Abstract
Objective:
The aim of this meta-analysis was to investigate the association between malnutrition assessed by the controlling nutritional status (CONUT) score and all-cause mortality in patients with heart failure.
Design:
Systematic review and meta-analysis.
Settings:
A comprehensively literature search of PubMed and Embase databases was performed until 30 November 2020. Studies reporting the utility of CONUT score in prediction of all-cause mortality among patients with heart failure were eligible. Patients with a CONUT score ≥2 are grouped as malnourished. Predictive values of the CONUT score were summarized by pooling the multivariable-adjusted risk ratios (RR) with 95 % CI for the malnourished v. normal nutritional status or per point CONUT score increase.
Participants:
Ten studies involving 5196 patients with heart failure.
Results:
Malnourished patients with heart failure conferred a higher risk of all-cause mortality (RR 1·92; 95 % CI 1·58, 2·34) compared with the normal nutritional status. Subgroup analysis showed the malnourished patients with heart failure had an increased risk of in-hospital mortality (RR 1·78; 95 % CI 1·29, 2·46) and follow-up mortality (RR 2·01; 95 % CI 1·58, 2·57). Moreover, per point increase in CONUT score significantly increased 16% risk of all-cause mortality during the follow-up.
Conclusions:
Malnutrition defined by the CONUT score is an independent predictor of all-cause mortality in patients with heart failure. Assessment of nutritional status using CONUT score would be helpful for improving risk stratification of heart failure.
Keywords: Controlling nutritional status, Heart failure, All-cause mortality, Meta-analysis
Heart failure remains a growing public health burden(1). Despite advancements in medical care, heart failure is still the main cause of mortality, morbidity and hospital readmission(2). Malnutrition is a common problem for heart failure(3). Heart failure patients with malnutrition are associated with poor prognosis than those with normal nutrition(4,5). Considering nutritional status can affect disease progression, early detection of malnutrition may improve risk classification of heart failure patients.
Controlling nutritional status (CONUT) score depending on the blood parameters of albumin, total cholesterol and lymphocyte counts is a simple screening tool for evaluation of nutritional status of inpatients(6). According to the CONUT score, individuals with a CONUT score ≥2 are grouped into malnourished (mild 2–4; moderate, 5–8; and severe 9–12).Malnutrition assessed by the CONUT score was correlated with heart failure severity and rehospitalisation in acute heart failure(7). The predictive utility of malnutrition defined by the CONUT score has attracted much attention in patients with heart failure(8,9,10,11,12,13,14,15). However, there are conflicting results on the predictive value of CONUT score in these studies(13,14,15).
No previous systematic review and meta-analysis has systematically addressed the predictive value of CONUT score in patients with heart failure. The aim of this systematic review and meta-analysis was to examine the association of malnutrition defined by the CONUT score with all-cause mortality in patients with heart failure.
Methods
Literature search
The current meta-analysis is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines(16). Two independent authors comprehensively searched the medical databases including PubMed and Embase until 30 November 2020. The following keywords in combination were applied for literature search: ‘Controlling nutritional status’ OR ‘CONUT’ AND ‘heart failure’ AND ‘mortality’ OR ‘death’ (see online Supplemental Text S1). Reference lists of relevant articles were manually scanned to identify any additional studies.
Inclusion and exclusion criteria
Inclusion criteria were as follows: (1) population: patients with heart failure; (2) exposure: baseline CONUT score; (3) comparison: patients with malnutrition defined by the CONUT score ≥2 v. those with normal nutritional status; (4) outcome measures: all-cause mortality; (5) study design: prospective or retrospective observational studies and (6) reported multivariable-adjusted risk estimate of survival outcome for the malnourished v. normal nutritional status or per point CONUT score increase. The exclusion criteria included (1) studies did not select the normal nutrition (CONUT score 0–1) as reference; (2) outcome measures were not of interest and (3) lack of detailed risk summary for the outcome or reported unadjusted risk estimate.
Data extraction and quality assessment
The following data of each study were abstracted surname of the first author, publication year, study design (retrospective or prospective), country, type of patients, sample size, percentage of men, mean/median age or age range, left ventricular ejection fraction (LVEF) at baseline, categorical or continuous analysis of CONUT score, most fully adjusted risk estimate, follow-up duration and adjusted variables. The Newcastle–Ottawa Scale was applied to assess the methodological quality of the included studies(17). Studies with a total score with 7 points or higher were considered as high quality. Two independent authors conducted the data extraction and quality assessment. Any disagreements were settled through discussion.
