Abstract
Background
COVID-19 sequelae can affect about 15% of patients with cancer who survive the acute phase of SARS-CoV-2 infection and can substantially impair their survival and continuity of oncological care. We aimed to investigate whether previous immunisation affects long-term sequelae in the context of evolving variants of concern of SARS-CoV-2.
Methods
OnCovid is an active registry that includes patients aged 18 years or older from 37 institutions across Belgium, France, Germany, Italy, Spain, and the UK with a laboratory-confirmed diagnosis of COVID-19 and a history of solid or haematological malignancy, either active or in remission, followed up from COVID-19 diagnosis until death. We evaluated the prevalence of COVID-19 sequelae in patients who survived COVID-19 and underwent a formal clinical reassessment, categorising infection according to the date of diagnosis as the omicron (B.1.1.529) phase from Dec 15, 2021, to Jan 31, 2022; the alpha (B.1.1.7)–delta (B.1.617.2) phase from Dec 1, 2020, to Dec 14, 2021; and the pre-vaccination phase from Feb 27 to Nov 30, 2020. The prevalence of overall COVID-19 sequelae was compared according to SARS-CoV-2 immunisation status and in relation to post-COVID-19 survival and resumption of systemic anticancer therapy. This study is registered with ClinicalTrials.gov, NCT04393974.
Findings
At the follow-up update on June 20, 2022, 1909 eligible patients, evaluated after a median of 39 days (IQR 24–68) from COVID-19 diagnosis, were included (964 [50·7%] of 1902 patients with sex data were female and 938 [49·3%] were male). Overall, 317 (16·6%; 95% CI 14·8–18·5) of 1909 patients had at least one sequela from COVID-19 at the first oncological reassessment. The prevalence of COVID-19 sequelae was highest in the pre-vaccination phase (191 [19·1%; 95% CI 16·4–22·0] of 1000 patients). The prevalence was similar in the alpha–delta phase (110 [16·8%; 13·8–20·3] of 653 patients, p=0·24), but significantly lower in the omicron phase (16 [6·2%; 3·5–10·2] of 256 patients, p<0·0001). In the alpha–delta phase, 84 (18·3%; 95% CI 14·6–22·7) of 458 unvaccinated patients and three (9·4%; 1·9–27·3) of 32 unvaccinated patients in the omicron phase had sequelae. Patients who received a booster and those who received two vaccine doses had a significantly lower prevalence of overall COVID-19 sequelae than unvaccinated or partially vaccinated patients (ten [7·4%; 95% CI 3·5–13·5] of 136 boosted patients, 18 [9·8%; 5·8–15·5] of 183 patients who had two vaccine doses vs 277 [18·5%; 16·5–20·9] of 1489 unvaccinated patients, p=0·0001), respiratory sequelae (six [4·4%; 1·6–9·6], 11 [6·0%; 3·0–10·7] vs 148 [9·9%; 8·4–11·6], p=0·030), and prolonged fatigue (three [2·2%; 0·1–6·4], ten [5·4%; 2·6–10·0] vs 115 [7·7%; 6·3–9·3], p=0·037).
Interpretation
Unvaccinated patients with cancer remain highly vulnerable to COVID-19 sequelae irrespective of viral strain. This study confirms the role of previous SARS-CoV-2 immunisation as an effective measure to protect patients from COVID-19 sequelae, disruption of therapy, and ensuing mortality.
Funding
UK National Institute for Health and Care Research Imperial Biomedical Research Centre and the Cancer Treatment and Research Trust.
Research in context.
Evidence before this study
Post-COVID-19 condition (also known as long COVID) affects a substantial proportion of patients with cancer who survive the acute phase of SARS-CoV-2 infection and can impair their survival and oncological continuity of care. Evidence suggests that infection resulting from the omicron (B.1.1.529) variant is less severe than that from previous variants in patients who received previous SARS-CoV-2 vaccination. We searched PubMed from database inception to Sept 27, 2022, for articles published in English on the effect of omicron-related COVID-19 sequelae on patients with cancer using the search terms (“COVID-19” OR “SARS-CoV-2”) AND (“oncology” OR “cancer” OR “malignancy”) AND (“Omicron” OR “B.1.1.529”) AND (“sequelae” OR “long COVID” OR “post COVID-19 syndrome”). Although registry studies have provided evidence of direct proportionality between severity of previous COVID-19 and prevalence of COVID-19 sequelae in patients with cancer, no evidence exists to clearly infer whether or not previous SARS-CoV-2 immunisation can prevent omicron-related COVID-19 sequelae in this especially vulnerable population.
Added value of this study
In this study, we document that patients with cancer diagnosed with COVID-19 during the omicron outbreak in Europe had a significantly lower prevalence of COVID-19 sequelae than those diagnosed in previous phases of the pandemic. However, in unvaccinated patients diagnosed with omicron infection, the prevalence of COVID-19 sequelae was not significantly lower than that recorded in previous phases of the pandemic. Previous SARS-CoV-2 immunisation emerges as the strongest determinant of protection against COVID-19 sequelae among patients with cancer who survive COVID-19, independent of patient demographics and oncological features.
Implications of all the available evidence
COVID-19 sequelae can impair post-COVID-19 survival and resumption of oncological continuity of care in patients with cancer who survive the acute phase of the disease. Despite the tendency of omicron to elude vaccinal protection against infection, our study provides original evidence to suggest that previous immunisation is protective against omicron-related COVID-19 sequelae, further highlighting the importance of universal vaccination in reducing long-term consequences from COVID-19, maintaining optimal delivery of systemic therapy, and preserving optimal oncological care throughout the evolving phases of the pandemic.
Introduction
Post-COVID-19 condition (also known as long COVID) is known to affect a substantial proportion of patients who survive the acute phase of SARS-CoV-2 infection and is characterised by various symptoms, including respiratory impairment, prolonged fatigue, and neurocognitive sequelae.1 Between 13% and 60% of COVID-19 survivors are at risk of developing COVID-19 sequelae.2, 3, 4, 5 Among pathogenetic mechanisms, systemic inflammatory and neuroinflammatory processes, endothelial damage, and reactivation of latent pathogens have been proposed as underlying mechanisms.
Evidence suggests that a pro-inflammatory diathesis and a history of severe COVID-19 are strong determinants of long-term sequelae after resolution of SARS-CoV-2 infection.6 These findings support the role of a full SARS-CoV-2 vaccination course as a protective measure against the post-acute sequelae from the disease.
Evidence from the OnCovid study suggests that up to 15% of patients with cancer can have persistent symptoms after COVID-19 recovery,7, 8, 9 which significantly affect their survival independent of background oncological prognosis.8
In a global scenario where coexistence with SARS-CoV-2 has become the norm, strategies aimed at preventing COVID-19 sequelae are of utmost importance in patients with cancer to ensure their oncological continuity of care. Limited, but plausible, evidence suggests that previous SARS-CoV-2 vaccination might reduce the prevalence of COVID-19 sequelae in patients with cancer.10
However, novel SARS-CoV-2 variants are capable of evading vaccinal immunity, and new vaccines have now been developed to overcome immune evasion.11, 12 Although recent evidence suggests that overall SARS-CoV-2 infections diagnosed during the outbreak of the omicron (B.1.1.529) variant are associated with improved COVID-19 outcomes in comparison to previous phases of the pandemic,13 we showed that improvement in mortality was mainly driven by previous vaccinal immunity, arguing against the hypothesis of an inherently reduced pathogenicity of the omicron variant in patients with cancer.13
Although vaccination has become a universally endorsed principle in the prevention of adverse outcomes from COVID-19 in vulnerable patients, it remains to be shown whether, alongside a reduction in mortality, vaccination against SARS-CoV-2 might be beneficial in protecting individuals from the long-term sequelae in the context of highly infectious novel variants of concerns.
In this follow-up study from the OnCovid registry, we sought to determine the proportion of COVID-19 sequelae in patients with cancer diagnosed with COVID-19 during the more recent omicron phase of the pandemic (from Dec 15, 2021, to Jan 31, 2022). With the benefit of longer follow-up of omicron infections, we intended to assess whether previous SARS-CoV-2 immunisation and receipt of booster doses were correlated with a reduced proportion of COVID-19 sequelae in patients with cancer.
