As of October, 13th 2022, a dozen articles reported a COVID-19 ‘rebound/recurrence’ in patients treated by nirmatrelvir/ritonavir (Table 6 ) [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27].
Table 6.
Summary of recurrence phenomena published in scientific literature.
| Main author | Reference | Type of article | Number of reports | Rebound or recurrence? | Description | Median time after end of Paxlovid® (days) |
|---|---|---|---|---|---|---|
| Coulson JM | [24] | Case series | 3 | Recurrence | On viral load & symptoms | 5 |
| Boucau J | [20] | Prospective study | 7 | Recurrence | On viral load & symptoms | 4 |
| Epling BP | [25] | Prospective study | 6 | Recurrence | On viral load & symptoms | 9 |
| Ranganath N | [27] | Retrospective cohort study | 4 | Recurrence | On symptoms | 9 |
| Carlin AF | [21] | Case report | 1 | Recurrence | On viral load & symptoms | 5 |
| Antonelli G | [18] | Case series | 2 | Recurrence | On viral load & symptoms | 5.5 |
| Rubin R | [28] | Case report | 1 | Recurrence | On viral load & symptoms | 6 |
| Alshanqeeti S | [16] | Case series | 2 | Recurrence | On symptoms | 6 |
| Epling BP | [26] | Case series | 6 | Recurrence | On viral load & symptoms | 6.5 |
| Charness ME | [23] | Case series | 3 | Recurrence | On viral load & symptoms | 5 |
| Anderson AS | [17] | EPIC-HR clinical study | 23 | Recurrence | On viral load | 9 |
| Birabarahan M | [19] | Case report | 1 | Recurrence | On symptoms | 3 |
Discussion
Analysis of reports reported during the investigation period (from January, 28th 2022 to December, 3rd 2022) confirms the safety profile of nirmatrelvir/ritonavir mentioned in the current SmPC, notably its complex drug-drug interaction profile linked to ritonavir. In addition, this analysis has also allowed identifying the unexpected adverse events that would need to be investigated more accurately to assess drug causality as follows: high blood pressure, confusion, acute renal failure and various cutaneous eruptions.
More than 10% of reports retrieved in the French Pharmacovigilance database are DDI reports. Apart from 2 reports of hematoma discovered during heparin therapy (as a relay of an oral anticoagulant) and the INR variation during fluindione treatment, all currently reported DDI were known and therefore expected taking into account the known pharmacokinetic interaction profile of ritonavir. No particular signal is raised. Indeed, a pharmacokinetic interaction between nirmatrelvir/ritonavir and heparin is not theoretically expected. Moreover, onset of the hematoma was not dated and it may have occurred during oral anticoagulant treatment and before the antiviral therapy. In the event of a DDI, the hypothesis could be a pharmacodynamic interaction (i.e., increased risk of bleeding) between heparin and oral anticoagulant due to a delay in its elimination related to the antiviral treatment.
Concerning VKA, the risk of DDI is expected only with acenocoumarol and warfarin (via a probable metabolic induction). Nevertheless, the expected clinico-biological impact of this DDI is low and in the direction of an INR decrease. Therefore, the association of a VKA with nirmatrelvir/ritonavir is possible without initial dose adjustment but with a close monitoring of INR. Concerning fluindione, the impact on INR is less obvious, since its metabolism is not dependent on a single cytochrome (i.e., 2C9), but potentially also on CYP1A2 [28].
Interestingly, the DDI between dapsone and ritonavir is expected, even if it is not explicitly mentioned in their respective SmPCs [29], [30]. Indeed, the methemoglobinemia induced by dapsone is mainly mediated by the formation of a metabolite (i.e., dapsone hydroxylamine) mediated by CYP2C9 and 2C19 [10]. These 2 CYP isoforms are induced by ritonavir, resulting in an increase in the production of this metabolite and then methemoglobin.
