Abstract
Introduction K i -67 is often used as a proliferation index to evaluate how aggressive a tumor is and its likelihood of recurrence. Vestibular schwannomas (VS) are a unique benign pathology that lends itself well to evaluation with K i -67 as a potential marker for disease recurrence or progression following surgical resection.
Methods All English language studies of VSs and K i -67 indices were screened. Studies were considered eligible for inclusion if they reported series of VSs undergoing primary resection without prior irradiation, with outcomes including both recurrence/progression and K i -67 for individual patients. For published studies reporting pooled K i -67 index data without detailed by-patient values, we contacted the authors to request data sharing for the current meta-analysis. Studies reporting a relationship between K i -67 index and clinical outcomes in VS for which detailed patients' outcomes or K i -67 indices could not be obtained were incorporated into the descriptive analysis, but excluded from the formal (i.e., quantitative) meta-analysis.
Results A systematic review identified 104 candidate citations of which 12 met inclusion criteria. Six of these studies had accessible patient-specific data. Individual patient data were collected from these studies for calculation of discrete study effect sizes, pooling via random-effects modeling with restricted maximum likelihood, and meta-analysis. The standardized mean difference in K i -67 indices between those with and without recurrence was calculated as 0.79% (95% confidence interval [CI]: 0.28–1.30; p = 0.0026).
Conclusion K i -67 index may be higher in VSs that demonstrate recurrence/progression following surgical resection. This may represent a promising means of evaluating tumor recurrence and potential need for early adjuvant therapy for VSs.
Keywords: histopathology, vestibular schwannoma, acoustic neuroma, recurrence, progression, K i -67 , index, systematic review, meta-analysis
Introduction
Management strategies for vestibular schwannoma (VS) have evolved substantially over time, in particular with the advent of new technologies, such as advanced neuromonitoring, stereotactic radiosurgery (SRS), and an enhanced understanding of the natural history of the disease. Although the ideal operative outcome for patients undergoing surgical resection remains gross total resection (GTR) when feasible, improved understanding of the adverse impact on patient function and well-being that are associated with facial nerve injury have shifted the paradigm away from the more aggressive posture of prioritizing GTR for these benign lesions. 1 Correspondingly, a higher fraction of patients emerge from surgery with near-total or subtotal resections (NTR or STR, respectively). 2 3 When coupled to the increasing actuarial survival of the population at large, this results in a complicated prognostic calculus regarding the risk of recurrence and optimal timing of adjuvant treatments, such as SRS. At present, no reliable serologic or radiographic markers have been validated to better inform this complex decision-making.
K i -67 is a widely available immunohistochemical test that stains cells in the premitotic or mitotic proliferative phases of the cell cycle. This antigen has been repeatedly validated as a proliferative index for meningiomas, with similarly positive results reported across a range of other cranial and spinal neoplasms. 4 5 6 Preliminary results in VS have included correlations between K i -67 index and preoperative tumor growth rates, as well as clinical symptoms; however, these studies have been limited by small sample sizes, methodologic concerns, and vulnerability to various sources of bias. Furthermore, essentially, no data are available to answer the specific clinical question of postoperative management after resection. The goal of the current study is to perform a systematic review and meta-analysis of all studies reporting postoperative VS tumor control outcomes as a function of K i -67 index.
Materials and Methods
Search Strategy
The study was conducted by adhering to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement. 7 A comprehensive search of several databases from inception to December 22, 2020, limited to English language and excluding animal studies, was conducted. The databases included Ovid MEDLINE(R) and the Epub Ahead of Print, In-Process and Other Non-Indexed Citations and Daily, Ovid Embase, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, and Scopus. The search strategy was designed and conducted by an expert medical research librarian, under direction from the investigators to identify studies reporting K i -67 outcomes in patients undergoing VS resection ( Appendix 1 ).
Table 1. Studies that met criteria for inclusion in the descriptive analysis.
