Abstract
The incidence of age-related dementia is increasing as the world population ages and due to lack of effective treatments for dementia. Vascular contributions to cognitive impairment and dementia are increasing as the prevalence of pathologies associated with cerebrovascular disease rise, including chronic hypertension, diabetes and ischemic stroke. The hippocampus is a bilateral deep brain structure that is central to learning, memory and cognitive function and highly susceptible to hypoxic/ischemic injury. Compared to cortical brain regions such as the somatosensory cortex, less is known about the function of the hippocampal vasculature that is critical in maintaining neurocognitive health. This review focuses on the hippocampal vascular supply, presenting what is known about hippocampal hemodynamics and blood-brain barrier function during health and disease, and discusses evidence that supports its contribution to vascular cognitive impairment and dementia. Understanding vascular-mediated hippocampal injury that contributes to memory dysfunction during healthy aging and cerebrovascular disease is essential to develop effective treatments to slow cognitive decline. The hippocampus and its vasculature may represent one such therapeutic target to mitigate the dementia epidemic.
Introduction
Alzheimer’s disease (AD) is the leading cause of clinically diagnosed dementia, with vascular contributions to cognitive impairment and dementia being the second leading cause of age-related dementia.1 However, emerging evidence suggests that the majority of patients with AD have mixed dementia, with both classic AD neurodegenerative pathology as well as cerebrovascular pathology.2 Therefore, the incidence of vascular-related cognitive impairment may be underestimated. Vascular cognitive impairment (VCI) is a broad term that encompasses a spectrum of cognitive dysfunction across multiple cognitive domains due to cerebrovascular pathology, including ischemic stroke (i.e., strategic infarcts) and/or cerebral small vessel disease that damages the cerebral microvasculature, resulting in white matter lesions and microinfarcts.1, 3–6 Therefore, understanding cerebrovascular function in health and disease is important, particularly in brain regions central to learning, memory and cognition, such as the hippocampus.
Pathologies that impact the cerebral circulation and contribute to VCI have been extensively studied, including chronic hypertension, diabetes and ischemic stroke.1, 7 Importantly, the vast majority of animal studies have been completed almost exclusively in the vasculature supplying the sensorimotor cortex and striatum (e.g. lenticulostriatal branches of the middle cerebral artery, MCA). However, reductions in hippocampal blood flow occur during healthy aging, and contribute to several pathologies that involve hippocampal neuronal atrophy and memory decline, including VCI, demonstrating a need to better understand hippocampal vascular function in health and disease.8–12 Despite the importance of the hippocampus in memory function and its high susceptibility to injury, we know remarkably little about the function of the hippocampal vasculature. VCI has recently been reviewed extensively elsewhere.1, 6, 13 For the purposes of this review, the term VCI (vascular cognitive impairment) will be used as it includes mild cognitive impairment due to cerebrovascular disease up to its most severe form, vascular dementia.1, 6 Although recent criteria no longer require memory impairment for a VCI diagnosis which is more characteristic of AD, memory is an important cognitive domain that can be disrupted, having a tremendous impact of the patient’s quality of life.14 Further, the hippocampus and its vasculature may be particularly important in dementia patients with mixed vascular and AD pathologies. This review will focus on the hippocampal vascular supply and discuss evidence that supports its contribution to VCI.
The purpose of this review is to introduce the hippocampal vascular supply as a potential contributor to VCI. An in-depth review of what is known about hippocampal arteriole (HA) function, hippocampal hemodynamics and blood-brain barrier (BBB) function, including properties that are unique from other cerebrovascular territories that increase the susceptibility of the hippocampus to hypoxic/ischemic injury will be discussed. The structure and function of the hippocampal vasculature under physiological (e.g. healthy aging) and pathological conditions will be presented, predominantly focusing on chronic hypertension and ischemic stroke that are primary contributors to VCI.
The Vascular Supply of the Hippocampus
The hippocampus is part of the archicortex and is a bilateral brain structure deep within the temporal lobes centrally involved in learning and memory. The blood supply to the hippocampus is dependent primarily upon the collateral branches of the posterior cerebral artery (PCA) with some contributions coming from the anterior choroidal artery (AChA).15–17 Common vascularization patterns of the hippocampus have been identified through histological studies, neurosurgical autopsy studies and more recently by an in vivo study using MRA (magnetic resonance angiography). Erdem et al. (1993) microsurgically characterized 30 fixed brain hemispheres post-mortem and reported that the most frequent pattern of vascularization of the hippocampus was a mixed supply of branches of the PCA and AChA, occurring in 17/30 hemispheres (57 %).15 Spallazzi et al. (2019) used high resolution 7 Tesla time-of-flight MRA to assess hippocampal vascularization patterns in 62 hemispheres in vivo in healthy, young adults that were 19 – 34 years old. They found that 31 (50 %) of hemispheres had a mixed vascular supply from both the PCA and AChA.17 The next most common patterns of the origin of the hippocampal arteries identified were predominantly PCA branches, including inferior temporal branches of the PCA (27 – 34 %) and the anterior inferior temporal branch of the PCA (10 – 11 %).15, 17 The least common vascularization pattern was the hippocampus being supplied only by branches of the AChA, occurring in 3 – 5 % of subjects.15, 17 Demené et al. (2016) used ultrafast Doppler tomography with high resolution imaging to reconstruct the rat brain vasculature in 3D (Figure 1A).18 Figure 1B shows the bilateral hippocampal vasculature in blue as it sits deep within the brain compared to the cortical vasculature shown in red.18
Figure 1.
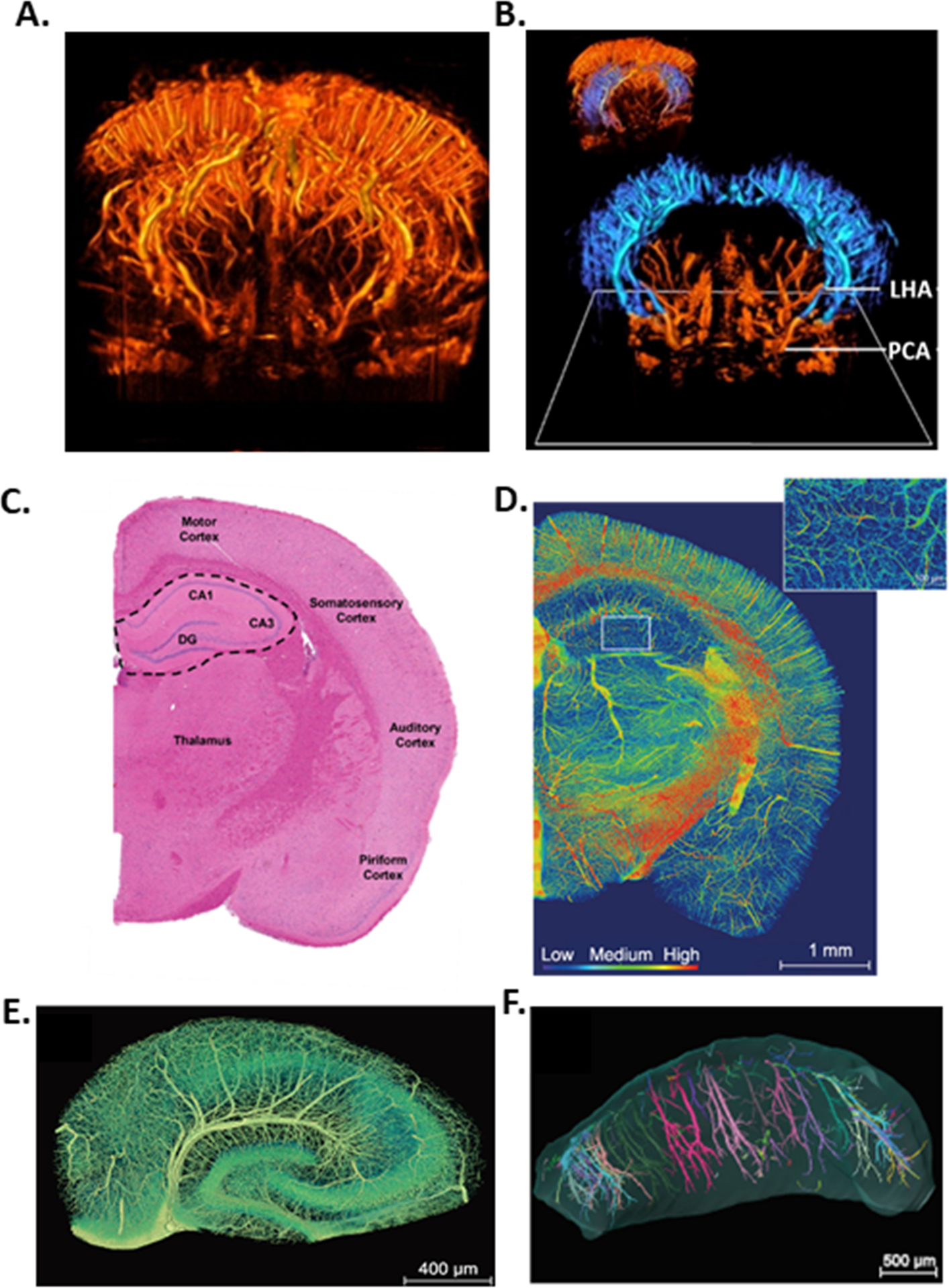
(A) High-quality 3D reconstruction of the rat brain vasculature. (B) The bilateral hippocampal vasculature (blue) deep within the brain, originating from the posterior cerebral artery (PCA) giving rise to the longitudinal hippocampal artery (LHA) to perfuse the hippocampi. (C) Half of a coronal section of rodent brain stained with H&E (hematoxylin and eosin). The hippocampal formation is outlined and tightly packed pyramidal neurons are visible comprising the hippocampal subfields (DG, dentate gyrus; CA, cornu ammonis). (D) Half of a coronal section of mouse brain with a heat map visualization of the vascular volumetric density coded by color scale. Inset in the hippocampus showing reduced vascular density demonstrated by cooler colors. (E) Coronal section through the hippocampus showing the internal transverse hippocampal artery in the hippocampal sulcus and its long arch-like arteriole branches that perfuse CA1 and CA3 subfields. (F) Longitudinal view of bilateral hippocampi along the septotemporal axis, with the temporal pole (ventral hippocampus) on the left and septal pole (dorsal hippocampus) on the right. Colors represent individual vessels. Panels A and B contain data and images provided by Iconeus, France and are adapted from NeuroImage, 127, Demené et al., 4D microvascular imaging based on ultrafast Doppler tomography, page 481, Copyright © 2016, with permission from Elsevier. Panels D-F are adapted from Zhang et al., 2019 Nat Sci Rev doi: 10.1093/nsr/nwz124, an open access journal https://creativecommons.org/licenses/by/4.0/.
