Abstract
OBJECTIVES
Develop a predictive model for transcatheter aortic valve replacement (TAVR)-related coronary obstruction in native aortic stenosis (AS).
BACKGROUND
TAVR-related coronary artery obstruction prediction remains unsatisfactory despite high mortality and novel preventative therapies.
METHODS
Pre-procedure computed tomography (CT) and fluoroscopy images of patients in whom TAVR caused coronary artery obstruction were collected. Central laboratories made measurements, which were compared to unobstructed patients from a single-center database. A multivariate model was developed and validated against a 1:1 propensity-matched sub-selection of the unobstructed cohort.
RESULTS
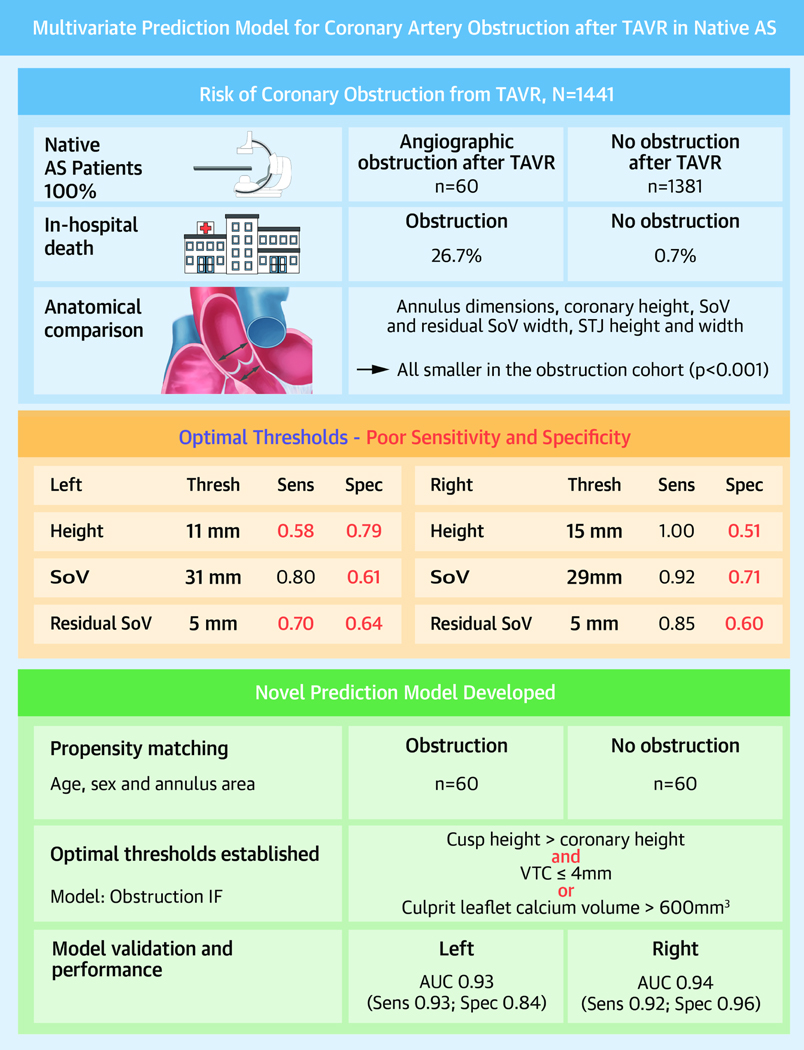
Sixty patients with angiographically confirmed coronary obstruction and 1,381 without obstruction were included. In-hospital death was higher in the obstruction cohort (26.7% versus 0.7%, p<0.001). Annular area and perimeter, coronary height, sinus width, and sinotubular junction height and width were all significantly smaller in the obstructed cohort. Obstruction was most common on the left side (78.3%) and at the level of the coronary artery ostium (92.1%). Coronary artery height and sinus width, but not annulus area, were significant risk factors for obstruction by logistic regression but performed poorly in predicting obstruction. The new multivariate model (coronary obstruction IF cusp height > coronary height, AND virtual valve to coronary (VTC) distance ≤4 mm OR culprit leaflet calcium volume >600 mm3) performed well, with an area under the curve of 0.93 (sensitivity=0.93, specificity=0.84) for the left coronary artery and 0.94 (sensitivity=0.92, specificity=0.96) for the right.
CONCLUSIONS
A novel CT-based multivariate prediction model that can be implemented routinely in real-world practice predicted coronary artery obstruction from TAVR in native AS.
Keywords: Screening, Computed tomography, BASILICA, Aortic stenosis, Transcatheter aortic valve replacement
INTRODUCTION
The incidence of coronary artery obstruction in native transcatheter aortic valve replacement (TAVR) is 0.6% with a 30-day mortality of 40% to 50% despite attempted rescue revascularization(1). Although the risk of obstruction is higher in valve-in-valve (VIV) TAVR (2.3%)(2), the incidence is higher in native aortic stenosis, as it is the more common procedure.
Coronary obstruction occurs when the diseased native or bioprosthetic leaflets are displaced toward the coronary artery ostia (coronary ostial obstruction) or the sinotubular junction (STJ; sinus sequestration). The covered skirt of the transcatheter heart valve (THV) may also directly obstruct the coronary artery. Predicting this risk requires a multidimensional understanding of the relationship between the aortic leaflets and the coronary arteries and STJ, as well as the dimensions of the intended THV to be implanted.
