Abstract
Background
Aerosolised Ad5-nCoV is the first approved mucosal respiratory COVID-19 vaccine to be used as a booster after the primary immunisation with COVID-19 vaccines. This study aimed to evaluate the safety and immunogenicity of aerosolised Ad5-nCoV, intramuscular Ad5-nCoV, or inactivated COVID-19 vaccine CoronaVac given as the second booster.
Methods
This is an open-label, parallel-controlled, phase 4 randomised trial enrolling healthy adult participants (≥18 years) who had completed a two-dose primary immunisation and a booster immunisation with inactivated COVID-19 vaccines (CoronaVac only) at least 6 months before, in Lianshui and Donghai counties, Jiangsu Province, China. We recruited eligible participants from previous trials in China (NCT04892459, NCT04952727, and NCT05043259) as cohort 1 (with the serum before and after the first booster dose available), and from eligible volunteers in Lianshui and Donghai counties, Jiangsu Province, as cohort 2. Participants were randomly assigned at a ratio of 1:1:1, using a web-based interactive response randomisation system, to receive the fourth dose (second booster) of aerosolised Ad5-nCoV (0·1 mL of 1·0 × 1011 viral particles per mL), intramuscular Ad5-nCoV (0·5 mL of 1·0 × 1011 viral particles per mL), or inactivated COVID-19 vaccine CoronaVac (0·5 mL), respectively. The co-primary outcomes were safety and immunogenicity of geometric mean titres (GMTs) of serum neutralising antibodies against prototype live SARS-CoV-2 virus 28 days after the vaccination, assessed on a per-protocol basis. Non-inferiority or superiority was achieved when the lower limit of the 95% CI of the GMT ratio (heterologous group vs homologous group) exceeded 0·67 or 1·0, respectively. This study was registered with ClinicalTrials.gov, NCT05303584 and is ongoing.
Findings
Between April 23 and May 23, 2022, from 367 volunteers screened for eligibility, 356 participants met eligibility criteria and received a dose of aerosolised Ad5-nCoV (n=117), intramuscular Ad5-nCoV (n=120), or CoronaVac (n=119). Within 28 days of booster vaccination, participants in the intramuscular Ad5-nCoV group reported a significantly higher frequency of adverse reactions than those in the aerosolised Ad5-nCoV and intramuscular CoronaVac groups (30% vs 9% and 14%, respectively; p<0·0001). No serious adverse events related to the vaccination were reported. The heterologous boosting with aerosolised Ad5-nCoV triggered a GMT of 672·4 (95% CI 539·7–837·7) and intramuscular Ad5-nCoV triggered a serum neutralising antibody GMT of 582·6 (505·0–672·2) 28 days after the booster dose, both of which were significantly higher than the GMT in the CoronaVac group (58·5 [48·0–71·4]; p<0·0001).
Interpretation
A heterologous fourth dose (second booster) with either aerosolised Ad5-nCoV or intramuscular Ad5-nCoV was safe and highly immunogenic in healthy adults who had been immunised with three doses of CoronaVac.
Funding
National Natural Science Foundation of China, Jiangsu Provincial Science Fund for Distinguished Young Scholars, and Jiangsu Provincial Key Project of Science and Technology Plan.
Introduction
Emerging SARS-CoV-2 variants and the waning of vaccine efficacy against SARS-CoV-2 infection pose substantial barriers to the control of the COVID-19 pandemic.1, 2 Previous evidence has shown that booster vaccines enhance waning immunity and expand the breadth of immunity against SARS-CoV-2 variants of concern in the initial vaccination regimen.2, 3 Studies in many countries that have rapidly deployed the third dose of COVID-19 vaccine to their populations have shown that the third vaccine dose improves humoral and cellular immunity and provides increased short-term protection against symptomatic infections with mutant strains of concern, including omicron, compared with two-dose regimens.4, 5, 6 However, protection against symptomatic infections has been shown to rapidly wane after the third doses of COVID-19 vaccination. As of April 2022, several countries, including Israel, Germany, and the UK, have offered a fourth dose of booster vaccine to their populations.7, 8, 9
Research in context.
Evidence before this study
We searched PubMed and preprint servers SSRN and medRxiv for papers of clinical trials in adults, using the terms (“SARS-CoV-2” OR “COVID-19”) AND (“trial” OR “clinical trial”) AND (“fourth dose” OR “second booster”) AND (“inactivated vaccine” OR “CoronaVac”) in the title or abstract, published between database inception and Dec 19, 2022, with no language restrictions applied. Only one non-randomised trial done in a small number of health-care workers who received a homologous booster of inactivated vaccine (BBIBPCorV) 6 months after the third dose was identified, the results of which showed that the fourth dose had distinct effects on humoral responses to different antigens, with the peak antibody response to the receptor-binding domain induced by the fourth dose being inferior to that after the third dose. Before this study, we had reported two heterologous prime-boost trials with either aerosolised Ad5-nCoV or intramuscular Ad5-nCoV as the first booster administrated to healthy adults who had been primed with two-dose CoronaVac. The heterologous prime-boost regimens were safe and highly immunogenic.
Added value of this study
This is, to our knowledge, the first randomised trial of aerosolised Ad5-nCoV and intramuscular Ad5-nCoV versus CoronaVac as the second booster dose given 6–11 months after immunisation with three doses of inactivated COVID-19 vaccine. This trial shows that all three vaccines have acceptable side-effect profiles, although intramuscular Ad5-nCoV was more reactogenic than the others. The peaking titre of neutralising antibodies induced by the fourth dose of CoronaVac was lower compared with that after the third dose of CoronaVac, indicating that the homologous boost regimen could not further boost an efficient immune response. Heterologous boost with aerosolised Ad5-nCoV or intramuscular Ad5-nCoV induced significantly higher titres of humoral immunity against wild-type SARS-CoV-2 as well as omicron variants BA.4–5 than did a homologous boost with CoronaVac. For neutralising antibodies against omicron, the proportion of participants with seroconversion was over 60% in both heterologous boosting groups, but only around 5% in the homologous CoronaVac group.
Implications of all the available evidence
All these results indicate that the heterologous boost regimens containing Ad5-nCoV were superior to the homologous schedule with CoronaVac. Policy makers and national immunisation advisory committees in China launched a mass immunisation campaign with the fourth dose (second booster) in mainland China, including aerosolised Ad5-nCoV, intramuscular Ad5-nCoV, and CoronaVac. Our results indicate that the vaccination strategy matters. The heterologous boost regimen could induce an efficient immune response to omicron, whereas the homologous boost with CoronaVac might not. Nevertheless, efficacy or effectiveness of this heterologous prime-boost immunisation still needs to be shown in future studies.
