Abstract
In order to keep up with a changing environment, mobile organisms must be capable of deciding both where and when to move. This precision necessitates a strong sense of time, as otherwise we would fail in many of our movement goals. Yet, despite this intrinsic link, only recently have researchers begun to understand how these two features interact. Primarily, two effects have been observed: movements can bias time estimates, but they can also make them more precise. Here we review this literature and propose that both effects can be explained by a Bayesian cue combination framework, in which movement itself affords the most precise representation of time, which can influence perception in either feedforward or active sensing modes.
Time and movement are intertwined
Continuous interaction with a dynamic world requires organisms to make precise temporal measurements of sensory input and directed motor output. As such, sensorimotor coupling is vital in multiple levels of organization in the nervous system, from spinal reflexes to complex motor sequences [1]. These intertwined sensorimotor processes have potential implications in the study of time perception [2]; however, the link between action and time perception has, until recently [3], been relatively unexplored. Current studies demonstrate that action exerts a wide range of effects on subjective time by biasing (i.e., affecting accuracy; see Glossary) or improving sensitivity (i.e., precision) of estimates and that these effects are not restricted to concurrent movement, but can exhibit robust effects before and after movement. Notably, a unifying account does not yet exist for these effects - a challenge for current models of timing (Box 1). Here, we propose a basic framework to base future studies upon; specifically, we suggest that movement provides a new channel of temporal information that is (i) evaluated by its reliability and (ii) integrated into temporal estimates optimally, in line with Bayesian cue combination (Box 2). This is a prominent idea in multisensory research that has been demonstrated previously in time perception across distinct sensory modalities [4].
Box 1. Models of time perception.
Numerous models have been developed over the past 50 years to describe and explain timing behavior. Of these, the most influential has been Scalar Expectancy Theory (SET), which proposes that timing results from a tripartite process in which an internal clock emits and accumulates pacemaker pulses, proceeding at a particular rate and variability, then compares the accumulated amount to a remembered criterion before making a ratio-based decision [131]. An alternative to this is the Behavioral and Learning Theory of Timing (BeT & LeT), which instead suggest that temporal accumulation emerges as a successive chaining of distinct behavioral ‘states’ with variable intervals between them [132,133]. More recent work conceives timing as a time-adaptive opponent drift-diffusion model (TopDDM) process, in which opponent populations of neurons generate a rate-adaptable process that elapses with the perceived interval [134]. Alternatively, recurrent neural network (RNN) models suggest that activity develops across stereotyped state-space trajectories, allowing for the measurement of distinct time intervals [108,109,135]. Across all of these models, bias and variability can be seen as arising from shifts in pacemaker rate or neural firing trajectories, as well as the variance/shape of those trajectories. For example, SET would posit a change in pacemaker rate/variance, whereas BeT/LeT might suggest changes in the number of states, or the variance of inter-state intervals. Similarly, TopDDM might suggest a shift in drift rate or its variance while timing, whereas RNN models would suggest changes in the speed or variance of the trajectory. Thus, a critical question for models of timing that posit a sensorimotor component, is if they can recapitulate movement-related changes in timing, both in conditions where movement is critical for the timed response (motor timing) or not critical (perceptual timing).
Box 2. Bayesian cue combination.
Bayesian Cue combination is a proposed mechanism by which the brain can combine noisy sensory estimates from different modalities. It serves as an extension of Bayesian Decision Theory, in which noisy sensory estimates are perceived as draws from a likelihood function with a certain mean (μ) and variance (σ2) that are then combined with a prior distribution of previous estimates to form a posterior estimate of the stimulus. In the case where two sensory modalities are presented, the sensory estimates are combined optimally using Maximum Likelihood Estimation (MLE), in which the variance of the multisensory estimate distribution is the product of the unisensory variances divided by their sum (see Figure 4B in main text). Further, the mean of the multisensory estimate is determined by a weighted sum of the unisensory means, where the weights are crucially, and inversely, related to the unisensory variances. In this way, the multisensory estimate mean is always 1) closer to the unisensory estimate of whichever modality is more precise, and 2) itself more precise than either of the unisensory estimates alone. Applications of Bayesian Cue Combination have been used to explain how the brain combines estimates of visual and haptic stimuli [136], and auditory and visual stimuli [137]. For time perception, Bayesian Cue Combination has also, to varying degrees of success, described and predicted behavior in the timing of auditory and visual stimuli [104], and visual and tactile stimuli [138] in humans. Applications of Bayesian Cue Combination have also been used to explain temporal averaging in rats [139]. For movement-related effects, this framework can explain both precision and biasing effects (see Figure 4C in main text).
Motor systems are invoked during time perception
Given the necessity of time and movement in everyday interactions, it is not surprising that the brain areas involved in these processes greatly overlap (Figure 1A). Timing relies on several brain systems traditionally thought to be dedicated to motor control [5,6], and that are activated during timing tasks where no overtly timed motor response is required (Figure 1A). The main cortical area implicated in temporal processing is the supplementary motor area (SMA). The SMA is often split into two main components by the anterior commissure: SMA-proper, bordering the primary motor cortex, and pre-SMA, located more anteriorly [7] and has been shown to be chronotopically organized in a rostrocaudal gradient, in that rostral clusters of neurons encode shorter durations and caudal clusters encode longer durations ([8]; see Figure 1B). More specifically, the SMA-proper is implicated in motor timing tasks, timing of single events [9], timing of shorter durations, stimulus-driven movements, and motor preparation [7], whereas the pre-SMA is implicated in perceptual timing tasks [10,11], sequence processing [12], self-initiated movements, response inhibition, timing of longer durations, and task switching. The SMA also appears to have a gradient for time and space, with SMA-proper neurons encoding longer-duration actions in navigating the environment [13]. Further, recordings in the SMA of non-human primates demonstrate duration tuning of individual neurons [14], as well as neurons that encode the categorical boundary between duration ranges that are predictive of temporal categorization [15].
Figure 1. The motor system is involved in time perception.
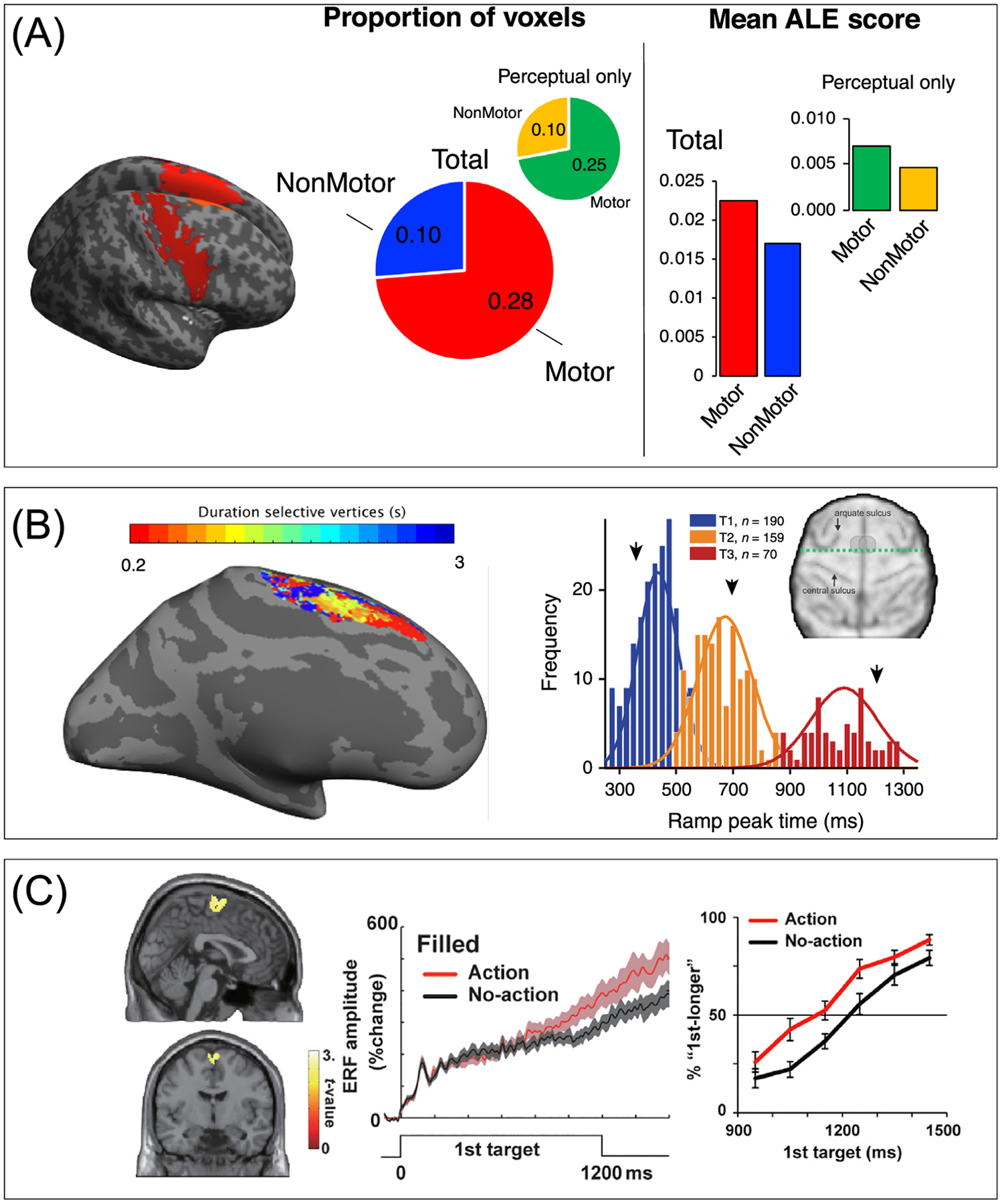
(A) Results from a recent meta-analysis of neuroimaging time perception studies (results adapted, with permission, from [5]) exhibiting greater activation likelihood in motor cortical regions (red highlights, from AAL atlas for SMA and precentral gyri) over nonmotor ones. Specifically, motor regions exhibit a higher mean proportion of significant voxels than non-motor regions, even when motor timing tasks are excluded (inset); additionally, mean activation likelihood estimation (ALE) values within motor regions are typically higher than those in non-motor regions (right side), again when excluding motor timing studies (inset). (B) The SMA exhibits tuning for duration and categorization. Left, high-resolution 7T fMRI ‘chronotopy’ of SMA in a time discrimination task reveal rostrocaudal gradients of activation. Adapted, with permission, from [8]; right, single-unit recordings of SMA neurons in primates performing a time bisection task reveal tuning preferences for the bisection point of three different interval ranges. Adapted, with permission, from [15]. (C) Movement-induced changes in time perception, while a subject engages in an action, are linked to increases in SMA activity. Adapted, with permission, from [29].
