Abstract
Purpose
Translocator protein (TSPO) positron emission tomography (PET) using 18F-GE-180 shows high tumor-to-brain contrast in high-grade glioma (HGG), even in areas without magnetic resonance imaging (MRI) contrast enhancement. Until now, the benefit of 18F-GE-180 PET in primary radiation therapy (RT) and reirradiation (reRT) treatment planning for patients with HGG has not been assessed.
Methods and Materials
The possible benefit of 18F-GE-180 PET in RT and reRT planning was retrospectively evaluated through post hoc spatial correlations of PET-based biological tumor volumes (BTVs) with conventional MRI-based consensus gross tumor volumes (cGTVs). To find the ideal threshold for BTV definition in RT and reRT treatment planning, tumor-to-background activity thresholds of 1.6, 1.8, and 2.0 were applied. Spatial overlap of PET- and MRI-based tumor volumes was measured by the Sørensen-Dice coefficient (SDC) and the conformity index (CI). Additionally, the minimal margin to include the entire BTV into the expanded cGTV was determined.
Results
Thirty-five primary RT and 16 reRT cases were examined. BTV1.6, BTV1.8, and BTV2.0 were significantly larger than corresponding cGTV volumes in primary RT (median volumes: 67.4, 50.7, and 39.1, respectively, vs 22.6 cm3; P < .001, P < .001, and P = .017, respectively; Wilcoxon test) and reRT cases (median volumes: 80.5, 55.0, and 41.6, respectively, vs 22.7 cm3; P = .001, P = .005, and P = .144, respectively; Wilcoxon test). BTV1.6, BTV1.8, and BTV2.0 showed low but increasing conformity with cGTVs in the primary RT (SDC: 0.51, 0.55, and 0.58, respectively; CI: 0.35, 0.38, and 0.41, respectively) and reRT setting (SDC: 0.38, 0.40, and 0.40, respectively; CI: 0.24, 0.25, and 0.25, respectively). The minimal margin required to include the BTV within the cGTV was significantly smaller in the RT versus the reRT setting for thresholds 1.6 and 1.8 but not significantly different for threshold 2.0 (median margin: 16, 12, and 10, respectively, vs 21.5, 17.5, and 13 mm, respectively; P = .007, P = .031, and P = .093, respectively; Mann-Whitney U test).
Conclusions
18F-GE-180 PET provides valuable information in RT treatment planning for patients with HGG. 18F-GE-180-based BTVs with a threshold of 2.0 were most consistent in primary and reRT.
Introduction
In previous studies, the mitochondrial translocator protein (TSPO) tracer 18F-GE-180 has shown promising results in the visualization of active glioma before radiation therapy (RT) and reirradiation (reRT).1, 2, 3 High tumor-to-brain contrast as well as uptake within and outside of contrast-enhancement on magnetic resonance imaging (MRI) make 18F-GE-180 positron emission tomography (PET) an interesting imaging modality for RT planning.1, 2, 3
To date, research on 18F-GE-180 PET has focused on the imaging properties of the tracer itself, such as the rate of detectable 18F-GE-180 tracer uptake in high- versus low-grade glioma or differences of 18F-GE-180 to 18F-FET tracer uptake.2 To the best of our knowledge, this is the first study focusing on the target volume delineation in high-grade glioma (HGG) treatment planning using 18F-GE-180 PET information additionally to conventional contrast-enhanced MRI. In the present planning study, we investigated the concordance of MRI-based gross tumor volumes (GTVs) with threshold-based biological tumor volumes (BTV) of 18F-GE-180 PET in RT and reRT planning of patients with primary and recurrent glioma.
Hereby, we aimed to gain knowledge of possible benefits of the novel 18F-GE-180 TSPO imaging for HGG treatment planning. Our special interest in this treatment planning study was to find out the most widely applicable threshold value for the BTVs in radiation treatment planning, as no preferred threshold value is defined in HGG 18F-GE-180 TSPO imaging as of yet.1, 2, 3 For this purpose, we assessed the degree of conformity of these standardized BTVs with consensus MRI-based GTVs in the RT and reRT setting and evaluated the margin needed to encompass the whole BTV within the consensus GTV.
Methods and Materials
Ethics approval
The retrospective assessment of radiation treatment plans was reviewed by the ethics committee of the medical faculty of LMU Munich and granted approval (reference no. 20-255).
Inclusion criteria
Treatment plans of patients with histologically verified high-grade intracranial glioma treated with RT in the primary setting or reRT at time of recurrence at the Department of Radiation Oncology, University Hospital, LMU Munich from August 2016 to February 2019 were included into the analysis. Only treatment plans of patients with a macroscopic tumor present in the RT treatment planning contrast-enhanced MRI were included. Radiation treatment plans of all patients, who met the inclusion criteria, were included in the study.
Histopathologic examinations
Integrated diagnosis was performed according to the World Health Organization (WHO) classification of central nervous system tumors of 2016.4 Mutational status of the isocitrate dehydrogenase 1 and 2 (IDH1/2) genes was available for all patients and methylation status of the O6-alkylguanine-DNA alkyltransferase (MGMT) promotor was available for all patients except 1.