Inactivated COVID-19 vaccine has been widely used for primary immunisation or boosting in more than 45 countries.10, 11 In China, more than 71·7% of the whole population have been immunised with three doses of inactivated COVID-19 vaccines.12 However, the initial evidence indicated that the primary series of two doses of inactivated COVID-19 vaccine (CoronaVac [Sinovac]) plus a third (booster) dose of CoronaVac offered low neutralisation responses against the omicron variant.13, 14 The low serum immunity concentration of the population against the omicron variants in China, combined with the high infectivity and ability of omicron variants to escape vaccine-induced neutralising antibodies, mean that an effective booster immunisation strategy is an urgent issue at hand.15, 16, 17
Previously reported trials have shown that a heterologous prime-boost schedule can be more immunogenic than a homologous schedule, and had more benefits for extending the breadth and longevity of protection provided by the available vaccines.18 Administration of a heterologous third dose (booster) of an intramuscular adenovirus type-5 vector-based COVID-19 vaccine (Ad5-nCoV; Convidecia [CanSino]) or orally administered aerosolised Ad5-nCoV after the two-dose priming with Sinovac CoronaVac showed strong enhancement of humoral and cellular immune responses compared with homologous administration of a third dose of CoronaVac.17, 19 However, vaccinating with Ad5-nCoV as a fourth dose (second booster) following three doses of inactivated COVID-19 vaccines has not yet been investigated.
Here, we report the safety and immunogenicity of a heterologous boost with the aerosolised Ad5-nCoV or intramuscular Ad5-nCoV versus a homologous boost with inactivated COVID-19 vaccine CoronaVac in Chinese adults who have been primed with three doses of inactivated COVID-19 vaccine.
Methods
Study design and participants
We did an open-label, parallel-controlled, phase 4, randomised trial to evaluate the safety and immunogenicity of heterologous boost immunisation with aerosolised Ad5-nCoV or intramuscular Ad5-nCoV as a fourth dose, compared with a homologous booster dose of CoronaVac, in healthy adults aged 18 years or older who had completed three doses of vaccination with inactivated COVID-19 vaccine at least 6 months before at the Centers for Disease Control and Prevention in Lianshui and Donghai counties, in Jiangsu Province, China. In cohort 1, eligible participants were recruited from previous trials (NCT04892459, NCT04952727, and NCT05043259), in which the serum of participants before and after receiving the third dose (first booster) of CoronaVac were available. In cohort 2, eligible participants were recruited from a pool of volunteers. The study was advertised through local media channels and prospective participants came to the Centers for Disease Control and Prevention to volunteer for the trial.
The trial protocol was reviewed and approved by the Research Ethics Committee of the Jiangsu Provincial Center for Disease Control and Prevention, and no changes to the protocol were made after the initiation of the study. The protocol is provided in the appendix, with a full list of inclusion and exclusion criteria. Written informed consent was obtained from each participant before screening. This trial was done following the principles of the Declaration of Helsinki, Good Clinical Practice guidelines, and local guidelines.
Randomisation
We used a web-based interactive response randomisation system for stratified randomisation of the participants in cohorts 1 and 2. Eligible participants in each cohort were randomly assigned in a 1:1:1 ratio to receive aerosolised Ad5-nCoV, intramuscular Ad5-nCoV, or intramuscular CoronaVac. The randomisation lists were generated by an independent statistician using SAS (version 9.4).
This is an open-label trial. Thus, both investigators and participants were aware of their treatment allocations. However, laboratory staff who were responsible for measuring immune responses remained masked during the study period.
Procedures
CoronaVac (Vero Cell; Beijing Sinovac Research & Development, Beijing, China) is an inactivated whole-virion vaccine of wild-type SARS-CoV-2 with aluminium hydroxide as the adjuvant, which was administered intramuscularly at 0·5 mL per dose. The Ad5-nCoV vaccine (CanSino Biologics, Tianjin, China) is a replication-defective Ad5-vectored vaccine expressing the full-length spike gene of wild-type SARS-CoV-2 (Wuhan-Hu-1), supplied as a liquid formulation at 1·5 mL per vial, at a concentration of 1·0 × 1011 viral particles per mL. The intramuscular Ad5-nCoV was administered into the upper arm at 0·5 mL, and the aerosolised Ad5-nCoV was administered at 0·1 mL by oral inhalation. A vapouring unit (Aerogen, Galway, Ireland) integrated by Suzhou Weiqi Biological Technology (Suzhou City, China) was used to aerosolise Ad5-nCoV and the aerosolised droplets of vaccine were poured into a disposable suction cup and inhaled orally. Following the booster vaccination, all participants were observed at the clinics for 30 min for any immediate vaccine-related reactions. We collected data regarding administration-site and systemic solicited adverse events and unsolicited adverse events up to 28 days after vaccination. In the grading of adverse events, we used standard guidelines issued by the State Food and Drug Administration of China. Serious adverse events were collected for the 6-month trial duration and are reported up to day 28 in this Article.
Blood and salivary samples were collected from all participants for immunogenicity assessments on day 0 (before booster vaccination) and on days 14 and 28 after boosting (the fourth dose). Participants in cohort 1, also had serum samples from before, and 14 and 28 days after, the third booster dose. Neutralising antibody against live SARS-CoV-2 virus was measured with cytopathic effect-based microneutralisation assay with a wild-type SARS-CoV-2 virus isolate, BetaCoV/Jiangsu/JS02/2020 (GISAID EPI_ISL_411952), following the procedures reported previously.17 Serum dilutions used for the microneutralisation test ranged from 1:4 to 1:8192 and were then mixed with the same volume of virus solution to achieve a 50% tissue culture infectious dose of 100 per well. The reported titre is the reciprocal of the highest sample dilution that protects at least 50% of the cells from cytopathic lesions, as observed under an inverted microscope. The WHO international standard (NIBSC code 20/136) was used as the reference for the cytopathic effect-based microneutralisation assay. Seropositivity for neutralising antibody against live SARS-CoV-2 virus is defined as a titre ≥1:4. Seroconversion was defined as at least a 4-times increase in the antibody titre after vaccination. The receptor-binding domain (RBD)-specific IgG and N-specific IgG titres against SARS-CoV-2 were measured by ELISA by means of a commercially available anti-SARS-CoV-2 RBD IgG ELISA kit (Vazyme Medical Technology, Nanjing, China), with a cutoff titre of 1:10.17 RBD-specific binding secretory (sIgA) antibodies in saliva before, and 14 and 28 days after, booster vaccination were measured by means of an anti-SARS-CoV-2 RBD sIgA ELISA kit (Vazyme Medical Technology). The detectable sIgA response was defined as a concentration of at least 5 RU/mL. Pseudovirus neutralising antibody titres against omicron variants BA.4–5 were measured by means of pseudovirus neutralisation tests (a vesicular stomatitis virus pseudovirus system expressing the spike glycoprotein), with a cutoff titre of 1:30.20 Pre-existing anti-Ad5 neutralising antibodies in serum at baseline in the participants were also measured, as described previously.21
In addition, peripheral blood mononuclear cells (PBMCs) were isolated from whole blood before the booster vaccination and at day 14 post-vaccination. Specific T-cell responses in terms of cytokine secretion from T helper type 1 cells (Th1; interferon-γ [IFN-γ], tumour necrosis factor-α [TNF-α], and interleukin [IL-2]) were quantified with enzyme-linked immunospot (ELISpot) assay (Mabtech, Stockholm, Sweden) by means of fresh PBMCs stimulated with overlapping spike glycoprotein peptide pools for about 12–24 h before detection, and expressed as the number of positive spot-forming cells per 106 cells.