Although it is generally accepted that the majority of the hippocampal blood supply comes from the PCA, there are variations in the regional contributions of blood flow to the PCA and therefore the hippocampus due to heterogeneity of the Circle of Willis. Smaller mammals (e.g. cats, sheep, gerbils) tend to have a greater proportion of hippocampal blood flow from the anterior circulation, with the PCA being largely supplied by the carotids via the posterior communicating artery.19, 20 In humans, the PCA is more often supplied by the vertebrobasilar system, making the posterior circulation the dominant source of hippocampal blood supply. However, it is estimated that ~ 20 – 30 % of the population has a fetal PCA in which the posterior communicating artery is larger than the P1 segment of the PCA, or a hypoplastic P1 segment, resulting in the anterior circulation supplying the majority of blood flow to the PCA.21–23 Thus, there is variability in the contributions of anterior vs. posterior circulation to distal branches of the PCA perfusing the hippocampus. A transient MCA occlusion (tMCAO) in gerbils and mice that lack a P1 segment of PCA and rely more heavily on anterior circulation for hippocampal blood flow have infarction that includes in portions of the hippocampal region.24, 25 Thus, the source of PCA blood flow becomes important if/when these people with an anterior-dominated PCA have a cerebral ischemic stroke. A large vessel occlusion in the carotid system or MCA could disrupt blood flow to the hippocampus, resulting in hippocampal ischemia and post-stroke memory loss. This is likely compounded by the fact that the hippocampus is one of the most susceptible brain regions to hypoxic and ischemic injury.26, 27 Thus, maintenance of appropriate hippocampal perfusion, including basal blood flow and activity-dependent changes in blood flow is critical to preserving healthy memory and cognitive function.
The Microarchitecture of the Hippocampal Vasculature Promotes Hypoxia and Ischemia
The hippocampus is distinct from cortical brain regions comprising the neocortex (i.e. sensorimotor cortex) from architectural and functional standpoints, both of which are important to consider to understand regional differences in susceptibility to hypoxia and ischemia. The hippocampal formation is formed by the infolding of the dentate gyrus, forming the hippocampal sulcus, the hippocampus proper cornu ammonis (CA), a seahorse-shaped structure containing the hippocampal subfields CA1–4, and the subiculum.28 The dentate gyrus and hippocampus proper form two C-shaped rings that interlock, with the subiculum connecting them. While the neocortex and hippocampus are both laminar structures, the neocortex is organized horizontally into six neuronal layers whereas the hippocampus is organized into three layers, with its tightly packed large pyramidal neuron cell bodies in the middle layer (Figure 1C). Unlike the somatosensory cortex, for example, that is also organized vertically into cortical columns of neurons that correspond to the same peripheral receptive field, no such columnar organization occurs in the hippocampus.29 Large groups of adjacent hippocampal pyramidal neurons (i.e. place cells) are not active all at once such as in cortical brain regions. Thus, the local energy demand in active hippocampus is likely less than in active cortex, and mediators involved in matching neuronal metabolic demand with appropriate increases in local blood flow (e.g. neurovascular coupling; NVC) may be distinct from other more well-studied cortical brain regions.27, 30
From a vascular standpoint, vascular density in the hippocampus is a fraction of the vast capillary network in the neocortex. Several studies have exquisitely mapped the microvasculature of the rodent brain and consistently report the hippocampus has less dense vascularization than other brain regions.31–35 Figure 1D shows a heat map visualization of the vascular volumetric density of a coronal section of mouse brain coded by color scale, with deep blue representing no vascularization.35 The cortex is highly vascularized with abundant arterioles penetrating the cortex giving rise to a robust microvascular network. In the hippocampus, arterioles penetrate perpendicularly to the coronal plane, and the microvascular network is substantially more sparse, demonstrated by the hippocampus being visually cooler in color than other brain regions (Figure 1D).35 Further, Ji et al. (2021) showed that the hippocampus is second only to corpus callosum in having the lowest vascular density, with there being fewer microvessels that were more widely spaced, requiring oxygen to diffuse further.34 Despite lower vascular density, the metabolic demand in the hippocampus was estimated to be matched appropriately, likely due to fewer spatially organized neurons being active at the same time than in cortical brain regions.34 However, the substantial reduction in capillary density, relatively long distance between microvessels, and tightly packed pyramidal neurons suggests the hippocampus may not be well suited to maintain necessary perfusion under pathological conditions. Diseases that cause even modest reductions in hippocampal blood flow, potentially due to capillary rarefaction, hyperconstriction and/or inward remodeling of HAs, would likely have a tremendous impact on neuronal function, memory and cognition.
The Hippocampal Vasculature is Critical to Neurocognitive Health
Reductions in hippocampal perfusion occur during healthy aging, and also underlie many pathologies that involve hippocampal volume changes (e.g. atrophy) and cognitive decline.8, 10 In fact, the vascular reserve of the hippocampus is now considered a primary contributing factor to cognitive performance,36 and understanding hippocampal vascular function is therefore central to protecting neurocognitive health. In older adults (~ 70 years old), greater blood flow to the hippocampus was positively correlated with memory performance, as measured using flow-enhanced signal intensity fMRI during a spatial memory task.12 Further, older adults that had at least one hippocampus with a mixed vascular supply from both the PCA and AChA had both higher average memory performance and higher learning rate during a memory task than individuals with no mixed vascular supply.36 Patients with cerebral small vessel disease, most often a result of chronic hypertension and a primary contributor to VCI, performed worse on most cognitive tasks compared to healthy individuals.36 However, of these patients, those that had a mixed hippocampal vascular supply performed better on memory tasks than those with a single vascular supply.36 These findings highlight the importance of hippocampal perfusion in healthy aging and directly implicate the hippocampal vasculature in cognitive health. Further, they suggest that greater hippocampal vascular reserve and improved perfusion may be protective against VCI.
Hippocampal Arterioles are Functionally Unique
The vasculature in the hippocampus forms a rake-like pattern comprised of a series of consistently spaced, alternating arcs of arterioles and venules.31 The internal transverse hippocampal artery runs in the hippocampal sulcus that sends long arch-like branches of arterioles deep into the hippocampus to perfuse CA1 and CA3 subfields (Figure 1E and 1F).31, 35 Until relatively recently, functional studies of arterioles perfusing the hippocampus have been limited compared to arterioles perfusing more accessible cortical brain regions. However, pressure myography has now been used to study isolated HAs independently of neuronal and glial influences under physiologically pressurized conditions.37–39 Figure 2 shows an illustration of the vasculature of the rat hippocampus, and a representative photomicrograph of a HA cannulated and pressurized in an arteriograph chamber for functional pressure myography studies.37, 40
Figure 2.
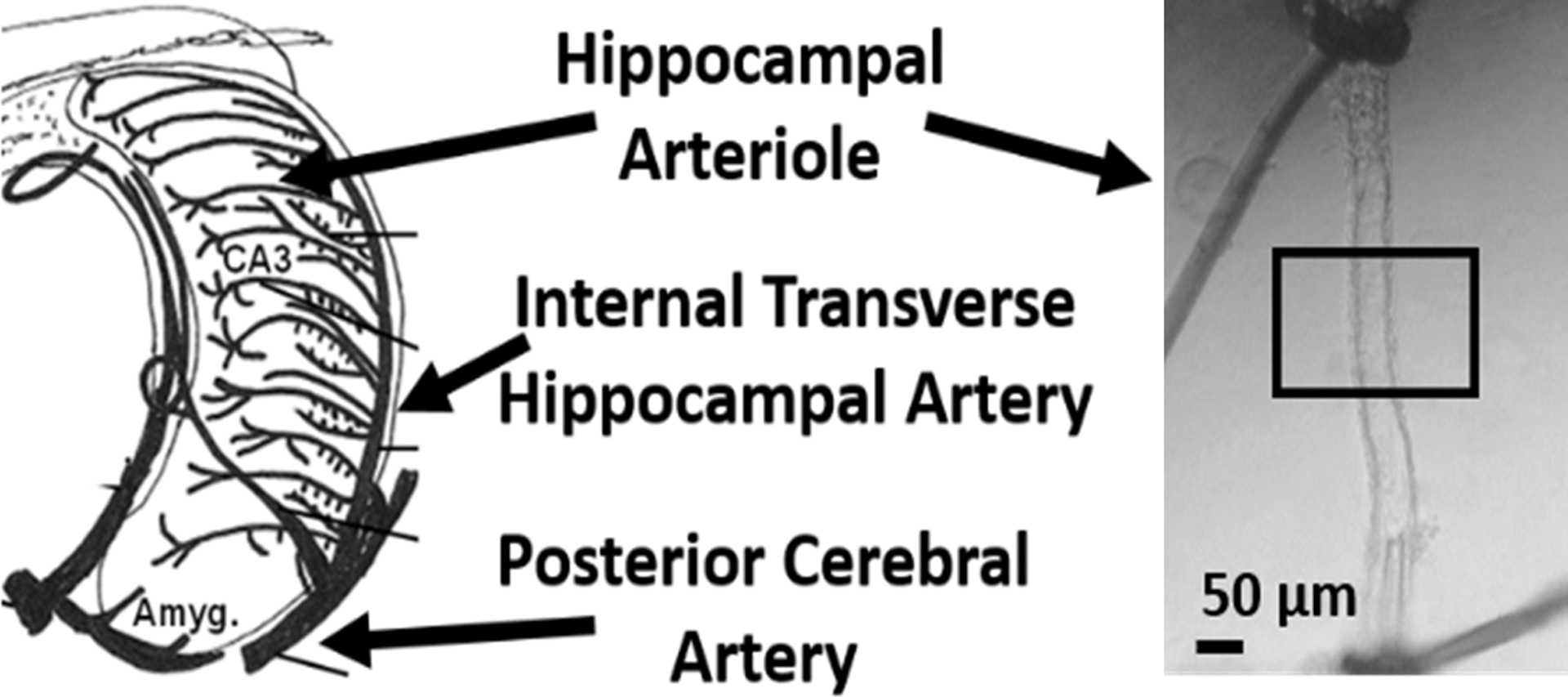
The arterial blood supply of the rat hippocampus and an isolated and pressurized segment of a hippocampal arteriole cannulated in an arteriograph chamber at 40 mmHg intravascular pressure. Adapted from Johnson & Cipolla, JCBFM, Volume 37, Issue 8, Page 2860, Copyright © 2016, with permission of SAGE Publications.