Radiation treatment protocols
Primary RT was performed according to the European Society for Radiation Oncology–Advisory Committee for Radiation Oncology Practice guideline on target delineation of glioblastomas5 and reRT as described in detail in previous publications.6,7
In brief, in the primary RT setting, the GTV including all visible macroscopic tumor on contrast-enhanced MRI was encompassed by a 20-mm clinical target volume margin, extended by the surrounding edema and then adapted anatomically at the scull and falx cerebri. A 3-mm planning target volume margin was added to account for setup uncertainties. In the reRT setting, the GTV was also based on all visible macroscopic tumor on contrast-enhanced MRI and then encompassed by a 5-mm clinical target volume and a 3-mm planning target volume margin. For patients treated with a simultaneous integrated boost (SIB) in the reRT setting, the SIB volume was based on the GTV with a 3-mm margin.
Dose prescription was 60 Gy (2 Gy × 30)8 or 40.05 Gy (2.67 Gy × 15)9 for primary RT and 36 Gy (2 Gy × 18) with or without a SIB volume (2.4 Gy × 18)6,7 for reRT.
18F-GE-180 PET
18F-GE-180 PET images were obtained before primary RT and reRT at the Department of Nuclear Medicine, University Hospital, LMU Munich as described in detail previously.1 In brief, summation images were acquired on a Biograph 64 PET/computed tomography scanner (Siemens, Erlangen, Germany) beginning at 60 minutes and ending 80 minutes after intravenous injection of 18F-GE-180 with an activity of approximately 180 MBq.
MRI- and 18F-GE-180 TSPO PET-based target volume delineation
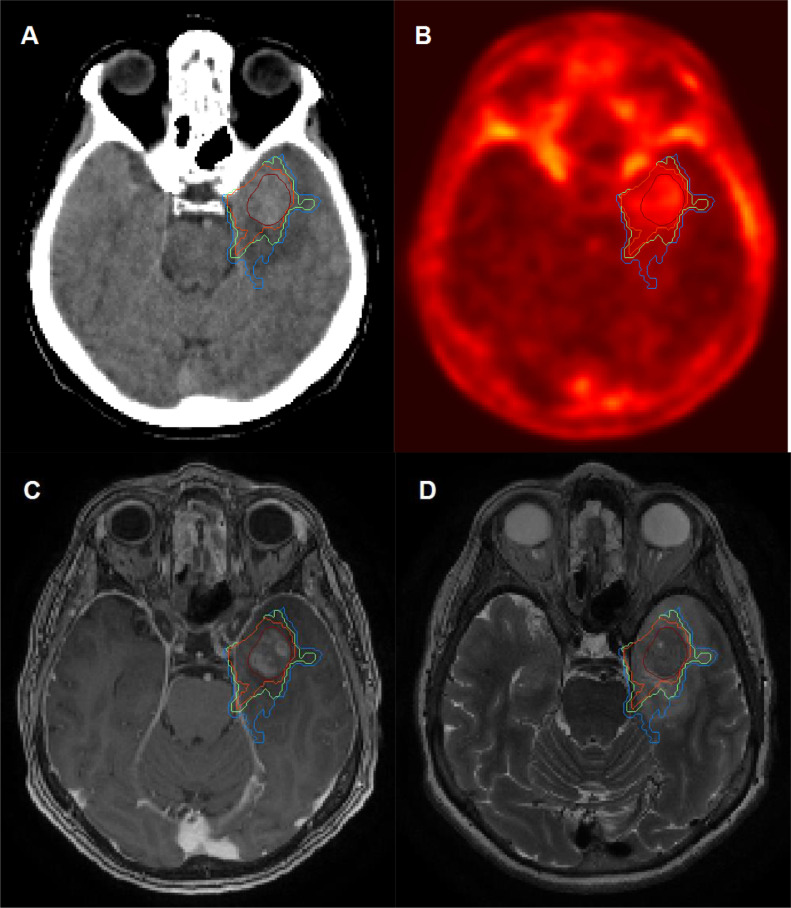
In the retrospective treatment planning analysis, MRI-based GTVs were independently delineated by 4 experienced radiation oncologists. An interrater consensus GTV (cGTV) structure was generated on basis of these 4 GTVs by using an implementation of the simultaneous truth and performance level estimation algorithm10 in the research treatment planning system Computational Environment for Radiotherapy Research11 within MATLAB (The MathWorks, Natick, MA). PET-based BTVs enclosed all areas exceeding the background activity, which was consistently assessed in the healthy brain tissue,12 by the factor 1.6, 1.8, and 2.0 (denoted as BTV1.6, BTV1.8, and BTV2.0, respectively) by guidance of a nuclear medicine expert. An example case is shown in Fig. 1.
Figure 1.
Example case. (A) Planning computed tomography imaging; (B) 18F-GE-180 translocator protein PET; (C) contrast-enhanced magnetic resonance imaging (MRI); and (D) T2 MRI with MRI-based consensus gross target volume in dark red and 18F-GE-180 PET-based biological tumor volumes with thresholds of 1.6 (blue), 1.8 (green), and 2.0 (orange). Abbreviation: PET = positron emission tomography.
Conformity assessment between MRI- and 18F-GE-180 TSPO PET-based target volumes
The Sørensen-Dice coefficient (SDC) was calculated by (2 × |cGTVK ∩ BTVx|) / (|cGTV|+|BTVx|)13 and the conformity index (CI) by (cGTV ∩ BTVx) / (cGTV ∪ BTVx)14 to assess the conformity between the cGTVs and the BTV1.6, BTV1.8, and BTV2.0 volumes.
