Abstract
Introduction
The study assessed the relationship between COVID-19 and influenza (flu) vaccination and voting patterns during the pandemic and the time trends between flu vaccination and voting patterns.
Methods
Flu and COVID-19 vaccination coverage were analyzed using National Immunization Surveys for flu (Years 2010–2022) and COVID-19 (National Immunization Surveys Adult COVID-19 Module 2021–2022), Centers for Disease Control and Prevention surveillance of COVID-19 vaccination coverage (2021–2022) and U.S. COVID-19 Trends and Impact Survey (2021–2022). The study described the correlations between state-level COVID-19 and flu vaccination coverage, examined individual-level characteristics of vaccination for COVID-19 and for flu using logistic regression (COVID-19 Trends and Impact Survey May–June 2022), and analyzed flu vaccination coverage by age (National Immunization Surveys for flu 2010–2022) and its relationship with voting patterns.
Results
There was a strong correlation between state-level COVID-19 vaccination coverage and voting share for the Democratic candidate in the 2020 presidential elections. COVID-19 vaccination coverage in June 2022 was higher than flu vaccination coverage, and it had a stronger correlation with voting patterns (R=0.90 vs R=0.60 in COVID-19 Trends and Impact Survey). Vaccinated people were more likely to be living in a county where the majority voted for the Democratic candidate in 2020 elections both for COVID-19 (adjusted OR=1.77, 95% CI=1.71, 1.84) and for flu (adjusted OR=1.27, 95% CI=1.23, 1.31). There is a longstanding correlation between voting patterns and flu vaccination coverage, which varies by age, with the strongest correlation in the youngest ages.
Conclusions
There are existing prepandemic patterns between vaccination coverage and voting patterns. The findings align with research that has identified an association between adverse health outcomes and the political environment in the U.S.
INTRODUCTION
Politicization of attitudes toward coronavirus disease 2019 (COVID-19) vaccination is suggested to have contributed to geographic heterogeneities in vaccination coverage in the U.S.1 The national strategy emphasizes vaccines as the main tool in their COVID-19 response.2 Jurisdictions with higher support for the Democratic candidate in the last presidential election have achieved higher COVID-19 vaccination coverage.3 Voting patterns have also been associated with differences in mobility and attitudes toward mitigation measures during the pandemic.4, 5, 6 In the winter of 2022, the U.S. has been affected by surges of influenza (flu), COVID-19, and respiratory syncytial virus.7 Understanding the heterogeneous coverage of vaccines available for flu and COVID-19 can aid the efforts to mitigate respiratory diseases.
The observed relationship between voting patterns and COVID-19 vaccination coverage may reproduce prepandemic associations seen for other vaccines: states where the Democratic candidate received the majority vote in the 2012 presidential election had a higher average vaccination coverage than states where the Republican candidate received the majority, for human papillomavirus vaccine (63.4% vs 56.0% for first dose in girls), meningococcal conjugate vaccine (90.1% vs 84.8%), and tetanus vaccine (79.3% vs 72.8%).8 During the H1N1 flu pandemic, acceptability of the H1N1 vaccine and attitudes toward mass vaccination resulted in divisive discourse; party politics and media were seen to influence opinions.9
The cross-sectional nature of earlier studies on vaccination coverage and voting patterns is a limitation, and longer time trends remain underexplored. This study aimed to answer 3 research questions: how has the relationship between COVID-19 vaccination and voting patterns changed by month during the pandemic, what characteristics are associated with having received COVID-19 vaccination and flu vaccination, and what do time trends in flu vaccination and voting patterns reveal about longer-term associations between vaccination coverage and voting patterns? The study can contribute to identifying emergent versus existing phenomena that contribute to low vaccination coverage in the U.S.
METHODS
Study Sample
The U.S. Surgeon General recommended annual influenza vaccination for at-risk people in 1960.10 Annual flu vaccine is currently recommended by the Centers for Disease Control and Prevention (CDC) for all people aged ≥6 months.11 The following 2 data sources were used to examine flu vaccination coverage:
-
1.
CDC data source compiles estimate from National Immunization Surveys (NIS) and Behavioral Risk Factor Surveillance System (BRFSS), nationally representative telephone-based interviews. Data are available for people aged ≥6 months (Years 2010–2022 included).12
-
2.
The U.S. COVID-19 Trends and Impact Survey (CTIS), an online survey, implemented by the Delphi Group at Carnegie Mellon University; using the Facebook active user base of people aged ≥18 years (2020–2021 and 2021–2022 flu seasons included).13
COVID-19 vaccination started in December 2020, and vaccination is now recommended for all people aged ≥6 months.14 The following 3 data sources of COVID-19 vaccination coverage were used (Years 2021–2022 included):
-
1.
