Abstract
Objective:
To determine the association between ultra-processed food (UPF) intake and all-cause mortality in a representative sample of Spanish population.
Design:
Prospective cohort design in which follow-up lasted from baseline (1991) to mortality date or 31 December 2017, whichever was first. Dietary information was collected using a validated frequency questionnaire and categorised following the NOVA classification according to the extent of food processing. The association between consumption of UPF and mortality was analysed using Cox models. Isoenergetic substitution models were constructed to compare the health effects of the NOVA groups.
Setting:
Cohort from the Diet and Risk of Cardiovascular Diseases (CVD) in Spain (DRECE) study, representative of the Spanish population
Participants:
Totally, 4679 subjects between 5 and 59 years old.
Results:
Average consumption of UPF was 370·8 g/d (24·4 % of energy intake). After a median follow-up of 27 years, 450 deaths occurred. Those who consumed the highest amount of UPF had higher risk of mortality. For every 10 % of the energy intake from UPF consumption, an increase of 15 % in the hazard of all-cause mortality was observed (HR 1·15; (95 % CI 1·03, 1·27); P-value = 0·012). Substitution of UPF with minimally processed foods was significantly associated with a decreased risk of mortality.
Conclusions:
An increase in UPF consumption was associated with higher risk of all-cause mortality in a representative sample of the Spanish population. Moreover, the theoretical substitution of UPF with unprocessed or minimally processed foods leads to a decrease in mortality. These results support the need to promote diets based on unprocessed or minimally processed foods.
Keywords: Ultra-processed food, NOVA classification, All-cause mortality, Isoenergetic substitution
Dietary habits have changed in recent decades. One of the most important changes is the increase in the ultra-processed foods (UPF) consumption around the world(1–5). UPF are industrial formulations made from substances derived from food or synthesised in laboratories (dyes, flavourings and other additives) usually containing little or no whole food(6). These are characterised by being food products with a low nutritional quality(7–12). It has been estimated that UPF intake is currently increasing, contributing from values below 20 % to above 60 % of total energy intake depending on the country and age range(13,14). Available evidence suggests that it is the high availability, low cost and extensive marketing of ready-to-consume food items that result in an excessive intake(15). These changes in dietary habits have been paralleled to an increase in non-communicable diseases (NCD)(16). The low nutritional content of these foods, coupled with excessive consumption patterns, is known to lead to these chronic NCD(17,18).
As the global incidence of NCD continues to grow, it is crucial to study the impact of UPF in health. The WHO has, therefore, developed the European Action Plans for Food and Nutrition Policy with the aim of improving upon existing national policies. Specifically, they have developed a Global Action Plan on NCD(19,20) to achieve a reduction in global mortality from the four major NCD (CVD, cancer, chronic respiratory disease and diabetes) by 2025. Global modelling of the impact of risk factors on mortality, such as UPF, could provide important information to achieve goals like this. The beneficial effects of fresh or minimally processed foods consumption on mortality are known(21), but few studies have examined the harmful effects of consumption of UPF. NutriNet-Santé cohort in France(22), the National Health and Nutrition Examination Survey (NHANES) cohort in the USA(23) and the Moli-Sani study in Italy(24) found a positive association between consumption of UPF and all-cause mortality. SUN cohort(25) and a cohort selected from the ENRICA study(26) in Spain also report similar findings, but participants were highly selected (only university graduates) and had a relatively short follow-up period ( approximately 8 years), respectively.
This study, carried out on a representative sample of the Spanish population after 27 years of follow-up, aimed to determine the association of UPF consumption with all-cause mortality.
Methods
Design and study population
The multicentre study Diet and Risk of Cardiovascular Diseases (CVD) in Spain (DRECE) was used as a substrate for analysis. DRECE was designed in 1991 to know the real situation of the Spanish population upon the risk of developing CVD, based on the prevalence of risk factors and their relationship with dietary habits. The details of the study (background, study population and methods of the survey) are reported elsewhere(27,28). Briefly, a cohort of 4787 people between 5 and 59 years of age was included, stratified by sex and age, randomly selected throughout the national territory, both rural and urban. At baseline, in 1991, a FFQ was carried out, designed and validated for epidemiological studies in the Spanish population(29,30).
Dietary assessment and classification of ultra-processed food
The estimation of UPF consumption was carried out through the data collected in the FFQ. The first step was to classify all foods into four groups according to the NOVA classification, developed in Brazil and used internationally in research(31). These four groups were: Group 1 describes unprocessed/minimally processed foods; Group 2 comprises processed culinary ingredients; Group 3 includes processed products and Group 4 collects all UPF. The full list of the recorded foods in the FFQ and their NOVA classification is shown in Supplemental Table 1. Subsequently, the kcal/d consumed from each of the four NOVA groups and the percentage they represented with respect to the total intake were determined for better interpretation. As some foods do not provide energy, the calculation was also considered in relation to the weight of the product and not only to the energy intake(22). As grams can be easily interpreted and measured, they were determined as an absolute value (g/d). Responses with extreme total energy intakes (<200 kcal and >5000 kcal) were excluded from the analysis. The consumption of the different nutrients in the diet (carbohydrates, proteins, fats and fibre) was also calculated (in g/d). All the information for the calculation of these data was obtained from the Spanish Food Composition Database (BEDCA)(32).
Mortality ascertainment
Mortality in this cohort was monitored from 1991 to December 2017 through an annual agreement with the National Institute of Statistics (INE). The vital status and cause of mortality were provided by the INE, according to the registered death certificates. In this way, all-cause mortality was obtained.
Statistical analysis
All statistical analyses were performed using SAS© software, Version 9.4 of the SAS System for Windows. Descriptive data were presented as mean and standard deviation for continuous variables and categorical variables were expressed as absolute or relative frequencies.
Correlations between nutritional characteristics (carbohydrates, proteins, fats and fibre), age and UPF consumption were analysed using Pearson’s correlation coefficient (ρ).