The most powerful determinant of hippocampal blood flow is the lumen diameter of its arterioles. This is because resistance to flow is inversely proportional to the radius to the 4th power, such that small decreases in lumen diameter can drastically increase vascular resistance and reduce perfusion, and vice versa.41 Lumen diameter is a result of a balance between vasoconstrictive and vasodilatory factors, and can rapidly change to control blood flow. In the cerebral circulation, one of the most influential vasoconstrictive stimuli is intravascular pressure. Vascular smooth muscle (VSM) of cerebral arteries and arterioles has the intrinsic ability to constrict or dilate in response to increased or decreased intravascular pressure, respectively.41, 42 This myogenic response is central to maintaining vascular resistance and cerebral hemodynamics. HAs from healthy male and female rats develop ~ 40 % basal myogenic tone at 40 mmHg intravascular pressure that is similar to penetrating or parenchymal arterioles branching off the MCA to perfuse the striatum and sensorimotor cortex.38, 43
In addition to VSM, the endothelium of cerebral arterioles also plays an important role in cerebral hemodynamics by producing vasoactive mediators that influence lumen diameter, vascular resistance and blood flow.44 In endothelial cells of pial arteries and penetrating arterioles in the cortex, nitric oxide (NO), a potent vasodilator, is basally produced through activity of endothelial NO synthase (eNOS) that counters myogenic tone.44, 45 NOS inhibition causes robust vasoconstriction of most cerebral arteries and arterioles, demonstrating basal production and release of NO that is important for limiting vasoconstriction due to myogenic and other influences, contributing to regulation of cerebral blood flow.45–47 In the hippocampus, however, neuronal (n)NOS-derived NO is considered the primary source of basal NO production. Studies show nNOS-containing nerve fibers are located in close proximity to HAs that generate NO and suggest tonic activity of this neuronal network influences lumen diameter of HAs, with little role for eNOS.48, 49 In support of this, recent pressure myography studies investigating HA function independently of neuronal influences show that the endothelium does not appear to basally produce NO that is in striking contrast to parenchymal arterioles supplying the sensorimotor cortex.37, 43, 44 Figure 3 compares the constriction of isolated and pressurized PCAs (upstream of HAs), cortical parenchymal arterioles and HAs in response to NOS inhibition with L-NAME (NG-nitro-L-arginine methyl ester). All vessels had a healthy level of myogenic tone prior to L-NAME treatment. PCA and cortical parenchymal arterioles underwent a 30 – 40 % vasoconstriction when NOS was inhibited, indicating basal NO production in the endothelium of these vascular segments that reduces myogenic tone. However, lumen diameter did not change and there was no constriction of HAs with NOS inhibition (Figure 3A and 3B), supporting that endothelium does not appear to produce basal NO in the hippocampus.37, 38
Figure 3.
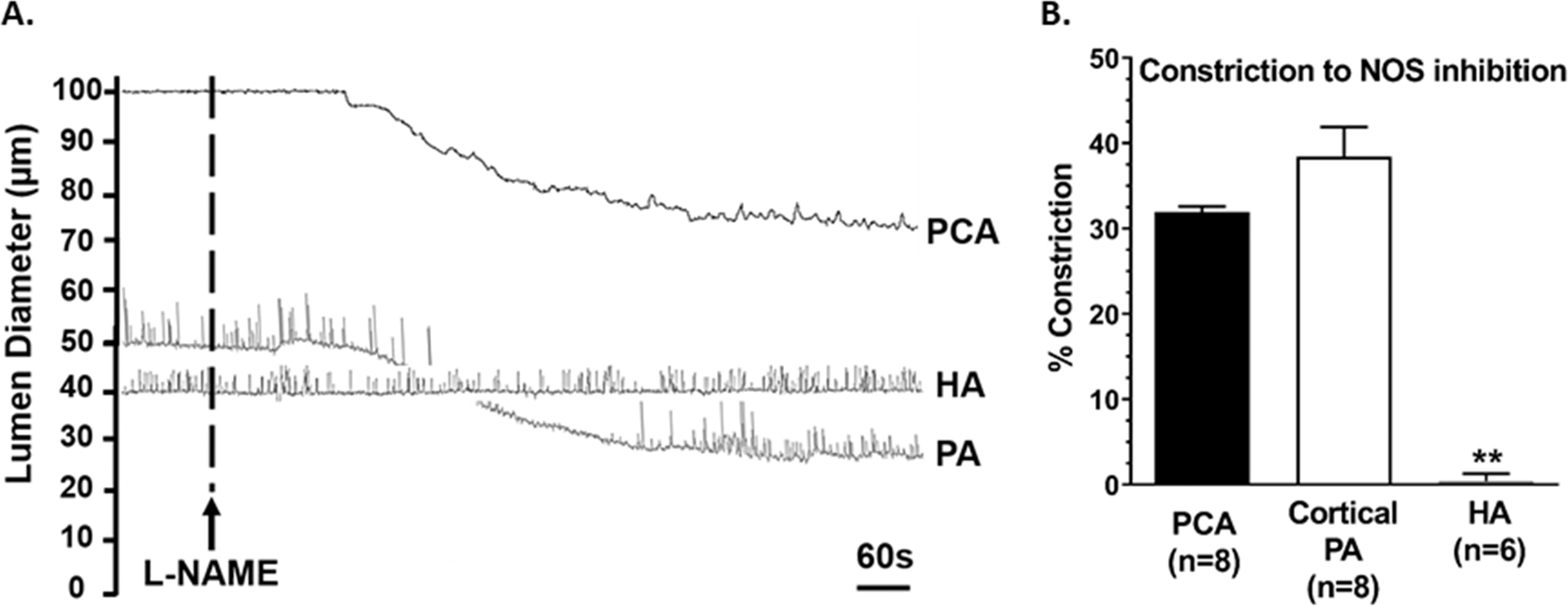
(A) Representative lumen diameter tracings of an isolated and pressurized posterior cerebral artery (PCA) at 75 mmHg intravascular pressure with ~30% tone, cortical parenchymal arteriole (PA) at 40 mmHg with ~40 % tone and hippocampal arteriole (HA) at 40 mmHg intravascular pressure with ~ 40% tone from male Wistar rats before and after treatment with the nitric oxide synthase (NOS) inhibitor NG-nitro-L-arginine methyl ester (L-NAME, 10−3M). (B) Percent constriction to NOS inhibition. **p<0.01 vs. PCA and PA by one-way ANOVA with post-hoc Bonferroni test.
The data presented in Figure 3 were obtained from studies performed in normotensive male rats. However, the lack of response to L-NAME also occurs in HAs from both normotensive and hypertensive male and female rats.37, 50 The well-established endothelial-dependent and NO-dependent vasodilator acetylcholine also did not elicit a change in HA diameter even at high concentrations known to cause vasodilation of other cerebrovascular segments.51, 52 It should be noted, however, that the isolated vessel experiments shown in Figure 3 were done under static conditions with no flow through the lumen. It is possible that flow-mediated vasodilation occurs in vivo that could involve an eNOS-generated NO component influencing myogenic tone and blood flow in the hippocampus. Whether eNOS is expressed in HAs remains unclear. Regardless, its basal activity is distinct in the hippocampus. Relatively little is known about HA function compared to other, well-studied segments of the cerebrovasculature, including the role, if any, of eNOS in influencing lumen diameter, vascular resistance and hippocampal blood flow.
Additional endothelium-dependent vasodilatory pathways exist that differentially affect vascular resistance depending upon the cerebrovascular segment. Small- and intermediate-conductance calcium-activated potassium (SKCa and IKCa) channels are expressed in the endothelium of cortical parenchymal arterioles and contribute to endothelium-derived hyperpolarization.44 In cortical parenchymal arterioles, but not upstream pial arteries, SKCa and IKCa channels are basally active to reduce myogenic tone and regulate resting cerebral blood flow.43, 44, 53 In HAs, SKCa and IKCa channels are expressed and cause robust vasodilation when activated pharmacologically, however, it is not yet clear if these channels are basally active.37–39 Roles for additional endothelial-dependent vasoactive pathways (e.g. cyclooxygenase) in basal tone remain to be determined.44 Further, the constellation of ion channels present in VSM cells that contribute to lumen diameter of HAs remains to be investigated. Understanding endothelial and VSM function in the hippocampus is important given that hippocampal perfusion is critical to neurocognitive health and that endothelial and VSM function is compromised in many disease states, including those associated with VCI.
Hippocampal Vascular Function, Perfusion, and Memory in Chronic Hypertension
The risk for dementia is up to 60% higher in those with mid-life hypertension compared to normotensive individuals.54–57 This is in part due to hypertension increasing the incidence of ischemic stroke and therefore post-stroke dementia.1 However, hypertension itself is a substantial contributor to steeper cognitive decline and worse cognitive performance, independently of stroke.1, 54, 56–58 In fact, elevated mid-life blood pressure correlates with reductions in hippocampal volume that likely contributes to hypertension-induced memory dysfunction, as hippocampal volumetry can be used to grade cognitive decline.56, 57 Hypoperfusion of the hippocampus is thought to be a primary mechanism by which neuropathological changes occur during chronic hypertension, including hippocampal atrophy, and is a predictor of cognitive decline.8, 9 Therefore, understanding how chronic hypertension affects hippocampal vascular function and perfusion may allow novel therapeutic targets to be identified in the hopes of slowing memory decline.
A recent pressure myography study investigated myogenic reactivity of HAs in response to changes in intravascular pressure during chronic hypertension using an established genetic model of hypertension, the spontaneously hypertensive rat (SHR).50 At physiological pressures, HAs from male normotensive rats had ~ 40% myogenic tone. Interestingly, HAs from 6-month-old male SHR were hyperconstricted with smaller lumen diameters compared to arterioles from normotensive Wistar rats and ~ 60 % myogenic tone.50 Absolute hippocampal blood flow was measured in vivo using the hydrogen (H2) clearance method where the half-life of H2 clearance was obtained from tissue desaturation measurements and used to calculate absolute blood flow (Figure 4A).37, 50, 59 Hippocampal perfusion was similar between 4-month-old Wistar rats and SHR; however, at 6 months of age when HAs were hyperconstricted, SHR had a substantial reduction in hippocampal blood flow (Figure 4B). Hippocampal-dependent long-term memory function was investigated using a novel object recognition task in which cognitively intact rats recognize a familiar object and spend the majority of the time investigating a novel object (recognition index > 0.50). Recognition indices were similar between Wistar rats and 4-month-old SHR, indicating similar levels of memory function (Figure 4C). However, memory was impaired in SHR at 6 months of age, with a recognition index of ~ 0.50 indicating no preference for the novel object (Figure 4C). Thus, HA hyperconstriction during hypertension was associated with hippocampal hypoperfusion and memory dysfunction. With a two month age difference in adult rats being equivalent to ~ 8–9 years, these findings demonstrate hemodynamic and cognitive consequences of hypertension-induced HA dysfunction in adulthood that may progress with age.60
Figure 4.