Subcortical motor systems, such as the basal ganglia (BG) and cerebellum also play a prominent role in timing. Innervation of the BG by dopamine neurons has demonstrated a close link with timing functions [16,17]. In animals, dopamine availability is strongly linked to the direction of temporal distortions; artificially increasing or decreasing striatal dopamine leads to overestimation and underestimation of time intervals, respectively [18]. Recent work has demonstrated a dissociation between these sub-cortical structures (BG and cerebellum) for timing in rhythmic and single-interval contexts [19]; further, both structures have been found to impact activity putatively within the SMA [20,21]. These findings suggest that time perception is instantiated in motor circuits between the SMA and subcortical regions, via cortical-striato-thalamic connections.
Given the strong overlap between motor and timing regions in the brain, it should be expected that disorders of movement also exhibit timing impairments [21,22]. Importantly, these impairments persist in perceptual timing tasks when movement is irrelevant or unnecessary [23]. For example, Parkinson’s Disease is associated with timing that is both less precise and less accurate, although deficits are heterogenous [24]. Huntington’s Disease patients display similar timing deficits, including greater response variability in interval (i.e., absolute) and beat-based (i.e., relative) timing [23]. Additional timing deficits have been observed in patients with Tourette’s Syndrome [25], Essential Tremor [26], Cerebellar Degeneration [20], Dystonia [27], and those with lesion damage affecting motor regions [28].
Concurrent movements bias time perception
Despite the involvement of motor regions in time perception, until recently movements were not specifically studied in the context of timing. Models of time perception typically set movements to represent the end-point of a timed process, with motor variance existing as a nuisance to be partialed out (Box 1). Yet, early evidence of this relationship came in the form of the chronostasis phenomenon, wherein saccading to a target induced a stereotyped expansion of visual time [30]. Post-action temporal expansion was also found for tactile feedback after manual movement to a target [31], auditory stimuli following a saccade [32], and spontaneous blinking [33]. A separate finding, intentional binding, describes a process by which an action and its sensory consequence are ‘pulled’ towards each other in time [34,35]. More recent work suggests that both phenomena may reflect distinct aspects of the same underlying process [36]; that is, the combination of disparate sensory and motor representations [37]. These representations may be combined in a manner that is also optimal with respect to Bayesian computations [38]. Yet, we note that both are examples in which timing is causally tied to the sensory consequences of actions, which we suggest are distinct from studies of explicit timing [39].
Within the realm of explicit timing, initial evidence that actions could lead to shifts in perceived time came from work demonstrating that, when asked to perform a time reproduction task, the length of that interval is longer when its initiation is triggered by a subject’s action [40]. Yet, while this established a link between motor timing and movements, a connection with perceptual timing would not come until a later study [41] in which the duration of tactile vibrations applied to the finger while performing a large single-movement lift were dilated compared to being at rest. Several follow-up experiments replicated this effect, demonstrating that the duration of an avatar hand performing a finger movement that was congruent with the movement was similarly dilated. This latter finding was similar to another motor timing study in which subjects reproduced auditory durations encoded while watching videos of broad hand gestures; longer-distance hand movements were associated with longer reproduced intervals [42].
An additional number of studies have also found that concurrent movements during time perception can compress or dilate time estimates (Figure 2) depending on various factors; the most significant contributor seems to be the influence of magnitude. It is implicitly understood that short, fast, or near movements take less time, and long, slow, or far movements take more time. This implicit knowledge may be at least partially instantiated in motor circuitry [43] and is necessary to evaluate one’s movements in the environment [44]. Therefore, studies that use actions that imply a particular movement magnitude could bias the perception of other magnitudes. For example, sensory events that appear in reachable space are perceived as having a shorter duration than those in unreachable spaces [45].
Figure 2. Concurrent movements bias time perception.
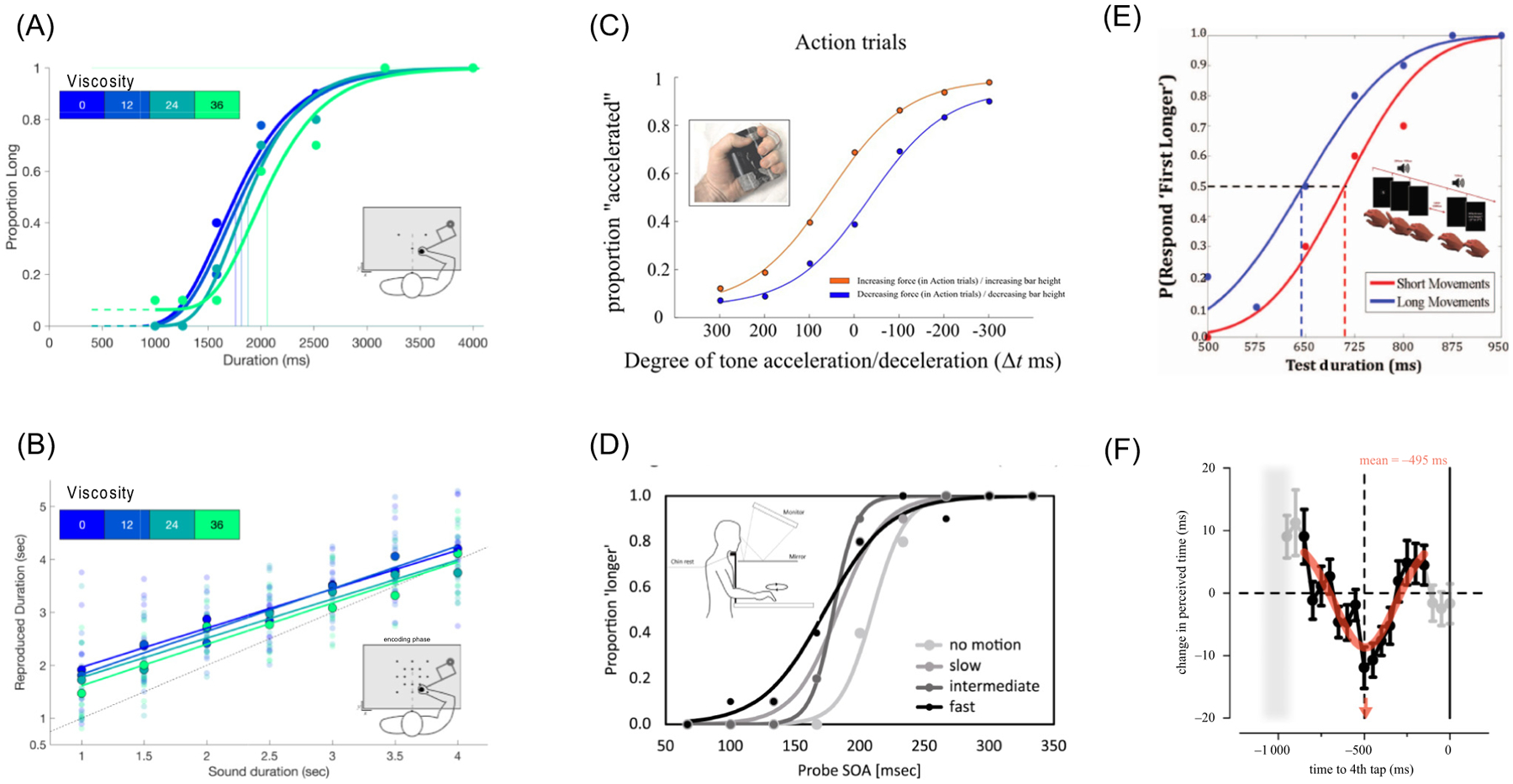
(A) While categorizing auditory tone lengths, increasing the viscosity of a concurrently moved robotic arm leads to shorter perceived durations. (B) Similarly, reproduced time intervals experienced with greater viscosity are also shortened. (C) Holding a force gripper with increasing/decreasing force while judging tone sequences as accelerating/decelerating enhances the separation between perceptual judgments. (D) When judging time intervals while performing circular movements behind an occluder at different speeds, faster speeds lead to shortened perceived time intervals. (E) When performing a finger hyperextension at different speeds, longer movement lengths lead to longer perceived intervals. (F) Similarly, when tapping with the index finger, concurrently presented time intervals are lengthened at the halfway point between finger taps, but shortened at times closest to the preceding or upcoming finger tap. Data from (A,B): [51]; (C): [55]; (D): [47]; (E) [46]; and (F) [52], with permission.