Methods
Study design and participants
OnCovid is a registry study collecting data from consecutive patients aged 18 years and older from 37 institutions across Belgium, France, Germany, Italy, Spain, and the UK with a real-time PCR-confirmed diagnosis of COVID-19 and a history of solid or haematological malignancy, either active or in remission, at the time of COVID-19 diagnosis.
Core study data were collated from electronic medical records into a case report form designed using the Research Electronic Data Capture software (REDCap, Vanderbilt University, Nashville, TN, USA). Multi-site access and data curation were coordinated by the Medical Statistics Unit in Novara, Italy.
By the previous data lock of Feb 4, 2022, the registry included 3820 patients with cancer diagnosed with COVID-19 between Feb 27, 2020, and Jan 31, 2022. For this specific analysis, we launched a follow-up update of previously entered patients with a new data lock of June 30, 2022. To ensure consecutive accrual and comparability of outcomes, we excluded data from centres that did not actively enter new information from the March, 2021, and February, 2022, data locks, randomly resulting in the exclusion of all centres from Belgium, France, and Germany.
Study details and procedures, patients' eligibility, and clinical endpoints have already been extensively presented7, 8, 9, 10, 13, 14, 15, 16, 17 and are summarised in the appendix (pp 2–3). A list of participating centres with eligible patients for the present analysis is provided in the appendix (p 4).
OnCovid was granted central approval by the UK Health Research Authority (20/HRA/1608) and by the corresponding research ethics committees at each participating institution. Full waiver of consent due to the retrospective nature of the study was granted by the Health Research Authority.
Procedures
The overarching main objective of this analysis was to describe the prevalence of COVID-19 sequelae in patients with cancer diagnosed with COVID-19 during the omicron outbreak in comparison to previous phases of the pandemic and to assess whether or not previous SARS-CoV-2 immunisation could have a protective role against COVID-19 sequelae following omicron infections. For this purpose, we focused on patients who survived COVID-19 and underwent a formal clinical reassessment in clinic after COVID-19 recovery at each participating institution.7, 8, 9
In view of the negative prognostic effect of persistent COVID-19 symptoms on clinical outcomes following COVID-19 recovery in the study population,8, 9 as a secondary objective we additionally evaluated whether or not vaccinal immunity was associated with patient survival, after the exclusion of patients without a subsequent follow-up update following the first clinical reassessment.
Last, in view of the fact that COVID-19 sequelae can affect the subsequent delivery of systemic anticancer therapy and affect survival,8 we did a separate analysis in patients who were on systemic anticancer therapy within 4 weeks before diagnosis of COVID-19 to evaluate post-COVID-19 survival in patients who resumed or continued systemic anticancer therapy without changes, those who resumed systemic anticancer therapy following a dose or regimen adjustment, and those who permanently discontinued the treatment. Furthermore, we described the associations between the emergence of COVID-19 sequelae, SARS-CoV-2 vaccination status, demographics, and disease characteristics, and systemic anticancer therapy resumption (unchanged vs adjustments or permanent discontinuations).
In terms of data collection, we reported baseline demographics, disease characteristics, and surrogates of previous COVID-19 severity categorised according to the presence of COVID-19 sequelae. Subsequently, we reported the overall prevalence of sequelae across previously validated time periods,13 defined as the pre-vaccination phase (Feb 27, 2020, to Nov 30, 2020), the alpha–delta phase (Dec 1, 2020, to Dec 14, 2021),18 and the omicron phase (Dec 15, 2020, to Jan 31, 2022).19
Because SARS-CoV-2 vaccines were made available only in late 2020, we further assessed the prevalence of overall COVID-19 sequelae across alpha–delta and omicron phases according to vaccination status and compared the prevalence of sequelae of unvaccinated patients diagnosed during the omicron phase with that of patients diagnosed during the pre-vaccination phase, in an attempt to discriminate the protective role of previous vaccination from differences in virulence of the different variants. We also compared the prevalence of COVID-19 sequelae between vaccinated patients (ie, those who received either a two doses or a booster dose before a diagnosis of COVID-19) and unvaccinated patients (including partially vaccinated patients) within the alpha–delta and omicron phases separately.
After exclusion of patients with unknown vaccination status, we reported demographics and disease characteristics stratified by previous SARS-CoV-2 vaccination status. To reduce bias in assessing the effect of vaccinal immunity on clinical outcomes of interest, we adopted a two-tiered approach. First, we did univariable and multivariable analyses to evaluate the rate of overall COVID-19 sequelae and specific sequelae grouped by type (respiratory sequelae, residual fatigue, weight loss, neurocognitive sequelae, and others) categorising patients into vaccinated patients (ie, those who received either a double dose or a booster dose before being diagnosed with COVID-19) and unvaccinated patients (including those receiving an incomplete vaccine course). Subsequently, we compared the prevalence of overall COVID-19 sequelae between unvaccinated patients and those who received two vaccine doses and a booster dose separately.
Outcomes
We elected the prevalence of overall COVID-19 sequelae as the main clinical endpoint.8 For the purpose of the analysis, COVID-19 sequelae were defined as any ongoing symptom or instrumental abnormality detected between 4 weeks and 12 weeks after the start of acute SARS-CoV-2 infection and that could not be explained by an alternative diagnosis. Sequelae could be of new onset following initial recovery, or defined as persisting from the initial illness or fluctuating over time.20, 21 COVID-19 sequelae were further categorised according to the system or organ involved into: respiratory symptoms (including dyspnoea and chronic chough), residual fatigue, weight loss, neurocognitive sequelae (including cognitive, visual impairment, anosmia or dysosmia, ageusia or dysgeusia, headache, confusion, and lethargy), and other organ disfunctions (including renal and hepatic disfunctions, residual fever, muscle cramps, arthralgia, and skin conditions).20, 21
Post-COVID-19 survival was chosen as secondary endpoint of interest and was defined as the time interval from the date of the post COVID-19 clinical reassessment to the date of patients' death or last follow-up. Additional details of study procedures and methods are provided in the appendix (pp 2–3).
Statistical analysis
Patient observation started from the date of SARS-CoV-2 infection until a patient's death or loss to follow-up, although this analysis focused on the time window starting from the post-COVID-19 reassessment. Patients were marked as censored if they were alive at their last follow-up, and they were marked as lost to follow-up when they, for any reason, did not attend planned appointments. Although patients without a subsequent follow-up update following the first clinical reassessment were excluded from the post-COVID-19 survival analysis, they were included for the analysis of prevalence of COVID-19 sequelae. Patients lost to follow-up after COVID-19 were censored at the date of last clinical assessment to maximise the sample size.
Baseline characteristics were summarised as categorical variables and reported using descriptive statistics. We tested associations between categorical variables using Fisher's exact test and the Pearson χ2 test as appropriate. Prevalence of COVID-19 sequelae was presented as a crude rate with a 95% CI.
To compare the COVID-19 sequelae rate of unvaccinated patients from the omicron phase and those from the pre-vaccination phase, we used propensity score matching with a 1:4 ratio and a caliper of 0·2 to include as many cases as possible and elicit the lowest bias by pairing them with four optional controls,22 in view of the low number of events23 and the largely unbalanced sample size. The balancing ability of propensity score matching was estimated through the standardised mean differences of the matched characteristics.