| Reference no. | Study | Year | n | Primary end point | Outcome |
|---|---|---|---|---|---|
| 18 | Prueter et al | 2019 | 74 | Tumor recurrence | Correlated |
| 12 | Graffeo et al | 2018 | 46 | Postoperative tumor regrowth after subtotal resection | Not correlated |
| 17 | Panigrahi et al | 2018 | 144 | Tumor recurrence | Correlated |
| 2 | Iannella et al | 2017 | 17 | Postoperative tumor regrowth | Correlated |
| 14 | Jabbour et al | 2016 | 180 | Younger age | Correlated |
| 9 | Cafer et al | 2008 | 34 | Tumor size at resection | Not correlated |
| 19 | Steinhart et al | 2003 | 50 | Preoperative tumor growth rate | Correlated |
| 13 | Hwang et al | 2002 | 30 | Postoperative tumor regrowth after subtotal resection, including selective adjunct radiosurgery | Correlated |
| 11 | Gomez-Brouchet et al | 2001 | 30 | Tumor size at resection | Not correlated |
| 16 | Niemczyk et al | 2000 | 43 | Preoperative tumor growth rate | Correlated |
| 20 | Yokoyama et al | 1996 | 18 | Postoperative tumor regrowth | Correlated |
| 10 | Charabi et al | 1993 | 21 | Preoperative symptom duration | Correlated |
| 15 | Lesser et al | 1991 | 8 | Preoperative tumor growth rate | Correlated |
Study Question, Inclusions, and Exclusions
Our Population, Intervention, Comparison, Outcomes, and Study (PICOS) -format study question was: among patients with sporadic VS (P) who had high K i -67 staining on resection histopathology (I), as compared with sporadic VS patients with low K i -67 staining (C), was there a difference in rates of postoperative tumor growth/recurrence (O), in original research articles reporting patient-level data (S). Studies were considered eligible for inclusion if they reported series of VS undergoing primary resection without prior irradiation and with outcomes including both recurrence/progression and K i -67 index for individual patients. For published studies reporting, pooled K i -67 index data without detailed by-patient values, we contacted the authors to request data sharing for the current meta-analysis. Studies reporting a relationship between K i -67 index and clinical outcomes in VS for which detailed patients' outcomes or K i -67 indices could not be obtained were incorporated into the descriptive analysis but excluded from the formal (i.e., quantitative) meta-analysis. For publications describing overlapping cohorts, only the most current/complete manuscripts were included.
Manuscript Screening, Review, and Data Abstraction
Titles and abstracts were screened from the retrieved literature search by two independent reviewers (K.V. and H.H.); all candidate citations flagged for possible inclusion by either reviewer underwent full-text assessment to determine eligibility ( Fig. 1 ). They then assessed the full text to decide on eligibility via detailed comparison to the above-detailed criteria. For studies requiring supplemental information from outside investigators, a standardized e-mail was sent detailing the proposed study and data sharing request; authors were allowed 2 months to respond, and at least 2 attempts were made to contact a representative for all such studies. Individual patient K i -67 indices and tumor control outcomes as of last follow-up were captured from all included studies. Included studies were graded using the modified Newcastle–Ottawa Scale ( Appendix 2 ).
Fig. 1.
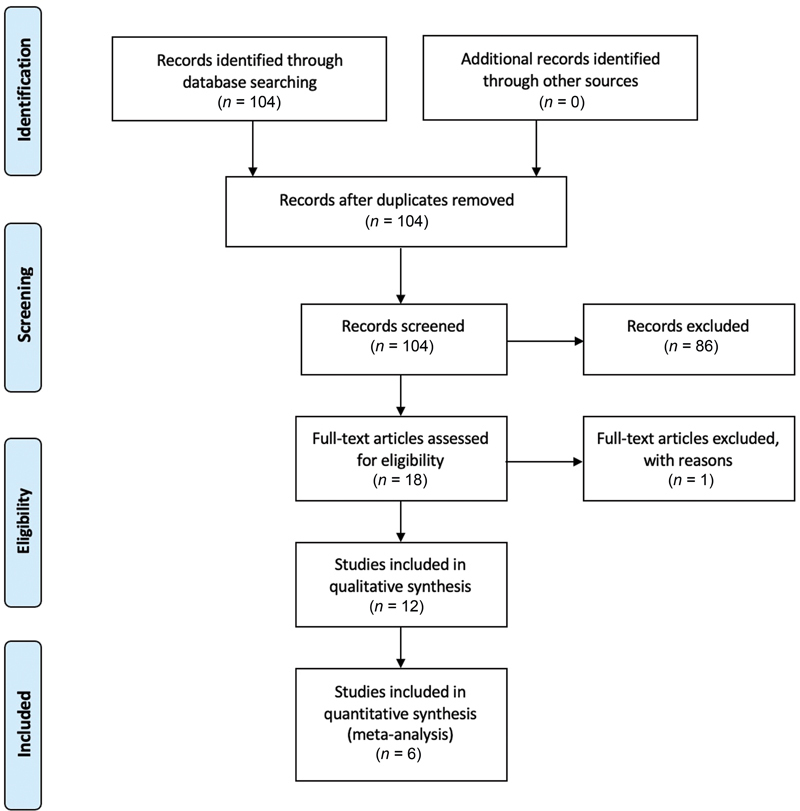
PRISMA flow diagram. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses.
Table 2. Studies included in the quantitative meta-analysis.
| Reference no. | Study | Year | n | Mean follow-up period (mo) | GTR rate (%) | Recurrence rate (%) | Average K i -67 (%) recurrence | Average K i -67 (%) no recurrence |
|---|---|---|---|---|---|---|---|---|
| 18 | Prueter et al | 2019 | 74 | 29.2 | 30.1 | 17.5 | 1.9 | 1.2 |
| 12 | Graffeo et al | 2018 | 46 | 41.0 | 0 | 28.2 | 1.4 | 1.2 |
| 17 | Panigrahi et al | 2018 | 144 | 38.0 | 36.1 | 12.5 | 4.8 | 1.9 |
| 2 | Iannella et al | 2017 | 17 | 80.4 | 0 | 23.5 | 3.2 | 1.4 |
| 13 | Hwang et al | 2002 | 30 | 38.0 | NA | 9.7 | 2.3 | 0.6 |
| 20 | Yokoyama et al | 1996 | 38 | 54.0 | 28.9 | 47.3 | 2.1 | 1.3 |
Abbreviations: GTR, gross-total resection; NA, not available.