NIS Adult COVID-19 Module (NIS-ACM) estimates of COVID-19 vaccination coverage. Public data are available for people aged ≥18 years.15
-
2.
CTIS self-reported COVID-19 vaccination coverage, among people aged ≥18 years.16
-
3.
CDC administrative data on COVID-19 vaccination at the state level using the percentage of the total population (all ages) with at least 1 dose on the basis of the jurisdiction where the recipient lives.13
Presidential election vote share data at the state and county levels were obtained from the MIT Data Lab.17 Rural−urban classification for counties was obtained from National Center for Health Statistics 2013 Urban-Rural Classification Scheme for Counties.18 The data are publicly available, except for CTIS microdata: Summary of vaccination data in Appendix Table 1, (available online). Analytic codes for analyses using publicly available data are available.19 Analyses were done in R.
Measures
COVID-19 vaccination coverage was defined as having received at least 1 dose of COVID-19 vaccine, the most inclusive definition of achieved vaccination coverage. For COVID-19 vaccination, coverage measures were calculated by month and for flu by flu season. In CTIS data, the cross-tabulation between flu and COVID-19 vaccination status was evaluated with the following categories: not vaccinated for COVID-19 nor for flu (none), only received flu vaccine (only flu), only received COVID-19 vaccine (only COVID-19), or vaccinated against both COVID-19 and flu (both vaccinations). Weighted estimates, adjusting for sampling and nonresponse in CTIS, are presented. Vote share was defined as the percentage of votes for the main Democratic candidate over the total votes given in that geographic area. Vote share reflects the average preference of adults eligible to vote in a given geographic area.
Statistical Analysis
Correlation measures presented represent Pearson correlation coefficient. Fixed-effects logistic regression analyses were performed on CTIS data to examine the individual-level factors associated with vaccination status for COVID-19 (at least 1 dose, by the survey date) and for flu (flu season 2021–2022). The models were run independently with the same explanatory variables: demographic variables expected to be associated with vaccination coverage (age, gender, race/ethnicity) and socioeconomic correlates with vaccination and healthcare access (level of education, financial worry, employment status).20 For voting patterns in the 2020 Presidential election at the county level, counties were categorized as ≤50% and >50% of total votes in a county given to the Democratic candidate, respectively. Rural−urban county classification (6 levels) was also included. The models were additionally adjusted for the state of the respondent. Analysis was restricted to responses in the CTIS survey May–June 2022, and responses from Alaska were excluded given the differences between voting districts and boroughs. R survey package was used to account for the survey design.21
Flu trends over the years were analyzed using NIS/BRFSS data. State-level trends were analyzed using Pearson correlation coefficient to compare the correlation between vote share and vaccination coverage by year and age. Flu vaccination coverage was predicted for 2020–2022 using data from the previous flu seasons. This was done to examine whether the relationships between vaccination coverage and vote share differed for the flu seasons during the pandemic. The linear model had state-level flu vaccination coverage as the outcome, random intercept by age−state dummy variable, and other variables were included as fixed effects: flu season as a centered year variable, presidential vote share of the previous presidential election, and state and age as categorical variables. Washington DC was excluded as an outlier given its >90% vote share for the Democratic candidate. The model was used to predict the expected vaccination coverage for flu seasons 2020–2021 and 2021–2022 using the 2020 presidential vote share.
RESULTS