To assess the association between all-cause mortality and UPF consumption at baseline, Cox regression models were fitted with the time variable covering from baseline in 1991 to the date of death (provided by the INE) or to the end of follow-up on 31 December 2017, whichever occurred first. Hazard ratios were estimated along with Wald’s 95 % CI. The models were adjusted for potential confounders defined a priori. Potential confounders were identified based on previous existing literature rather than on statistical criteria(33). The following potential confounders were included as covariates in the multivariate models: age; sex; baseline BMI (continuous); total energy intake (kcal/d, continuous); alcohol consumption (servings/d, continuous); smoking status (yes/no); physical activity (yes/no); family history of CVD (yes/no); history of diabetes, hypertension or CVD at baseline (yes/no).
Cox models were built with three successive levels of additional adjustments for potential confounders: model 1 was adjusted for age and sex; model 2 was additionally adjusted for lifestyle and model 3 was additionally adjusted for clinical factors (history of chronic conditions). The likelihood of the three models was compared using the Akaike information criterion. This criterion penalises the likelihood of the model, and the one with the lowest value is the most likely model. Also, Harrell’s C index was used to evaluate the performance of the models. The proportionality of hazards was verified with the Schoenfeld residuals test. In addition, smoothing by penalised splines was used to calculate the possible non-linear association between UPF consumption and all-cause mortality. All analyses were carried out using UPF consumption measured as percentage of total energy intake and g/d. Additionally, we performed subgroup analyses separated by sex in model 3.
Sensitivity analyses were also performed by rerunning the best model (model 3) under five different a priori assumptions: (1) excluding participants with prevalent diabetes at baseline; (2) excluding participants with prevalent hypertension at baseline; (3) excluding participants aged less than 18 years; (4) excluding the first 2 years of follow-up for all participants to reduce the possibility of attribution of deaths to health conditions at baseline and (5) excluding deaths from cancer, deaths from CVD and deaths from injuries.
Isoenergetic substitution models were constructed with the aim of comparing the effects of substituting UPF by different NOVA groups (either processed foods and processed culinary ingredients or unprocessed or minimally processed foods) in vital status(34). In these models, the total energy intake, the percentages of energy or grams derived from processed foods and processed culinary ingredients or unprocessed/minimally processed foods, as appropriate, were simultaneously included, in addition to the confounding variables studied in model 3. The coefficients obtained in these models can be interpreted as the estimated effect of substituting a certain percentage of energy or absolute values of grams from UPF with the equivalent processed foods or unprocessed or minimally processed foods, while holding constant the intake of total energy and the energy or grams from the corresponding non-replaced NOVA group.
Results
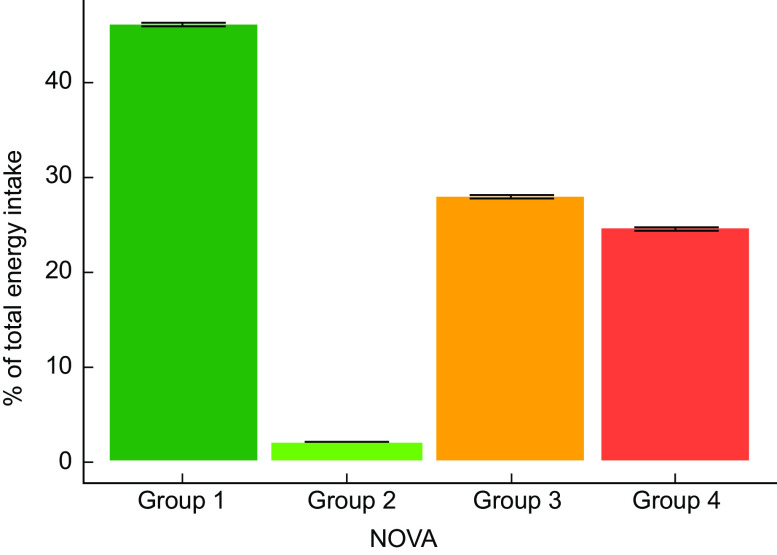
Of the 4787 study participants, 108 were excluded due to inconsistent dietary data (total daily energy intake outside the range of 200–5000 kcal/d). The final sample size was 4679. A total of 2288 (48·9 %) males and 2391 (51·1 %) females were included in this analysis. Mean age at baseline was 30·5 (sd 15·6) years. Table 1 shows the baseline characteristics of participants. After a median of 27 years and 122 134 person-years followed up, 450 deaths due to any cause occurred. An average consumption of UPF of 370·8 g/d was found, corresponding to 24·4 % of the total energy intake (Fig. 1). A total of 1·9 % of the sample did not consumed any UPF on a daily basis.
Table 1.
Baseline characteristics of the cohort participants
| Characteristics | All participants n 4679 | SD |
|---|---|---|
| Mean | ||
| Age, years | 30·5 | 15·6 |
| Sex (female) | ||
| n | 2391 | |
| % | 51·1 % | |
| BMI, kg/m2 | 24·2 | 5·0 |
| Family history of CVD | ||
| n | 701 | |
| % | 14·9 % | |
| History of diabetes | ||
| n | 73 | |
| % | 1·5 % | |
| History of hypertension | ||
| n | 183 | |
| % | 3·9 % | |
| History of angina | ||
| n | 44 | |
| % | 0·9 % | |
| History of myocardial infarction | ||
| n | 24 | |
| % | 0·5 % | |
| History of atherosclerosis | ||
| n | 114 | |
| % | 2·4 % | |
| Current smokers | ||
| n | 1190 | |
| % | 25·4 % | |
| Alcohol intake, servings/d | 2·1 | 1·1 |
| Physical activity | ||
| n | 2813 | |
| % | 60·9 % | |
| Moderate, d/week | 2·3 | 2·7 |
| Intense, d/week | 4·1 | 2·2 |
| Total energy intake, kcal/d | 2025 | 727·1 |
| Total grams intake, g/d | 1882 | 642·2 |
| Carbohydrates, g/d | 303·4 | 118·8 |
| Protein, g/d | 118·5 | 41·5 |
| Fat, g/d | 122·9 | 52·5 |
| Saturated fat, g/d | 45·7 | 19·2 |
| Fibre, g/d | 25·1 | 10·5 |
| Cholesterol, mg/d | 547·3 | 235·1 |
| NOVA classification | ||
| Group 1 | ||
| % of energy | 45·9 | 13·3 |
| g/d | 968·1 | 375·2 |
| Group 2 | ||
| % of energy | 1·9 | 2·3 |
| g/d | 14·3 | 19·9 |
| Group 3 | ||
| % of energy | 27·8 | 12·2 |
| g/d | 309·5 | 310·4 |
| Group 4 | ||
| % of energy | 24·4 | 13·9 |
| g/d | 370·7 | 328·6 |
Fig. 1.