Further, when subjects performed either short and long, or slow and fast finger hyperextensions, as well as near and far hand reaching movements while judging the duration of auditory stimuli, perceived time was dilated when longer, slower, or farther movements were performed ([46]; Figure 2E). By contrast, another study found when subjects performed either slow, medium, or fast hand circles while judging the duration of visual stimuli, faster hand movements were associated with a compression of perceived time [47]. However, in this case, subjects were restricted to making movements within a constrained area. As such, faster hand movements may have led to compression due to a reduced sampling of the environment; indeed, slow, deliberate movements have been shown to provide more information than faster ones [48,49], within a certain limit [50]. Other studies have also shown that, within the same area of space, slower movements lead to time dilation effects [42,46]. More recently, we performed a series of experiments in which subjects were required to move a robotic handle to explore a flat workspace while performing either a temporal bisection task or time reproduction task with auditory intervals [51]. Crucially, increasing resistance (i.e., viscosity) of the robotic handle resulted in shorter movement paths and subsequently shorter perceived durations. Computational modelling further suggested that the time compression effects occurred at the level of perception, rather than decision or production levels.
Other possible explanations for movement-related temporal biases include at what point during the movement the interval occurred [52], the type of stimuli [53], and the sense of agency of the movement [54]. For example, when subjects performed a synchronization-continuation task using finger taps-during which a separate visual temporal interval to be judged occurred randomly during the continuation sequence- the direction and strength of the bias depended on the point during the finger tap the interval occurred ([52]; Figure 2F).
Concurrent movements improve time perception
In addition to the work cited above showing the biasing effects of movement, there is also evidence that concurrent movements can enhance timing (Figure 3). This secondary finding is an essential component of Bayesian cue combination, in which greater precision leads to greater bias.
Figure 3. Concurrent movements enhance timing.
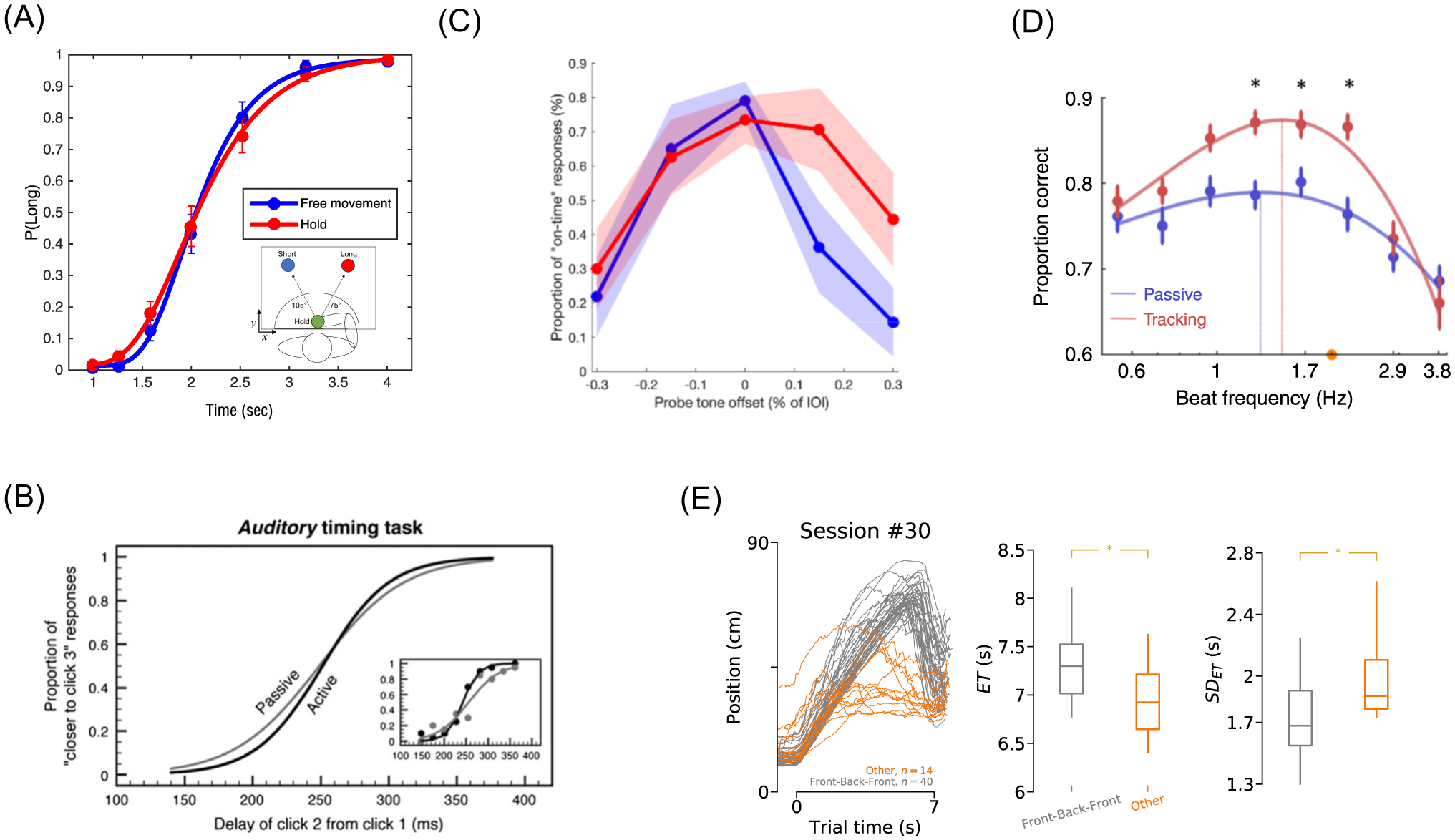
(A) When freely allowed to move a robotic arm while categorizing auditory time intervals, the perception of those intervals is more precise (lower coefficient of variation) than when the arm is held in place during listening. (B) When discriminating auditory intervals, perception is more precise when interval onset is initiated by the subject than when it is presented at a fixed onset. (C) When tapping along with an auditory beat (blue trace), subjects are more precise at detecting deviant tone intervals of different durations. (D) Action improvements in beat perception additionally show greater accuracy and reduced variability at a preferred tempo of 1.5Hz. (E) In rats, when trained to walk along a moving treadmill to a target at fixed time (7s), rats that develop a stereotyped movement pattern (gray traces) exhibit improved accuracy and greater precision of the entry time (ET). Asterisks refer to significant effects at P < 0.05, from the original sources. Data from (A): [57]; (B) [56] (C) [64]; (D) [67]; and (E) [59], with permission.
In one study [56], subjects were more precise timing auditory intervals when the stimulus onset was determined by the subject (Figure 3B). Further studies [57] found that subjects were more precise timing auditory tones when they freely moved compared to remaining stationary. In both bisection and reproduction experiments, precision improved without affecting accuracy (Figure 3A), independent of movement strategies or proximity to choice targets. Further, time estimates could be decoded from the movements, suggesting that movements were actively engaged in the timing process, rather than reflecting its output. A similar finding was recently observed for facial muscles recorded during a visual temporal bisection task [58].
Similarly, in a recent experiment, rats learned to approach a goal location after a fixed interval (7 seconds in most experiments) to obtain a reward. They were positioned on a small treadmill that began moving backward during the interval period. Over many trials, rats learned to keep time by employing a stereotyped ‘wait-and-run’ strategy - they started at the front of the treadmill, allowed it to take them backwards, and ran to the goal location. Rats using this strategy displayed higher accuracy and precision than rats not using the strategy (Figure 3E). Additionally, accuracy and precision were lower when treadmill movement was omitted; however, trials with more movement were more accurate, demonstrating that movement improved timing overall, but performance was highest when temporal regularities in the external environment could be used in tandem with movement [59]. In another rodent experiment, rats performing a temporal bisection task adopted stereotyped head movements as measured by motion tracking, with virtually no between-subject similarities. Importantly, movement patterns, even early in the trial or prior to stimulus onset, were predictive of the eventual choice [60]. A similar finding in humans is that when tracking a moving target with the hand, the perceived duration is also more precise [61], particularly when the target moves in a compatible way with human movement [62].