Data synthesis
The predictive value of CONUT score was expressed by pooling multivariable-adjusted risk ratios (RR) with 95 % CI for the CONUT score ≥2 v. CONUT score 0–1 or per point CONUT score increase. The I 2 statistics and Cochran’s Q test were applied to investigate the heterogeneity between studies. Value of I 2 statistics ≥50 % or P < 0·10 of the Cochran Q test revealed the presence of significant heterogeneity, and then a random effects model was selected. We selected a fixed-effect model in case of without significant heterogeneity. The robustness of the pooling risk summary was investigated by leave-out one study sensitivity analysis. Potential publication bias was checked by funnel plots if more than ten studies analyzed in the outcome. Subgroup analyses were conducted by the study design, patients’ number, mean/median age, follow-up duration, type of heart failure and baseline LVEF. Data analyses were conducted using Stata 12.0 (Stata Corp., College Station, TX).
Results
Search results and studies characteristics
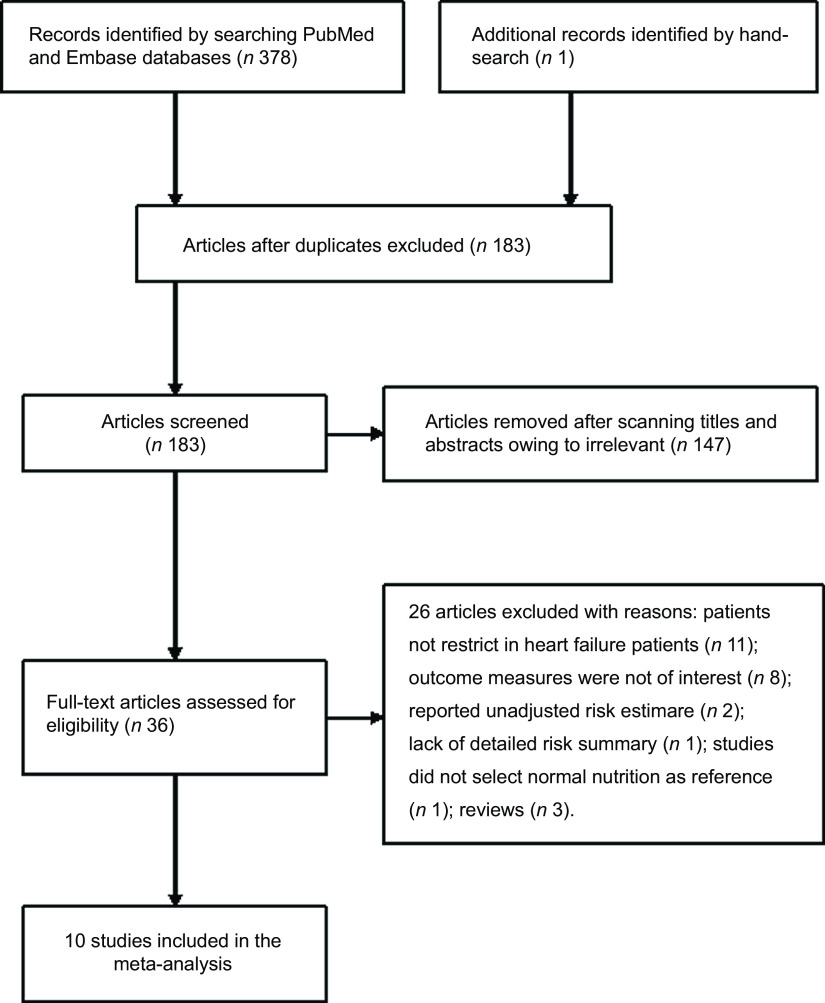
A total of 183 potentially relevant articles were identified after the removal of duplications. Of which, 147 articles were excluded after scanning the titles and abstracts. Thirty-six articles were retrieved for full-text evaluation. After applying our pre-defined inclusion and exclusion criteria, twenty-six articles were further removed for different reasons (Fig. 1). Thus, ten studies(8,9,10,11,13,14,18,19,20,21) were ultimately included in this meta-analysis.