COVID-19 vaccination coverage showed a strong correlation with state-level 2020 vote share in CDC surveillance, NIS-ACM, and CTIS (Appendix Figure 1, available online). Between May 2021 and June 2022, the relationship remained relatively stable in NIS-ACM and CDC surveillance (Pearson correlation coefficient ranged between 0.76 and 0.88) and in CTIS (range=0.89–0.92). The observed relationship between vaccination coverage and vote share implied an average of 8.6%, 5.8%, and 4.7% percentage point change in vaccination coverage for every 10% percentage point change in Democratic vote share in June 2022 on the basis of data from CDC surveillance, NIS-ACM, and CTIS surveys, respectively. The relationship between increase in vaccination coverage and vote share was more variable over time. In CDC surveillance, the largest monthly increases in COVID-19 vaccination coverage took place between February and May 2021, and the coverage increased more in states with higher vote share for the Democratic candidate until June 2021 (Appendix Figures 1B and Figure 2, available online). From July to September 2021, the coverage increased more in states with lower vote share for the Democratic candidate. Between October 2021 and February 2022, larger increases in coverage were in states with higher vote share for the Democratic candidate in all months except in January 2022. All states offered COVID-19 vaccination to all adults by April 19, 2021.22 On October 29, 2021, Food and Drug Administration authorized emergency use of the Pfizer/BioNTech COVID-19 Vaccine for children aged 5–11 years.23
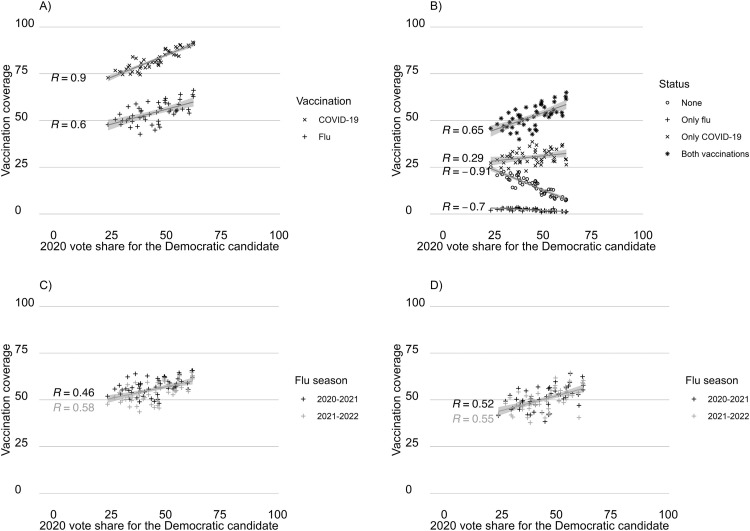
COVID-19 vaccination coverage was compared with flu vaccination coverage among responses in CTIS in June 2022 (Figure 1A ). COVID-19 vaccination coverage was higher in all states, and the correlation with the 2020 vote share was stronger for COVID-19 than for flu (R=0.90 vs R=0.60). For flu vaccination, there was an average 3.1% percentage point change in coverage for every 10% percentage point change in Democratic vote share. Most people who were vaccinated had received both COVID-19 and flu vaccines, and the proportion of people with both vaccines was higher than expected if uptake of the 2 vaccines was independent (Appendix Figure 3, available online). Reporting not having received either of the vaccines was negatively correlated with the 2020 Democratic vote share (Figure 1B), and in the states with the lowest vote share for the Democratic candidate, over 20% of respondents reported not having received either vaccine. There was a small reduction in flu vaccination coverage from 2020–2021 to 2021–2022 flu season in CTIS data but less so in NIS/BRFSS data (Figure 1C and D). In CTIS, the reduction in flu vaccination coverage was larger in states with the lowest vote share for the Democratic candidate but not in NIS/BRFSS (Appendix Figure 4, available online).
Figure 1.
Vaccination coverage for flu and COVID-19, stratified by state and state's vote share for the Democratic candidate in the 2020 presidential election. (A) Flu and COVID-19 vaccination coverage. (B) Vaccination status. (C) Flu vaccination coverage by flu season. (D) Flu vaccination coverage by flu season. CTIS data for June 2022 are shown in panels A and B, and CTIS and NIS/BRFSS data by flu seasons (2020–2021, 2021–2022) are shown in panels C and D, respectively. Washington DC was excluded from the figure.
Vaccination status defined as 4 discreet states: persons who reported receiving neither flu nor COVID-19 vaccine, only received flu vaccine, only received COVID-19 vaccine, and those who received both vaccines.
DC, District of Columbia.
In the analysis of CTIS responses for May–June 2022, increased age was associated with both COVID-19 and flu vaccination (Table 1 ). Men were less likely than women to be vaccinated for COVID-19 and for flu: adjusted OR (AOR) of 0.57 (95% CI=0.56, 0.59) and AOR of 0.71 (95% CI=0.69, 0.72), respectively. People who reported being very worried about their household finances were less likely to be vaccinated for COVID-19 and flu (AOR=0.69, 95% CI=0.67, 0.72 and AOR=0.71, 95% CI=0.69, 0.73) than those reporting some or no worry over household finances. Similarly, people who had not completed high school were less likely to be vaccinated for COVID-19 and flu (AOR=0.58, 95% CI=0.56, 0.60 and AOR=0.62, 95% CI=0.60, 0.64) than those with high school or higher educational attainment. Employment status in the past 4 weeks was not associated with COVID-19 vaccination status and only weakly for flu vaccination.
Table 1.