Outcomes
The co-primary outcomes were safety and immunogenicity. The primary endpoint for safety was the incidence of adverse reactions occurring within 28 days after the fourth dose, whereas the primary endpoint for immunogenicity was geometric mean titres (GMTs) for neutralising antibodies against the live SARS-CoV-2 virus at day 28. The secondary endpoints for safety were the incidences of adverse events within 28 days and serious adverse events at 6 months after booster immunisation. The secondary endpoints of immunogenicity were the seroconversion rate, geometric mean fold increase (GMFI) for neutralising antibodies against the live SARS-CoV-2 virus at day 28, and the seroconversion rate, GMTs, and GMFI for neutralising antibodies against the live SARS-CoV-2 virus as well as receptor-binding domain (RBD)-specific IgG at 14 days, 3 months and 6 months after booster immunisation.
Statistical analysis
Sample size calculation was based on the assumption that heterologous booster vaccinations (aerosolised Ad5-nCoV or intramuscular Ad5-nCoV) following a three-dose inactivated vaccine regimen would elicit non-inferior and superior concentrations of neutralising antibodies to the homologous booster dose with CoronaVac. Power Analysis and Sample Size software (LLC, USA version 11.0.7) was used.
We assumed that administration of the heterologous fourth dose vaccinations with aerosolised Ad5-nCoV or intramuscular Ad5-nCoV could induce antibody titres at least 2-times higher than the peak GMT of neutralising antibodies after the homologous fourth dose immunisation, and a SD of 0·6 at log10 scale for GMTs of neutralising antibodies. The study needed to recruit 101 participants in each group to achieve 90% power to identify non-inferiority, with a margin of greater than a 0·67-times (0·174 at log10 scale) difference, and superiority with a margin of greater than a 1·0-times difference. Thus, we decided on a total sample size of 360 with 120 for each group after adjusting for an attrition rate of 15% due to loss to follow-up.
The safety analysis population included all randomly assigned participants who received the fourth dose vaccination. However, the primary analysis of wild-type SARS-CoV-2 neutralising antibodies was carried out in a per-protocol cohort, a subsample from which the primary immunogenic endpoint data were available and had no protocol deviations. The GMT ratio was calculated as the antilogarithm of the difference between the mean of the log10 transformed wild-type SARS-CoV-2 neutralising antibodies in the heterologous group and that in the homologous group (as the reference). Non-inferiority or superiority was achieved when the lower limit of the 95% CI of the GMT ratio (heterologous group vs homologous group) exceeded 0·67 or 1·0, respectively. The χ2 test or Fisher's exact test was used to analyse categorical data. ANOVA was used to analyse log-transformed antibody titres and the Student-Newman-Keuls test for multiple comparisons was used if a significant difference between treatment groups was noted. We used the Wilcoxon rank sum test to analyse data that did not follow a normal distribution. Pearson correlation analysis was used for the association between log-transformed antibody neutralisation titres and RBD-specific binding IgG titres. Stratified analyses were done according to participants' pre-existing Ad5 neutralising antibody titres, age, and study site. The dynamic changes of the antibody responses following the third dose (first booster) and fourth dose (second booster) were as described for cohort 1. Statistical analysis was done by means of SAS (version 9.4), R (version 4.2.1), or GraphPad Prism (LLC, USA, version 9.0.0).
Role of the funding source
The funders of the study had no role in protocol design, data collection, statistical analysis, data interpretation, or writing of the report.
Results
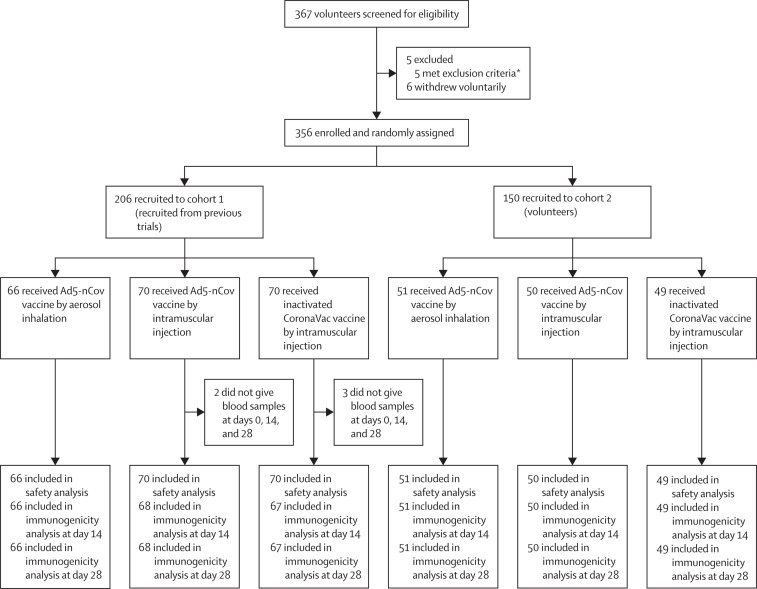
Between April 23, and May 23, 2022, we recruited a total of 367 participants, of whom 11 participants were excluded, and 356 participants met eligibility criteria and received a dose of aerosolised Ad5-nCoV (n=117), intramuscular Ad5-nCoV (n=120), or CoronaVac (n=119) as the fourth dose (figure 1 ). The median age of participants was 45·0 years (IQR 37·0–53·0), with 201 (57%) female participants. 52 (15%) participants in the study were aged 60 years or older. Baseline characteristics were well balanced across the three groups (table 1 ).
Figure 1.
Trial profile
*Excluded due to severe hypertension (blood pressure of above 221/142 mm Hg), positive urinary pregnancy test, urticaria, psoriasis, or because they had undergone cholecystectomy within the past 14 days. The previous iSARS-CoV-2 infection status of participants was confirmed by asking the participants and by checking their recent medical visits.
Table 1.
