Abstract
Activation of the EphB2 receptor tyrosine kinase by clustered ephrin-B1 induces growth cone collapse and neurite retraction in differentiated NG108 neuronal cells. We have investigated the cytoplasmic signaling events associated with EphB2-induced cytoskeletal reorganization in these neuronal cells. We find that unlike other receptor tyrosine kinases, EphB2 induces a pronounced downregulation of GTP-bound Ras and consequently of the extracellular signal-regulated kinase (ERK) mitogen-activated protein kinase (MAPK) pathway. A similar inhibition of the Ras-MAPK pathway was observed on stimulation of endogenous EphB2 in COS-1 cells. Inactivation of Ras, induced by ephrin B1 stimulation of NG108 neuronal cells, requires EphB2 tyrosine kinase activity and is blocked by a truncated form of p120-Ras GTPase-activating protein (p120-RasGAP), suggesting that EphB2 signals through the SH2 domain protein p120-RasGAP to inhibit the Ras-MAPK pathway. Suppression of Ras activity appears functionally important, since expression of a constitutively active variant of Ras impaired the ability of EphB2 to induce neurite retraction. In addition, EphB2 attenuated the elevation in ERK activation induced by attachment of NG108 cells to fibronectin, indicating that the EphB2 receptor can modulate integrin signaling to the Ras GTPase. These results suggest that a primary function of EphB2, a member of the most populous family of receptor tyrosine kinases, is to inactivate the Ras-MAPK pathway in a fashion that contributes to cytoskeletal reorganization and adhesion responses in neuronal growth cones.
External signals that control cellular behavior in metazoan organisms are often transduced at the cell surface by receptor tyrosine kinases (RTKs). Eph receptors comprise the largest family of mammalian RTKs, with 14 members. The family has apparently undergone a striking expansion during the evolution of multicellular animals, since only a single Eph receptor has been identified in Caenorhabditis elegans (30) or Drosophila (65), suggesting that these receptors might be involved in controlling complex cellular interactions. The ligands for Eph receptors, termed ephrins, are themselves anchored to the plasma membrane, either via a glycosylphosphatidylinositol linkage (A class) or through a transmembrane sequence (B class) (21, 28, 39). Consequently, signaling generally requires direct contact between ephrin- and Eph receptor-expressing cells. The Eph receptors are also classified into A and B groups on the basis of sequence homology and ephrin-binding ability (27). Although the binding of receptors to ephrins is generally nonselective within a given class, different combinations of receptors and ligands interact with distinct affinities, while EphA4 can bind both classes of ephrins (28).
In C. elegans, the VAB-1 Eph receptor and corresponding ephrins regulate a series of morphogenetic cell movements important for development (14, 30, 72). In mammals, Eph receptors and ephrins are expressed in reciprocal compartments of the developing embryo (28, 33) and are important for axon guidance and topographic map formation in the central nervous system (7, 19, 25, 34, 74), neural crest cell migration (18), patterning of the hindbrain and paraxial mesoderm (28), and vascular network assembly (1, 29, 31, 71). For both invertebrates and vertebrates, there are data to suggest that Eph receptors have both kinase-dependent and kinase-independent functions, with the latter potentially reflecting either an ability of Eph-ephrin interactions to mediate cell adhesion or an intrinsic ephrin-signaling activity (13, 22, 23, 37).
In the guidance of axons in the nervous system, and in cell migrations, ephrin-Eph receptor signaling commonly has a repulsive effect on cell movement (11, 53, 54, 57). In vitro, the activation of Eph receptors in neuronal cells induces deadhesive responses and collapse of neural growth cones (6), correlating with axon and neural crest cell repulsion from ephrins displayed on cells or isolated membranes (53, 54). Although ephrins and Eph receptors clearly activate repellant responses in many cells, there is increasing evidence that specific ligand-receptor pairs can also initiate an attractive response in some cell types, for example by eliciting endothelial cell sprouting (1), increased cellular adhesion (8, 22, 23, 41), neural tube closure (40), and projection of vomeronasal axons (47). This resembles the ability of several other guidance molecules to induce either attraction or repulsion (56).
The intracellular signaling pathways that mediate the biological effects of Eph receptors and ephrins are only starting to emerge. Activated receptors become autophosphorylated at multiple sites, including two absolutely conserved tyrosine residues in the juxtamembrane region and a tyrosine within the activation segment of the kinase domain (6, 44). Interestingly, prior to phosphorylation, the juxtamembrane tyrosines (Y604 and Y610 in EphB2) repress receptor kinase activity, but following phosphorylation they are released to serve as docking sites for SH2 domain proteins (6). RTKs commonly signal through cytoplasmic proteins with SH2 domains, which bind either directly to phosphotyrosine (pTyr) sites on the activated receptor or to phosphorylated docking proteins. Both mechanisms may be used by Eph receptors. A variety of SH2 proteins have been identified as potential Eph receptor-binding partners, including the Fyn and Src tyrosine kinases (15, 26, 35, 75), the p120-Ras GTPase-activating protein (p120-RasGAP) (see interaction ID:123 at www.BIND.ca [35, 38]), the Nck and Crk adaptors (35, 69), SHEP1 (24), the Ras-binding protein AF6 (36), and the Src-like adaptor protein SLAP (58). Which of these targets are relevant to the biological functions of Eph receptors remain uncertain. In addition, we and others have found that activated Eph receptors preferentially phosphorylate the p62dok-1 docking protein in neuronal and endothelial cells (4, 38). p62dok-1 has an N-terminal pleckstrin homology (PH) domain followed by a phosphotyrosine-binding (PTB) domain and multiple tyrosine phosphorylation sites which engage the SH2 domains of p120-RasGAP and Nck (73). In NG108 neuronal cells expressing EphB2 and stimulated with clustered ephrin-B1, p62dok-1 is the most prominently tyrosine-phosphorylated protein other than the receptor itself (38).
The Ras-mitogen-activated protein kinase (MAPK) pathway is commonly activated by RTKs, and indeed is viewed as a hallmark of RTK signaling (16). Autophosphorylation of RTKs such as the epidermal growth factor, platelet-derived growth factor (PDGF), or insulin receptors leads to the recruitment (either directly or indirectly) of the Grb2-Sos1 complex, which in turn induces the exchange of GDP for GTP on Ras proteins, and the association of Ras with the Raf serine/threonine protein kinase (59). Raf phosphorylates the dual-specificity protein kinases MEK1 and MEK2, which consequently activate the MAPKs extracellular signal-related kinases 1 and 2 (ERK1/2). This core biochemical pathway is regulated by many different signals in numerous cell types, raising the issue of how such a widespread signaling pathway generates distinct biological responses in different cells or following stimulation by different ligands. In metazoans, each cell is simultaneously exposed to multiple extracellular signals and must integrate these inputs to initiate the appropriate outcome. The combined nature of these external signals, together with regulators expressed within the target cell, may therefore determine the extent and duration of Ras-MAPK activation, which in turn can determine how the cell responds (51).
Unlike other RTKs, Eph receptors appear inefficient at stimulating cell proliferation in fibroblasts or epithelial cells (10, 12), and the role of MAPK signaling downstream of activated Eph receptors remains unclear. Here, we show that EphB2 tyrosine kinase activity down regulates Ras and ERK1/2 MAPKs in a neuronal cell culture system. Our data suggest that p120-RasGAP contributes to Ras inhibition by Eph receptors and indicate that Ras activation interferes with neurite retraction induced by ephrin-B1.
MATERIALS AND METHODS
EphB2 constructs, mutagenesis, and reagents.
Full-length murine EphB2 cDNA was cloned into the mammalian expression vector pcDNA3 (Invitrogen) as previously described (38). Mutations and truncations in EphB2 were made using PCR-based site-directed mutagenesis. For juxtamembrane mutations, a BglII fragment encompassing the tyrosine residues was replaced with the identical fragment with tyrosines 604 (JX1) and 610 (JX2) mutated either singly to phenylalanine or together to phenylalanine or glutamate. EphB2-ΔC was synthesized by mutation of V952 in the SAM domain to a stop codon. The presence of the mutations was confirmed by sequence analysis. The YFP-Actin cDNA (Clontech) was cloned into the retroviral PMX-Puro vector (60). For cloning, the entire coding region was amplified by PCR with EcoRI sites flanking the start and stop codons. The 5′-primer CCCGAATTCGCTAGCGCTACCGGTCGCCACCATG and the 3′-primer CCCGAATTCCTTAAGATACATTGATGAGTTTGGAC were used. Cloning of a truncated p120-RasGAP encoding the N-terminal hydrophobic region and including the SH2-SH3-SH2 domains (GAP-N) was previously described (52). pcDNA3-HA-RasV12 used in live imaging of cells was a kind gift of A. Guha. PDGF-BB was purchased from PeproTech Inc.
Cell culture and EphR stimulation.
Rat2 cells were maintained in Dulbeco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS) (Sigma). NG108-15 (NG108) cells were routinely cultured in DMEM containing 10% FBS and 1x hypoxanthine-aminopterin-thymidine (HAT) (Gibco). Stable cell lines in NG108 cells were produced as previously described (38). Briefly, parental NG108 cells were transfected with 10 μg of cDNA using Lipofectin reagent and OptiMEM medium (Gibco) as specified by the manufacturer. Cells were then grown in the presence of 400 μg of G418 (Gibco) per ml to select stable transfectants. Transient transfections into these cell lines were performed using Lipofectin or ExGEN 500 (MBI Fermentas) reagents. Stimulation of EphB2 expressing cells was carried out as previously described (38) with 2 μg of aggregated Fc-ephrin-B1 per ml unless otherwise indicated. For ERK and MEK activation experiments, NG-EphB2 cells were seeded into six-well plates 24 h prior to stimulation at 3 × 105 cells/well.
Immunoprecipitation and Western blotting.