The data and understanding of coronary obstruction come from two seminal registries comprising 27 cases with computed tomography (CT) of native leaflet coronary obstruction(1) and 20 cases with CT of VIV coronary obstruction(2). In the native aortic stenosis group, patients with obstruction had lower left coronary artery height (<12 mm) and smaller left sinus width (<30 mm). In the VIV group, patients with obstruction had a smaller virtual THV to coronary distance (VTC ≤4 mm). The small numbers in these studies limit their predictive power, and thresholds were only validated for the left coronary artery. Moreover, the measurements assessed were unidimensional and, in the case of native obstruction, did not account for the intended THV to be implanted.
We aimed to develop and validate a multivariable prediction model for TAVR-related coronary obstruction in native aortic stenosis based on sophisticated contemporary and novel multidimensional assessments on pre-procedure multidetector CT.
METHODS
Sources of data
The CO-TAVR (Coronary Obstruction with Transcatheter Aortic Valve Replacement) and COBRA (Coronary Obstruction Risk Assessment) global registries were established specifically to answer this study question. Pre-procedure electrocardiogram (ECG)-gated multidetector CT and procedure fluoroscopy images of patients in whom TAVR caused coronary artery obstruction were collected.
A control group of patients who had TAVR without coronary obstruction was compiled from the MedStar Aortic Valve Database.
Study population
Patients were included if they developed angiographically confirmed obstruction to flow at the ostium or sinus of one or both coronary arteries after TAVR for native aortic stenosis. The obstruction had to be a result of TAVR and had to be confirmed angiographically with supporting clinical signs and symptoms prior to discharge from hospital. Patients who underwent VIV TAVR or those who did not have high-quality pre-procedure CT were excluded.
Patients in the control group were included if they underwent TAVR for native aortic stenosis without coronary obstruction. Patients in whom mitigating strategies such as snorkel stenting and BASILICA (bioprosthetic or native aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction) were used were excluded because these would confound the a priori risk of coronary obstruction.
Study oversight
This was an investigator-initiated multicenter study with central review of images. The MedStar Washington Hospital Center and Emory University Institutional Review Boards approved this investigation. Research on de-identified data was performed within the MedStar Heart and Vascular Institute and Emory University Hospitals. The participating centers approved transmission of de-identified images, waiver of consent, and transmission of de-identified electronic data collection forms.
Outcome assessment
Fluoroscopy images were reviewed at central laboratories at MedStar and Emory to confirm coronary artery obstruction and determine whether obstruction was at the level of the coronary ostium or at the STJ. Site investigators reported on hemodynamic, ECG, echocardiographic, and biomarker evidence of coronary obstruction via an online data collection form.
CT measurements
Multidetector CT images from the CO-TAVR and COBRA databases were analyzed at central laboratories at MedStar and Emory, and standard measurements were made of the annulus and aortic root dimensions. Coronary artery height was measured as the vertical distance from the annulus to the bottom of the coronary ostium. Residual sinus width was calculated by subtracting the sinus width from the nominal valve size for balloon-expandable and mechanically expandable valves and from the waist diameter for self-expanding valves. Waist diameter was chosen because it better correlates with CT measurements of post-TAVR VTC distance(3). These standardized measurements were also available for the large control group of patients without coronary obstruction from the MedStar Aortic Valve Database.
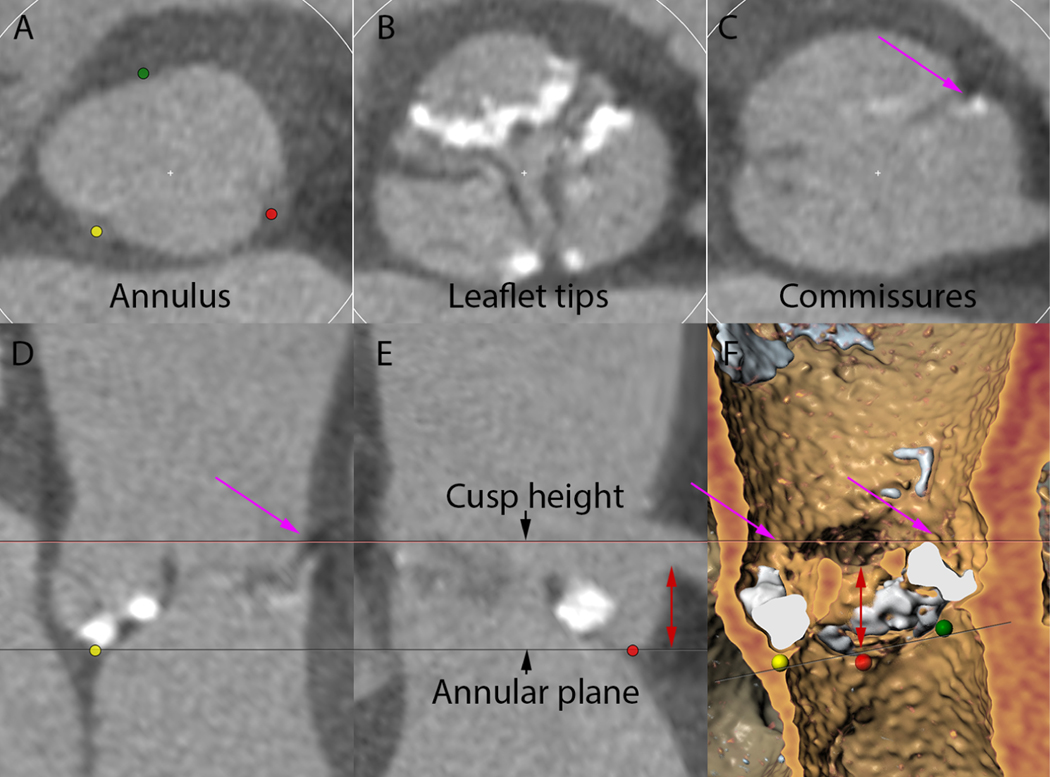