Baseline characteristics of participants in the intention-to-treat cohort
| Aerosolised Ad5-nCoV group (n=117) | Intramuscular Ad5-nCoV group (n=120) | CoronaVac group (n=119) | ||
|---|---|---|---|---|
| Sex | ||||
| Female | 62 (53%) | 72 (60%) | 67 (56%) | |
| Male | 55 (47%) | 48 (40%) | 52 (44%) | |
| Age, years | ||||
| 60–70 | 20 (17%) | 16 (13%) | 16 (13%) | |
| 18–59 | 97 (83%) | 104 (87%) | 103 (87%) | |
| Mean* | 47·2 (11·3) | 46·4 (11·2) | 43·5 (12·2) | |
| Height, cm | 164·3 (8·3) | 163·8 (7·8) | 163·8 (7·1) | |
| Weight, kg | 70·8 (13·3) | 68·5 (13·2) | 67·3 (12·3) | |
| Time since the last priming dose of inactivated vaccine, months | 6·6 (6·5–8·6) | 6·6 (6·4–8·6) | 6·6 (6·4–8·6) | |
| Pre-existing Ad5-neutralising antibodies† | ||||
| Geometric mean titre | 182·6 (127·8–260·7) | 139·5 (95·2–204·3) | 166·0 (115·2–239·3) | |
| Participants with titre ≤1:200 | 48 (41%) | 54/118 (46%) | 54/116 (47%) | |
| Participants with titre >1:200 | 69 (59%) | 64/118 (54%) | 62/116 (53%) | |
Data are n (%), mean (SD), median (IQR,) or geometric mean titre (95% CI). The analysis was based on the intention-to-treat cohort, with some participants reclassified into the correct groups according to the vaccines that they received.
Significant differences were noted among all groups, with p=0·034.
Two in the intramuscular Ad5-nCoV group and three in the CoronaVac group failed to collect blood and therefore did not do the Ad5 neutralising antibody assay.
Among these eligible participants, 206 were recruited into cohort 1 and 150 were recruited into cohort 2 (figure 1). All eligible participants received the designated booster vaccine. The median interval of time between the fourth dose and the third was 6·6 (IQR 6·5–8·6) months. We obtained serum samples from 351 participants on day 0, 14, and 28. The distribution of pre-existing Ad5 antibodies was similar across groups of participants at enrolment, before receiving the fourth dose, with 195 (56%) participants showing high pre-existing titre greater than 1:200.
Within 28 days of booster vaccination, participants in the intramuscular Ad5-nCoV group reported a significantly higher frequency of overall adverse reactions than those in the aerosolised Ad5-nCoV and intramuscular CoronaVac groups (30% vs 9% vs 14%, respectively; p <0·0001; table 2 ). Participants receiving intramuscular Ad5-nCoV had a higher incidence of administration-site adverse reactions (23%) than those receiving aerosolised Ad5-nCoV (7%) or intramuscular CoronaVac (11%). A similar occurrence was seen for systemic adverse reactions. The most common adverse reaction in the intramuscular Ad5-nCoV and CoronaVac groups was administration-site pain, which was reported by 26 (22%) participants, and 12 (10%) participants, respectively. In addition, the most common adverse reaction reported by participants receiving the aerosolised Ad5-nCoV was xerostomia (six [5%]). All adverse reactions were generally mild or moderate in severity and usually resolved within 1 or 2 days. The only grade 3 adverse reaction was fever, which occurred in four (3%) participants following the administration of the intramuscular Ad5-nCoV. The incidences of unsolicited adverse events within 28 days were low and similar across the treatment groups (appendix p 2). No serious adverse events were documented in any group within 28 days after the boost vaccination.
Table 2.
Adverse reactions within 28 days after booster vaccination
| Aerosolised Ad5-nCoV group (n=117) | Intramuscular Ad5-nCoV group (n=120) | CoronaVac group (n=119) | p value* | |
|---|---|---|---|---|
| Adverse reactions within 28 days | ||||
| Total (any) | 11 (9%) | 36 (30%) | 17 (14) | <0·0001 |
| Grade 3 | 0 | 4 (3%) | 0 | 0·019 |
| Solicited administration-site adverse reactions | ||||
| Total (any) | 8 (7%) | 27 (23%) | 13 (11%) | 0·0012 |
| Redness† (any) | 0 | 3 (3%) | 0 | 0·083 |
| Xerostomia (any) | 6 (5%) | 0 | 0 | .. |
| Oral ulcer (any) | 1 (1%) | 0 | 0 | .. |
| Itch† (any) | 0 | 4 (3%) | 4 (3%) | 0·99 |
| Hoarseness (any) | 1 (1%) | 0 | 0 | − |
| Pain† (any) | 0 | 26 (22%) | 12 (10%) | 0·014 |
| Throat pain (any) | 4 (3%) | 0 | 0 | .. |
| Induration† (any) | 0 | 4 (3%) | 2 (2%) | 0·41 |
| Swelling† (any) | 0 | 3 (3%) | 0 | 0·083 |
| Solicited systemic adverse reactions | ||||
| Total (any) | 8 (7%) | 18 (15%) | 6 (5%) | 0·016 |
| Nausea (any) | 0 | 2 (2%) | 1 (1%) | 0·37 |
| Fever (any) | 1 (1%) | 12 (10%) | 0 | <0·0001 |
| Grade 3 | 0 | 4 (3%) | 0 | 0·019 |
| Muscle pain (any) | 1 (1%) | 4 (3%) | 0 | 0·075 |
| Diarrhoea (any) | 1 (1%) | 3 (3%) | 2 (2%) | 0·62 |
| Joint pain (any) | 0 | 3 (3%) | 1 (1%) | 0·18 |
| Cough (any) | 4 (3%) | 2 (2%) | 0 | 0·13 |
| Runny nose (any) | 3 (3%) | 2 (2%) | 1 (1%) | 0·60 |
| Vomiting (any) | 1 (1%) | 1 (1%) | 0 | 0·60 |
| Sneeze (any) | 2 (2%) | 1 (1%) | 0 | 0·36 |
| Fatigue (any) | 2 (2%) | 8 (7%) | 2 (2%) | 0·049 |
| Headache (any) | 3 (3%) | 7 (6%) | 1 (1%) | 0·077 |
| Thoracalgia (any) | 1 (1%) | 0 | 0 | 0·36 |
| Loss of appetite (any) | 0 | 1 (1%) | 0 | 0·37 |
| Throat pain (any) | 0 | 1 (1%) | 0 | 0·37 |
| Unsolicited adverse reactions | ||||
| Itchy pharynx (any) | 1 (1%) | 0 | 0 | 0·36 |
Data are n (%). Any=all the participants with any grade adverse reactions or events. The analysis was based on the intention-to-treat cohort.
Calculated with χ2 test or Fisher's exact test.
p value shows the result of the comparison between the intramuscular Ad5-nCoV group and the CoronaVac group.