Unless otherwise indicated, cells were serum starved overnight in DMEM containing 1X HAT. For ERK, MEK, and antiphosphotyrosine (anti-pTyr) Western blots, cells were stimulated with Fc-ephrin-B1 as indicated and lysed directly in 2× sodium dodecyl sulfate (SDS) loading buffer. For immunoprecipitation experiments, cells were washed twice in phosphate-buffered saline (PBS) and lysed as previously described (38). Protein levels were equalized using a bicinchoninic acid protein assay kit (Pierce), and the proteins were immunoprecipitated as indicated. They were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to a polyvinylidene difluoride membrane (Millipore), and blotted with the appropriate antibodies. Antibodies against EphB2 have been previously described (33). Phospho-ERK1/2 and phospho-MEK1 antibodies were purchased from Cell Signaling Technologies, and Grb2 antibodies were purchased from Transduction Laboratories. All other antibodies were from Santa Cruz. Immunoblots were visualized using a linear enhanced chemiluminescence (ECL) substrate (ECL Plus; Amersham) as specified by the manufacturer. All blots were exposed to either X-Omat imaging film (Kodak) or Fluor-S MultiImager and visualized using QuantityOne software (Bio-Rad).
Assay for activated Ras.
The Ras-binding domain of c-Raf (Raf-RBD) is thought to have a high affinity for GTP-bound Ras, and has been used as a probe for activated Ras (61). After treatment with clustered ephrin-B1 ligand, the cells were washed twice with HEPES-buffered saline (HBS) (25 mM HEPES [pH 7.5], 150 mM NaCl), and lysates were prepared in p21Ras lysis buffer (PLB) (25 mM HEPES [pH 7.5], 150 mM NaCl, 1% NP-40, 1 mM EDTA, 10% glycerol, 25 mM NaF, 10 mM MgCl2, 1 mM sodium vanadate, 10 μg of leupeptin per ml, 10 μg of aprotinin per ml, 0.1 mM phenylmethylsulfonyl fluoride). Total-cell lysates were equalized for protein content and were incubated with 20 μg of purified glutathione S-transferase (GST)–Raf-RBD on glutathione beads (Pharmacia) for 60 minutes. Samples were resolved by SDS-PAGE and immunoblotted with anti-Ras antibodies (Quality Biotech) as indicated above.
Neurite retraction assays.
NG-EphB2 cells were transfected as indicated 56 h prior to the retraction assay. YFP-Actin-expressing cells were plated onto DeltaT3 dishes (Bioptechs) and mounted on a temperature-controlled stage adaptor. The temperature was maintained at 37°C, and the cells were imaged using an inverted Olympus IX-70 fluorescence microscope equipped with Deltavision deconvolution software (Applied Precision). For neurite retraction assays, ligand was added to the culture medium and the cells were immediately imaged every 30 s for 40 min.
RESULTS
Kinase activity of EphB2 downregulates ERK1/2 and MEK1 phosphorylation.
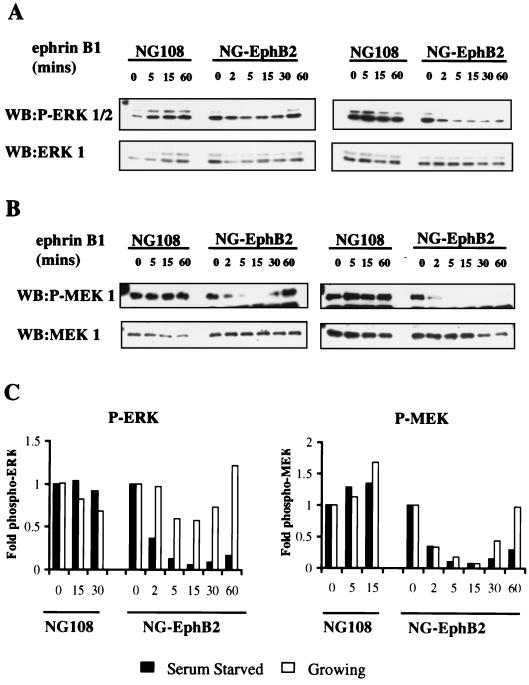
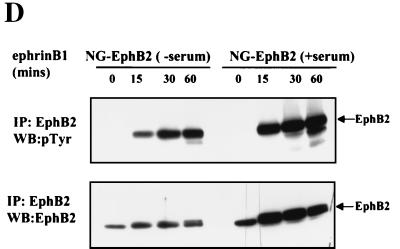
To investigate the role of Eph RTKs in regulating the MAPK pathway in a neuronal cell type, we have used a derivative of the NG108-15 (NG108) cell line stably expressing wild-type EphB2 (NG-EphB2) (6, 38). The parental NG108 cells do not express detectable Eph receptors and do not respond to ephrin-B1 stimulation. In contrast, although neurite outgrowth is induced normally in the NG-EphB2 cells by cyclic AMP, ephrin-B1 induces neurite retraction of these cells in a fashion that depends on EphB2 kinase activity (6). Ephrin-B1 stimulation of NG-EphB2 cells leads to a rapid increase in the tyrosine phosphorylation of EphB2 and a number of cellular proteins, most notably p62dok-1 (6, 38). Growing or serum-starved NG108 parental and NG-EphB2 cells were stimulated with clustered Fc-ephrin-B1 ligand for different times ranging from 2 to 60 min, and total-cell proteins were assayed for activated ERK and MEK using antibodies directed against the phosphorylated, activated forms of these kinases. Both growing (in 10% FBS) and serum-starved NG-EphB2 cells exhibited a marked decrease in activated, phospho-ERK1/2 (Fig. 1A, upper panel), as well as activated, phospho-MEK1 (Fig. 1B, upper panel), although the absolute levels of these proteins did not decrease significantly (Fig. 1A and B, lower panels). In contrast, stimulation of parental NG108 cells with ephrin-B1 showed no appreciable difference in phospho-ERK1/2 or phospho-MEK1 levels compared to total protein levels. Furthermore, the decrease in the phosphorylated forms of both MEK and ERK was more pronounced and occurred for a longer duration under serum-starved conditions (Fig. 1C). This is most probably due to the presence of compensating mitogenic factors in serum, since we found significantly elevated levels of tyrosine phosphorylated EphB2 up to 60 min after ephrin-B1 stimulation in the presence or absence of serum (Fig. 1D).
FIG. 1.
Ephrin-B1 stimulation of EphB2 leads to the down regulation of the ERK1/2 MAPK signaling pathway. (A and B) Serum-starved (right panels) or growing (10% FBS) (left panels) parental NG108 or NG-EphB2 cells were stimulated with 2 μg of clustered Fc-ephrin-B1 per ml for the indicated time points and lysed directly in 2x SDS-PAGE sample buffer. The lysates were electrophoresed and blotted (WB) with antibodies against phosphorylated ERK1/2 (A, top panels), or phosphorylated MEK1 (B, top panels). The blots were stripped and reprobed for total ERK1 or MEK1 (bottom panels, A and B, respectively). (C) Graphical representation of phospho-ERK1/2 and phospho -MEK1 from panels A and B relative to total levels. (D) Time course of EphB2 tyrosine phosphorylation in NG108 cells. NG108 cells were serum starved (lanes −serum) or grown in the presence of serum (lanes +serum) and stimulated with 2 μg of clustered Fc-ephrin-B1 per ml as indicated. Immunoprecipitated (IP) EphB2 was then resolved by SDS-PAGE and probed with anti-pTyr antibodies (upper panel). Blots were subsequently stripped and reprobed with crude anti-EphB2 sera (lower panel).
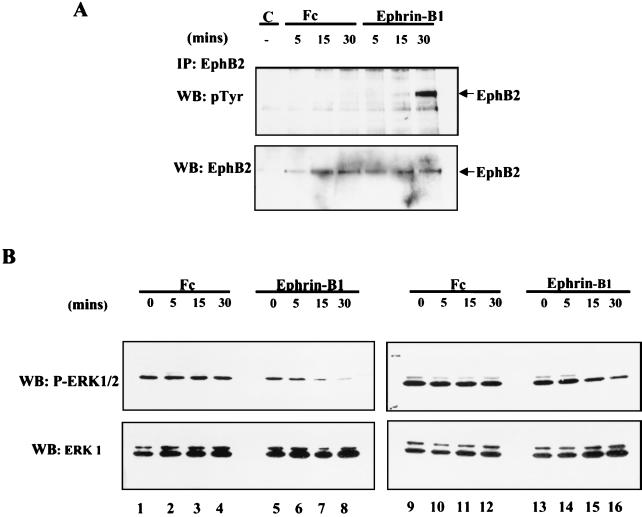
Unlike NG108 cells, COS-1 cells express endogenous EphB2, which becomes phosphorylated on tyrosine residues subsequent to stimulation with clustered Fc-ephrin-B1 (Fig. 2A). In agreement with the observations made in NG-EphB2 cells, stimulation of COS-1 cells with clustered ephrin-B1 led to a time-dependent decrease in ERK phosphorylation in cells that had been serum starved (Fig. 2B, left panels), as well as in cells grown in the presence of 10% FBS (Fig. 2B, right panels). In contrast, treatment with clustered Fc alone did not significantly alter the ERK1/2 phosphorylation status in COS 1 cells (Fig. 2B, lanes 1 to 4 and 9 to 12). These results indicate that activation of the EphB2 RTK in two distinct cell types of neuronal and epithelial origin induces downregulation in ERK phosphorylation.
FIG. 2.
Ephrin-B1 stimulation of COS-1 cells leads to tyrosine phosphorylation of endogenous EphB2 and down regulation of ERK1/2 phosphorylation. (A) COS-1 cells were serum starved for 14 h and were either left untreated (lane C) or stimulated with 8 μg of clustered human Fc (lanes Fc) or Fc-ephrin-B1 (lanes Ephrin-B1) per ml. The cells were subsequently washed twice with PBS and lysed in PLC buffer as indicated in Materials and Methods. Lysates equalized for protein concentration were subjected to immunoprecipitation (IP) with anti-EphB2 antibodies, and immunoprecipitates were subsequently separated by SDS-PAGE and blotted with anti-pTyr antibodies (upper panel). The membranes were then stripped and reprobed with anti-EphB2 antibodies (bottom panel). (B) Serum-starved (left panels) and growing (right panels) COS-1 cells were challenged for the indicated time with 8 μg/ml clustered Fc or Fc-ephrin-B1 and subsequently lysed and immunoblotted (WB) with anti-phospho-ERK1/2 antibodies as indicated in Fig. 1. The membranes were then stripped and reprobed with anti-ERK1 antibodies (B, bottom panels).
