Abstract
Wearable devices, such as smartwatches and activity trackers, are commonly used by patients in their everyday lives to manage their health and wellbeing. These devices collect and analyze long-term continuous data on measures of behavioral or physiologic function, which may provide clinicians with a more comprehensive view of a patients’ health compared to the traditional sporadic measures captured by office visits and hospitalizations. Wearable devices have a wide range of potential clinical applications ranging from arrhythmia screening of high-risk individuals to remote management of chronic conditions such as heart failure or peripheral artery disease. As the use of wearable devices continues to grow, we must adopt a multifaceted approach with collaboration amongst all key stakeholders to effectively and safely integrate these technologies into routine clinical practice. In this Review, we summarize the features of wearable devices and associated machine learning techniques. We describe key research studies that illustrate the role of wearable devices in the screening and management of cardiovascular conditions, and we identify directions for future research. Lastly, we highlight the challenges that are currently hindering the widespread use of wearable devices in cardiovascular medicine, and we provide short-term and long-term solutions to promote increased use of wearable devices in clinical care.
INTRODUCTION:
The evolution of digital health technologies has allowed individuals to assume an active role in the management of their cardiovascular health and facilitated increased patient-provider contact. Wearable devices are digital medicine tools that process data captured by mobile sensors to generate measures of behavioral and/or physiological function such as physical activity, heart rate, heart rhythm, and sleep 1. The wearable device market is now a multi-billion dollar industry 2. A large population survey indicates that many individuals use a wearable device to promote healthy behaviors or to ‘manage a diagnosed condition’ 3. While the clinical integration of wearable devices remains in the earliest stages, their use has the potential to broadly impact cardiovascular medicine through lifestyle modifications for primary prevention, arrhythmia screening of at-risk individuals, and remote management of patients with established heart failure (HF) or peripheral artery disease (PAD), among other chronic cardiovascular conditions. There are several factors limiting the clinical use of wearables including concerns about data privacy, device accuracy, regulatory and reimbursement policies, and lack of dedicated clinical staff to monitor, interpret, and respond to the vast amounts of data generated by devices. Our work aims to provide an overview of and expand upon the previously published reviews on wearable devices in cardiovascular medicine 3–7. In this Review, we describe the features of consumer-grade, medical-grade, and research grade devices, the analytical machine learning techniques that augment the clinical utility of wearable devices, the role of wearables in the diagnosis and management of common cardiac conditions, and the challenges that are limiting more widespread clinical adoption of wearables. Lastly, we propose a short-term and long-term roadmap to maximize the value of wearable devices in clinical care and management of cardiovascular disease.
FEATURES OF WEARABLE DEVICES:
Overview of Wearable Devices:
Wearable devices have several forms including: smartwatches, bands, patches, rings, medical ear buds, and clothing-embedded devices. They use motion and biometric sensors to capture several physiologic parameters including step count, activity intensity, heart rate, heart rhythm, blood pressure (BP), oxygen saturation (SpO2), sleep, maximum oxygen uptake (VO2 max), and temperature. As illustrated in Figure 1, wearable devices have many potential clinical applications to enhance the screening and management of cardiovascular conditions.
Figure 1: Overview of Wearable Devices.
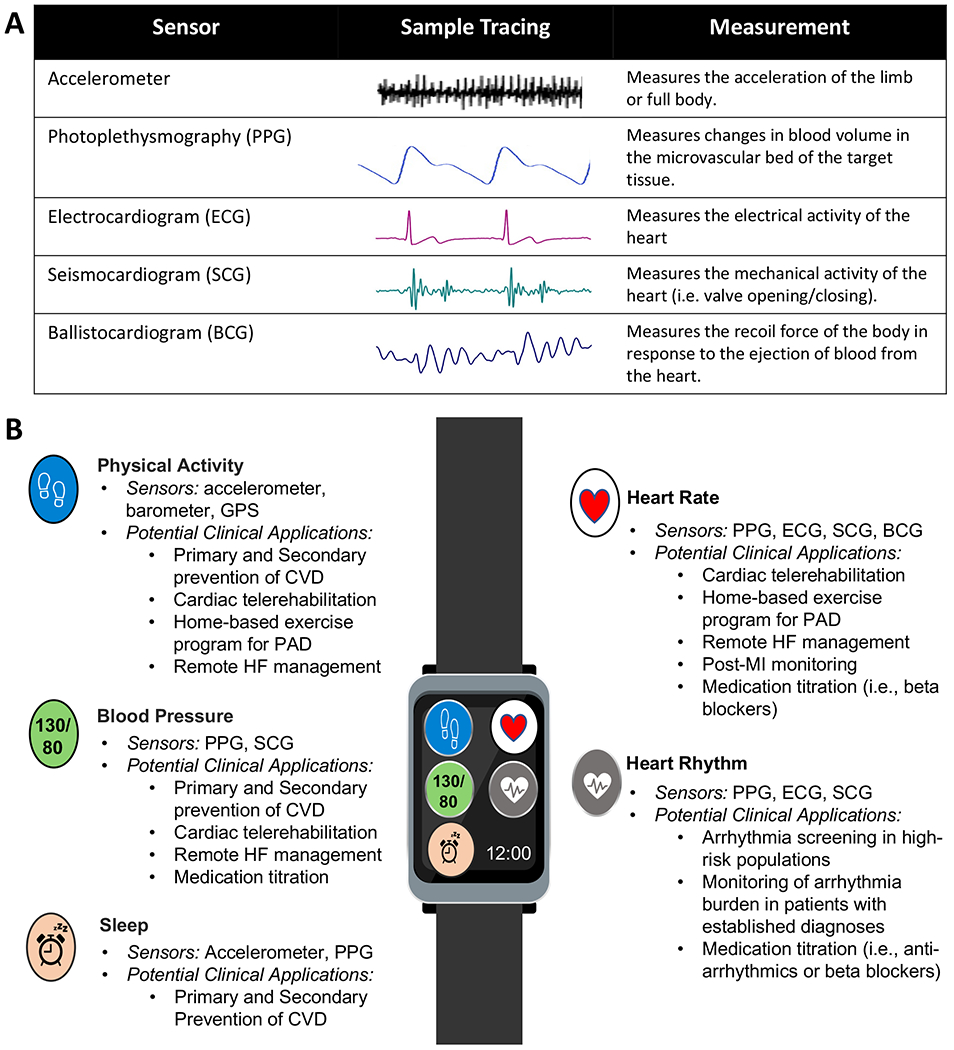
A) Common sensor modalities utilized by wearable devices to generate physiologic measurements. B) Key physiologic measures, sensors, and potential clinical applications in cardiovascular medicine. Abbreviations: ballistocardiogram (BCG), cardiovascular disease (CVD), electrocardiogram (ECG), Global Positioning System (GPS), heart failure (HF), myocardial infarction (MI), peripheral artery disease (PAD), photoplethysmography (PPG), seismocardiogram (SCG).
* Continuous glucose monitoring (CGM) devices use biochemical sensors to measure glucose levels and have important clinical applications for type 1 and type 2 diabetes. However, they are difficult to integrate into consumer-grade wearables and are usually a stand-alone device.
Wearables may be divided into consumer-grade, medical-grade, or research-grade devices. A consumer-grade wearable is available on the market, advertised toward the broader population, and intended to function for fitness, wellbeing, or entertainment purposes. A research-grade wearable may or may not be available on the market, is targeted for research scientists, and is intended to collect data for research purposes. A medical grade wearable is available on the market, but often only accessible with a prescription from a clinician, is advertised toward patients and clinicians, and intended for medical applications. Importantly, with our above definitions, the designation of a wearable may change depending on the context of use. This is particularly true for wearables with intended use for health-related measurements, where the line between consumer, medical, and research uses can become blurred. An example of this is the Fitbit smartwatch, which has been used frequently in clinical trials, medical, and consumer contexts, for each context falling under our designation of research-, medical-, and consumer-grade wearables, respectively. Another aspect of medical-grade devices is that they fall under FDA oversight. A single wearable may have multiple functions, some of which fall under regulatory oversight and others of which do not, making it difficult to classify the entire device into just one category (for example, irregular heart rhythm detection versus SpO2 measurement, which are regulated differently but available together on multiple devices at the time of writing). Thus, as the field evolves, it may become appropriate to rethink the nomenclature to classify wearables.
Sensor Technology:
This section highlights the common sensing modalities utilized in wearable devices as well as some novel sensing modalities that have demonstrated potential in this space (Figure 1).
Accelerometry:
Accelerometers are microelectromechanical system (MEMS) sensors that measure the acceleration of an object through capacitive, piezoresistive, and piezoelectric effects 8. Accelerometers are widely used in wearable devices to measure the volume and intensity of physical activity and energy expenditure 8. Accelerometers can be placed in various locations of the body (i.e. torso, arm, ankle, etc.), but most smartwatches and activity trackers use the wrist position due to user comfort.
Photoplethysmogram:
The photoplethysmogram (PPG) signal is an optical measurement of the changes in blood volume in the microvascular tissue bed, which can be measured by shining a light on the skin and collecting the reflected or transmitted wave of the light with a photodetector 9. PPG is widely used in wearable devices to track heart rate, heart rhythm, and pulse oximetry 10. PPG may ultimately have other healthcare applications including monitoring blood pressure and vascular aging 11.
Electrocardiogram:
The electrocardiogram (ECG) signal captures the propagation of electrical action potentials in the heart. The ECG measures the difference in electrical potential between various points on the body with skin-mounted electrodes and depicts the depolarization and repolarization of the heart 12. The ECG can be used to monitor heart rate, detect arrhythmias, and identify myocardial ischemia or infarction, among other applications. Wearable devices commonly use a patch for arrhythmia detection in the outpatient setting 13.
Seismocardiogram:
The seismocardiogram (SCG) signal is the local vibration of the chest wall that occurs with every cardiac cycle including: movement of the heart, opening and closing of the heart valves, and movement of blood in the aorta. SCG can be recorded using an accelerometer or gyroscope placed on the mid-sternum 14. Multiple studies have demonstrated the potential for SCG to estimate cardiac time intervals14, changes in hemodynamics14, and characterize whether heart failure patients are compensated or decompensated15.
Ballistocardiogram:
The ballistocardiogram (BCG) signal is a measurement of the recoil forces of the body in response to the cardiac ejection of blood into the vasculature. BCG is generally recorded in the longitudinal (head-to-foot) direction using a modified weighing scale, bed or table-based systems14. Researchers have demonstrated the potential for BCG to estimate heart rate14, and assess the clinical state of patients with HF 9.
Continuous Glucose Monitoring:
Continuous glucose monitoring (CGM) is a method of measuring glucose levels in the body in real-time through a small sensor under the skin, typically on the abdomen or arm16. The sensor measures the glucose levels in interstitial fluid, which is connected to a transmitter that sends the glucose readings to a monitoring device or mobile phone. Users can track their glucose over time and receive alerts when their glucose levels are too high or too low, which can help to prevent dangerous complications such as hypoglycemia or hyperglycemia.
Artificial Intelligence:
The enormous volume of information from wearable devices requires intelligent algorithms and computational power to generate meaningful information. Machine learning (ML) is a method of data analysis that automates analytical model building, which evolved to recognize patterns in data without explicitly programming for particular tasks. The advantages of ML are adaptability, scalability, automation, and the ability to handle multidimensional and multivariate data. ML can be divided into supervised, semi-supervised, unsupervised, and reinforcement learning (Table 1). In supervised learning, the outcome variable is known and the algorithm attempts to learn the relationship between the outcome variable (i.e. atrial fibrillation) and the estimating variables (i.e. raw ECG data and summary ECG metrics) using parametric and non-parametric algorithms. Supervised learning can be further classified for the intended tasks: regression (where the target variable is continuous, i.e. heart rate), classification (where the target variable is discrete, i.e. arrhythmia existence or not; type of arrhythmia), and forecasting (predicting future outcomes based on the past and present data, i.e. prognosis in HF). Unsupervised learning is used to investigate groupings or patterns in the data where the label is not known, and semi-supervised learning is used when labels are known for only a portion of the data. Reinforcement learning algorithms focus on trial and error, where the model learns from past experiences and begins to adapt its approach in response to the situation to achieve the best possible result, which can be used for remote home monitoring and just-in-time adaptive interventions (JITAI).