In the other reports of DDI, the main involved drugs were immunosuppressants (notably tacrolimus) for which the recommendations of the SmPC and the French Society of Pharmacology & Therapeutics are not always followed (recommendations applied in only 1/3 of the reports) [7]. In addition, to comply with the proposed recommendations, the interest of monitoring blood/plasma/serum concentrations (for immunosuppressants but also for any drug for which therapeutic drug monitoring is necessary in current practice) is major when feasible. Ideally, for immunosuppressants, this monitoring should include at least a sample taken during the treatment (between the 2nd and 3rd day of treatment) and after (within 3 days) the end of treatment. This surveillance optimizes the immunosuppressant adjustment and the prevention of adverse effects related to overexposure.
The systematic search for potential DDI before any initiation of nirmatrelvir/ritonavir is essential. As mentioned in the SmPC, a multidisciplinary approach (involving physicians and specialists in clinical pharmacology) is recommended for the management of DDI in some patients receiving multiple concomitant medications. For that purpose, in France, the General Directorate for Health in collaboration with the French Society of Pharmacology & Therapeutics, the national network of regional pharmacovigilance centers and the network of pharmacology laboratories, has put in place a device through a toll-free phone number to support the prescribing of Paxlovid® and to help prescribers and dispensers for the management of DDI [31].
Regarding elevated blood pressure (which is a listed adverse effects in the Norvir® SmPC) [30], the seven reported reports in the French Pharmacovigilance database were mostly not serious and transient with a spontaneous regression after nirmatrelvir/ritonavir discontinuation, without symptomatic treatment. No expected DDI to date was identified as a potential cause of this effect. Given the potential severity and the evocative timeline in some reports, these events represent a potential safety signal that need to be investigated, all the more that a discrepancy in the rates of hypertension events was observed in the pivotal EPIC-HR study between Paxlovid® and placebo arms (0.6% vs. 0.2% respectively) [32].
The reported reports of cutaneous reactions (excluding immediate hypersensitivity reactions) are generally not sufficiently documented and do not allow, to date to identify a common type of manifestation or to precisely determine the nirmatrelvir/ritonavir causality.
Reports of confusion (n = 5) occurred in elderly patients and/or patients with comorbidities. The chronology is compatible with nirmatrelvir/ritonavir as onset and improvement of these confusions coincided with the initiation and discontinuation of the antiviral treatment. Furthermore no other identified etiology (except COVID-19 itself) was identified. A least no other suspected drugs or DDI were identified to explain these manifestations. Although the occurrence of confusion is not currently a signal, these events require specific attention.
Regarding AKI, this effect usually occurred after another identified cause such as tacrolimus overexposure or severe dehydration following important diarrhea and/or vomiting. From our point of view, these AKI reports do not constitute to date a potential signal, knowing that acute renal failure may also be a complication of COVID-19 itself.
No report of COVID-19 recurrence or virological rebound were reported or identified in this period in France, despite a broad literature on this subject. The currently published reports of Covid-19 “recurrence” report the reappearance of clinical symptoms after an initial improvement, with a positive SARS-CoV-2 PCR test after negativation, during (or immediately after) a nirmatrelvir/ritonavir treatment. Some authors mention a “rebound effect” which implies an increase in manifestations after an initial improvement. However, this terminology does not correspond to the reported reports where the intensity (symptoms and/or viral load) is not described or suggestive of a worsening compared to the initial state. The term “recurrence” is therefore more appropriate, specifying the nature (clinical and/or virological) of the manifestations, the time to onset compared to the treatment withdrawal and to ensure that there has been a real phase of post-treatment improvement. In pathophysiological considerations, some authors hypothesize that these recurrence phenomena are not related to a failure of antiviral therapy, but to the natural history of COVID-19 [33]. The results of the EPIC-HR study support this hypothesis, with viral load rebound observed to be similar (in terms of incidence and intensity) between the treatment (nirmatrelvir/ritonavir) and placebo groups and not associated with more severe symptoms neither emergence of resistance to nirmatrelvir [16]. Finally, the less likely hypothesis of a viral reinfection also remains possible.