AOR and 95% CIs From Logistic Regression Models
| Variable | COVID-19 | Flu | ||
|---|---|---|---|---|
| AOR | 95% CI | AOR | 95% CI | |
| Sample size | 386,846 | 388,193 | ||
| Race/ethnicity | ||||
| White NH | 1.00 | 1.00 | ||
| Hispanic | 1.34 | 1.28–1.41 | 0.83 | 0.80–0.86 |
| Black NH | 1.21 | 1.14–1.29 | 0.69 | 0.66–0.71 |
| NH multiple/other | 0.47 | 0.45–0.50 | 0.62 | 0.60–0.65 |
| NH Asian/NH NHPI | 4.09 | 3.53–4.75 | 1.26 | 1.18–1.34 |
| NH AI/AN | 0.76 | 0.67–0.86 | 0.73 | 0.67–0.81 |
| Age group (years) | ||||
| 18–24 | 1.00 | 1.00 | ||
| 25–34 | 1.05 | 0.98–1.12 | 1.14 | 1.08–1.21 |
| 35–44 | 1.26 | 1.18–1.35 | 1.44 | 1.36–1.52 |
| 45–54 | 1.55 | 1.45–1.65 | 1.66 | 1.57–1.75 |
| 55–64 | 2.20 | 2.06–2.35 | 2.41 | 2.28–2.55 |
| 65–74 | 3.82 | 3.56–4.10 | 4.27 | 4.03–4.52 |
| 75+ | 5.43 | 5.00–5.89 | 5.59 | 5.25–5.95 |
| Sex | ||||
| Female | 1.00 | 1.00 | ||
| Male | 0.57 | 0.56–0.59 | 0.71 | 0.69–0.72 |
| Very worried about finances for next montha | ||||
| No | 1.00 | 1.00 | ||
| Yes | 0.69 | 0.67–0.72 | 0.71 | 0.69–0.73 |
| Educationb | ||||
| HS or more | 1.00 | 1.00 | ||
| Less than HS | 0.58 | 0.56–0.60 | 0.62 | 0.60–0.64 |
| In paid employment in the past 4 weeksc | ||||
| Yes | 1.00 | 1.00 | ||
| No | 0.99 | 0.96–1.02 | 1.09 | 1.06–1.11 |
| Aggregate 2020 vote share at county leveld | ||||
| ≤50% for the Democratic Party candidate | 1.00 | 1.00 | ||
| >50% for Democratic Party candidate | 1.77 | 1.71–1.84 | 1.27 | 1.24–1.31 |
| Urban−rural classification for countiese | ||||
| Large central metropolitan | 1.00 | 1.00 | ||
| Large fringe metropolitan | 0.83 | 0.79–0.87 | 0.95 | 0.92–0.98 |
| Medium metropolitan | 0.80 | 0.76–0.84 | 0.95 | 0.92–0.98 |
| Small metropolitan | 0.67 | 0.64–0.71 | 0.87 | 0.84–0.90 |
| Micropolitan | 0.59 | 0.55–0.62 | 0.76 | 0.73–0.80 |
| Noncore | 0.48 | 0.45–0.51 | 0.67 | 0.64–0.70 |
Note: Outcome is vaccination status for COVID-19 or flu. Data of responses in Wave 13, during May–June 2022 in CTIS, are presented. The models were additionally adjusted for the state of the respondent; the estimates for states are presented in Appendix Table 2 (available online).
Survey question: How worried are you about your household's finances for the next month? Categorized as very worried (yes), somewhat worried, not too worried, or not worried at all (no).
Survey question: What is the highest degree or level of school you have completed? Categorized as less than HS (yes), HS graduate or equivalent, and higher with multiple categories (no).
Survey question: In the past 4 weeks, did you do any kind of work for pay? Yes or No.
2020 Presidential election vote-share average at the county level representing living in a Democratic (>50% vote share for Democrats) or Republican (£50% vote share for Democrats) voting county.
Large central metropolitan counties are part of a metropolitan area with at least 1 million population (inner cities), large fringe metropolitan counties are suburban areas of large central metropolitan, medium metropolitan counties are part of metropolitan areas that contain at least 250,000 residents, small metropolitan counties are part of metropolitan areas that contain <250,000 residents, noncore areas are rural.
AI/AN, American Indian or Alaska Native; AOR, adjusted OR; CTIS, COVID-19 Trends and Impact Survey; HS, high school; NH, Non-Hispanic; NHPI, Native Hawaiian or Pacific Islander.
Among CTIS responses, COVID-19 and flu vaccination patterns differed by race/ethnicity. COVID-19 vaccination was lower among those reporting multiple races or other race/ethnicity (AOR=0.47, 95% CI=0.45, 0.50) and among American Indian or Alaska Native (AOR=0.76, 95% CI=0.67, 0.86) respondents than among non-Hispanic White respondents. Hispanic, non-Hispanic Black and non-Hispanic Asian respondents had a higher AOR for being vaccinated against COVID-19 than non-Hispanic White respondents. Flu vaccination was lower in all other race/ethnicity categories except for non-Hispanic Asian respondents than in non-Hispanic White respondents (AOR=1.26, 95% CI=1.18, 1.34).