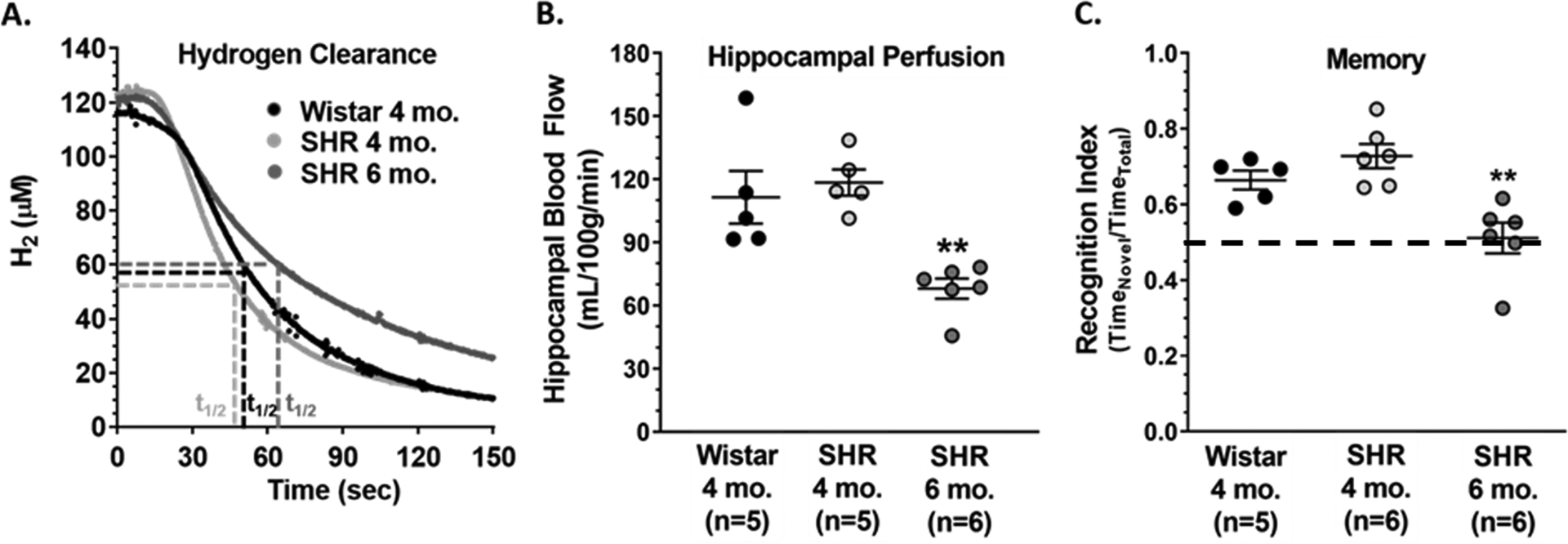
(A) Representative traces of hydrogen (H2) clearance used to calculate (B) absolute hippocampal blood flow. (C) Recognition indices of Wistar rats and spontaneously hypertensive rats (SHR) at 4 and 6 months of age. **p<0.01 vs. 4-month-old Wistar and SHR by one-way ANOVA with post-hoc Bonferroni test.
It is well established that during chronic hypertension, decreased bioavailability of NO causes endothelial dysfunction that increases tone and causes hyperconstriction of pial arteries and cortical parenchymal areterioles.7, 44, 61, 62 However, as NO does not appear to be similarly produced in endothelium of HAs as in other cerebrovascular segments, the underlying mechanism(s) by which HAs are hyperconstricted in chronic hypertension remain unknown but are likely distinct from hypertension-induced endothelial dysfunction in other brain regions. Also in contrast to other cerebrovascular segments that undergo hypertension-induced inward hypertrophic remodeling (smaller lumen diameter and thicker vascular wall) that reduces vasodilatory capacity and contributes to hypoperfusion, lumen diameters of fully relaxed HAs were similar between groups.7, 50 Thus, reductions in hippocampal perfusion in 6-month-old SHR was likely due to hyperconstriction of HAs and not inward remodeling, although arteriolar remodeling in the hippocampus may occur at older ages in SHR. That hyperconstriction rather than remodeling contributes to hippocampal hypoperfusion in mid-life drastically narrows the scope of therapeutic interventions to focus on drugs that cause relaxation and vasodilation of HAs in the hopes to restore perfusion and improve memory function.
CADASIL (Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy) is a monogenic form of cerebral small vessel disease and the most frequent genetic cause of VCI.63 While CADASIL largely shares the clinical and pathological characteristics of hypertension- and age-related sporadic forms of cerebral small vessel disease, it occurs at younger ages and in normotensive patients.63 In the normotensive TgNotch3R169C genetic mouse model of CADASIL that demonstrates impaired learning and memory, VSM depolarization and myogenic tone of HAs were decreased compared to arterioles from wild-type mice.64 This finding was similar to what occurred in pial arteries and cortical penetrating arterioles from TgNotch3R169C mice, with blunted pressure-induced contractility responses due to increased VSM expression of voltage-gated potassium channels that oppose myogenic tone and impair cerebral hemodynamics.64–66 These studies suggest that any disruption of HA function may contribute to VCI, whether it be hypertension-induced hyperconstriction or hypoconstriction associated with CADASIL, and highlights the therapeutic potential of targeting the hippocampal vasculature to slow cognitive decline and prevent vascular dementia.
NVC in the Hippocampus
NVC is the dynamic communication between neurons, glia and the vasculature to cause local vasodilation in response to neuronal activation to match the metabolic demand of neurons with appropriate blood flow and clear metabolic waste.7 NVC is a critical component in maintaining neuronal health, and impaired NVC in the cerebral cortex contributes to cognitive decline during healthy aging and in disease states associated with VCI.1 Many NVC studies in rodents have been performed in the relatively easily accessible somatosensory cortex, primarily measuring relative changes in blood flow in the barrel cortex in response to whisker stimulation or sensory cortex in response to paw pinch. Mechanisms involved in NVC have yet to be fully elucidated, but many signaling molecules, neurotransmitters and metabolites have been shown to be involved in the cortex. However, it is important to consider that these mechanisms may differ based on the brain region being studied, particularly in brain regions with unique vascular and neuronal organization and activity patterns like the hippocampus. Investigating NVC in the hippocampus in vivo proves challenging due to its deep location and requiring cognitive tasks to stimulate it physiologically. Although studies are relatively limited, NVC in the hippocampus appears to be distinct from the somatosensory cortex. For example, whisker stimulation causes a rapid local increase in blood flow within a few seconds in the barrel cortex largely through elevated extracellular K+ activating cortical capillary inward rectifying potassium channels causing hyperpolarization and vasodilation.67 In the hippocampus, in vitro and in vivo studies have determined diffusion of nNOS-derived NO to be the critical mediator translating neuronal activity into local increases in hippocampal blood flow that occurs more slowly than in the cortex.48, 68 A recent study by Shaw et al. (2021) assessed hippocampal NVC in vivo by recording neuronal activity and HA diameters in conscious, head-fixed mice during a virtual spatial navigation task using two-photon microscopy.33 In response to a similar level of neuronal firing, HAs dilated less than arterioles in the visual cortex that resulted in blunted increase in local blood flow. Given less hippocampal neurons are simultaneously active compared to visual cortex, this lower hyperemic response occurring in the hippocampus was likely sufficient to meet metabolic demand. However, during robust hippocampal neuronal activity (e.g. epileptic seizures), the hippocampus is less capable of maintaining blood flow than regions of the cerebral cortex, resulting in a metabolic crisis that potentiates seizure-induced injury.69 Together with the ~ 37 % reduction in hippocampal capillary density, these findings suggest the hippocampus has less intrinsic ability to match energy demand with appropriate blood flow that may increase the vulnerability of the hippocampus to hypoxic/ischemic injury under pathological conditions.32, 33
Much of what is known about NVC in the hippocampus has been determined in anesthetized rodents using an experimental paradigm that involves measuring NO and O2 levels and simultaneous changes in blood flow in response to stimulated neuronal activity via a microinjection of glutamate.68, 70–72 Using this methodology to assess age-related changes in healthy male Fisher 344 rats, Lourenco et al., (2018) determined that hippocampal NVC and spatial memory impairment occurred at ~ 20 months of age.72 Interestingly, NVC appeared to be impaired due to age-related blunted vascular responses to NO, but not changes in nNOS-derived NO production in response to neuronal activation.72 These findings suggest impaired hippocampal NVC and HA dysfunction are involved in healthy cognitive aging that may be accelerated during pathologies that promote dementia. A study in the 3xTg-AD triple transgenic mouse model of AD reported NVC was impaired in the hippocampus in vivo due to vascular dysfunction rather than changes in NO production or signaling between neurons and the microvasculature.70, 71 Importantly, this vascular-mediated disruption in NVC preceded cognitive dysfunction, demonstrating hippocampal vascular dysfunction and impaired NVC may have a causal role in memory decline.71 In a non-obese rat model of type 2 diabetes with impaired hippocampal-dependent spatial memory, reduced NO bioavailability blunted functional hyperemia in the hippocampus in response to activation of glutamatergic neurons, suggesting NVC was impaired due to oxidative stress but not vascular dysfunction.73 Thus, disease states can have differential effects on critical aspects involved in NVC in the hippocampus. Studies investigating hippocampal NVC in vivo are limited at this point in time, however, they are gaining momentum as technology advances to allow simultaneous neuronal activity and perfusion measurements to be made in conscious rodents in this deep brain region.
The ability of isolated and pressurized HAs to vasodilate to mediators of NVC such as NO have been investigated as an indirect assessment of hippocampal NVC, as this is a critical component of increasing local blood flow. Pressure myography studies have revealed that hypertensive disorders may impair NVC via HA dysfunction to contribute to memory dysfunction. Preeclampsia (PE) is a hypertensive disorder of pregnancy that has long-lasting consequences, and is a major risk factor for hypertension, stroke and VCI later in life.74, 75 In a hypercholesterolemia rat model of PE, HAs demonstrated endothelial dysfunction and impaired vasodilation to NO several months after pregnancy that was associated with impaired long-term memory.76 Interestingly, memory was intact late in gestation, demonstrating that cognitive decline occurred as a function of time after PE pregnancy.77 These findings suggest vascular mechanisms of NVC are impaired in the hippocampus after PE contributing to declining memory function and VCI.