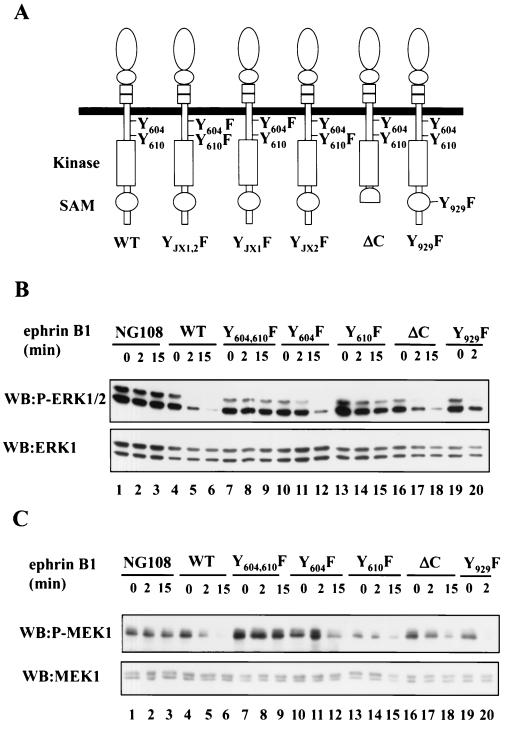
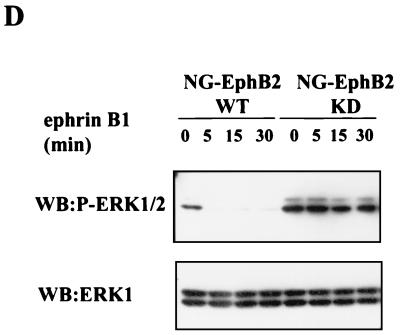
To investigate the features of EphB2 that are important for the downregulation of ERK signaling, we constructed stable NG108 cell lines expressing EphB2 with truncations or mutations in specific residues (Fig. 3A). Several protein interaction motifs are conserved among Eph family receptors and have been implicated in the downstream signaling (39). The juxtamembrane tyrosines 604 and 610 (JX1 and JX2, respectively, in Fig. 3A) regulate kinase activity (6) and serve as docking sites for SH2 domains once phosphorylated, whereas the C-terminal SAM domain is implicated in receptor oligomerization (67, 70) but may also serve a docking function through phosphorylation of Y929 (69). The EphB2-ΔC construct ends at V951 in the SAM domain, effectively eliminating this domain, as well as the C-terminal PDZ domain-binding site. Similar to wild-type (WT) EphB2, stimulation of NG108 cells expressing EphB2-ΔC or EphB2-Y929F caused a rapid decrease in the levels of phosphorylated ERK1/2 and MEK1, which could be detected within 2 min (Fig. 3B and C). These observations suggest that neither a functional SAM domain nor the C-terminal PDZ domain-binding motif are required for the decrease in EphB2 stimulated MAPK signaling, and, indeed, the C-terminal region of EphB2 is not required for neurite retraction (S. Holland and T. Pawson, unpublished results). In contrast, replacement of either of the two conserved juxtamembrane tyrosines with phenylalanine, which impairs kinase activity and neurite retraction, resulted in a smaller decrease in ERK1/2 and MEK1 phosphorylation following ephrin-B1 challenge than that of WT EphB2. In particular, the rate of EphB2-YJX2F-induced down regulation of ERK1/2 phosphorylation was significantly lower in comparison to WT EphB2 (Fig. 3B, upper panel, compare lanes 5 and 6 with lanes 14 and 15). The double substitution of EphB2-YJX1,2F effectively eliminated the decrease in phosphorylated ERK1/2 (Fig. 3B, upper panel, compare lanes 8 and 9 with lanes 5 and 6) after stimulation with ephrin-B1 ligand, consistent with the observation that this double substitution abrogates ephrin-B1-induced kinase activity and NG108 neurite retraction (6). Similarly, ephrin-B1 treatment of cells expressing a mutant EphB2 rendered kinase inactive through a mutation in the catalytic domain (EphB2-KD, substitution at Lys 611 to Met) induced no appreciable decline in phospho-ERK levels (Fig. 3D). Together, these results indicate that activation of EphB2 in the NG108 cells attenuates the phosphorylation and thus the activation of the MEK1 and ERK1/2 kinases and show that this inhibition of the MAPK signaling pathway requires EphB2 kinase activity.
FIG. 3.
Kinase activity of EphB2 is required for down regulation of ERK1/2 signaling. (A) Schematic representation of the EphB2 mutant structures, indicating juxtamembrane tyrosines JX1 (Y604), JX2 (Y610) and the conserved SAM domain tyrosine (Y929). (B and C) EphB2-ΔC, a C-terminal truncation, ends at V951 in the SAM domain. NG108 or NG-EphB2 clones expressing WT or mutant receptors were stimulated with 2 μg of clustered Fc-ephrin-B1 per ml for the indicated time points. The cells were harvested directly in 2× SDS sample buffer, and lysates were electrophoresed and immunoblotted (WB) with antibodies against phosphorylated ERK1/2 (B, top panel) or phosphorylated MEK1 (C, top panel). Immunoblots were stripped and reprobed with anti-ERK1 (B, bottom panel) or anti-MEK1 (C, bottom panel). (D) NG108 cells expressing WT EphB2 and kinase-inactive EphB2 (KD) were stimulated with clustered Fc-ephrin-B1 and treated as indicated for panels B and C. Lysates were then probed with anti-phospho-ERK1/2 (upper panel), and anti-ERK1 (lower panel).
Activation of EphB2 in NG108 cells leads to a decrease in the levels of GTP-bound Ras.
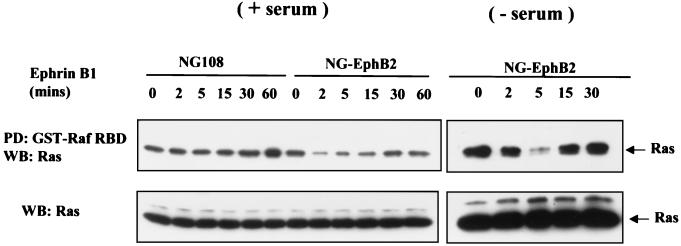
The small GTPase Ras is an important upstream regulator of the MAPK cascade and a common downstream effector of RTK signaling (59). To study the effect of EphB2 on Ras activation, parental NG108 and stable EphB2 cell lines were stimulated with clustered ephrin-B1 and cell lysates were incubated with the Ras-binding domain of c-Raf expressed as a GST fusion protein (GST–Raf-RBD) and immobilized on glutathione-Sepharose beads. The Raf-RBD has a higher affinity for the GTP-bound form of Ras than for the GDP bound form and can thus be used as a probe for activated Ras (61). Stimulation of NG-EphB2 cells in the presence of serum with clustered Fc-ephrin-B1 ligand led to a transient decrease in the levels of Ras-GTP that was restored to the basal level by 30 mins after stimulation (Fig. 4, left panels). In the absence of serum, the decrease in the Ras-GTP level was more dramatic (Fig. 4, right panels). In contrast, parental NG108 cells showed no changes in the levels of GTP-bound Ras. These observations are in good agreement with the decrease in ERK1/2 and MEK1 phosphorylation shown in Fig. 1. Collectively, these results imply that activation of EphB2 in NG108 cells leads to a decrease in the levels of activated Ras and subsequently a more prolonged decrease in ERK activation, even in the presence of serum.
FIG. 4.
EphB2 activation by ephrin-B1 causes a transient decrease in the levels of activated p21Ras. (A) Parental NG108 or NG-EphB2 cells in the presence of serum (left panels) or serum starved (right panels) were stimulated for the indicated times with Fc-ephrin-B1 clusters (2 μg/ml), and lysates were mixed with GST-Raf RBD immobilized on glutathione beads in a pull-down (PD) assay. Samples were electrophoresed and immunoblotted (WB) for Ras (upper panel). To demonstrate equivalent protein levels at different time points, equal volumes of whole-cell lysate were electrophoresed and immunoblotted for Ras (lower panel).
p120-RasGAP participates in EphB2-induced downregulation of the Ras-MAP kinase pathway.
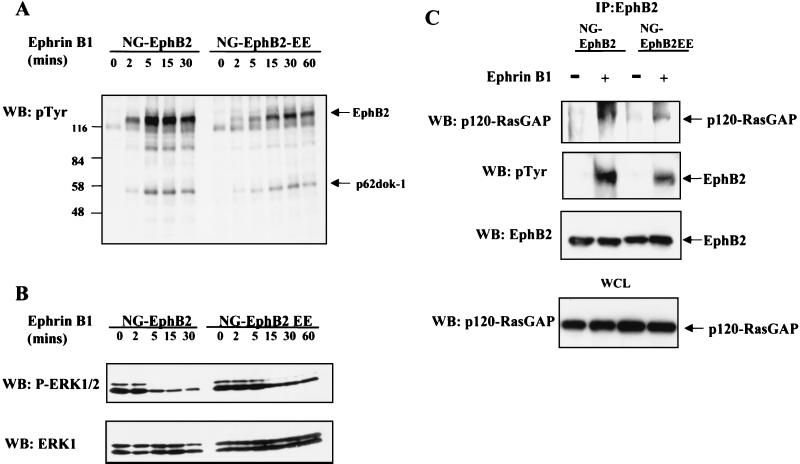
Replacement of Y604 and Y610 in the EphB2 juxtamembrane region by phenylalanine locks the receptor in an autoinhibited state, but their replacement by glutamate allows for receptor activation while impairing the subsequent binding of SH2 domain proteins to the juxtamembrane sequence (6, 76). To test the consequences of mutating the EphB2 juxtamembrane tyrosines to glutamate on the inhibition of Ras-MAPK activation, lines of NG108 cells stably expressing a Tyr604/610Glu mutant (EphB2-EE) were isolated. Ephrin-B1 stimulation of NG-EphB2 and NG-EphB2-EE cells resulted in a similar profile of tyrosine phosphorylation, notably of the p120-RasGAP-associated protein p62dok-1, although phosphorylation was induced with slower kinetics in the EphB2-EE cells (Fig. 5A). Ephrin B1 stimulation of NG-EphB2-EE cells caused a significant decrease in ERK1/2 phosphorylation; however, this down regulation was attenuated compared with that for cells expressing WT EphB2 (Fig. 5B). Ephrin-B1 stimulation of cells expressing EphB2 or EphB2-EE induced the association of both WT and mutant receptors with p120-RasGAP, although this interaction was markedly reduced for EphB2-EE (Fig. 5C). Thus, the EphB2-EE mutant can still down regulate ERK activation, but this activity is less potent than that of WT EphB2, corresponding to a decreased ability of the mutant to access p120-RasGAP, either directly or through p62dok-1 phosphorylation.