People living in less urban counties were less likely to be vaccinated than people living in more urban counties. People living in a noncore (rural) county were half as likely to be vaccinated for COVID-19 as people living in large central metropolitan counties (AOR=0.48, 95% CI=0.45, 0.51), whereas for flu, the association was somewhat weaker (AOR=0.67, 95% CI=0.64, 0.70). Living in a county where the majority voted for the Democratic candidate in 2020 elections was more strongly associated with having been vaccinated against COVID-19 (AOR=1.77, 95% CI=1.71, 1.84) than against flu (AOR=1.27, 95% CI=1.24, 1.31) than living in a county where fewer than 50% of people voted for the Democratic candidate.
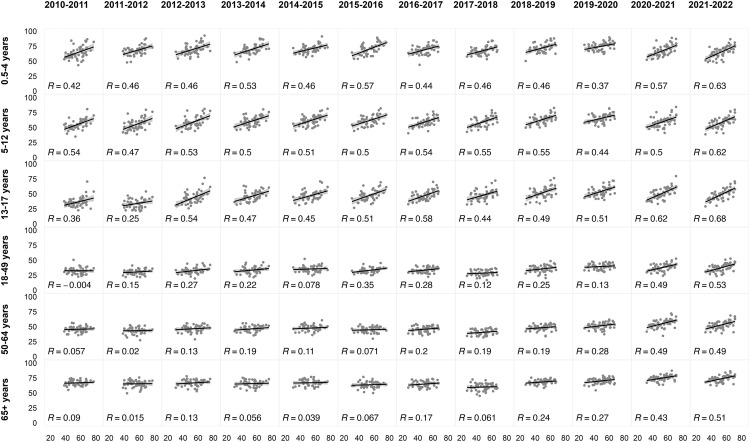
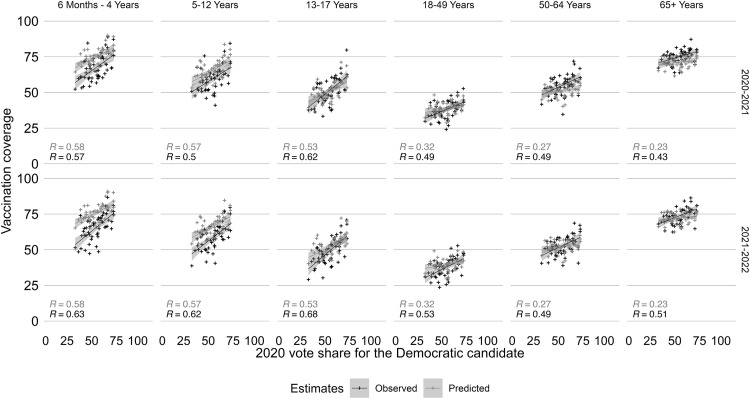
When examining longer time trends for flu, the correlations between flu vaccination coverage and voting patterns differed by age (Figure 2 ). People aged 50–64 and ≥65 years had the highest flu vaccination coverage and the weakest correlation with state-level vote share (R ≤0.2 before 2018–2019 and R ≥0.2 for 2019–2020 and 2020–2021). There was a stronger correlation between voting patterns (of voting-aged individuals) and flu vaccination coverage for younger age groups, with a correlation coefficient of approximately 0.5 among age groups ≤17 years. In the 2010–2011 flu season, the strongest correlation coefficient was among children aged 5–12 years (R=0.54), and this remained similar in 2020–2021 and 2021–2022 (R=0.50 and R=0.62). Since the 2017–2018 flu season, there has been an increase in flu vaccination coverage in the adult population, and this increase in coverage has been more pronounced in states with higher vote share for the Democratic candidate (Appendix Figure 5, available online). During the 2020–2021 and 2021–2022 flu seasons, the observed and predicted vaccination coverage were similar in their correlation with vote share (Figure 3 ). For people aged ≥18 years, the observed correlation between vaccination coverage and vote share was higher than the predicted correlation, with R=0.23–0.32 in the predicted estimates compared with R=0.43–0.49 and R=0.49–0.53 in the observed estimates in 2020–2021 and 2021–2022, respectively.
Figure 2.
State-level correlation between 2020 vote share for the Democratic Party candidate in 2020 presidential elections (x-axis, in percentage) and flu vaccine coverage (y-axis, in percentage) by age (rows) and by flu season (columns). Washington DC was excluded from the figure.
DC, District of Columbia.
Figure 3.
Predicted and observed flu vaccination coverage by age for the 2020–2021 and 2021–2022 flu seasons.
Correlation between flu vaccine coverage and vote share for the Democratic Party candidate by age is shown. Predicted values are shown in light gray, and observed values are shown in darker gray. Washington DC was excluded from the analysis.
DC, District of Columbia.