Fig. 1.
Flow chart showing studies selection process
The major characteristics of the included studies are summarised in Table 1. These studies were published from 2017 to 2020 and conducted in UK, Italy, Turkey, Japan, China and Taiwan. Seven studies(8,9,10,11,13,14,20,21) were retrospective designs and two(18,19) were prospective studies. The number of patients of the eligible studies ranged from 170 to 1120, with a total of 5196 heart failure patients. The mean/median age of the patients ranged from 61 to 80 years old. Follow-up duration was up to 3·4 years. Regarding the methodological quality, eight studies(8,9,11,13,14,19,20,21) were grouped as high quality (see online Supplemental Table S1).
Table 1.
Main characteristic of the included studies
| Author/year | Region | Study design | Patients (% male) | Age (years) | LVEF (%) | Cut-off value of CONUT | Outcomes/RR | 95 % CI | Follow-up (years) | Adjusted for variables | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Iwakami 2017(8) | Japan | R | AHF 635 | 62 | 75 | 12 | 38 | 17 | CONUT ≥ 2 Per point increase | Total death 2·01 1·23 |
1·08, 3·72*
1·08, 1·39 |
1·0 | Age, sex, SBP, Hb, eGFR, serum sodium, BMI, statin use, history of malignancy, liver disease, reactive airway disease and depression |
| Sze 2017(13) | UK | R | HF 265 | 62 | 80 | 72–86 | NP | Per point increase | Total death 1·06 |
0·89, 1·27 | 1·6 | Age, sex, Hb, atrial fibrillation, NT-proBNP, creatinine, Na and presence of CAD | |
| Nishi 2017(9) | Japan | R | HF 482 | 61·8 | 71·7 | 13·6 | 40·5 | 15·2 | CONUT ≥ 2 Per point increase | Total death 2·38 1·14 |
1·39, 4·06 1·04, 1·25 |
1·5 | Age, sex for categorical analysis; age, sex, BMI, history of HF hospitalisation, Hb, eGFR, BNP and therapeutic agents |
| La Rovere 2017(10) | Italy | R | HF 466 | 86 | 61 | 11 | 33·7 | 10·6 | Per point increase | Total death 1·42 |
1·11, 1·81 | 1·0 | Multivariable analysis |
| Shirakabe 2017(14) | Japan | R | AHF 458 | 66 | 76 | 67–82 | 40 | 28–53 | CONUT ≥ 2 | Total death 1·49 |
0·99, 2·22* | 1·0 | Multivariable analysis |
| Chien 2019(11) | Taiwan | R | AHF 1120 | 39·4 | 77·2 | 12·6 | ≥50 | Per point increase | Total death 1·08 |
1·02, 1·13 | 3·4 | Age, sex, BMI, SBP, heart rate, prior HF, hypertension, CVD, DM, atrial fibrillation, hyperlipidaemia, eGFR and BNP | |
| Geng 2019(18) | China | P | AHF 505 | 64 | 72 | 14 | NP | CONUT ≥ 2 | Total death 1·77 |
1·20, 3·01 | In-hospital | Multivariable analysis | |
| Sze 2020(19) | UK | P | HF 467 | 67 | 76 | 69–82 | 45 | 35–54 | CONUT ≥ 2 Per point increase | Total death 3·05 1·28 |
1·58, 5·85 1·13, 1·45 |
1·5 | Age, BMI, cardiac rhythm, NYHA class, Charlson score, NT-proBNP, Hb and eGFR |
| Uemura 2020(20) | Japan | R | AHF 170 | 59·4 | 67·6 | 15·1 | 48 | 32–61 | CONUT ≥ 2 Per unit increase | Total death 2·31 1·13 |
1·16, 4·58 1·00, 1·29 |
3·0 | Age, sex, BMI, history of HF admission, CAD, Hb, eGFR and LVEF |
| Alatas 2020(21) | Turkey | R | AHF 628 | 53·7 | 74·7 | 11·8 | NP | CONUT ≥ 5 | Total death 1·79 |
1·13, 2·83 | In-hospital | Age, sex, NT-proBNP and presence of CAD | |
LVEF, left ventricular ejection fraction; CONUT, controlling nutritional status; RR, risk ratio; R, retrospective; AHF, acute heart failure; SBP, systolic blood pressure; eGFR, estimated glomerular filtration rate; HF heart failure; NP, not provided; NT-proBNP, amino-terminal pro-brain natriuretic peptide; BNP, brain natriuretic peptide; DM, diabetes mellitus; P, prospective; NYHA, New York Heart Association; CAD, coronary artery disease.