UPF consumption in Spain according to the NOVA classification. Error bars denote standard error of the mean. UPF, ultra-processed food
The main food groups contributing to the intake of UPF were sugar-sweetened beverages (i.e. soft drinks) (18·4 %), milkshakes and juice boxes (17·5 %), meat and meat products (16·3 %), dairy products (13·5 %), cakes and pastries (10·7 %) and sweets and cookies (9·8 %) (Table 2). Participants who consumed the highest amount of UPF had significantly higher intakes of total energy (ρ = 0·40, P-value <0·0001), carbohydrates (ρ = 0·34, P-value <0·0001), total fat (ρ = 0·30, P-value <0·0001), saturated fat (ρ = 0·39, P-value <0·0001), and dietary cholesterol (ρ = 0·27, P-value <0·001), and lower protein (ρ = -0·13, P-value <0·0001) and fibre (ρ = -0·06, P-value <0·0001) intake. In addition, the individuals who consumed more UPF were younger (ρ = -0·53, P-value <0·0001).
Table 2.
Relative contribution of the dietary intake of food groups to UPF (NOVA group 4)
| Characteristics | All participants n 4679 |
|---|---|
| Total energy intake, kcal/d | |
| Mean | 2025 |
| sd | 727·1 |
| Energy from unprocessed/minimally processed, kcal/d | |
| Mean | 878·9 |
| sd | 289·9 |
| Energy from processed culinary ingredients, kcal/d | |
| Mean | 36·7 |
| sd | 46·8 |
| Energy from processed food, kcal/d | |
| Mean | 573·8 |
| sd | 324·7 |
| Energy from ultra-processed food, kcal/d | |
| Mean | 535·7 |
| sd | 346·7 |
| Ultra-processed food consumption, g/d | |
| Mean | 370·7 |
| sd | 328·6 |
| Energy from ultra-processed food, % of total energy | |
| Mean | 24·4 |
| sd | 13·9 |
| Ultra-processed food by food groups (% ultra-processed energy) | |
| Sugar-sweetened beverages | 18·4 % |
| Milkshakes and juice boxes | 17·5 % |
| Meat and meat products* | 16·3 % |
| Dairy products† | 13·5 % |
| Cakes and pastries‡ | 10·7 % |
| Sweets and cookies§ | 9·8 % |
| Breakfast cereals | 4·5 % |
| Ultra-processed cheese | 3·0 % |
| Liquors | 2·3 % |
| Sauces and dressings | 2·0 % |
| Salty snacks | 1·0 % |
| Margarine | 1·0 % |
Includes ham, cold cuts, sausages and hamburgers.
Includes yogurts and fermented milk, ice cream, and petit suisse.
Includes doughnuts, muffins or other non-hand-made pastries.
Includes biscuits, chocolate cookies, candies and chocolates.
In all the models, the participants who consumed the highest amount of UPF had a higher risk of mortality. When covariates were added to the models, the association did not change substantially. Model 3 was the one with the best Harrell’s C index and the lowest Akaike information criterion. In model 3, every 10 % absolute increment of the energy intake from UPF had a relatively 15 % higher hazard of all-cause mortality (multivariable-adjusted hazard ratio 1·15; (95 % CI 1·03, 1·27); P-value = 0·012) (Table 3). The corresponding mortality risk when UPF consumption was expressed in g/d was (1·04 (95 % CI 1·01, 1·10); P-value = 0·018; see online Supplemental Table 2).
Table 3.
Cox proportional hazard ratios (Wald’s 95 % CI) for all-cause mortality according to ultra-processed food consumption (measured as % of total energy intake) in the DRECE study
| Variables | P-value | HR | 95 % CI |
|---|---|---|---|
| Model 1 † AIC = 6697·52; C-index = 0·84 (445 deaths) | |||
| Age, years | <0·0001 | 1·10 | 1·09, 1·11 |
| Sex (female) | <0·0001 | 0·42 | 0·35, 0·52 |
| Energy from ultra-processed food, % of total energy* | 0·0122 | 1·11 | 1·02, 1·20 |
| Schoenfeld’s global test | 0·2980 | ||
| Model 2 ‡ AIC = 6324·05; C-index = 0·84 (424 deaths) | |||
| Age, years | <0·0001 | 1·10 | 1·09, 1·11 |
| Sex (female) | <0·0001 | 0·47 | 0·37, 0·59 |
| BMI, kg/m2 | <0·0001 | 1·05 | 1·02, 1·07 |
| Physical activity (yes) | 0·2972 | 0·90 | 0·74, 1·09 |
| Alcohol intake, servings/d | 0·3802 | 1·01 | 0·99, 1·03 |
| Smokers (yes) | <0·0001 | 1·63 | 1·31, 2·04 |
| Total energy intake, 1000 kcal/d | 0·1540 | 0·87 | 0·73, 1·05 |
| Energy from ultra-processed food, % of total energy* | 0·0012 | 1·16 | 1·06, 1·26 |
| Schoenfeld’s global test | 0·6830 | ||
| Model 3 § AIC = 4342·15 C-index = 0·85 (303 Deaths) | |||
| Age, years | <0·0001 | 1·10 | 1·08, 1·11 |
| Sex (female) | <0·0001 | 0·45 | 0·34, 0·59 |
| BMI, kg/m2 | 0·0027 | 1·04 | 1·01, 1·07 |
| Physical activity (yes) | 0·1048 | 0·82 | 0·65, 1·04 |
| Alcohol intake, servings/d | 0·9914 | 1·00 | 0·98, 1·02 |
| Smokers (yes) | 0·0004 | 1·60 | 1·23, 2·08 |
| Total energy intake, 1000 kcal/d | 0·7556 | 0·97 | 0·78, 1·19 |
| Family history of CVD | 0·3781 | 1·12 | 0·87, 1·46 |
| History of diabetes | <0·0001 | 2·73 | 1·78, 4·17 |
| History of hypertension | 0·3022 | 1·20 | 0·85, 1·68 |
| History of angina | 0·4448 | 1·34 | 0·63, 2·82 |
| History of myocardial infarction | 0·0032 | 3·86 | 1·57, 9·45 |
| History of atherosclerosis | 0·0252 | 1·66 | 1·06, 2·58 |
| Energy from ultra-processed food, % of total energy* | 0·0129 | 1·15 | 1·03, 1·27 |
| Schoenfeld’s global test | 0·0928 | ||
AIC, Akaike information criterion.