FIG. 5.
Role for p120-RasGAP in signaling from EphB2 in NG108 cells. (A) NG-EphB2 and NG-EphB2-EE clones were stimulated with aggregated Fc-ephrinB1 ligand for the indicated times, and whole-cell lysates were separated by SDS-PAGE and immunoblotted (WB) for anti-pTyr. Tyrosine-phosphorylated EphB2 and p62dok-1 are indicated by arrows. (B) Cell lysates from (panel A) were immunoblotted for phosphorylated ERK1/2 (upper panel) and reprobed for ERK1 (lower panel). (C) Serum-starved NG-EphB2 and NG-EphB2-EE cells were incubated in the presence or absence of 2 μg of aggregated ephrin-B1 per ml for 20 min and lysed as indicated in Materials and Methods. EphB2 immunoprecipitates were blotted for p120-RasGAP (upper panel) and reprobed with anti-pTyr antibodies (second panel) and anti-EphB2 (third panel). For control, equivalent amounts of whole-cell lysate (WCL) were immunoblotted for p120-RasGAP to indicate equal input (bottom panel).
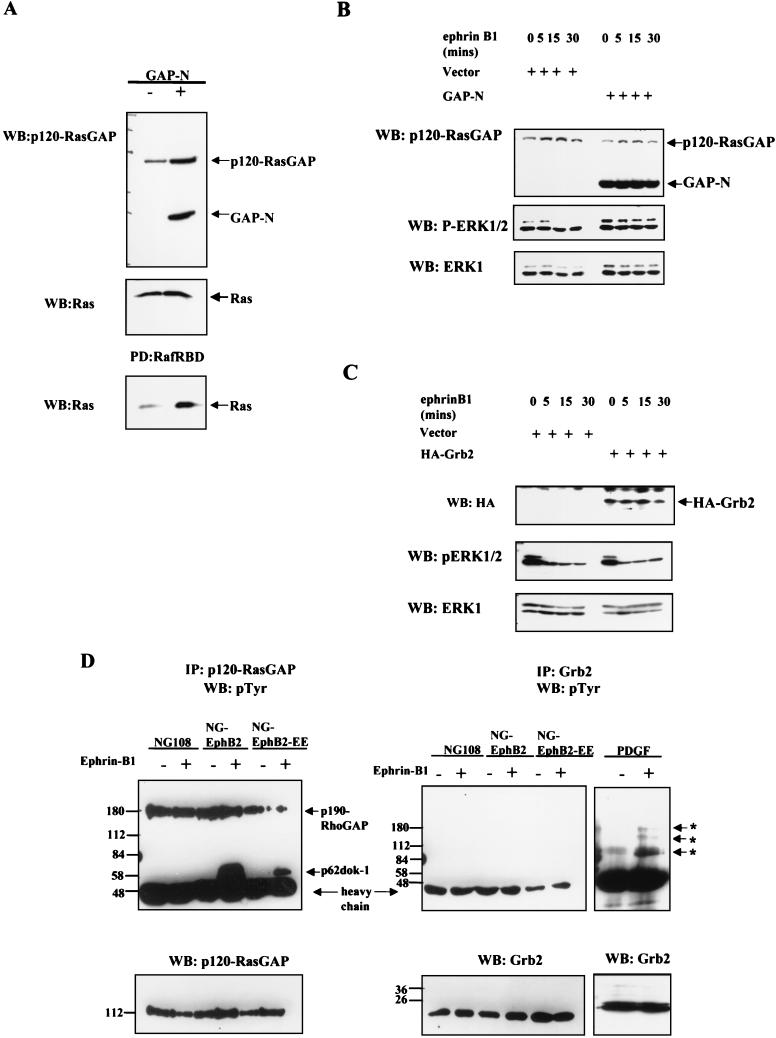
To pursue the possible role of p120-RasGAP in EphB2-mediated Ras-MAPK downregulation, we have used a truncated form of p120-RasGAP lacking the C-terminal GTPase-activating domain but retaining the N-terminal SH2 and SH3 protein binding motifs (GAP-N) (52). In fibroblasts stably transfected with this truncated p120-RasGAP, the levels of activated Ras are upregulated, indicating that GAP-N acts as a dominant negative (Fig. 6A). NG-EphB2 cells were transfected with either vector control or the GAP-N construct and were challenged with clustered Fc-ephrin-B1 48 h after transfection. Immunoblotting of whole-cell lysates with p120-RasGAP antibodies raised against the N-terminal region (52) revealed a 49-kDa band in the GAP-N-transfected cells that was not present in the control cells and corresponds to the expected molecular weight of GAP-N (Fig. 6B, upper panel). In the control NG-EphB2 cells, stimulation of EphB2 led to a time-dependent decrease in phospho-ERK1/2 levels whereas there was no apparent decrease in these levels in the GAP-N-transfected cells (Fig. 6B, middle panel). In contrast, NG-EphB2 cells overexpressing the Grb2 adaptor protein showed no apparent difference in the kinetics of ephrin-induced ERK downregulation compared to cells transfected with the vector control (Fig. 6C). These results argue that Grb2 does not play a significant role in signaling downstream of EphB2 in NG108 cells and indicate that the dominant inhibitory effect of GAP-N is specific and not simply due to overexpression of an SH2/SH3 domain polypeptide. These results show that overexpression of GAP-N ablates the EphB2-induced downregulation of ERK signaling in NG108 cells, suggesting that the GTPase domain of p120-RasGAP may play an important role in this process. Interestingly, and in agreement with the results presented above, we were unable to detect association of tyrosine-phosphorylated proteins with Grb2 in ephrin-B1-stimulated NG-EphB2 or NG-EphB2-EE lysates, whereas ephrin-B1 stimulation led to the recruitment of tyrosine-phosphorylated p62dok-1 to p120-RasGAP in both of these cell lines (38) (Fig. 6D). Collectively, these data suggest that the primary role of EphB2 in NG108 cells in relation to the Ras-MAPK pathway is the transmission of negative signals through p120-RasGAP rather than of positive signals through the Grb2/Sos1 complex.
FIG. 6.
p120-RasGAP but not Grb2 participates in signaling from EphB2 in NG-108 cells. (A) Lyates from WT Rat2 cells or Rat2 cells stably expressing GAP-N (top panel) were mixed with GST-Raf RBD immobilized on glutathione beads. Samples were electrophoresed and immunoblotted (WB) for Ras (bottom panel). Equivalent aliquots of whole-cell lysate were probed for Ras to demonstrate equal input (middle panel). (B and C) NG-EphB2 cells were transfected with empty vector or GAP-N, as indicated (B), or in parallel empty vector and HA-Grb2 (C). At 48 h after transfection, the cells were challenged with 2 μg of clustered Fc-ephrin-B1 per ml and assayed for expression of p120-RasGAP and GAP-N (B, upper panel), HA-Grb2 (C, upper panel), and phospho-ERK1/2 (B and C, middle panel), then reprobed for total ERK1 (B and C, bottom panel). (D) Parental NG108, NG-EphB2 and NG-EphB2-EE cells were incubated in the absence (−) or presence (+) of clustered Fc-ephrin-B1 for 20 mins. For the Grb2 immunoprecipitation (IP) control, Rat2 cells were incubated in the absence (−) or presence (+) of PDGF for 20 min. Lysates were subsequently immunoprecipitated with either p120-RasGAP (left panel) or Grb2 (middle and right panels) antibodies, and immunoblots were probed with anti-pTyr antibodies. Asterisks indicate tyrosine-phosphorylated proteins in the Grb2 immunoprecipitation control. Lower panels indicate total protein levels.
Constitutive Ras activation impairs EphB2-mediated neurite retraction in NG108 cells.
Ephrin-B1 stimulation of differentiated, neurite-bearing NG-EphB2 cells leads to the collapse of polymerized actin structures and to neurite retraction, as visualized by using rhodamine-phalloidin staining (6). Using a conjugated yellow fluorescence protein-actin construct (YFP-actin [see Materials and Methods]), we have identified a similar series of events using time lapse imaging of living cells. Parental NG108 or NG-EphB2 cells were transfected with YFP-actin, and neurite outgrowth was induced 24 h after transfection. In both NG108 and NG-EphB2 cells, YFP-actin protein was localized throughout the cell body and was particularly rich in the protruding filopodial projections and growth cones (Fig. 7A and data not shown), in good agreement with the distribution patterns of endogenous F-actin in NG108 cells (38). Imaging of untreated parental and NG-EphB2 cells revealed basal levels of cytoskeletal movement, including dynamic but small projections and retractions in actin microspikes (data not shown). After stimulation with 2 μg of clustered ephrin-B1 per ml, NG-EphB2 cells exhibited a rapid reorganization of polymerized actin structures, as visualized by disassembly of the filopodial projections and retraction of neurite extensions (compare arrows in Fig. 7A to F) (time-lapse videography of the neurite retraction can be seen on our website at www.mshri.on.ca/pawson/home.html). The collapse of organized actin structures was accompanied by shrinkage and compaction of the cell bodies. In contrast, ephrin-B1 stimulation of YFP-actin transfected parental NG108 cells induced no significant collapse of neurite or microspike extensions and the cells retained their differentiated morphology at the end of the 40-min time course (data not shown).
FIG. 7.