Statistical and Sensitivity Analyses
The meta-analysis was conducted using a random-effects model to calculate a standardized mean difference in K i -67 indices between patients who experienced recurrence/progression and those with durable tumor control. 8 Due to the small sample sizes, no adjusted multivariable analyses could be performed; however, variables that were significant on univariate analysis and associated with the main independent variable (i.e., K i -67 index) underwent secondary study via stratified analyses. The Cochran Q test, which is typically used to check for differences on a dichotomous dependent variable between three or more related groups, was conducted as a preliminary test of heterogeneity. High levels of heterogeneity were identified, which were likely attributable to small sample sizes. To strengthen interpretation of the study results in the context of marked heterogeneity, we performed a sensitivity analysis by excluding the two studies with the least precise effect estimates and repeating the meta-analysis for this smaller population of studies. Statistical analyses were performed using R version 4.0.3. for Windows (R Foundation for Statistical Computing, Vienna, Austria), all pertinent tests were two-sided, and an α threshold of 0.05 was used to define statistical significance.
Results
Literature Search
The systematic review identified 104 candidate citations of which 18 underwent full-text review. Twelve studies met criteria for inclusion in the descriptive analysis, six of these studies also had accessible by-patient data and were correspondingly included in the quantitative meta-analysis ( Tables 1 and 2 ).
Descriptive Analysis
A total of 12 studies were identified as reporting a specific analysis of K i -67 index as a predictor of clinical outcomes after resection for VS. 2 9 10 11 12 13 14 15 16 17 18 19 20 Among these studies, six specifically assessed the relationship between K i -67 index and tumor recurrence/progression, with five identifying a statistically significant relationship between increased K i -67 positivity and treatment failure. Another five studies studied a variety of primary endpoints in relation to K i -67 indices, including preoperative tumor size, preoperative tumor growth rate, and preoperative symptoms. One study assessed the relationship between K i -67 staining and patient age at the time of resection. We were able to gather individual patient-level data for 349 patients from six studies ( Table 2 ). 2 12 13 17 18 20 The range of K i -67 index values for patients with recurrence was 1.4 to 4.8%, whereas the range for patients without recurrence was 0.6 to 1.9%.
Meta-analysis
Six studies quantitatively analyzed the relationship between K i -67 index and postoperative VS recurrence/progression. Individual patient data were collected from these studies for calculation of discrete study effect sizes, pooling via random-effects modeling with restricted maximum likelihood and meta-analysis. The standardized mean difference in K i -67 indices between those with and without recurrence was calculated as 0.79% (95% confidence interval [CI]: 0.28–1.30; p = 0.0026; Fig. 2 ).
Fig. 2.
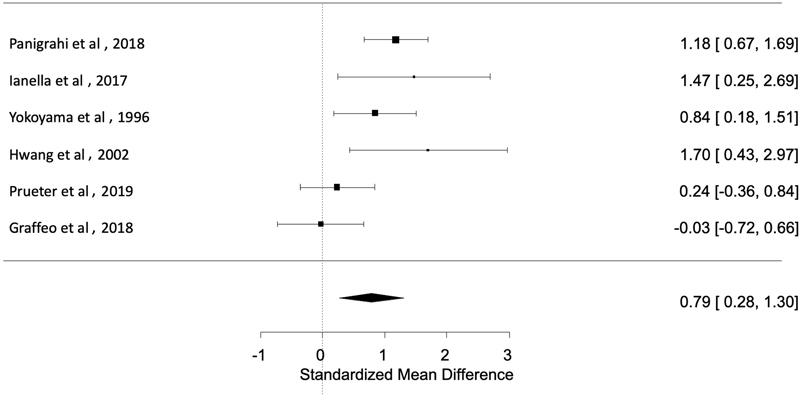
Forest plot of six studies showing standardized mean difference in K i -67 index between those with and without recurrence was calculated as 0.79 (95% CI: 0.28–1.30; p = 0.0026). CI, confidence interval.
Heterogeneity and Sensitivity Analysis
The Cochran Q test demonstrated significant heterogeneity between the incorporated studies ( p = 0.01). Due to the relatively high expected and observed heterogeneity, we proceeded with a sensitivity analysis in which the primary meta-analysis was repeated after removing the two studies with the lowest precision. 2 13 In this iteration, the standardized mean difference retained statistically significance at a value of 0.58% (95% CI: 0.03–1.13; p = 0.04; Fig. 3 ).
Fig. 3.