Table 1:
Common Machine Learning Algorithms and Potential Clinical Applications in Cardiovascular Medicine.
| Type | Tasks | Algorithms | Clinical Utility | Potential Clinical Applications |
|---|---|---|---|---|
| Supervised Learning | Regression | • Linear Regression • Polynomial Regression • Support Vector Regression • Decision Tree Regression • Random Forest Regression • Lasso Regression • Ridge Regression • Boosting Algorithms |
Estimating a numeric and continuous clinical outcome | • Estimating cardiorespiratory fitness (VO2) 102 • Predicting measurements such as resting heart rate, skin temperature, fasting plasma glucose, Hemoglobin A1C. 103 |
| Classification | • Support Vector Machine • Logistic Regression • Random Forest • Boosting Algorithms (i.e. Gradient Boosting Machine, AdaBoost) • Deep Learning models (i.e. Deep Neural Network, Convolutional Neural Network) |
Estimating a discrete/binary clinical outcome | • Arrhythmia detection 104 • Assessment of LV function 105 • Compensated vs decompensated HF 9, 15, 102 |
|
| Forecasting | • Prophet • Recurrent Neural Network (i.e. Long-Short-Term Memory Network) |
Outcome prediction | • Prediction of CAD, HF, stroke, and arrhythmias 106 | |
| Semi-Supervised Learning | Classification where labels are only available for a portion of the data. | • Generative Adversarial Network • Long-Short-Term Memory Network |
Training on a small pool of data, which is labeled for use on a larger pool of data to label/cluster the unlabeled data. | • CVD risk prediction from wearable data combined with EHR data 107 |
| Unsupervised Learning | Clustering | • K-means Clustering • Spectral Clustering • Hierarchical Clustering • Gaussian Mixture Model |
Identifies groups/clusters in the data. It can be used as a preprocessing step for supervised learning. | • Outlier removal from wearable data108 |
| Dimension Reduction | • Principal Component Analysis • Isomap Embedding • Spectral Embedding • Locally Linear Embedding • T-Distributed Stochastic Neighbor Embedding • Multidimensional Scaling |
Visualizing multimodal wearable features in a low dimensional space | Visualizing wearable data against outcome variable such as SCG and BCG trends with changes in cardiac contractility due to exercise. 109 | |
| Reinforcement Learning | Automate decision-making | • Deep Q Networks | Personalized Interventions | Remote monitoring and personalized management of comorbidities (i.e. type 2 diabetes), which may include just-in-time adaptive interventions 110. |
Abbreviations: ballistocardiography (BCG), coronary artery disease (CAD), cardiovascular disease (CVD), electronic health record (EHR), heart failure (HF), left ventricle (LV), myocardial infarction (MI), seismocardiography (SCG).
Deep learning (DL) models, a branch of supervised ML, have become increasingly popular in recent years and have achieved excellent performance in many biomedical applications, particularly in detecting and predicting cardiovascular diseases such as arrhythmias, myocardial infarction (MI), HF, and coronary artery disease (CAD) 17. As opposed to more traditional ML models (i.e. random forest, support vector machine), DL models are more complex in nature. Although DL models have demonstrated superior performance in cardiovascular disease detection, these models cannot easily learn from small datasets. More importantly, they require intelligent design and often result in very opaque algorithms that are challenging to interpret and have a higher chance of overfitting. In addition, these algorithms learn and save millions of parameters, which requires extensive computational power to train the models. As such, DL models are difficult to deploy on wearable hardware, which are usually very limited in computation power and storage space.
Traditional ML and DL algorithms can reduce noise in the data, particularly wearable data (i.e. motion artifact, intra- and inter-subject variabilities), and extract relevant information from high-dimensional data. Representation learning is a method used in ML/DL to extract useful information from raw data18. The goal of representation learning is to find a compact and informative representation of the data, in a way that captures the most important information and discards the noise or irrelevant information. This can be done by training a neural network to learn a set of features from the data, or by using unsupervised learning techniques such as clustering or dimensionality reduction. Once the data has been transformed into a more useful representation, it can be used for various tasks such as classification, regression, or generation. Representation learning is particularly useful when the data is high-dimensional, unstructured, or difficult to understand, as it can make it easier to extract insights or make predictions.
WEARABLES IN CLINICAL CARE
This is not designed as a systematic review. Instead, the aim of this section is to highlight the pivotal clinical trials or novel proof-of-concept studies on the use of wearable devices in the screening and management of cardiovascular conditions (Table 2). Primary literature was acquired by searching for articles with keywords such as wearables, smartwatch, mHealth, telemonitoring, etc. The referenced articles are not exhaustive, but were selected based on the level of evidence or number of citations with some additional studies included at the authors’ discretion to illustrate findings that may help inform future directions.
Table 2:
Selected Clinical Studies of Cardiovascular Management with Wearable Devices
| Study (year) | Study Design (n) | Wearable Device | Measurement | Major Findings |
|---|---|---|---|---|
| Physical Activity | ||||
| Lee et al. 2019 20 | Prospective cohort study (n = 16,741) | Actigraph GT3X+ Accelerometer (Actigraph Corp) | Daily step counts and step intensity | - As daily steps increased, the mortality rates decreased until it plateaued around 7,500 steps/day in a large cohort of older women. |
| Brickwood et al. 2019 23 | SR/MA of RCTs (n = 3,646) | Variety of wearable activity trackers | Daily step counts and step intensity | - Multifaceted interventions were more effective compared to interventions that relied solely on the wearable device. |
| Hodkinson et al. 202124 | SR/MA of RCTs (n = 4,203) | Variety of wearable activity trackers | Daily step counts and step intensity | - Interventions that combine activity trackers and consultations with health care professionals can significantly improve the PA level in patients with cardiometabolic conditions. |
| ENGAGE study 2021 33 | RCT (n = 500) | Fitbit Alta or Fitbit Inspire (Fitbit Inc) | Daily step counts and step intensity | - There was a greater increase in PA when individuals selected their goals (rather than assigned) with immediate implementation in economically disadvantaged adults. |
| Ferguson et al. 2022 2 | Umbrella review (n = 163,992) | Variety of wearable activity trackers | Daily step counts and step intensity | - Wearables increase activity by an average of 1800 steps/ day, 6 min of MVPA, and promote 1kg of weight loss with sustainable effects for at least 6 months. |
| Atrial Fibrillation | ||||
| REHEARSE-AF Study 2017 111 | RCT (n=1,001) | AliveCor Kardia device | Single-lead iECG | - Twice-weekly iECG screening led to nearly fourfold increase in new AF diagnosis in patients ≥ 65 y.o |
| mSToPS study 2018 13 | RCT and observational cohort study (n=2,659) | Zio Patch (iRhythm Technologies) | Single-lead ECG | - Monitored individuals had higher rates of AF diagnosis, AC use, and healthcare utilization. |
| Apple Heart Study. 2019. 38 | Prospective, multicenter, study. (n=419,297) |
Apple Watch (Apple Inc) | PPG | - Irregular pulse notifications occurred in 0.52% of the population cohort, and the PPV of the notification was 0.84 for atrial fibrillation detection. |
| Huawei Heart Study 201939 | Prospective cohort study (n=187,912) | Honor Band 4, Huawei Watch GT, Honor Watch (Huawei Tech.) | PPG | - PPG identified “suspected AF” in 0.2% of the cohort, and the PPV of the notification was 91.6%. |
| EPACS Study. 2019. 40 | RCT (n=116) | Zio Patch (iRhythm Technologies) | Single-lead ECG | - Continuous ECG monitoring after an index stroke/TIA resulted in significantly higher AF detection and AC use compared to Holter monitor. |
| SEARCH-AF Study. 2021 41 | RCT (n= 336) | SEEQ (Medtronic) or CardioSTAT (Icentia) chest patch | Single-lead ECG | - Continuous ECG monitoring increased the rate of AF detection by 17.9% within 30 days of hospital discharge after cardiac surgery. |
| SCREEN-AF Study 2021 112 | RCT (n = 856) | Zio Patch (iRhythm Technologies) and Watch BP-HomeA Monitor (Microlife) | Single-lead ECG and Oscillometric Screening |
- Continuous ECG monitoring increased AF detection 10-fold and led to AC initiation in 75% of patients ≥ 75 y.o. BP monitor screening was inferior compared to continuous ECG. |
| Heart Failure | ||||
| TIM-HF 2011. 46 | RCT (n=710) | Unspecified ECG and BP monitor | 3-lead ECG and BP | - Telemonitoring with physician interventions did not reduce all-cause mortality compared to usual care in ambulatory patients with stable HFrEF. |
| BEAT-HF study 2016 47 | RCT (n=1,437) | Unspecified BP + HR monitor | Unspecified | - Telemonitoring with health coaching did not reduce readmission or mortality rates after a HF hospitalization. |
| TIM-HF 2 Study 2018 45 | RCT (n= 1,571) | PhysioMem PM 1000 (GETEMED) and UA 767 PBT BP Device (A & D company) | 3-lead ECG and BP | - Telemonitoring reduced number of days lost to CV admissions and all-cause mortality in patients with HFrEF, no depression, and recent HF admission (subgroup of TIM-HF study). |
| LINK-HF study 2020 49 | Prospective multicenter study (n=100) | Multisensor wearable chest patch (Vital Connect) | PA, ECG, skin impedance, temperature | - ML algorithms detected rehospitalization with 76-88% sensitivity and 85% specificity, similar to the accuracy of implanted devices. - Alerts preceded the readmission by 6.5 days. |
| Cardiac Rehabilitation | ||||
| Telerehab II study 2015. 57 | RCT (n=80) | Yorbody Triaxial Accelerometer (Yorbody) | Daily step counts and activity intensity | - The telemonitoring program combined with conventional CR increased the peak VO2 more effectively than the control at 18 weeks in patients with CAD. |
| Maddison et al. 2019 58 | Non-inferiority RCT (n=162) | BioHarness 3 (Zephyr Technology) | HR, RR, single lead ECG, and PA | - Remotely monitored exercise-based cardiac telerehabilitation was similarly effective and had reduced program delivery costs compared to traditional center-based cardiac rehabilitation. |
| SmartCare-CAD study 2021 59, 60 | RCT (n=300) | Mio Alpha HR monitor (Physical Enterprises, Inc) and Actigraph wGT3x-BT triaxial accelerometer (Actigraph Corp) | Physical Activity Intensity and HR | - Cardiac telerehabilitation with on-demand coaching was similarly effective to center-based CR for improvement in PA and QOL. - The intervention was likely to be cost-effective. |
| Nagatomi et al. 2022 61 | RCT (n = 30) | Fitbit Inspire (Fitbit Inc.) | Daily steps | - The home based CR program was a safe and effective approach that improved 6MWD and muscle strength in HF patients with frailty. |
| Peripheral Artery Disease | ||||
| Gardner et al. 2011 63 | RCT. (n = 119) | StepWatch 3 Activity Monitor (Cyma Inc). | Daily step counts and activity intensity | - The home-based exercise program had high adherence and similarly improved claudication measures compared to a standard supervised program. |
| GOALS study 2013 113 | RCT (n = 194) | Caltrac Vertical Accelerometer (Muscle Dynamics Fitness Network) | Activity units | - The home based exercise program with a cognitive behavioral intervention improved objective and subjective measures of PA. |
| HONOR study 2018 64 | RCT (n = 200) | Fitbit Zip (Fitbit Inc) | Daily step counts | - The home-based exercise program without onsite visits did not improve 6MWD compared to usual care. |
| LITE study 2021. 65 | RCT (n=305) | Unspecified Accelerometer | Activity units | - Low-intensity home-based exercise was significantly less effective than the high-intensity group for improving 6MWD. |
| Diabetes: | ||||
| Beck et al. 2017 68 | RCT (n = 158) | Dexcom G4 Platinum | Glucose levels and amount of time in range | - In patients with type 2 DM on basal-bolus insulin, CGM significantly reduced hemoglobin A1c at 24 weeks compared to usual care with an adjusted difference in mean change of −0.3%. - There was high satisfaction and adherence to CGM use. |
| MOBILE study 2021 69 | RCT (n = 175) | Dexcom G6 | Glucose levels and amount of time in range | - In primary care patients with type 2 DM on basal insulin only, the CGM group had significantly improved hemoglobin A1c at 8 months (adjusted difference −0.4%) compared to fingerstick testing. |
| FLASH-UK study 202267 | RCT (n = 156) | Freestyle Libre 2 | Glucose levels and amount of time in range | - In patients with type 1 DM, intermittently scanned CGM with optional alerts improved hemoglobin A1c by 0.5% compared to fingerstick testing. - The CGM group had more time in range and lower burden of hypoglycemia. |
Abbreviations: anticoagulation (AC), atrial fibrillation (AF), blood pressure (BP), cardiovascular (CV), cardiac rehabilitation (CR), continuous glucose monitoring (CGM), coronary artery disease (CAD), diabetes mellitus (DM), electrocardiogram (ECG), ejection fraction (EF), heart failure (HF), heart rate (HR), heart failure with preserved ejection fraction (HFpEF), heart failure with reduced ejection fraction (HFrEF), machine learning (ML), meta-analysis (MA), moderate to vigorous physical activity (MVPA) myocardial infarction (MI), peripheral artery disease (PAD), photoplethysmography (PPG), physical activity (PA), positive predictive value (PPV), quality of life (QOL), randomized controlled trial (RCT), respiratory rate (RR), systematic review (SR), transient ischemic attack (TIA), 6 minute walk distance (6MWD).