Chronic psychological stress disrupts cognitive function, particularly when it occurs in mid-life, increasing the risk for age-related dementia by 2.5-fold.78, 79 Chronic stress is associated with reduced hippocampal blood flow and hippocampal atrophy, suggesting the hippocampal vasculature is damaged that could contribute to memory dysfunction and VCI.80–82 In a rat model of chronic neuroendocrine stress, chronic activation of the hypothalamic-pituitary-adrenal axis caused stress-induced cardiovascular symptoms including hypertension. Long-term memory was impaired that was associated with HA endothelial dysfunction and blunted vasoreactivity to NO.39 Interestingly, stress-induced memory and HA dysfunction were more robust in male rats than female rats. Further, male rats, regardless of chronic exposure to the neuroendocrine components of the stress response, had lower vascular density and increased distance between capillaries in the hippocampal CA1 region compared to female rats.39 Thus, impaired vascular responses to mediators of NVC in the hippocampus coupled with sparser vascularization could underlie the sex-dependent increased susceptibility to stress-induced hippocampal dysfunction. These findings suggest chronic stress has deleterious effects on the hippocampus and its vasculature, including potentially impaired NVC that may represent underlying mechanisms by which stress-related disorders contribute to VCI.
A Role for the Hippocampal Vasculature in Post-Stroke Dementia (PSD)
In addition to hypertensive disorders having deleterious effects on the hippocampal vasculature that contribute to memory decline, chronic hypertension is also a leading cause of cerebral ischemic stroke. PSD, defined as dementia occurring within six months after stroke, occurs in up to 80% of ischemic stroke survivors and is a major subtype of vascular dementia, the most severe form of VCI.1, 83 PSD is increasing in prevalence due to increased life expectancy and decreased stroke-related mortality and involves an array of, and often multiple cognitive domains. Importantly, ~ 70% of stroke patients that achieved successful clinical recovery (e.g. no functional disability 3 months after stroke) had significant impairment in at least one cognitive domain, with memory being one of the most commonly affected domains.83 Thus, despite easing the physical burden, the remaining cognitive burden can drastically limit the quality of life of stroke survivors. The focus on the cognitive consequences of stroke is increasing, as evidenced by the landmark DISCOVERY (Determinants of Incident Stroke Cognitive Outcomes and Vascular Effects on RecoverY) study beginning in 2019 investigating mechanisms of PSD with the goal of developing targets for personalized medicine to improve post-stroke cognition.
The spectrum of cognitive impairment after stroke can often be explained by the location of the infarction; however, that does not seem to be the case with post-stroke memory impairment. Ischemic stroke in brain regions involved in memory (e.g. medial temporal lobe and hippocampus) are rare, yet PSD is associated with impaired memory, decreased hippocampal functionality on fMRI and hippocampal neurodegeneration.84, 85 While this cognitive consequence of stroke is not well understood, it suggests that brain regions involved in memory, such as the hippocampus, undergo damage after stroke despite being outside of the primarily affected brain region that may be vascular in nature.
Cerebrovascular dysfunction, including impaired cerebral blood flow autoregulation, is known to occur after ischemic stroke.86 Vascular dysfunction can occur not only within the ischemic region, but also in the non-ischemic hemisphere, suggesting widespread cerebrovascular dysfunction after focal cerebral ischemia that could impact the hippocampus.51, 87–89 In rodents, tMCAO results in memory dysfunction by disrupting hippocampal neuroplasticity, the cellular mechanism of learning and memory.90–92 Importantly, impaired hippocampal neuroplasticity occurs bilaterally.91, 92 Underlying mechanisms by which hippocampal neuronal network function is disrupted after ischemic stroke remain unclear, but are likely vascular in nature and may involve circulating factors elevated after cerebral ischemia. Such circulating factors include vasoactive peptides, like the potent vasoconstrictor endothelin-1, that increase constriction of otherwise healthy pial arteries.93–95 Thus, focal cerebral ischemic stroke may indirectly cause HA hyperconstriction and reduce hippocampal perfusion, particularly during chronic hypertension when preexisting HA hyperconstriction and hypoperfusion may be exacerbated and potentiate PSD.38 Further, circulating levels of proinflammatory cytokines elevated after cerebral ischemia could damage the BBB that could cause hippocampal neuroinflammation well known to contribute to VCI.96
The Hippocampal BBB
The BBB is a complex interface between systemically circulating factors and the delicate brain milieu. The BBB plays a critical role in maintaining neuronal homeostasis, and its breakdown is considered an early biomarker of human cognitive dysfunction.97 Importantly, the BBB in the hippocampus is more susceptible to disruption than other brain regions, thereby increasing the impact on cognition and memory.97–100 In patients with mild cognitive impairment, increased BBB permeability in the hippocampus occurred earlier and to a greater extent than in cortical brain regions, as measured via dynamic contrast-enhanced MRI.97, 98 Further, a study in rodents showed that increasing BBB permeability in the hippocampus for less than 48 hours caused impaired spatial memory that persisted for seven days.101 These findings suggest that even transient disruption of hippocampal BBB function contributes to prolonged memory impairment, and highlights the importance of BBB integrity in the hippocampus in maintaining neurocognition.101
The hippocampal BBB may play a central role in PSD. The acute inflammatory response to cerebral ischemic stroke includes activation of peripheral immune cells that produce proinflammatory cytokines such as tumor necrosis factor alpha (TNFα) that is damaging to the BBB.93 TNFα increases BBB permeability via downregulation and degradation of BBB tight junction proteins.102 BBB disruption can have long-lasting effects on memory function through activation of microglia, the resident immune cells in the brain, resulting in local neuroinflammation.101, 103 Activated microglia can become cytotoxic (e.g. M1 microglia) and secrete high levels of TNFα. TNFα then exacerbates neuroinflammation, further potentiating BBB degradation and directly impairing hippocampal neuronal network dynamics causing memory dysfunction.103–106 This neuroinflammatory cascade in the hippocampus contributes to cognitive dysfunction in models of AD that may also be an underlying mechanism of PSD.105, 106 Treatment with the anti-inflammatory agent minocycline decreased neuroinflammation in the hippocampus that improved spatial learning and memory six weeks after tMCAO in healthy rats.107 In a high-fat diet/low-dose streptozotocin rat model of diabetes, memory was impaired two weeks after tMCAO due to hippocampal neuroinflammation that was associated with increased hippocampal BBB permeability.108, 109 These studies suggest that the hippocampal BBB may play a central role in post-stroke memory dysfunction, and that protecting the BBB protects from PSD.
In addition to tight junction degradation, TNFα also disrupts the critical biochemical/selective component of the BBB comprised of specific transport proteins known as efflux transporters.110 Efflux transporters contained within the luminal BBB are powerful ATP-driven pumps that actively efflux ligands (e.g. cytokines, steroids, xenobiotics) back into the circulation, thereby preventing passage in to the brain.111, 112 Efflux transporters at the hippocampal BBB are implicated in the pathogenesis of AD, and may also have a role in PSD.113 Importantly, BBB efflux transporters in capillaries in the hippocampus are more susceptible to inhibition than in the cerebral cortex.99, 100, 111 This not only suggests regional differences in barrier function, but more importantly suggests that such differences selectively increase the susceptibility of the hippocampus to damage in the context of acute efflux transporter failure. In fact, TNFα is one of a few cytokines that directly inhibits p-glycoprotein, the most abundant BBB efflux transporter.112 Therefore, the BBB in the hippocampus may be selectively disrupted under conditions when circulating TNFα is elevated, such as after cerebral ischemic stroke. Hippocampal BBB components including efflux transporters may represent novel therapeutic targets to mitigate the cognitive consequences associated with focal cerebral ischemic stroke and cognitive impairment associated with inflammatory states including chronic hypertension and diabetes.
Conclusions
Overall, the function of the hippocampal vasculature and hippocampal hemodynamics are critical to neurocognitive health and are compromised under several pathological conditions contributing to VCI. It is possible that treatments that restore arteriole function may protect against age- and disease-related reductions in basal hippocampal blood flow and NVC to improve memory function. Further, evidence suggest that protecting the injury-susceptible hippocampal BBB, specifically efflux transporter function, may represent a novel therapeutic target to minimize the deleterious neuroinflammatory cascade that occurs during systemic inflammation and ameliorate PSD. Figure 5 shows a summary of critical aspects of hippocampal neurovascular function impaired under pathological conditions known to accelerate age-related cognitive decline and contribute to VCI. There is still much to learn about this unique and understudied vascular territory responsible for maintaining basal and activity-dependent perfusion of the injury-prone hippocampus. There are currently no treatments for VCI, only prevention strategies to modify lifestyle risk factors, which have no impact on the healthy aging process. Therefore, it is critical for basic science and clinical studies to further our understanding of pathological mechanisms of age-related dementias to identify potential therapeutic targets. The hippocampal vasculature may be one such target, including arteriole and BBB function, potentially holding currently untapped therapeutic potential to help alleviate VCI and other dementias.
Figure 5.