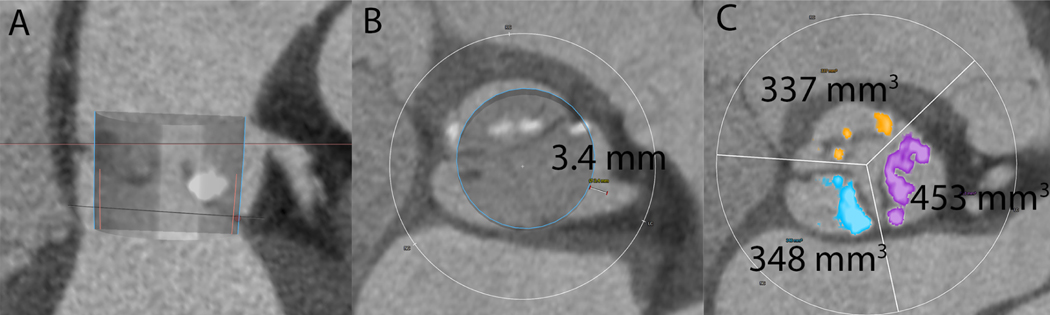
Specialized measurements using dedicated software (3mensio) included VTC, virtual THV-to-STJ distance (VTSTJ), calcium volume per leaflet, cusp height, and leaflet length (Figures 1 and 2). Calcium volume measurements represented the actual volume of calcium on the leaflet, unlike the Agatston score, which represents both attenuation and size. The Hounsfield Unit threshold for distinguishing calcium from luminal contrast was automatically determined by the software and adjusted by the user as needed. The novel parameter of cusp height was measured as the vertical distance from the annular plane to the top of the cusp commissural attachment.
Figure 1. CT analysis for aortic cusp height.
A-C demonstrates the relationship of the aortic cusps and coronary arteries in cross section at the level of the aortic annulus (A), leaflet tips (B), and top of the commissures (C). D-E is a “stretched” vessel view constructed around the aortic root centerline demonstrating top of the cusp commissure in long axis (D) and then rotated to show the relation to the coronary artery (E). F is a three-dimensional reconstruction showing the relation of the left cusp height to the left coronary artery. Red dot = nadir of left coronary cusp; green dot = nadir of right coronary cusp; yellow dot = nadir of non-coronary cusp; magenta arrow = top of left cusp; red double-headed arrow = coronary artery height. CT = computed tomography.
Figure 2. CT analysis for VTC and leaflet calcium volume.
Panel A is a multiplanar reconstruction of the aortic root with a virtual Sapien 3 valve simulated in position. The blue outline represents the valve and the pink outline represents the valve skirt. B demonstrates the virtual valve to coronary distance (VTC) measurement from the edge of the virtual valve to the left coronary ostium. Panel C demonstrates left (purple), right (orange), and non (blue) aortic leaflet calcium volume measurements.
Anatomical predictors
Aortic annulus and root measurements between obstructed and non-obstructed cohorts were compared. Predictors of obstruction were determined by multivariate regression, and optimal thresholds were defined.
Multivariate model development
A multivariate prediction model was developed to improve prediction of coronary obstruction. The model used a novel measure of aortic cusp height. This was defined as the vertical distance from the nadir of the aortic cusp to the top of the leaflet commissural attachments (Figure 1). This measure is likely more reproducible than leaflet length and may more accurately predict the level of the native leaflets after TAVR with respect to the coronary arteries and STJ. The cusp height likely approximates the upper limit of leaflet extension, and therefore, we hypothesize that the cusp height must be greater than the coronary artery height for TAVR-related obstruction to occur.
Secondly, the extent of lateral leaflet displacement toward the coronaries depends on the width of the sinus of Valsava, as well as the type and size of THV. This can be estimated by calculating residual sinus width, by subtracting the THV diameter from the sinus of Valsava width. However, this calculation makes geometric assumptions of the aortic root and further assumptions on the positioning of the THV in the case of self-expanding valves. A more accurate measure may be VTC, determined by simulating a virtual valve in the aortic root and measuring the distance from the valve edge to the coronary ostium (Figure 2). For VIV TAVR, a VTC ≤ 4 mm predicted increased risk of coronary obstruction(2). An optimal cutoff for VTC was derived for native aortic stenosis from these data and imputed into the model.
In addition, VTC may underestimate the risk of coronary obstruction in the presence of extremely bulky leaflet calcification. A calcium volume threshold for coronary obstruction risk has not been described in the literature. Therefore, data from these registries were used to define an optimal calcium volume per leaflet cutoff that would predict coronary obstruction and imputed into the prediction model.