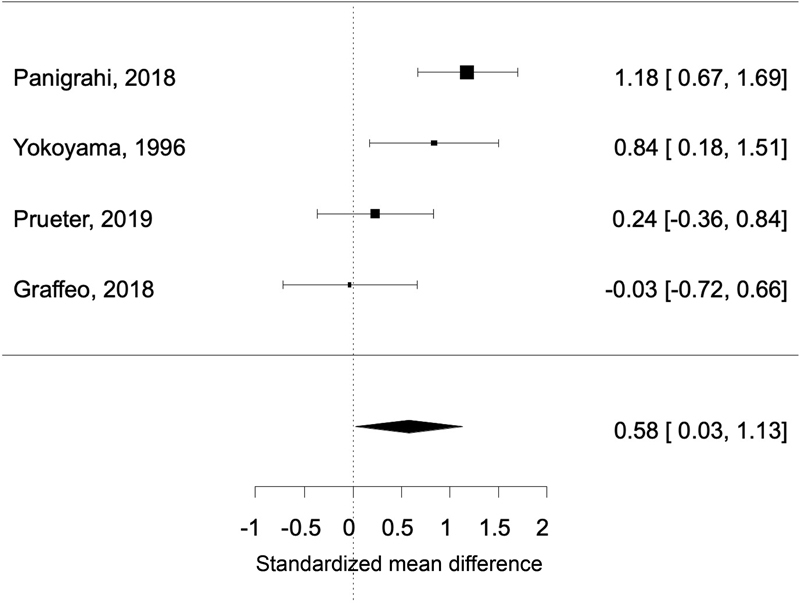
Forest plot of four studies (after removing two studies for low precision) showing standardized mean difference retained statistical significance at a value of 0.58 (95% CI: 0.03–1.13; p = 0.04). CI, confidence interval.
Discussion
We report the first systematic review and meta-analysis of K i -67 index as a predictor of tumor control outcomes after primary surgical resection for sporadic VS. Our results indicate that an elevated K i -67 index appears to be associated with increased risk of recurrence/progression after surgery, a finding that is reproduced throughout the majority of the preceding literature on VS, as well as several parallel disease processes, including meningioma. 4 5 6 21 Because of the heterogeneity of the datasets, generating a standard deviation and creating ranges that are sufficient to guide clinical practice is limited. With the generated K i -67 index values based on calculated ranges from these patients with and without recurrence, we believe that patients with a K i -67 index of <1.4% have a lower chance of recurrence. On the other hand, patients with a K i -67 index of >1.9% are associated with higher risk of recurrence which may warrant more aggressive serial imaging and adjuvant therapy. Patients with K i -67 index values that fall between 1.4 and 1.9% remain in the gray zone and may pose a continued management challenge. We anticipate that these findings will help inform several key aspects of patient care and counseling, while simultaneously providing key insights that may guide impactful translational studies in the future.
Stereotactic Radiosurgery in Management of Vestibular Schwannoma Recurrence/Progression
Considerable controversy exists in the literature regarding the differential influence of proactive versus delayed SRS following recurrence/progression after primary microsurgery, with some studies indicating that early treatment may be associated with durable tumor control. 22 23 24 By contrast, others suggest equivalent outcomes if treatment is withheld until serial tumor growth is confirmed, particularly for patients who have preserved hearing after surgery, or those with significant facial weakness. 25 26 Correspondingly, a critical role for potential prognostic parameters such as K i -67 index in the VS treatment paradigm would be the identification of candidate patients for up-front SRS or individuals who may warrant closer surveillance if early SRS is deferred.
K i -67 Index and the Role for Prognostication in Vestibular Schwannoma Management
K i -67 is a nuclear protein expressed in actively proliferating cells of vertebrates, 27 and the K i -67 index (also known as mitotic index) is used to quantify the percentage of mitotic activity by counting the number of K i -67-positive tumor cells in a 10 high-power field under the microscope. 28 Although nonspecific, the K i -67 index may be a promising prognostic factor in VS management for several reasons. Principally, the K i -67 antigen and associated index calculations have been validated for several related disease processes—most importantly, meningioma—as well as across numerous institutions and patient populations that lend robustness to the generalization of its utility. 2 9 10 12 14 15 16 17 18 19 20 29 Additionally, phenotypically aggressive disease behavior has been associated with increased K i -67 staining in several other central nervous system neoplasms, such as dural infiltration and associated disease recurrence in pituitary adenomas. 30
Within the domain of VS, Lesser et al reported a small cohort study in which tumor growth rates were categorized as very slow (0.02 cm/year), slow (0.2 cm/year), or rapid (1.0 cm/year) which were differentially associated with increasing levels of K i -67 positivity. 15 This preliminary analysis was compromised by a small sample size and a limited focus on preoperative growth rates rather than postoperative outcomes, restricting its clinical impact. However, based on the congruency with findings of the current study, these early results now provide additional motivation for the on-going analysis of K i -67 index as a predictor of outcome after VS resection.