DISCUSSION
States with higher vote share for the Democratic candidate in the last Presidential election saw larger monthly increases in vaccination coverage for COVID-19, most prominently in the early phases of the COVID-19 vaccine roll out in early 2021 but also later when vaccination eligibility expanded to younger age groups. When comparing state-level COVID-19 vaccination with flu vaccination, state-level COVID-19 vaccination coverage had approximately 50% higher correlation coefficient with the vote share than flu vaccination coverage. In June 2022, states with the lowest proportion of votes for the Democratic candidate had gaps in vaccination coverage, and this was apparent for both flu and COVID-19, and a high proportion of people reported not having received either of the vaccines. Voting patterns and flu vaccination coverage have been correlated since 2010 to an extent that has varied across age groups, with the strongest correlation in the youngest age groups. In individual-level data analysis, living in a county with a majority vote share for the Democratic candidate remained more strongly associated with being vaccinated against COVID-19 than being vaccinated against flu when adjusting for individual-level demographic, socioeconomic variables, and urbanicity of the county of the respondent.
This study identified a decreasing adjusted odds of vaccination for both COVID-19 and for flu in less urban counties, which is similar to findings for COVID-19 vaccination coverage by rural−urban scale.24 Also observed was a lower adjusted odds of vaccination for COVID-19 among the combined category of American Indian or Alaska Native people than among non-Hispanic White people, whereas higher vaccination coverage has been reported in tribal communities.25 Nationally, the reporting of COVID-19 vaccination coverage among American Indian or Alaska Native people is limited,26 and broad race/ethnicity categories can mask disparities within the category.27
In a global analysis, higher trust in the government was identified as having less severe COVID-19 infection and mortality outcomes at the country level.28 Higher levels of interpersonal trust and government trust were also associated with higher COVID-19 vaccination coverage. People in trusted leadership positions and their messages have a key role in the acceptability of vaccines.29 Willingness to get vaccinated is dynamic, influenced by perceived risk, safety, and prevailing norms.29 Mistrust and blame toward the media and the elites with political power have been reported by people across political affiliations with similar anxiety expressed about growing inequality.30
The vote share used in this study is by default a proxy measure of more distal factors. A strong correlation has been observed between vaccine hesitancy and vote share,31 but access has also been identified to contribute to heterogeneity in vaccination coverage.32 Warraich et al. found an association between adverse health outcomes and political environment in the U.S. Higher mortality rates persist in counties with lower vote share for the Democratic candidate, and the gap in mortality rates has grown during 2001–2019.33 Diverse health outcomes, including heart disease, cancer, chronic lower respiratory tract diseases, unintentional injuries, and suicide, were contributing to the differences in county-level mortality rates.33 Potential ways in which voting patterns may be associated with differences in health outcomes and vaccination coverage are through political decision making, which influences health and social welfare policies through federal legislature and funding, and through decisions about healthcare legislature and budgeting at the state level, such as state-level expansion of Medicaid eligibility.34 Vaccination requirements by institution and employment also differ by state.
Limitations
Measures of voting patterns are at ecologic level and reflect living in a state or county with an average vote share for either of the main party. The aggregate measure is an imprecise estimate of complex processes. The relative recency of the COVID-19 pandemic makes identifying emerging trends challenging. Healthcare resources have been more constrained since COVID-19 pandemic began, which could impact flu vaccination programs during active COVID-19 vaccination roll out, and obtaining multiple vaccinations may influence decision making at the individual level in favor of COVID-19 vaccination over the flu vaccine. During the 2020–2021 and 2021–2022 flu seasons, this study did not observe clear signs of population-level divergence in flu vaccination coverage in relation to voting patterns compared with that of prepandemic flu seasons. Self-reported vaccination status is subject to social desirability bias. For COVID-19, self-reported and administrative vaccination coverage showed a similar correlation regarding voting patterns. Only self-reported data were available for flu. In a study from Spain by Jiménez-García et al.,35 flu vaccination coverage was higher in self-reported data than in registry-based data among men, people with immigrant status, younger people, and people without comorbidities. If similar patterns of overestimation occur in the self-reported data in this study, this could underestimate the differences in vaccination coverage by gender and by age. Norms around vaccination acceptance could impact the direction of social desirability bias. Further research is needed on reporting behaviors and how they are changing over time.
CONCLUSIONS
This study showed a prepandemic association between flu vaccination coverage and voting patterns. During the pandemic, a consistent association with voting patterns was observed across data and for both COVID-19 and flu vaccines. Findings of lower vaccination coverage for COVID-19 and for flu among people who were very worried about their finances and people who lived in rural counties suggest that access is contributing to disparities observed in vaccination coverage. For long-term management of COVID-19 and flu, monitoring and understanding heterogeneities in vaccination coverage is needed to address gaps in the utilization of key prevention tools.