The names of the different models constructed are shown in bold. The Schoenfeld’s global test for each model is shown in italic.
Calculated for every 10 %.
Model 1 was adjusted for age and sex.
Model 2 was adjusted for the variables in model 1 plus BMI, physical activity, alcohol intake, smoking status and total energy intake.
Model 3 was adjusted for the variables in model 2 plus family history of CVD, history of diabetes, hypertension, angina, myocardial infarction and atherosclerosis.
The proportionality of hazards was verified with a test based on Schoenfeld residuals; the non-significant result (P-value >0·05) suggested that there was no evidence against the proportionality assumption in all models (corresponding P-values in Table 3 and see online Supplemental Table 2). The linearity assumptions between UPF consumption and all-cause mortality were confirmed by the restricted cubic spline (model 3 non-linear association in percentage P-value = 0·470; in g/d P-value = 0·101).
Sensitivity analyses were performed by repeating the multivariable-adjusted Cox regression model 3 under different scenarios. All point estimates showed a direct association between consumption of UPF and higher mortality. Results did not substantially change in any of these alternative scenarios, suggesting that the direct association between consumption of UPF and mortality was robust (Fig. 2(a) and (b)). When UPF consumption was measured as percentage of total energy intake (Fig. 2(a)), the association when excluding cases of prevalent hypertension at baseline was non-significant. In contrast, the association became stronger after excluding deaths from cancer, CVD and injuries. The association remained significant after excluding cases of prevalent diabetes at baseline, excluding participants aged <18 years and excluding the first 2 years of follow-up. When UPF consumption was measured as g/d (Fig. 2(b)), some associations were no longer significant, in particular under the scenarios of excluding participants aged less than 18 years, excluding the first 2 years of follow-up and after excluding deaths from CVD and injuries. The association became slightly higher after excluding cases of prevalent hypertension and diabetes at baseline and after excluding deaths from cancer. Subgroup analysis was performed in model 3. According to Supplemental Fig. 1, the association between UPF energy contribution and all-cause mortality was stronger and only significant among males. On the other hand, associations between UPF gram intake and all-cause mortality in males and females were non-significant, probably due to lack of statistical power. The interaction between sex and UPF consumption was non-significant for both energy and gram consumption (P-value = 0·054 and P-value = 0·511, respectively).
Fig. 2.

Sensitivity analyses for association between consumption of ultra-processed foods and all-cause mortality performed in model 3. a) Cox proportional hazard ratios (Wald’s 95% CI) for all-cause mortality of UPF consumption measured as percentage of total energy intake and b) Cox proportional hazard ratios (Wald’s 95% CI) for all-cause mortality of UPF consumption measured as grams/day. UPF, ultra-processed food
After performing the isoenergetic substitution in model 3, the hazard ratio when UPF were substituted by processed foods was less than 1 but did not achieve statistical significance (neither in percentage of energy nor in grams). However, when UPF were replaced by unprocessed or minimally processed foods, an inverse pattern was observed. A reduction of 14 % (in percentage of energy) and 6 % (in g) in all-cause mortality was estimated (P-value = 0·018 and P-value = 0·002, respectively) (see Table 4).
Table 4.
Isoenergetic substitution. Hazard ratios derived from Cox multiple regression model 3 in which processed foods or unprocessed/minimally processed foods replaced UPF
| HR | 95 % CI | P-value | |
|---|---|---|---|
| Replace 10 % energy of Group 4 with 10 % energy of: | |||
| Processed foods (Group 3 + Group 2) | 0·88 | 0·78, 1·01 | 0·0506 |
| Unprocessed or minimally processed foods (Group 1) | 0·86 | 0·77, 0·98 | 0·0180 |
| Replace 100 g of Group 4 with 100 g of: | |||
| Processed foods (Group 3 + Group 2) | 0·90 | 0·83, 1·00 | 0·1045 |
| Unprocessed or minimally processed foods (Group 1) | 0·94 | 0·91, 0·98 | 0·0027 |
UPF, ultra-processed food.
Discussion
In this longitudinal cohort study of a representative Spanish population, a significant association between higher consumption of UPF and an increased risk of all-cause mortality over a median follow-up of 27 years was found. This association remained significant after adjusting for socio-demographic factors, lifestyle and clinical factors.