Physical Activity:
Physical inactivity increases the likelihood of premature mortality or morbidity from CAD, type 2 diabetes, and mental illness 19. For example, a prospective cohort study of 16,741 older women demonstrated an inverse relationship between daily steps at enrollment, measured with an accelerometer for 7 consecutive days, and all-cause mortality as higher daily step totals were associated with lower mortality rates until the benefit plateaued around 7,500 steps/day 20. A longitudinal cohort study leveraged the All of Us Research Program to demonstrate an association between activity levels and incident chronic diseases with commercial wearable devices that were linked to an individual’s electronic health record (EHR) 21. In a cohort of 6,042 participants with a total of 5.9 million person-days of monitoring, higher daily step counts were associated with a reduced risk of several chronic diseases including hypertension, diabetes, obesity, and sleep apnea. Given that many adults fail to reach suggested levels of physical activity, this is a relevant public health issue that warrants attention.
There are hundreds of RCTs testing the use of activity trackers in various populations. One umbrella review suggested that interventions that incorporate wearable devices increase physical activity (PA) by approximately 1,800 steps per day, walking by 40 minutes per day, and moderate-to-vigorous physical activity (MVPA) by 6 minutes per day 2. The umbrella review demonstrated an average weight loss of 1kg with wearable PA interventions. The impact on other clinical outcomes (i.e. blood pressure, lipid profile, and hemoglobin A1c) and quality of life (QOL) outcomes were modest and often statistically insignificant. However, changes in these clinical outcomes may be delayed relative to physical activity and may need longer follow-up to become apparent. The umbrella review was comprehensive and included a wide range of interventions. For example, one intervention aimed to improve PA in a worksite environment by automatically transmitting data from activity trackers to an internet-based program to provide employees with daily step goals through emails or text messages, which had been adapted based on their activity levels 22. While this stand-alone intervention was completely automated, there were other studies with interventions that combined the wearable device with counseling sessions to create a multifaceted intervention. Brickwood et al. demonstrated that multifaceted interventions (i.e. wearable device and group or individual counseling) improved PA more effectively than interventions that only utilized wearable-devices 23. One systematic review and meta-analysis focused specifically on interventions with wearable PA devices in patients with cardiometabolic conditions 24. The review analyzed 38 RCTs with 4,203 total patients and found the interventions that combined wearable PA devices and regular follow-up sessions with healthcare providers (face to face or remotely) were associated with increased activity levels.
Behavioral change techniques (BCTs) such as personalized text messages, gamification, or just-in-time adaptive interventions (JITAIs), may enhance the impact of wearable devices on PA. The mActive study demonstrated that the combination of automated, personalized text messages with activity trackers increased PA levels in outpatient cardiology patients. The text messages were either positive reinforcement if the individual was likely to reach the daily step goal (10,000 steps) or booster messages to provide motivation when they were unlikely to reach the daily goal 25. A similar study design led to an increase of more than 1,000 steps per day and improved quality of life in a population with pulmonary arterial hypertension 26. Gamification integrates elements such as points or achievements to promote competition and facilitate positive lifestyle modifications 27. For example, the BE FIT study of 200 adults comprising 94 families used a gamification intervention focused on behavioral economics principles to increase PA 27. Over the course of 12 weeks, the families were awarded 70 points at the start of each week. One family member was randomly selected each day as their team’s representative, and the family retained its points if that individual met their step goal on the previous day; otherwise, 10 points were deducted from the family’s total. This approach increased the average number of steps by approximately 1,700 steps/day and a higher proportion of participants in the intervention arm achieved their daily step goals. Gamification interventions have successfully increased PA in a variety of populations including adults with uncontrolled diabetes 28, veterans with BMI ≥ 25 29, and families in the general population 27. However, reports on the sustainability of PA improvements following gamification interventions have been mixed. A systematic review on gamification interventions on PA demonstrated a very small to small effect on PA 12-24 weeks after the intervention, and the long-term effect was weaker and decreased over time 30. The design of the gamification intervention seems to impact the sustainability of the results as one study in adults with obesity and type 2 DM demonstrated that a gamification with competition intervention sustained PA through 12 months more effectively compared to a gamification with support intervention in the same population 28. In contrast, a gamification intervention with social support and financial incentives in veterans with obesity demonstrated no sustainability over a 8 week period 29. Given the mixed results on sustainability, it emphasizes the importance of ongoing studies to identify the effective gamification elements for target populations. JITAIs are personalized interventions that integrate real-time information from wearable devices to deliver notifications at times that are most likely to impact behavior 31. For example, a JITAI promoting PA may be delivered when an individual is walking near a green space during leisure time as they are more likely to integrate the behavioral change during this time 32. A systematic review of JITAIs demonstrated mixed results on PA and sedentary behavior; however, it is important to note that studies in this review were underpowered and only six studies were randomized 32. Effective JITAIs require the use of evidence-based BCTs and the delivery of notifications in a timely manner, but this may be lacking in some of the published studies. For example, the systematic review highlighted the lack of behavioral science evidence in the design of many JITAIs and identified one study where 43% of the intervention messages were not just-in-time 32 The mixed results likely arose due to the variations in the design and delivery of the JITAI in underpowered studies, which should be addressed as future studies aim to grow the supporting evidence base.
Some important populations have been underrepresented in device-based PA studies including individuals of lower socioeconomic status and patients with mental illness 2. Adults from lower socioeconomic status have more difficulty increasing their PA levels compared to the general population 33. This is a complex phenomenon with numerous contributing factors such as inadequate leisure time arising from increased occupational responsibilities or the inability to access a safe exercise environment 34. The ENGAGE study 33 demonstrated that a novel gamification intervention with a wearable activity tracker significantly increased PA in economically disadvantaged adults with an atherosclerotic cardiovascular disease (ASCVD) condition or 10-year ASCVD risk score ≥ 7.5% in Philadelphia, Pennsylvania by using self-chosen goals (rather than assigned) that were implemented immediately (rather than gradually over an 8-week period). For example, the individuals were either assigned a goal of 2,000 step increase from baseline or they were able to select a goal between 1,000 and 3,000 steps above baseline with the flexibility to change their self-selected goal at any time. Future studies are needed to further investigate physical activity in geographically and socioeconomically diverse populations.
Lastly, there is a need for device-based PA studies with longer follow-up to further characterize the impact of PA on clinical and psychosocial outcomes as the current studies may have inadequate follow-up to capture the physiologic benefits that emerge after consistent changes in activity. Additionally, the duration of the current studies may be too short to effectively build a new habit. While the necessary follow-up duration remains unclear based on the current body of evidence, this will be an important variable to investigate in order to guide the design of future studies.
Atrial Fibrillation:
Atrial fibrillation (AF) is associated with an increased risk of all-cause mortality and significant morbidity from stroke and heart failure 35. Modern wearables represent a practical and effective tool to screen for AF, with potential to reduce stroke and mortality rates if therapeutic anticoagulation is initiated in eligible individuals.
The development of an adhesive single-lead ECG chest patch, such as the Zio patch (iRhythm Technologies, San Francisco, California), improved the practicality of ambulatory cardiac monitoring as the patch was smaller and less burdensome than traditional Holter monitors 4. In a large cohort study of 26,751 patients who wore the Zio patch for clinical indications, there was incremental diagnostic yield for all arrhythmia types beyond the first 48 hours of monitoring 36. The mSToPS study found that AF screening in asymptomatic, high-risk patients with an ECG patch compared with non-monitored controls led to increased rates of new AF diagnosis and anticoagulation initiation, but they also had higher health care resource utilization (i.e. cardiology or primary care clinic visits) at 1 year 13. The mSToPS group subsequently published follow-up data, and the individuals who underwent AF screening had a lower rate of clinical events at 3 years compared to routine care. However, the impact of early AF diagnosis on these findings remains unclear due to the observational nature of the study 37. Two large studies investigated the ability of an irregular pulse notification from a PPG-enabled smartwatch or band to identify a new diagnosis of AF in a predominantly middle-aged population 38, 39. These studies demonstrated a low prevalence of HR irregularity and the diagnostic algorithms performed well with a positive predictive value of 0.84 – 0.92 for AF. Additionally, RCTs have demonstrated increased AF detection rates with continuous ambulatory ECG monitoring in specific high-risk patient populations (i.e. cryptogenic stroke or post-cardiac surgery). 40, 41
There are important limitations with the existing body of literature, some of which are being addressed by ongoing studies. For example, it remains unclear whether improved AF detection will reduce hard clinical outcomes with an acceptable safety profile. Future studies should also investigate whether wearable devices can be used to optimize an outpatient medication regimen in patients with an established diagnosis of AF, such as the up-titration of beta blockers or the addition of another anti-arrhythmic medication if the patient has insufficient rate control. Additionally, there are limited validation studies investigating the use of these devices in clinical practice. Current literature has raised concern about the accuracy of wearables for AF detection as some validation studies have found notably worse performance compared to industry reported data 42, 43. This highlights the need for rigorous, real-world validation studies in clinical settings.
Heart Failure:
Studies using wearable devices to improve HF management have reported mixed results. Several RCTs have assessed whether non-invasive telemonitoring interventions decrease HF readmissions or all-cause mortality. Many of these studies used multifaceted telemonitoring interventions with home ECG devices, BP monitors, and weight scales along with increased provider follow-up. The TEMA-HF 1 44 and TIM-HF 2 45 studies showed improved clinical outcomes while the TIM-HF 46 and BEAT-HF 47 studies did not improve all-cause mortality or readmission rates. It is difficult to evaluate the impact of wearable devices on clinical outcomes in these telemonitoring studies as the benefit may have been due to the extensive clinical follow-up rather than the use of wearable devices. The mixed results across studies likely stems from differences in the study population and significant variation in the telemonitoring interventions such as devices used, follow-up frequency, study team composition (i.e. general practioners, cardiologists, nurses), and intensity of intervention (i.e. patient-education, health coaching, urgency/frequency of medication adjustments). The impact of the study cohort is seen in the TIM-HF46 and TIM-HF 2 45 studies as similar physician-led telemonitoring programs only yielded clinical benefit when applied to the well-defined HF population of TIM-HF 2 (i.e. hospitalization in past year and no major depression) compared to the broader TIM-HF study population (i.e. stable ambulatory HF patients).
Two studies investigated whether a ML algorithm that utilizes continuous data from wearable devices can predict HF decompensation. The MUSIC study 48 developed and validated a personalized algorithm to predict acute decompensation in 543 HF patients. The wearable chest patch continuously monitored heart rate, respiratory rate, activity, posture, and bioimpedance. The algorithm predicted HF decompensation with 63% sensitivity and 92% specificity in the validation cohort. However, the results of this study were limited as 42% of patients were excluded from statistical analyses, largely due to poor performance of the wearable device prototype. The LINK-HF study 49 is a multicenter, observational study of 100 patients who wore a chest patch that captured continuous ECG, accelerometry, impedance, and skin temperature. The ML algorithm used this data to predict impending HF hospitalization with 76 to 88% sensitivity and 85% specificity at a median time of 6.5 days before the admission. Since the clinical alerts preceded the hospitalization by nearly one week, this may provide adequate time to make medication adjustments focused on preventing hospitalization.
Small studies with novel biomechanical sensors show promise in the management of HF patients. Remote dielectric sensing (ReDS) uses electromagnetic energy technology in a wearable vest to measure lung fluid volume for assessment of the patient’s volume status. Two studies suggest that ReDS-guided management, such as diuretic adjustment, may reduce HF readmission rates. 50, 51 In a prospective study of 50 patients hospitalized for acute decompensated HF, the use of daily ReDS measurements to optimize outpatient HF therapies during a 90 day follow-up period reduced HF readmission rates 50. A retrospective cohort study of 220 patients who were seen for a follow-up clinic visit shortly after HF hospitalization demonstrated that patients who received point-of-care ReDS testing during the clinic visit had a lower 30 day CV readmission rate compared to patients who did not receive a ReDS assessment 51. SCG measures the local vibration of the chest wall that occurs during the cardiac cycle. When SCG was measured in the setting of submaximal exercise with the six minute walk test, this technology was able to differentiate between decompensated and compensated HF patients 15.
Wearable devices are also used as a research tool in several HF trials to measure outcomes, such as physical activity or sleep, in response to a pharmacologic intervention. The NEAT-HFpEF study tested the hypothesis that extended-release isosorbide mononitrate would improve daily activity levels, as measured by accelerometers, in patients with heart failure with preserved ejection fraction (HFpEF)52. In this study, patients with HFpEF who received isosorbide mononitrate had decreased activity levels compared to placebo. The AWAKE-HF study used wrist actigraphy to investigate the impact of sacubitril/valsartan on activity and sleep in patients with heart failure with reduced ejection fraction (HFrEF) 53, 54. They hypothesized that sacubitril/valsartan would improve PA levels and sleep compared to enalapril, but the study found no differences in either of these Actigraph-measured outcomes. Lastly, the HeartSleep Study used actigraph-measured sleep parameters, such as sleep efficiency and sleep duration, to demonstrate the sustained effects of cognitive behavioral therapy in patients with chronic HF and insomnia compared to a self-management program 55.