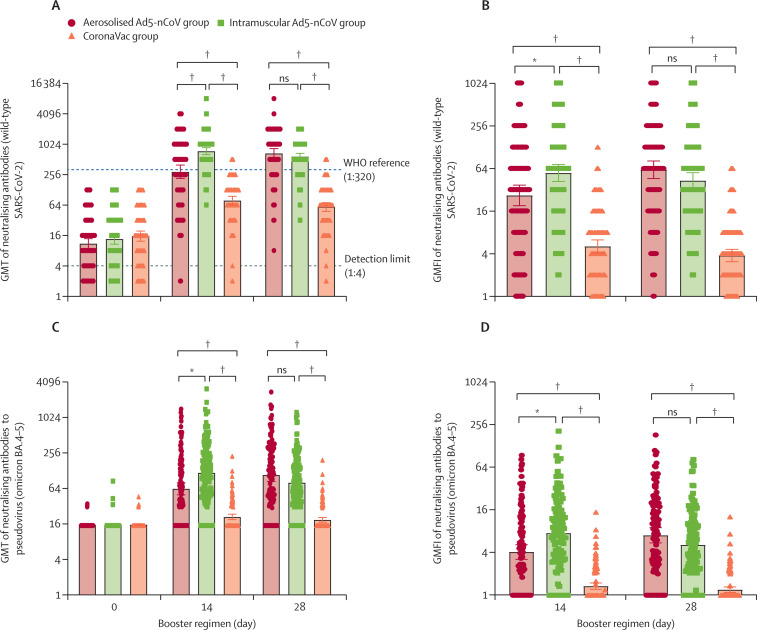
The neutralising antibody titres against wild-type SARS-CoV-2 observed before the fourth dose vaccination were low and similar in all groups: GMT of 11·0 (95% CI 8·8–13·8) in the aerosolised Ad5-nCoV group, 13·6 (10·8–17·1) in the intramuscular Ad5-nCoV group, and 15·5 (12·3–19·6) in the CoronaVac group (figure 2 ). Among participants immunised with three doses of CoronaVac, the post-vaccination GMTs of neutralising antibody against wild-type SARS-CoV-2 at day 14 and 28 in both the heterologous booster groups (291·6 [215·0–395·6] in the aerosolised Ad5-nCoV group, 745·7 [636·6– 873·4] in the intramuscular Ad5-nCoV group at day 14; 672·4 [539·7–837·7] in the aerosolised Ad5-nCoV group, 582·6 [505·0–672·2] in the intramuscular Ad5-nCoV group at day 28) were significantly higher than those in the homologous booster group (CoronaVac group, 78·4 [64·6–95·1] at day 14 and 58·5 [48·0–71·4] at day 28); appendix pp 3–6). However, participants receiving intramuscular Ad5-nCoV showed a faster increase of the antibody titres, which peaked (745·7 [95% CI 636·6–873·4]) at day 14 after the boost, whereas those receiving aerosolised Ad5-nCoV reached the peak antibody titre (672·4 [539·7–837·7]) at day 28. The GMT ratio between the intramuscular Ad5-nCoV group and the CoronaVac group was 10·0 (7·8–12·7), and that between the aerosolised Ad5-nCoV group and the CoronaVac group was 11·5 (8·6–15·0) at day 28, indicating that the GMTs of both the heterologous groups were superior to that of the homologous group. The proportions of participants with seroconversion were similar between the aerosolised Ad5-nCoV group (97·4% [95% CI 92·7–99·1]) and the intramuscular Ad5-nCoV group (99·2% [95·4–99·9]), but were significantly higher than that of the CoronaVac group (58·6% [49·5–67·2]). Similarly, both the heterologous boosters resulted in significantly higher GMFI of the neutralising antibody titres against wild-type SARS-CoV-2 compared with the homologous booster with CoronaVac (appendix p 3).
Figure 2.
Neutralising antibodies to wild-type SARS-CoV-2 and pseudovirus of omicron BA.4–5 before and after boosting
GMT of neutralising antibodies to wild-type SARS-CoV-2 and pseudovirus of omicron BA.4–5 (A and C). GMFI of neutralising antibodies to wild-type SARS-CoV-2 and pseudovirus of omicron BA.4–5 (B and D). Error bars indicate 95% CIs. Each point on the graphs represents a sample. GMFI=geometric mean titre fold increase. GMT=geometric mean titre. ns=not significantly different. *p<0·01. †p<0·0001.
Before the fourth booster, the pseudovirus neutralising antibodies against the SARS-CoV-2 omicron variants BA.4–5 were undetectable for most participants at baseline; however, after the booster, they were significantly higher in both the aerosolised Ad5-nCoV group (GMT 62·9 [95% CI 49·8–79·6] at day 14 and 108·2 [84·3–138·9] at day 28) and the intramuscular Ad5-nCoV group (117·1 [93·7–146·4) at day 14 and 79·9 [65·4–97·6] at day 28) than in the CoronaVac group (21·0 [18·8–23·3] at day 14 and 18·7 [17·1–20·5] at day 28; figure 2; appendix p 3). The overall titres of neutralising antibodies against omicron variants BA.4–5 were approximately 4·6–7·3-times lower than that of the neutralising antibody against wild-type SARS-CoV-2. However, similar to the dynamics of the neutralising antibody titres against wild-type SARS-CoV-2, the administration of intramuscular Ad5-nCoV elicited a faster antibody response against omicron variants BA.4–5 than did the aerosolised Ad5-nCoV. Seroconversion was achieved by 61·5% (95% CI 52·5–69·9) of participants in the aerosolised Ad5-nCoV group and 67·0% (58·1–74·8) of participants in the intramuscular Ad5-nCoV group at the peaking titres, versus 5·2% (2·4–10·9) in the CoronaVac group.
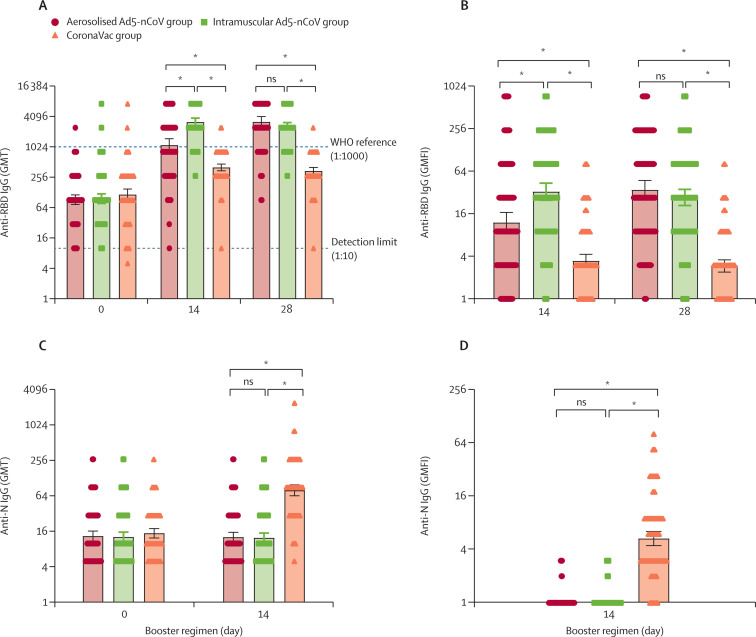
Similar to neutralising antibody titres against the wild-type SARS-CoV-2, the heterologous booster doses with aerosolised Ad5-nCoV and intramuscular Ad5-nCoV induced a significant increase in the wild-type RBD-specific IgG antibody responses, whereas the homologous booster dose with CoronaVac elicited a moderate increase (figure 3 ). The post-vaccination GMT of RBD-specific antibody against wild-type SARS-CoV-2 in both the heterologous booster aerosolised Ad5-nCoV group and the intramuscular Ad5-nCoV group was significantly higher than in the CoronaVac group at days 14 and 28. Strong correlations were found between the titres of RBD-specific IgG and neutralising antibody against wild-type SARS-CoV-2, with Pearson correlation coefficients of 0·7–0·9 (appendix p 10). However, the GMTs of N-specific IgG antibodies showed no increase 14 days after the heterologous vaccination with aerosolised Ad5-nCoV or intramuscular Ad5-nCoV (figure 3). Nevertheless, boosting with inactivated vaccine CoronaVac significantly increased N-specific IgG antibody responses with a GMFI of 5·3 (95% CI 4·4–6·4) at day 14. Detectable sIgA responses were noted in 45 (39%) and 49 (42%) participants in the aerosolised Ad5-nCoV group at days 14 and 28, respectively, which were significantly higher than those in the intramuscular Ad5-nCoV group (33 [28%] and 33 [28%]) and CoronaVac group (28 [24%] and 25 [22%]) at the same timepoints (appendix p 7). However, the differences in sIgA concentrations across the groups were only observed at day 14, not day 28.