Other groups looked at histopathological markers other than K i -67 where the role of the inflammatory microenvironment in VS growth appears to be significant. 31 Graffeo et al and Kontorinis et al showed that there are increased immune cells in VSs that have more rapid growth. 12 29 Graffeo et al demonstrated that tumors with higher S100 and CD68 macrophages tended to have a more aggressive growth pattern after STR. 12 These results have been recently validated by Lewis et al in a combined neuroimaging and pathology study where macrophages, rather than tumor cells, constituted most of the proliferating cells in growing VSs. 32 Kontorinis et al showed that an increased neutrophil-to-lymphocyte ratio is predictive of recurrence. 29 Even with other immune markers that may be good surrogates, these data are hard to interpret due to limited sample sizes and because these studies were performed only on patients with STR, offering a potentially higher correlation to preoperative tumor growth instead of postoperative recurrence rates. In addition, unlike meningiomas that have a homogenous cell population, VS can have more inflammatory cells than tumor cells which can affect the K i -67 indices of tumor cells per se and their relevance. Despite the heterogeneous cell populations of VS, however, it appears that a higher K i -67 index is correlated to a higher chance of recurrence.
Strengths and Limitations
The current study is the first formal systematic review and meta-analysis to address the question of K i -67 as a predictor of recurrence/progression after primary resection for sporadic VS, rendering it an important contribution to the shared knowledge base on this relatively prevalent disease and the need to identify quantifiable factors that can predict VS behavior. Our methodology is compliant with the PRISMA guidelines, based on a PICOS-formatted clinical question, and incorporates both heterogeneity and sensitivity analyses, lending reproducibility and robustness to the results. Further, we were able to ascertain individual patient-level K i -67 indices and clinical outcomes for six studies representing a total of 349 unique patients, markedly improving our ability to provide an objective analysis of the available data.
By token, a range of important limitations influence our interpretation of the current study, including those pertinent to any systematic review or meta-analysis of observational studies, as well as several specific to our methodological decisions. The K i -67 index, although well studied and widely available, is frequently assessed by manual means, resulting in substantial risk of bias, systematic error, and interobserver variability. 33 34 35 36 37 In some centers, automation has resulted in improved reliability and would be optimal for any clinical implementation of our results, particularly given that findings from such studies have almost universally reproduced those based on manual assessments of the K i -67 index. 21 38 39
As the current study is a meta-analysis, the quality, certainty, and reliability of the results by definition cannot exceed those of the included studies; these features are universally low in the included studies, due to the nature of observational studies with small samples and inconsistent methodologies. These same factors contribute to the high heterogeneity and wide confidence intervals, as we observed, which highlight the need for a guarded interpretation of our results. Although we were able to obtain K i -67 indices and outcome data for individual patients in six studies, those outcomes were almost universally captured and reported as binary parameters, rather than time-to-event data which would have allowed for a much more robust analysis including the calculation of hazard ratios, rather than simple mean differences. Similarly, other clinically relevant parameters were inconsistently reported; when combined with the small sample size and low event rate, this precluded the possibility of an adjusted, multivariable pooled analysis.
Conclusion
We report a novel meta-analysis of K i -67 index as a potential predictor of recurrence/progression after primary resection for sporadic VS. These results accord with much of the preceding literature on both meningioma and VS; however, the data are broadly equivocal, resulting in a highly qualified interpretation regarding the meaningful clinical utility of K i -67 index for VS patients. The current analysis indicates that high K i -67 index (>1.9%) may be associated with an increased risk of VS recurrence or progression following surgical resection, a clinically quantifiable factor that may impact the decision-making of whether adjuvant therapy or closer observation should be pursued. Patient counseling may similarly be informed by K i -67 index results after VS resection, provided that interpretation of the clinical data are made cautiously, and with appropriate patient education measures by their neurosurgical care team. More importantly, we recommend strong consideration for future studies of the K i -67 index in a large scale, prospective, and systematic fashion to more definitively determine its role in VS management and potentially look for correlations with various VS subtypes (cystic or solid).