Similarly,23 to optimise the unbalanced sample size when comparing vaccination subgroups, we performed separate inverse probability of treatment weighting (IPTW) procedures accounting for selected demographics and oncological characteristics for all the COVID-19 sequelae analyses. The balancing ability of each IPTW was evaluated through the distribution of the unweighted and weighted selected variables with relevant p values and standardised mean differences. A double adjustment criterion was adopted for both the propensity score matching and IPTW-fitted models when exploring clinical outcomes between the matched and weighted cohorts, with the inclusion of variables with a standardised mean difference of more than 0·10 in the multivariable analyses.24 Propensity score-weighted logistic regression models were used to compute odds ratios (ORs) and 95% CIs. The following covariates were used: country (the UK vs Spain vs Italy), sex (male vs female), number of comorbidities (0–1 vs ≥2), primary tumour, tumour stage at COVID-19 diagnosis (advanced vs non-advanced), tumour status at COVID-19 diagnosis (active vs non-active), receipt of systemic anticancer therapy within 4 weeks before COVID-19 diagnosis (yes vs no), and age with the 65 years cutoff that has been concordantly used in our registry (≥65 vs <65 years).7, 8, 9, 10, 13, 14 To obtain more balanced models, we included variables with missing data by grouping them as reference term in case of a less than 5% of missingness and as an “unknown” category in case of a 5% or more of missingness. Last, in view of the fact that sequelae evaluation and assessments were not predefined across centres, and that the data source consisted of 20 different institutions, all 95% CIs for COVID-19 sequelae comparisons were corrected following a clustered-robust adjustment for participating centre.
Post-COVID-19 survival was estimated with the Kaplan-Meier method and compared with the log-rank test. Considering the small number of events and the high proportion of censored patients, the median post-COVID-19 follow-up was estimated with the reverse Kaplan-Meier method. Acknowledging that the effect of COVID-19 sequelae and previous SARS-CoV-2 vaccination on the risk of death following COVID-19 recovery might not be proportional over time, and considering the differential observation period between vaccinated and unvaccinated groups, we first evaluated post-COVID-19 survival according to all the aforementioned characteristic, which are already known to influence clinical outcomes in patients with COVID-19 and cancer, with restricted mean survival time analyses at 2 months, 4 months, and 6 months. We then adopted fixed multivariable Cox proportional hazards regression models for the multivariable analysis of the risk of death, with results presented as hazard ratios (HRs) and 95% CIs.
All p values were two-sided and CIs set at the 95% level, with significance predefined to be at less than 0·05. Analyses were performed using R (version RStudio 2022.07.2+576) and MedCalc statistical software (version 20).
This study is registered with ClinicalTrials.gov, NCT04393974.
Role of the funding source
The sponsor and the funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
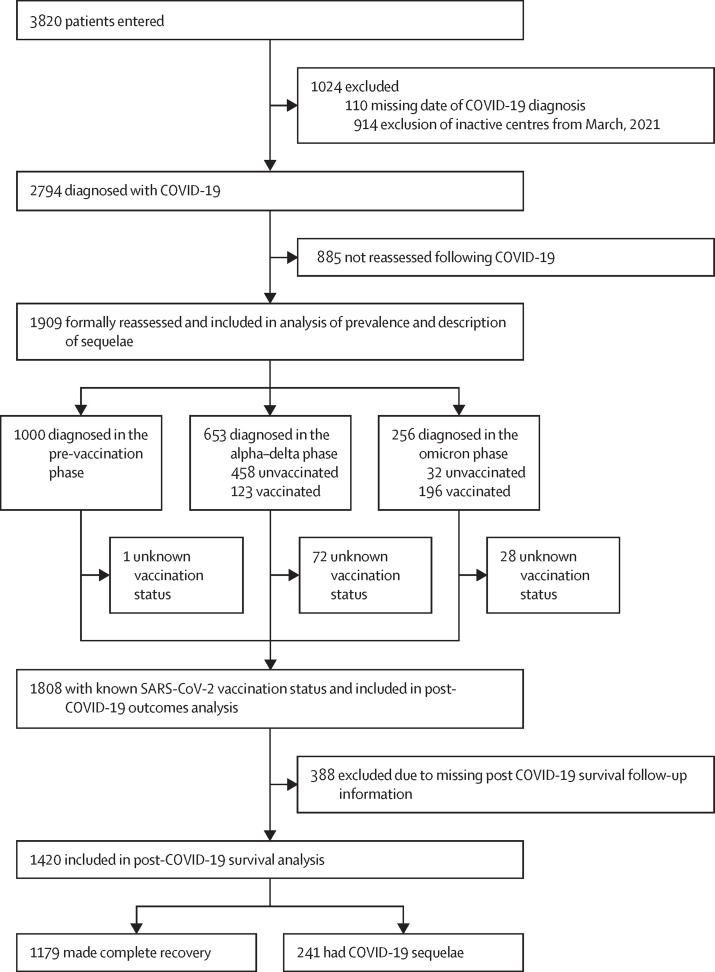
At the database lock on Feb 2, 2022, the registry included 3820 patients from 37 institutions including academic hospitals, research cancer centres, and community hospitals across six countries (Belgium, France, Germany, Italy, Spain, and the UK; appendix p 4). After the exclusion of 110 ineligible patients with missing data and 914 patients who previously entered from centres who did not participate in this post-COVID-19 follow-up update (all in Belgium, France, and Germany), the study population consisted of 2794 patients with cancer diagnosed with COVID-19 from Feb 27, 2020, to Jan 31, 2022. Overall, 1909 (68·3%) COVID-19 survivors were formally reassessed in clinic at the participating institutions after a median time of 39 days (IQR 24–68) from COVID-19 diagnosis; 1000 (52·4%) were diagnosed in the pre-vaccination phase, 653 (34·2%) in the alpha–delta phase, and 256 (13·4%) in the omicron phase (figure 1 ), with 28-day accrual rates of 113, 48, and 160 patients, respectively.
Figure 1.
Study flow diagram
Among 1909 patients, 317 (16·6%; 95% CI 14·8–18·5) had at least one sequela from COVID-19 at the first oncological reassessment, including 171 (9·0%) with respiratory sequelae, 133 (7·0%) with prolonged fatigue, 30 (1·6%) with weight loss, 55 (2·9%) with neurocognitive sequelae, and 25 (1·3%) reporting other categories of organ dysfunction. In keeping with previously reported evidence,8 patients with sequelae displayed increased features characteristic of worse COVID-19 severity, including a higher comorbidity burden, a positive smoking history, previous complications from COVID-19, hospitalisation due to COVID-19, the need for COVID-19-specific therapy, and oxygen therapy requirement (table 1 ). COVID-19 sequelae were also more frequent among patients entered from the UK and Spain than in those from Italy (table 1). Data on race and ethnicity were not available.
Table 1.