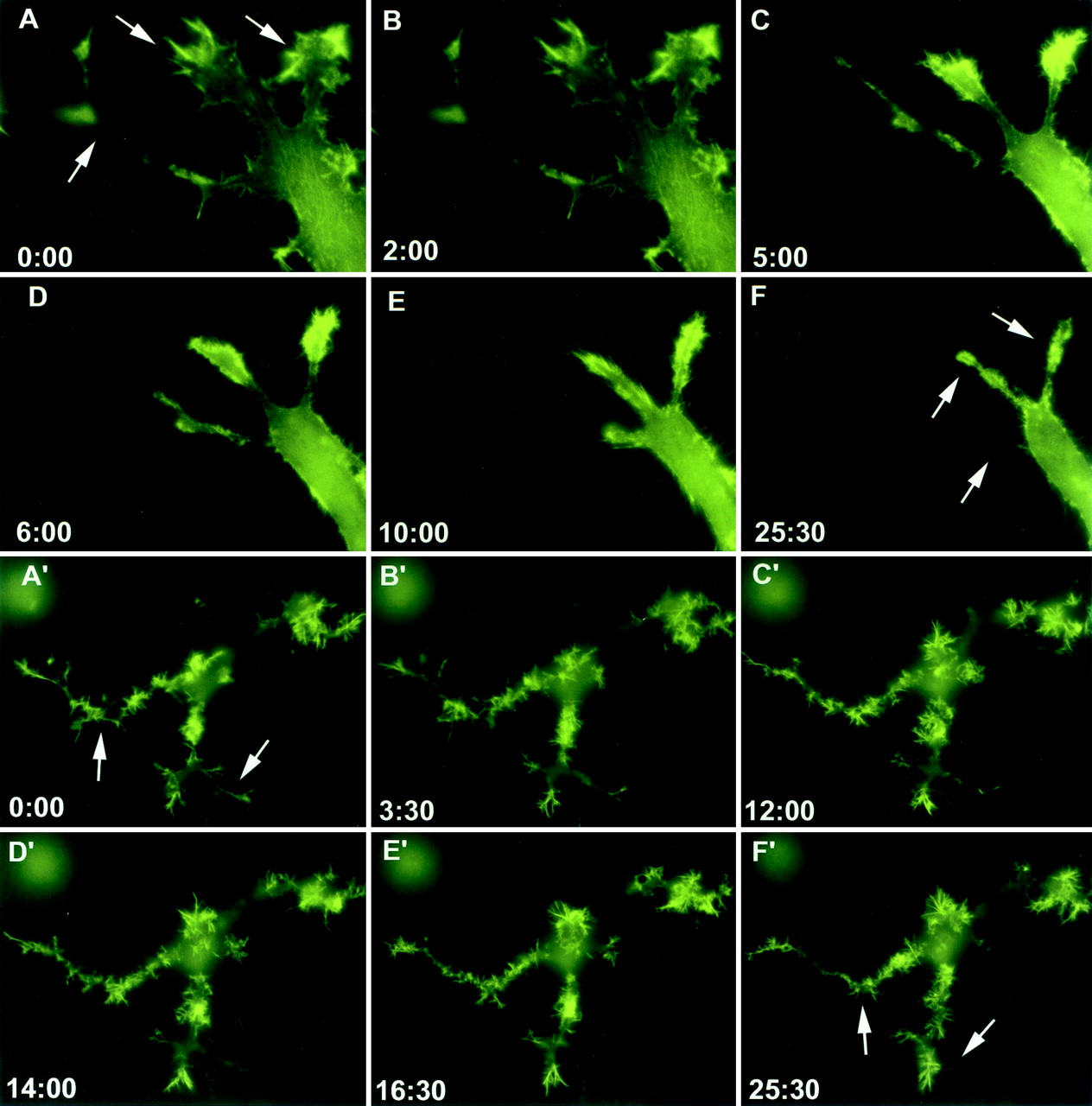
EphB2 down regulation of Ras signaling is required for neurite retraction in NG108 cells. Stable cell lines expressing EphB2 were transfected with either a YFP-actin (yellow fluorescence protein conjugated actin) construct alone (A to F) or YFP-actin and HAp21RasV12 (A′ to F′) and stimulated with 2 μg of clustered ephrin-B1 per ml. Live imaging of cells was performed using an inverted Olympus IX-70 fluorescence microscope for 25 min (A to F) or 40 min (A′ to F′), with frames taken at 30-s intervals. Time points in the stimulation are indicated in minutes:seconds.
To investigate the possible significance of EphB2-induced down regulation of the Ras-MAPK pathway for cytoskeletal remodeling, we analyzed the behavior of NG-EphB2 cells coexpressing YFP-actin and the RasV12 variant, which is constitutively active due to its insensitivity to the GTPase activating function of p120-RasGAP. If down regulation of GTP-bound Ras is important for EphB2-mediated cytoskeletal reorganization, we reasoned that constitutively active RasV12 might antagonize this response. NG-EphB2 cells were cotransfected with YFP-actin and RasV12 and were differentiated to form neurites 24 h after transfection. The resulting cells showed an accumulation of actin at the tip of microspike projections, much like NG-EphB2 cells (Fig. 7A′). However, in contrast to parental NG-EphB2 cells, stimulation of the cells expressing RasV12 with clustered Fc-ephrin-B1 resulted in the collapse of only a few neurites, while most microspikes remained extended and structured (Fig. 7A′ to F′). Together, these observations raise the possibility that down regulation of Ras activity may be required for EphB2-induced changes in cell morphology in NG108 cells.
EphB2 stimulation causes down regulation of fibronectin-induced ERK1/2 activation.
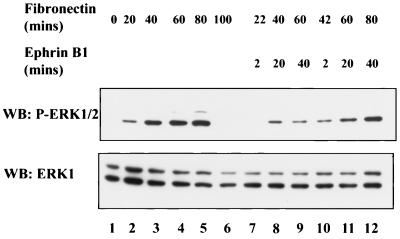
Several recent studies have implicated Eph receptors and ephrins in modulating integrin activity (55, 77). Integrin engagement of matrix ligands such as fibronectin causes transient activation of the ERK pathway in a Ras-dependent manner (64). To investigate the effect of EphB2 signaling on integrin-mediated ERK1/2 activation, NG-EphB2 cells were serum starved overnight and then held in suspension in the absence of serum to diminish ERK1/2 phosphorylation. Cells were subsequently seeded onto fibronectin-coated plates for various times to activate ERK1/2 signaling and then challenged with clustered ephrin-B1 ligand. As anticipated, plating of NG-EphB2 cells on fibronectin led to an increase in phospho-ERK1/2 levels in a time-dependent fashion, up to 80 min after plating (Fig. 8, left-hand five lanes). EphB2 stimulation with ligand for 2, 20, or 40 min caused a notable decrease in the integrin-stimulated phospho-ERK1/2 levels at all time points of integrin engagement tested, compared to untreated controls (Fig. 8, compare lanes 11 and 9 to lane 4 and compare lane 7 to lane 2). These data suggest that EphB2 can negatively regulate signaling from integrin receptors, leading to down regulation of ERK1/2 activation.
FIG. 8.
Integrin-induced stimulation of ERK1/2 phosphorylation is down regulated in response to EphB2 signaling. NG-EphB2 cells were serum starved overnight and subsequently detached and held in suspension for approximately 1 h in the absence of serum. The cells were subsequently plated onto fibronectin-coated six-well plates for the specified times. After incubation of the cells in the absence (lanes 1 to 6) or presence (lanes 7 to 12) of 2 μg of Fc-ephrin-B1 per ml as indicated, the cells were lysed directly in 2× SDS-PAGE sample buffer, separated by electrophoresis, and immunoblotted (WB) with anti-phospho-ERK1/2 antibodies (upper panel) or anti-ERK1 antibodies (lower panel).
DISCUSSION
Activated RTKs generally engage the Grb2/Sos1 complex, leading to activation of the Ras-MAPK pathway. However, in addition to recruiting SH2 domain proteins that enhance signaling events, receptors can bind SH2 proteins that attenuate cytoplasmic signaling pathways, such as p120-RasGAP. Since all RTKs studied to date stimulate the Ras-MAPK pathway, negative regulators have generally been viewed as playing a secondary role in receptor signaling by down regulating previously activated pathways and thereby controlling the duration of the cellular response to growth factors. However, in immune cells a number of receptors (such as FcγRIIB) which are substrates for cytoplasmic tyrosine kinases are dedicated to the inhibition of phosphotyrosine signaling pathways, which they achieve through the recruitment of SH2-containing inositol and tyrosine phosphatases (i.e. SHIP1 and Shp1) to ITIM motifs (17). Here, we show that in some cell types a primary effect of the EphB2 receptor, a member of the largest family of vertebrate RTKs, is to inactivate Ras and thereby inhibit the MAPK pathway. In particular, we show that activation of the EphB2 RTK in the neuronal cell line NG108 leads to inhibition of Ras and ERK1/2 in a p120-RasGAP-dependent manner and demonstrate that down regulation of activated Ras is required for EphB2 stimulated neurite retraction in NG108 cells.
To investigate the role of EphB2 in the activation of the ERK pathway in a neuronal context, we have used NG108 cells in which we have ectopically expressed WT or mutant EphB2 (38). These cells can be differentiated to extend elaborate neurite and filopodial structures reminiscent of motor neurons, a cell type in which endogenous Eph receptors are expressed during embryogenesis (33), and they can therefore be considered a physiologically relevant cell culture system. Using phosphospecific antibodies that detect the activated forms of MEK1 and ERK1/2, we have found that activation of EphB2 by clustered ephrin-B1 causes a dose-dependent (data not shown), and time-dependent (Fig. 1 A and B) decrease in the level of activated ERK1/2 and MEK1 in NG-EphB2 cells. This down regulation of MEK and ERK activation induced by ephrin-B1 is more pronounced and of longer duration in the absence of serum, suggesting that EphB2 inhibitory signaling can be partially counteracted by serum growth factors. Active EphB2 also down regulated the Ras-MAPK pathway in COS 1 cells (Fig. 2), suggesting that inhibitory Ras signaling by EphB2 operates in multiple cell types. However, we have not observed any Ras inhibition following ephrin-B1 stimulation of mouse embryo fibroblasts ectopically expressing EphB2 (data not shown). These results suggest that negative signaling from EphB2 to Ras is cell type dependent. This may explain the observation that overexpression of chicken EphB2 in 293 cells stimulates ERK2 activity (76).