Results from pooling the CONUT score subgroup in a fixed-effect model.
Categorical analysis of CONUT score on all-cause mortality
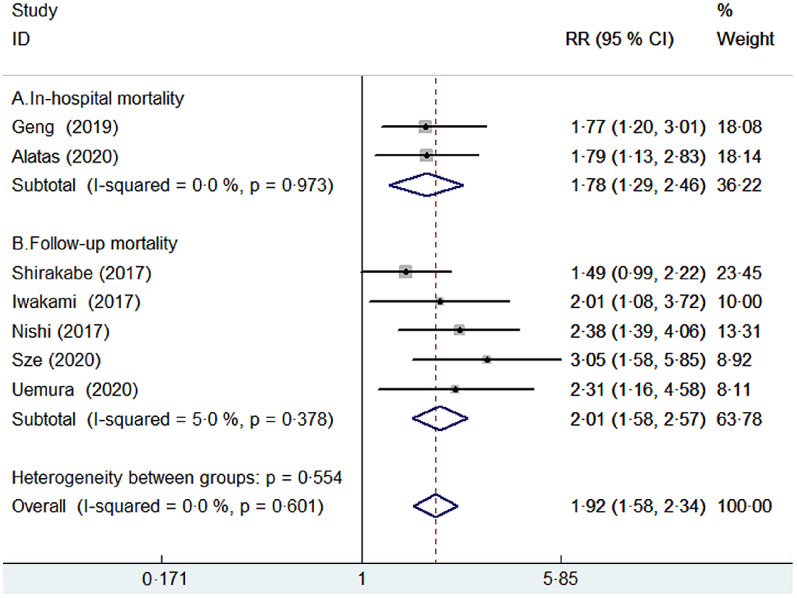
Seven studies(8,9,14,18,19,20,21) provided the categorical analysis of the CONUT score. A fixed-effect model meta-analysis indicated that the pooled RR of all-cause mortality was 1·92 (95 % CI 1·58, 2·34) for the CONUT score ≥2 v. CONUT score 0–1, without significant heterogeneity (I 2 = 0 %; P = 0·601; Fig. 2). Sensitivity analysis by excluding anyone study each time did not significantly alter the pooling risk summary (pooled RR varied from 1·84 to 2·08 and low 95 % CI varied from 1·50, 1·67). Subgroup analysis showed that malnutrition (CONUT score ≥2) was associated with an increased risk of in-hospital mortality (RR 1·78; 95 % CI 1·29, 2·46) and follow-up mortality (RR 2·01; 95 % CI 1·58, 2·57). In addition, significantly predictive values of malnutrition were consistently observed in each pre-defined subgroup (Table 2).
Fig. 2.
Forest plots showing pooled RR with 95% CI of all-cause mortality for patients with malnutrition v. those with normal nutritional status
Table 2.
Subgroup analyses on all-cause mortality by categorical analysis
| Subgroup | Number. of studies | Pooled RR | 95 % CI | Heterogeneity between studies |
|---|---|---|---|---|
| Study design | ||||
| Prospective | 2 | 2·12 | 1·45, 3·09 | P = 0·182; I 2 = 43·8 % |
| Retrospective | 5 | 1·86 | 1·48, 2·34 | P = 0·655; I 2 = 0·0 % |
| Sample sizes | ||||
| <500 | 4 | 1·83 | 1·37, 2·44 | P = 0·943; I 2 = 0·0 % |
| ≥500 | 3 | 2·01 | 1·54, 2·63 | P = 0·240; I 2 = 28·7 % |
| Median/mean age | ||||
| <75 years | 4 | 1·97 | 1·53, 2·55 | P = 0·787; I 2 = 0·0 % |
| ≥75 years | 3 | 1·86 | 1·38, 2·51 | P = 0·182; I 2 = 41·4 % |
| Type of HF | ||||
| AHF | 5 | 1·76 | 1·41, 2·20 | P = 0·837; I 2 = 0·0 % |
| All HF | 2 | 2·63 | 1·74, 3·98 | P = 0·566; I 2 = 0·0 % |
| Baseline LVEF | ||||
| <45 % | 3 | 1·82 | 1·36, 2·42 | P = 0·367; I 2 = 0·2 % |
| ≥45 % | 2 | 2·67 | 1·66, 4·29 | P = 0·566; I 2 = 0·0 % |
| Follow-up duration | ||||
| ≤1 year | 4 | 1·71 | 1·35, 2·16 | P = 0·857; I 2 = 0·0 % |
| >1 year | 3 | 2·54 | 1·78, 3·62 | P = 0·806; I 2 = 0·0 % |
RR, risk ratio; HF heart failure; AHF, acute heart failure; LVEF, left ventricular ejection fraction.