In light of advancements in device technology and AI capabilities, future studies should employ wearable devices in large, RCTs to further clarify the clinical utility of wearable devices in remote HF management. There is a wide range of potential clinical applications that warrant future investigation including innovative applications of novel sensors and ML algorithms as well as the optimization of traditional HF therapies with existing consumer-grade wearables. For example, studies could evaluate whether notifications from wearable devices can improve HF outcomes through increased adherence to standard medications such as diuretics and guideline-directed medical therapy. Additionally, the heart rate and blood pressure monitoring from wearable devices may provide clinicians with useful information to safely up-titrate guideline-directed medical therapy. Lastly, the existing body of evidence supports the ongoing use of wearable devices in research studies to assess PA and sleep outcomes, which are relevant measures in HF patients.
Cardiac Rehabilitation:
Cardiac rehabilitation (CR) incorporates patient education, lifestyle modification, and exercise training for secondary prevention of cardiovascular conditions 56. CR has been historically underutilized due to access and cost issues, so home-based CR programs were introduced to improve participation. The Telerehab II 57 study was a RCT that added an 18 week PA telemonitoring intervention to conventional CR. The telemonitoring intervention utilized a triaxial accelerometer and weekly personalized automated feedback via email or text to encourage the patient to increase their step count by 10% each week. The intervention increased the patient’s physical fitness (peak VO2) and showed a trend toward fewer re-hospitalizations compared to the standard CR group. Several RCTs demonstrated that home-based CR and center-based CR similarly improved clinical outcomes 56. For example, a randomized controlled non-inferiority trial demonstrated that a telerehabilitation program that provided real-time, remote exercise monitoring with a chest-worn sensor and individualized coaching was similarly effective and reduced program delivery costs compared to center-based CR 58. The wearable device measured heart and respiratory rates, single lead ECG, and accelerometry during the exercise training sessions, which were transmitted to a CR specialist in real-time for review. Based on the wearable data, the CR specialist provided real-time individualized coaching, feedback, and support during the exercise sessions. The SmartCare-CAD study 59, 60 evaluated the efficacy of a novel cardiac telerehabilitation intervention that focused on preventing relapse into a sedentary lifestyle compared to center-based CR. The intervention included 6 supervised sessions, home based training with wearable devices, and weekly video meetings. The study used both a wrist-worn heart rate monitor and a hip-worn triaxial accelerometer, which was uploaded for review and feedback by an exercise specialist during weekly video meetings for 3 months. After 3 months, the weekly meetings stopped but patients continued to upload data from their wearable devices on a weekly basis to identify when relapses occurred. Relapses were defined as nonadherence (i.e. no data uploaded) or a 50% reduction in PA, and these prompted video consultations for additional coaching. The telerehabilitation program similarly improved PA and quality of life (QOL) compared to center-based CR in this population of low-risk CAD patients.
Many of the existing studies used research-grade devices for PA monitoring, but recent studies have started to incorporate consumer-grade wearables. Nagatomi et al 61 randomized 30 HF patients with physical frailty to a home-based CR program with exercise and nutrition coaching via a Fitbit device. The intervention improved 6 Minute Walk Distance (6MWD) and muscle strength compared to standard care without a CR component. The body of evidence for CR with wearable devices continues to grow and ongoing studies are investigating ways to maximize the durability of CR 31.
Peripheral Artery Disease:
The initial management for patients with symptomatic peripheral artery disease (PAD) is a supervised exercise program to improve functional capacity and QOL with guidelines supporting both center-based or home-based exercise programs (class IIa recommendation)62. Similar to CR, the center-based programs are underutilized due to access issues and the burden of traveling to the medical center on a regular basis, which highlights the importance of developing effective home-based programs. In a single-center RCT, a home-based exercise program with a StepWatch3 activity monitor improved claudication measures to a similar degree as a standard supervised program 63. The patients quantified their home exercise sessions with the step activity monitor and the data was analyzed every two weeks by an exercise specialist in order to provide coaching and feedback during individualized meetings. The HONOR study 64 was the first RCT to investigate a home-based exercise program without any periodic medical center visits. Patients used a Fitbit Zip activity tracker to monitor their prescribed exercise regimen, and the data from the wearable device was available to the patient and their coach to facilitate discussions during their remote coaching sessions. The intervention did not improve 6MWD, which suggests there may be a clinical benefit from intermittent visits to the medical center when patients are enrolled in the home based programs. Notably, participants only adhered to 79% of the remote coaching sessions, which may have influenced the results as well. Recent studies have aimed to further refine the optimal design of home-based exercise programs. The LITE study 65 randomized 305 patients to a home-based exercise program with varying intensities: low-intensity (does not induce ischemic leg symptoms), high-intensity (induces ischemic leg symptoms), or non-exercise controls. Participants wore an accelerometer to capture exercise intensity, and were called weekly by a coach who used activity data to promote adherence. The low-intensity program was significantly less effective than the high-intensity program, and there were no appreciable differences between the low intensity and non-exercise control. As the evidence supporting home-based exercise programs continues to grow, future studies should aim to determine the most effective design and intensity for home-based exercise programs that are reproducible, sustainable, and generalizable to large patient populations.
Coronary Artery Disease / Myocardial Infarction:
The recovery period after an acute myocardial infarction (MI) is associated with an increased risk of complications such as arrhythmias or need for readmission. With the exception of CR (described above), the existing literature on wearable devices in the post-MI recovery period is limited. Treskes et al 66 evaluated the efficacy and feasibility of smart technology to control home blood pressure in 200 patients after an acute type 1 MI compared to usual care. The intervention utilized smartphone-compatible devices to measure daily single-lead ECGs, daily BP and weight, and continuous step measurements. Patients were contacted if their BP measurements exceeded pre-specified thresholds or if the ECG identified a new arrhythmia. The intervention also replaced some of the in-person follow-up visits with telemedicine visits. While this intervention was feasible and acceptable to patients, it did not improve blood pressure control. Large prospective clinical trials are currently lacking for all aspects of CAD management.
Diabetes:
Patients with type 1 diabetes or type 2 diabetes have an increased risk of cardiovascular conditions such as: CAD, stroke, PAD, and HF. Continuous glucose monitoring (CGM) devices have several benefits as they can provide the patients with safety alerts (i.e. hypoglycemia) and promote self-management of insulin dosing, while also providing clinicians with longitudinal data to optimize long-term glycemic control. Evidence supports the use of CGM for patients with type 1 or type 2 diabetes to improve glycemic control. For example, in a multicenter, RCT of 156 patients with type 1 diabetes on insulin infusions or multiple daily injections, the patients who intermittently scanned their CGM device (Freestyle Libre 2) with optional alarms for hyperglycemia or hypoglycemia had significantly lower hemoglobin A1c levels compared to the usual care group with traditional fingerstick testing 67. A multicenter RCT of 158 patients with type 2 diabetes on basal-bolus insulin regimens demonstrated that CGM (Dexcom G4 Platinum) reduced Hemoglobin A1c more effectively at 24 weeks compared to intermittent fingerstick readings 68. Similar results were seen in a cohort of 175 primary care patients with type 2 diabetes who were only managed with basal insulin, as the use of CGM (Dexcom G6) significantly reduced Hemoglobin A1c levels at 8 months compared to intermittent fingerstick testing69. In each study, there were no statistically significant differences in the total daily insulin dose between the CGM and the fingerstick control groups.
While the ability for CGM to improve glycemic control is now well-established, recent studies have started to investigate associations with clinical outcomes. CGM produces several measures of glycemic control, of which, time in range (TIR) likely represents a key metric in diabetes management. In a prospective cohort study of 6,225 patients with type 2 diabetes 70, TIR was inversely associated with long-term risk of all-cause and CV mortality. TIR was assessed by CGM over a 72 hour period during a hospitalization and patients were subsequently followed for a median follow-up of 6.9 years. In a cross-sectional study of 510 patients with diabetes and AF, patients underwent CGM for 72 hours after a hospitalization and there was an inverse relationship between TIR and stroke risk 71. Future studies are needed to further investigate CGM metrics, such as TIR, and hard clinical outcomes.
CHALLENGES, OPPORTUNITIES, and FUTURE DIRECTIONS:
This section summarizes many of the challenges that are currently hindering the widespread use of wearable devices in clinical care (Table 3) and we identify some short-term and long-term solutions to transform the digital health landscape and improve cardiovascular care (Figure 2).
Table 3:
Advantages and challenges associated with wearable devices in cardiovascular medicine: patient and healthcare provider perspectives
| Advantages | Challenges | |
|---|---|---|
|
Patient Perspectives |
• Active role in monitoring and managing chronic conditions • Promotion of healthy lifestyle choices • Telemonitoring may reduce the number of office visits, travel, and time commitment • Increased screening and earlier detection of disease • Sense of ‘safety’ from continuous monitoring |
• Difficulty navigating the wearable device technology; exacerbated by cognitive, visual, or hearing impairments • Poor understanding of the device’s clinical role and inability to contextualize clinical significance of measurements • Cost of device/software • Inconsistent internet access • Concern for data security • Social isolation by decreasing in-person visits • Lack of culturally diverse interventions 74 • Lack of non-English resources |
|
Healthcare Provider Perspectives |
• Improved screening and management of cardiac conditions • Earlier detection of clinical decompensation that may be amenable to intervention to prevent hospitalization • Objective assessment of patient adherence to exercise or medication regimen • Additional data for telemedicine visits |
• Insufficient clinical infrastructure and staff to handle wearables data • Wearable use may be patient-initiated, though providers are asked to interpret findings and provide guidance • Integration of wearables data into electronic health record • Lack of standardized reimbursement policies • Need for large randomized controlled trials to build supporting evidence to define meaningful use criteria and clinical guidelines • Constantly changing technology and poorly defined regulatory oversight • Concerns about device accuracy and validity • Data privacy and medicolegal concerns. • Concerns about potentially creating a new health disparity |
Figure 2: Short and Long-Term Roadmap to Increase the Use of Wearable Devices in Clinical Care.
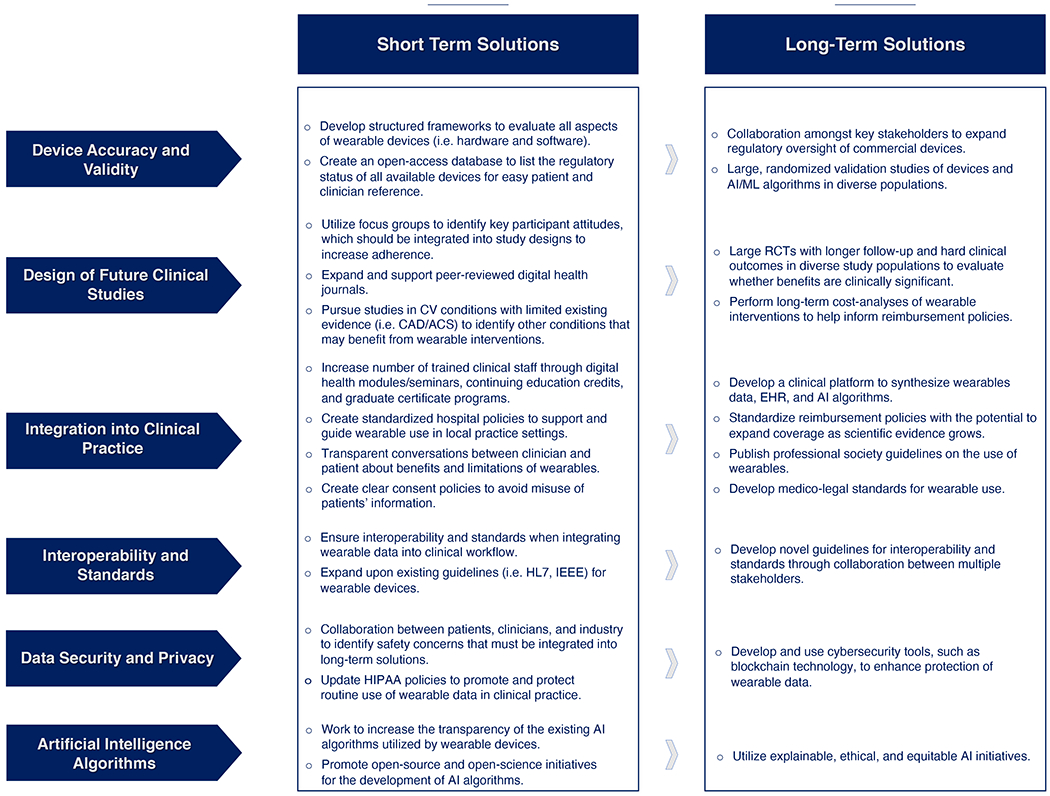
Abbreviations: acute coronary syndrome (ACS), artificial intelligence (AI), cardiovascular (CV), coronary artery disease (CAD), electronic health record (EHR), Health Insurance Portability and Accountability Act (HIPAA), Institute of Electrical and Electronics Engineers (IEEE), machine learning (ML), randomized controlled trial (RCT)
Avoiding Unintended Health Disparities:
The term digital divide describes the growing chasm between groups as a result of unequal access to digital technologies ranging from computers and the internet to smartphones and wearables. Groups who are most affected by the digital divide include people who are elderly, handicapped, of low socioeconomic status, and/or live in rural areas, with intersectionality often compounding the challenges. Apart from access, a lack of digital literacy can impede equitable implementation of digital technologies. Digital literacy describes an individual’s ability to use digital technology to access, understand, or communicate information through digital means. Given the rapidly changing technology landscape, including the continuous emergence of new types of devices, interfaces, and information, digital literacy remains a moving target.