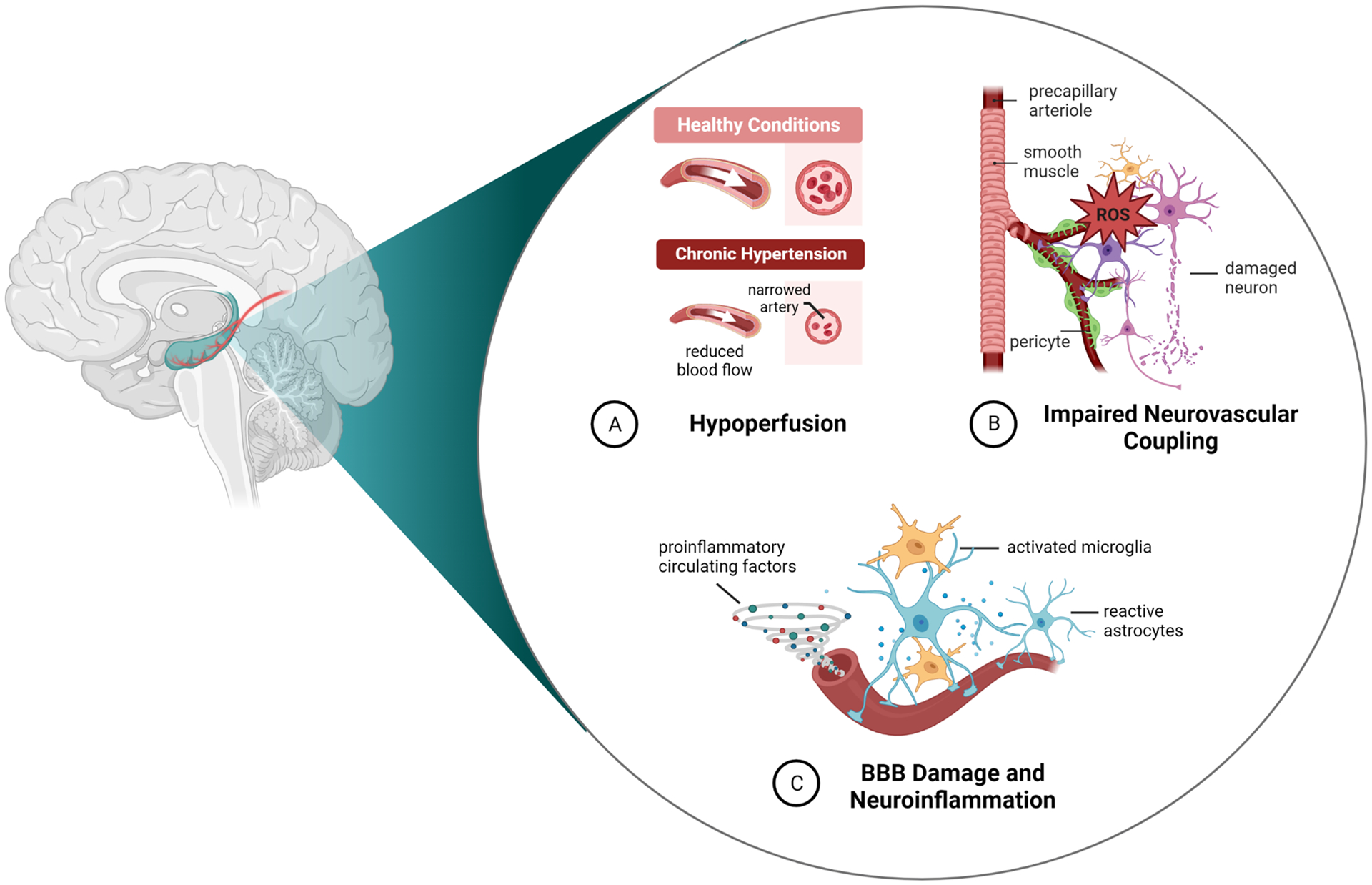
Summary of how pathological conditions known to accelerate age-related cognitive decline may affect the hippocampal vasculature and neurovascular function to contribute to VCI.
Sources of Funding
Portions of this work were supported by the NIH National Institute of Neurological Disorders and Stroke R01 NS127284, the American Heart Association Career Development Award 20CDA35310239 and the Cardiovascular Research Institute of Vermont.
Non-standard Abbreviations and Acronyms
- AChA
anterior choroidal artery
- AD
Alzheimer’s disease
- BBB
blood-brain barrier
- CA
cornu ammonis
- CADASIL
Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy
- eNOS
endothelial nitric oxide synthase
- HA
hippocampal arteriole
- IKCa
intermediate-conductance calcium-activated potassium channel
- L-NAME
NG-nitro-L-arginine methyl ester
- MCA
middle cerebral artery
- nNOS
neuronal nitric oxide synthase
- NO
nitric oxide
- NOS
nitric oxide synthase
- NVC
neurovascular coupling
- PCA
posterior cerebral artery
- PE
preeclampsia
- PSD
post-stroke dementia
- SHR
spontaneously hypertensive rat
- SKCa
small-conductance calcium-activated potassium channel
- tMCAO
transient middle cerebral artery occlusion
- TNFα
tumor necrosis factor alpha
- VCI
vascular cognitive impairment
- VSM
vascular smooth muscle
Footnotes
Disclosures
None.
References
- 1.Iadecola C, Duering M, Hachinski V, Joutel A, Pendlebury ST, Schneider JA, Dichgans M. Vascular cognitive impairment and dementia: Jacc scientific expert panel. Journal of the American College of Cardiology. 2019;73:3326–3344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Power MC, Mormino E, Soldan A, James BD, Yu L, Armstrong NM, Bangen KJ, Delano-Wood L, Lamar M, Lim YY, et al. Combined neuropathological pathways account for age-related risk of dementia. Ann Neurol. 2018;84:10–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hooghiemstra AM, Leeuwis AE, Bertens AS, Biessels GJ, Bots ML, Brunner-La Rocca HP, Greving JP, Kappelle LJ, van Oostenbrugge RJ, van Rossum AC, et al. Frequent cognitive impairment in patients with disorders along the heart-brain axis. Stroke. 2019;50:3369–3375 [DOI] [PubMed] [Google Scholar]
- 4.Sengupta P, Ganguly J, Pal S, Ghosal M. Pattern of cognitive deficits in vascular dementia. Indian J Med Res. 2019;149:503–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao L, Biesbroek JM, Shi L, Liu W, Kuijf HJ, Chu WW, Abrigo JM, Lee RK, Leung TW, Lau AY, et al. Strategic infarct location for post-stroke cognitive impairment: A multivariate lesion-symptom mapping study. J Cereb Blood Flow Metab. 2018;38:1299–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dichgans M, Leys D. Vascular cognitive impairment. Circ Res. 2017;120:573–591 [DOI] [PubMed] [Google Scholar]
- 7.Iadecola C, Gottesman RF. Neurovascular and cognitive dysfunction in hypertension. Circ Res. 2019;124:1025–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chao LL, Buckley ST, Kornak J, Schuff N, Madison C, Yaffe K, Miller BL, Kramer JH, Weiner MW. Asl perfusion mri predicts cognitive decline and conversion from mci to dementia. Alzheimer Dis Assoc Disord. 2010;24:19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glodzik L, Rusinek H, Tsui W, Pirraglia E, Kim HJ, Deshpande A, Li Y, Storey P, Randall C, Chen J, et al. Different relationship between systolic blood pressure and cerebral perfusion in subjects with and without hypertension. Hypertension. 2019;73:197–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jack CR Jr., Petersen RC, Xu Y, O’Brien PC, Smith GE, Ivnik RJ, Boeve BF, Tangalos EG, Kokmen E. Rates of hippocampal atrophy correlate with change in clinical status in aging and ad. Neurology. 2000;55:484–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gemmell E, Bosomworth H, Allan L, Hall R, Khundakar A, Oakley AE, Deramecourt V, Polvikoski TM, O’Brien JT, Kalaria RN. Hippocampal neuronal atrophy and cognitive function in delayed poststroke and aging-related dementias. Stroke. 2012;43:808–814 [DOI] [PubMed] [Google Scholar]
- 12.Heo S, Prakash RS, Voss MW, Erickson KI, Ouyang C, Sutton BP, Kramer AF. Resting hippocampal blood flow, spatial memory and aging. Brain Res. 2010;1315:119–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zlokovic BV, Gottesman RF, Bernstein KE, Seshadri S, McKee A, Snyder H, Greenberg SM, Yaffe K, Schaffer CB, Yuan C, et al. Vascular contributions to cognitive impairment and dementia (vcid): A report from the 2018 national heart, lung, and blood institute and national institute of neurological disorders and stroke workshop. Alzheimers Dement. 2020;16:1714–1733 [DOI] [PubMed] [Google Scholar]
- 14.Skrobot OA, Black SE, Chen C, DeCarli C, Erkinjuntti T, Ford GA, Kalaria RN, O’Brien J, Pantoni L, Pasquier F, et al. Progress toward standardized diagnosis of vascular cognitive impairment: Guidelines from the vascular impairment of cognition classification consensus study. Alzheimers Dement. 2018;14:280–292 [DOI] [PubMed] [Google Scholar]
- 15.Erdem A, Yasargil G, Roth P. Microsurgical anatomy of the hippocampal arteries. J Neurosurg. 1993;79:256–265 [DOI] [PubMed] [Google Scholar]
- 16.Haegelen C, Berton E, Darnault P, Morandi X. A revised classification of the temporal branches of the posterior cerebral artery. Surg Radiol Anat. 2012;34:385–391 [DOI] [PubMed] [Google Scholar]
- 17.Spallazzi M, Dobisch L, Becke A, Berron D, Stucht D, Oeltze-Jafra S, Caffarra P, Speck O, Duzel E. Hippocampal vascularization patterns: A high-resolution 7 tesla time-of-flight magnetic resonance angiography study. Neuroimage Clin. 2019;21:101609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demene C, Tiran E, Sieu LA, Bergel A, Gennisson JL, Pernot M, Deffieux T, Cohen I, Tanter M. 4d microvascular imaging based on ultrafast doppler tomography. Neuroimage. 2016;127:472–483 [DOI] [PubMed] [Google Scholar]
- 19.Seal JB, Buchh BN, Marks JD. New variability in cerebrovascular anatomy determines severity of hippocampal injury following forebrain ischemia in the mongolian gerbil. Brain Res. 2006;1073–1074:451–459 [DOI] [PubMed] [Google Scholar]
- 20.Goetzen B, Sztamska E. Comparative anatomy of the arterial vascularization of the hippocampus in man and in experimental animals (cat, rabbit and sheep). Neuropatol Pol. 1992;30:173–184 [PubMed] [Google Scholar]
- 21.Menshawi K, Mohr JP, Gutierrez J. A functional perspective on the embryology and anatomy of the cerebral blood supply. J Stroke. 2015;17:144–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Raamt AF, Mali WP, van Laar PJ, van der Graaf Y. The fetal variant of the circle of willis and its influence on the cerebral collateral circulation. Cerebrovasc Dis. 2006;22:217–224 [DOI] [PubMed] [Google Scholar]
- 23.Van Overbeeke JJ, Hillen B, Tulleken CA. A comparative study of the circle of willis in fetal and adult life. The configuration of the posterior bifurcation of the posterior communicating artery. J Anat. 1991;176:45–54 [PMC free article] [PubMed] [Google Scholar]
- 24.Qian B, Rudy RF, Cai T, Du R. Cerebral artery diameter in inbred mice varies as a function of strain. Front Neuroanat. 2018;12:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Du XY, Zhu XD, Dong G, Lu J, Wang Y, Zeng L, Zhao TY, Ye HH, Li RS, Bai JY, et al. Characteristics of circle of willis variations in the mongolian gerbil and a newly established ischemia-prone gerbil group. ILAR J. 2011;52:E1–7 [DOI] [PubMed] [Google Scholar]
- 26.Nikonenko AG, Radenovic L, Andjus PR, Skibo GG. Structural features of ischemic damage in the hippocampus. Anat Rec (Hoboken). 2009;292:1914–1921 [DOI] [PubMed] [Google Scholar]
- 27.Zhang H, Roman RJ, Fan F. Hippocampus is more susceptible to hypoxic injury: Has the rosetta stone of regional variation in neurovascular coupling been deciphered? Geroscience. 2022;44:127–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schultz C, Engelhardt M. Anatomy of the hippocampal formation. Front Neurol Neurosci. 2014;34:6–17 [DOI] [PubMed] [Google Scholar]
- 29.Mountcastle VB. The columnar organization of the neocortex. Brain. 1997;120 (Pt 4):701–722 [DOI] [PubMed] [Google Scholar]
- 30.Ekstrom AD. Regional variation in neurovascular coupling and why we still lack a rosetta stone. Philos Trans R Soc Lond B Biol Sci. 2021;376:20190634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirst C, Skriabine S, Vieites-Prado A, Topilko T, Bertin P, Gerschenfeld G, Verny F, Topilko P, Michalski N, Tessier-Lavigne M, et al. Mapping the fine-scale organization and plasticity of the brain vasculature. Cell. 2020;180:780–795 e725 [DOI] [PubMed] [Google Scholar]
- 32.Nair V, Palm D, Roth LJ. Relative vascularity of certain anatomical areas of the brain and other organs of the rat. Nature. 1960;188:497–498 [DOI] [PubMed] [Google Scholar]