Continuous analysis of CONUT score on all-cause mortality
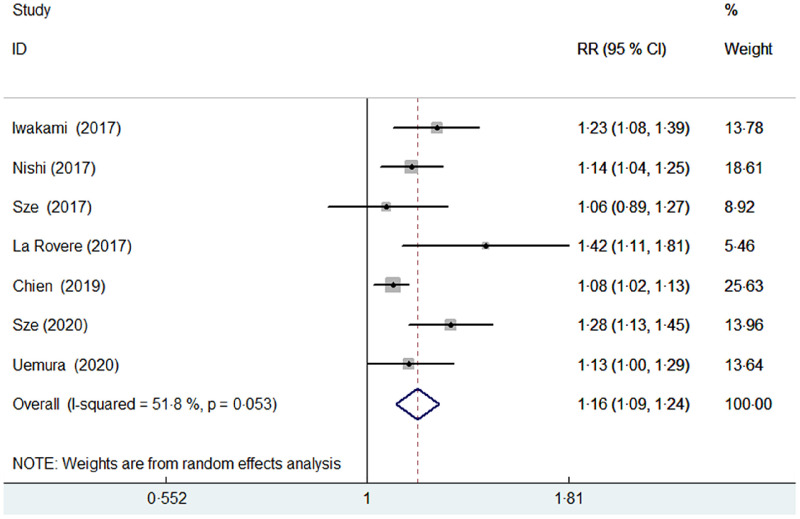
Seven studies(8,9,10,11,13,19,20) reported the predictive value of the CONUT score by continuous analysis. A random effect model meta-analysis indicated that the pooled RR of all-cause mortality was 1·16 (95 % CI 1·09, 1·24) for per point CONUT score increase, with significant heterogeneity (I 2 = 51·8 %; P = 0·053; Fig. 3). Sensitivity analysis by removal of individual study each turn did not significantly change the pooling risk summary (pooled RR varied from 1·14 to 1·19 and low 95 % CI varied from 1·07, 1·11).
Fig. 3.
Forest plots showing pooled RR with 95% CI of all-cause mortality for per point increase in CONUT score
Publication bias
Owing to less than recommended arbitrary number of ten studies, we did not construct the funnel plots or perform Begg’s and Egger’s test to check the publication bias(22).
Discussion
To the best of our knowledge, this is the first systematic review and meta-analysis to assess the association of malnutrition assessed by the CONUT score with all-cause mortality in heart failure patients. This meta-analysis demonstrated that malnutrition assessed by the CONUT score ≥2 was associated with higher risk of all-cause mortality. Heart failure patients with malnutrition had a 1·92-fold exaggerated risk of all-cause mortality. Moreover, when analysed the predictive role value of CONUT score as a continuous variable, per point increase in CONUT score was associated with 16 % exaggerated risk of all-cause mortality. The current meta-analysis confirms the available evidence for nutritional evaluation by the CONUT score in risk classification of patients with heart failure. Additionally, each category increase in CONUT score conferred 37 % higher risk of all-cause mortality in hospitalised patients with heart failure(23).