Multivariate model validation
Specialized measurements, including cusp height, VTC, and calcium volume, were performed in a subgroup of 60 patients without coronary obstruction. Patients in this group were propensity-score matched for age, sex, and annulus area. Annulus area was not a predictor for coronary obstruction by multivariate regression analysis but was significantly smaller in the obstruction cohort; therefore, we chose to match for annulus area to enrich the subgroup.
Statistical analysis
Categorical variables were expressed in counts and percentages. Continuous variables were expressed as mean ± SD. Categorical variables were compared using the χ2 test, and continuous variables were compared using Student’s t-test. Logistic regressions are built with respect to coronary obstruction. Odds ratio estimates and confidence intervals, receiver operating characteristic (ROC) curve and area under the curve (AUC), and the optimal cutoff points and their sensitivity and specificity, as well as their prediction plots, are presented for univariate models. To account for the dependence among coronary artery height, sinus width, and annulus area in modeling probability of obstruction, principal factor analysis is conducted and compared to the corresponding multivariate model. Propensity score is estimated using sex, age, and annulus area. Matching is performed via nearest-neighbor matching, with a caliper width of 0.5 and controlling for sex. A p-value below 0.05 was considered significant. All analyses were conducted using SAS 9.2 (SAS Institute, Cary, NC, USA).
Data are presented in accordance with the Transparent Reporting of a Multivariable Prediction Model of Individual Prognosis or Diagnosis (TRIPOD) guidelines(4).
RESULTS
The Central Illustration summarizes the study results.
Central Illustration.
Development and validation of a multivariate prediction model for coronary artery obstruction after TAVR in native AS.
Participants
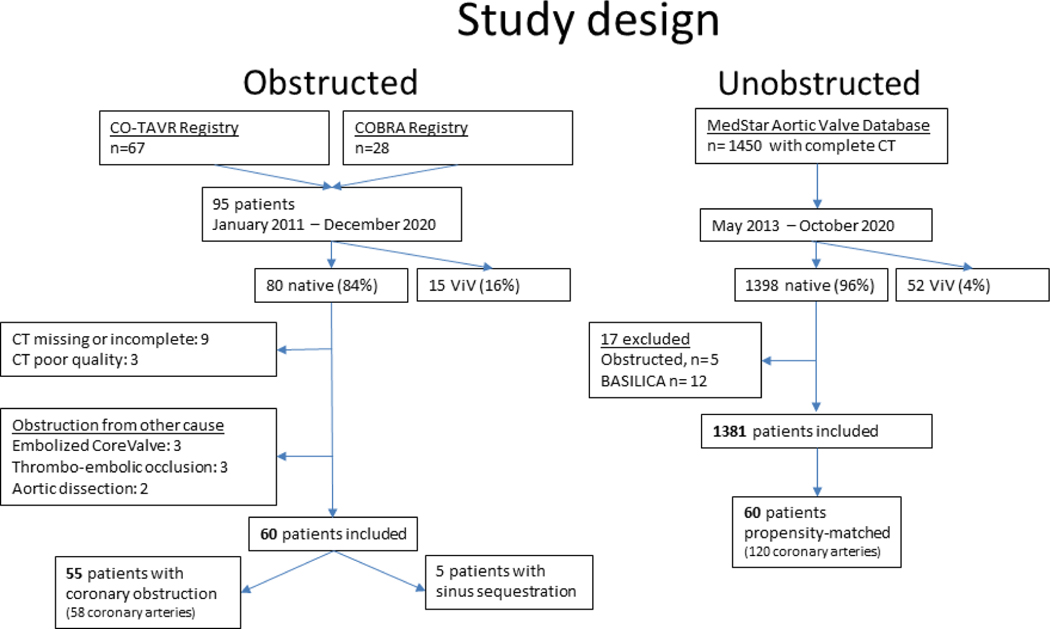
Twenty-five centers from 12 countries enrolled 95 patients in the CO-TAVR and COBRA registries (Supplemental Table 1). Figure 3 shows the flow of participants in the study in the obstruction and control arms.
Figure 3. Study design.
Flow chart of patients included in both arms of the study.
Table 1 shows the baseline patient characteristics. There was a trend toward more females in the obstruction cohort, with no difference in THV type or size between the obstructed and unobstructed cohorts.
Table 1.
Baseline characteristics
| Obstruction (n=60) | No obstruction (n=1,381) | p-value | |
|---|---|---|---|
| Female | 35 (58.3%) | 650 (47.1%) | 0.09 |
| Age (years) | 79.6 ± 8.1 | 79.3 ± 10 | 0.75 |
| Balloon-expandable THV | 28 (46.6%) | 601 (43.5%) | 0.82 |
| Self-expanding THV | 28 (46.6%) | 725 (52.5%) | 0.53 |
| Mechanically expanding THV | 4 (6.9%) | 55 (4%) | 0.30 |
| THV nominal size (mm) | 24.6 ± 2.2 | 24.4 ± 2.4 | 0.65 |
Reported as n (%) or mean ± SD. THV = transcatheter heart valve
Coronary obstruction outcomes
Table 2 shows the distribution in site of coronary obstruction, with the majority occurring at the left coronary artery, at the level of the coronary ostium. In-hospital death was 26.7% in the obstructed cohort compared to 0.7% in the non-obstructed cohort (p<0.001).