The average consumption of UPF in Spain was 24·4 % of the total energy intake. These data are in line with those provided by the ENRICA cohort study(26). Spain is a country with low UPF consumption when compared with other countries, such as Canada (48 %)(7), the USA (57·9 %)(35), the UK (56·8 %)(10), Belgium (about 30 %)(36), France (35·9 %)(37) and even in developing countries like Brazil (21·5 %)(38). One of the possible causes for these differences may be the period of time in which these data were collected; our data are based on the diet assessed in 1991, where there was probably a lower intake of UPF. This also could be due to the Mediterranean diet, which is characterised by a high consumption of plant-based foods, a low consumption of red meat and other processed foods, the use of olive oil as the main source of fat, and a moderate intake of wine during meals(39). In addition, the Mediterranean diet is mainly based on cooking at home, so the consumption of ready-to consume UPF is smaller compared to other countries. However, it is also known that in recent decades. the Spanish population has been moving away from this traditional diet pattern to adopt a less healthy diet(40), especially the younger population(41). These dietary changes support estimates that the consumption of UPF will continue to increase as will their sales in Spain(14,42).
The present study builds on previous longitudinal studies that examined the association between consumption of UPF (using the NOVA classification) and NCD. These studies found associations between UPF consumption and different adverse health outcomes such as obesity, dyslipidemia, hypertension and cancer at mid-life (≥40 years)(43–46). Our results appear to be consistent with those studies and reflect the adverse effects associated with UPF consumption.
There are also other studies under the NOVA framework that evaluate the association between UPF intake and all-cause mortality, such as the NutriNet-Santé (≥45 years) in France(22), the US National Health and Nutrition Examination Survey (NHANES)(23), Moli-sani study in Italy(24), SUN cohort(25) and a cohort selected from the ENRICA study(26) in Spain. NHANES, Moli-sani, SUN and ENRICA cohort(23–26) categorise the consumption of UPF into quartiles and study the risk associated with the fourth quartile v. the first quartile. Based on the existing literature(47,48), we consider that in epidemiological studies in which it is intended to evaluate the effect of an exposure on a response, the magnitude or direction of such effect may be biased as a consequence of the categorisation of the variable. To avoid possible biases or loss of information, we decided not to categorise the variable and use it as continuous. In this sense, these previous studies report a much higher effect of UPF on all-cause mortality than our study. However, they only take into account the first and fourth quartiles of consumption adjusted for different covariates (NHANES: HR = 1·30; (95 % CI 1·08, 1·57); P-value = 0·001); (Moli-sani: HR = 1·26; (95 % CI 1·09, 1·46); P-value <0·050); (SUN: HR = 1·62; (95 % CI 1·13, 2·33); P-value = 0·005) and (ENRICA: HR = 1·44; (95 % CI 1·01, 2·07); P-value = 0·030). The information provided by the second and third quartiles is not reflected in these results. Specifically, the SUN cohort is the one that finds a greater effect of the UPF on all-cause mortality, a 62 % relatively higher hazard of mortality. This may be due to the fact that the SUN cohort is a relatively young population of university graduates, and it is known that young people have a less healthy diet(41). As these participants were non-representative of the population, these results cannot be extrapolated to the general Spanish population. NutriNet-Santé Cohort(22) in France studies the consumption of UPF as a continuous variable, and their reports are very similar to ours, and the HR per 10 % increment of UPF consumed was (1·14 (95 % CI 1·04, 1·27); P-value = 0·008). However, the population of this study is older than 45 years, which reduces the generalisability of their results. Similar results in different populations, with several methods for assessing dietary exposures and different age ranges, support a robust association. Moreover, the results obtained from isoenergetic replacement are in line with different studies that estimated through national household data the contribution of dietary trends to the risk of CVD mortality. In the UK, if the consumption of processed and ultra-processed products were reduced to the levels of unprocessed and minimally processed foods, there could be a substantial 10–13 % decrease in cardiovascular deaths from CVD(49). In Brazil, with a similar approach, assuming a 50 % reduction in UPF, replacing this reduction in consumption with a 50 % increase in the consumption of unprocessed or minimally processed foods, plus an additional reduction of 50 % in processed culinary ingredients, an 11 % reduction in cardiovascular mortality was estimated(50). As far as we know, no previous studies have performed the isoenergetic substitution of UPF for all-cause mortality. Although the studies published so far provide results for CVD deaths, they support the results we found for all-cause mortality.
The increased risk of all-cause mortality associated with consumption of UPF depends on several factors. UPF cause overconsumption due to the high energy density they provide which is less satiating. This inadvertent overconsumption has been associated with mortality(51). Several of these UPF contain high amounts of salt, and high Na intake has been associated with mortality and cardiovascular deaths(52,53). Likewise, UPF provide added sugars(35), which contributes to the excessive consumption of added sugars that has been associated with increased CVD mortality(54). Processed meats and sugar-sweetened beverages have been consistently linked to mortality in prospective studies(55–58) and may therefore be important factors in the association found between UPF and mortality. In contrast, UPF tend to be low in fibre and dietary fibre has been associated with a lower risk of mortality(59,60). UPF are characterised by being food products of low nutritional quality and their high consumption is associated with unhealthy dietary patterns(7,61,62). Such unhealthy patterns could lead to develop NCD, contributing to an increased risk of mortality(63,64). Finally, UPF often contain additives in their composition (such as titanium dioxide, artificial sweeteners, emulsifiers,etc), and several studies have raised concerns about the health consequences of those additives(65,66). Other studies have found associations between the consumption of UPF and urinary concentrations of phthalates and bisphenol(67). These chemicals are present in food packaging and have been associated with harmful health effects. However, more research is needed to know how and to which extent UPF could affect health(68–70).
All these findings reinforce the detrimental effects of UPF. Consistent with the evidence cited, our findings support the negative impact of UPF on the overall incidence of NCD and all-cause mortality in Spain; they also highlight the importance of implementing new nutritional policies and guidelines as soon as possible to address this impact in the population.
Strengths and limitations of this study
The strengths of this study are its prospective design, long follow-up and the large sample size, representative of Spanish population, which broadens the generalisation of the results and also the use of validated methods and the adjustment for a wide range of potential confounders in the analysis. The most important novelty is that we did not categorise the consumption of UPF and use as a continuous variable, which provides richer information and avoids biases. As well, to avoid underestimating the consumption of UPF (since some foods do not provide energy), all the analyses were considered in relation to the weight of the product and not only to the energy contribution. Lastly, the performances of several sensitivity analyses support the robustness of the results.