Distribution of baseline patient, tumour, and COVID-19 characteristics among the reassessed patients according to whether or not they had COVID-19 sequelae
| Overall population (n=1909) | Without COVID-19 sequelae (n=1592) | With COVID-19 sequelae (n=317) | p value | ||
|---|---|---|---|---|---|
| Country | .. | .. | .. | <0·0001 | |
| UK | 695 (36·4%) | 558 (35·1%) | 137 (43·2%) | .. | |
| Spain | 688 (36·0%) | 558 (35·1%) | 130 (41·0%) | .. | |
| Italy | 526 (27·6%) | 476 (29·9%) | 50 (15·8%) | .. | |
| Sex | .. | .. | .. | 0·20 | |
| Male | 938/1902 (49·3%) | 793/1587 (50·0%) | 145/315 (46·0%) | .. | |
| Female | 964/1902 (50·7%) | 794/1587 (50·0%) | 170/315 (54·0%) | .. | |
| Missing | 7 | 5 | 2 | .. | |
| Age, years | .. | .. | .. | 0·72 | |
| <65 | 902/1896 (47·6%) | 754/1579 (47·8%) | 148 (46·7%) | .. | |
| ≥65 | 994/1896 (52·4%) | 825/1579 (52·2%) | 169 (53·3%) | .. | |
| Missing | 13 | 13 | .. | .. | |
| Comorbidities | .. | .. | .. | 0·0067 | |
| 0–1 | 1142 (59·8%) | 974 (61·2%) | 168 (53·0%) | .. | |
| ≥2 | 767 (40·2%) | 618 (38·8%) | 149 (47·0%) | .. | |
| Smoking history | .. | .. | .. | 0·0011 | |
| Never smokers | 807/1604 (50·3%) | 697/1337 (52·1%) | 110/267 (41·2%) | .. | |
| Former or current smokers | 797/1604 (49·7%) | 640/1337 (47·9%) | 157/267 (58·8%) | .. | |
| Missing | 305 | 255 | 50 | .. | |
| Primary tumour | .. | .. | .. | 0·12 | |
| Breast | 352/1892 (18·6%) | 301/1575 (19·1%) | 51 (16·1%) | .. | |
| Gastrointestinal | 474/1892 (25·1%) | 410/1575 (26·0%) | 64 (20·2%) | .. | |
| Gynaecological or genitourinary | 345/1892 (18·2%) | 279/1575 (17·7%) | 66 (20·8%) | .. | |
| Thoracic | 274/1892 (14·5%) | 221/1575 (14·0%) | 53 (16·7%) | .. | |
| Other solid tumours | 131/1892 (6·9%) | 108/1575 (6·9%) | 23 (7·3%) | .. | |
| Haematological | 316/1892 (16·7%) | 256/1575 (16·3%) | 60 (18·9%) | .. | |
| Missing | 17 | 17 | − | .. | |
| Tumour stage | .. | .. | .. | 0·051 | |
| Local or locoregional | 818/1670 (49·0%) | 666/1390 (47·9%) | 152/280 (54·3%) | .. | |
| Advanced | 852/1670 (51·0%) | 724/1390 (52·1%) | 128/280 (45·7%) | .. | |
| Missing | 239 | 202 | 37 | .. | |
| Tumour status at COVID-19 diagnosis | .. | .. | .. | 0·89 | |
| Remission or non-measurable disease | 853/1896 (45·0%) | 712/1585 (44·9%) | 141/311 (45·3%) | .. | |
| Active malignancy | 1043/1896 (55·0%) | 873/1585 (55·1%) | 170/311 (54·7%) | .. | |
| Missing | 13 | 7 | 6 | .. | |
| Systemic anticancer therapy at COVID-19 diagnosis | .. | .. | .. | 0·057 | |
| No | 968/1806 (53·6%) | 795/1511 (52·6%) | 173/295 (58·6%) | .. | |
| Yes | 838/1806 (46·6%) | 716/1511 (47·4%) | 122/295 (41·4%) | .. | |
| Missing | 103 | 81 | 22 | .. | |
| Complicated COVID-19 | .. | .. | .. | <0·0001 | |
| No | 1468 (76·9%) | 1316 (82·7%) | 152 (47·9%) | ||
| Yes | 441 (23·1%) | 276 (17·3%) | 165 (52·1%) | ||
| Hospitalisation | .. | .. | .. | <0·0001 | |
| Not required | 672/1887 (35·6%) | 630/1572 (40·1%) | 42/315 (13·3%) | .. | |
| Due to COVID-19 | 771/1887 (40·9%) | 562/1572 (35·8%) | 209/315 (66·3%) | .. | |
| Pre-existing | 444/1887 (23·5%) | 380/1572 (24·2%) | 64/315 (20·3%) | .. | |
| Missing | 22 | 20 | 2 | .. | |
| COVID-19 therapy requirement | .. | .. | .. | <0·0001 | |
| No | 921/1784 (51·6%) | 846/1483 (57·0%) | 75/301 (24·9%) | .. | |
| Yes | 863/1784 (48·4%) | 637/1483 (43·0%) | 226/301 (75·1%) | ||
| Missing | 125 | 109 | 16 | .. | |
| Oxygen therapy requirement | .. | .. | .. | <0·0001 | |
| No | 1155/1782 (64·8%) | 1060/1484 (71·4%) | 95/298 (31·9%) | .. | |
| Yes | 627/1782 (35·2%) | 424/1484 (28·6%) | 203/298 (68·1%) | .. | |
| Missing | 127 | 108 | 19 | .. | |
Data are n (%) or n unless otherwise stated.
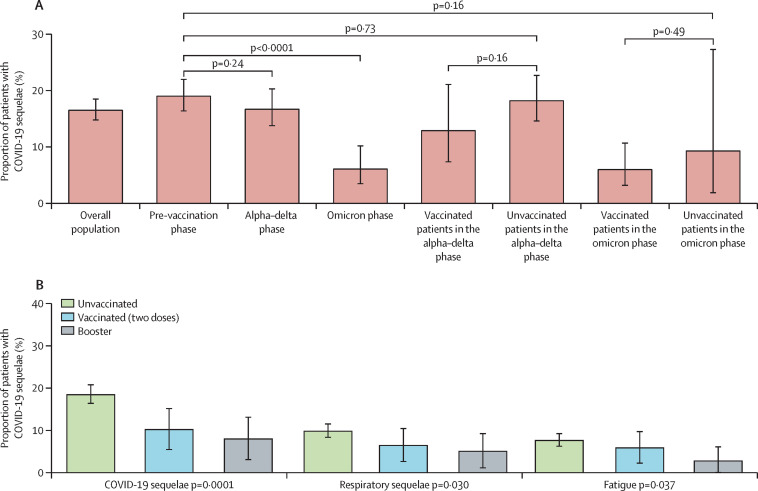
Compared with the pre-vaccination phase, in which 191 (19·1%; 95% CI 16·4–22·0) of 1000 patients had COVID-19 sequelae, a similar proportion of patients diagnosed during the alpha–delta phase had COVID-19 sequelae (110 [16·8%; 13·8–20·3] of 653, p=0·24), whereas the proportion of patients with sequelae diagnosed during the omicron phase was significantly lower (16 [6·2%; 3·5–10·2] of 256, p<0·0001; figure 2A ; appendix p 5).
Figure 2.
Histogram plot summarising the univariable analysis of prevalence of overall COVID-19 sequelae
(A) COVID-19 sequelae across pandemic phases (appendix p 5). (B) Overall COVID-19 sequelae by vaccination status (data are reported in full in table 2).
After the exclusion of 72 patients with unknown vaccination status from the alpha–delta phase and 28 patients with unknown vaccination status from the omicron phase, the prevalence of sequelae reported for patients diagnosed during the pre-vaccination phase was similar to that in unvaccinated patients from the alpha–delta phase (84 [18·3%; 95% CI 14·6–22·7] of 458, p=0·73). No significant difference was reported for unvaccinated patients from the omicron phase (three [9·4%; 95% CI 1·9–27·3] of 32, p=0·1659) in comparison to patients from the pre-vaccination phase. Finally, no difference between vaccinated and unvaccinated patients in the omicron phase (p=0·49) and the alpha–delta phase (p=0·16) were reported (figure 2A; appendix p 5).
After propensity score matching, 30 unvaccinated patients from the omicron phase were matched with 118 patients from the pre-vaccination phase (appendix p 5). The propensity score matching-fitted multivariable logistic regression analysis adjusted for country, sex, number of comorbidities, primary tumour, tumour stage at COVID-19 diagnosis, tumour status at COVID-19 diagnosis, and the receipt of systemic anticancer therapy at COVID-19 diagnosis, and age following the clustered-robust correction for participating centre confirmed that the risk of COVID-19 sequelae for unvaccinated patients from the omicron phase was not significantly lower than for patients diagnosed during the pre-vaccination phase (adjusted OR 0·35 [95% CI 0·08–3·64]; appendix p 6).
Next, we examined whether or not previous vaccination status was associated with the development of COVID-19 sequelae. After exclusion of 101 (5·3%) patients with unknown vaccination status, 1808 patients were included in the COVID-19 sequelae and vaccination analysis, of whom 1489 (82·3%) were unvaccinated patients, 65 (3·6%) were partially vaccinated, 183 (10·1%) had received two doses, and 136 (7·5%) had received a booster dose (appendix p 6). Unvaccinated patients were assessed at a median interval of 40 days (IQR 25–67), partially vaccinated patients 50 days (28–68), doubly vaccinated patients 38 days (23–68), and boosted patients 34 days (22–67) after COVID-19 diagnosis.