Table 2.
Site of obstruction
| Coronary artery obstructed | Per-patient analysis (n=60) |
|---|---|
|
| |
| Left | 47 (78.3%) |
| Right | 10 (16.7%) |
| Both | 3 (5%) |
|
| |
| Level of obstruction | Per-vessel analysis (n=63) |
|
| |
| Coronary ostium | 58 (92.1%) |
| STJ | 5 (7.9%) |
STJ = sinotubular junction
Aortic root anatomy
Table 3 shows the anatomical aortic root measurement differences between the obstructed and unobstructed cohorts. Annular area and perimeter, coronary height, sinus width, and STJ height and width were all significantly smaller in the obstructed cohort. Residual sinus diameter, measured by subtracting the THV diameter from the sinus width, was also significantly smaller in the obstructed cohort.
Table 3.
Aortic root measurements
| Obstruction (n=60) | No obstruction (n=1,381) | p-value | |
|---|---|---|---|
| Annulus area (mm2) | 415 ± 89 | 468 ± 103 | <0.001 |
| Annulus perimeter (mm) | 70.6 ± 13.7 | 77 ± 8.1 | <0.001 |
| Coronary artery height (left) (mm) | 10.8 ± 3.3 | 13.1 ± 3 | <0.001 |
| Coronary artery height (right) (mm) | 12.4 ± 1.7 | 15.5 ± 3.4 | <0.001 |
| Sinus diameter (left) (mm) | 29.8 ± 3.4 | 32.7 ± 4.1 | <0.001 |
| Sinus diameter (right) (mm) | 26.3 ± 2.6 | 31.1 ± 4 | <0.001 |
| STJ height left (mm) | 17.5 ± 2.9 | 21.9 ± 4.2 | <0.001 |
| STJ height right (mm) | 26.2 ± 3.1 | 22.4 ± 4.2 | <0.001 |
| STJ diameter (mm) | 26.2 ± 3.1 | 29.6 ± 3.7 | <0.001 |
| Left Sinus – THV diameter (mm) | 5 ± 3.2 | 8.2 ± 3.7 | <0.001 |
| Right Sinus – THV diameter (mm) | 2.7 ± 3.5 | 6.6 ± 3.7 | <0.001 |
THV = transcatheter heart valve; STJ = sinotubular junction
Only 5 patients had obstruction from sinus sequestration. They had a median VTSTJ of 0.9 mm (range 0–2.2 mm) and the median height from the top of the aortic cusp to the STJ was 0.5 mm (range 0–0.7 mm). Because of small numbers, and to avoid confounding from a different mechanism of obstruction, these patients were excluded from further analysis.
In a multivariate regression model, reduced coronary artery height and reduced sinus width increased the odds of coronary obstruction, but annulus area did not demonstrate a significant impact (Table 4). A primary component analysis of these three variables was also significant.
Table 4.
Multivariate regression and primary component analysis
| Odds ratio for left obstruction | Odds ratio for right obstruction | |||
|---|---|---|---|---|
| Coronary height (mm) | 0.79 (0.70–0.88) | p<0.0001 | 0.80 (0.64–0.99) | p=0.039 |
| Sinus width (mm) | 0.86 (0.78–0.94) | p=0.001 | 0.82 (0.71–0.94) | p=0.005 |
| Annulus area (mm2) | 0.98 (0.85 – 1.12) | p=0.72 | 0.90 (0.74 – 1.11) | p=0.32 |
| Primary component analysis | 0.86 (0.81–0.91) | p<0.0001 | 0.83 (0.73 – 0.89) | p<0.0001 |
Odds ratios are calculated for 1 unit (mm or mm2, respectively) increments of the continuous variables
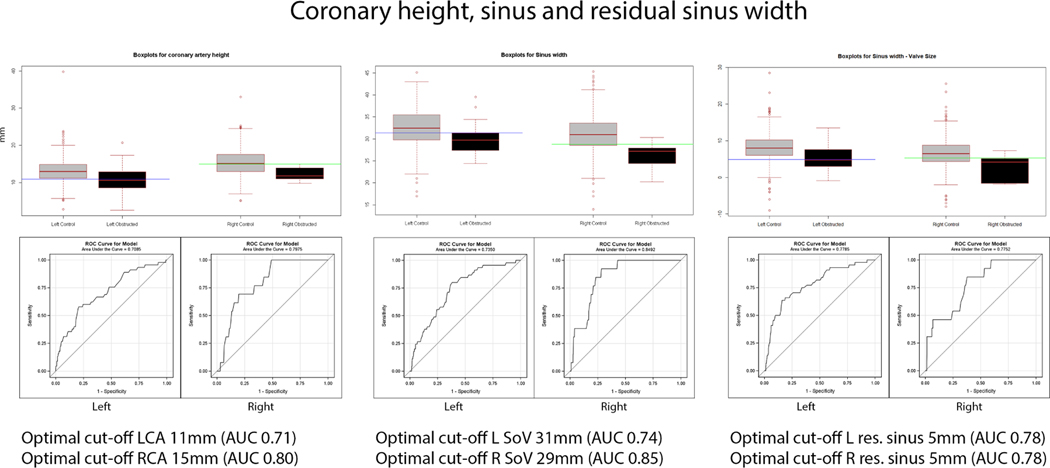
The optimal threshold for coronary artery height was <11 mm for the left (sensitivity 0.58, specificity 0.79) and <15 mm for the right (sensitivity 1, specificity 0.51); for sinus width, it was <31 mm for the left (sensitivity 0.8, specificity 0.61) and <29 mm for the right (sensitivity 0.92, specificity 0.71); and for residual sinus width, it was <5 mm for both left (sensitivity 0.7, specificity 0.64) and right (sensitivity 0.85, specificity 0.6). The ROC curves are shown in Figure 4.