Following the observation that ephrin-B1 inhibits ERK activation, we have analyzed the regulation of upstream elements of the MAPK pathways. In contrast to the Ras activation seen after stimulation of most RTKs, ephrin-B1 causes a decrease in the levels of active, GTP-bound Ras that mimics the time course of EphB2 activation, as well as the decrease in MEK1 and ERK1/2 phosphorylation (Fig. 1A and B). These data indicate that stimulation of EphB2 in NG108 cells leads to a decrease in the levels of GTP-Ras and thus to the attenuation of MAPK signaling. This inhibition of the MAPK cascade requires phosphorylation at the conserved juxtamembrane tyrosine residues of EphB2, since their replacement by phenylalanine (either singly or together) resulted in progressive loss of MAPK inhibitory signaling (Fig. 3B and C). We have previously shown that these juxtamembrane tyrosines in their unphosphorylated state repress kinase activity and that the double phenylalanine mutant is locked in an autoinhibited state and therefore unable to induce neurite retraction (6). In agreement with these results, we also demonstrate that a kinase-inactive mutant of EphB2 is unable to attenuate ERK phosphorylation after ephrin stimulation (Fig. 3D). These results indicate that EphB2 autophosphorylation and consequent kinase activation are required to downregulate Ras.
A direct mechanism by which activated EphB2 could down regulate Ras is through p120-RasGAP. Indeed, p120-RasGAP can bind directly through its SH2 domains to the autophosphorylated EphB2 juxtamembrane region and also to p62dok-1, the principal substrate for EphB2 in NG-EphB2 cells (38) (Fig. 6D). Previous work has shown that p62dok-1 is involved in negative signaling from cell surface receptors to inhibit the MAPK pathway (43, 50, 73). We have found that overexpression of a truncated form of p120-RasGAP lacking the GTPase domain inhibited the decrease in ERK1/2 phosphorylation induced by ephrin-B1 in NG-EphB2 cells (Fig. 6B).
A role for Ras in remodeling neuronal growth cones has been previously proposed (3, 9, 63). When microinjected into PC12 cells or primary embryonic neurons, Ras stimulates morphological differentiation, including neurite outgrowth (3). Our data suggest that decreased levels of activated Ras are necessary for neurite retraction in ephrin-B1-stimulated NG-EphB2 cells, since forced expression of the activated variant RasV12 significantly blocked cytoskeletal changes in response to ligand stimulation (Fig. 7). How down regulation of Ras activity contributes to neurite retraction in these cells remains to be established. Several Ras effectors and Rho family members have been implicated in growth cone development and guidance. Inhibition of phosphatidylinositol (PI) 3-kinase signaling by wortmanin or a dominant negative form of p85 can block nerve growth factor-induced neurite outgrowth (42, 45), and activated PI 3-kinase leads to the formation of neurite-like structures in PC12 cells (46, 48). Furthermore, in N1E-115 neuroblastoma cells, PI-3 kinase appears to be an integral mediator of Ras-induced neurite outgrowth, in a Cdc42- and Rac1-dependent manner, while RhoA competes with Ras to inhibit neurite extension (49, 63). Interestingly, recent work suggests mutual cross talk between the Ras and Rho signaling pathways in transformed cells, with Ras acting to specifically inhibit the effects of Rho-GTP on the actin cytoskeleton by blocking activation of the Rho kinase ROCK (62). Since Rho can also potentially be activated during Eph receptor signaling through guanine nucleotide exchange factors such as Ephexin (66), down regulation of Ras may be important for Rho to exert an effect on the actin cytoskeleton during neurite retraction.
In addition to mediating the signaling functions of ephrins, Eph receptors are increasingly implicated as modulators of other cell surface receptors. For example, EphB2 physically associates with the N-methyl-d-aspartate receptor in synapses (20) and with the Ryk receptor (32). It is therefore possible that one role of EphB2 inhibitory signaling is to attenuate the ability of other receptors to activate the Ras-MAPK pathway. As a case in point, a number of recent reports have implicated Eph RTKs in modulating integrin signaling. Activation of EphA2 in PC-3 cells inhibits integrin-mediated adhesion and causes the dephosphorylation of focal adhesion kinase in a ligand-dependent manner (55). Zou et al. suggest that in NIH 3T3 cells, EphB2 inhibits cell adhesion through phosphorylation of R-Ras (another member of the Ras family of small GTPases), which suppresses the ability of R-Ras to support integrin activity (77). Here, we show that phosphorylation of ERK1/2 induced by integrin engagement in NG-EphB2 cells is suppressed in the presence of clustered ephrin-B1, potentially through p120-RasGAP-induced downregulation of GTP-Ras. These data argue that ephrin-Eph receptor signaling may modify the cellular response to other receptors.
While the role of Eph receptors in axon guidance and growth cone migration is well established, it is not completely understood. The neuronal growth cone is a specialized structure responsible for pathfinding in the developing nervous system (2, 5). It guides the extending neurite toward its target by constantly protruding and retracting filopodia and lamellipodia. We have demonstrated that activation of EphB2 in the neuronal cell line NG108 leads to retraction of filopodial extensions and collapse of neurite structures. Moreover, we show here that activation of EphB2 leads to attenuation of Ras-MAPK signaling and that the decrease in levels of activated Ras is required for the observed changes in the actin cytoskeleton. Our results raise an interesting issue concerning the specificity of RTK signaling. Although RTKs as a general rule signal through the Grb2 SH2/SH3 adaptor to activate the Ras-MAPK pathway, Eph receptors can preferentially signal through p120-RasGAP to inhibit Ras signaling in some cell types. Since both Grb2 and p120-RasGAP are controlled through recruitment of their SH2 domains to pTyr-containing motifs, it appears that distinct RTKs can transmit opposing signals to the Ras pathway through their ability to engage distinct targets.
ACKNOWLEDGMENTS
We thank Kathleen Binns for critical reading of the manuscript and members of the Pawson laboratory for many helpful discussions. We also thank Suzanne Del Rizzo for purification of Fc-ephrin-B1.
This work was supported by a grant from the Canadian Institute of Health Research (CIHR) and a Howard Hughes Medical Institute International Research Scholar award to T.P. S.E. was supported by an Ontario Graduate Scholarship. T.P. is a Distinguished Scientist of the CIHR.
REFERENCES
- 1.Adams R H, Wilkinson G A, Weiss C, Diella F, Gale N W, Deutsch U, Risau W, Klein R. Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev. 1999;13:295–306. doi: 10.1101/gad.13.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aletta J M, Greene L A. Growth cone configuration and advance: a time-lapse study using video-enhanced differential interference contrast microscopy. J Neurosci. 1988;8:1425–1435. doi: 10.1523/JNEUROSCI.08-04-01425.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bar-Sagi D, Feramisco J R. Microinjection of the ras oncogene protein into PC12 cells induces morphological differentiation. Cell. 1985;42:841–848. doi: 10.1016/0092-8674(85)90280-6. [DOI] [PubMed] [Google Scholar]
- 4.Becker E, Huynh-Do U, Holland S, Pawson T, Daniel T O, Skolnik E Y. Nck-interacting Ste20 kinase couples Eph receptors to c-Jun N-terminal kinase and integrin activation. Mol Cell Biol. 2000;20:1537–1545. doi: 10.1128/mcb.20.5.1537-1545.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bentley D, O'Connor T P. Cytoskeletal events in growth cone steering. Curr Opin Neurobiol. 1994;4:43–48. doi: 10.1016/0959-4388(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 6.Binns K L, Taylor P P, Sicheri F, Pawson T, Holland S J. Phosphorylation of tyrosine residues in the kinase domain and juxtamembrane region regulates the biological and catalytic activities of Eph receptors. Mol Cell Biol. 2000;20:4791–4805. doi: 10.1128/mcb.20.13.4791-4805.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birgbauer E, Cowan C A, Sretavan D W, Henkemeyer M. Kinase independent function of EphB receptors in retinal axon pathfinding to the optic disc from dorsal but not ventral retina. Development. 2000;127:1231–1241. doi: 10.1242/dev.127.6.1231. [DOI] [PubMed] [Google Scholar]
- 8.Bohme B, VandenBos T, Cerretti D P, Park L S, Holtrich U, Rubsamen-Waigmann H, Strebhardt K. Cell-cell adhesion mediated by binding of membrane-anchored ligand LERK-2 to the EPH-related receptor human embryonal kinase 2 promotes tyrosine kinase activity. J Biol Chem. 1996;271:24747–24752. doi: 10.1074/jbc.271.40.24747. [DOI] [PubMed] [Google Scholar]
- 9.Borasio G D, John J, Wittinghofer A, Barde Y A, Sendtner M, Heumann R. Ras p21 protein promotes survival and fiber outgrowth of cultured embryonic neurons. Neuron. 1989;2:1087–1096. doi: 10.1016/0896-6273(89)90233-x. [DOI] [PubMed] [Google Scholar]