Compared with unvaccinated patients, vaccinated patients were more likely to be current or former smokers (148 [56·7%] of 261 vs 606 [48·1%] of 1260, p=0·011), with an advanced-stage (179 [61·9%] of 289 vs 619 [48·2%] of 1285, p<0·0001) and active malignancy at COVID-19 diagnosis (202 [63·5%] of 318 vs 775 [52·5%] of 1477, p=0·0003). They were also more likely to have been recruited from the UK or Spain than from Italy (125 [39·2%] of 319 vs 130 [40·8%] vs 64 [20·1%], p=0·018) and to be receiving systemic anticancer therapy at the time of COVID-19 diagnosis (172 [57·9%] of 297 vs 619 [43·8%] of 1414, p<0·0001; appendix p 7).
Table 2 and figure 2B summarise the univariable analysis of COVID-19 sequelae according to the vaccination status. Patients who received a booster and those who received two vaccine doses had a significantly lower prevalence of overall COVID-19 sequelae than unvaccinated patients (ten [7·4%] of 136 and 18 [9·8%] of 183 vs 277 [18·5%] of 1489, p=0·0001), respiratory sequelae (six [4·4%] and 11 [6·0%] vs 148 [9·9%], p=0·030), and prolonged fatigue (three [2·2%] and ten [5·4%] vs 115 [7·7%], p=0·037; table 2). No significant difference in terms of weight loss, neurocognitive sequelae, and other sequelae according to vaccination status were found.
Table 2.
Overall COVID-19 sequelae according to SARS-CoV-2 vaccination status
| Overall population (n=1909) | Unvaccinated or partially vaccinated patients n=1489) | Patients who received two doses (n=183) | Patients who received a booster (n=136) | p value | ||
|---|---|---|---|---|---|---|
| COVID-19 sequelae | 317 (16·6%; 14·8–18·5) | 277 (18·5%; 16·5–20·9) | 18 (9·8%; 5·8–15·5) | 10 (7·4%; 3·5–13·5) | 0·0001 | |
| Respiratory sequelae | 171 (9·0%; 7·6–10·4) | 148 (9·9%; 8·4–11·6) | 11 (6·0%; 3·0–10·7) | 6 (4·4%; 1·6–9·6) | 0·030 | |
| Fatigue | 133 (7·0%; 5·8–8·2) | 115 (7·7%; 6·3–9·3) | 10 (5·4%; 2·6–10·0) | 3 (2·2%; 0·1–6·4) | 0·037 | |
| Weight loss | 30 (1·6%; 1·1–2·2) | 29 (1·9%; 1·3–2·8) | 0 | 1 (0·7%; 0·1–4·1) | 0·10 | |
| Neurological sequelae | 55 (2·9%; 2·2–3·7) | 46 (3·1%; 2·2–4·1) | 4 (2·1%; 0·1–5·6) | 2 (1·5%; 0·1–5·3) | 0·46 | |
| Other organ disfunctions | 25 (1·3%; 0·1–1·9) | 23 (1·5%; 0·1–2·3) | 0 | 1 (0·7%; 0·1–4·1) | 0·18 | |
Data are n (%; 95% CI) unless otherwise stated.
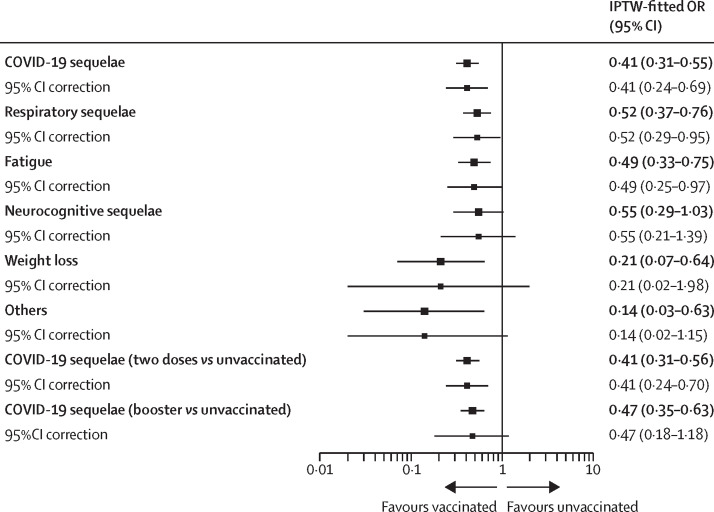
Patients' characteristics before and after the IPTW between unvaccinated vaccinated and vaccinated patients are reported in the appendix (p 8). IPTW-fitted logistic regressions following the clustered-robust correction for participating centres confirmed that compared with unvaccinated patients, vaccinated patients displayed a lower risk of overall COVID-19 sequelae (OR 0·41 [95% CI 0·24–0·69]), respiratory sequelae (0·52 [0·29–0·95]), and prolonged fatigue (0·49 [0·25–0·97]; figure 3 ). The appendix reports patients' characteristics before and after the IPTW between unvaccinated patients and patients who received two vaccine doses (p 8) and those who received a booster dose separately (p 9). At the IPTW-fitted clustered-robust corrected analysis, patients who received two doses had a reduced prevalence of sequelae compared with unvaccinated patients (OR 0·41 [95% CI 0·24–0·70]), whereas there was no difference in the prevalence of sequelae between unvaccinated patients and who received a booster dose (adjusted OR 0·47 [95% CI 0·18–1·18]; figure 3; appendix p 9).
Figure 3.
Forest plot summarising the IPTW-fitted logistic regression analysis with log10 transformation of odds ratios and 95% CIs
Comparison between vaccinated patients (either two doses or a booster dose) and unvaccinated patients (including partially vaccinated patients) for overall COVID-19 sequelae, respiratory sequelae, prolonged fatigue, neurocognitive sequelae, weight loss, and other sequelae is reported with no adjusting covariate included given the optimal balancing ability of the IPTW procedure. Comparison between patients who received two vaccine doses and unvaccinated patients included no adjusting covariate given the optimal balancing ability of the IPTW procedure. Comparison between patients who received a booster dose and unvaccinated patients was adjusted for country of origin (multivariable model reported in the appendix p 9). 95% CIs are provided for both before and after the clustered-robust correction for participating centre. IPTW=inverse probability of treatment weighting.
A follow-up survival update after the post-COVID-19 reassessment was available for 1420 patients. With 419 deaths, the post-COVID-19 survival was not reached at a median post-COVID-19 follow-up of 12·6 months (95% CI 12·0–13·4). Given the time dependence in delivering vaccinations, the median post-COVID-19 follow-up was shorter for vaccinated patients (4·8 months [95% CI 4·6–5·1]) than for unvaccinated patients (14·8 months [13·9–15·7]).
We confirmed that patients who had any sequelae (n=241) had an increased risk of death compared with those who did not have any COVID-19 sequelae (n=1179; HR 1·61 [95% CI 1·28–2·01]; appendix p 12).
The restricted mean survival time analysis of post-COVID-19 survival is summarised in the appendix (p 10) . Although COVID-19 sequelae have a consistent detrimental effect at 2 months (restricted mean survival time difference –0·13; p<0·0001), 4 months (–0·34; p<0·0001), and 6 months (–0·54; p<0·0001), previous SARS-CoV-2 vaccination did not show a proportional effect over time (appendix p 10); therefore, it was not included in the fixed multivariable regression, which confirmed that after adjustment for country, sex, age, number of comorbidities, primary tumour, tumour stage and status, and the receipt of systemic anticancer therapy at the time of COVID-19 diagnosis, COVID-19 sequelae were associated with an increased risk of death after the acute phase of the disease (HR 1·39 [95% CI 1·10–1·74]; table 3 ).
Table 3.