Figure 4. Coronary height, sinus and residual sinus width.
Box and whiskers plots for coronary height, sinus width, and residual sinus volume, with the box encompassing the interquartile range, the centerline within the box representing the median, and the whiskers representing 1.5x the interquartile range and extremes plotted outside these boundaries. Optimal cutoffs and ROC curves for coronary height, sinus width, and residual sinus width are illustrated.
Multivariate prediction model specification
A multivariate model was developed using cusp height, VTC, and culprit leaflet calcium volume. Optimal thresholds in the model were 4 mm for VTC and 600 mm3 for leaflet calcium volume.
The cusp height was greater than the coronary height in 55/58 (95%) of cases (Supplemental Figure 1).
The VTC was >4 mm in 14/58 (24%) arteries where coronary ostial obstruction was evident. These cases would be missed if VTC alone was used in the prediction model. However, the majority of these obstructing leaflets had an extremely high calcium volume (>600 mm3) (Supplemental Figure 2). Therefore, adding calcium volume to the model significantly improved model sensitivity.
As a result, a multivariate model was defined as predicting coronary obstruction if cusp height was greater than coronary artery height AND VTC ≤4 mm OR culprit leaflet calcium volume >600 mm3.
Model performance
Comparison with the enriched 1:1 subgroup of unobstructed patients is shown in Supplemental Table 2. Univariate logistic regression for the individual model parameters is presented for left and right coronary arteries in Supplemental Table 3 with ROC curves in Supplemental Figure 3.
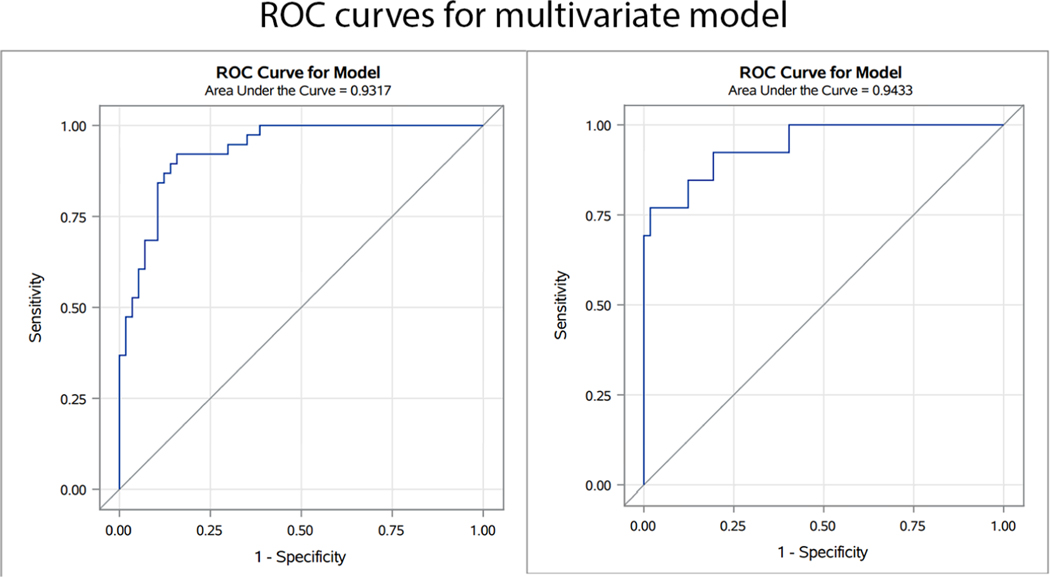
The multivariate regression model for left and right coronary arteries is presented in Table 5 and ROC curves in Figure 5. The combined model performed well, with an AUC of 0.93 for the left coronary artery and 0.94 for the right. Using the optimal cutoffs as defined, coronary artery obstruction could be predicted with a sensitivity of 0.93 and specificity of 0.84 for the left coronary artery and sensitivity of 0.92 and specificity of 0.96 for the right.
Table 5.
Multivariate logistic model
| Left coronary | Odds ratio | 95% CI | p-value |
|---|---|---|---|
| Cusp height – coronary height (mm) | 1.95 | 1.38–2.75 | 0.0002 |
| VTC (mm) | 0.27 | 0.15–0.50 | <0.0001 |
| Leaflet calcium volume (mm3) | 1.01 | 1.00–1.01 | 0.0009 |
| Right coronary | Odds ratio | 95% CI | p-value |
| Cusp height – coronary height (mm) | 2.25 | 1.34–3.76 | 0.002 |
| VTC (mm) | 0.26 | 0.11–0.61 | 0.002 |
| Leaflet calcium volume (mm3) | 1.00 | 1.00–1.01 | 0.04 |
Odds ratios are calculated for 1 unit (mm or mm3, respectively) increments of the continuous variables
VTC = virtual transcatheter heart valve to coronary distance
Figure 5. ROC curves for the multivariate prediction model.