However, the study also has some limitations. First, dietary information was collected only at baseline, assuming no changes over time in dietary intake. Furthermore, the dietary data were collected in 1991, a time frame that was likely characterised by less UPF intake, which probably underestimates the true impact of UPF consumption on mortality nowadays. Second, although the NOVA classification has been sometimes questioned(71), it is clear and simply to apply, no better alternative has yet been proposed. Third, the FFQ was not designed to collect data on consumption of UPF according to the NOVA classification. Each food product was classified in the most likely NOVA group, but we cannot rule out misclassification of some foods. Finally, due to the observational design of this study, the hypothesis of residual confounding cannot be ruled out.
Conclusions
Our results suggest that an increased consumption of UPF is associated with a higher hazard of all-cause mortality. Furthermore, the theoretical isoenergetic substitution of UPF with unprocessed or minimally processed foods would lead to a reduction in the risk of mortality. More studies are needed to confirm these findings in different populations and to unravel the mechanisms by which UPF can affect human health. Likewise, the evidence on the harmful effects on health of the intake of UPF highlights the need for new public health policies, such as the development of nutritional guidelines, to limit the consumption of UPF and to promote the consumption of unprocessed or minimally processed foods.
Acknowledgements
Acknowledgements: The authors thank all collaborators for their involvement in the DRECE study. Financial support: This study was funded by Instituto de Salud Carlos III FIS 03/0014, FIS 08/90643; and by Fundación MMA de Investigación biomédica P-MMA2004/19, P-MMA2008/88. Conflict of interest: The authors declare that they have no conflict of interest. Authorship: All authors contributed to the study conception and design. Funding acquisition and supervision were carried out by A.G.D.L.C. and P.C.N. Material preparation, data collection and analysis were performed by C.R.F., D.L.P. and C.M.-A.A. The first draft of the manuscript was written by Carmen Romero Ferreiro and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. Ethics of human subject participation: The present study was approved by the Research Ethics Committee of the Hospital Universitario 12 de Octubre on 05 November 2010 (ref. 10/292) and has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its latter amendments.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980021003256.
click here to view supplementary material
References
- 1. Pan American Health Organization (2015) Ultra-Processed Food and Drink Products in Latin America: Trends, Impact on Obesity, Policy Implications. https://iris.paho.org/handle/10665.2/7699 (accessed November 2020).
- 2. Stuckler D, McKee M, Ebrahim S et al. (2012) Manufacturing epidemics: the role of global producers in increased consumption of unhealthy commodities including processed foods, alcohol, and tobacco. PLoS Med 9, e1001235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Monteiro CA & Cannon G (2012) The impact of transnational ‘big food’ companies on the South: a view from Brazil. PLoS Med 9, e1001252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moodie R, Stuckler D, Monteiro C et al. (2013) Profits and pandemics: prevention of harmful effects of tobacco, alcohol, and ultra-processed food and drink industries. Lancet 381, 670–679. [DOI] [PubMed] [Google Scholar]
- 5. Monteiro CA, Moubarac J-C, Cannon G et al. (2013) Ultra-processed products are becoming dominant in the global food system. Obes Rev 14, Suppl. 2, S21–S28. [DOI] [PubMed] [Google Scholar]
- 6. Monteiro CA, Cannon G, Levy R et al. (2016) NOVA. The star shines bright. World Nutr 7, 28–38. [Google Scholar]
- 7. Moubarac J-C, Batal M, Louzada ML et al. (2017) Consumption of ultra-processed foods predicts diet quality in Canada. Appetite 108, 512–520. [DOI] [PubMed] [Google Scholar]
- 8. Martínez Steele E, Popkin BM, Swinburn B et al. (2017) The share of ultra-processed foods and the overall nutritional quality of diets in the US: evidence from a nationally representative cross-sectional study. Popul Health Metr 15, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Louzada ML da C, Ricardo CZ, Steele EM et al. (2018) The share of ultra-processed foods determines the overall nutritional quality of diets in Brazil. Public Health Nutr 21, 94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rauber F, da Costa Louzada ML, Steele EM et al. (2018) Ultra-processed food consumption and chronic non-communicable diseases-related dietary nutrient profile in the UK (2008–2014). Nutrients 10, 587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marrón-Ponce JA, Flores M, Cediel G et al. (2019) Associations between consumption of ultra-processed foods and intake of nutrients related to chronic non-communicable diseases in Mexico. J Acad Nutr Diet 119, 1852–1865. [DOI] [PubMed] [Google Scholar]
- 12. Parra DC, da Costa-Louzada ML, Moubarac J-C et al. (2019) Association between ultra-processed food consumption and the nutrient profile of the Colombian diet in 2005. Salud Publica Mex 61, 147–154. [DOI] [PubMed] [Google Scholar]
- 13. Elizabeth L, Machado P, Zinöcker M et al. (2020) Ultra-processed foods and health outcomes: a narrative review. Nutrients 12, 1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kelly B & Jacoby E (2018) Public Health Nutrition special issue on ultra-processed foods. Public Health Nutr 21, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Swinburn BA, Sacks G, Hall KD et al. (2011) The global obesity pandemic: shaped by global drivers and local environments. Lancet 378, 804–814. [DOI] [PubMed] [Google Scholar]
- 16. Mendis S, Davis S & Norrving B (2015) Organizational update: the World Health Organization global status report on noncommunicable diseases 2014; one more landmark step in the combat against stroke and vascular disease. Stroke 46, e121–e122. [DOI] [PubMed] [Google Scholar]
- 17. Canella DS, Levy RB, Martins APB et al. (2014) Ultra-processed food products and obesity in Brazilian households (2008–2009). PLoS One 9, e92752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tavares LF, Fonseca SC, Garcia Rosa ML et al. (2012) Relationship between ultra-processed foods and metabolic syndrome in adolescents from a Brazilian Family Doctor Program. Public Health Nutr 15, 82–87. [DOI] [PubMed] [Google Scholar]
- 19. United Nations (2011) Political Declaration of the High-Level Meeting of the General Assembly on the Prevention and Control of Non-Communicable Diseases. https://digitallibrary.un.org/record/710899/ (accessed November 2020).