Appendix 1 Actual search strategies
OVID
Database(s): Ovid MEDLINE(R) 1946 to Present and Epub Ahead of Print, In-Process and Other Non-Indexed Citations and Ovid MEDLINE(R) Daily, EBM Reviews - Cochrane Central Register of Controlled Trials December 2020, EBM Reviews—Cochrane Database of Systematic Reviews 2005 to December 22, 2020, Embase 1974 to 2020 December 21
Search Strategy:
| No. | Searches |
|---|---|
| 1 | exp Neuroma, Acoustic/ |
| 2 | ((acoustic or vestibular or acusticus or auditory or ear) adj1 (schwannoma* or neuroma* or neurinoma* or neurofibroma*)).ti,ab,hw,kw. |
| 3 | (“acoustic nerve” adj1 (cancer or tumor or tumor)).ti,ab,hw,kw. |
| 4 | (neurofibromatosis adj1 “2”).ti,ab,hw,kw. |
| 5 | or/1–4 |
| 6 | K i -67 Antigen/ |
| 7 | “ki-67.”ti,ab,hw,kw. |
| 8 | 6 or 7 |
| 9 | 5 and 8 |
| 10 | 9 not ((exp animals/ or exp nonhuman/) not exp humans/) |
| 11 | limit 10 to english language [Limit not valid in CDSR; records were retained] |
| 12 | (conference abstract or conference review or editorial or erratum or note or addresses or autobiography or bibliography or biography or blogs or comment or dictionary or directory or interactive tutorial or interview or lectures or legal cases or legislation or news or newspaper article or patient education handout or periodical index or portraits or published erratum or video-audio media or webcasts).mp. or conference abstract.st. |
| 13 | 11 not 12 |
| 14 | remove duplicates from 13 |
SCOPUS
| 1 | TITLE-ABS-KEY ((acoustic or vestibular or acusticus or auditory or ear) w/1 (schwannoma* or neuroma* or neurinoma* or neurofibroma*)) |
| 2 | TITLE-ABS-KEY (“acoustic nerve” w/1 (cancer or tumor or tumor)) |
| 3 | TITLE-ABS-KEY (neurofibromatosis w/1 2) |
| 4 | 1 or 2 or 3 |
| 5 | “ki-67” |
| 6 | 4 and 5 |
| 7 | INDEX(embase) OR INDEX(medline) OR PMID(0* OR 1* OR 2* OR 3* OR 4* OR 5* OR 6* OR 7* OR 8* OR 9*) |
| 8 | 6 not 7 |
| 9 | DOCTYPE(ed) OR DOCTYPE(bk) OR DOCTYPE(er) OR DOCTYPE(no) OR DOCTYPE(sh) OR DOCTYPE(ch) |
| 10 | 8 not 9 |
| 11 | LANGUAGE(english) |
| 12 | 10 and 11 |
Appendix 2 Quality assessment by modified Newcastle–Ottawa scale
This scale assesses articles based on a predefined set of five criteria listed below modified from the original Newcastle-Ottawa Scale (NOS) assessment for noncomparative case series which constituted all studies included in this meta-analysis. The items are scored in response to each question as either 1 in the positive, and 0 in the negative, to lead to a maximum score of 5. Overall quality was assessed based on impression of criteria scoring (low, 0–2; moderate, 3–4; and high, 5).
Question 1. Did the patient(s) represent the whole case(s) of the medical center? Cases included represented the general population of walled-of-necrosis; question 2: was the diagnosis correctly made? Based on the revised Atlanta criteria; question 3: was follow-up long enough for outcomes to occur? Reported adequate follow-up time; question 4: were all important data cited in the report? Reported resolution and at least two outcomes; question 5: was the outcome correctly ascertained? Provided definition of resolution. (d) Not available.
Criteria
Did the patient(s) represent the whole case(s) of the medical center?
Was the correct diagnosis made?
Was follow-up long enough for outcomes to occur?
Were all important data cited in the report?
Was the outcome correctly ascertained?
| Criterion | |||||||
|---|---|---|---|---|---|---|---|
| Study (year) | 1 | 2 | 3 | 4 | 5 | Total | Overall quality |
| Prueter et al (2019) 18 | 1 | 1 | 1 | 1 | 1 | 5 | High |
| Graffeo et al (2018) 12 | 0 | 1 | 1 | 1 | 1 | 4 | Moderate |
| Panigrahi et al (2018) 17 | 1 | 1 | 0 | 1 | 1 | 4 | Moderate |
| Iannella et al (2017) 2 | 1 | 1 | 0 | 1 | 1 | 4 | Moderate |
| Hwang et al (2002) 13 | 1 | 1 | 1 | 0 | 1 | 4 | Moderate |
| Yokoyama et al (1996) 20 | 1 | 1 | 0 | 0 | 1 | 4 | Moderate |
Acknowledgments
We thank Leslie Hassett, MLS, for performing a comprehensive search to allow for a thorough literature review.
Footnotes
Conflict of Interest None declared.