In addition to enhancing precision for interval timing, research on beat-based timing demonstrates several advantages of sensorimotor synchronization in timing rhythmic stimuli [63]. More specifically, actively tapping along with a rhythm leads to greater accuracy [64–66] and precision [67] at detecting rhythmic deviants.
These considerations are additionally interesting between movements that have explicit temporal properties versus emergent temporal properties, whereby rhythmic properties in the former require ‘event timing’ -explicit temporal representations of each cycle (e.g., tapping) -and in the latter, temporal properties emerge as a byproduct of other motor goals (e.g., maintaining constant velocity during circle drawing; [68]). Further, emergent timing (in a rhythmic context) is thought to evolve from an initial explicit representation (e.g., during the first cycle of circle-drawing) that is thereafter replaced by emergent timing [69]. So far, we have highlighted instances of emergent motor ‘strategies’ that aided in concurrent interval timing, and although these timing distinctions have not been investigated in the majority of studies, they are an interesting future research direction.
Of note, improved timing abilities during action have additionally been observed in individuals with highly trained motor skills, such as elite athletes [70], expert drummers [71], and professional dancers [72]. This highlights the plasticity of time-keeping that evolves with motor experience; indeed, research in children also suggests that temporal concepts are built upon an understanding of actions and their consequences [73]. Further, movement training also enhances other perceptual discrimination abilities [74,75]. Conversely, subjects trained to discriminate a time interval become better at reproducing it with a motor action [76].
Pre-action and post-action effects on timing
In addition to the effects of concurrent action, there are also distinct effects on perceived duration during pre-action and post-action periods, yet with notable inconsistencies. For pre-action periods, in some cases durations are compressed [77–79] and in others dilated [29,80,81]. Various factors lead to these differential effects, including whether the temporal stimulus is a filled interval or an empty interval [29,81] as well as the intended direction of a planned movement [79].
It has been suggested that pre-action visual processing is accelerated to maximize opportunities for stopping or changing a planned action, thus leading to longer perceived intervals. This idea was originally supported when subjects perceived filled visual intervals as longer during preparation of ballistic reaching movements during a temporal discrimination task [80]. Additionally, when viewing flickering visual stimuli during the same period, flicker frequencies were perceived as slower, and subjects displayed increased detection rates for flickering letter displays compared to when passively viewing the stimuli. Notably, this effect appears to only extend to filled intervals, whereas empty intervals are not affected [81]. In addition, magnetoencephalography (MEG) activity revealed ramping activity in the SMA during filled intervals only (Figure 1C) as well as asymmetric suppression of visual 10Hz alpha rhythms. This interaction between motor and visual factors reveals a selective role of alpha rhythm for neural communications across visual, motor, and time-processing regions [29].
In contrast to these pre-action effects, temporal shifts are also observed after actions are initiated [30,32,82]. Often, motor plans are executed with a goal and predicted sensory consequences. As such, these consequences are initially processed separately from externally-generated sensory signals in the brain [83]. Additionally, sensory feedback is used to evaluate the accuracy of goal-directed movements and calibrate internal models to perform future movements more effectively [84–87]. These specialized evaluation mechanisms accompanied by selective suppression or amplification of sensory signals may have implications in time perception.
Taken together, the discussed temporal shifts induced during pre- and post-action periods are indicative of dynamic neural encoding of time with respect to action onset. This is compatible with general principles of motor control, such as anticipatory and predictive pre-action signals, and evaluative and corrective post-action signals [86] - measures that have rarely been examined in the context of explicit timing. Given the nuances of action-locked temporal distortions, dynamical system analysis techniques may elucidate trial-by-trial contributions of evolving movement states to perceived time. These have been used in motor control to understand the evolution of motor ‘states’, both behaviorally [88] and neurally [89]. Dynamical systems models have also been used to understand interval timing [90] and beat-based timing [91], but only recently to study the interactions of movement and time perception [3,92]. This would be a productive area for future research, and highlights the necessity of collaborative efforts with complementary motor control and timing expertise.
A framework for movement and time
As described above, movement effects on explicit time estimates, both perceived and produced, can lead to both biases and enhancements under particular conditions. Additionally, these effects are predicted using a Bayesian cue combination framework (Box 2). Yet, cue combination has traditionally been formulated as one in which disparate channels of sensory information are combined to a unified percept. In our framework, movement itself serves as an additional channel for duration information. Accordingly, the brain integrates the statistics of body movements, which are affected by the speed, length, direction, and area covered. As such, the sampling of positional information in space provides a vector [93] ‘readout’ of elapsed time across a neuronal population. Further, given the high temporal fidelity and precision of motor movements, with some estimates below 10ms [94,95], the variance of movement time estimates is also predicted to be very low. Indeed, motor neurons have demonstrated tuning properties related to both upcoming and preceding movement in time at different lags relative to the present moment [96]. Further, SMA firing rates themselves exhibit multidimensional trajectories that provide tracking and scaling of movements [97], suggesting this region is continuously evaluating the temporal statistics of the environment. This final point is critical; previous studies have demonstrated a ‘modality-appropriateness’ effect in time perception, such that, when measuring a time interval presented with multiple sensory modalities, time estimates gravitate towards the modality with the lower variance [98]. Across sensory modalities, auditory time estimates have been shown as more precise than visual time estimates [99], with tactile estimates in between [100]. Yet, movements have been found to affect visual, tactile, and auditory estimates, suggesting that movements afford a precision that is better or at least as precise as any other sensory domain.
We suggest that a Bayesian cue combination process can explain disparate findings across the literature. In some ways, these effects appear to be universal to time estimates; movement-related effects have been found to influence sensory time estimates across sensory domains, task designs, and also at both sub- and supra-second intervals. The latter effect is particularly relevant, given that sub-second interval timing has been shown to rely more on subcortical structures traditionally associated with movement [5]. By contrast, many common movements span the multi-second domain, and so movement-related effects on longer intervals may not be surprising. Nevertheless, the above literature highlights the fact that not all movements are equal with respect to influencing time. Indeed, we suggest time can be affected differently across movement direction, rhythmicity, effector, and vigor. These differences may arise from the natural variance of particular movements [101], which may relate to how commonly they are employed [102]. Notably, these differential effects of movement parameters may arise as a result of magnitude-based priors leading to a bias in time estimates in certain directions (e.g., shorter movements take less time than longer movements; [103]).
As stated above and in Box 2, Bayesian cue combination can provide a method by which noisy sensory estimates are combined optimally. In this case, movements shift timing by pulling estimates towards their (more precise) duration. No studies that we are aware of have investigated how well humans can time the duration of their own movements, however, according to MLE predictions, movement timing should have a precision that is equal to or better than auditory timing precision. A further prediction is that, when the more precise modality becomes less reliable, its weighting in the combined estimate will decrease. Thus, if movements are made less reliable or uncertain, they should have less of an influence on time estimates from other modalities. Similarly, the cue combination framework for multisensory timing holds most strongly when the sensory modalities demarcating time intervals are filled rather than ‘empty’ [104]. This distinction may explain why movement effects are inconsistent or absent when the interval being timed is also empty [81]. By contrast, during an empty interval, the motor system is likely still active, and so one may predict that empty intervals should instead lead to stronger movement-related effects. The finding that this prediction does not hold has implications for the neural instantiation of cue combination effects for movement and time.
Neural implementation
If Bayesian cue combination can explain movement-related effects on time, then how are these effects implemented in the brain? We outline two possibilities (Figure 4D, Key figure). First, as described above, motor structures predominate neural timing systems, and so one possibility is that movements lead to changes within these motor circuits themselves, most notably the SMA, possibly by sharpening duration or category-tuned neurons, which are driven by sensory and motor responses, or by altering the speed and variance of state-space trajectories. This possibility, which we term Feedforward Enhancement, suggests that motor effects on time occur within motor regions themselves in an adjunctive manner [29]. Indeed, given that the motor system is active during timing, regardless of whether subjects are moving or not [11,105], timing computations may be intrinsically driven and shaped by motor activity. Previous work demonstrating timing responses in motor and premotor cortices has also suggested that the motor system may develop internal models of predictable events in time [106], for use in guiding future movements with increased temporal precision [87].
Additional evidence for Feedforward Enhancement comes from neural recordings in non-human primates, demonstrating motor system trajectories of neural firing rates that are used to encode the duration of intervals [90,107]. These trajectories may be employed across a variety of timing tasks and domains [108,109]. Yet, given this involvement, why would concurrent movements enhance timed responses, rather than hinder them [110]? One possibility is that, when remaining immobile, subjects are faced with a variety of possible, competing movement trajectories, whereas during movement the brain can exploit corollary feedback from movements to dynamically update neural trajectories [111]. Movement preparation offers a similar reason for enhancement, as the specific movement to be made is being prepared over alternatives, which shifts motor cortex activity into a more active state [112]; recent work further suggests movement preparation shifts spontaneous M1 activity into an optimal subspace for engaging in the intended movement [111]. Further, recent modeling work using feedforward neural networks has demonstrated that reinforcement learning agents can learn to time intervals by self-generating idiosyncratic movement patterns that can be exploited as a temporal measurement [113].