Treatment failures are not considered as unexpected events based on clinical assessments and primary endpoints used in clinical studies, i.e., reduction in hospitalization and death rates. Nevertheless, the analysis of these reports is important to identify any potential cause (such as underlying drug interaction, medication error, etc.), to specify their nature (symptomatology, hospitalization, death, etc.) and their reporting frequency with respect to the treated population. Moreover, recent articles describe possible viral mutations of SARS-CoV-2 main protease that could induce resistance to nirmatrelvir which argues in favor of continuing the monitoring of these “inefficiencies” in the nirmatrelvir/ritonavir national pharmacovigilance survey.
The main limitations of our analysis are those of retrospective pharmacovigilance studies. Indeed, current post-marketing pharmacovigilance is strongly based on spontaneous notification and presents well-known bias [34]. Among these, the main bias concerns the lack of information and the under-notification. Nevertheless, at this stage, the main limitation of our study lies in the volume of reported reports, which remains quite low, in particular for certain clinical situations and events of interest. The reports reported to date in pharmacovigilance in France are generally well documented, but the quality of this documentation is qualitatively heterogeneous, including for some serious situations.
Conclusion
According to data collected to date in France by post-marketing pharmacovigilance, the safety profile of nirmatrelvir/ritonavir is compliant with SmPC of Paxlovid®. However, blood pressure elevation, confusion, cutaneous reactions and AKI are the main unexpected ADRs of interest to monitor. However, these potential pharmacovigilance signals require confirmation with a qualitative approach over time and new reports.
Although the general benefit/risk ratio of this antiviral drug seems favorable, estimating individual benefit is sometimes difficult, especially in view of the major risk of DDI. Therefore, the SmPC and expert recommendations should be consulted systematically before the initiation of this drug, which is particularly indicated in polypharmacy patients. A case-by-case analysis must be carried out by including a specialized pharmacological advice in those complex situations.
Authors’ contributions
KB made substantial contributions to the conception, the design and the interpretation of data. KB made substantial contributions to the acquisition and analysis of data. KB was involved in drafting the manuscript. LL, FL, AD, SF, MA, AV, AP and BLV made substantial contributions in the critical revising of the manuscript to obtain an important intellectual content. All authors approved the final version of the manuscript.
Funding
None.
Disclosure of interest
KB, LL, AD, SF, MA, AV, AP and BLV declare that they have no competing interest. FL received research grants (paid to institution) from Astellas Pharma, Chiesi and Sandoz Pharma and fees to attend meetings from Chiesi, Sandoz, Pfizer, Gilead and Viiv.
Acknowledgments
This publication uses data collected from all Regional Centers of Pharmacovigilance (RCPV) – organized in a French network of RCPV. The information comes from a variety of sources, and the probability that the suspected adverse effect is drug-related is not the same in all report.
References
- 1.World Health Organization.Coronavirus disease (COVID-19) general informations. Web page. 2023. https://www.who.int/health-topics/coronavirus#tab=tab_1 [Accessed 7 February 2023].
- 2.European Medicines Agency. Nirmatrelvir/ritonavir informations. Last update on January 25, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/paxlovid [Accessed 7 February 2023].
- 3.European Medicines Agency.Summary of product characteristics. Paxlovid. 2022. https://www.ema.europa.eu/en/documents/product-information/paxlovid-epar-product-information_en.pdf [Accessed 7 February 2023 (44 pp)].
- 4.European Medicines Agency.Warning about monoclonal antibodies against emerging strains of SARS-CoV-2. December 09, 2022. https://www.ema.europa.eu/en/news/etf-warns-monoclonal-antibodies-may-not-be-effective-against-emerging-strains-sars-cov-2 [Accessed 7 February 2023].
- 5.Miremont-Salamé G., Théophile H., Haramburu F., Bégaud B. Causality assessment in pharmacovigilance: the French method and its successive updates. Therapie. 2016;71:179–186. doi: 10.1016/j.therap.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Montastruc J.L. Pharmacovigilance and drug safety: fair prescribing and clinical research. Therapie. 2022;77:261–263. doi: 10.1016/j.therap.2022.03.001. [DOI] [PubMed] [Google Scholar]
- 7.EPI-PHARE. Paxlovid oral antiviral use study from February 4 to June 29, 2022. French. https://www.epi-phare.fr/rapports-detudes-et-publications/utilisation-paxlovid/ [Accessed 7 February 2023 (27 pp)].