Trends from the early stages of the COVID-19 pandemic highlighted significant inequities in access to telemedicine care. A cohort study of more than 148,000 patients in primary care and subspecialty clinics demonstrated that older age, Asian race, and non-English speaking were associated with fewer telemedicine visits completed, and older age, female sex, black race, Latinx ethnicity, and lower income were associated with less video use 72. Internet access is being increasingly recognized as a social determinant of health as this allows patients to participate in telemedicine visits, make appointments, and obtain test results 73.
Racial and ethnic minorities suffer from a higher prevalence of and mortality due to cardiometabolic conditions 74. These populations may stand to benefit from wearable device interventions to improve their cardiovascular health. However, there are several barriers that limit the efficacy of wearable devices in this population. There are currently a lack of non-English tools 3, limited culturally diverse mHealth interventions 74, and the social networks of these communities may not prioritize the adoption of wearable technology for health promotion75. Finally, the lower adoption of wearables in these groups may lead to their data being underrepresented in the large datasets used by ML models, which can further worsen disparities through data absenteeism 76.
Wearable devices offer several potential benefits to older adults, but they are not widely used by this population. In a survey of 1,481 older adults, aged 65 or above, only 17.5% of these individuals used a wearable device. Of the older adults who used a wearable device, the majority (80%) were agreeable to sharing the data with healthcare providers 77. Some of the barriers to older adults using wearable devices include: technology anxiety and resistance to change 78, visual, hearing, and motor impairments with increased age 77, and difficulty troubleshooting the device or user interface complexity 79. The current barriers illustrate that ease of use will be an important factor to increase the percentage of older adults who integrate wearable devices into their daily lives.
The cost of wearable devices is quite variable depending on technological capabilities. Lower income may be an important driver of disproportionate use of wearable devices based on a survey of 4,272 adults in which wearable use was three times higher for individuals earning over $75,000 compared to individuals earning less than $30,000 5. Since adults with lower socioeconomic status experience greater difficulty increasing their PA compared to the general population 33, this warrants novel strategies to increase the accessibility of wearable devices in this population.
As we move to expand the clinical use of wearable devices, we must recognize the existing inequities, and work to design studies and clinical practices that achieve health equity rather than exacerbating current disparities. Efforts to address these disparities include improving broadband internet access, affordability of digital tools, language accessibility (i.e. through translations), and form factor accessibility (i.e. large text or voice-based agents for visual and hearing impairments, respectively). Recognizing internet access as a social determinant of health has been a critical first step 73, and efforts to advance broadband connectivity are already underway, for example through the Connect2HealthFCC Task Force (C2H Task Force). Another effort by the U.S. Department of Health & Human Services in 2022 awarded over $55M to HRSA-funded health centers to enhance telehealth, digital patient tools, and health information technology to support underserved communities. Continued efforts to address the digital divide and digital literacy in the context of the evolving technology landscape will go a long way toward increasing the equitability of digital health.
Design of Future Clinical Studies:
While the initial studies highlight the exciting potential for wearable devices, the current body of evidence still has several limitations. The generalizability of many existing studies is limited as there are several populations that are underrepresented including economically disadvantaged individuals, older adults, non-English speaking, and patients with mental illnesses. The reproducibility of studies is limited by a large degree of heterogeneity in the study design, interventions, and wearable devices used, which partially arises from the everchanging technological advancements and the abundance of available wearable devices. Focus groups and pilot studies may be used to ensure that participant attitudes are incorporated into the study design to optimize adherence. Additionally, the future studies need to identify whether wearable devices and associated interventions lead to clinically meaningful results, which can be accomplished through long follow-up periods and hard clinical outcomes. Moving forward, we also need to evaluate the accuracy and validity of the wearable devices that are being used in these clinical studies through structured frameworks. Coravos et al. 80 proposed a robust framework for the assessment of the hardware and software components of wearable devices based on five key aspects: validation, security practices, data rights and governance, utility and usability, and economic feasibility. These evaluation frameworks will promote the use of safe and effective wearable devices in research studies and clinical practice, while identifying the devices that have suboptimal efficacy or the potential to cause harm. These frameworks may address some of the legal and ethical issues, such as privacy and data sharing policies and the lack of transparent wearable device algorithms, which must first be addressed before wearable devices can maximize their contributions to the Big Data movement designed to advance the field of cardiology. 81 Lastly, the FDA was able to provide the pharmaceutical industry with guidance and mandate large, long-term cardiovascular outcome trials (CVOTs) for the development of medications for certain conditions, such as type 2 diabetes 82, but this may prove more challenging for the wearables field since a consumer-grade wearable may not be under FDA oversight with the existing regulatory policies. While these limitations and challenges are not easily overcome, a close collaboration between clinicians, scientists, industry, and community members, especially from historically underrepresented populations, may allow for the design of large reproducible and generalizable trials needed to strengthen the existing data.
Integration of Wearables into EHR:
Wearable device integration with electronic health record (EHR) systems facilitates automatic transfer of real-time data on clinical measurements (i.e. heart rate or blood glucose) and behavior measures (i.e. physical activity or sleep) from a wearable device to an EHR system within the healthcare ecosystem. This integration of wearable data within the EHR is a promising approach to provide a more comprehensive view of patients’ health as the clinical measurements may predict or identify a new diagnosis or help guide the management of a chronic condition. Since patients can observe these clinical measurements in real-time, it also fosters shared decision making and improves patients’ engagement in their care. A recent study 21 leveraged the longitudinal Fitbit and EHR data from the All of Us Research Program to examine the association between daily steps and incident disease that can occur across the entire human phenome. This study illustrates the potential clinical value of linking wearables data to the EHR since it may provide valuable and actionable information to healthcare professionals and help advance personalized care.
However, the integration of wearables into EHR systems is currently in the infancy stage given technical and privacy barriers 83. Medical systems may not have sufficient technical infrastructure to support systems interoperability or to establish scalable workflows to protect patients’ confidentiality. Another important consideration for integrating wearables into the EHR is to ensure that the patients consent to sharing their data. This consent process should utilize clear documentation to avoid any misuse of a patient’s personal health information 84. Therefore, there is a need for additional policies to protect patient’s confidentiality and minimize the risk of information misuse by third parties. EHR systems must be able to manage the large amount of longitudinal data from wearable devices in order to utilize this information in a clinically meaningful manner. Clinicians will need to interpret the wearables data and tailor their recommendations to the individual patient, but it will be essential that EHR systems have the ability to identify the situations that warrant clinical intervention while minimizing false alarms to avoid an unintended increase in clinical workload. Lastly, there is a need for user-friendly interfaces to engage both the providers and the patients in the routine use of wearables in clinical practice.
The integration of wearable data into the EHR will be a challenging endeavor that requires strong interdisciplinary teams to identify and implement solutions into clinical practice. Some of the initial steps that clinics and hospitals may take to enhance the integration of wearables include the development of educational resources for patients and clinicians, the identification of clinician champions, and to ensure that the clinical team has individuals capable of providing technological support 85. The educational resources and clinician champions should be transparent about the benefits and limitations of wearables data in the EHR, and recognize that the information will likely need to be adapted as the use of wearables evolves over time. Managing the extensive data from wearables is critical, and solutions can be generated around FAIR guiding principles – Findability, Accessibility, Interoperability, and Reusability 86. For instance, machine learning and AI algorithms 87 may serve as potential solutions to identify the meaningful and actionable parameters for clinical care. A scoping review by Dinh-Le et al.83 reported that nearly 10 start-up companies are working with 16 health systems to develop workflows to meaningfully integrate wearables into EHR systems. To further illustrate the value of multidisciplinary collaboration, a consortium of more than 130 individuals from 60+ organizations (i.e. industry, academia, policymakers, clinicians, and patients) established the Integration of Continuous Glucose Monitoring Data into the Electronic Health Record (iCoDE) project 88. The 2022 iCoDE Report discusses numerous practical considerations (i.e. clinical workflows, team compositions, EHR data displays, etc), a template for a Project Implementation Guide, and a list of formal recommendations from the iCoDE steering committee. The iCoDE project aims to overcome many of the limitations hindering the integration of CGM data into the EHR, and this will likely serve as a useful framework for other wearable devices (i.e smartwatches) to build upon.
Clinical Workflow:
For wearable devices to reach its full potential, we need to expand the number of clinical staff who are trained and capable of managing the constant influx of data. This requires dedicated training on digital technology. In the short-term, the training may involve modules and seminars for all existing clinical staff followed by the creation of digital technology curriculum for nursing and medical schools to provide a sustainable, long-term approach. Additionally, it will be essential that we create strategies to allow these clinicians to easily identify actionable data while ignoring the “noise” that the abundant wearable data will inevitably generate. By developing a common clinical platform that synthesizes information from wearables, EHR, and AI algorithms, we may be able to establish prespecified thresholds that identifies the meaningful data that warrants clinical attention. This will certainly require close collaboration amongst clinicians, patients, and technology specialists, and these collaborative efforts may benefit from leveraging the expertise of existing companies that already provide implementation services and support for digital health technologies 3.
In response to the COVID-19 pandemic, the United States Food and Drug Administration (FDA) and Centers for Medicare and Medicaid Services (CMS) modified some requirements for remote physiologic monitoring (RPM) to increase access to care and provide greater reimbursement flexibility for clinicians 89, 90. RPM collects and analyzes physiologic data (i.e. heart rate, weight, pulse oximetry) from non-invasive medical devices, such as wearables, to guide the treatment of an acute or chronic condition. Currently, the reimbursement policies are more straightforward for certain aspects of RPM, such as vital signs, compared to variables such as PA 5. Current Procedural Terminology (CPT) codes include: a one-time reimbursement for the initial set-up of a RPM device (CPT 99453), data collection and interpretation (CPT 99091), and development/implementation of a treatment plan through interactive communication with the patient based on remotely collected data from devices, such as wearables (CPT 99457 and 99458) 90. As we move beyond the current pandemic, we may consider expanded reimbursement policies to increase the clinical use of wearable devices if the growing body of scientific evidence proves to be beneficial and cost-effective for the management of chronic conditions. For example, we may reach the point where evidence supports a novel reimbursement strategy for a clinician who reviews wearable data, such as physical activity, and prescribes wearable-guided interventions. 5
Costs Associated with Clinical Use:
In order to support the integration of wearable devices into routine clinical care, we must demonstrate that this is a cost-effective approach for patients and payers. For example, screening efforts that use wearables to identify new diagnoses, such as atrial fibrillation, must ensure that the screening improves clinical outcomes without increasing costs or harms that may arise from false positives or confirmatory follow-up testing 91. Currently, the data on cost-effectiveness of wearable interventions is limited. One recent systematic review examined the impact of wearables on health care outcomes in patients with chronic diseases, but only a small number of these studies examined costs 92. A simulated model suggested that atrial fibrillation screening with wearable devices is cost-effective compared to no screening or traditional methods (i.e. pulse palpation and 12-lead ECG)91, and an economic evaluation of a cardiac telerehabilitation program suggested that it was likely to be cost-effective compared to traditional center-based CR60. As the existing body of evidence for wearables in cardiovascular medicine grows, it will be important for these studies to also publish cost-analyses. The cost-analyses should measure Quality Adjusted Life Years (QALYs) and incremental cost-effectiveness ratios (ICERs) as these variables will help guide future discussions about wearable technologies in clinical care.
Many of the key stakeholders have already demonstrated a commitment to promote a healthy lifestyle as health insurance companies offer incentive programs for healthy behaviors and employers provide workplace wellness programs. Wearable devices are frequently incorporated into these incentive or wellness programs. For example, Fitbit Health Solutions, has already established programs to work with employers, health plans, and health systems to encourage PA, sleep, nutrition, and stress management. Additionally, policymakers have demonstrated a willingness to modify reimbursement policies, such as RPM reimbursement during the COVID-19 pandemic, when needed to support new avenues of healthcare delivery. Similarly, Medicare and private health insurance plans now provide coverage for CGM devices for patients with type 1 and type 2 diabetes, so the payers may embrace a similar approach towards other wearable devices (i.e. smartwatches) if the growing body of evidence demonstrates long-term benefits. Lastly, all of these stakeholders will play a pivotal role in establishing the necessary infrastructure and payment models to overcome the initial startup costs associated with integrating new wearable devices into clinical care in order to achieve the potential long-term benefits and decreased healthcare costs that wearables may offer.