Fixed multivariable regression model for post COVID-19 survival, including COVID-19 sequelae, in 840 patients
| Hazard ratio (95% CI) | |
|---|---|
| COVID-19 sequelae | |
| No | 1 (ref) |
| Yes | 1·39 (1·10–1·74) |
| Country | |
| UK | 1 (ref) |
| Spain | 0·65 (0·52–0·82) |
| Italy | 0·42 (0·32–0·57) |
| Sex | |
| Female | 1 (ref) |
| Male | 1·25 (1·03–1·53) |
| Age | |
| <65 years | 1 (ref) |
| ≥65 years | 1·39 (1·13–1·72) |
| Comorbidities | |
| 0–1 | 1 (ref) |
| ≥2 | 1·11 (0·89–1·37) |
| Primary tumour | |
| Breast | 1 (ref) |
| Gastrointestinal | 1·21 (0·90–1·63) |
| Gynaecological or genitourinary | 1·46 (1·06–2·01) |
| Thoracic | 0·96 (0·67–1·38) |
| Other solid tumours | 1·09 (0·69–1·71) |
| Haematological | 1·13 (0·81–1·58) |
| Tumour stage at COVID-19 diagnosis | |
| Local or locoregional | 1 (ref) |
| Advanced | 1·08 (0·85–1·35) |
| Unknown | 1·08 (0·78–1·49) |
| Tumour status at COVID-19 diagnosis | |
| Remission or non-measurable disease | 1 (ref) |
| Active malignancy | 0·92 (0·74–1·15) |
| Systemic anticancer therapy at COVID-19 diagnosis | |
| No | 1 (ref) |
| Yes | 1·02 (0·83–1·25) |
| Unknown | 1·26 (0·81–1·97) |
Only 840 patients were included in the final model due to missing covariate data.
Among the 838 patients who were on systemic anticancer therapy within 4 weeks before COVID-19 diagnosis, 506 (60·4%) resumed or continued the treatment without changes, 243 (30·0%) resumed systemic anticancer therapy following a regimen adjustment, and 89 (10·5%) permanently discontinued oncological treatments (appendix p 11).
We confirmed that the presence of at least one COVID-19 sequela of any kind during follow-up was associated with disruptions of post-COVID-19 oncological care (61 [18·4%] of 332 vs 61 [12·1%] of 506, p=0·011), and surrogates of previous COVID-19 severity, including the need for COVID-19-specific therapy (p=0·0014) and oxygen therapy (p=0·0090), complications from COVID-19 (p=0·0002), and hospitalisation (p=0·0005; appendix p 11). Country of origin (p=0·0154), primary tumour (p=0·0081), and tumour status (p=0·034) were also associated with adjustments to or discontinuation of systemic anticancer therapy.
Finally, we confirmed that patients who resumed treatment with systemic anticancer therapy after a regimen adjustment had similar post-COVID-19 survival to those who resumed or continued their treatment without changes (HR 0·92 [95% CI 0·71–1·21]), whereas those who permanently discontinued experienced a significantly increased risk of death (3·17 [2·29–4·38]; appendix p 13).
Discussion
In this study, we demonstrate that omicron infections are associated with a reduced persistence of sequelae (6·2%) in comparison with infections contracted during previous phases of the pandemic (19·1% in the prevaccination phase and 16·8% in the alpha–delta phase).
When stratifying patients according to previous vaccination status, we demonstrated a substantial uniformity in the prevalence of sequelae between unvaccinated patients from the alpha–delta phase and that from the pre-vaccination phase, along with a numerically, and potentially clinically meaningful, reduced prevalence of sequelae for unvaccinated patients from the omicron phase (9·4%), which did not reach the threshold of significance when compared with that of the pre-vaccination phase (>19%), as a possible consequence of its reduced sample size. These results support vaccinal immunity as one of the determinants of the reduction of post-COVID-19 symptoms over time, in addition to other factors including improved health-care resilience, clinical management, and testing capacity. In fact, in a previous analysis of our registry, we demonstrated a time-dependent reduction in COVID-19 severity even before the advent of SARS-CoV-2 vaccines.14
Despite widespread adoption of SARS-CoV-2 vaccines and improved disease management including the use of antivirals and antibody therapy in vulnerable patients, post-COVID-19 condition continues to represent a substantial challenge to the increasing number of patients who survive COVID-19. In patients with cancer, sequelae can cause deferral of oncological care, leading to a detrimental effect on overall survival.8
We previously showed that SARS-CoV-2 infection diagnosed during the omicron outbreak is associated with reduced disease severity in Europe.13 With the ease of international restrictions and the reduced emphasis on widespread community testing against COVID-19,25 SARS-CoV-2 remains a highly prevalent, albeit less lethal, pathogen that has carved itself a co-existence niche during the year 2022.
To our knowledge, this study is the first to confirm that previous vaccination is independently associated with improved COVID-19 outcomes even during the post-acute phase of the disease, despite the presumed lower pathogenicity of the omicron variant. Although we cannot mechanistically prove the protective role of SARS-CoV-2 vaccines against COVID-19 sequelae, the underlying causative link is biologically plausible and represents a potentially practice-informing finding despite possible sources of heterogeneity, including selection and ascertainment bias.
We reported a concordantly decreasing prevalence of sequelae for patients who received two doses and patients who received a booster vaccination, whereas our two-tiered analysis clearly indicates that vaccinal immunity before COVID-19 infection is significantly associated with a decreased risk of overall sequelae, respiratory sequelae, and prolonged fatigue.
Despite evidence of the immune-escape potential of the omicron variant,26 this study provides complementary evidence to earlier reports from OnCovid showing that previous vaccination protects from omicron-related morbidity and mortality.13
The development of sequelae was independent of major oncological features describing our patient population, being more strongly associated with features pertaining to a worse course of SARS-CoV-2 infection as previously observed,7, 8 including baseline comorbidity burden, smoking status, emergence of COVID-19 complications, need for hospitalisation, and need for COVID-19-specific therapy.
We confirmed that the occurrence of COVID-19 sequelae was associated with decreased post-COVID-19 survival and with SARS-CoV-2-induced disruption of the oncological continuity of care among patients on systemic anticancer therapy at COVID-19. Moreover, we further confirmed that discontinuation of systemic anticancer therapy in particular emerged as a strong negative prognostic driver among patients on active oncological therapy.
Overall, these findings collectively highlight the importance of previous vaccinal immunity against SARS-CoV-2 as an efficacious preventive measure to protect patients with cancer beyond the acute phase of the disease and suggest a protective role in ensuring the post-COVID-19 oncological continuity of care.
An aspect to be considered in interpreting our analysis is that the distribution of SARS-CoV-2 vaccinations reflects prioritisation of frailer categories of individuals, since in our study population the vaccinated subgroup is significantly enriched in patients with advanced-stage and active tumours receiving systemic anticancer therapy at the time of COVID-19 diagnosis. The small number of vaccinated patients in our study cohort is inherently associated with the time-dependent delivery of immunisation campaigns during the alpha–delta and omicron phases, rather than to vaccine hesitancy given the very high vaccination rate reported in European countries.27 Additionally, the protective effect of first-generation vaccines against the delta and omicron variants28, 29 is likely to have reduced our capacity to recruit breakthrough infections following full vaccination. However, the low percentages of fully vaccinated patients led to unavoidable dispersion of our data, limiting subgroup sample sizes and the power of the propensity score matching analysis. Including only COVID-19 survivors, our study population is also enriched in patients with gastrointestinal, breast, and gynaecological or genitourinary malignancies, which probably reflects the increased vulnerability of patients with thoracic and haematological malignancies to COVID-19.30
An important limitation of our analysis is related to the definition of COVID-19 sequelae and the precise attribution of these to previous SARS-CoV-2 infection. Although prospectively reported, the occurrence of sequelae was assessed locally by treating physicians during routine consultations and not centrally reviewed. This might affect reliability and reproducibility of the results, and the absence of standardised time intervals for assessments and a unified definition of sequelae leads to a considerable risk of under-reporting of less symptomatic sequelae. Additionally, sequelae were primarily defined symptomatically during clinical consultation, rather than on the basis of pre-planned assessments. Underreporting of asymptomatic or minimally symptomatic sequelae and imprecise causal attribution to either COVID-19 or underlying malignancy are sources of bias that we could not fully control for.8 Routine clinical care cannot by definition be standardised per protocol; therefore, reduced hospital capacity during earlier phases of the pandemic could also have affected patient reassessment. However, a recent update of the OnCovid registry has reported a 16% prevalence of COVID-19 sequelae in patients with non-advanced cancer and therefore with a lower burden of symptoms attributable to active malignancy,7 and although sequelae can resolve over time,7 we consistently reported a prevalence of COVID-19 sequelae of around 15% at the first oncological reassessment across previous updates of the registry, which ranged from 1·5 months to 2·3 months following COVID-19.7, 8 Despite this, we acknowledge the limited external validity of our findings, given the scarcity of evidence replicating this results in independent cohort studies with similar study populations.
