Abstract
Nerves and immunologic mediators play pivotal roles in body homeostasis by interacting with each other through diverse mechanisms. The spread of nerves in the tumor microenvironment increases tumor cell proliferation and disease progression, and this correlates with poor patient outcomes. The effects of sympathetic and parasympathetic nerves on cancer regulation are being investigated. Recent findings demonstrate the possibility of developing therapeutic strategies that target the tumor microenvironment and its components such as immune cells, neurotransmitters, and extracellular vesicles. Therefore, examining and understanding the mechanisms and pathways associated with the sympathetic and parasympathetic nervous systems, neurotransmitters, cancer-derived mediators and their interactions with the immune system in the tumor microenvironment may lead to the development of new cancer treatments. This review discusses the effects of nerve cells, immune cells, and cancer cells have on each other that regulate neurogenesis, cancer progression, and dissemination.
Keywords: Cancer, Neuroimmune axis, Sympathetic nervous system, Parasympathetic nervous system, Tumor microenvironment
1. Introduction
Cancer is an extremely complex disease to fight because of its social and psychological aspects as well as the physical pain and destruction that it causes, and its incidence is increasing worldwide. In 2020, about 10 million deaths worldwide were cancer deaths (Sung et al., 2021). In clinical management of cancer, the most widely preferred treatment methods are insufficient to meet patient needs because of several limitations such as associated toxicity, adverse effects, high cost, and drug resistance. Therefore, discovering new mechanisms and biomarkers to aid in the diagnosis and treatment of advanced cancers is needed to achieve better patient outcomes.
Cancer cells divide and grow uncontrollably and spread to other parts of the body. This can result from many mechanisms, such as the activation of proto-oncogenes by translocations and point mutations and the inactivity of genes that normally limit cell proliferation. Although previous research focused on the ways in which tumor cell-intrinsic pathways (e.g., deregulation of proliferative signals, activation of oncogenes) contribute to the progression of cancer, growing evidence suggests that tumor cell-extrinsic mechanisms heavily influence the tumor microenvironment, which is an essential but complex setting for cancer cell dissemination that includes nerves and specific molecular signals (Amit, Na’ara, & Gil, 2016). Studies conducted in recent years have shown that tumor development is supported by factors such as the immune system and perineural invasion (Liebig, Ayala, Wilks, Berger, & Albo, 2009) that are significant players in the tumor microenvironment. Neurons that interact with immune and structural cells in the perineural niche drive tumorigenesis for several cancer types, including pancreatic, gastric, hematopoietic, and non-melanoma skin cancers (Hunt, Andujar, Silverman, & Amit, 2021). Immune cells such as lymphocytes, macrophages, and natural killer (NK) cells have a prominent role in antitumor responses and markedly regulate tumor proliferation and cancer metabolism (Leone & Powell, 2020). Therefore, the role of the neuroimmune axis in cancer is an intriguing research field.
Recent studies have shown that the relationship between nerves and cancer is not unilateral but rather bidirectional. Although the sympathetic and parasympathetic nervous systems play a role in the behavior of tumor cells, investigators have shown that neurotrophic growth factor, axon guidance molecules, vascular endothelial growth factor (VEGF), and chemical messengers secreted by tumor cells increase neoneurogenesis, axonogenesis, and angiogenesis (O’Donnell, Chance, & Bashaw, 2009; Palm & Entschladen, 2007; Zhang et al., 2021). The tumor-infiltrating components of tissues include dense inflammatory molecules and various cell types such as endothelial cells, immune cells, smooth muscle cells, epithelial cells, adipocytes, and fibroblasts, that maintain homeostasis in the tumor microenvironment or promote tumor development (Kamiya, Hiyama, Fujimura, & Yoshikawa, 2021; Scheff & Saloman, 2021). This neuroimmune interaction plays a significant role in the mechanisms of treatment resistance, growth of cancer cell population, and angiogenesis (Kamiya et al., 2021; Scheff & Saloman, 2021).