Data Accuracy/Validity:
Accelerometry devices such Actigraph and ActivPal are being increasingly used in research to quantify clinical measurements such as activity, sedentary behavior, or sleep. In addition, these devices are also being used to assess the validity and reliability of consumer-grade wearables 93–95. However, as hardware, software, and algorithmic properties of wearables continue to evolve, the number of wearables available on the market will also continue to grow. In turn, there may be potential risks related to data accuracy and validation 80, 96. Since consumer-grade wearables are not under FDA oversight, there is no regulatory agency surveying their accuracy. Additionally, algorithms used for wearables are often not open-sourced. Given the lack of transparency in the AI algorithms and concerns about inaccurate data from unregulated devices, key challenges remain for healthcare professionals to interpret findings, such alarms for atrial fibrillation, which hinders the widespread adoption of wearables in healthcare settings.
Data Security/Privacy:
AI algorithms rely heavily on personal data for their decision-making process, but the software and hardware used in these systems may have security vulnerabilities that could result in the theft of personal information. For that reason, significant effort is required to maintain the privacy and security of personal and identifiable data. We must also limit the number of individuals who have access to the raw identifiable data. Federated learning is a newer technique in AI that allows for a decentralized approach to training algorithms, thus addressing issues related to data safety and privacy 97. Unlike traditional methods, which involve centralizing data from multiple sources to train an algorithm, federated learning trains the algorithm locally on devices or servers, without sharing any data. Instead, only the model parameters are shared and optimized through iteration. This approach keeps data safe and private by not sharing it. Finally, data ownership is an ongoing issue, which may result in unauthorized sharing of user data without explicit and prior approval.
Artificial Intelligence/Machine Learning:
AI and ML technologies are faced with various challenges such as fairness and bias, transparency and accountability, and data drift. These can occur when data and algorithms reflect, reinforce, or perpetuate biases, or when the data used to train AI is incomplete or inherently biased, which can lead to further disparities and biases, particularly in underserved and underrepresented communities. Additionally, some models may be more accurate but opaque (i.e. complex non-linear models over simple linear models), making it difficult to understand how the algorithm reached its conclusions. This can be particularly problematic in healthcare, as it can affect a clinician’s medical decision-making and a patient’s treatment. Lastly, AI and ML algorithms often assume that data from the past will be representative of future data, which is not always the case in real-world settings, causing changes in the data to affect the model’s behavior and accuracy.
Although some ML, particularly DL, algorithms can outperform humans in certain tasks, such as detecting arrhythmias from ECG data 98 or diagnosing cardiovascular diseases from radiology images 99, AI technologies are more effective when used to augment rather than replace human capabilities. Additionally, AI can reduce human errors, enhance knowledge capacity, and save time previously spent on repetitive tasks. However, it is important that we recognize both the exciting potential as well as the challenges and concerns of AI/ML as we apply these tools to healthcare 100. As technological advancements in wearable sensor hardware and AI algorithms accelerate personalized medicine, care should be taken to ensure that these advancements do not worsen health-related disparities or create new inequities in care or health-related outcomes. Future research in this field should address equity and fairness of the development and application of AI in cardiovascular medicine, as well as the interpretability and generalizability of AI models 101.
CONCLUSIONS:
Wearable devices have the potential to revolutionize the prevention, diagnosis, and management of cardiovascular conditions. While patients frequently use wearable devices in their everyday lives, there remain several challenges that prevent the widespread integration of wearable technologies in clinical practice. A collaborative, multidisciplinary approach with patients, clinicians, scientists, policymakers, and industry leaders will be needed to transform the digital health landscape and allow wearable devices to achieve their full clinical benefit.
DISCLOSURES:
Evan Brittain has an Investigator Initiated grant to study activity in pulmonary hypertension from United Therapeutics
ABBREVIATIONS AND ACRONYMS:
- 6MWD
6 Minute Walk Distance
- ACS
Acute Coronary Syndrome
- AC
Anticoagulation
- AI
Artificial Intelligence
- ASCVD
Atherosclerotic Cardiovascular Disease
- AF
Atrial Fibrillation
- BCG
Ballistocardiogram
- BCT
Behavioral Change Technique
- BP
Blood Pressure
- BMI
Body Mass Index
- CR
Cardiac Rehabilitation
- CV
Cardiovascular
- CVD
Cardiovascular Disease
- CVOT
Cardiovascular Outcome Trials
- CMS
Centers for Medicare and Medicaid Services
- CGM
Continuous Glucose Monitoring
- CAD
Coronary Artery Disease
- CPT
Current Procedural Terminology
- DL
Deep Learning
- DM
Diabetes Mellitus
- ECG
Electrocardiogram
- HER
Electronic Health Record
- FDA
Food and Drug Administration
- GPS
Global Positioning System
- HIPAA
Health Insurance Portability and Accountability Act
- HRSA
Health Resources and Services Administration
- HF
Heart Failure
- HFpEF
Heart Failure with Preserved Ejection Fraction
- HFrEF
Heart Failure with Reduced Ejection Fraction
- HR
Heart Rate
- ICER
Incremental Cost Effectiveness Ratios
- IEEE
Institute of Electrical and Electronics Engineers
- JITAI
Just-in-time Adaptive Intervention
- LV
Left Ventricle
- ML
Machine Learning
- VO2 max
Maximum Oxygen Uptake
- MA
Meta Analysis
- MVPA
Moderate-to-Vigorous Physical Activity
- MI
Myocardial Infarction
- SpO2
Oxygen Saturation
- PAD
Peripheral Artery Disease
- PPG
Photoplethysmogram
- PA
Physical Activity
- PPV
Positive Predictive Value
- QALY
Quality Adjusted Life Years
- QOL
Quality of Life
- RCT
Randomized Controlled Trial
- ReDS
Remote Dielectric Sensing
- RPM
Remote Physiologic Monitoring
- RR
Respiratory Rate
- SCG
Seismocardiogram
- SR
Systematic Review
- TIR
Time in Range
- TIA
Transient Ischemic Attack
REFERENCES:
- 1.Goldsack JC, Coravos A, Bakker JP, Bent B, Dowling AV, Fitzer-Attas C, Godfrey A, Godino JG, Gujar N, Izmailova E, et al. Verification, analytical validation, and clinical validation (V3): the foundation of determining fit-for-purpose for Biometric Monitoring Technologies (BioMeTs). NPJ Digit Med. 2020;3:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferguson T, Olds T, Curtis R, Blake H, Crozier AJ, Dankiw K, Dumuid D, Kasai D, O’Connor E, Virgara R, et al. Effectiveness of wearable activity trackers to increase physical activity and improve health: a systematic review of systematic reviews and meta-analyses. Lancet Digit Health. 2022;4:e615–e626. [DOI] [PubMed] [Google Scholar]
- 3.Leclercq C, Witt H, Hindricks G, Katra RP, Albert D, Belliger A, Cowie MR, Deneke T, Friedman P, Haschemi M, et al. Wearables, telemedicine, and artificial intelligence in arrhythmias and heart failure: Proceedings of the European Society of Cardiology: Cardiovascular Round Table. Europace. 2022. [DOI] [PubMed] [Google Scholar]
- 4.Sana F, Isselbacher EM, Singh JP, Heist EK, Pathik B and Armoundas AA. Wearable Devices for Ambulatory Cardiac Monitoring: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75:1582–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bayoumy K, Gaber M, Elshafeey A, Mhaimeed O, Dineen EH, Marvel FA, Martin SS, Muse ED, Turakhia MP, Tarakji KG, et al. Smart wearable devices in cardiovascular care: where we are and how to move forward. Nat Rev Cardiol. 2021;18:581–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krittanawong C, Rogers AJ, Johnson KW, Wang Z, Turakhia MP, Halperin JL and Narayan SM. Integration of novel monitoring devices with machine learning technology for scalable cardiovascular management. Nat Rev Cardiol. 2021;18:75–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacKinnon GE and Brittain EL. Mobile Health Technologies in Cardiopulmonary Disease. Chest. 2020;157:654–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang CC and Hsu YL. A review of accelerometry-based wearable motion detectors for physical activity monitoring. Sensors (Basel). 2010;10:7772–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aydemir VB, Nagesh S, Shandhi MMH, Fan J, Klein L, Etemadi M, Heller JA, Inan OT and Rehg JM. Classification of Decompensated Heart Failure From Clinical and Home Ballistocardiography. IEEE Trans Biomed Eng. 2020;67:1303–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castaneda D, Esparza A, Ghamari M, Soltanpur C and Nazeran H. A review on wearable photoplethysmography sensors and their potential future applications in health care. Int J Biosens Bioelectron. 2018;4:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kyriacou PA and Allen J. Photoplethysmography: Technology, Signal Analysis and Applications: Academic Press; 2021. [Google Scholar]
- 12.Becker DE. Fundamentals of electrocardiography interpretation. Anesth Prog. 2006;53:53–63; quiz 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steinhubl SR, Waalen J, Edwards AM, Ariniello LM, Mehta RR, Ebner GS, Carter C, Baca-Motes K, Felicione E, Sarich T, et al.Effect of a Home-Based Wearable Continuous ECG Monitoring Patch on Detection of Undiagnosed Atrial Fibrillation: The mSToPS Randomized Clinical Trial. Jama. 2018;320:146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inan OT, Migeotte PF, Park KS, Etemadi M, Tavakolian K, Casanella R, Zanetti J, Tank J, Funtova I, Prisk GK, et al. Ballistocardiography and seismocardiography: a review of recent advances. IEEE J Biomed Health Inform. 2015;19:1414–27. [DOI] [PubMed] [Google Scholar]
- 15.Inan OT, Baran Pouyan M, Javaid AQ, Dowling S, Etemadi M, Dorier A, Heller JA, Bicen AO, Roy S, De Marco T, et al. Novel Wearable Seismocardiography and Machine Learning Algorithms Can Assess Clinical Status of Heart Failure Patients. Circ Heart Fail. 2018;11:e004313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Danne T, Nimri R, Battelino T, Bergenstal RM, Close KL, DeVries JH, Garg S, Heinemann L, Hirsch I, Amiel SA, et al. International Consensus on Use of Continuous Glucose Monitoring. Diabetes Care. 2017;40:1631–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang JD, Wang J, Ramsey E, Leavey G, Chico TJA and Condell J. Applying Artificial Intelligence to Wearable Sensor Data to Diagnose and Predict Cardiovascular Disease: A Review. Sensors (Basel). 2022;22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bengio Y, Courville A and Vincent P. Representation learning: a review and new perspectives. IEEE Trans Pattern Anal Mach Intell. 2013;35:1798–828. [DOI] [PubMed] [Google Scholar]