For assessment of the minimal margin around the cGTVs required to include the BTV1.6, BTV1.8, and BTV2.0 volumes, margins between 1 and 20 mm were semiautomatically added to the cGTV in the Computational Environment for Radiotherapy Research platform for subsequent visual evaluation.
Statistics
The SPSS Statistics software package, version 25 (IBM, Armonk, NY), was used for descriptive and comparative statistical analysis. A Wilcoxon signed-rank test was used to compare the BTV1.6, BTV1.8, and BTV2.0 volumes with the cGTVs in the primary RT and reRT setting. A Mann-Whitney U test was applied to compare the BTV1.6, BTV1.8, and BTV2.0 volumes between the primary RT and the reRT setting. No correction for multiple testing was applied.
Results
Patient characteristics
Treatment plans of 51 patients (34 male, 17 female) with HGG treated with primary RT (n = 35) or reRT (n = 16) were analyzed. Median age of the patients was 62 years (range, 26-81) in the primary RT cohort and 55 years (range, 30-73) in the reRT cohort. WHO grade before RT was grade 3 in 8 (22.9%) and grade 4 in 27 (77.1%) cases. WHO grade before reRT was grade 3 in 1 (6.25%) and grade 4 in 15 (93.25%) cases. IDH1/2 mutation was present in 4 (11.4%) and 3 (18.75) cases, and MGMT promotor methylation was present in 15 (42.9%) and 10 (62.5%) cases, respectively.
Treatment at primary and recurrent treatment setting
Three of 35 patients underwent incomplete surgical resection before primary RT and 8 of 16 patients before reRT. Prescription of the primary RT was 60 Gy (2 Gy × 30) for 20 of 35 (51.7%) or 40.05 (2.67 Gy × 15) for 15 of 35 patients (42.9%) in the primary RT cohort. Primary RT prescriptions of the reRT cohort are listed in detail in Table 1. ReRT was prescribed with 43.2 Gy to a SIB volume (2.4 Gy × 18) and 36 Gy to an extended volume (2 Gy × 18) in 8 of 16 cases (50%) and 36 Gy (2 Gy × 18) in 8 of 16 cases (50%).
Table 1.
Patient characteristics
| All patients (N = 51) | Primary RT cohort (n = 35) | ReRT cohort (n = 16) | |
|---|---|---|---|
| Characteristic | No. (%) or median (range) | No. (%) or median (range) | No. (%) or median (range) |
| Sex | |||
| Male | 34 (66.7%) | 23 (65.7%) | 11 (68.75%) |
| Female | 17 (33.3%) | 12 (34.3%) | 5 (31.25%) |
| Age (y) | 61 (26-81) | 62 (26-81) | 55 (30-73) |
| Diagnosis before RT | |||
| Anaplastic astrocytoma | 10 (19.6%) | 8 (22.9%) | 2 (12.5%) |
| Glioblastoma | 41 (80.4%) | 27 (77.1%) | 14 (87.5%) |
| WHO grade before RT | |||
| WHO grade 3 | 10 (19.6%) | 8 (22.9%) | 2 (12.5%) |
| WHO grade 4 | 41 (80.4%) | 27 (77.1%) | 14 (87.5%) |
| WHO grade before reRT | |||
| WHO grade 3 | - | NA | 1 (6.25%) |
| WHO grade 4 | - | NA | 15 (93.25%) |
| IDH1/2 mutational status | |||
| IDH1/2 mutated | 7 (13.7%) | 4 (11.4%) | 3 (18.75%) |
| IDH1/2-wildtype | 44 (86.3%) | 31 (88.6%) | 13 (81.25%) |
| MGMT promotor status | |||
| MGMT methylated | 25 (49%) | 15 (42.9%) | 10 (62.5%) |
| MGMT unmethylated | 25 (49%) | 20 (57.1%) | 5 (31.25%) |
| Unknown | 1 (2%) | 0 | 1 (6.25%) |
| Neurosurgical intervention before primary RT | |||
| Resection | 11 (21.6%) | 3 (8.6%) | 8 (50%) |
| Stereotactical biopsy | 40 (78.4%) | 32 (91.4%) | 8 (50%) |
| Prescribed primary RT dose | |||
| 61.2 Gy (1.8 Gy × 34) | 1 (2%) | 0 | 1 (6.25%) |
| 60 Gy (2 Gy × 30) | 32 (62.7%) | 20 (57.1%) | 12 (75%) |
| 55 Gy (2.2 Gy × 25) | 1 (2%) | 0 | 1 (6.25%) |
| 40.05 Gy (2.67 Gy × 15) | 17 (33.3%) | 15 (42.9%) | 2 (12.5%) |
| Neurosurgical intervention before reRT | |||
| Resection | - | NA | 3 (18.75%) |
| Stereotactical biopsy | - | NA | 5 (31.25%) |
| None | - | NA | 8 (50%) |
| Prescribed reRT dose | |||
| 43.2 Gy (2.4 Gy × 18) | - | NA | 8 (50%) |
| 36 Gy (2 Gy × 18) | - | NA | 8 (50%) |
Abbreviations: IDH = isocitrate dehydrogenase; MGMT = O6-alkylguanine-DNA alkyltransferase; NA = not applicable; reRT = reirradiation; RT = radiation therapy; WHO = World Health Organization.
Patients were examined with GE-180 translocator protein positron emission tomography before RT or reRT.