Figure 3.
RBD-specific or N-specific IgG antibody titres before and after boosting
GMT of anti-RBD IgG (A) and anti-N IgG (C). GMFI of anti-RBD IgG (B) and anti-N IgG (D). Error bars indicate 95% CIs. Each point on the graphs represents a sample. GMFI=geometric mean titre fold increase. GMT=geometric mean titre. RBD=receptor-binding domain. ns=not significantly different. *p<0·0001.
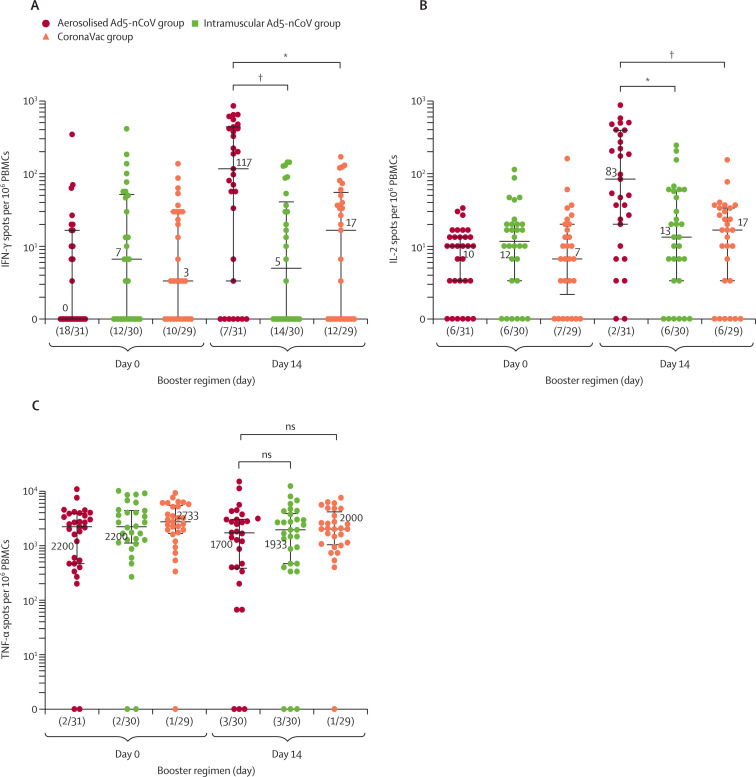
The administration of the fourth dose with aerosolised Ad5-nCoV showed a significant cellular response as measured by IFN-γ and IL-2 ELISpot at day 14 (figure 4 ). Participants in the aerosolised Ad5-nCoV group had median IFN-γ+ spot counts of 117 (IQR 3–437) and IL-2+ spot counts of 83 (20–393) per 106 PBMCs, whereas, both intramuscular Ad5-nCoV and CoronaVac immunisation as the fourth dose showed lower responses. Although the number of TNF-α secretion T cells was relatively high at baseline before the boost, it did not increase significantly after boosting across the treatment groups.
Figure 4.
SARS-CoV-2 spike-specific T-cell cytokine responses before and after boosting
IFN-γ (A), IL-2 (B), and TNF-α (C) cytokine concentrations. Data are the median (IQR) of positive spot counts per 106 PBMCs and (n/N), where n indicates the number of negatives for T-cell responses of the participants, and N indicates the total number of participants. Each point on the graphs represents a sample. IFN-γ=interferon-γ. IL-2=interleukin-2. ns=not significantly different. TNF-α=tumour necrosis factor-α PBMCs=peripheral blood mononuclear cells. *p<0·01. †p<0·001.
Similar neutralising antibody titres were observed in participants in cohort 1 and cohort 2 after the fourth dose (appendix pp 5–6). We present dynamic trends of neutralising antibody titres against wild-type SARS-CoV-2 in cohort 1 after the third and fourth doses (appendix pp 8–9). All these participants were primed with two doses of CoronaVac, and showed low GMTs of neutralising antibody of around 3·5–4·0, before receiving an immunisation with CoronaVac as the third dose (first booster). After the third dose, the GMTs of neutralising antibody were increased to 84·7–117·3 at day 14 and then declined swiftly to 33·6–44·8 at day 28. The neutralising antibody titres against wild-type SARS-CoV-2 of these participants continued to wane during the next 6–11 months, showing a low titre around 11·0–15·5 before immunisation with the fourth dose. Participants who received intramuscular Ad5-nCoV as the fourth dose showed the fastest-growing trend, peaking at 14 days after the fourth dose, whereas those who received aerosolised Ad5-nCoV showed a more moderate but longer growth, with the neutralising antibody titres peaking at day 28 after the fourth booster. Both of the heterologous boost groups with aerosolised Ad5-nCoV or intramuscular Ad5-nCoV could further boost the humoral immune response and had more robust neutralising antibody responses after the fourth dose compared with the third dose. However, the fourth dose of homologous booster with CoronaVac induced only a mild increase of the neutralising antibody, which was even lower than the peaking titre after the third dose.