References
- 1.Nakatomi H, Jacob J T, Carlson M L.Long-term risk of recurrence and regrowth after gross-total and subtotal resection of sporadic vestibular schwannoma J Neurosurg 2017. May 19;1–7. [DOI] [PubMed] [Google Scholar]
- 2.Iannella G, de Vincentiis M, Di Gioia C. Subtotal resection of vestibular schwannoma: evaluation with Ki-67 measurement, magnetic resonance imaging, and long-term observation. J Int Med Res. 2017;45(03):1061–1073. doi: 10.1177/0300060516686873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacob J T, Carlson M L, Driscoll C L, Link M J. Volumetric analysis of tumor control following subtotal and near-total resection of vestibular schwannoma. Laryngoscope. 2016;126(08):1877–1882. doi: 10.1002/lary.25779. [DOI] [PubMed] [Google Scholar]
- 4.Choi Y, Lim D H, Yu J I. Prognostic value of Ki-67 labeling index and postoperative radiotherapy in WHO grade II meningioma. Am J Clin Oncol. 2018;41(01):18–23. doi: 10.1097/COC.0000000000000224. [DOI] [PubMed] [Google Scholar]
- 5.Liu N, Song S Y, Jiang J B, Wang T J, Yan C X. The prognostic role of Ki-67/MIB-1 in meningioma: a systematic review with meta-analysis. Medicine (Baltimore) 2020;99(09):e18644. doi: 10.1097/MD.0000000000018644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mirian C, Skyrman S, Bartek J., Jr. The Ki-67 proliferation index as a marker of time to recurrence in intracranial meningioma. Neurosurgery. 2020;87(06):1289–1298. doi: 10.1093/neuros/nyaa226. [DOI] [PubMed] [Google Scholar]
- 7.Page M J, McKenzie J E, Bossuyt P M. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372(71):n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barili F, Parolari A, Kappetein P A, Freemantle N. Statistical primer: heterogeneity, random- or fixed-effects model analyses? Interact Cardiovasc Thorac Surg. 2018;27(03):317–321. doi: 10.1093/icvts/ivy163. [DOI] [PubMed] [Google Scholar]
- 9.Cafer S, Bayramoglu I, Uzum N, Yilmaz M, Memis L, Uygur K. Expression and clinical significance of Ki-67, oestrogen and progesterone receptors in acoustic neuroma. J Laryngol Otol. 2008;122(02):125–127. doi: 10.1017/S0022215107000229. [DOI] [PubMed] [Google Scholar]
- 10.Charabi S, Engel P, Jacobsen G K, Tos M, Thomsen J. Growth rate of acoustic neuroma expressed by Ki-67 nuclear antigen versus symptom duration. Ann Otol Rhinol Laryngol. 1993;102(10):805–809. doi: 10.1177/000348949310201013. [DOI] [PubMed] [Google Scholar]
- 11.Gomez-Brouchet A, Delisle M B, Cognard C. Vestibular schwannomas: correlations between magnetic resonance imaging and histopathologic appearance. Otol Neurotol. 2001;22(01):79–86. doi: 10.1097/00129492-200101000-00016. [DOI] [PubMed] [Google Scholar]
- 12.Graffeo C S, Perry A, Raghunathan A. Macrophage density predicts facial nerve outcome and tumor growth after subtotal resection of vestibular schwannoma. J Neurol Surg B Skull Base. 2018;79(05):482–488. doi: 10.1055/s-0038-1627474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hwang S K, Kim D G, Paek S H.Aggressive vestibular schwannomas with postoperative rapid growth: clinicopathological analysis of 15 cases Neurosurgery 200251061381–1390., discussion 1390–1391 [PubMed] [Google Scholar]
- 14.Jabbour J, Earls P, Biggs N, Gracie G, Fagan P, Bova R. Role of cyclins D1 and D3 in vestibular schwannoma. J Laryngol Otol. 2016;130 01:S2–S10. doi: 10.1017/S0022215115001735. [DOI] [PubMed] [Google Scholar]
- 15.Lesser T H, Janzer R C, Kleihues P, Fisch U. Clinical growth rate of acoustic schwannomas: correlation with the growth fraction as defined by the monoclonal antibody ki-67. Skull Base Surg. 1991;1(01):11–15. doi: 10.1055/s-2008-1056973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niemczyk K, Vaneecloo F M, Lecomte M H. Correlation between Ki-67 index and some clinical aspects of acoustic neuromas (vestibular schwannomas) Otolaryngol Head Neck Surg. 2000;123(06):779–783. doi: 10.1067/mhn.2000.111356. [DOI] [PubMed] [Google Scholar]
- 17.Panigrahi M, Kumar D, Vooturi S, Madigubba S. MIB index as predictor of recurrence in sporadic vestibular schwannomas. World Neurosurg. 2018;120:e1203–e1207. doi: 10.1016/j.wneu.2018.09.039. [DOI] [PubMed] [Google Scholar]
- 18.Prueter J, Norvell D, Backous D. Ki-67 index as a predictor of vestibular schwannoma regrowth or recurrence. J Laryngol Otol. 2019;133(03):205–207. doi: 10.1017/S0022215119000549. [DOI] [PubMed] [Google Scholar]
- 19.Steinhart H, Wigand M E, Fahlbusch R, Triebswetter F, Gress H, Iro H. Facial nerve schwannoma in the inner auditory canal and geniculate ganglion [in German] HNO. 2003;51(08):640–645. doi: 10.1007/s00106-002-0788-4. [DOI] [PubMed] [Google Scholar]
- 20.Yokoyama M, Matsuda M, Nakasu S, Nakajima M, Handa J.Clinical significance of Ki-67 staining index in acoustic neurinoma Neurol Med Chir (Tokyo) 19963610698–702., discussion 702–703 [DOI] [PubMed] [Google Scholar]