- 19.Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN and Katzmarzyk PT. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. 2012;380:219–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee IM, Shiroma EJ, Kamada M, Bassett DR, Matthews CE and Buring JE. Association of Step Volume and Intensity With All-Cause Mortality in Older Women. JAMA Intern Med. 2019;179:1105–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Master H, Annis J, Huang S, Beckman JA, Ratsimbazafy F, Marginean K, Carroll R, Natarajan K, Harrell FE, Roden DM, et al. Association of step counts over time with the risk of chronic disease in the All of Us Research Program. Nat Med. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poirier J, Bennett WL, Jerome GJ, Shah NG, Lazo M, Yeh HC, Clark JM and Cobb NK. Effectiveness of an Activity Tracker- and Internet-Based Adaptive Walking Program for Adults: A Randomized Controlled Trial. J Med Internet Res. 2016;18:e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brickwood KJ, Watson G, O’Brien J and Williams AD. Consumer-Based Wearable Activity Trackers Increase Physical Activity Participation: Systematic Review and Meta-Analysis. JMIR Mhealth Uhealth. 2019;7:e11819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hodkinson A, Kontopantelis E, Adeniji C, van Marwijk H, McMillian B, Bower P and Panagioti M. Interventions Using Wearable Physical Activity Trackers Among Adults With Cardiometabolic Conditions: A Systematic Review and Meta-analysis. JAMA Netw Open. 2021;4:e2116382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin SS, Feldman DI, Blumenthal RS, Jones SR, Post WS, McKibben RA, Michos ED, Ndumele CE, Ratchford EV, Coresh J, et al. mActive: A Randomized Clinical Trial of an Automated mHealth Intervention for Physical Activity Promotion. J Am Heart Assoc. 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hemnes AR, Silverman-Lloyd LG, Huang S, MacKinnon G, Annis J, Whitmore CS, Mallugari R, Oggs RN, Hekmat R, Shan R, et al. A Mobile Health Intervention to Increase Physical Activity in Pulmonary Arterial Hypertension. Chest. 2021;160:1042–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel MS, Benjamin EJ, Volpp KG, Fox CS, Small DS, Massaro JM, Lee JJ, Hilbert V, Valentino M, Taylor DH, et al. Effect of a Game-Based Intervention Designed to Enhance Social Incentives to Increase Physical Activity Among Families: The BE FIT Randomized Clinical Trial. JAMA Intern Med. 2017;177:1586–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel MS, Small DS, Harrison JD, Hilbert V, Fortunato MP, Oon AL, Rareshide CAL and Volpp KG. Effect of Behaviorally Designed Gamification With Social Incentives on Lifestyle Modification Among Adults With Uncontrolled Diabetes: A Randomized Clinical Trial. JAMA Netw Open. 2021;4:e2110255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agarwal AK, Waddell KJ, Small DS, Evans C, Harrington TO, Djaraher R, Oon AL and Patel MS. Effect of Gamification With and Without Financial Incentives to Increase Physical Activity Among Veterans Classified as Having Obesity or Overweight: A Randomized Clinical Trial. JAMA Netw Open. 2021;4:e2116256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mazeas A, Duclos M, Pereira B and Chalabaev A. Evaluating the Effectiveness of Gamification on Physical Activity: Systematic Review and Meta-analysis of Randomized Controlled Trials. J Med Internet Res. 2022;24:e26779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeganathan VS, Golbus JR, Gupta K, Luff E, Dempsey W, Boyden T, Rubenfire M, Mukherjee B, Klasnja P, Kheterpal S, et al. Virtual AppLication-supported Environment To INcrease Exercise (VALENTINE) during cardiac rehabilitation study: Rationale and design. Am Heart J. 2022;248:53–62. [DOI] [PubMed] [Google Scholar]
- 32.Hardeman W, Houghton J, Lane K, Jones A and Naughton F. A systematic review of just-in-time adaptive interventions (JITAIs) to promote physical activity. Int J Behav Nutr Phys Act. 2019;16:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel MS, Bachireddy C, Small DS, Harrison JD, Harrington TO, Oon AL, Rareshide CAL, Snider CK and Volpp KG. Effect of Goal-Setting Approaches Within a Gamification Intervention to Increase Physical Activity Among Economically Disadvantaged Adults at Elevated Risk for Major Adverse Cardiovascular Events: The ENGAGE Randomized Clinical Trial. JAMA Cardiol. 2021;6:1387–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schultz WM, Kelli HM, Lisko JC, Varghese T, Shen J, Sandesara P, Quyyumi AA, Taylor HA, Gulati M, Harold JG, et al. Socioeconomic Status and Cardiovascular Outcomes: Challenges and Interventions. Circulation. 2018;137:2166–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18:1609–1678. [DOI] [PubMed] [Google Scholar]
- 36.Turakhia MP, Hoang DD, Zimetbaum P, Miller JD, Froelicher VF, Kumar UN, Xu X, Yang F and Heidenreich PA. Diagnostic utility of a novel leadless arrhythmia monitoring device. Am J Cardiol. 2013;112:520–4. [DOI] [PubMed] [Google Scholar]
- 37.Steinhubl SR, Waalen J, Sanyal A, Edwards AM, Ariniello LM, Ebner GS, Baca-Motes K, Zambon RA, Sarich T and Topol EJ. Three year clinical outcomes in a nationwide, observational, siteless clinical trial of atrial fibrillation screening-mHealth Screening to Prevent Strokes (mSToPS). PLoS One. 2021;16:e0258276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perez MV, Mahaffey KW, Hedlin H, Rumsfeld JS, Garcia A, Ferris T, Balasubramanian V, Russo AM, Rajmane A, Cheung L, et al. Large-Scale Assessment of a Smartwatch to Identify Atrial Fibrillation. N Engl J Med. 2019;381:1909–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo Y, Wang H, Zhang H, Liu T, Liang Z, Xia Y, Yan L, Xing Y, Shi H, Li S, et al. Mobile Photoplethysmographic Technology to Detect Atrial Fibrillation. J Am Coll Cardiol. 2019;74:2365–2375. [DOI] [PubMed] [Google Scholar]
- 40.Kaura A, Sztriha L, Chan FK, Aeron-Thomas J, Gall N, Piechowski-Jozwiak B and Teo JT. Early prolonged ambulatory cardiac monitoring in stroke (EPACS): an open-label randomised controlled trial. Eur J Med Res. 2019;24:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ha ACT, Verma S, Mazer CD, Quan A, Yanagawa B, Latter DA, Yau TM, Jacques F, Brown CD, Singal RK, et al. Effect of Continuous Electrocardiogram Monitoring on Detection of Undiagnosed Atrial Fibrillation After Hospitalization for Cardiac Surgery: A Randomized Clinical Trial. JAMA Netw Open. 2021;4:e2121867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seshadri DR, Bittel B, Browsky D, Houghtaling P, Drummond CK, Desai MY and Gillinov AM. Accuracy of Apple Watch for Detection of Atrial Fibrillation. Circulation. 2020;141:702–703. [DOI] [PubMed] [Google Scholar]
- 43.Ford C, Xie CX, Low A, Rajakariar K, Koshy AN, Sajeev JK, Roberts L, Pathik B and Teh AW. Comparison of 2 Smart Watch Algorithms for Detection of Atrial Fibrillation and the Benefit of Clinician Interpretation: SMART WARS Study. JACC Clin Electrophysiol. 2022;8:782–791. [DOI] [PubMed] [Google Scholar]
- 44.Dendale P, De Keulenaer G, Troisfontaines P, Weytjens C, Mullens W, Elegeert I, Ector B, Houbrechts M, Willekens K and Hansen D. Effect of a telemonitoring-facilitated collaboration between general practitioner and heart failure clinic on mortality and rehospitalization rates in severe heart failure: the TEMA-HF 1 (TElemonitoring in the MAnagement of Heart Failure) study. Eur J Heart Fail. 2012;14:333–40. [DOI] [PubMed] [Google Scholar]
- 45.Koehler F, Koehler K, Deckwart O, Prescher S, Wegscheider K, Kirwan BA, Winkler S, Vettorazzi E, Bruch L, Oeff M, et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): a randomised, controlled, parallel-group, unmasked trial. Lancet. 2018;392:1047–1057. [DOI] [PubMed] [Google Scholar]
- 46.Koehler F, Winkler S, Schieber M, Sechtem U, Stangl K, Böhm M, Boll H, Baumann G, Honold M, Koehler K, et al. Impact of remote telemedical management on mortality and hospitalizations in ambulatory patients with chronic heart failure: the telemedical interventional monitoring in heart failure study. Circulation. 2011;123:1873–80. [DOI] [PubMed] [Google Scholar]
- 47.Ong MK, Romano PS, Edgington S, Aronow HU, Auerbach AD, Black JT, De Marco T, Escarce JJ, Evangelista LS, Hanna B, et al. Effectiveness of Remote Patient Monitoring After Discharge of Hospitalized Patients With Heart Failure: The Better Effectiveness After Transition -- Heart Failure (BEAT-HF) Randomized Clinical Trial. JAMA Intern Med. 2016;176:310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anand IS, Tang WH, Greenberg BH, Chakravarthy N, Libbus I and Katra RP. Design and performance of a multisensor heart failure monitoring algorithm: results from the multisensor monitoring in congestive heart failure (MUSIC) study. J Card Fail. 2012;18:289–95. [DOI] [PubMed] [Google Scholar]
- 49.Stehlik J, Schmalfuss C, Bozkurt B, Nativi-Nicolau J, Wohlfahrt P, Wegerich S, Rose K, Ray R, Schofield R, Deswal A, et al. Continuous Wearable Monitoring Analytics Predict Heart Failure Hospitalization: The LINK-HF Multicenter Study. Circ Heart Fail. 2020;13:e006513. [DOI] [PubMed] [Google Scholar]
- 50.Amir O, Ben-Gal T, Weinstein JM, Schliamser J, Burkhoff D, Abbo A and Abraham WT. Evaluation of remote dielectric sensing (ReDS) technology-guided therapy for decreasing heart failure re-hospitalizations. Int J Cardiol. 2017;240:279–284. [DOI] [PubMed] [Google Scholar]
- 51.Lala A, Barghash MH, Giustino G, Alvarez-Garcia J, Konje S, Parikh A, Ullman J, Keith B, Donehey J, Mitter SS, et al. Early use of remote dielectric sensing after hospitalization to reduce heart failure readmissions. ESC Heart Fail. 2021;8:1047–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Redfield MM, Anstrom KJ, Levine JA, Koepp GA, Borlaug BA, Chen HH, LeWinter MM, Joseph SM, Shah SJ, Semigran MJ, et al. Isosorbide Mononitrate in Heart Failure with Preserved Ejection Fraction. N Engl J Med. 2015;373:2314–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khandwalla RM, Grant D, Birkeland K, Heywood JT, Fombu E, Owens RL and Steinhubl SR. The AWAKE-HF Study: Sacubitril/Valsartan Impact on Daily Physical Activity and Sleep in Heart Failure. Am J Cardiovasc Drugs. 2021;21:241–254. [DOI] [PubMed] [Google Scholar]
- 54.Owens RL, Birkeland K, Heywood JT, Steinhubl SR, Dorn J, Grant D, Fombu E and Khandwalla R. Sleep Outcomes From AWAKE-HF: A Randomized Clinical Trial of Sacubitril/Valsartan vs Enalapril in Patients With Heart Failure and Reduced Ejection Fraction. J Card Fail. 2021;27:1466–1471. [DOI] [PubMed] [Google Scholar]
- 55.Redeker NS, Yaggi HK, Jacoby D, Hollenbeak CS, Breazeale S, Conley S, Hwang Y, Iennaco J, Linsky S, Nwanaji-Enwerem U, et al. Cognitive behavioral therapy for insomnia has sustained effects on insomnia, fatigue, and function among people with chronic heart failure and insomnia: the HeartSleep Study. Sleep. 2022;45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thomas RJ, Beatty AL, Beckie TM, Brewer LC, Brown TM, Forman DE, Franklin BA, Keteyian SJ, Kitzman DW, Regensteiner JG, et al. Home-Based Cardiac Rehabilitation: A Scientific Statement From the American Association of Cardiovascular and Pulmonary Rehabilitation, the American Heart Association, and the American College of Cardiology. J Am Coll Cardiol. 2019;74:133–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Frederix I, Van Driessche N, Hansen D, Berger J, Bonne K, Alders T and Dendale P. Increasing the medium-term clinical benefits of hospital-based cardiac rehabilitation by physical activity telemonitoring in coronary artery disease patients. Eur J Prev Cardiol. 2015;22:150–8. [DOI] [PubMed] [Google Scholar]
- 58.Maddison R, Rawstorn JC, Stewart RAH, Benatar J, Whittaker R, Rolleston A, Jiang Y, Gao L, Moodie M, Warren I, et al. Effects and costs of real-time cardiac telerehabilitation: randomised controlled non-inferiority trial. Heart. 2019;105:122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brouwers RWM, Kraal JJ, Regis M, Spee RF and Kemps HMC. Effectiveness of Cardiac Telerehabilitation With Relapse Prevention: SmartCare-CAD Randomized Controlled Trial. J Am Coll Cardiol. 2021;77:2754–2756. [DOI] [PubMed] [Google Scholar]
- 60.Brouwers RWM, van der Poort EKJ, Kemps HMC, van den Akker-van Marle ME and Kraal JJ. Cost-effectiveness of Cardiac Telerehabilitation With Relapse Prevention for the Treatment of Patients With Coronary Artery Disease in the Netherlands. JAMA Netw Open. 2021;4:e2136652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nagatomi Y, Ide T, Higuchi T, Nezu T, Fujino T, Tohyama T, Nagata T, Higo T, Hashimoto T, Matsushima S, et al. Home-based cardiac rehabilitation using information and communication technology for heart failure patients with frailty. ESC Heart Fail. 2022;9:2407–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, Fleisher LA, Fowkes FG, Hamburg NM, Kinlay S, et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e686–e725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gardner AW, Parker DE, Montgomery PS, Scott KJ and Blevins SM. Efficacy of quantified home-based exercise and supervised exercise in patients with intermittent claudication: a randomized controlled trial. Circulation. 2011;123:491–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McDermott MM, Spring B, Berger JS, Treat-Jacobson D, Conte MS, Creager MA, Criqui MH, Ferrucci L, Gornik HL, Guralnik JM, et al. Effect of a Home-Based Exercise Intervention of Wearable Technology and Telephone Coaching on Walking Performance in Peripheral Artery Disease: The HONOR Randomized Clinical Trial. Jama. 2018;319:1665–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McDermott MM, Spring B, Tian L, Treat-Jacobson D, Ferrucci L, Lloyd-Jones D, Zhao L, Polonsky T, Kibbe MR, Bazzano L, et al. Effect of Low-Intensity vs High-Intensity Home-Based Walking Exercise on Walk Distance in Patients With Peripheral Artery Disease: The LITE Randomized Clinical Trial. Jama. 2021;325:1266–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Treskes RW, van Winden LAM, van Keulen N, van der Velde ET, Beeres S, Atsma DE and Schalij MJ. Effect of Smartphone-Enabled Health Monitoring Devices vs Regular Follow-up on Blood Pressure Control Among Patients After Myocardial Infarction: A Randomized Clinical Trial. JAMA Netw Open. 2020;3:e202165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leelarathna L, Evans ML, Neupane S, Rayman G, Lumley S, Cranston I, Narendran P, Barnard-Kelly K, Sutton CJ, Elliott RA, et al. Intermittently Scanned Continuous Glucose Monitoring for Type 1 Diabetes. N Engl J Med. 2022;387:1477–1487. [DOI] [PubMed] [Google Scholar]