Discussion
Our findings show that the heterologous booster regimen with aerosolised Ad5-nCoV or intramuscular Ad5-nCoV and the homologous booster regimen with CoronaVac as the fourth dose were generally safe. However, the frequency of adverse reactions reported in the intramuscular Ad5-nCoV group was significantly higher than those of the other two groups, and grade 3 adverse reactions (fever) were noted only after the administration of intramuscular intramuscular Ad5-nCoV (3%). The heterologous regimens with aerosolised Ad5-nCoV or intramuscular Ad5-nCoV induced significantly higher titres of neutralising antibody against wild-type SARS-CoV-2, as well as pseudovirus neutralising antibody against the SARS-CoV-2 omicron variants BA.4–5, than did the homologous booster schedule with CoronaVac, in individuals who had been vaccinated with three doses of inactivated COVID-19 vaccine, indicating that the heterologous boost regimens containing Ad5-nCoV were superior to the homologous schedule. We chose GMT of neutralising antibody as the primary endpoint of immunogenicity because neutralising antibody is reported to be protective against SARS-CoV-2 virus in most studies, and could act as a correlate of protection to suggest that the boost has induced a response associated with vaccine efficacy.22 Additionally, the T-cell response across all groups was greatest with the aerosolised Ad5-nCoV boost regimen, which might be important in terms of durability of protection against new SARS-CoV-2 variants.23 Surprisingly, the peaking titre of neutralising antibody following the homologous second boost with CoronaVac was even lower than that after the first boost with CoronaVac, indicating an impaired recall of humoral immune responses on elevating neutralising antibodies, in line with a previous report.24 Nevertheless, the heterologous Ad5-nCoV-containing boosting schedules were more immunogenic than the homologous CoronaVac immunisation.
Although aerosolised Ad5-nCoV and intramuscular Ad5-nCoV showed a similar peaking titre of the neutralising antibodies post-vaccination, participants receiving a dose of intramuscular Ad5-nCoV boost had a more rapid increase of the neutralising antibodies, which peaked at day 14, whereas those receiving aerosolised Ad5-nCoV had a peak at day 28. This pattern of increasing antibody responses was consistent with the previous study with aerosolised Ad5-nCoV,19 which indicated that the vaccination administration targets on lymphoid tissues of both the upper and lower respiratory tract need longer response times to reach the peak compared with intramuscular administration. Although data in this study do not show any convincing evidence of additional benefits of the protection associated with mucosal immune activity, the aerosolised boosting schedule dose was associated with a higher proportion of participants with positive sIgA in their saliva. In addition, the immunisation with aerosolised Ad5-nCoV by inhaling through the mouth used a dose only one-fifth that of intramuscular Ad5-nCoV and was associated with a reduced occurrence of adverse reactions following the vaccination.
Crucially, this study includes aerosolised or intramuscular Ad5-nCoV and CoronaVac vaccines manufactured by CanSino and Sinovac, respectively, which are extensively used in low-income and middle-income countries and potentially more likely to rely on mixed schedules. These data are the first from a randomised controlled trial of COVID-19 vaccines of heterologous aerosolised Ad5-nCoV and intramuscular Ad5-nCoV boost following three doses of immunisation with CoronaVac.
This study has several limitations. First, only healthy adults aged between 18 and 70 years were included, included; we excluded those older than 70 years, immunocompromised individuals, and those with co-existing disease, which might result in a poorer serological response and higher percentage of unresponsiveness, as some studies have reported.25 Second, because this was an immunogenicity and reactogenicity study, we did not assess the efficacy of the booster immunisation regimen for symptomatic or severely ill patients with COVID-19, and thus the protective effect associated with such a heterologous vaccination regimen remains uncertain. However, several efficacy or effectiveness studies have found that heterologous booster vaccination significantly increased neutralisation antibody titres against SARS-CoV-2 variants (including omicron), reducing the risk of infection and the disease severity and mortality associated with COVID-19, consistent with the results of the immunogenicity studies.8, 9, 26 Third, the small sample size was not sufficient to determine the potential increased risk of some rare but serious adverse reactions, such as vaccine-induced immune thrombotic thrombocytopenia. Fourth, there is a lack of data on the neutralisation antibodies to other current omicron subvariants such as BF.7, BQ.1, BA.2.75.2, and XBB, which might partly limit the generalisability of immunogenicity results in this study. In addition, the persistence of immune responses after booster dosing is unknown since only the data up to 28 days are reported here. However, we are confident that the heterologous vaccination regimens maintain neutralising activity for a longer period after the boost, since the much higher peak titre of neutralising antibodies induced by the heterologous vaccination and the decreased rates of neutralising antibodies elicited by various schedules in other studies was broadly similar.27 Follow-up of participants in this study for 6-month safety and immunogenicity is ongoing.
In conclusion, a heterologous fourth dose (second booster) with aerosolised Ad5-nCoV or intramuscular Ad5-nCoV as the second booster was safe and highly immunogenic. Aerosolised Ad5-nCoV shows a more favourable safety profile and an enhanced T-cell response versus intramuscular Ad5-nCoV in healthy adults who have been immunised with three doses of CoronaVac. The substantially higher antibody titres with heterologous boosting are noteworthy and encouraging. However, the relatively mild boosting effect of the heterologous fourth dose against omicron BA.4–5, compared with the effect against wild-type SARS-CoV-2 is a concern. Our findings support the heterologous administration of aerosolised Ad5-nCoV or intramuscular Ad5-nCoV vaccines and provide support for accelerated booster rollout.
Data sharing
All data will be made available from the corresponding author on reasonable request. All code used to produce the results will be made available from the corresponding author on reasonable request.
Declaration of interests
J-BG is an employee of CanSino Biologics. Tao Zhu owns stock in CanSino Biologics. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
This work is funded by National Natural Science Foundation of China, Jiangsu Provincial Science Fund for Distinguished Young Scholars, and Jiangsu Provincial Key Project of Science and Technology Plan. CanSino Biologics contributed in providing investigational vaccines and the Continuous Vapouring System for this study. We thank members of the CanSino COVID-19 study group Tao Zhu, Hai-Tao Huang, Xiao-Long Li, Xue Wang, and Peng Wan (employees of CanSino Biologics) for their assistance with the study.
Acknowledgments
Contributors
J-XLi is the principal investigator of this trial. J-XLi, B-SW, RT, and J-BG designed the trial and the study protocol. HZ drafted the manuscript. J-XLi and RT interpreted the data and revised the manuscript. HZ did the statistical analysis. H-XPan supervised the study. X-QC, JZ, J-HZ, and Z-ZS led and participated in the site work, including the recruitment, follow-up, and data collection. X-LG, F-JS, YC, Z-PLi, X-YZ, J-XLiu, S-PWu, and X-YX were responsible for laboratory analyses. J-BG monitored the trial. J-XLi, L-HH, and F-CZ critically reviewed the manuscript. All authors had full access to and verified the data. All authors had final responsibility for the decision to submit for publication.