Primary RT target volumes
Median cGTV volumes (22.6 cm3; range, 3-146.5) were smaller than the corresponding volumes of BTV1.6 (67.4 cm3; range, 1.1-180.9), BTV1.8 (50.7 cm3; range, 0.9-153.8), and BTV2.0 (39.1 cm3; range, 0-131.4) in the retrospective treatment planning analysis of the primary RT setting. The difference between the cGTV to the BTV volumes was statistically significant in the Wilcoxon test for BTV1.6, BTV1.8, and BTV2.0 (P < .001, P < .001, P = .017, respectively). See Table 2 for the target volumes of each case of the primary RT cohort.
Table 2.
Primary radiation therapy target volumes
| No. | BTV 1.6 | BTV 1.8 | BTV 2.0 | cGTV | SDC 1.6 | SDC 1.8 | SDC 2.0 | CI 1.6 | CI 1.8 | CI 2.0 | cGTV margin BTV 1.6 | cGTV margin BTV 1.8 | cGTV margin BTV 2.0 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 117.5 | 94.5 | 81.2 | 44.8 | 0.52 | 0.57 | 0.59 | 0.35 | 0.39 | 0.42 | 24 | 20 | 18 |
| 2 | 43.4 | 37.1 | 31.1 | 29.8 | 0.72 | 0.74 | 0.75 | 0.56 | 0.58 | 0.59 | 9 | 7 | 5 |
| 3 | 80.0 | 70.4 | 63.2 | 76.2 | 0.78 | 0.79 | 0.78 | 0.63 | 0.66 | 0.64 | 9 | 6 | 5 |
| 4 | 125.0 | 100.2 | 85.8 | 45.0 | 0.47 | 0.51 | 0.54 | 0.31 | 0.34 | 0.37 | 33 | 27 | 21 |
| 5 | 23.7 | 17.9 | 14.3 | 3.0 | 0.12 | 0.14 | 0.16 | 0.07 | 0.08 | 0.09 | 27 | 22 | 19 |
| 6 | 84.3 | 68.9 | 45.0 | 36.1 | 0.23 | 0.24 | 0.23 | 0.13 | 0.13 | 0.13 | 29 | 28 | 25 |
| 7 | 97.2 | 82.9 | 72.9 | 35.8 | 0.54 | 0.60 | 0.65 | 0.37 | 0.43 | 0.49 | 17 | 15 | 13 |
| 8 | 92.5 | 79.8 | 68.5 | 27.5 | 0.46 | 0.49 | 0.52 | 0.30 | 0.32 | 0.35 | 19 | 15 | 13 |
| 9 | 2.9 | 0.9 | 0.2 | 15.6 | 0.26 | 0.10 | 0.03 | 0.15 | 0.05 | 0.02 | 0 | 0 | 0 |
| 10 | 12.4 | 10.3 | 8.9 | 4.6 | 0.35 | 0.36 | 0.35 | 0.21 | 0.22 | 0.21 | 10 | 10 | 9 |
| 11 | 1.1 | 0.9 | 0.0 | 4.9 | 0.28 | 0.24 | 0.01 | 0.16 | 0.14 | 0.00 | 0 | 0 | 0 |
| 12 | 149.7 | 111.7 | 86.1 | 63.4 | 0.31 | 0.32 | 0.35 | 0.19 | 0.19 | 0.21 | 22 | 20 | 18 |
| 13 | 19.0 | 13.9 | 10.3 | 3.8 | 0.33 | 0.42 | 0.52 | 0.20 | 0.27 | 0.35 | 19 | 11 | 8 |
| 14 | 9.9 | 8.2 | 6.6 | 10.8 | 0.74 | 0.72 | 0.69 | 0.59 | 0.56 | 0.52 | 4 | 3 | 2 |
| 15 | 172.3 | 139.9 | 112.3 | 125.7 | 0.70 | 0.70 | 0.68 | 0.54 | 0.54 | 0.52 | 14 | 11 | 8 |
| 16 | 115.7 | 97.5 | 84.0 | 74.1 | 0.69 | 0.70 | 0.69 | 0.53 | 0.53 | 0.53 | 27 | 19 | 17 |
| 17 | 40.2 | 30.7 | 24.1 | 40.1 | 0.70 | 0.68 | 0.64 | 0.54 | 0.51 | 0.47 | 12 | 8 | 7 |
| 18 | 18.0 | 10.2 | 6.4 | 45.5 | 0.50 | 0.35 | 0.24 | 0.33 | 0.21 | 0.14 | 6 | 0 | 0 |
| 19 | 44.2 | 33.4 | 28.3 | 20.6 | 0.61 | 0.70 | 0.73 | 0.44 | 0.54 | 0.58 | 19 | 9 | 7 |
| 20 | 16.1 | 12.1 | 8.9 | 21.6 | 0.59 | 0.55 | 0.48 | 0.42 | 0.38 | 0.32 | 6 | 4 | 3 |
| 21 | 68.7 | 50.7 | 36.9 | 12.8 | 0.31 | 0.40 | 0.51 | 0.19 | 0.25 | 0.34 | 32 | 28 | 22 |
| 22 | 35.5 | 31.2 | 27.1 | 16.9 | 0.64 | 0.70 | 0.73 | 0.47 | 0.54 | 0.57 | 10 | 8 | 7 |
| 23 | 51.5 | 43.3 | 36.6 | 21.8 | 0.59 | 0.66 | 0.69 | 0.42 | 0.49 | 0.53 | 12 | 9 | 8 |
| 24 | 13.4 | 9.0 | 5.9 | 9.4 | 0.56 | 0.60 | 0.59 | 0.39 | 0.43 | 0.42 | 11 | 9 | 3 |
| 25 | 69.2 | 63.4 | 58.9 | 16.5 | 0.38 | 0.41 | 0.43 | 0.23 | 0.25 | 0.27 | 19 | 17 | 16 |
| 26 | 104.5 | 91.1 | 79.9 | 21.8 | 0.34 | 0.38 | 0.42 | 0.21 | 0.24 | 0.27 | 20 | 19 | 18 |
| 27 | 76.2 | 64.4 | 54.0 | 75.8 | 0.79 | 0.77 | 0.74 | 0.65 | 0.63 | 0.59 | 7 | 6 | 5 |
| 28 | 51.6 | 44.3 | 39.1 | 8.3 | 0.28 | 0.32 | 0.35 | 0.16 | 0.19 | 0.21 | 19 | 17 | 16 |