- 20. World Health Organization (2013) Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020. https://apps.who.int/iris/handle/10665/94384 (accessed November 2020).
- 21. Wang X, Ouyang Y, Liu J et al. (2014) Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 349, g4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schnabel L, Kesse-Guyot E, Allès B et al. (2019) Association between ultraprocessed food consumption and risk of mortality among middle-aged adults in France. JAMA Intern Med 179, 490–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim H, Hu EA & Rebholz CM (2019) Ultra-processed food intake and mortality in the USA: results from the Third National Health and Nutrition Examination Survey (NHANES III, 1988–1994). Public Health Nutr 22, 1777–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bonaccio M, Di Castelnuovo A, Costanzo S et al. (2021) Ultra-processed food consumption is associated with increased risk of all-cause and cardiovascular mortality in the Moli-Sani Study. Am J Clin Nutr 113, 446–455. [DOI] [PubMed] [Google Scholar]
- 25. Rico-Campà A, Martínez-González MA, Alvarez-Alvarez I et al. (2019) Association between consumption of ultra-processed foods and all cause mortality: SUN prospective cohort study. BMJ 365, l1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Blanco-Rojo R, Sandoval-Insausti H, López-Garcia E et al. (2019) Consumption of ultra-processed foods and mortality: a national prospective cohort in Spain. Mayo Clin Proc 94, 2178–2188. [DOI] [PubMed] [Google Scholar]
- 27. Gómez Gerique JA, Herrera R, Gómez de la Cámara A et al. (2011) Capítulo 2. El proyecto DRECE. Med Clín 12, 3–5. [Google Scholar]
- 28. Gómez Gerique JA, Rubio Herrera MA, Gómez de la Cámara A et al. (2011) Capítulo 3. DRECE I (1991). Med Clín 12, 6–15. [Google Scholar]
- 29. Martin-Moreno JM, Boyle P, Gorgojo L et al. (1993) Development and validation of a food frequency questionnaire in Spain. Int J Epidemiol 22, 512–519. [DOI] [PubMed] [Google Scholar]
- 30. Rodríguez IT, Ballart JF, Pastor GC et al. (2008) Validation of a short questionnaire on frequency of dietary intake: reproducibility and validity. Nutr Hosp 23, 242–252. [PubMed] [Google Scholar]
- 31. Monteiro CA, Cannon G, Levy RB et al. (2019) Ultra-processed foods: what they are and how to identify them. Public Health Nutr 22, 936–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. BEDCA (2006) Base de Datos BEDCA. https://www.bedca.net/bdpub/index.php (accessed November 2020).
- 33. Hernán MA, Hernández-Díaz S, Werler MM et al. (2002) Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology. Am J Epidemiol 155, 176–184. [DOI] [PubMed] [Google Scholar]
- 34. Willett W (2012) Issues in Analysis and Presentation of Dietary Data. En: Nutritional Epidemiology. New York: Oxford University Press; available at https://oxford.universitypressscholarship.com/10.1093/acprof:oso/9780199754038.001.0001/acprof-9780199754038-chapter-13 (accessed December 2020).
- 35. Martínez Steele E, Baraldi LG, Louzada ML da C et al. (2016) Ultra-processed foods and added sugars in the US diet: evidence from a nationally representative cross-sectional study. BMJ Open 6, e009892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vandevijvere S, De Ridder K, Fiolet T et al. (2019) Consumption of ultra-processed food products and diet quality among children, adolescents and adults in Belgium. Eur J Nutr 58, 3267–3278. [DOI] [PubMed] [Google Scholar]
- 37. Julia C, Martinez L, Allès B et al. (2018) Contribution of ultra-processed foods in the diet of adults from the French NutriNet-Santé study. Public Health Nutr 21, 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Costa Louzada ML da, Martins APB, Canella DS et al. (2015) Ultra-processed foods and the nutritional dietary profile in Brazil. Rev Saude Publica 49, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Willett WC, Sacks F, Trichopoulou A et al. (1995) Mediterranean diet pyramid: a cultural model for healthy eating. Am J Clin Nutr 61, Suppl. 6, S1402–S1406. [DOI] [PubMed] [Google Scholar]
- 40. León-Muñoz LM, Guallar-Castillón P, Graciani A et al. (2012) Adherence to the Mediterranean diet pattern has declined in Spanish adults. J Nutr 142, 1843–1850. [DOI] [PubMed] [Google Scholar]
- 41. García-Meseguer MJ, Burriel FC, García CV et al. (2014) Adherence to Mediterranean diet in a Spanish university population. Appetite 78, 156–164. [DOI] [PubMed] [Google Scholar]
- 42. Latasa P, Louzada MLDC, Martinez Steele E et al. (2018) Added sugars and ultra-processed foods in Spanish households (1990–2010). Eur J Clin Nutr 72, 1404–1412. [DOI] [PubMed] [Google Scholar]
- 43. Rauber F, Campagnolo PDB, Hoffman DJ et al. (2015) Consumption of ultra-processed food products and its effects on children’s lipid profiles: a longitudinal study. Nutr Metab Cardiovasc Dis 25, 116–122. [DOI] [PubMed] [Google Scholar]
- 44. Mendonça RD, Pimenta AM, Gea A et al. (2016) Ultraprocessed food consumption and risk of overweight and obesity: the University of Navarra Follow-Up (SUN) cohort study. Am J Clin Nutr 104, 1433–1440. [DOI] [PubMed] [Google Scholar]
- 45. Mendonça RD, Lopes ACS, Pimenta AM et al. (2017) Ultra-processed food consumption and the incidence of hypertension in a Mediterranean cohort: the Seguimiento Universidad de Navarra Project. Am J Hypertens 30, 358–366. [DOI] [PubMed] [Google Scholar]
- 46. Fiolet T, Srour B, Sellem L et al. (2018) Consumption of ultra-processed foods and cancer risk: results from NutriNet-Santé prospective cohort. BMJ 360, k322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Altman DG (1994) Problems in dichotomizing continuous variables. Am J Epidemiol 139, 442–445. [DOI] [PubMed] [Google Scholar]