Our subgroup analysis revealed that the value of malnutrition assessed by the CONUT score in predicting follow-up mortality was stronger than in-hospital death. This finding suggested that the impact of malnutrition on all-cause mortality appeared to strengthen with the lengthening of the follow-up. Furthermore, subgroup analysis by types of heart failure indicated that the predictive performance of CONUT score may be different in acute and chronic heart failure. According to the results of subgroup analysis, malnutrition assessed by the CONUT score was associated with 1·76-fold and 2·63-fold higher risk of all-cause mortality in acute heart failure and total heart failure. These findings indicated that the impact of malnutrition on all-cause mortality may be stronger in chronic heart failure. In addition, the baseline LVEF may also affect the predictive value of malnutrition assessed by the CONUT score in the stratified analysis. The impact of malnutrition on all-cause mortality appeared to be stronger in patients with preserved LVEF than those with reduced LVEF. However, it should be emphasised that the results of subgroup analysis should be interpreted with caution because of the limited number of studies analysed.
A number of simple nutritional screening tools, such as prognostic nutritional index, geriatric nutritional risk index and CONUT score, have been introduced in the assessment of nutritional status in the clinical practice(24). However, which index is superior nutritional assessment method for estimating prognosis has yet been established in heart failure patients. Previous meta-analysis has showed that malnutrition assessed by the geriatric nutritional risk index (calculated by the albumin, body weight and height) was associated with higher risk of all-cause mortality (RR 2·11; 95 % CI 1·72, 2·58) in heart failure patients(25). However, geriatric nutritional risk index was calculated by the albumin, body weight and height. Body weight of the heart failure patients can be affected by oedema and use of diuretics, which is potentially inaccurate in these patients. Another meta-analysis showed that Mini Nutritional Assessment score exhibited the best value in predicting risk of mortality (RR 4·32; 95 % CI 2·30, 8·11) in heart failure patients than other tools(26). However, Mini Nutritional Assessment was difficult to apply in routine clinical setting because this screening tool required a multidimensional evaluation of psychological problems, residential status, mobility, body weight, diets and medications(27).
The exact mechanisms of predictive values of CONUT score in heart failure remain unclear. CONUT score is derived from serum albumin, total cholesterol and lymphocyte count. The parameters of the CONUT score reflect protein and lipid metabolism as well as immune defences. Both hypoalbuminemia(28) and low total cholesterol(29) have been considered as a predictor for worse outcomes in patients with heart failure. Peripheral lymphocyte counts as surrogate biomarker of immune status could independently predict 1-year mortality in patients with acute heart failure(30). Therefore, the combination of inflammatory and nutritional status can synergetic improve the predictive significance.
Although malnutrition assessed by the CONUT score increased all-cause mortality in patients with heart failure, the type of death is largely unclear. Conflicting findings have reported on the association of CONUT score with cardiovascular death(8,9). There may be different mechanistic association between malnutrition and mortality in acute or chronic heart failure. Malnutrition was mainly correlated with reduced cardiac output and subsequent hypoperfusion-associated neurohormonal and inflammatory activity as well as anorexia/malabsorption in patients with acute heart failure(8). While in patients with chronic heart failure, malnutrition was correlated with systemic inflammation, renal dysfunction, immunocompetence and anaemia(31). Future well-designed studies are required to investigate whether there are different mechanistic relationships between nutrition and cardiovascular death or all-cause mortality.
Over 20 % of hospitalised patients with heart failure had moderate-to-severe malnutrition assessed by CONUT score, prognostic nutritional index and geriatric nutritional risk index(23). Loss of appetite and malabsorption induced by heart failure can lead to malnutrition. Considering the higher prevalence and adverse impact of malnutrition on the prognosis, heart failure patients should be routinely assessed for nutritional status(32). Our meta-analysis highlights the importance to evaluate the nutritional status using CONUT score in patients with heart failure. Heart failure patients with higher CONUT score should be accepted more closely monitoring and active nutritional interventions. On the other hand, a multicentre, randomised, controlled clinical trial supported the survival benefit of nutritional intervention in malnourished patients with heart failure(33). Future well-designed clinical trials are warranted to evaluate whether nutritional interventions could potentially improve outcomes in heart failure patients with malnutrition.
This meta-analysis had several potential limitations. First, majority of the analysed studies were retrospective designs, and the inherent selection bias may be existed in these studies. Second, cholesterol level is affected by the use of statins therapy, which could have confounded the assessment of nutritional status using the CONUT score. Third, predictive value of different degree of malnutrition defined by the CONUT score was not evaluated in the current meta-analysis due to insufficient data. Fourth, lack of adjusting both measured and not measured residual confounding variables such as baseline LVEF, Hb, BMI or therapeutic agents may have led to over-estimate the predictive values of the CONUT score. Finally, potential publication bias may affect the pooling risk estimates. However, we did not test the publication bias because of less than recommended arbitrary number of studies analysed.