- 8.French Society of Pharmacology and Therapeutics (SFPT). Therapeutic recommandations for drug combination with nirmatrelvir/ritonavir. Version of November 25, 2022. https://sfpt-fr.org/recospaxlovid [Accessed 7 February 2023].
- 9.Lemaitre F., Grégoire M., Monchaud C., Bouchet S., Saint-Salvi B., Polard E., et al. Management of drug-drug interactions with nirmatrelvir/ritonavir in patients treated for Covid-19: guidelines from the French Society of Pharmacology and Therapeutics (SFPT) Therapie. 2022;77:509–521. doi: 10.1016/j.therap.2022.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bluhm R., Adedoyin A., Mccarver D., Branch R. Development of dapsone toxicity in patients with inflammatory dermatoses: activity of acetylation and hydroxylation of dapsone as risk factors. Clin Pharmacol Ther. 1999;65:598–605. doi: 10.1016/S0009-9236(99)90081-4. [DOI] [PubMed] [Google Scholar]
- 11.Corallo C., Coutsouvelis J., Avery S., Morgan S., Morrissey O. Dapsone and azole interactions: a clinical perspective. J Oncol Pharm Pract. 2018;24:637–640. doi: 10.1177/1078155217722048. [DOI] [PubMed] [Google Scholar]
- 12.European Medicines Agency.Summary of product characteristics. Kyprolis. https://www.ema.europa.eu/en/documents/product-information/kyprolis-epar-product-information_en.pdf [Accessed 7 February 2023 (60 pp)].
- 13.Sassolas B., Haddad C., Mockenhaupt M., Dunant A., Liss Y., Bork K., et al. ALDEN, an algorithm for assessment of drug causality in Stevens-Johnson syndrome and toxic epidermal necrolysis: comparison with case-control analysis. Clin Pharmacol Ther. 2010;88:60–68. doi: 10.1038/clpt.2009.252. [DOI] [PubMed] [Google Scholar]
- 14.Young C., Papiro T., Greenberg J.H. Elevated tacrolimus levels after treatment with nirmatrelvir/ritonavir (Paxlovid) for COVID-19 infection in a child with a kidney transplant. Pediatr Nephrol. 2023;38:1387–1388. doi: 10.1007/s00467-022-05712-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prikis M., Cameron A. Paxlovid (nirmatelvir/ritonavir) and tacrolimus drug-drug interaction in a kidney transplant patient with SARS-2-CoV infection: a case report. Transplant Proc. 2022;54:1557–1560. doi: 10.1016/j.transproceed.2022.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alshanqeeti S., Bhargava A. COVID-19 rebound after Paxlovid treatment: a case series and review of literature. Cureus. 2022;14:e26239. doi: 10.7759/cureus.26239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson A.S., Caubel P., Rusnak J.M., Trial EPIC-HR., Investigators Nirmatrelvir-ritonavir and viral load rebound in COVID-19. N Engl J Med. 2022;387:1047–1049. doi: 10.1056/NEJMc2205944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antonelli G., Focosi D., Turriziani O., Tuccori M., Brandi R., Fillo S., et al. Virological and clinical rebounds of COVID-19 soon after nirmatrelvir/ritonavir discontinuation. Clin Microbiol Infect. 2022;28:1657–1658. doi: 10.1016/j.cmi.2022.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Birabaharan M., Martin T.C.S. Acute pulmonary emboli following rebound phenomenon after Nirmatrelvir/Ritonavir treatment for COVID-19. Am J Emerg Med. 2022;61 doi: 10.1016/j.ajem.2022.08.012. [235.e5-235.e6] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boucau J., Uddin R., Marino C., Regan J., Flynn J.P., Choudhary M.C., et al. Characterization of virologic rebound following nirmatrelvir-ritonavir treatment for COVID-19. Clin Infect Dis. 2023;76:e526–e529. doi: 10.1093/cid/ciac512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carlin A.F., Clark A.E., Chaillon A., Garretson A.F., Bray W., Porrachia M., et al. Virologic and immunologic characterization of COVID-19 