| 29 | 81.7 | 69.0 | 51.5 | 29.9 | 0.48 | 0.52 | 0.49 | 0.31 | 0.35 | 0.32 | 18 | 17 | 17 |
| 30 | 26.0 | 21.4 | 18.3 | 7.0 | 0.42 | 0.48 | 0.54 | 0.27 | 0.32 | 0.37 | 16 | 13 | 11 |
| 31 | 48.3 | 41.0 | 34.9 | 20.2 | 0.51 | 0.57 | 0.63 | 0.35 | 0.40 | 0.46 | 15 | 13 | 10 |
| 32 | 89.1 | 77.5 | 68.1 | 36.7 | 0.58 | 0.63 | 0.68 | 0.41 | 0.46 | 0.52 | 13 | 12 | 11 |
| 33 | 67.4 | 53.7 | 44.2 | 22.6 | 0.49 | 0.55 | 0.58 | 0.32 | 0.38 | 0.41 | 18 | 16 | 15 |
| 34 | 77.8 | 68.9 | 61.2 | 52.0 | 0.79 | 0.83 | 0.84 | 0.65 | 0.72 | 0.73 | 9 | 7 | 6 |
| 35 | 180.9 | 153.8 | 131.4 | 146.5 | 0.63 | 0.62 | 0.59 | 0.46 | 0.44 | 0.42 | 17 | 14 | 12 |
| Median | 67.4 | 50.7 | 39.1 | 22.6 | 0.51 | 0.55 | 0.58 | 0.35 | 0.38 | 0.41 | 16 | 12 | 10 |
Abbreviations: BTV = biological tumor volume; CI = conformity index; cGTV = consensus gross tumor volume; SDC = sørensen-dice coefficient.
BTVs with thresholds 1.6, 1.8, and 2.0, cGTV, SDC and CI for BTVs with cGTV, and margin to include the whole BTV into the cGTV.
ReRT target volumes
Median cGTV volumes (22.7 cm3; range, 6.4-94.6) were also smaller than the corresponding volumes of BTV1.6 (80.5 cm3; range, 7.8-369.5), BTV1.8 (55.0 cm3; range, 6.6-128.9), and BTV2.0 (41.6 cm3; range, 4.3-101) in the retrospective treatment planning analysis of the reRT setting. The difference between the cGTV to the BTV volumes was statistically significant in the Wilcoxon test for BTV1.6 and BTV1.8 but not for BTV2.0 (P = .001, P = .005, P = .144, respectively). See Table 3 for the target volumes of each case of the reRT cohort.
Table 3.
Reirradiation target volumes
| No. | BTV 1.6 | BTV 1.8 | BTV 2.0 | cGTV | SDC 1.6 | SDC 1.8 | SDC 2.0 | CI 1.6 | CI 1.8 | CI 2.0 | cGTV margin BTV1.6 | cGTV margin BTV1.8 | cGTV margin BTV2.0 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 99.4 | 75.1 | 55.3 | 27.9 | 0.41 | 0.49 | 0.57 | 0.25 | 0.32 | 0.40 | 27 | 25 | 20 |
| 2 | 89.7 | 70.5 | 54.9 | 62.8 | 0.36 | 0.36 | 0.35 | 0.22 | 0.22 | 0.21 | 14 | 12 | 10 |
| 3 | 36.1 | 25.2 | 17.8 | 14.9 | 0.50 | 0.56 | 0.61 | 0.33 | 0.39 | 0.43 | 36 | 35 | 33 |
| 4 | 65.5 | 43.8 | 31.3 | 44.7 | 0.48 | 0.47 | 0.45 | 0.31 | 0.31 | 0.29 | 19 | 14 | 10 |
| 5 | 134.2 | 102.4 | 79.6 | 19.3 | 0.20 | 0.22 | 0.23 | 0.11 | 0.12 | 0.13 | 47 | 43 | 40 |
| 6 | 7.8 | 6.6 | 5.2 | 7.6 | 0.63 | 0.62 | 0.60 | 0.46 | 0.45 | 0.43 | 6 | 5 | 3 |
| 7 | 74.6 | 41.2 | 29.0 | 7.6 | 0.15 | 0.21 | 0.23 | 0.08 | 0.12 | 0.13 | >50 | >50 | >50 |
| 8 | 143.9 | 116.1 | 88.7 | 28.8 | 0.26 | 0.23 | 0.22 | 0.15 | 0.13 | 0.12 | 43 | 41 | 37 |
| 9 | 86.4 | 66.1 | 50.0 | 26.1 | 0.42 | 0.50 | 0.56 | 0.27 | 0.33 | 0.39 | 18 | 16 | 11 |
| 10 | 98.6 | 81.5 | 66.8 | 18.8 | 0.29 | 0.34 | 0.40 | 0.17 | 0.21 | 0.25 | 31 | 29 | 26 |
| 11 | 55.7 | 42.9 | 33.2 | 29.7 | 0.66 | 0.71 | 0.73 | 0.49 | 0.55 | 0.57 | 13 | 10 | 8 |
| 12 | 369.5 | 109.0 | 73.7 | 13.8 | 0.06 | 0.20 | 0.27 | 0.03 | 0.11 | 0.16 | >50 | >50 | 43 |
| 13 | 23.6 | 13.0 | 8.4 | 12.7 | 0.40 | 0.44 | 0.40 | 0.25 | 0.28 | 0.25 | 21 | 8 | 6 |
| 14 | 22.3 | 10.8 | 4.3 | 6.4 | 0.26 | 0.29 | 0.25 | 0.15 | 0.17 | 0.15 | 14 | 9 | 6 |
| 15 | 57.6 | 24.5 | 6.7 | 67.0 | 0.36 | 0.30 | 0.13 | 0.22 | 0.18 | 0.07 | 18 | 9 | 3 |
| 16 | 165.7 | 128.9 | 101.0 | 94.6 | 0.65 | 0.72 | 0.75 | 0.49 | 0.56 | 0.61 | 22 | 19 | 15 |