ROC curves demonstrate a good fit for both left and right coronary artery obstruction.
This model outperformed the equivalent model with cusp height substituted for leaflet length (Supplemental Table 4).
DISCUSSION
This study comprised the largest CT dataset comparing patients with and without TAVR-related coronary artery obstruction. The key findings are: (1) TAVR-related coronary obstruction in native aortic stenosis occurred more commonly at the left coronary artery, and the primary mechanism was the native leaflet obstructing the coronary ostium; (2) Low coronary artery height and narrow sinus width were associated with increased risk for coronary artery obstruction in a multivariate logistic regression analysis but were poor predictors of coronary artery obstruction; (3) The novel prediction algorithm combining cusp height, coronary artery height, VTC, and leaflet calcium volume performed well in predicting coronary artery obstruction; and (4) Optimal cutoffs for VTC and leaflet calcium volume were 4 mm and 600 mm3, respectively.
Previous multicenter registries found female sex, low left coronary artery height (<12 mm), narrow left sinus (<30 mm), VIV procedures, and a VTC <4 mm in the setting of VIV TAVR to be associated with increased coronary obstruction and associated mortality(1,2). A multicenter registry looking at delayed coronary obstruction found an association with VIV procedures, use of self-expanding valves, narrow sinuses (<30 mm), and a small residual sinus (<3 mm)(5).
This study confirmed that coronary artery height and sinus width were strongly correlated with increased risk of obstruction and demonstrated the association for both left and right coronary arteries. The study also found residual sinus width to be strongly correlated with acute coronary obstruction after TAVR. Smaller annulus area was associated with coronary obstruction but was not found to be a significant predictor after regression analysis. New cutoffs for coronary artery height, sinus width, and residual sinus width were defined for left and right coronary obstruction risk. However, these measurements still performed poorly in predicting coronary artery obstruction risk in native AS. Therefore, coronary artery height and sinus width should not be used in isolation to estimate the risk of coronary artery obstruction.
In addition, a low STJ height and narrow STJ diameter were also associated with increased risk of coronary obstruction. These patients were found to have a short distance from the aortic cusp summit to the STJ and a small VTSTJ.
Previous studies have not found an association between leaflet length and coronary obstruction in native aortic stenosis(1). Cusp height may be a more reproducible measure and may correlate better with leaflet upper extension post-TAVR. The hypothesis that a higher cusp height than coronary artery height is required for coronary obstruction was supported by this analysis.
The study suggests that accounting for extreme leaflet calcification (>600 mm3 on the left or right leaflet) may capture cases at risk of coronary obstruction that would otherwise be missed by measuring VTC alone.
A simple multivariate prediction model was developed using aortic cusp height and coronary artery height, VTC, and leaflet calcium volume. The model performed well in predicting coronary artery obstruction and is simple to implement in real-world practice.
Implications
This study demonstrated a 26% absolute increase in in-hospital mortality in patients who developed coronary artery obstruction despite attempted bailout. Accurate screening for TAVR-related coronary obstruction is paramount so patients at risk undergo either surgical aortic valve replacement (SAVR) or transcatheter preventative strategies with BASILICA or snorkel stenting(3,6–8). Furthermore, several dedicated devices are in the pipeline and this study should inform US Food and Drug Administration benchmarks for these new devices.
This algorithm is easy to implement in routine clinical practice. The first-line assessment for coronary obstruction risk should be to determine the relationship between aortic cusp height and coronary artery height. If the commissural attachment of the aortic cusp is higher than the bottom of the coronary artery, then an assessment of VTC and leaflet calcium volume should be made. If the VTC ≤4 mm or culprit leaflet calcium volume >600 mm3, then the patient should be considered at high risk of coronary obstruction with TAVR. In such cases, either surgery or BASILICA should be considered. Snorkel stenting may be considered in palliative cases when the risk of stent thrombosis and lack of future coronary access may be acceptable.
Limitations
The VTC and calcium volume cutoffs used in this model were generated using the obstruction dataset. These data were also used for multivariate model validation, increasing the risk of overfitting and possible optimistic estimate of performance. Therefore, the model could be further improved with external validation. Calcium volume was calculated using contrast-enhanced CT, and these measurements are less comparable than non-contrast CT (Agatston Units). There is a potential for bias, as the highest-risk patients may have undergone BASILICA or snorkel stenting and were excluded from this analysis.
Propensity-score matching was performed using three key variables to define a subset of unobstructed patients in whom detailed anatomical measurements could be made, but there remains a risk of undetected mismatch between groups.
CONCLUSIONS
A new prediction model for TAVR-related coronary obstruction in native aortic stenosis was developed and validated. This model outperformed existing standard measurements. This model can be used in clinical practice to determine coronary obstruction risk and guide the decision between SAVR and TAVR and the use of adjunctive therapies for coronary protection. Furthermore, this study may inform the benchmark for approval of new dedicated devices.
Supplementary Material
CLINICAL PERSPECTIVE.
What is known?
Coronary artery height and sinus width have poor sensitivity and specificity for predicting coronary obstruction after transcatheter aortic valve replacement (TAVR).
What is new?
New cutoffs for computed tomography (CT) anatomical measures for coronary obstruction in native aortic stenosis are needed.