Another limitation of our study is that the only inclusion criteria to enter the analysis was the availability of a post-COVID-19 reassessment in clinics at the participating institutions, with risks of under-reporting for patients with less severe illness who require fewer hospital visits, and the inherently different follow-up period of patients entered during the different phases of the pandemic, which partially impairs the post-COVID-19 survival analysis. Furthermore, the lack of viral genomic sequences at the point of PCR testing is an important limitation, although we adopted strict epidemiological definition for pandemic phases as previously reported,13 which relies on the temporal dynamics of transmission of the omicron variant in Europe in late 2021 and early 2022.19
Although evidence now consistently shows the negative prognostic effect of sequelae and disruption of post-COVID-19 oncological care in the prognosis of patients with cancer,8, 9 retrospective cohort studies remain inevitably exposed to the risk of selection bias, such as ascertainment and recollection bias, which might have flawed the analysis even beyond the COVID-19 sequelae definition, leading to an increased overall heterogeneity of our data, including geographical differences. In fact, beside the country-based differences in the delivery of immunisation campaigns and infection control policies, we already described how patients from the UK tend to have worse COVID-19 outcomes than patients in other European countries, as a likely result of their inherent older age and increased burden of comorbidities.16
Despite the acknowledged limitations, the significant association with reduced prevalence of overall COVID-19 sequelae, respiratory sequelae, and prolonged fatigue clearly indicates that previous SARS-CoV-2 immunisation and booster doses with original strain-based vaccines is a key strategy for protecting patients with cancer from adverse outcomes beyond COVID-19-related mortality.
Although emerging variants of COVID-19 such as omicron sublineages BA.2.12.1, BA.4, BA.5, and the newest BA.2.75, with immune-escaping potential to both natural and vaccinal immunity, will continue to pose a threat to patients with cancer in terms of risk of infection, universal immunisation, including those with novel vaccines targeted to newly emerging variants of concern,11 should continue to be promoted to protect this especially vulnerable population against both acute COVID-19 morbidity and mortality and post-COVID-19 condition in order to ensure a prompt resumption of their oncological care.
For more on MedCalc see https://www.medcalc.org
Data sharing
Individual, de-identified participant data and data dictionary can be made available at the request of investigators whose proposed use of the data has been approved by the OnCovid consortium. Requests can be made to DJP (david.pinato@imperial.ac.uk). Additional related documents are available at: https://clinicaltrials.gov/ct2/show/NCT04393974.
Declaration of interests
AC has received consulting fees from MSD, Bristol Myers Squibb, AstraZeneca, and Roche, and speakers' fee from AstraZeneca, MSD, Novartis, and Eisai. ML acted as a consultant for Roche, Novartis, Lilly, AstraZeneca, Exact Sciences, MSD, Pfizer, and Seagen, and received speaker honoraria from Roche, Novartis, Lilly, Pfizer, Takeda, Ipsen, and Sandoz outside the submitted work. AG declares consulting or advisory roles for Roche, MSD, Eli Lilly, Pierre Fabre, Eisai, and Daichii Sankyo; speakers' fees for Eisai, Novartis, Eli Lilly, Roche, Teva, Gentili, Pfizer, AstraZeneca, Celgene, and Daichii Sankyo; and research funds from Eisai, Eli Lilly, and Roche. CM-V has received travel grants and other honoraria from Bristol Myers Squibb, MSD, Novartis, and Roche. JB has declared consulting or advisory roles for MSD and AstraZeneca, and support for attending meetings and travel from GlaxoSmithKline. OM reports personal fees from Grupo Pacifico, Kyowa Kirin, Roche, and ROVI, and travel support from Almirall, Kyowa Kirin, and Sanofi. JT reports personal financial interest in the form of scientific consultancy roles for Array Biopharma, AstraZeneca, Bayer, Boehringer Ingelheim, Chugai, Daiichi Sankyo, F Hoffmann-La Roche, Genentech, HalioDX SAS, Hutchison MediPharma International, Ikena Oncology, Inspirna, IQVIA, Lilly, Menarini, Merck Serono, Merus, MSD, Mirati, Neophore, Novartis, Ona Therapeutics, Orion Biotechnology, Peptomyc, Pfizer, Pierre Fabre, Samsung Bioepis, Sanofi, Scandion Oncology, Scorpion Therapeutics, Seattle Genetics, Servier, Sotio Biotech, Taiho, Tessa Therapeutics, and TheraMyc; stocks in Oniria Therapeutics; and educational collaboration with Imedex/HMP, Medscape Education, MJH Life Sciences, PeerView Institute for Medical Education, and Physicians Education Resource. LRi reports receiving consulting fees from Amgen, ArQule, AstraZeneca, Basilea, Bayer, Bristol Myers Squibb, Celgene, Eisai, Exelixis, Genenta, Hengrui, Incyte, Ipsen, IQVIA, Lilly, MSD, Nerviano Medical Sciences, Roche, Sanofi, Servier, Taiho Oncology, and Zymeworks; lecture fees from AbbVie, Amgen, Bayer, Eisai, Gilead, Incyte, Ipsen, Lilly, Merck Serono, Roche, Sanofi, and Servier; travel expenses from AstraZeneca; and institutional research funding from Agios, ARMO BioSciences, AstraZeneca, BeiGene, Eisai, Exelixis, Fibrogen, Incyte, Ipsen, Lilly, MSD, Nerviano Medical Sciences, Roche, and Zymeworks. AD'A has received educational support for congress attendance and consultancy fees from Roche. DJP has received lecture fees from ViiV Healthcare, Bayer Healthcare, BMS, Roche, Eisai, and the Falk Foundation; travel expenses from Bristol Myers Squibb and Bayer Healthcare; consulting fees for Mina Therapeutics, Eisai, Roche, DaVolterra, and Astra Zeneca; and institutional research funding from MSD and Bristol Myers Squibb. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
OnCovid is sponsored by Imperial College London and received direct project funding and infrastructural support by the UK National Institute for Health and Care Research (NIHR) Imperial Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care. AC is supported by the NIHR Imperial Biomedical Research Centre. DJP is supported by grant funding from the Wellcome Trust Strategic Fund (PS3416) and from the Associazione Italiana per la Ricerca sul Cancro (AIRC MFAG grant identification number 25697) and acknowledges support by the NIHR Imperial Biomedical Research Centre, the Imperial Experimental Cancer Medicine Centre, and the Imperial College Tissue Bank. AG is supported by the AIRC IG Grant, number 14230, Associazione Italiana per la Ricerca sul Cancro Foundation, Milan, Italy; she also acknowledges support from the UPO Aging Project. AD'A is supported by the National Institute for Health Research (NIHR) Imperial BRC, by grant funding from the European Association for the Study of the Liver (Andrew Burroughs Fellowship) and from Cancer Research UK (RCCPDB- Nov21/100008).
Contributors
AC and DJP were responsible for the study concept and design, analysis and interpretation of the data, and drafting of the manuscript (and any substantially modified version that involves the author's contribution to the study). AC and DF did the statistical analyses. All authors were involved in acquisition of data and manuscript review and approval. DJP obtained funding and supervised the study. AC, DF, and DJP had full access to and verified all the data. All authors had full access to the data underlying this study and had final responsibity for the decision to submit for publication.