| Median | 80.5 | 55.0 | 41.6 | 22.7 | 0.38 | 0.40 | 0.40 | 0.24 | 0.25 | 0.25 | 21.5 | 17.5 | 13 |
Abbreviations: BTV = biological tumor volume; CI = conformity index; cGTV = consensus gross tumor volume; SDC = Sørensen-Dice coefficient.
BTVs with thresholds 1.6, 1.8, and 2.0, cGTV, SDC and CI for BTVs with cGTV, and margin to include the whole BTV into the cGTV.
Conformity assessments in the primary RT setting
Conformity between the cGTV and BTV contours was low in the RT setting as calculated by the SDC and the CI with rising median levels from BTV1.6 (0.51; 0.35) and BTV1.8 (0.55; 0.38) to BTV2.0 (0.58; 0.41). The median minimal margin required to include the entire BTV volume within the cGTV volume was 16 mm (range, 0-33) for BTV1.6, 12 mm (range, 0-28) for BTV1.8, and 10 mm (range, 0- 25) for BTV2.0.
Conformity assessments in the reRT setting
Conformity rates between the cGTV and BTV contours in the reRT setting were even lower both in the SDC and the CI, again with rising median levels from BTV1.6 (0.38; 0.24) and BTV1.8 (0.40; 0.25) to BTV2.0 (0.40; 0.25). The median minimal margin required to include the entire BTV volume within the expanded cGTV volume was 21.5 mm (range, 6 to >50) for BTV1.6, 17.5 mm (range, 5 to >50) for BTV1.8, and 13 mm (range, 3 to >50 mm) for BTV2.0.
Evaluation of the stability of threshold-based BTVs
Comparing the minimal margins required to include the entire BTV setting showed statistically significant larger minimal margins for BTV1.6 and BTV1.8 in the reRT compared with the primary RT setting, whereas for BTV2.0 no statistically significant difference was detected (P = .007, P = .031, P = .093, respectively; Mann-Whitney U test).
Discussion
Despite extensive research on molecular imaging of HGG, the role of PET imaging in RT planning remains poorly defined.15, 16, 17, 18, 19 Current guidelines on target volume delineation do not include PET imaging in the workup because PET examination facilities are not universally available and data of structured analyses on the benefits of PET-based RT planning for patients with HGG are still sparse.5 As 18F-GE-180 PET showed promising imaging properties in high-grade patients,1, 2, 3 the aim of the current study was to assess its use in RT and reRT planning.
To allow a valid comparability between MRI- and PET-based tumor volumes, the respective volumes were created with the highest level of standardization in a structured RT treatment planning study. The aim of the study was to answer the question, “To what extent does 18F-GE-180 PET improve the target volume delineation in high-grade patients, and what is the optimal BTV threshold for this purpose?”
Interestingly, 18F-GE-180 PET-based BTV volumes were significantly larger in this retrospective treatment planning study than the corresponding conventional MRI-based cGTVs with possible consequences for appropriate RT treatment planning. The BTVs with the highest threshold of 2.0 were the most comparable to the MRI-based volumes, with a median size of 39.1 versus 22.6 cm3 in the RT and 41.6 versus 22.7 cm3 in the reRT setting. The generation of BTVs with higher threshold values was omitted because 1 case did not show an increased tracer accumulation with the threshold level of 2.0 compared with the background activity.