- 68.Beck RW, Riddlesworth TD, Ruedy K, Ahmann A, Haller S, Kruger D, McGill JB, Polonsky W, Price D, Aronoff S, et al. Continuous Glucose Monitoring Versus Usual Care in Patients With Type 2 Diabetes Receiving Multiple Daily Insulin Injections: A Randomized Trial. Ann Intern Med. 2017;167:365–374. [DOI] [PubMed] [Google Scholar]
- 69.Martens T, Beck RW, Bailey R, Ruedy KJ, Calhoun P, Peters AL, Pop-Busui R, Philis-Tsimikas A, Bao S, Umpierrez G, et al. Effect of Continuous Glucose Monitoring on Glycemic Control in Patients With Type 2 Diabetes Treated With Basal Insulin: A Randomized Clinical Trial. Jama. 2021;325:2262–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lu J, Wang C, Shen Y, Chen L, Zhang L, Cai J, Lu W, Zhu W, Hu G, Xia T, et al. Time in Range in Relation to All-Cause and Cardiovascular Mortality in Patients With Type 2 Diabetes: A Prospective Cohort Study. Diabetes Care. 2021;44:549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guo J, Wang J, Zhao Z and Yu L. Association between glycemic control assessed by continuous glucose monitoring and stroke in patients with atrial fibrillation and diabetes mellitus. Ann Palliat Med. 2021;10:9157–9164. [DOI] [PubMed] [Google Scholar]
- 72.Eberly LA, Kallan MJ, Julien HM, Haynes N, Khatana SAM, Nathan AS, Snider C, Chokshi NP, Eneanya ND, Takvorian SU, et al. Patient Characteristics Associated With Telemedicine Access for Primary and Specialty Ambulatory Care During the COVID-19 Pandemic. JAMA Netw Open. 2020;3:e2031640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rubin R Internet Access as a Social Determinant of Health. Jama. 2021;326:298. [DOI] [PubMed] [Google Scholar]
- 74.Brewer LC, Fortuna KL, Jones C, Walker R, Hayes SN, Patten CA and Cooper LA. Back to the Future: Achieving Health Equity Through Health Informatics and Digital Health. JMIR Mhealth Uhealth. 2020;8:e14512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee EW, McCloud RF and Viswanath K. Designing Effective eHealth Interventions for Underserved Groups: Five Lessons From a Decade of eHealth Intervention Design and Deployment. J Med Internet Res. 2022;24:e25419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee EWJ and Viswanath K. Big Data in Context: Addressing the Twin Perils of Data Absenteeism and Chauvinism in the Context of Health Disparities Research. J Med Internet Res. 2020;22:e16377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chandrasekaran R, Katthula V and Moustakas E. Too old for technology? Use of wearable healthcare devices by older adults and their willingness to share health data with providers. Health Informatics J. 2021;27:14604582211058073. [DOI] [PubMed] [Google Scholar]
- 78.Hoque R and Sorwar G. Understanding factors influencing the adoption of mHealth by the elderly: An extension of the UTAUT model. Int J Med Inform. 2017;101:75–84. [DOI] [PubMed] [Google Scholar]
- 79.Abouzahra M and Ghasemaghaei M. The antecedents and results of seniors’ use of activity tracking wearable devices. Health Policy and Technology. 2020;9:213–217. [Google Scholar]
- 80.Coravos A, Doerr M, Goldsack J, Manta C, Shervey M, Woods B and Wood WA. Modernizing and designing evaluation frameworks for connected sensor technologies in medicine. NPJ Digit Med. 2020;3:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dai H, Younis A, Kong JD, Puce L, Jabbour G, Yuan H and Bragazzi NL. Big Data in Cardiology: State-of-Art and Future Prospects. Front Cardiovasc Med. 2022;9:844296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cefalu WT, Kaul S, Gerstein HC, Holman RR, Zinman B, Skyler JS, Green JB, Buse JB, Inzucchi SE, Leiter LA, et al. Cardiovascular Outcomes Trials in Type 2 Diabetes: Where Do We Go From Here? Reflections From a Diabetes Care Editors’ Expert Forum. Diabetes Care. 2018;41:14–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dinh-Le C, Chuang R, Chokshi S and Mann D. Wearable Health Technology and Electronic Health Record Integration: Scoping Review and Future Directions. JMIR Mhealth Uhealth. 2019;7:e12861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Banerjee S, Hemphill T and Longstreet P. Wearable devices and healthcare: Data sharing and privacy. The Information Society. 2018;34:49–57. [Google Scholar]
- 85.Smuck M, Odonkor CA, Wilt JK, Schmidt N and Swiernik MA. The emerging clinical role of wearables: factors for successful implementation in healthcare. NPJ Digit Med. 2021;4:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wilkinson MD, Dumontier M, Aalbersberg IJ, Appleton G, Axton M, Baak A, Blomberg N, Boiten JW, da Silva Santos LB, Bourne PE, et al. The FAIR Guiding Principles for scientific data management and stewardship. Sci Data. 2016;3:160018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Baig MM, GholamHosseini H, Moqeem AA, Mirza F and Lindén M. A Systematic Review of Wearable Patient Monitoring Systems - Current Challenges and Opportunities for Clinical Adoption. J Med Syst. 2017;41:115. [DOI] [PubMed] [Google Scholar]
- 88.Espinoza J, Klonoff D, Vidmar A, Tut M, Corathers S, Seigel R, Yeung A, Xu N, Shah P, Babaei M, et al. 2022 iCoDE Report: CGM-EHR Integration Standards and Recommendations. Diabetes Technology Society. . 2022. [Google Scholar]
- 89.Dey P, Jarrin R, Mori M, Geirsson A and Krumholz HM. Leveraging Remote Physiologic Monitoring in the COVID-19 Pandemic to Improve Care After Cardiovascular Hospitalizations. Circ Cardiovasc Qual Outcomes. 2021;14:e007618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee GS K AAMC Regulatory Resource: 2021 Medicare Coverage of Remote Physiologic Monitoring (RPM). 2021. [Google Scholar]
- 91.Chen W, Khurshid S, Singer DE, Atlas SJ, Ashburner JM, Ellinor PT, McManus DD, Lubitz SA and Chhatwal J. Cost-effectiveness of Screening for Atrial Fibrillation Using Wearable Devices. JAMA Health Forum. 2022;3:e222419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mattison G, Canfell O, Forrester D, Dobbins C, Smith D, Töyräs J and Sullivan C. The Influence of Wearables on Health Care Outcomes in Chronic Disease: Systematic Review. J Med Internet Res. 2022;24:e36690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Feehan LM, Geldman J, Sayre EC, Park C, Ezzat AM, Yoo JY, Hamilton CB and Li LC. Accuracy of Fitbit Devices: Systematic Review and Narrative Syntheses of Quantitative Data. JMIR Mhealth Uhealth. 2018;6:e10527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brooke SM, An HS, Kang SK, Noble JM, Berg KE and Lee JM. Concurrent Validity of Wearable Activity Trackers Under Free-Living Conditions. J Strength Cond Res. 2017;31:1097–1106. [DOI] [PubMed] [Google Scholar]
- 95.Tedesco S, Sica M, Ancillao A, Timmons S, Barton J and O’Flynn B. Validity Evaluation of the Fitbit Charge2 and the Garmin vivosmart HR+ in Free-Living Environments in an Older Adult Cohort. JMIR Mhealth Uhealth. 2019;7:e13084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Herkert C, Kraal JJ, van Loon EMA, van Hooff M and Kemps HMC. Usefulness of Modern Activity Trackers for Monitoring Exercise Behavior in Chronic Cardiac Patients: Validation Study. JMIR Mhealth Uhealth. 2019;7:e15045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li T, Sahu AK, Talwalkar A and Smith V. Federated learning: Challenges, methods, and future directions. IEEE Signal Processing Magazine. 2020;37:50–60. [Google Scholar]
- 98.Trayanova NA, Popescu DM and Shade JK. Machine Learning in Arrhythmia and Electrophysiology. Circ Res. 2021;128:544–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Litjens G, Ciompi F, Wolterink JM, de Vos BD, Leiner T, Teuwen J and Išgum I. State-of-the-Art Deep Learning in Cardiovascular Image Analysis. JACC Cardiovasc Imaging. 2019;12:1549–1565. [DOI] [PubMed] [Google Scholar]
- 100.Gerke S, Minssen T and Cohen G. Ethical and legal challenges of artificial intelligence-driven healthcare Artificial intelligence in healthcare: Elsevier; 2020: 295–336. [Google Scholar]
- 101.Amann J, Blasimme A, Vayena E, Frey D and Madai VI. Explainability for artificial intelligence in healthcare: a multidisciplinary perspective. BMC Med Inform Decis Mak. 2020;20:310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shandhi MMH, Hersek S, Fan J, Sander E, De Marco T, Heller JA, Etemadi M, Klein L and Inan OT. Wearable Patch-Based Estimation of Oxygen Uptake and Assessment of Clinical Status during Cardiopulmonary Exercise Testing in Patients With Heart Failure. J Card Fail. 2020;26:948–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dunn J, Kidzinski L, Runge R, Witt D, Hicks JL, Schüssler-Fiorenza Rose SM, Li X, Bahmani A, Delp SL, Hastie T, et al. Wearable sensors enable personalized predictions of clinical laboratory measurements. Nat Med. 2021;27:1105–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hannun AY, Rajpurkar P, Haghpanahi M, Tison GH, Bourn C, Turakhia MP and Ng AY. Cardiologist-level arrhythmia detection and classification in ambulatory electrocardiograms using a deep neural network. Nat Med. 2019;25:65–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Attia ZI, Kapa S, Lopez-Jimenez F, McKie PM, Ladewig DJ, Satam G, Pellikka PA, Enriquez-Sarano M, Noseworthy PA, Munger TM, et al. Screening for cardiac contractile dysfunction using an artificial intelligence-enabled electrocardiogram. Nat Med. 2019;25:70–74. [DOI] [PubMed] [Google Scholar]
- 106.Krittanawong C, Virk HUH, Bangalore S, Wang Z, Johnson KW, Pinotti R, Zhang H, Kaplin S, Narasimhan B, Kitai T,et al. Machine learning prediction in cardiovascular diseases: a meta-analysis. Sci Rep. 2020;10:16057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ballinger B, Hsieh J, Singh A, Sohoni N, Wang J, Tison GH, Marcus GM, Sanchez JM, Maguire C and Olgin JE. DeepHeart: semi-supervised sequence learning for cardiovascular risk prediction. Thirty-Second AAAI Conference on Artificial Intelligence. 2018. [Google Scholar]
- 108.Shandhi MMH, Fan J, Heller JA, Etemadi M, Klein L and Inan OT. Estimation of Changes in Intracardiac Hemodynamics Using Wearable Seismocardiography and Machine Learning in Patients With Heart Failure: A Feasibility Study. IEEE Trans Biomed Eng. 2022;69:2443–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shandhi MMH, Semiz B, Hersek S, Goller N, Ayazi F and Inan OT. Performance Analysis of Gyroscope and Accelerometer Sensors for Seismocardiography-Based Wearable Pre-Ejection Period Estimation. IEEE J Biomed Health Inform. 2019;23:2365–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zheng H, Ryzhov IO, Xie W and Zhong J. Personalized Multimorbidity Management for Patients with Type 2 Diabetes Using Reinforcement Learning of Electronic Health Records. Drugs. 2021;81:471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Halcox JPJ, Wareham K, Cardew A, Gilmore M, Barry JP, Phillips C and Gravenor MB. Assessment of Remote Heart Rhythm Sampling Using the AliveCor Heart Monitor to Screen for Atrial Fibrillation: The REHEARSE-AF Study. Circulation. 2017;136:1784–1794. [DOI] [PubMed] [Google Scholar]
- 112.Gladstone DJ, Wachter R, Schmalstieg-Bahr K, Quinn FR, Hummers E, Ivers N, Marsden T, Thornton A, Djuric A, Suerbaum J, et al. Screening for Atrial Fibrillation in the Older Population: A Randomized Clinical Trial. JAMA Cardiol. 2021;6:558–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.McDermott MM, Liu K, Guralnik JM, Criqui MH, Spring B, Tian L, Domanchuk K, Ferrucci L, Lloyd-Jones D, Kibbe M,et al. Home-based walking exercise intervention in peripheral artery disease: a randomized clinical trial. Jama. 2013;310:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]