recrudescence after nirmatrelvir/ritonavir treatment. Clin Infect Dis. 2023;76:e530–e532. doi: 10.1093/cid/ciac496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charness M.E., Gupta K., Stack G., Strymish J., Adams E., Lindy D.C., et al. Rebound of SARS-CoV-2 infection after nirmatrelvir-ritonavir treatment. N Engl J Med. 2022;387:1045–1047. doi: 10.1056/NEJMc2206449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coulson J.M., Adams A., Gray L.A., Evans A. COVID-19 “Rebound” associated with nirmatrelvir/ritonavir pre-hospital therapy. J Infect. 2022;85:436–480. doi: 10.1016/j.jinf.2022.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Epling B.P., Rocco J.M., Boswell K.L., Laidlaw E., Galindo F., Kellogg A., et al. COVID-19 redux: clinical, virologic, and immunologic evaluation of clinical rebound after nirmatrelvir/ritonavir. medRxiv. 2022 [2022.06.16.22276392] [Google Scholar]
- 25.Epling B.P., Rocco J.M., Boswell K.L., Laidlaw E., Galindo F., Kellogg A., et al. Clinical, virologic, and immunologic evaluation of symptomatic coronavirus disease 2019 rebound following nirmatrelvir/ritonavir treatment. Clin Infect Dis. 2023;76:573–581. doi: 10.1093/cid/ciac663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ranganath N., O’Horo J.C., Challener D.W., Tulledge-Scheitel S.M., Pike M.L., Michael O’Brien R., et al. Rebound phenomenon after nirmatrelvir/ritonavir treatment of coronavirus disease-2019 in high-risk persons. Clin Infect Dis. 2023;76:e537–e539. doi: 10.1093/cid/ciac481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubin R. From positive to negative to positive again – the mystery of why COVID-19 rebounds in some patients who take paxlovid. JAMA. 2022;327:2380–2382. doi: 10.1001/jama.2022.9925. [DOI] [PubMed] [Google Scholar]
- 28.Verstuyft C., Delavenne X., Rousseau A., Robert A., Tod M., Diquet B., et al. A pharmacokinetic-pharmacodynamic model for predicting the impact of CYP2C9 and VKORC1 polymorphisms on fluindione and acenocoumarol during induction therapy. Clin Pharmacokinet. 2012;51:41–53. doi: 10.2165/11595560-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 29.Ministère des solidarités et de la santé. Summary of product characteristics. Disulone. Last update on June 09, 2022. https://base-donnees-publique.medicaments.gouv.fr/affichageDoc.php?specid=67654911&typedoc=R [Accessed 7 February 2023].
- 30.European Medicines Agency.Summary of product characteristics. Norvir. https://www.ema.europa.eu/en/documents/product-information/norvir-epar-product-information_en.pdf [Accessed 7 February 2023 (113 pp)].
- 31.Direction générale de la santé.Support device for prescription of Paxlovid. October 12, 2022. https://sante.gouv.fr/IMG/pdf/dgs-urgent_no2022_80_-_dispositif_d_appui_a_la_prescription_de_paxlovid.pdf [Accessed 7 February 2023 (3 pp)].
- 32.European Medicines Agency.Paxlovid assessment report. January 27, 2022. https://www.ema.europa.eu/en/documents/assessment-report/paxlovid-epar-public-assessment-report_en.pdf [Accessed 7 February 2023 (18 pp)].
- 33.Deo R., Choudhary M.C., Moser C., Ritz J., Daar E.S., Wohl D.A., et al. Viral and symptom rebound in untreated COVID-19 infection. medRxiv. 2022 [2022.08.01.22278278] [Google Scholar]
- 34.Bihan K., Lebrun-Vignes B., Funck-Brentano C., Salem J.E. Uses of pharmacovigilance databases: an overview. Therapie. 2020;75:591–598. doi: 10.1016/j.therap.2020.02.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The use of confidential, electronically processed patient data was approved by the French national commission for data protection and liberties (Commission nationale de l’informatique et des libertés; reference number, 1735841).