- 48. Cumsille F & Bangdiwala SI (2000) Categorizing variables in the statistical analysis of data: consequences for interpreting the results. Rev Panam Salud Publica 8, 348–354. [DOI] [PubMed] [Google Scholar]
- 49. Moreira PVL, Baraldi LG, Moubarac J-C et al. (2015) Comparing different policy scenarios to reduce the consumption of ultra-processed foods in UK: impact on cardiovascular disease mortality using a modelling approach. PLoS One 10, e0118353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Moreira PV, Hyseni L, Moubarac J-C et al. (2018) Effects of reducing processed culinary ingredients and ultra-processed foods in the Brazilian diet: a cardiovascular modelling study. Public Health Nutr 21, 181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nagai M, Ohkubo T, Miura K et al. (2016) Association of total energy intake with 29-year mortality in the Japanese: NIPPON DATA80. J Atheroscler Thromb 23, 339–354. [DOI] [PubMed] [Google Scholar]
- 52. Mozaffarian D, Fahimi S, Singh GM et al. (2014) Global sodium consumption and death from cardiovascular causes. N Engl J Med 371, 624–634. [DOI] [PubMed] [Google Scholar]
- 53. Graudal N, Jürgens G, Baslund B et al. (2014) Compared with usual sodium intake, low- and excessive-sodium diets are associated with increased mortality: a meta-analysis. Am J Hypertens 27, 1129–1137. [DOI] [PubMed] [Google Scholar]
- 54. Yang Q, Zhang Z, Gregg EW et al. (2014) Added sugar intake and cardiovascular diseases mortality among US adults. JAMA Intern Med 174, 516–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Abete I, Romaguera D, Vieira AR et al. (2014) Association between total, processed, red and white meat consumption and all-cause, CVD and IHD mortality: a meta-analysis of cohort studies. Br J Nutr 112, 762–775. [DOI] [PubMed] [Google Scholar]
- 56. Larsson SC & Orsini N (2014) Red meat and processed meat consumption and all-cause mortality: a meta-analysis. Am J Epidemiol 179, 282–289. [DOI] [PubMed] [Google Scholar]
- 57. Schwingshackl L, Schwedhelm C, Hoffmann G et al. (2017) Food groups and risk of all-cause mortality: a systematic review and meta-analysis of prospective studies. Am J Clin Nutr 105, 1462–1473. [DOI] [PubMed] [Google Scholar]
- 58. Wang X, Lin X, Ouyang YY et al. (2016) Red and processed meat consumption and mortality: dose-response meta-analysis of prospective cohort studies. Public Health Nutr 19, 893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yang Y, Zhao L-G, Wu Q-J et al. (2015) Association between dietary fiber and lower risk of all-cause mortality: a meta-analysis of cohort studies. Am J Epidemiol 181, 83–91. [DOI] [PubMed] [Google Scholar]
- 60. Veronese N, Solmi M, Caruso MG et al. (2018) Dietary fiber and health outcomes: an umbrella review of systematic reviews and meta-analyses. Am J Clin Nutr 107, 436–444. [DOI] [PubMed] [Google Scholar]
- 61. Luiten CM, Steenhuis IH, Eyles H et al. (2016) Ultra-processed foods have the worst nutrient profile, yet they are the most available packaged products in a sample of New Zealand supermarkets – CORRIGENDUM. Public Health Nutr 19, 530–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Poti JM, Mendez MA, Ng SW et al. (2015) Is the degree of food processing and convenience linked with the nutritional quality of foods purchased by US households? Am J Clin Nutr 101, 1251–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Micha R, Peñalvo JL, Cudhea F et al. (2017) Association between dietary factors and mortality from heart disease, stroke, and type 2 diabetes in the United States. JAMA 317, 912–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Murray CJL, Atkinson C, Bhalla K et al. (2013) The state of US health, 1990–2010: burden of diseases, injuries, and risk factors. JAMA 310, 591–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. International Agency for Research on Cancer (2010) Carbon Black, Titanium Dioxide, and Talc. IARC monographs on the evaluation of carcinogenic risks to humans. https://pubmed.ncbi.nlm.nih.gov/21449489/ (accessed December 2020). [PMC free article] [PubMed]
- 66. Chang X, Zhang Y, Tang M et al. (2013) Health effects of exposure to nano-TiO2: a meta-analysis of experimental studies. Nanoscale Res Lett 8, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Martínez Steele E, Khandpur N, da Costa Louzada ML et al. (2020) Association between dietary contribution of ultra-processed foods and urinary concentrations of phthalates and bisphenol in a nationally representative sample of the US population aged 6 years and older. PLoS One 15, e0236738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zota AR, Phillips CA & Mitro SD (2016) Recent fast food consumption and bisphenol a and phthalates exposures among the U.S. population in NHANES, 2003–2010. Environ Health Perspect 124, 1521–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rudel RA, Gray JM, Engel CL et al. (2011) Food packaging and bisphenol A and bis(2-ethyhexyl) phthalate exposure: findings from a dietary intervention. Environ Health Perspect 119, 914–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Varshavsky JR, Morello-Frosch R, Woodruff TJ et al. (2018) Dietary sources of cumulative phthalates exposure among the U.S. general population in NHANES 2005–2014. Environ Int 115, 417–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gibney MJ, Forde CG, Mullally D et al. (2017) Ultra-processed foods in human health: a critical appraisal. Am J Clin Nutr 106, 717–724. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980021003256.
click here to view supplementary material