ACKNOWLEDGMENTS
The use of the U.S. COVID-19 Trends and Impact Survey for analysis of COVID-19 symptoms and related behaviors was exempt from review by Harvard's IRB (Protocol Number IRB20-0592). Other data used in the study are publicly available aggregate estimates.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the funders or the authors’ affiliated institutions.
JAS, NAM, and MMR were supported by the Council of State and Territorial Epidemiologists Grant Number NU38OT000297-03 (to JAS). MMR was additionally supported by Harvard Data Science Initiative Trust in Science Exploratory Award.
No financial disclosures were reported by the authors of this paper.
CrediT AUTHOR STATEMENT
Minttu M. Rönn: Conceptualization, Methodology, Data Curation, Formal analysis, Visualization, Writing – original draft, Funding Acquisition. Nicolas A. Menzies: Methodology, Writing – review and editing, Funding Acquisition. Joshua A. Salomon: Methodology, Writing – review and editing, Funding Acquisition.
Footnotes
Supplemental materials associated with this article can be found in the online version at https://doi.org/10.1016/j.amepre.2023.03.001.
Appendix. SUPPLEMENTAL MATERIAL
REFERENCES
- 1.Sharfstein JM, Callaghan T, Carpiano RM, et al. Uncoupling vaccination from politics: a call to action. Lancet. 2021;398(10307):1211–1212. doi: 10.1016/S0140-6736(21)02099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The White House. National COVID-19 preparedness plan. https://www.whitehouse.gov/covidplan/. Accessed December 19, 2022.
- 3.Kates J, Tolbert J, Orgera K. The red/blue divide in COVID-19 vaccination rates. KFF. https://www.kff.org/policy-watch/the-red-blue-divide-in-covid-19-vaccination-rates/. Accessed January 2, 2022.
- 4.Clinton J, Cohen J, Lapinski J, Trussler M. Partisan pandemic: how partisanship and public health concerns affect individuals’ social mobility during COVID-19. Sci Adv. 2021;7(2) doi: 10.1126/sciadv.abd7204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grossman G, Kim S, Rexer JM, Thirumurthy H. Political partisanship influences behavioral responses to governors’ recommendations for COVID-19 prevention in the United States. Proc Natl Acad Sci U S A. 2020;117(39):24144–24153. doi: 10.1073/pnas.2007835117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang J, Chen E, Yan S, Lerman K, Ferrara E. Political polarization drives online conversations about COVID-19 in the United States. Hum Behav Emerg Technol. 2020;2(3):200–211. doi: 10.1002/hbe2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanne JH. US faces triple epidemic of flu, RSV, and covid. BMJ. 2022;379:o2681. doi: 10.1136/bmj.o2681. [DOI] [PubMed] [Google Scholar]
- 8.Bernstein S, North A, Schwartz J, Niccolai LM. State-level voting patterns and adolescent vaccination coverage in the United States, 2014. Am J Public Health. 2016;106(10):1879–1881. doi: 10.2105/AJPH.2016.303381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baum MA. Red state, blue state, flu state: media self-selection and partisan gaps in swine flu vaccinations. J Health Polit Policy Law. 2011;36(6):1021–1059. doi: 10.1215/03616878-1460569. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Influenza historic timeline. https://www.cdc.gov/flu/pandemic-resources/pandemic-timeline-1930-and-beyond.htm. Accessed January 8, 2022.
- 11.Grohskopf LA, Alyanak E, Ferdinands JM, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices, United States, 2021–22 influenza season. MMWR Recomm Rep. 2021;70(5):1–28. doi: 10.15585/MMWR.RR7005A1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. Influenza vaccination coverage for all ages (6+ months).https://data.cdc.gov/Flu-Vaccinations/Influenza-Vaccination-Coverage-for-All-Ages-6-Mont/vh55-3he6. Accessed March 28, 2022.
- 13.Centers for Disease Control and Prevention. COVID-19 vaccinations in the United States,jurisdiction.https://data.cdc.gov/Vaccinations/COVID-19-Vaccinations-in-the-United-States-Jurisdi/unsk-b7fc. Accessed March 28, 2022.
- 14.Centers for Disease Control and Prevention. Stay up to date with your COVID-19 vaccines. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/stay-up-to-date.html. Accessed April 1, 2022.
- 15.Centers for Disease Control and Prevention. National Immunization Survey Adult COVID Module (NIS-ACM): vaccination status and intent by demographics. https://data.cdc.gov/Vaccinations/National-Immunization-Survey-Adult-COVID-Module-NI/iwxc-qftf. Accessed March 28, 2022.
- 16.Salomon JA, Reinhart A, Bilinski A, et al. The US COVID-19 Trends and Impact Survey: continuous real-time measurement of COVID-19 symptoms, risks, protective behaviors, testing, and vaccination. Proc Natl Acad Sci U S A. 2021;118(51) doi: 10.1073/PNAS.2111454118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MIT Election Data Science Lab. Data. https://electionlab.mit.edu/data. Accessed March 28, 2022.