- 33.Shaw K, Bell L, Boyd K, Grijseels DM, Clarke D, Bonnar O, Crombag HS, Hall CN. Neurovascular coupling and oxygenation are decreased in hippocampus compared to neocortex because of microvascular differences. Nature Communications. 2021;12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ji X, Ferreira T, Friedman B, Liu R, Liechty H, Bas E, Chandrashekar J, Kleinfeld D. Brain microvasculature has a common topology with local differences in geometry that match metabolic load. Neuron. 2021;109:1168–1187 e1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang X, Yin X, Zhang J, Li A, Gong H, Luo Q, Zhang H, Gao Z, Jiang H. High-resolution mapping of brain vasculature and its impairment in the hippocampus of alzheimer’s disease mice. National Science Review. 2019;6:1223–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perosa V, Priester A, Ziegler G, Cardenas-Blanco A, Dobisch L, Spallazzi M, Assmann A, Maass A, Speck O, Oltmer J, et al. Hippocampal vascular reserve associated with cognitive performance and hippocampal volume. Brain. 2020;143:622–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson AC, Cipolla MJ. Altered hippocampal arteriole structure and function in a rat model of preeclampsia: Potential role in impaired seizure-induced hyperemia. J Cereb Blood Flow Metab. 2017;37:2857–2869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson AC, Miller JE, Cipolla MJ. Memory impairment in spontaneously hypertensive rats is associated with hippocampal hypoperfusion and hippocampal vascular dysfunction. J Cereb Blood Flow Metab. 2019:271678X19848510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson AC, Uhlig F, Einwag Z, Cataldo N, Erdos B. The neuroendocrine stress response impairs hippocampal vascular function and memory in male and female rats. Neurobiol Dis. 2022;168:105717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coyle P Vascular patterns of the rat hippocampal formation. Exp Neurol. 1976;52:447–458 [DOI] [PubMed] [Google Scholar]
- 41.Bayliss WM. On the local reactions of the arterial wall to changes of internal pressure. J Physiol. 1902;28:220–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Osol G, Brekke JF, McElroy-Yaggy K, Gokina NI. Myogenic tone, reactivity, and forced dilatation: A three-phase model of in vitro arterial myogenic behavior. Am J Physiol Heart Circ Physiol. 2002;283:H2260–2267 [DOI] [PubMed] [Google Scholar]
- 43.Cipolla MJ, Smith J, Kohlmeyer MM, Godfrey JA. Skca and ikca channels, myogenic tone, and vasodilator responses in middle cerebral arteries and parenchymal arterioles: Effect of ischemia and reperfusion. Stroke. 2009;40:1451–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andresen J, Shafi NI, Bryan RM Jr., Endothelial influences on cerebrovascular tone. J Appl Physiol. 2006;100:318–327 [DOI] [PubMed] [Google Scholar]
- 45.Faraci FM, Brian JE Jr., Nitric oxide and the cerebral circulation. Stroke. 1994;25:692–703 [DOI] [PubMed] [Google Scholar]
- 46.Iadecola C, Pelligrino DA, Moskowitz MA, Lassen NA. Nitric oxide synthase inhibition and cerebrovascular regulation. J Cereb Blood Flow Metab. 1994;14:175–192 [DOI] [PubMed] [Google Scholar]
- 47.Toda N, Ayajiki K, Okamura T. Cerebral blood flow regulation by nitric oxide: Recent advances. Pharmacological reviews. 2009;61:62–97 [DOI] [PubMed] [Google Scholar]
- 48.Lovick TA, Brown LA, Key BJ. Neurovascular relationships in hippocampal slices: Physiological and anatomical studies of mechanisms underlying flow-metabolism coupling in intraparenchymal microvessels. Neuroscience. 1999;92:47–60 [DOI] [PubMed] [Google Scholar]
- 49.Brown LA, Key BJ, Lovick TA. Fluorescent imaging of nitric oxide production in neuronal varicosities associated with intraparenchymal arterioles in rat hippocampal slices. Neuroscience letters. 2000;294:9–12 [DOI] [PubMed] [Google Scholar]
- 50.Johnson AC, Miller JE, Cipolla MJ. Memory impairment in spontaneously hypertensive rats is associated with hippocampal hypoperfusion and hippocampal vascular dysfunction. J Cereb Blood Flow Metab. 2020;40:845–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Winters A, Taylor JC, Ren M, Ma R, Liu R, Yang SH. Transient focal cerebral ischemia induces long-term cerebral vasculature dysfunction in a rodent experimental stroke model. Translational stroke research. 2012;3:279–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang S, Zhang H, Liu Y, Li L, Guo Y, Jiao F, Fang X, Jefferson JR, Li M, Gao W, et al. Sex differences in the structure and function of rat middle cerebral arteries. Am J Physiol Heart Circ Physiol. 2020;318:H1219–H1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hannah RM, Dunn KM, Bonev AD, Nelson MT. Endothelial sk(ca) and ik(ca) channels regulate brain parenchymal arteriolar diameter and cortical cerebral blood flow. J Cereb Blood Flow Metab. 2011;31:1175–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gottesman RF, Schneider AL, Albert M, Alonso A, Bandeen-Roche K, Coker L, Coresh J, Knopman D, Power MC, Rawlings A, et al. Midlife hypertension and 20-year cognitive change: The atherosclerosis risk in communities neurocognitive study. JAMA Neurol. 2014;71:1218–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gottesman RF, Albert MS, Alonso A, Coker LH, Coresh J, Davis SM, Deal JA, McKhann GM, Mosley TH, Sharrett AR, et al. Associations between midlife vascular risk factors and 25-year incident dementia in the atherosclerosis risk in communities (aric) cohort. JAMA Neurol. 2017;74:1246–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Korf ES, White LR, Scheltens P, Launer LJ. Midlife blood pressure and the risk of hippocampal atrophy: The honolulu asia aging study. Hypertension. 2004;44:29–34 [DOI] [PubMed] [Google Scholar]
- 57.Yano Y, Reis JP, Levine DA, Bryan RN, Viera AJ, Shimbo D, Tedla YG, Allen NB, Schreiner PJ, Bancks MP, et al. Visit-to-visit blood pressure variability in young adulthood and hippocampal volume and integrity at middle age: The cardia study (coronary artery risk development in young adults). Hypertension. 2017;70:1091–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walker KA, Sharrett AR, Wu A, Schneider ALC, Albert M, Lutsey PL, Bandeen-Roche K, Coresh J, Gross AL, Windham BG, et al. Association of midlife to late-life blood pressure patterns with incident dementia. JAMA. 2019;322:535–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haining JL, Turner MD, Pantall RM. Measurement of local cerebral blood flow in the unanesthetized rat using a hydrogen clearance method. Circ Res. 1968;23:313–324 [DOI] [PubMed] [Google Scholar]
- 60.Sengupta P The laboratory rat: Relating its age with human’s. Int J Prev Med. 2013;4:624–630 [PMC free article] [PubMed] [Google Scholar]
- 61.Iadecola C, Davisson RL. Hypertension and cerebrovascular dysfunction. Cell Metab. 2008;7:476–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pires PW, Dams Ramos CM, Matin N, Dorrance AM. The effects of hypertension on the cerebral circulation. Am J Physiol Heart Circ Physiol. 2013;304:H1598–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chabriat H, Joutel A, Dichgans M, Tournier-Lasserve E, Bousser MG. Cadasil. Lancet Neurol. 2009;8:643–653 [DOI] [PubMed] [Google Scholar]
- 64.Fontaine JT, Rosehart AC, Joutel A, Dabertrand F. Hb-egf depolarizes hippocampal arterioles to restore myogenic tone in a genetic model of small vessel disease. Mech Ageing Dev. 2020;192:111389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dabertrand F, Kroigaard C, Bonev AD, Cognat E, Dalsgaard T, Domenga-Denier V, Hill-Eubanks DC, Brayden JE, Joutel A, Nelson MT. Potassium channelopathy-like defect underlies early-stage cerebrovascular dysfunction in a genetic model of small vessel disease. Proc Natl Acad Sci U S A. 2015;112:E796–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baron-Menguy C, Domenga-Denier V, Ghezali L, Faraci FM, Joutel A. Increased notch3 activity mediates pathological changes in structure of cerebral arteries. Hypertension. 2017;69:60–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Longden TA, Dabertrand F, Koide M, Gonzales AL, Tykocki NR, Brayden JE, Hill-Eubanks D, Nelson MT. Capillary k+-sensing initiates retrograde hyperpolarization to increase local cerebral blood flow. Nat Neurosci. 2017;20:717–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lourenco CF, Santos RM, Barbosa RM, Cadenas E, Radi R, Laranjinha J. Neurovascular coupling in hippocampus is mediated via diffusion by neuronal-derived nitric oxide. Free Radic Biol Med. 2014;73:421–429 [DOI] [PubMed] [Google Scholar]
- 69.Choy M, Wells JA, Thomas DL, Gadian DG, Scott RC, Lythgoe MF. Cerebral blood flow changes during pilocarpine-induced status epilepticus activity in the rat hippocampus. Exp Neurol. 2010;225:196–201 [DOI] [PubMed] [Google Scholar]
- 70.Lourenco CF, Ledo A, Dias C, Barbosa RM, Laranjinha J. Neurovascular and neurometabolic derailment in aging and alzheimer’s disease. Front Aging Neurosci. 2015;7:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lourenco CF, Ledo A, Barbosa RM, Laranjinha J. Neurovascular uncoupling in the triple transgenic model of alzheimer’s disease: Impaired cerebral blood flow response to neuronal-derived nitric oxide signaling. Exp Neurol. 2017;291:36–43 [DOI] [PubMed] [Google Scholar]
- 72.Lourenco CF, Ledo A, Caetano M, Barbosa RM, Laranjinha J. Age-dependent impairment of neurovascular and neurometabolic coupling in the hippocampus. Front Physiol. 2018;9:913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goncalves JS, Seica RM, Laranjinha J, Lourenco CF. Impairment of neurovascular coupling in the hippocampus due to decreased nitric oxide bioavailability supports early cognitive dysfunction in type 2 diabetic rats. Free Radic Biol Med. 2022 [DOI] [PubMed] [Google Scholar]