Altogether, Feedforward Enhancement would predict that movement-related timing effects depend on motor cortex activity and, crucially, on corollary feedback from movements. One prediction from this hypothesis is that altering proprioceptive information about a subjects movements should alter or eliminate movement-related timing effects. For example, if a subject were moved automatically without any volition, then no movement-related effects should exist. Similarly, if a subject lost all access to feedback regarding movements, then movement-related effects should similarly be reduced. Previous work with deafferented patients has shown a disruption in motor timing abilities which is stabilized in presence of visual feedback [114,115]. Further, movement-related effects on time persist even when limb position is occluded [51,57].
However, while the presence of motor activity during non-motor timing is evident, this does not necessarily mean that motor-related effects on timing are occurring there. Indeed, work with recurrent neural networks demonstrates that timed responses can be ‘learned’ by any neural region [116,117]. As such, motor activity during perceptual timing may be a byproduct of the way in which time intervals are learned [73], owing to the development of the motor system in primates as a critical node for acquiring cognitive skills [118]. Therefore, a second possibility is that motor activity provides recurrent influence back on the sensory regions themselves, thus leading to more precise, yet biased neural firing in sensory cortices. This possibility, termed Active Sensing [119], has more empirical support from other domains, where motor movements have been found to alter and enhance [120,121] responses in sensory regions (Figure 4D).
Under an Active Sensing framework, sensory responses will be altered by motor activity evoked by concurrent movements while estimating time, which will in turn further influence motor-related timing activity. This hypothesis more closely matches experimental data from other domains, in which movements lead to substantial changes in sensory activity [122] that can further enhance neural responses to sensory stimuli [123]. Further work in humans has demonstrated tight linkage between sensory detection and motor activity [124]. For example, walking in an open environment can enhance peripheral visual contrast sensitivity [125], and recent work has demonstrated that movement plans can be decoded from sensory regions associated with the consequence of those movements [126].
For movement-related effects on explicit timing, the Active Sensing hypothesis matches some experimental findings. For example, the Active Sensing hypothesis predicts that movement-related effects critically depend on activity within sensory cortices. This prediction explains why movement-related effects appear to be strongest for time intervals that are ‘filled’ rather than ‘empty’ [29]. Further, recent evidence demonstrating that motor regions rhythmically enhance sensory responses to perceived rhythms according to preferred frequency bands, additionally supports the Active Sensing hypothesis [67]. Yet, movement-related effects have also been observed for empty intervals, although less consistently across different modalities [56]. Additionally, interesting work has shown that the sensory consequences of planned movements elicit less sensory-evoked activity [127], which should therefore lead to shorter perceived durations [128], whereas the opposite is actually observed. These inconsistencies suggest that both Feedforward Enhancement and Active Sensing hypotheses need to be further examined to determine which can best instantiate cue combination and movement-related effects (see Outstanding questions).
Outstanding questions.
If the spatial properties of movement cause temporal magnitudes to be biased, is it the size of the area in which the movements occur, the trajectory length of the movement, or both causing the bias?
How is the reliability (i.e., ‘noisiness’) of a motor signal evaluated during optimal integration? For example, are erroneous, premature, or discontinuous (e.g., intermittent starting and stopping) movements considered noisier? Do these parameters alter how movements impact time?
How do movements of different effectors (i.e., hand, finger, foot, head, full body) differentially influence the perception of time, and can these be studied together in ecological settings?
Do movement goals (or lack thereof) influence perceptual timing? That is, do movements have to be task-relevant to enhance timing? Will uncertainty of movements bias timing?
How does the volitional control of movement versus passive movement affect the perception of time?
Can the link between movement and timing be used in diagnosis and treatment of movement disorders and psychiatric conditions with parallel cognitive and motor symptoms (e.g., schizophrenia)?
How does short- and long-term motor training transfer to improved perceptual timing mechanisms? Do elite athletes and expert musicians improve in the same way?
Where are movement-related changes in timing occurring in the brain? Can these responses distinguish between feedforward and active sensing hypotheses?
How does stimulating different brain regions influence movement timing interactions.
What role do neural oscillations play in coordinating between motor and sensory regions during movement-related changes in time perception?
Concluding remarks
In this review we have introduced a basic framework of how movement information can bias timing while improving its precision, from the overlap of neural systems to behavioral correlates. We note that this area of study is in its early stages: most movement-timing studies have examined effects of brief [53], discrete [30], or ballistic movements [80], and have tightly controlled motor variables of interest in laboratory settings. Naturally, these come with some limitations. The multi-effector and multisensory qualities of our everyday experiences call for more ecologically valid experimental paradigms. For example, future studies could focus on time perception outcomes for longer-scale continuous movements, movements with varying velocity profiles (e.g., biological vs. non-biological motion), and movements with uncertainty or competition between possible action plans [129]. Additionally, it would be worthwhile to study how timing relates to whole-body movement parameters (e.g., locomotion/gait, rotation, and head movement) and multi-effector motor sequences. High-level movement characteristics like these have not yet been investigated in detail for timing, and with the emergence of sophisticated motion tracking and virtual reality environments, the feasibility of studies with greater ecological validity is increasing [130].
Key figure
Motor movements provide a privileged channel for duration information
Figure 4.
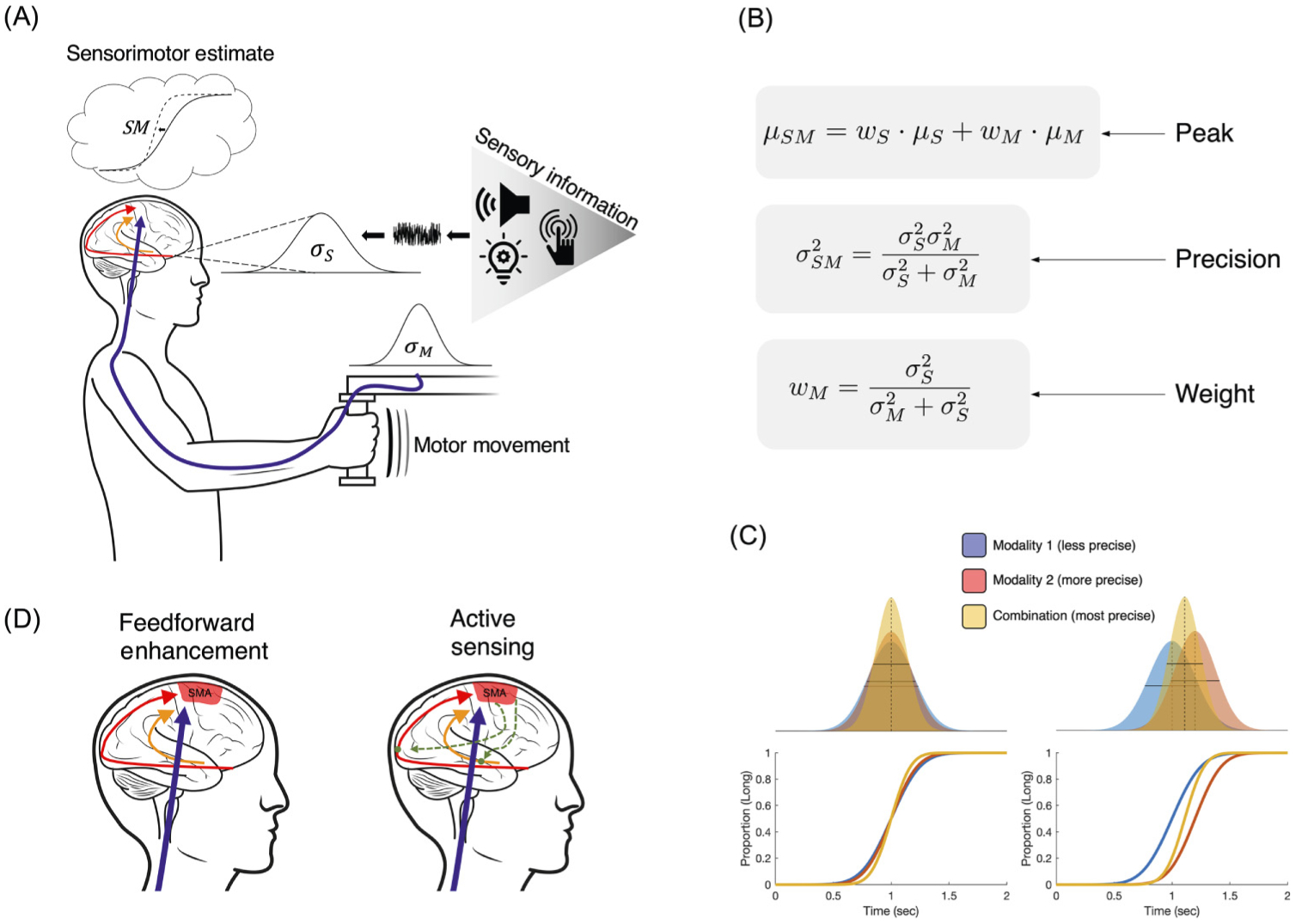
(A) Enhancement and bias effects in time perception can be explained by a cue combination framework (Box 1), in which movement provides the greatest temporal fidelity. When subjects measure sensory time intervals (S), presented in different modalities (auditory, visual, or somatosensory), this information is transformed into a noisy estimate with variance σS that is transferred along sensory pathways to the SMA and accompanying motor circuitry. Concurrent movements, here of a robotic arm, confer a second duration estimate (M) that is perceived with greater reliability and lower variance σM. (B) Both estimates are combined via cue combination equations to form a sensorimotor estimate of duration (SM) that is both more precise, yet biased towards the movement duration estimate. (C) Cue combination framework for combining two signals from different modalities with different levels of precision (top: likelihood distributions for each modality and combination; bottom: psychometric curves for a standard time bisection task). Left panels display the case when both modalities have the same mean; right panels display the case with different means. (D) In a Feedforward Enhancement mode, movement directly influences time estimates occurring within the SMA and associated motor regions; Conversely, in an Active Sensing mode, sensory responses are influenced via feedback from movement-related regions (green broken traces).