Contributor Information
CanSino COVID-19 Study Group:
Hai-Tao Huang, Xiao-Long Li, Xue Wang, Peng Wan, and Tao Zhu
Supplementary Material
References
- 1.Feikin DR, Higdon MM, Abu-Raddad LJ, et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet. 2022;399:924–944. doi: 10.1016/S0140-6736(22)00152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrews N, Stowe J, Kirsebom F, et al. Covid-19 vaccine effectiveness against the omicron (B.1.1.529) variant. N Engl J Med. 2022;386:1532–1546. doi: 10.1056/NEJMoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muik A, Lui BG, Wallisch AK, et al. Neutralization of SARS-CoV-2 omicron by BNT162b2 mRNA vaccine-elicited human sera. Science. 2022;375:678–680. doi: 10.1126/science.abn7591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. Effect of mRNA vaccine boosters against SARS-CoV-2 omicron infection in Qatar. N Engl J Med. 2022;386:1804–1816. doi: 10.1056/NEJMoa2200797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Servellita V, Syed AM, Morris MK, et al. Neutralizing immunity in vaccine breakthrough infections from the SARS-CoV-2 omicron and delta variants. Cell. 2022;185:1539. doi: 10.1016/j.cell.2022.03.019. 48.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zuo F, Abolhassani H, Du L, et al. Heterologous immunization with inactivated vaccine followed by mRNA-booster elicits strong immunity against SARS-CoV-2 Omicron variant. Nat Commun. 2022;13 doi: 10.1038/s41467-022-30340-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grewal R, Kitchen SA, Nguyen L, et al. Effectiveness of a fourth dose of covid-19 mRNA vaccine against the omicron variant among long term care residents in Ontario, Canada: test negative design study. BMJ. 2022;378 doi: 10.1136/bmj-2022-071502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Regev-Yochay G, Gonen T, Gilboa M, et al. Efficacy of a fourth dose of Covid-19 mRNA vaccine against omicron. N Engl J Med. 2022;386:1377–1380. doi: 10.1056/NEJMc2202542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munro APS, Feng S, Janani L, et al. Safety, immunogenicity, and reactogenicity of BNT162b2 and mRNA-1273 COVID-19 vaccines given as fourth-dose boosters following two doses of ChAdOx1 nCoV-19 or BNT162b2 and a third dose of BNT162b2 (COV-BOOST): a multicentre, blinded, phase 2, randomised trial. Lancet Infect Dis. 2022;22:1131–1141. doi: 10.1016/S1473-3099(22)00271-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO Evidence assessment: Sinopharm/BBIBP COVID-19 vaccine, 2021. Microsoft PowerPoint - 2- SAGE critical evidence of Sinopharm .pptx (who.int) https://cdn.who.int/media/docs/default-source/immunization/sage/2021/april/2_sage29apr2021_critical-evidence_sinopharm.pdf
- 11.WHO Evidence assessment: CoronaVac COVID-19 vaccine. 2021. https://cdn.who.int/media/docs/default-source/immunization/sage/2021/april/5_sage29apr2021_critical-evidence_sinovac.pdf
- 12.New China News Agency The immunisation coverage with the full primary series of COVID-19 vaccines in China is 89.7% http://www.news.cn/politics/2022-07/23/c_1128857426.htm
- 13.Jiang HD, Guo XL, Jin PF, Tang R, Li JX, Zhu FC. Head-to-head comparisons of the neutralizing antibody against SARS-CoV-2 variants elicited by four priming-boosting regimens. Emerg Microbes Infect. 2022;11:1751–1753. doi: 10.1080/22221751.2022.2095931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin L, Tang R, Wu S, et al. Antibody persistence and safety after heterologous boosting with orally aerosolised Ad5-nCoV in individuals primed with two-dose CoronaVac previously: 12-month analyses of a randomized controlled trial. Emerg Microbes Infect. 2023;12:1–20. doi: 10.1080/22221751.2022.2155251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu L, Mok BWY, Chen LL, et al. Neutralization of severe acute respiratory syndrome coronavirus 2 omicron variant by sera from BNT162b2 or CoronaVac vaccine recipients. Clin Infect Dis. 2022;75:e822–e826. doi: 10.1093/cid/ciab1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pérez-Then E, Lucas C, Monteiro VS, et al. Neutralizing antibodies against the SARS-CoV-2 Delta and Omicron variants following heterologous CoronaVac plus BNT162b2 booster vaccination. Nat Med. 2022;28:481–485. doi: 10.1038/s41591-022-01705-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J, Hou L, Guo X, et al. Heterologous AD5-nCOV plus CoronaVac versus homologous CoronaVac vaccination: a randomized phase 4 trial. Nat Med. 2022;28:401–409. doi: 10.1038/s41591-021-01677-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atmar RL, Lyke KE, Deming ME, et al. Homologous and heterologous Covid-19 booster vaccinations. N Engl J Med. 2022;386:1046–1057. doi: 10.1056/NEJMoa2116414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li JX, Wu SP, Guo XL, et al. Safety and immunogenicity of heterologous boost immunisation with an orally administered aerosolised Ad5-nCoV after two-dose priming with an inactivated SARS-CoV-2 vaccine in Chinese adults: a randomised, open-label, single-centre trial. Lancet Respir Med. 2022;10:739–748. doi: 10.1016/S2213-2600(22)00087-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu FC, Li YH, Guan XH, et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395:1845–1854. doi: 10.1016/S0140-6736(20)31208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sprangers MC, Lakhai W, Koudstaal W, et al. Quantifying adenovirus-neutralizing antibodies by luciferase transgene detection: addressing preexisting immunity to vaccine and gene therapy vectors. J Clin Microbiol. 2003;41:5046–5052. doi: 10.1128/JCM.41.11.5046-5052.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilbert PB, Donis RO, Koup RA, Fong Y, Plotkin SA, Follmann D. A Covid-19 milestone attained - a correlate of protection for vaccines. N Engl J Med. 2022;387:2203–2206. doi: 10.1056/NEJMp2211314. [DOI] [PubMed] [Google Scholar]
- 23.Stuart ASV, Shaw RH, Liu X, et al. Immunogenicity, safety, and reactogenicity of heterologous COVID-19 primary vaccination incorporating mRNA, viral-vector, and protein-adjuvant vaccines in the UK (Com-COV2): a single-blind, randomised, phase 2, non-inferiority trial. Lancet. 2022;399:36–49. doi: 10.1016/S0140-6736(21)02718-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J, Deng C, Liu M, et al. A fourth dose of the inactivated SARS-CoV-2 vaccine redistributes humoral immunity to the N-terminal domain. Nat Commun. 2022;13 doi: 10.1038/s41467-022-34633-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cristelli MP, Nakamura MR, Viana LA, Tedesco-Silva H, Medina-Pestana J. The fourth dose of CoronaVac vaccine results in a small increase of seroconversion and antibody values among kidney transplant recipients. Transplantation. 2022;106:e420–e421. doi: 10.1097/TP.0000000000004219. [DOI] [PubMed] [Google Scholar]
- 26.Intawong K, Chariyalertsak S, Chalom K, et al. Reduction in severity and mortality in COVID-19 patients owing to heterologous third and fourth-dose vaccines during the periods of delta and omicron predominance in Thailand. Int J Infect Dis. 2023;126:31–38. doi: 10.1016/j.ijid.2022.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin L, Tang R, Wu S, et al. Antibody persistence and safety after heterologous boosting with orally aerosolised Ad5-nCoV in individuals primed with two-dose CoronaVac previously: 12-month analyses of a randomized controlled trial. Emerg Microbes Infect. 2023;12 doi: 10.1080/22221751.2022.2155251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data will be made available from the corresponding author on reasonable request. All code used to produce the results will be made available from the corresponding author on reasonable request.