Looking at the conformity of the BTV volumes to the cGTVs, the overall rate was low, with highest values achieved by the BTV2.0 volumes with SDC values of 0.58 and 0.41 and CI values of 0.4 and 0.25 in the RT and reRT setting, respectively. In the comparative analysis of the minimal margin needed to encompass the BTV volumes within the cGTV volumes, BTV1.6 and BTV1.8 needed large margins, both in the RT setting with 16 and 12 mm and in the reRT setting with 21.5 and 17.5 mm, respectively. In contrast, for BTV2.0, reasonable margins of 10 and 13 mm were detected. BTV2.0 also showed the most stable results with no statistically significant difference in the size of the minimal margin needed to include the whole cGTV when comparing the primary RT and the reRT setting, whereas significantly larger minimal margins were detected in the reRT setting for BTV1.6 and BTV1.8. From these results, we conclude that a threshold value of 2.0 might be the most appropriate in target volume delineation in RT and reRT treatment planning for patients with HGG.
Lower conformity levels and larger deviations of the localization of the BTVs from the cGTVs in the reRT treatment setting could be explained by the fact that the number of interfering effects — triggered, for example, by neurosurgical interventions and systemic therapy — increases over the course of treatment. Therefore, the patient group with inoperable HGG tumors treated with RT may benefit most from 18F-GE-180 PET imaging. In this patient group, 18F-GE-180 PET imaging could also be helpful for detecting the area with highest tumor activity for the preparation of the stereotactical biopsy for histopathologic diagnosis confirmation.
It has to be noted, that the question whether 18F-GE-180 PET represents solely tumor or also tumor-associated microglia and macrophages is still a subject of scientific investigation. Additionally, it has been questioned in the past, whether 18F-GE-180 tracer uptake is driven by blood-brain barrier (BBB) leakage.20 To address this question, a recent voxel-vise analysis examined the hotspots of 18F-GE-180 and 18F-fluoroethyltyrosine (18F-FET) tracer uptake and the hotspots of contrast enhancement on MRI reflecting BBB leakage. The average Hausdorff distance measures between the 18F-GE-180 PET hotspots from the hotspots of the relative contrast enhancement and the 18F-FET PET images were 12 ± 13 and 9 ± 10 mm, respectively and 14 ± 12 mm between the hotspots of the relative contrast enhancement and the 18F-FET PET images. These results with spatial differences between all modalities led to the conclusion that BBB leakage does not dominantly affect 18F-GE-180 tracer uptake.21
As this is, to the best of our knowledge, the first study to evaluate the novel 18F-GE-180 PET for its use in RT treatment planning, many more steps need to be taken to provide a conclusive evaluation of the value of 18F-GE-180 PET in radiation planning. It is of note that 18F-GE-180 PET gives the clinician insight of the metabolically active areas surrounding the contrast enhancement of MRI, which, for example, could represent the tumor extension in nearby white matter tracts as well as dural and leptomeningeal extension of the tumor. Because the areas of increased 18F-GE-180 uptake exceed the areas of contrast enhancement in the T1 images but are smaller than the T2 alterations, mere edema without increased metabolic activity seems not to be associated with an increase in 18F-GE-180 signal. Therefore, TSPO PET with 18F-GE-180 may help to delineate the metabolically active tumor volume with greater detail. Especially when using SIB concepts, the information of 18F-GE-180 PET could help delineate boost volumes, whereas T2 fluid-attenuated inversion recovery MRI cannot be used for this purpose. Regarding the optimal threshold of the BTVs, 2.0 seems to be most practicable because the median conformity measured by SDC and CI was manageable both in the RT (0.58, 0.41) and the reRT (0.40, 0.25) setting, resulting in union volumes of GTV and BTV with reasonable sizes. As BTVs with thresholds of 1.6 and 1.8 had even lower conformity in both settings, they seem to be not feasible for delineation of boost volumes. Due to the lower resolution of 18F-GE-180 in comparison to the resolution of MRI, it must be taken into consideration that spatial uncertainties increase for larger volumes, again strengthening the role of a rather strict threshold at 2.0.
The next steps will include histologic verification studies, which are currently ongoing, and recurrence pattern analyses after RT and reRT, which as of yet are only available for 18F-FET.22,23 Long-term prospective treatment planning studies are needed to provide level 1 evidence for PET-based RT planning, which as of yet is still pending for 18F-FET.19
Conclusion
TSPO PET with18F-GE-180 provides valuable complementary information to MRI in RT treatment planning for patients with HGG. 18F-GE-180-based BTVs with a threshold of 2.0 were the most consistent volumes both in the primary and the reRT treatment setting and may help to further guide target volume delineation.
Footnotes
Presented virtually at the 2021 American Society for Radiation Oncology Annual Meeting.
Sources of support: The German Cancer Consortium provided funding for publication costs.
Disclosures: All authors are members of the Comprehensive Cancer Center Neuro-Oncology, Ludwig Maximilians University, Munich, Germany. No other disclosures were reported.
All data generated or analyzed during this study are included in this published article.