A novel prediction model using cusp height, virtual transcatheter heart valve to coronary distance, and leaflet calcium volume performs well in predicting coronary obstruction.
What is next?
This novel and simple CT-based prediction algorithm could be adopted routinely in clinical practice to improve coronary obstruction risk stratification in TAVR.
ACKNOWLEDGEMENTS:
Jason Wermers, MedStar Cardiovascular Research Network, for assistance in preparing the manuscript and Megan Rowland, MedStar Cardiovascular Research Network, for administrative assistance for the study.
FUNDING:
Research grant from Medtronic to MedStar Cardiovascular Research Network and MedStar Health.
RELATIONSHIPS WITH INDUSTRY:
Norihiko Kamioka – Proctor for Edwards Lifesciences.
Sebastian Ludwig was supported by a grant from the German Heart Foundation (DHS) and has received travel compensation from Edwards Lifesciences.
Dr. Sinning has received speaker honoraria and research grants from Abbott, Abiomed, Medtronic, Boston Scientific, and Edwards Lifesciences and is proctor for Medtronic and Boston Scientific.
Yasushi Fuku – Proctor for Edwards and Medtronic.
Vijay S. Iyer – Proctor: Edwards Lifesciences, Medtronic, and Boston Scientific.
Roberto Rodriguez – Speaker for Medtronic, Abbott, and Edwards.
Matteo Montorfano: Proctor for Edwards Lifesciences, Boston Scientific, and Abbott.
Marco B. Ancona: Received consultant fees from Abbott and Abiomed.
Hasan Ahmad – Proctor for Edwards Lifesciences.
Nicholas J. Ruggiero – Abbott: Proctor, Consultant; Ancora Heart: Research Support; Bard: Research Support; Edwards: Proctor, Consultant; RegenX-Bio: Consultant.
Gaby Weissman – No personal disclosures. Director of an academic cardiac computed tomography core lab with institutional contracts with Ancora Heart and LivaNova.
Ron Waksman reports serving on the advisory boards of Abbott Vascular, Boston Scientific, Medtronic, Philips IGT, and Pi-Cardia Ltd.; being a consultant for Abbott Vascular, Biotronik, Boston Scientific, Cordis, Medtronic, Philips IGT, Pi-Cardia Ltd., Swiss Interventional Systems/SIS Medical AG, Transmural Systems Inc., and Venous MedTech; receiving institutional grant support from Amgen, Biotronik, Boston Scientific, Chiesi, Medtronic, and Philips IGT; and being an investor in MedAlliance and Transmural Systems Inc.
Toby Rogers being a proctor and consultant for Boston Scientific, Edwards Lifesciences, and Medtronic; serving on the Advisory Board of Medtronic; and holding equity interest in Transmural Systems Inc.
ABBREVIATIONS:
- BASILICA
Bioprosthetic or native Aortic Scallop Intentional Laceration to prevent Iatrogenic Coronary Artery Obstruction
- COBRA
Coronary Obstruction Risk Assessment
- CO-TAVR
Coronary Obstruction with Transcatheter Aortic Valve Replacement
- CT
computed tomography
- STJ
sinotubular junction
- TAVR
transcatheter aortic valve replacement
- THV
transcatheter heart valve
- VIV
valve-in-valve
- VTC
virtual transcatheter heart valve to coronary distance
- VTSTJ
virtual transcatheter heart valve to sinotubular junction distance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ribeiro HB, Webb JG, Makkar RR et al. Predictive factors, management, and clinical outcomes of coronary obstruction following transcatheter aortic valve implantation: insights from a large multicenter registry. J Am Coll Cardiol 2013;62:1552–62. [DOI] [PubMed] [Google Scholar]
- 2.Ribeiro HB, Rodes-Cabau J, Blanke P et al. Incidence, predictors, and clinical outcomes of coronary obstruction following transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves: insights from the VIVID registry. Eur Heart J 2018;39:687–695. [DOI] [PubMed] [Google Scholar]
- 3.Khan JM, Greenbaum AB, Babaliaros VC et al. The BASILICA Trial: Prospective Multicenter Investigation of Intentional Leaflet Laceration to Prevent TAVR Coronary Obstruction. JACC Cardiovasc Interv 2019;12:1240–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collins GS, Reitsma JB, Altman DG, Moons KG, TRIPOD Group. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. The TRIPOD Group. Circulation 2015;131:211–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jabbour RJ, Tanaka A, Finkelstein A et al. Delayed Coronary Obstruction After Transcatheter Aortic Valve Replacement. J Am Coll Cardiol 2018;71:1513–1524. [DOI] [PubMed] [Google Scholar]
- 6.Mercanti F, Rosseel L, Neylon A et al. Chimney Stenting for Coronary Occlusion During TAVR: Insights From the Chimney Registry. JACC Cardiovasc Interv 2020;13:751–761. [DOI] [PubMed] [Google Scholar]
- 7.Khan JM, Babaliaros VC, Greenbaum AB et al. Preventing Coronary Obstruction During Transcatheter Aortic Valve Replacement: Results From the Multicenter International BASILICA Registry. JACC Cardiovasc Interv 2021;14:941–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Westermann D, Ludwig S, Kalbacher D et al. Prevention of coronary obstruction in patients at risk undergoing transcatheter aortic valve implantation: the Hamburg BASILICA experience. Clin Res Cardiol 2021;110:1900–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.