Highlights.
Time perception and movement are linked through interactions with the environment.
Recent psychophysical studies have begun to demonstrate that movements, while previously construed as an endpoint for timing, informs and shapes the perception of time.
These studies demonstrate that movements can bias our perception of time, but also enhance it in some circumstances.
A Bayesian cue combination framework, in which movement provides its own timing signal can explain both bias and enhancement effects.
Cue combination effects can be mediated neurally either via feedforward or active sensing modes.
Acknowledgments
The two first authors (RD & KAG) contributed equally to this work. Funding to support this work was provided by the National Science Foundation (Award # 1849067 to MW & WMJ and #1922598 to KAG). This work was also supported in part by the National Institute Of Mental Health of the National Institutes of Health (Award T32MH112507).
Glossary
- Accuracy
refers to the closeness of measurements to a particular value and can be seen as the correctness of measurements.
- Chronostasis
a temporal illusion in which the perceived duration of an event or interval is dilated immediately following a saccade (i.e., quick eye movement). Has also been shown to occur with other types of actions (i.e., arm movements) and with various types of stimuli (i.e., visual, auditory).
- Empty intervals
time intervals presented as the difference between two brief sensory events.
- Explicit timing
tasks in which subjects are specifically told to measure or attend the passage of time.
- Filled intervals
time intervals presented as continuous, sustained sensory events.
- Intentional binding
a phenomenon in which stimuli that occur as a consequence of an action are perceived to occur earlier in time (closer to the action) than stimuli that do not occur as a consequence of an action; can be used as an implicit measure of a sense of agency.
- Magnitude
size, extent, or intensity along a continuous scale; in this case referring to the size of something either in regards to space (length, distance), numerosity (number), or time (duration).
- Motor timing
timing tasks in which a motor action determines the to-be-timed interval (temporal reproduction and synchronization-continuation task are both examples of this).
- Perceptual timing
timing tasks in which no overtly timed motor action is required and subjects may passively view the stimulus and estimate its duration (bisection and discrimination are both examples of this).
- Post-action period
refers to when the to-be-timed interval occurs immediately after the execution of a manual movement.
- Pre-action period
refers to when the to-be-timed interval occurs while a subject is preparing to make a manual movement.
- Precision
refers to the closeness of measurements to each other and can be seen as the consistency of measurements.
- Synchronization-continuation task
subjects are to tap along to a rhythmic beat (synchronization) and then continue tapping at the same rhythm after the beat stops (continuation).
- Temporal bisection task
subjects are presented with time intervals which they must classify into ‘long’ and ‘short’ categories. This categorization can occur relative to previously trained anchor points, or to a running average of all presented intervals.
- Temporal discrimination task
similar to temporal bisection tasks except subjects are presented with two (or more) intervals within a given trial and must compare their relative durations.
- Time reproduction task
subjects are presented with a sample duration and are required to reproduce that duration. The manner in which this is done is dependent on the experimental context; in some cases it is pressing and holding a button for the length of the duration, pressing a button to terminate the length of the duration, or moving for the length of the duration.
Footnotes
Declaration of Interests
No interests are declared.
References
- 1.Franklin DW and Wolpert DM (2011) Computational mechanisms of sensorimotor control. Neuron 72, 425–442 [DOI] [PubMed] [Google Scholar]
- 2.Merchant H and Yarrow K (2016) How the motor system both encodes and influences our sense of time. Curr. Opin. Behav. Sci 8, 22–27 [Google Scholar]
- 3.Balasubramaniam R et al. (2021) Neural encoding and representation of time for sensorimotor control and learning. J. Neurosci 41, 866–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alais D and Burr D (2019) Cue Combination Within a Bayesian Framework. Multisensory Processes 10.1007/978-3-030-10461-0_2 [DOI] [Google Scholar]
- 5.Nani A et al. (2019) The neural correlates of time: a meta-analysis of neuroimaging studies. J. Cogn. Neurosci 31, 1796–1826 [DOI] [PubMed] [Google Scholar]
- 6.Teghil A et al. (2019) Neural substrates of internally-based and externally-cued timing: An activation likelihood estimation (ALE) meta-analysis of fMRI studies. Neurosci. Biobehav. Rev 96, 197–209 [DOI] [PubMed] [Google Scholar]
- 7.Nachev P et al. (2008) Functional role of the supplementary and pre-supplementary motor areas. Nat. Rev. Neurosci 9, 856–869 [DOI] [PubMed] [Google Scholar]
- 8.Protopapa F et al. (2019) Chronotopic maps in human supplementary motor area. PLoS Biol 17, e3000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwartze M et al. (2012) Functional dissociation of pre-SMA and SMA-proper in temporal processing. Neuroimage 60, 290–298 [DOI] [PubMed] [Google Scholar]
- 10.Macar F et al. (2006) The supplementary motor area in motor and perceptual time processing: fMRI studies. Cogn. Process 7, 89–94 [DOI] [PubMed] [Google Scholar]
- 11.Coull JT et al. (2004) Functional anatomy of the attentional modulation of time estimation. Science 303, 1506–1508 [DOI] [PubMed] [Google Scholar]
- 12.Cona G and Semenza C (2017) Supplementary motor area as key structure for domain-general sequence processing: A unified account. Neurosci. Biobehav. Rev 72, 28–42 [DOI] [PubMed] [Google Scholar]
- 13.Cona G et al. (2021) From ATOM to GradiATOM: Cortical gradients support time and space processing as revealed by a meta-analysis of neuroimaging studies. Neuroimage 224, 117407. [DOI] [PubMed] [Google Scholar]
- 14.Merchant H et al. (2013) Interval tuning in the primate medial premotor cortex as a general timing mechanism. J. Neurosci 33, 9082–9096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mendoza G et al. (2018) Neural basis for categorical boundaries in the primate pre-SMA during relative categorization of time intervals. Nat. Commun 9, 1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiener M et al. (2014) Individual differences in the morphometry and activation of time perception networks are influenced by dopamine genotype. Neuroimage 89, 10–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coull JT et al. (2012) Dopamine precursor depletion impairs timing in healthy volunteers by attenuating activity in putamen and supplementary motor area. J. Neurosci 32, 16704–16715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soares S et al. (2016) Midbrain dopamine neurons control judgment of time. Science 354, 1273–1277 [DOI] [PubMed] [Google Scholar]
- 19.Breska A and Ivry RB (2018) Double dissociation of single-interval and rhythmic temporal prediction in cerebellar degeneration and Parkinson’s disease. Proc. Natl. Acad. Sci. U. S. A 115, 12283–12288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Breska A and Ivry RB (2020) Context-specific control over the neural dynamics of temporal attention by the human cerebellum. Sci. Adv 6 Published online December 2, 2020. 10.1126/sciadv.abb1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh A et al. (2021) Timing variability and midfrontal 4Hz rhythms correlate with cognition in Parkinson’s disease. NPJ Park. Dis 7, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Avanzino L et al. (2016) Time Processing and Motor Control in Movement Disorders. Front. Hum. Neurosci 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cope TE et al. (2014) The basal ganglia in perceptual timing: timing performance in Multiple System Atrophy and Huntington’s disease. Neuropsychologia 52, 73–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merchant H et al. (2008) Interval timing and Parkinson’s disease: heterogeneity in temporal performance. Exp. Brain Res 184, 233–248 [DOI] [PubMed] [Google Scholar]
- 25.Vicario CM et al. (2016) Timing recalibration in childhood Tourette syndrome associated with persistent pimozide treatment. J. Neuropsychol 10, 211–222 [DOI] [PubMed] [Google Scholar]
- 26.Pedrosa DJ et al. (2016) Time reproduction deficits in essential tremor patients. Mov. Disord 31, 1234–1240 [DOI] [PubMed] [Google Scholar]
- 27.Martino D et al. (2015) Temporal processing of perceived body movement in cervical dystonia. Mov. Disord 30, 1005–1007 [DOI] [PubMed] [Google Scholar]
- 28.Gooch CM et al. (2011) Temporal discrimination of sub- and suprasecond time intervals: a voxel-based lesion mapping analysis. Front. Integr. Neurosci 5, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iwasaki M et al. (2019) Neural correlates of time distortion in a preaction period. Hum. Brain Mapp 40, 804–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yarrow K et al. (2001) Illusory perceptions of space and time preserve cross-saccadic perceptual continuity. Nature 414, 302–305 [DOI] [PubMed] [Google Scholar]