References
- 1.Albert NL, Unterrainer M, Fleischmann DF, et al. TSPO PET for glioma imaging using the novel ligand (18)F-GE-180: First results in patients with glioblastoma. Eur J Nucl Med Mol Imaging. 2017;44:2230–2238. doi: 10.1007/s00259-017-3799-9. [DOI] [PubMed] [Google Scholar]
- 2.Unterrainer M, Fleischmann DF, Diekmann C, et al. Comparison of (18)F-GE-180 and dynamic (18)F-FET PET in high grade glioma: a double-tracer pilot study. Eur J Nucl Med Mol Imaging. 2019;44:580–590. doi: 10.1007/s00259-018-4166-1. [DOI] [PubMed] [Google Scholar]
- 3.Unterrainer M, Fleischmann DF, Vettermann F, et al. Tumour grading and molecular genetics in histologically verified glioma: A correlative (18)F-GE-180 PET study. Eur J Nucl Med Mol Imaging. 2020;47:1368–1380. doi: 10.1007/s00259-019-04491-5. [DOI] [PubMed] [Google Scholar]
- 4.Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 5.Niyazi M, Brada M, Chalmers AJ, et al. ESTRO-ACROP guideline “target delineation of glioblastomas”. Radiother Oncol. 2016;118:35–42. doi: 10.1016/j.radonc.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Fleischmann DF, Jenn J, Corradini S, et al. Bevacizumab reduces toxicity of reirradiation in recurrent high-grade glioma. Radiother Oncol. 2019;138:99–105. doi: 10.1016/j.radonc.2019.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Schnell O, Thorsteinsdottir J, Fleischmann DF, et al. Re-irradiation strategies in combination with bevacizumab for recurrent malignant glioma. J Neurooncol. 2016;130:591–599. doi: 10.1007/s11060-016-2267-x. [DOI] [PubMed] [Google Scholar]
- 8.Stupp R, Mason WP, van den Bent MJ. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 9.Perry JR, Laperriere N, O'Callaghan CJ, et al. Short-course radiation plus temozolomide in elderly patients with glioblastoma. N Engl J Med. 2017;376:1027–1037. doi: 10.1056/NEJMoa1611977. [DOI] [PubMed] [Google Scholar]
- 10.Warfield SK, Zou KH, Wells WM. Simultaneous truth and performance level estimation (STAPLE): An algorithm for the validation of image segmentation. IEEE Trans Med Imaging. 2004;23:903–921. doi: 10.1109/TMI.2004.828354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deasy JO, Blanco AI, Clark VH. CERR: A computational environment for radiotherapy research. Med Phys. 2003;30:979–985. doi: 10.1118/1.1568978. [DOI] [PubMed] [Google Scholar]
- 12.Unterrainer M, Vettermann F, Brendel M, et al. Towards standardization of 18F-FET PET imaging: Do we need a consistent method of background activity assessment? EJNMMI Res. 2017;7:48. doi: 10.1186/s13550-017-0295-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dice LR. Measures of the amount of ecologic association between species. Ecology. 1945;26:297–302. [Google Scholar]
- 14.Feuvret L, Noël G, Mazeron JJ, Bey P. Conformity index: A review. Int J Radiat Oncol Biol Phys. 2006;64:333–342. doi: 10.1016/j.ijrobp.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 15.Grosu AL, Weber WA, Franz M, et al. Reirradiation of recurrent high-grade gliomas using amino acid PET (SPECT)/CT/MRI image fusion to determine gross tumor volume for stereotactic fractionated radiotherapy. Int J Radiat Oncol Biol Phys. 2005;63:511–519. doi: 10.1016/j.ijrobp.2005.01.056. [DOI] [PubMed] [Google Scholar]
- 16.Albert NL, Weller M, Suchorska B, et al. Response Assessment in Neuro-Oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro Oncol. 2016;18:1199–1208. doi: 10.1093/neuonc/now058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Unterrainer M, Eze C, Ilhan H, et al. Recent advances of PET imaging in clinical radiation oncology. Radiation oncology (London. England) 2020;15:88. doi: 10.1186/s13014-020-01519-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galldiks N, Niyazi M, Grosu AL, et al. Contribution of PET imaging to radiotherapy planning and monitoring in glioma patients - A report of the PET/RANO group. Neuro Oncol. 2021;23:881–893. doi: 10.1093/neuonc/noab013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oehlke O, Mix M, Graf E, et al. Amino-acid PET versus MRI guided re-irradiation in patients with recurrent glioblastoma multiforme (GLIAA) - Protocol of a randomized phase II trial (NOA 10/ARO 2013-1) BMC Cancer. 2016;16:769. doi: 10.1186/s12885-016-2806-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zanotti-Fregonara P, Veronese M, Pascual B, Rostomily RC, Turkheimer F, Masdeu JC. The validity of 18F-GE180 as a TSPO imaging agent. Eur J Nucl Med Mol Imaging. 2019;46:1205–1207. doi: 10.1007/s00259-019-4268-4. [DOI] [PubMed] [Google Scholar]
- 21.Kaiser L, Holzgreve A, Quach S, et al. Differential spatial distribution of TSPO or amino acid PET signal and MRI contrast enhancement in gliomas. Cancers. 2021;14:53. doi: 10.3390/cancers14010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pauleit D, Floeth F, Hamacher K, et al. O-(2-[18F]fluoroethyl)-L-tyrosine PET combined with MRI improves the diagnostic assessment of cerebral gliomas. Brain. 2005;128:678–687. doi: 10.1093/brain/awh399. [DOI] [PubMed] [Google Scholar]
- 23.Fleischmann DF, Unterrainer M, Schon R, et al. Margin reduction in radiotherapy for glioblastoma through (18)F-fluoroethyltyrosine PET? – A recurrence pattern analysis. Radiother Oncol. 2020;145:49–55. doi: 10.1016/j.radonc.2019.12.005. [DOI] [PubMed] [Google Scholar]