- 10.Brambilla R, Schnapp A, Casagranda F, Labrador J P, Bergemann A D, Flanagan J G, Pasquale E B, Klein R. Membrane-bound LERK2 ligand can signal through three different Eph-related receptor tyrosine kinases. EMBO J. 1995;14:3116–3126. doi: 10.1002/j.1460-2075.1995.tb07314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brennan C, Monschau B, Lindberg R, Guthrie B, Drescher U, Bonhoeffer F, Holder N. Two Eph receptor tyrosine kinase ligands control axon growth and may be involved in the creation of the retinotectal map in the zebrafish. Development. 1997;124:655–664. doi: 10.1242/dev.124.3.655. [DOI] [PubMed] [Google Scholar]
- 12.Bruce V, Olivieri G, Eickelberg O, Miescher G C. Functional activation of EphA5 receptor does not promote cell proliferation in the aberrant EphA5 expressing human glioblastoma U-118 MG cell line. Brain Res. 1999;821:169–176. doi: 10.1016/s0006-8993(99)01112-9. [DOI] [PubMed] [Google Scholar]
- 13.Bruckner K, Pasquale E B, Klein R. Tyrosine phosphorylation of transmembrane ligands for Eph receptors. Science. 1997;275:1640–1643. doi: 10.1126/science.275.5306.1640. [DOI] [PubMed] [Google Scholar]
- 14.Chin-Sang I D, George S E, Ding M, Moseley S L, Lynch A S, Chisholm A D. The ephrin VAB-2/EFN-1 functions in neuronal signaling to regulate epidermal morphogenesis in C. elegans. Cell. 1999;99:781–790. doi: 10.1016/s0092-8674(00)81675-x. [DOI] [PubMed] [Google Scholar]
- 15.Choi S, Park S. Phosphorylation at Tyr-838 in the kinase domain of EphA8 modulates Fyn binding to the Tyr-615 site by enhancing tyrosine kinase activity. Oncogene. 1999;18:5413–5422. doi: 10.1038/sj.onc.1202917. [DOI] [PubMed] [Google Scholar]
- 16.Cobb M H. MAP kinase pathways. Prog Biophys Mol Biol. 1999;71:479–500. doi: 10.1016/s0079-6107(98)00056-x. [DOI] [PubMed] [Google Scholar]
- 17.Coggeshall K M. Inhibitory signaling by B cell Fc gamma RIIb. Curr Opin Immunol. 1998;10:306–312. doi: 10.1016/s0952-7915(98)80169-6. [DOI] [PubMed] [Google Scholar]
- 18.Conover J C, Doetsch F, Garcia-Verdugo J M, Gale N W, Yancopoulos G D, D. G, Alvarez-Buylla A. Disruption of Eph/ephrin signaling affects migration and proliferation in the adult subventricular zone. Nat Neurosci. 2000;3:1091–1097. doi: 10.1038/80606. [DOI] [PubMed] [Google Scholar]
- 19.Cowan C A, Yokoyama N, Bianchi L M, Henkemeyer M, Fritzsch B. EphB2 guides axons at the midline and is necessary for normal vestibular function. Neuron. 2000;26:417–430. doi: 10.1016/s0896-6273(00)81174-5. [DOI] [PubMed] [Google Scholar]
- 20.Dalva M B, Takasu M A, Lin M Z, Shamah S M, Hu L, Gale N W, Greenberg M E. EphB receptors interact with NMDA receptors and regulate excitatory synapse formation. Cell. 2000;103:945–956. doi: 10.1016/s0092-8674(00)00197-5. [DOI] [PubMed] [Google Scholar]
- 21.Davis S, Gale N W, Aldrich T H, Maisonpierre P C, Lhotak V, Pawson T, Goldfarb M, Yancopoulos G D. Ligands for EPH-related receptor tyrosine kinases that require membrane attachment or clustering for activity. Science. 1994;266:816–819. doi: 10.1126/science.7973638. [DOI] [PubMed] [Google Scholar]
- 22.Davy A, Gale N W, Murray E W, Klinghoffer R A, Soriano P, Feuerstein C, Robbins S M. Compartmentalized signaling by GPI-anchored ephrin-A5 requires the Fyn tyrosine kinase to regulate cellular adhesion. Genes Dev. 1999;13:3125–3135. doi: 10.1101/gad.13.23.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davy A, Robbins S M. Ephrin-A5 modulates cell adhesion and morphology in an integrin-dependent manner. EMBO J. 2000;19:5396–5405. doi: 10.1093/emboj/19.20.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dodelet V C, Pazzagli C, Zisch A H, Hauser C A, Pasquale E B. A novel signaling intermediate, SHEP1, directly couples Eph receptors to R-Ras and Rap1A. J Biol Chem. 1999;274:31941–31946. doi: 10.1074/jbc.274.45.31941. [DOI] [PubMed] [Google Scholar]
- 25.Drescher U, Kremoser C, Handwerker C, Loschinger J, Noda M, Bonhoeffer F. In vitro guidance of retinal ganglion cell axons by RAGS, a 25 kDa tectal protein related to ligands for Eph receptor tyrosine kinases. Cell. 1995;82:359–370. doi: 10.1016/0092-8674(95)90425-5. [DOI] [PubMed] [Google Scholar]
- 26.Ellis C, Kasmi F, Ganju P, Walls E, Panayotou G, Reith A D. A juxtamembrane autophosphorylation site in the Eph family receptor tyrosine kinase. Sek, mediates high affinity interaction with p59fyn. Oncogene. 1996;12:1727–1736. [PubMed] [Google Scholar]
- 27.Eph Nomenclature Committee. Unified nomenclature for Eph family receptors and their ligands, the ephrins. Cell. 1997;90:403–404. doi: 10.1016/s0092-8674(00)80500-0. [DOI] [PubMed] [Google Scholar]
- 28.Gale N W, Holland S J, Valenzuela D M, Flenniken A, Pan L, Ryan T E, Henkemeyer M, Strebhardt K, Hirai H, Wilkinson D G, Pawson T, Davis S, Yancopoulos G D. Eph receptors and ligands comprise two major specificity subclasses and are reciprocally compartmentalized during embryogenesis. Neuron. 1996;17:9–19. doi: 10.1016/s0896-6273(00)80276-7. [DOI] [PubMed] [Google Scholar]
- 29.Gale N W, Baluk P, Pan L, Kwan M, Holash J, DeChiara T, McDonald D, Yancopoulos G. Ephrin-B2 selectively marks arterial vessels and neovascularization sites in the adult, with expression in both endothelial and smooth-muscle cells. Dev Biol. 2001;230:151–160. doi: 10.1006/dbio.2000.0112. [DOI] [PubMed] [Google Scholar]
- 30.George S E, Simokat K, Hardin J, Chisholm A D. The VAB-1 Eph receptor tyrosine kinase functions in neural and epithelial morphogenesis in C. elegans. Cell. 1998;92:633–643. doi: 10.1016/s0092-8674(00)81131-9. [DOI] [PubMed] [Google Scholar]
- 31.Gerety S S, Wang H U, Chen Z F, Anderson D J. Symmetrical mutant phenotypes of the receptor EphB4 and its specific transmembrane ligand ephrin-B2 in cardiovascular development. Mol Cell. 1999;4:403–414. doi: 10.1016/s1097-2765(00)80342-1. [DOI] [PubMed] [Google Scholar]
- 32.Halford M M, Armes J, Buchert M, Meskenaite V, Grail D, Hibbs M L, Wilks A F, Farlie P G, Newgreen D F, Hovens C M, Stacker S A. Ryk-deficient mice exhibit craniofacial defects associated with perturbed Eph receptor crosstalk. Nat Genet. 2000;25:414–418. doi: 10.1038/78099. [DOI] [PubMed] [Google Scholar]
- 33.Henkemeyer M, Marengere L E, McGlade J, Olivier J P, Conlon R A, Holmyard D P, Letwin K, Pawson T. Immunolocalization of the Nuk receptor tyrosine kinase suggests roles in segmental patterning of the brain and axonogenesis. Oncogene. 1994;9:1001–1014. [PubMed] [Google Scholar]
- 34.Henkemeyer M, Orioli D, Henderson J T, Saxton T M, Roder J, Pawson T, Klein R. Nuk controls pathfinding of commissural axons in the mammalian central nervous system. Cell. 1996;86:35–46. doi: 10.1016/s0092-8674(00)80075-6. [DOI] [PubMed] [Google Scholar]
- 35.Hock B, Bohme B, Karn T, Feller S, Rubsamen-Waigmann H, Strebhardt K. Tyrosine-614, the major autophosphorylation site of the receptor tyrosine kinase HEK2, functions as multi-docking site for SH2-domain mediated interactions. Oncogene. 1998;17:255–260. doi: 10.1038/sj.onc.1201907. [DOI] [PubMed] [Google Scholar]
- 36.Hock B, Bohme B, Karn T, Yamamoto T, Kaibuchi K, Holtrich U, Holland S, Pawson T, Rubsamen-Waigmann H, Strebhardt K. PDZ-domain-mediated interaction of the Eph-related receptor tyrosine kinase EphB3 and the ras-binding protein AF6 depends on the kinase activity of the receptor. Proc Natl Acad Sci USA. 1998;95:9779–84. doi: 10.1073/pnas.95.17.9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holland S J, Gale N W, Mbamalu G, Yancopoulos G D, Henkemeyer M, Pawson T. Bidirectional signalling through the EPH-family receptor Nuk and its transmembrane ligands: Nature. 1996;383:722–725. doi: 10.1038/383722a0. [DOI] [PubMed] [Google Scholar]
- 38.Holland S J, Gale N W, Gish G D, Roth R A, Songyang Z, Cantley L C, Henkemeyer M, Yancopoulos G D, Pawson T. Juxtamembrane tyrosine residues couple the Eph family receptor EphB2/Nuk to specific SH2 domain proteins in neuronal cells. EMBO J. 1997;16:3877–3888. doi: 10.1093/emboj/16.13.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holland S J, Peles E, Pawson T, Schlessinger J. Cell-contact-dependent signalling in axon growth and guidance: Eph receptor tyrosine kinases and receptor protein tyrosine phosphatase beta. Curr Opin Neurobiol. 1998;8:117–127. doi: 10.1016/s0959-4388(98)80015-9. [DOI] [PubMed] [Google Scholar]
- 40.Holmberg J, Clarke D L, Frisén J. Regulation of repulsion versus adhesion by different splice forms of an Eph receptor. Nature. 2000;408:203–206. doi: 10.1038/35041577. [DOI] [PubMed] [Google Scholar]
- 41.Huai J, Drescher U. An ephrin-A-dependent signaling pathway controls integrin function and is linked to the tyrosine phosphorylation of a 120-kDa protein. J Biol Chem. 2001;276:6689–6694. doi: 10.1074/jbc.M008127200. [DOI] [PubMed] [Google Scholar]