- 31.Yarrow K and Rothwell JC (2003) Manual chronostasis: tactile perception precedes physical contact. Curr. Biol 13, 1134–1139 [DOI] [PubMed] [Google Scholar]
- 32.Melcher D et al. (2020) The role of action intentionality and effector in the subjective expansion of temporal duration after saccadic eye movements. Sci. Rep 10, 16922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Terhune DB et al. (2016) Time dilates after spontaneous blinking. Curr. Biol 26, R459–R460 [DOI] [PubMed] [Google Scholar]
- 34.Haggard P et al. (2002) Voluntary action and conscious awareness. Nat. Neurosci 5, 382–385 [DOI] [PubMed] [Google Scholar]
- 35.Wenke D and Haggard P (2009) How voluntary actions modulate time perception. Exp. Brain Res 196, 311–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suzuki K et al. (2019) Intentional binding without intentional action. Psychol. Sci 30, 842–853 [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto K (2020) Cue integration as a common mechanism for action and outcome bindings. Cognition 205, 104423. [DOI] [PubMed] [Google Scholar]
- 38.Legaspi R and Toyoizumi T (2019) A Bayesian psychophysics model of sense of agency. Nat. Commun 10, 4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coull J and Nobre A (2008) Dissociating explicit timing from temporal expectation with fMRI. Curr. Opin. Neurobiol 18, 137–144 [DOI] [PubMed] [Google Scholar]
- 40.Park J et al. (2003) Voluntary action expands perceived duration of its sensory consequence. Exp. Brain Res 149, 527–529 [DOI] [PubMed] [Google Scholar]
- 41.Press C et al. (2014) Moving time: the influence of action on duration perception. J. Exp. Psychol. Gen 143, 1787–1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cai ZG et al. (2013) Time does not flow without language: Spatial distance affects temporal duration regardless of movement or direction. Psychon. Bull. Rev 20, 973–980 [DOI] [PubMed] [Google Scholar]
- 43.Anobile G et al. (2021) A sensorimotor numerosity system. Trends Cogn. Sci 25, 24–36 [DOI] [PubMed] [Google Scholar]
- 44.Walsh V (2003) A theory of magnitude: common cortical metrics of time, space and quantity. Trends Cogn. Sci 7, 483–488 [DOI] [PubMed] [Google Scholar]
- 45.Anelli F et al. (2015) The remapping of time by active tool-use. PLoS One 10, e0146175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yon D et al. (2017) Time on your hands: Perceived duration of sensory events is biased toward concurrent actions. J. Exp. Psychol. Gen 146, 182–193 [DOI] [PubMed] [Google Scholar]
- 47.Yokosaka T et al. (2015) Apparent time interval of visual stimuli is compressed during fast hand movement. PLoS One 10, e0124901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoon T et al. (2018) Control of movement vigor and decision making during foraging. Proc. Natl. Acad. Sci. U. S. A 115, E10476–E10485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trimmer PC et al. (2008) Mammalian choices: combining fast-but-inaccurate and slow-but-accurate decision-making systems. Proc. Biol. Sci 275, 2353–2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berret B and Jean F (2016) Why don’t we move slower? The value of time in the neural control of action. J. Neurosci 36, 1056–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Kock R et al. (2021) Slowing the body slows down time perception. eLife 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tomassini A et al. (2018) Rhythmic motor behaviour influences perception of visual time. Proc. Biol. Sci 285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ayhan I and Ozbagci D (2020) Action-induced changes in the perceived temporal features of visual events. Vis. Res 175, 1–13 [DOI] [PubMed] [Google Scholar]
- 54.Imaizumi S and Asai T (2017) My action lasts longer: Potential link between subjective time and agency during voluntary action. Conscious. Cogn 51, 243–257 [DOI] [PubMed] [Google Scholar]
- 55.Binetti N et al. (2015) Binding space and time through action. Proc. Biol. Sci 282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iordanescu L et al. (2013) Action enhances auditory but not visual temporal sensitivity. Psychon. Bull. Rev 20, 108–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wiener M et al. (2019) Movement improves the quality of temporal perception and decision-making. eNeuro 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fernandes AC and Garcia-Marques T (2019) The perception of time is dynamically interlocked with the facial muscle activity. Sci. Rep 9, 18737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Safaie M et al. (2020) Turning the body into a clock: Accurate timing is facilitated by simple stereotyped interactions with the environment. Proc. Natl. Acad. Sci. U. S. A 117, 13084–13093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gouvêa TS et al. (2014) Ongoing behavior predicts perceptual report of interval duration. Front. Neurorobot 8, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carlini A and French R (2014) Visual tracking combined with hand-tracking improves time perception of moving stimuli. Sci. Rep 4, 5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gavazzi G et al. (2013) Time perception of visual motion is tuned by the motor representation of human actions. Sci. Rep 3, 1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morillon B et al. (2014) Motor contributions to the temporal precision of auditory attention. Nat. Commun 5, 5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Manning F and Schutz M (2013) Moving to the beat improves timing perception. Psychon. Bull. Rev 20, 1133–1139 [DOI] [PubMed] [Google Scholar]
- 65.Manning FC and Schutz M (2016) Trained to keep a beat: movement-related enhancements to timing perception in percussionists and non-percussionists. Psychol. Res 80, 532–542 [DOI] [PubMed] [Google Scholar]
- 66.Manning FC et al. (2017) Temporal prediction abilities are mediated by motor effector and rhythmic expertise. Exp. Brain Res 235, 861–871 [DOI] [PubMed] [Google Scholar]
- 67.Zalta A et al. (2020) Natural rhythms of periodic temporal attention. Nat. Commun 11, 1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ivry RB et al. (2002) The cerebellum and event timing. Ann. N. Y. Acad. Sci 978, 302–317 [DOI] [PubMed] [Google Scholar]
- 69.Zelaznik HN et al. (2005) Timing variability in circle drawing and tapping: probing the relationship between event and emergent timing. J. Mot. Behav 37, 395–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen YH and Cesari P (2015) Elite athletes refine their internal clocks. Mot. Control 19, 90–101 [DOI] [PubMed] [Google Scholar]
- 71.Cicchini GM et al. (2012) Optimal encoding of interval timing in expert percussionists. J. Neurosci 32, 1056–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sgouramani H and Vatakis A (2014) Flash dance: how speed modulates perceived duration in dancers and non-dancers. Acta Psychol 147, 17–24 [DOI] [PubMed] [Google Scholar]
- 73.Coull JT and Droit-Volet S (2018) Explicit understanding of duration develops implicitly through action. Trends Cogn. Sci 22, 923–937 [DOI] [PubMed] [Google Scholar]
- 74.Guo J and Song JH (2019) Action fluency facilitates perceptual discrimination. Psychol. Sci 30, 1434–1448 [DOI] [PubMed] [Google Scholar]
- 75.Fautrelle L et al. (2015) Motor activity improves temporal expectancy. PLoS One 10, e0119187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Meegan DV et al. (2000) Motor timing learned without motor training. Nat. Neurosci 3, 860–862 [DOI] [PubMed] [Google Scholar]
- 77.Nazari MA et al. (2016) Time for action versus action in time: time estimation differs between motor preparation and execution. J. Cogn. Psychol 29, 129–136 [Google Scholar]
- 78.Yabe Y and Goodale MA (2015) Time flies when we intend to act: temporal distortion in a go/no-go task. J. Neurosci 35, 5023–5029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tomassini A and Morrone MC (2016) Perceived visual time depends on motor preparation and direction of hand movements. Sci. Rep 6, 27947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hagura N et al. (2012) Ready steady slow: action preparation slows the subjective passage of time. Proc. Biol. Sci 279, 4399–4406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Iwasaki M et al. (2017) Non-uniform transformation of subjective time during action preparation. Cognition 160, 51–61 [DOI] [PubMed] [Google Scholar]
- 82.Yarrow K et al. (2004) Consistent chronostasis effects across saccade categories imply a subcortical efferent trigger. J. Cogn. Neurosci 16, 839–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wolpert DM et al. (1995) An internal model for sensorimotor integration. Science 269, 1880–1882 [DOI] [PubMed] [Google Scholar]