Contributor Information
OnCovid study group:
Joanne S Evans, Judith Swallow, Georgina Hanbury, Chris Chung, Meera Patel, Gino Dettorre, Katherine Belessiotis, Dolly Saorise, Eleanor Jones, Eleanor Apthorp, Charlotte Moss, Beth Russell, Sarah Townsend, Amanda Jackson, Angela Loizidou, Martine Piccart, Fanny Pommeret, Emeline Colomba-Blameble, Aleix Prat, Claudia A Cruz, Roxana Reyes, Elia Segui, Javier Marco-Hernández, Margarita Viladot, Nadia Harbeck, Rachel Wuerstlein, Franziska Henze, Sven Mahner, Eudald Felip, Lorenza Scotti, Andrea Marrari, Federica Grosso, Vittorio Fusco, Sara Delfanti, Maura Rossi, Alberto Zambelli, Carlo Tondini, Lorenzo Chiudinelli, Michela Franchi, Michela Libertini, Salvatore Provenzano, Daniele Generali, Salvatore Grisanti, Alice Baggi, Valeria Tovazzi, Corrado Ficorella, Giampiero Porzio, Maristella Saponara, Marco Filetti, Marco Tucci, Rossana Berardi, Luca Cantini, Francesco Paoloni, Annalisa Guida, Sergio Bracarda, Maria Iglesias, Ana Sanchez de Torre, and Marco Tagliamento
Supplementary Material
References
- 1.Sandler CX, Wyller VBB, Moss-Morris R, et al. Long COVID and post-infective fatigue syndrome: a review. Open Forum Infect Dis. 2021;8 doi: 10.1093/ofid/ofab440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daugherty SE, Guo Y, Heath K, et al. Risk of clinical sequelae after the acute phase of SARS-CoV-2 infection: retrospective cohort study. BMJ. 2021;373 doi: 10.1136/bmj.n1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Logue JK, Franko NM, McCulloch DJ, et al. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med. 2021;27:626–631. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azzolini E, Levi R, Sarti R, et al. Association between BNT162b2 vaccination and long COVID after infections not requiring hospitalization in health care workers. JAMA. 2022;328:676–678. doi: 10.1001/jama.2022.11691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cortellini A, Salazar R, Gennari A, et al. Persistence of long-term COVID-19 sequelae in patients with cancer: an analysis from the OnCovid registry. Eur J Cancer. 2022;170:10–16. doi: 10.1016/j.ejca.2022.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinato DJ, Tabernero J, Bower M, et al. Prevalence and impact of COVID-19 sequelae on treatment and survival of patients with cancer who recovered from SARS-CoV-2 infection: evidence from the OnCovid retrospective, multicentre registry study. Lancet Oncol. 2021;22:1669–1680. doi: 10.1016/S1470-2045(21)00573-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cortellini A, Gennari A, Pommeret F, et al. COVID-19 sequelae and the host proinflammatory response: an analysis from the OnCovid registry. J Natl Cancer Inst. 2022;114:979–987. doi: 10.1093/jnci/djac057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinato DJ, Ferrante D, Aguilar-Company J, et al. Vaccination against SARS-CoV-2 protects from morbidity, mortality and sequelae from COVID19 in patients with cancer. Eur J Cancer. 2022;171:64–74. doi: 10.1016/j.ejca.2022.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.European Medicines Agency Adapted vaccine targeting BA.4 and BA.5 Omicron variants and original SARS-CoV-2 recommended for approval. Sept 12, 2022. https://www.ema.europa.eu/en/news/adapted-vaccine-targeting-ba4-ba5-omicron-variants-original-sars-cov-2-recommended-approval
- 12.Thorne LG, Bouhaddou M, Reuschl A-K, et al. Evolution of enhanced innate immune evasion by SARS-CoV-2. Nature. 2022;602:487–495. doi: 10.1038/s41586-021-04352-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinato DJ, Aguilar-Company J, Ferrante D, et al. Outcomes of the SARS-CoV-2 omicron (B.1.1.529) variant outbreak among vaccinated and unvaccinated patients with cancer in Europe: results from the retrospective, multicentre, OnCovid registry study. Lancet Oncol. 2022;23:865–875. doi: 10.1016/S1470-2045(22)00273-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.OnCovid Study Group Time-dependent COVID-19 mortality in patients with cancer: an updated analysis of the OnCovid registry. JAMA Oncol. 2022;8:114–122. doi: 10.1001/jamaoncol.2021.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinato DJ, Lee AJX, Biello F, et al. Presenting features and early mortality from SARS-CoV-2 infection in cancer patients during the initial stage of the COVID-19 pandemic in Europe. Cancers. 2020;12 doi: 10.3390/cancers12071841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinato DJ, Scotti L, Gennari A, et al. Determinants of enhanced vulnerability to coronavirus disease 2019 in UK patients with cancer: a European study. Eur J Cancer. 2021;150:190–202. doi: 10.1016/j.ejca.2021.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinato DJ, Zambelli A, Aguilar-Company J, et al. Clinical portrait of the SARS-CoV-2 epidemic in European cancer patients. Cancer Discov. 2020;10:1465–1474. doi: 10.1158/2159-8290.CD-20-0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walker AS, Vihta K-D, Gethings O, et al. Tracking the emergence of SARS-CoV-2 alpha variant in the United Kingdom. N Engl J Med. 2021;385:2582–2585. doi: 10.1056/NEJMc2103227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.European Centre for Disease Prevention and Control Assessment of the further emergence and potential impact of the SARS-CoV-2 omicron variant of concern in the context of ongoing transmission of the delta variant of concern in the EU/EEA, 18th update 2021. Dec 15, 2021. https://www.ecdc.europa.eu/sites/default/files/documents/covid-19-assessment-further-emergence-omicron-18th-risk-assessment-december-2021.pdf
- 20.WHO A clinical case definition of post COVID-19 condition by a Delphi consensus. Oct 6, 2021. https://apps.who.int/iris/bitstream/handle/10665/345824/WHO-2019-nCoV-Post-COVID-19-condition-Clinical-case-definition-2021.1-eng.pdf [DOI] [PMC free article] [PubMed]
- 21.Venkatesan P. NICE guideline on long COVID. Lancet Respir Med. 2021;9:129. doi: 10.1016/S2213-2600(21)00031-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linden A, Samuels SJ. Using balance statistics to determine the optimal number of controls in matching studies. J Eval Clin Pract. 2013;19:968–975. doi: 10.1111/jep.12072. [DOI] [PubMed] [Google Scholar]
- 23.Biondi-Zoccai G, Romagnoli E, Agostoni P, et al. Are propensity scores really superior to standard multivariable analysis? Contemp Clin Trials. 2011;32:731–740. doi: 10.1016/j.cct.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen TL, Collins GS, Spence J, et al. Double-adjustment in propensity score matching analysis: choosing a threshold for considering residual imbalance. BMC Med Res Methodol. 2017;17:78. doi: 10.1186/s12874-017-0338-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mathieu E, Ritchie H, Rodés-Guirao L, et al. Policy responses to the coronavirus pandemic. 2020. https://ourworldindata.org/policy-responses-covid
- 26.Mannar D, Saville JW, Zhu X, et al. SARS-CoV-2 omicron variant: antibody evasion and cryo-EM structure of spike protein–ACE2 complex. Science. 2022;375:760–764. doi: 10.1126/science.abn7760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.European Centre for Disease Prevention and Control COVID-19 vaccine tracker. 2022. https://vaccinetracker.ecdc.europa.eu/public/extensions/COVID-19/vaccine-tracker.html#uptake-tab
- 28.Hall V, Foulkes S, Insalata F, et al. Protection against SARS-CoV-2 after Covid-19 Vaccination and Previous Infection. N Engl J Med. 2022;386:1207–1220. doi: 10.1056/NEJMoa2118691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meggiolaro A, Sane Schepisi M, Farina S, et al. Effectiveness of vaccination against SARS-CoV-2 omicron variant infection, symptomatic disease, and hospitalization: a systematic review and meta-analysis. Expert Rev Vaccines. 2022;21:1831–1841. doi: 10.1080/14760584.2022.2130773. [DOI] [PubMed] [Google Scholar]
- 30.Lee LYW, Cazier J-B, Starkey T, et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. Lancet Oncol. 2020;21:1309–1316. doi: 10.1016/S1470-2045(20)30442-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Individual, de-identified participant data and data dictionary can be made available at the request of investigators whose proposed use of the data has been approved by the OnCovid consortium. Requests can be made to DJP (david.pinato@imperial.ac.uk). Additional related documents are available at: https://clinicaltrials.gov/ct2/show/NCT04393974.