- 21.Kim Y J, Romeike B F, Uszkoreit J, Feiden W. Automated nuclear segmentation in the determination of the Ki-67 labeling index in meningiomas. Clin Neuropathol. 2006;25(02):67–73. [PubMed] [Google Scholar]
- 22.Breivik C N, Varughese J K, Wentzel-Larsen T, Vassbotn F, Lund-Johansen M.Conservative management of vestibular schwannoma–a prospective cohort study: treatment, symptoms, and quality of life Neurosurgery 201270051072–1080., discussion 1080 [DOI] [PubMed] [Google Scholar]
- 23.Breshears J D, Chang J, Molinaro A M. Temporal dynamics of pseudoprogression after gamma knife radiosurgery for vestibular schwannomas-a retrospective volumetric study. Neurosurgery. 2019;84(01):123–131. doi: 10.1093/neuros/nyy019. [DOI] [PubMed] [Google Scholar]
- 24.Myrseth E, Møller P, Pedersen P H, Lund-Johansen M.Vestibular schwannoma: surgery or gamma knife radiosurgery? A prospective, nonrandomized study Neurosurgery 20096404654–661., discussion 661–663 [DOI] [PubMed] [Google Scholar]
- 25.Jacob J T, Pollock B E, Carlson M L, Driscoll C L, Link M J. Stereotactic radiosurgery in the management of vestibular schwannoma and glomus jugulare: indications, techniques, and results. Otolaryngol Clin North Am. 2015;48(03):515–526. doi: 10.1016/j.otc.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 26.Carlson M L, Jacob J T, Pollock B E. Long-term hearing outcomes following stereotactic radiosurgery for vestibular schwannoma: patterns of hearing loss and variables influencing audiometric decline. J Neurosurg. 2013;118(03):579–587. doi: 10.3171/2012.9.JNS12919. [DOI] [PubMed] [Google Scholar]
- 27.Gerdes J, Schwab U, Lemke H, Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983;31(01):13–20. doi: 10.1002/ijc.2910310104. [DOI] [PubMed] [Google Scholar]
- 28.Ueda T, Aozasa K, Tsujimoto M. Prognostic significance of Ki-67 reactivity in soft tissue sarcomas. Cancer. 1989;63(08):1607–1611. doi: 10.1002/1097-0142(19890415)63:8<1607::aid-cncr2820630827>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 29.Kontorinis G, Crowther J A, Iliodromiti S, Taylor W A, Locke R. Neutrophil to lymphocyte ratio as a predictive marker of vestibular schwannoma growth. Otol Neurotol. 2016;37(05):580–585. doi: 10.1097/MAO.0000000000001026. [DOI] [PubMed] [Google Scholar]
- 30.Liu X, Feng M, Zhang Y. Expression of Matrix metalloproteinase-9, pituitary tumor transforming gene, high mobility group A 2, and Ki-67 in adrenocorticotropic hormone-secreting pituitary tumors and their association with tumor recurrence. World Neurosurg. 2018;113:e213–e221. doi: 10.1016/j.wneu.2018.01.214. [DOI] [PubMed] [Google Scholar]
- 31.Hannan C J, Lewis D, O'Leary C. The inflammatory microenvironment in vestibular schwannoma. Neurooncol Adv. 2020;2(01):a023. doi: 10.1093/noajnl/vdaa023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewis D, Roncaroli F, Agushi E. Inflammation and vascular permeability correlate with growth in sporadic vestibular schwannoma. Neuro-oncol. 2019;21(03):314–325. doi: 10.1093/neuonc/noy177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nielsen L AG, Bangsø J A, Lindahl K H. Evaluation of the proliferation marker Ki-67 in gliomas: Interobserver variability and digital quantification. Diagn Pathol. 2018;13(01):38. doi: 10.1186/s13000-018-0711-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vörös A, Csörgő E, Nyári T, Cserni G. An intra- and interobserver reproducibility analysis of the Ki-67 proliferation marker assessment on core biopsies of breast cancer patients and its potential clinical implications. Pathobiology. 2013;80(03):111–118. doi: 10.1159/000343795. [DOI] [PubMed] [Google Scholar]
- 35.Furukawa T, Ozaka M, Takamatsu M. Ki-67 labeling index variability between surgically resected primary and metastatic hepatic lesions of gastroenteropancreatic neuroendocrine neoplasms. Int J Surg Pathol. 2021;29(05):475–481. doi: 10.1177/1066896921990715. [DOI] [PubMed] [Google Scholar]
- 36.Korean Breast Pathology Ki-67 Study Group . Chung Y R, Jang M H, Park S Y, Gong G, Jung W H. Interobserver variability of Ki-67 measurement in breast cancer. J Pathol Transl Med. 2016;50(02):129–137. doi: 10.4132/jptm.2015.12.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamamoto S, Chishima T, Mastubara Y. Variability in measuring the Ki-67 labeling index in patients with breast cancer. Clin Breast Cancer. 2015;15(01):e35–e39. doi: 10.1016/j.clbc.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 38.Feng M, Deng Y, Yang L. Automated quantitative analysis of Ki-67 staining and HE images recognition and registration based on whole tissue sections in breast carcinoma. Diagn Pathol. 2020;15(01):65. doi: 10.1186/s13000-020-00957-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang H Y, Li Z W, Sun W. Automated quantification of Ki-67 index associates with pathologic grade of pulmonary neuroendocrine tumors. Chin Med J (Engl) 2019;132(05):551–561. doi: 10.1097/CM9.0000000000000109. [DOI] [PMC free article] [PubMed] [Google Scholar]