- 84.Alhussein L et al. (2019) Dissociating effects of error size, training duration, and amount of adaptation on the ability to retain motor memories. J. Neurophysiol 122, 2027–2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhou W et al. (2017) The temporal stability of visuomotor adaptation generalization. J. Neurophysiol 118, 2435–2447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shadmehr R et al. (2010) Error correction, sensory prediction, and adaptation in motor control. Annu. Rev. Neurosci 33, 89–108 [DOI] [PubMed] [Google Scholar]
- 87.McNamee D and Wolpert DM (2019) Internal models in biological control. Annu. Rev. Control Robot. Auton. Syst 2, 339–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hogan N and Sternad D (2012) Dynamic primitives of motor behavior. Biol. Cybern 106, 727–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yu BM et al. (2009) Gaussian-process factor analysis for low-dimensional single-trial analysis of neural population activity. J. Neurophysiol 102, 614–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sohn H et al. (2019) Bayesian computation through cortical latent dynamics. Neuron 103, 934–947.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gámez J et al. (2019) The amplitude in periodic neural state trajectories underlies the tempo of rhythmic tapping. PLoS Biol 17, e3000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cannon JJ and Patel AD (2021) How beat perception co-opts motor neurophysiology. Trends Cogn. Sci 25, 137–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Leek EC and Johnston SJ (2009) Functional specialization in the supplementary motor complex. Nat. Rev. Neurosci 10, 78. [DOI] [PubMed] [Google Scholar]
- 94.Brenner E et al. (2012) Timing the moment of impact in fast human movements. Acta Psychol 141, 104–111 [DOI] [PubMed] [Google Scholar]
- 95.Doumas M et al. (2008) Interval timing and trajectory in unequal amplitude movements. Exp. Brain Res 189, 49–60 [DOI] [PubMed] [Google Scholar]
- 96.Hatsopoulos NG and Suminski AJ (2011) Sensing with the motor cortex. Neuron 72, 477–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Russo AA et al. (2020) Neural trajectories in the supplementary motor area and motor cortex exhibit distinct geometries, compatible with different classes of computation. Neuron 107, 745–758.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.van Wassenhove V et al. (2008) Distortions of subjective time perception within and across senses. PLoS One 3, e1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wiener M et al. (2014) Continuous carryover of temporal context dissociates response bias from perceptual influence for duration. PLoS One 9, e100803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jones LA et al. (2009) Good vibrations: human interval timing in the vibrotactile modality. Q. J. Exp. Psychol. (Hove) 62, 2171–2186 [DOI] [PubMed] [Google Scholar]
- 101.The role of execution noise in movement variability. J. Neurophysiol 91, 1050–1063 [DOI] [PubMed] [Google Scholar]
- 102.Wolpert DM et al. (2011) Principles of sensorimotor learning. Nat. Rev. Neurosci 12, 739–751 [DOI] [PubMed] [Google Scholar]
- 103.Petzschner FH et al. (2015) A Bayesian perspective on magnitude estimation. Trends Cogn. Sci 19, 285–293 [DOI] [PubMed] [Google Scholar]
- 104.Hartcher-O’Brien J et al. (2014) The duration of uncertain times: audiovisual information about intervals is integrated in a Statistically Optimal Fashion. PLoS One 9, e89339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Coull JT (2004) fMRI studies of temporal attention: allocating attention within or towards, time. Cogn. Brain Res 21, 216–226 [DOI] [PubMed] [Google Scholar]
- 106.Schubotz RI (2007) Prediction of external events with our motor system: towards a new framework. Trends Cogn. Sci 11, 211–218 [DOI] [PubMed] [Google Scholar]
- 107.Wang J et al. (2018) Flexible timing by temporal scaling of cortical responses. Nat. Neurosci 21, 102–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cueva CJ et al. (2020) Low-dimensional dynamics for working memory and time encoding. Proc. Natl. Acad. Sci. U. S. A 117, 23021–23032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bi Z and Zhou C (2020) Understanding the computation of time using neural network models. Proc. Natl. Acad. Sci. U. S. A 117, 10530–10540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mioni G et al. (2016) The impact of a concurrent motor task on auditory and visual temporal discrimination tasks. Atten. Percept. Psychophys 78, 742–748 [DOI] [PubMed] [Google Scholar]
- 111.Kao TC et al. (2021) Optimal anticipatory control as a theory of motor preparation: A thalamo-cortical circuit model. Neuron 109, 1567–1581.e12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lara AH et al. (2018) Conservation of preparatory neural events in monkey motor cortex regardless of how movement is initiated. eLife 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Deverett B et al. (2019) Interval timing in deep reinforcement learning agents. Proc. Adv. Neural Inf. Proces. Syst 32 (NeurIPS 2019) https://proceedings.neurips.cc/paper/2019/hash/2bf283c05b601f21364d052ca0ec798d-Abstract.html [Google Scholar]
- 114.Fleury M et al. (1994) Production of short timing responses: a comparative study with a deafferented patient. Neuropsychologia 32, 1435–1440 [DOI] [PubMed] [Google Scholar]
- 115.Stenneken P et al. (2006) The effect of sensory feedback on the timing of movements: evidence from deafferented patients. Brain Res 1084, 123–131 [DOI] [PubMed] [Google Scholar]
- 116.Zhou S et al. (2020) Neural sequences as an optimal dynamical regime for the readout of time. Neuron 108, 651–658.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Motanis H et al. (2018) Short-term synaptic plasticity as a mechanism for sensory timing. Trends Neurosci 41, 701–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mendoza G and Merchant H (2014) Motor system evolution and the emergence of high cognitive functions. Prog. Neurobiol 122, 73–93 [DOI] [PubMed] [Google Scholar]
- 119.Schroeder CE et al. (2010) Dynamics of active sensing and perceptual selection. Curr. Opin. Neurobiol 20, 172–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yon D et al. (2018) Action sharpens sensory representations of expected outcomes. Nat. Commun 9, 4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Niell CM and Stryker MP (2010) Modulation of visual responses by behavioral state in mouse visual cortex. Neuron 65, 472–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Busse L et al. (2017) Sensation during active behaviors. J. Neurosci 37, 10826–10834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Musall S et al. (2019) Single-trial neural dynamics are dominated by richly varied movements. Nat. Neurosci 22, 1677–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tomassini A et al. (2020) Visual detection is locked to the internal dynamics of cortico-motor control. PLoS Biol 18, e3000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cao L and Händel B (2019) Walking enhances peripheral visual processing in humans. PLoS Biol 17, e3000511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gale DJ et al. (2021) Motor planning modulates neural activity patterns in early human auditory cortex. Cereb. Cortex 31, 2952–2967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Harrison AW et al. (2021) Sensory attenuation is modulated by the contrasting effects of predictability and control. Neuroimage [DOI] [PubMed] [Google Scholar]
- 128.Noguchi Y and Kakigi R (2006) Time representations can be made from nontemporal information in the brain: an MEG study. Cereb. Cortex 16, 1797–1808 [DOI] [PubMed] [Google Scholar]
- 129.Gallivan JP et al. (2017) Rapid automatic motor encoding of competing reach options. Cell Rep 19, 890–893 [DOI] [PubMed] [Google Scholar]
- 130.Mobbs D et al. (2021) Promises and challenges of human computational ethology. Neuron [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gibbon J et al. (1984) Scalar timing in memory. Ann. N. Y. Acad. Sci 423, 52–77 [DOI] [PubMed] [Google Scholar]
- 132.Killeen PR and Fetterman JG (1988) A behavioral theory of timing. Psychol. Rev 95, 274–295 [DOI] [PubMed] [Google Scholar]
- 133.Machado A (1997) Learning the temporal dynamics of behavior. Psychol. Rev 104, 241–265 [DOI] [PubMed] [Google Scholar]
- 134.Simen P et al. (2011) A model of interval timing by neural integration. J. Neurosci 31, 9238–9253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Goudar V and Buonomano DV (2018) Encoding sensory and motor patterns as time-invariant trajectories in recurrent neural networks. eLife 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ernst MO and Banks MS (2002) Humans integrate visual and haptic information in a statistically optimal fashion. Nature 415, 429–433 [DOI] [PubMed] [Google Scholar]
- 137.Alais D and Burr D (2004) The ventriloquist effect results from near-optimal bimodal integration. Curr. Biol 14, 257–262 [DOI] [PubMed] [Google Scholar]
- 138.Ball DM et al. (2017) Weighted integration suggests that visual and tactile signals provide independent estimates about duration. J. Exp. Psychol. Hum. Percept. Perform 43, 868–880 [DOI] [PubMed] [Google Scholar]
- 139.De Corte BJ and Matell MS (2016) Interval timing, temporal averaging, and cue integration. Curr. Opin. Behav. Sci 8, 60–66 [DOI] [PMC free article] [PubMed] [Google Scholar]