Conclusion
This meta-analysis consolidates the current evidence that malnutrition assessed by the CONUT score was associated with higher risk of all-cause mortality in patients with heart failure. Assessment of nutritional status using CONUT score can improve risk classification of heart failure. More well-designed prospective studies with large sample sizes are required to demonstrate the current findings.
Acknowledgements
Acknowledgements: None. Financial support: This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors. Conflict of interest: The authors declare no conflicts of interest. Authorship: H.Y.L. and P.Z. searched the literature, selected the studies, extracted data and evaluated the quality. Y.K.Z. and H.C.N. analysed the data. H.Y.L. drafted the manuscript. X.P.L. revised the manuscript. J.L. contributed the study design and interpreted the results. Ethics of human subject participation: Not applicable.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980021002470.
click here to view supplementary material
References
- 1. Bowen RES, Graetz TJ, Emmert DA et al. (2020) Statistics of heart failure and mechanical circulatory support in 2020. Ann Transl Med 8, 827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Butrous H & Hummel SL (2016) Heart failure in older adults. Can J Cardiol 32, 1140–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kinugawa S & Fukushima A (2018) Malnutrition in heart failure: important but undervalued issue. JACC Heart Fail 6, 487–488. [DOI] [PubMed] [Google Scholar]
- 4. Rahman A, Jafry S, Jeejeebhoy K et al. (2016) Malnutrition and cachexia in heart failure. JPEN J Parenter Enteral Nutr 40, 475–486. [DOI] [PubMed] [Google Scholar]
- 5. Sze S, Pellicori P, Kazmi S et al. (2018) Prevalence and prognostic significance of malnutrition using 3 scoring systems among outpatients with heart failure: a comparison with body mass index. JACC Heart Fail 6, 476–486. [DOI] [PubMed] [Google Scholar]
- 6. Ignacio de Ulibarri J, Gonzalez-Madrono A, de Villar NG et al. (2005) CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp 20, 38–45. [PubMed] [Google Scholar]
- 7. Agra Bermejo RM, Gonzalez Ferreiro R, Varela Roman A et al. (2017) Nutritional status is related to heart failure severity and hospital readmissions in acute heart failure. Int J Cardiol 230, 108–114. [DOI] [PubMed] [Google Scholar]
- 8. Iwakami N, Nagai T, Furukawa TA et al. (2017) Prognostic value of malnutrition assessed by controlling nutritional status score for long-term mortality in patients with acute heart failure. Int J Cardiol 230, 529–536. [DOI] [PubMed] [Google Scholar]
- 9. Nishi I, Seo Y, Hamada-Harimura Y et al. (2017) Nutritional screening based on the controlling nutritional status (CONUT) score at the time of admission is useful for long-term prognostic prediction in patients with heart failure requiring hospitalization. Heart Vessels 32, 1337–1349. [DOI] [PubMed] [Google Scholar]
- 10. La Rovere MT, Maestri R, Olmetti F et al. (2017) Additional predictive value of nutritional status in the prognostic assessment of heart failure patients. Nutr Metab Cardiovasc Dis 27, 274–280. [DOI] [PubMed] [Google Scholar]
- 11. Chien SC, Lo CI, Lin CF et al. (2019) Malnutrition in acute heart failure with preserved ejection fraction: clinical correlates and prognostic implications. ESC Heart Fail 6, 953–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kato T, Yaku H, Morimoto T et al. (2020) Association with controlling nutritional status (CONUT) score and in-hospital mortality and infection in acute heart failure. Sci Rep 10, 3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sze S, Zhang J, Pellicori P et al. (2017) Prognostic value of simple frailty and malnutrition screening tools in patients with acute heart failure due to left ventricular systolic dysfunction. Clin Res Cardiol 106, 533–541. [DOI] [PubMed] [Google Scholar]
- 14. Shirakabe A, Hata N, Kobayashi N et al. (2018) The prognostic impact of malnutrition in patients with severely decompensated acute heart failure, as assessed using the Prognostic Nutritional Index (PNI) and Controlling Nutritional Status (CONUT) score. Heart Vessels 33, 134–144. [DOI] [PubMed] [Google Scholar]
- 15. Laura BS, Francesc F, Jonathan F et al. (2016) Prognostic mortality value of the nutritional index (CONUT) in hospitalized patients for acute heart failure. Nutr Clín Diet Hosp 36, 143–147. [Google Scholar]
- 16. Liberati A, Altman DG, Tetzlaff J et al. (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 62, e1–e34. [DOI] [PubMed] [Google Scholar]
- 17. Wells G, Shea B, O’Connell D et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (assessed March 2021).