- 18.National Center for Health Statistics. Urban rural classification scheme for counties. https://www.cdc.gov/nchs/data_access/urban_rural.htm. Published 2022. Accessed November 23, 2022.
- 19.Rönn MM, GitHub Repository. https://github.com/mintturonn/voting_patterns_vaccines. Accessed March 27, 2022.
- 20.Troiano G, Nardi A. Vaccine hesitancy in the era of COVID-19. Public Health. 2021;194(May):245–251. doi: 10.1016/j.puhe.2021.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lumley T. survey: analysis of complex survey samples. http://r-survey.r-forge.r-project.org/survey/. Published 2021. Accessed July 24, 2022.
- 22.Anthes E, Ngo M, All SE, States Have US. Met Biden's vaccine expansion deadline. New York Times. https://www.nytimes.com/2021/04/19/world/adults-eligible-covid-vaccine.html. Accessed August 18, 2022.
- 23.US Food & Drug Administration. FDA authorizes Pfizer-BioNTech COVID-19 vaccine for emergency use in children 5 through 11 years of age.https://www.fda.gov/news-events/press-announcements/fda-authorizes-pfizer-biontech-covid-19-vaccine-emergency-use-children-5-through-11-years-age. Accessed August 17, 2022.
- 24.Sun Y, Monnat SM. Rural-urban and within-rural differences in COVID-19 vaccination rates. J Rural Health. 2022;38(4):916–922. doi: 10.1111/jrh.12625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haroz EE, Kemp CG, O'Keefe VM, et al. Nurturing innovation at the roots: the success of COVID-19 vaccination in American Indian and Alaska native communities. Am J Public Health. 2022;112(3):383–387. doi: 10.2105/AJPH.2021.306635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skinner A, Raifman J, Ferrara E, Raderman W, Quandelacy TM. Disparities made invisible: gaps in COVID-19 data for American Indian and Alaska native populations. Health Equity. 2022;6(1):226–229. doi: 10.1089/heq.2021.0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohlsen EC, Yankey D, Pezzi C, et al. COVID-19 vaccination coverage, intentions, attitudes and barriers by race/ethnicity, language of interview, and nativity, National Immunization Survey Adult COVID Module, 22 April 2021–29 January 2022. Clin Infect Dis. 2022;75(suppl 2):S182–S192. doi: 10.1093/cid/ciac508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.COVID-19 National Preparedness Collaborators Pandemic preparedness and COVID-19: an exploratory analysis of infection and fatality rates, and contextual factors associated with preparedness in 177 countries, from Jan 1, 2020, to Sept 30, 2021. Lancet. 2022;399(10334):1489–1512. doi: 10.1016/S0140-6736(22)00172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sabahelzain MM, Hartigan-Go K, Larson HJ. The politics of Covid-19 vaccine confidence. Curr Opin Immunol. 2021;71:92–96. doi: 10.1016/j.coi.2021.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hardy LJ, Mana A, Mundell L, Neuman M, Benheim S, Otenyo E. Who is to blame for COVID-19? Examining politicized fear and health behavior through a mixed methods study in the United States. PLOS ONE. 2021;16(9) doi: 10.1371/journal.pone.0256136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu R, Li GM. Hesitancy in the time of coronavirus: temporal, spatial, and sociodemographic variations in COVID-19 vaccine hesitancy. SSM Popul Health. 2021;15 doi: 10.1016/j.ssmph.2021.100896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bor J, Assoumou SA, Lane K, et al. Inequities in COVID-19 vaccine and booster coverage across Massachusetts ZIP codes after the emergence of Omicron: a population-based cross-sectional study. PLOS Med. 2023;20(1) doi: 10.1371/journal.pmed.1004167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Warraich HJ, Kumar P, Nasir K, Joynt Maddox KEJ, Wadhera RK. Political environment and mortality rates in the United States, 2001–19: population based cross sectional analysis. BMJ. 2022;377 doi: 10.1136/BMJ-2021-069308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rocco P, Keller AC, Kelly AS. State politics and the uneven fate of Medicaid expansion. Health Aff (Millwood) 2020;39(3):494–501. doi: 10.1377/hlthaff.2019.01414. [DOI] [PubMed] [Google Scholar]
- 35.Jiménez-García R, Hernandez-Barrera V, Rodríguez-Rieiro C, et al. Comparison of self-report influenza vaccination coverage with data from a population based computerized vaccination registry and factors associated with discordance. Vaccine. 2014;32(35):4386–4392. doi: 10.1016/j.vaccine.2014.06.074. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.