- 42.Jackson T R, Blader I J, Hammonds-Odie L P, Burga C R, Cooke F, Hawkins P T, Wolf A G, Heldman K A, Theibert A B. Initiation and maintenance of NGF-stimulated neurite outgrowth requires activation of a phosphoinositide 3-kinase. J Cell Sci. 1996;109:289–300. doi: 10.1242/jcs.109.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jones N, Dumont D. Recruitment of Dok-R to the EGF receptor through its PTB domain is required for attenuation of Erk MAP kinase activation. Curr Biol. 1999;9:1057–1060. doi: 10.1016/s0960-9822(99)80458-8. [DOI] [PubMed] [Google Scholar]
- 44.Kalo M S, Pasquale E B. Multiple in vivo tyrosine phosphorylation sites in EphB receptors. Biochemistry. 1999;38:14396–14408. doi: 10.1021/bi991628t. [DOI] [PubMed] [Google Scholar]
- 45.Kimura K, Hattori S, Kabuyama Y, Shizawa Y, Takayanagi J, Nakamura S, Toki S, Matsuda Y, Onodera K, Fukui Y. Neurite outgrowth of PC12 cells is suppressed by wortmannin, a specific inhibitor of phosphatidylinositol 3-kinase. J Biol Chem. 1994;269:18961–18967. [PubMed] [Google Scholar]
- 46.Kita Y, Kimura K D, Kobayashi M, Ihara S, Kaibuchi K, Kuroda S, Ui M, Iba H, Konishi H, Kikkawa U, Nagata S, Fukui Y. Microinjection of activated phosphatidylinositol-3 kinase induces process outgrowth in rat PC12 cells through the Rac-JNK signal transduction pathway. J Cell Sci. 1998;111:907–915. doi: 10.1242/jcs.111.7.907. [DOI] [PubMed] [Google Scholar]
- 47.Knoll B, Zarbalis K, Wurst W, Drescher U. A role for the EphA family in the topographic targeting of vomeronasal axons. Development. 2001;128:895–906. doi: 10.1242/dev.128.6.895. [DOI] [PubMed] [Google Scholar]
- 48.Kobayashi M, Nagata S, Kita Y, Nakatsu N, Ihara S, Kaibuchi K, Kuroda S, Ui M, Iba H, Konishi H, Kikkawa U, Saitoh I, Fukui Y. Expression of a constitutively active phosphatidylinositol 3-kinase induces process formation in rat PC12 cells. Use of Cre/loxP recombination system. J Biol Chem. 1997;272:16089–16092. doi: 10.1074/jbc.272.26.16089. [DOI] [PubMed] [Google Scholar]
- 49.Kozma R, Sarner S, Ahmed S, Lim L. Rho family GTPases and neuronal growth cone remodelling: relationship between increased complexity induced by Cdc42Hs, Rac1, and acetylcholine and collapse induced by RhoA and lysophosphatidic acid. Mol Cell Biol. 1997;17:1201–1211. doi: 10.1128/mcb.17.3.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lemay S, Davidson D, Latour S, Veillette A. Dok-3, a novel adapter molecule involved in the negative regulation of immunoreceptor signaling. Mol Cell Biol. 2000;20:2743–2754. doi: 10.1128/mcb.20.8.2743-2754.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marshall C J. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 52.McGlade J, Brunkhorst B, Anderson D, Mhamalu G, Settleman J, Dedhar S, Rozakis-Adcock M, Chen L B, Pawson T. The N-terminal region of GAP regulates cytoskeletal structure and cell adhesion. EMBO J. 1993;12:3073–3081. doi: 10.1002/j.1460-2075.1993.tb05976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meima L, Kljavin I J, Moran P, Shih A, Winslow J W, Caras I W. AL-1-induced growth cone collapse of rat cortical neurons is correlated with REK7 expression and rearrangement of the actin cytoskeleton. Eur J Neurosci. 1997;9:177–188. doi: 10.1111/j.1460-9568.1997.tb01365.x. [DOI] [PubMed] [Google Scholar]
- 54.Meima L, Moran P, Matthews W, Caras I W. Lerk2 (ephrin-B1) is a collapsing factor for a subset of cortical growth cones and acts by a mechanism different from AL-1 (ephrin-A5) Mol Cell Neurosci. 1997;9:314–328. doi: 10.1006/mcne.1997.0621. [DOI] [PubMed] [Google Scholar]
- 55.Miao H, Burnett E, Kinch M, Simon E, Wang B. Activation of EphA2 kinase suppresses integrin function and causes focal-adhesion-kinase dephosphorylation. Nat Cell Biol. 2000;2:62–69. doi: 10.1038/35000008. [DOI] [PubMed] [Google Scholar]
- 56.Mueller B K. Growth cone guidance: first steps towards a deeper understanding. Annu Rev Neurosci. 1999;22:351–388. doi: 10.1146/annurev.neuro.22.1.351. [DOI] [PubMed] [Google Scholar]
- 57.Nakamoto M, Cheng H J, Friedman G C, McLaughlin T, Hansen M J, Yoon C H, O'Leary D D, Flanagan J G. Topographically specific effects of ELF-1 on retinal axon guidance in vitro and retinal axon mapping in vivo. Cell. 1996;86:755–766. doi: 10.1016/s0092-8674(00)80150-6. [DOI] [PubMed] [Google Scholar]
- 58.Pandey A, Shao H, Marks R M, Polverini P J, Dixit V M. Role of B61, the ligand for the Eck receptor tyrosine kinase, in TNF-alpha-induced angiogenesis. Science. 1995;268:567–569. doi: 10.1126/science.7536959. [DOI] [PubMed] [Google Scholar]
- 59.Pawson T, Saxton T M. Signaling networks—do all roads lead to the same genes? Cell. 1999;97:675–678. doi: 10.1016/s0092-8674(00)80779-5. [DOI] [PubMed] [Google Scholar]
- 60.Pear W S, Nolan G P, Scott M L, Baltimore D. Production of high titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci USA. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Rooij J, Bos J L. Minimal Ras-binding domain of Raf1 can be used as an activation-specific probe for Ras. Oncogene. 1997;14:623–625. doi: 10.1038/sj.onc.1201005. [DOI] [PubMed] [Google Scholar]
- 62.Sahai E, Olson M F, Marshall C J. Cross-talk between Ras and Rho signalling pathways in transformation favours proliferation and increased motility. EMBO J. 2001;20:755–766. doi: 10.1093/emboj/20.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sarner S, Kozma R, Ahmed S, Lim L. Phosphatidylinositol 3-kinase, Cdc42, and Rac1 act downstream of Ras in integrin-dependent neurite outgrowth in N1E-115 neuroblastoma cells. Mol Cell Biol. 2000;20:158–172. doi: 10.1128/mcb.20.1.158-172.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schlaepfer D D, Hunter T. Integrin signalling and tyrosine phosphorylation: just the FAKs? Trends Cell Biol. 1998;8:151–157. doi: 10.1016/s0962-8924(97)01172-0. [DOI] [PubMed] [Google Scholar]
- 65.Scully A L, McKeown M, Thomas J B. Isolation and characterization of Dek, a Drosophila eph receptor protein tyrosine kinase. Mol Cell Neurosci. 1999;13:337–347. doi: 10.1006/mcne.1999.0752. [DOI] [PubMed] [Google Scholar]
- 66.Shamah S M, Lin M Z, Goldberg J L, Estrach S, Sahin M, Hu L, Bazalakova M, Neve R L, Corfas G, Debant A, Greenberg M E. EphA receptors regulate growth cone dynamics through the novel guanine nucleotide exchange factor ephexin. Cell. 2001;105:233–244. doi: 10.1016/s0092-8674(01)00314-2. [DOI] [PubMed] [Google Scholar]
- 67.Stapleton D, Balan I, Pawson T, Sicheri F. The crystal structure of an Eph receptor SAM domain reveals a mechanism for modular dimerization. Nat Struct Biol. 1999;6:44–49. doi: 10.1038/4917. [DOI] [PubMed] [Google Scholar]
- 68.Stein E, Cerretti D P, Daniel T O. Ligand activation of ELK receptor tyrosine kinase promotes its association with Grb10 and Grb2 in vascular endothelial cells. J Biol Chem. 1996;271:23588–23593. doi: 10.1074/jbc.271.38.23588. [DOI] [PubMed] [Google Scholar]
- 69.Stein E, Hyunh-Do U, Lane A A, Cerretti D P, Daniel T O. Nck recruitment to Eph Receptor, EphB1/Elk, couples ligand activation to c-Jun kinase. J Biol Chem. 1998;273:1303–1308. doi: 10.1074/jbc.273.3.1303. [DOI] [PubMed] [Google Scholar]
- 70.Thanos C D, Faham S, Goodwill K E, Cascio D, Phillips M, Bowie J U. Monomeric structure of the human EphB2 sterile alpha motif domain. J Biol Chem. 1999;274:37301–37306. doi: 10.1074/jbc.274.52.37301. [DOI] [PubMed] [Google Scholar]
- 71.Wang H U, Chen Z F, Anderson D J. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- 72.Wang X, Roy P J, Holland S J, Zhang L W, Culotti J G, Pawson T. Multiple ephrins control cell organization in C. elegans using kinase-dependent and -independent functions of the VAB-1 Eph receptor. Mol Cell. 1999;4:903–913. doi: 10.1016/s1097-2765(00)80220-8. [DOI] [PubMed] [Google Scholar]
- 73.Yamanashi Y, Tamura T, Kanamori T, Yamane H, Nariuchi H, Yamamoto T, Baltimore D. Role of the rasGAP-associated docking protein p62(dok) in negative regulation of B cell receptor-mediated signaling. Genes Dev. 2000;14:11–16. [PMC free article] [PubMed] [Google Scholar]
- 74.Yokoyama N, Romero M I, Cowan C A, Galvan P, Helmbacher F, Charnay P, Parada L F, Henkemeyer M. Forward signaling mediated by ephrin-B3 prevents contralateral corticospinal axons from recrossing the spinal cord midline. Neuron. 2001;29:85–97. doi: 10.1016/s0896-6273(01)00182-9. [DOI] [PubMed] [Google Scholar]
- 75.Zisch A H, Kalo M S, Chong L D, Pasquale E B. Complex formation between EphB2 and Src requires phosphorylation of tyrosine 611 in the EphB2 juxtamembrane region. Oncogene. 1998;16:2657–2670. doi: 10.1038/sj.onc.1201823. [DOI] [PubMed] [Google Scholar]
- 76.Zisch A H, Pazzagli C, Freeman A, Schneller M, Hadman M, Smith J W, Ruoshlahti E, Pasquale E B. Replacing two conserved tyrosines of the EphB2 receptor with glutamic acid prevents binding of SH2 domains without abrogating kinase activity and biological responses. Oncogene. 2000;19:177–187. doi: 10.1038/sj.onc.1203304. [DOI] [PubMed] [Google Scholar]
- 77.Zou J X, Wang B, Kalo M S, Zisch A, Pasquale E B, Ruoshlahti E. An Eph receptor regulates integrin activity through R-Ras. Proc Natl Acad Sci USA. 1999;96:13813–13818. doi: 10.1073/pnas.96.24.13813. [DOI] [PMC free article] [PubMed] [Google Scholar]