- 18. Geng Y & Li XM (2019) Relationship between nutritional screening and short-term prognosis in patients with heart failure. Chin Heart J 31, 174–176. [Google Scholar]
- 19. Sze S, Pellicori P, Zhang J et al. (2020) The impact of malnutrition on short-term morbidity and mortality in ambulatory patients with heart failure. Am J Clin Nutr 113, 695–705. [DOI] [PubMed] [Google Scholar]
- 20. Uemura Y, Shibata R, Masuda A et al. (2020) Utility of the nutritional screening in predicting adverse outcome of patients with overweight/obesity and acute heart failure. J Card Fail 26, 566–573. [DOI] [PubMed] [Google Scholar]
- 21. Alatas OD, Biteker M, Yildirim B et al. (2020) Comparison of objective nutritional indexes for the prediction of in-hospital mortality among elderly patients with acute heart failure. Eur J Emerg Med 27, 362–367. [DOI] [PubMed] [Google Scholar]
- 22. Lau J, Ioannidis JP, Terrin N et al. (2006) The case of the misleading funnel plot. BMJ 333, 597–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yoshihisa A, Kanno Y, Watanabe S et al. (2018) Impact of nutritional indices on mortality in patients with heart failure. Open Heart 5, e000730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sze S, Pellicori P, Zhang J et al. (2020) Agreement and classification performance of malnutrition tools in patients with chronic heart failure. Curr Dev Nutr 4, nzaa071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li H, Cen K, Sun W et al. (2020) Prognostic value of geriatric nutritional risk index in elderly patients with heart failure: a meta-analysis. Aging Clin Exp Res. Published online: 6 August 2020. doi: 10.1007/s40520-020-01656-3. [DOI] [PubMed]
- 26. Lin H, Zhang H, Lin Z et al. (2016) Review of nutritional screening and assessment tools and clinical outcomes in heart failure. Heart Fail Rev 21, 549–565. [DOI] [PubMed] [Google Scholar]
- 27. Vellas B, Guigoz Y, Garry PJ et al. (1999) The mini nutritional assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition 15, 116–122. [DOI] [PubMed] [Google Scholar]
- 28. Peng W, Zhang C, Wang Z et al. (2019) Prediction of all-cause mortality with hypoalbuminemia in patients with heart failure: a meta-analysis. Biomarkers 24, 631–637. [DOI] [PubMed] [Google Scholar]
- 29. Yoon CH, Youn TJ, Ahn S et al. (2012) Low serum total cholesterol level is a surrogate marker, but not a risk factor, for poor outcome in patients hospitalized with acute heart failure: a report from the Korean Heart Failure Registry. J Card Fail 18, 194–201. [DOI] [PubMed] [Google Scholar]
- 30. Nunez J, Nunez E, Minana G et al. (2011) Effectiveness of the relative lymphocyte count to predict 1-year mortality in patients with acute heart failure. Am J Cardiol 107, 1034–1039. [DOI] [PubMed] [Google Scholar]
- 31. Nakagomi A, Kohashi K, Morisawa T et al. (2016) Nutritional status is associated with inflammation and predicts a poor outcome in patients with chronic heart failure. J Atheroscler Thromb 23, 713–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sargento L, Longo S, Lousada N et al. (2014) The importance of assessing nutritional status in elderly patients with heart failure. Curr Heart Fail Rep 11, 220–226. [DOI] [PubMed] [Google Scholar]
- 33. Bonilla-Palomas JL, Gamez-Lopez AL, Castillo-Dominguez JC et al. (2016) Nutritional intervention in malnourished hospitalized patients with heart failure. Arch Med Res 47, 535–540. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980021002470.
click here to view supplementary material