- 74.Miller EC. Preeclampsia and cerebrovascular disease. Hypertension. 2019;74:5–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Basit S, Wohlfahrt J, Boyd HA. Pre-eclampsia and risk of dementia later in life: Nationwide cohort study. BMJ. 2018;363:k4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Johnson AC, Tremble SM, Cipolla MJ. Experimental preeclampsia causes long-lasting hippocampal vascular dysfunction and memory impairment. Frontiers in physiology. 2022;13:889918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Johnson AC, Li Z, Orfila JE, Herson PS, Cipolla MJ. Hippocampal network dysfunction as a mechanism of early-onset dementia after preeclampsia and eclampsia. Progress in neurobiology. 2021;199:101938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stuart KE, Padgett C. A systematic review of the association between psychological stress and dementia risk in humans. J Alzheimers Dis. 2020;78:335–352 [DOI] [PubMed] [Google Scholar]
- 79.Johansson L, Guo X, Waern M, Ostling S, Gustafson D, Bengtsson C, Skoog I. Midlife psychological stress and risk of dementia: A 35-year longitudinal population study. Brain. 2010;133:2217–2224 [DOI] [PubMed] [Google Scholar]
- 80.Li Y, Qin J, Yan J, Zhang N, Xu Y, Zhu Y, Sheng L, Zhu X, Ju S. Differences of physical vs. Psychological stress: Evidences from glucocorticoid receptor expression, hippocampal subfields injury, and behavioral abnormalities. Brain Imaging Behav. 2019;13:1780–1788 [DOI] [PubMed] [Google Scholar]
- 81.Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C, Nair NP, Thakur M, McEwen BS, Hauger RL, Meaney MJ. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci. 1998;1:69–73 [DOI] [PubMed] [Google Scholar]
- 82.Endo Y, Nishimura JI, Kobayashi S, Kimura F. Chronic stress exposure influences local cerebral blood flow in the rat hippocampus. Neuroscience. 1999;93:551–555 [DOI] [PubMed] [Google Scholar]
- 83.Jokinen H, Melkas S, Ylikoski R, Pohjasvaara T, Kaste M, Erkinjuntti T, Hietanen M. Post-stroke cognitive impairment is common even after successful clinical recovery. Eur J Neurol. 2015;22:1288–1294 [DOI] [PubMed] [Google Scholar]
- 84.Snaphaan L, Rijpkema M, van Uden I, Fernandez G, de Leeuw FE. Reduced medial temporal lobe functionality in stroke patients: A functional magnetic resonance imaging study. Brain. 2009;132:1882–1888 [DOI] [PubMed] [Google Scholar]
- 85.Akinyemi RO, Allan LM, Oakley A, Kalaria RN. Hippocampal neurodegenerative pathology in post-stroke dementia compared to other dementias and aging controls. Front Neurosci. 2017;11:717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nogueira RC, Aries M, Minhas JS, N HP, Xiong L, Kainerstorfer JM, Castro P. Review of studies on dynamic cerebral autoregulation in the acute phase of stroke and the relationship with clinical outcome. J Cereb Blood Flow Metab. 2022;42:430–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dawson SL, Panerai RB, Potter JF. Serial changes in static and dynamic cerebral autoregulation after acute ischaemic stroke. Cerebrovasc Dis. 2003;16:69–75 [DOI] [PubMed] [Google Scholar]
- 88.Reinhard M, Roth M, Guschlbauer B, Harloff A, Timmer J, Czosnyka M, Hetzel A. Dynamic cerebral autoregulation in acute ischemic stroke assessed from spontaneous blood pressure fluctuations. Stroke. 2005;36:1684–1689 [DOI] [PubMed] [Google Scholar]
- 89.Cipolla MJ, McCall AL, Lessov N, Porter JM. Reperfusion decreases myogenic reactivity and alters middle cerebral artery function after focal cerebral ischemia in rats. Stroke. 1997;28:176–180 [DOI] [PubMed] [Google Scholar]
- 90.Lynch MA. Long-term potentiation and memory. Physiol Rev. 2004;84:87–136 [DOI] [PubMed] [Google Scholar]
- 91.Orfila JE, Grewal H, Dietz RM, Strnad F, Shimizu T, Moreno M, Schroeder C, Yonchek J, Rodgers KM, Dingman A, et al. Delayed inhibition of tonic inhibition enhances functional recovery following experimental ischemic stroke. J Cereb Blood Flow Metab. 2017:271678X17750761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li W, Huang R, Shetty RA, Thangthaeng N, Liu R, Chen Z, Sumien N, Rutledge M, Dillon GH, Yuan F, et al. Transient focal cerebral ischemia induces long-term cognitive function deficit in an experimental ischemic stroke model. Neurobiol Dis. 2013;59:18–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ramiro L, Simats A, Garcia-Berrocoso T, Montaner J. Inflammatory molecules might become both biomarkers and therapeutic targets for stroke management. Ther Adv Neurol Disord. 2018;11:1756286418789340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cipolla MJ, Sweet JG, Gokina NI, White SL, Nelson MT. Mechanisms of enhanced basal tone of brain parenchymal arterioles during early postischemic reperfusion: Role of et-1-induced peroxynitrite generation. J Cereb Blood Flow Metab. 2013;33:1486–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Canavero I, Sherburne HA, Tremble SM, Clark WM, Cipolla MJ. Effects of acute stroke serum on non-ischemic cerebral and mesenteric vascular function. Translational stroke research. 2016;7:156–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pasqualetti G, Brooks DJ, Edison P. The role of neuroinflammation in dementias. Curr Neurol Neurosci Rep. 2015;15:17. [DOI] [PubMed] [Google Scholar]
- 97.Nation DA, Sweeney MD, Montagne A, Sagare AP, D’Orazio LM, Pachicano M, Sepehrband F, Nelson AR, Buennagel DP, Harrington MG, et al. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nature medicine. 2019;25:270–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, Toga AW, Jacobs RE, Liu CY, Amezcua L, et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85:296–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Johnson AC, Hammer ES, Sakkaki S, Tremble SM, Holmes GL, Cipolla MJ. Inhibition of blood-brain barrier efflux transporters promotes seizure in pregnant rats: Role of circulating factors. Brain, behavior, and immunity. 2018;67:13–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kuntner C, Bankstahl JP, Bankstahl M, Stanek J, Wanek T, Stundner G, Karch R, Brauner R, Meier M, Ding X, et al. Dose-response assessment of tariquidar and elacridar and regional quantification of p-glycoprotein inhibition at the rat blood-brain barrier using (r)-[(11)c]verapamil pet. Eur J Nucl Med Mol Imaging. 2010;37:942–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cao Y, Ni C, Li Z, Li L, Liu Y, Wang C, Zhong Y, Cui D, Guo X. Isoflurane anesthesia results in reversible ultrastructure and occludin tight junction protein expression changes in hippocampal blood-brain barrier in aged rats. Neuroscience letters. 2015;587:51–56 [DOI] [PubMed] [Google Scholar]
- 102.Rochfort KD, Collins LE, Murphy RP, Cummins PM. Downregulation of blood-brain barrier phenotype by proinflammatory cytokines involves nadph oxidase-dependent ros generation: Consequences for interendothelial adherens and tight junctions. PloS one. 2014;9:e101815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kabba JA, Xu Y, Christian H, Ruan W, Chenai K, Xiang Y, Zhang L, Saavedra JM, Pang T. Microglia: Housekeeper of the central nervous system. Cell Mol Neurobiol. 2018;38:53–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Belarbi K, Jopson T, Tweedie D, Arellano C, Luo W, Greig NH, Rosi S. Tnf-alpha protein synthesis inhibitor restores neuronal function and reverses cognitive deficits induced by chronic neuroinflammation. J Neuroinflammation. 2012;9:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Samidurai M, Ramasamy VS, Jo J. Beta-amyloid inhibits hippocampal ltp through tnfr/ikk/nf-kappab pathway. Neurol Res. 2018;40:268–276 [DOI] [PubMed] [Google Scholar]
- 106.Gabbita SP, Srivastava MK, Eslami P, Johnson MF, Kobritz NK, Tweedie D, Greig NH, Zemlan FP, Sharma SP, Harris-White ME. Early intervention with a small molecule inhibitor for tumor necrosis factor-alpha prevents cognitive deficits in a triple transgenic mouse model of alzheimer’s disease. J Neuroinflammation. 2012;9:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu Z, Fan Y, Won SJ, Neumann M, Hu D, Zhou L, Weinstein PR, Liu J. Chronic treatment with minocycline preserves adult new neurons and reduces functional impairment after focal cerebral ischemia. Stroke. 2007;38:146–152 [DOI] [PubMed] [Google Scholar]
- 108.Ward R, Valenzuela JP, Li W, Dong G, Fagan SC, Ergul A. Poststroke cognitive impairment and hippocampal neurovascular remodeling: The impact of diabetes and sex. Am J Physiol Heart Circ Physiol. 2018;315:H1402–H1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ward R, Li W, Abdul Y, Jackson L, Dong G, Jamil S, Filosa J, Fagan SC, Ergul A. Nlrp3 inflammasome inhibition with mcc950 improves diabetes-mediated cognitive impairment and vasoneuronal remodeling after ischemia. Pharmacol Res. 2019;142:237–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Miller DS, Bauer B, Hartz AM. Modulation of p-glycoprotein at the blood-brain barrier: Opportunities to improve central nervous system pharmacotherapy. Pharmacological reviews. 2008;60:196–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Begley DJ. Abc transporters and the blood-brain barrier. Curr Pharm Des. 2004;10:1295–1312 [DOI] [PubMed] [Google Scholar]
- 112.Hartz AM, Bauer B, Fricker G, Miller DS. Rapid modulation of p-glycoprotein-mediated transport at the blood-brain barrier by tumor necrosis factor-alpha and lipopolysaccharide. Mol Pharmacol. 2006;69:462–470 [DOI] [PubMed] [Google Scholar]
- 113.Pereira CD, Martins F, Wiltfang J, da Cruz ESOAB, Rebelo S. Abc transporters are key players in alzheimer’s disease. J Alzheimers Dis. 2018;61:463–485 [DOI] [PubMed] [Google Scholar]