In this review article, we discuss recent developments in the relationship between neuroimmune cells and cancer by focusing on the mechanisms of the sympathetic and parasympathetic nervous systems, neurotransmitters, cancer-derived mediators, and the role of the neuroimmune axis in cancer progression.
2. Sympathetic nervous system
The sympathetic nervous system stimulates tumor growth and metastasis (Cole, Nagaraja, Lutgendorf, Green, & Sood, 2015) via epinephrine/norepinephrine, and catecholamines act directly on tumors via endothelial cells, immune cells, and fibroblasts. Likewise, sympathetic innervation plays a role in early cancer metastasis by activating endoneurial macrophage infiltration in the tumor microenvironment (Hunt et al., 2021; Jobling et al., 2015). Researchers have shown that the neurotransmitters of the sympathetic system promote the progression of prostate (Magnon et al., 2013; Zahalka et al., 2017), stomach (Liao et al., 2010; Lu et al., 2017), pancreatic (Ceyhan et al., 2009; Kim-Fuchs et al., 2014; Renz et al., 2018), breast (Campbell et al., 2012; Kamiya et al., 2019; Setordzi, Chang, Liu, Wu, & Zuo, 2021; Sloan et al., 2010; Tanaka & Sakaguchi, 2017; Yu et al., 2021), ovarian (Armaiz-Pena et al., 2013; Huang et al., 2016; Kang et al., 2016; Thaker et al., 2006), skin (Calvani et al., 2018; Calvani et al., 2019; Dal Monte et al., 2014; Vander Heiden, Cantley, & Thompson, 2009), brain (He et al., 2017; Venkatesh et al., 2015; Venkatesh et al., 2017), head and neck (Amit et al., 2020; Bernabe, Tamae, Biasoli, & Oliveira, 2011; Bravo-Calderon et al., 2020; Lopes-Santos, Bernabe, Miyahara, & Tjioe, 2021; Yamanaka et al., 2002), and colon (Chin et al., 2016; Ciurea et al., 2017; Hicks, Murray, Powe, Hughes, & Cardwell, 2013; Zhou et al., 2018) cancers.
Magnon et al., by performing surgical or chemical sympathectomy in a mouse model, showed that adrenergic signals have an important role in prostate cancer metastasis and tumor development. Loss of (β2-adrenergic receptors (β2-AR) and (β3-adrenergic receptors (β3-AR) causes decreased tumor development and dissemination (Magnon et al., 2013). Furthermore, (β2-AR signals maintain endothelial cell metabolism and promote angiogenesis by supporting aerobic glycolysis rather than oxidative phosphorylation (Zahalka et al., 2017). In gastric cancer cells, cyclooxygenase 2 (COX-2), VEGF, and matrix metalloprotease-2 and -9 (MMP-2/–9) levels were downregulated by exposure to the non-selective (β-adrenergic antagonist, propranolol, with inhibited expression of nuclear factor-κB (Liao et al., 2010). Using a human gastric cancer cell line, surgically resected tissue specimens, and a mouse model, researchers showed that isoprenaline increases neovascularization in gastric cancers by upregulating the expression of plexin-1 and VEGF receptor-2 (Lu et al., 2017).
Investigators found that neural density, neural hypertrophy, and norepinephrine levels were higher in pancreatic cancer tissue than in normal pancreatic tissue (Ceyhan et al., 2009). An increased norepinephrine level causes an increase in neurotrophic growth factor levels and induces axonogenesis via tropomyosin receptor kinase (Trk) receptors. Inhibition of (β-AR expression interrupts bidirectional interaction between cancer-derived neurotrophic factors and adrenergic signaling, reduces pancreatic tumorigenesis, and contributes to the prolongation of survival (Renz, Takahashi, et al., 2018). Another study showed that isoproterenol increased cyclic adenosine monophosphate (cAMP) levels in tumor cells, whereas MMP-2/–9 levels were increased twofold and fourfold, respectively, by β-adrenoceptor signaling (Kim-Fuchs et al., 2014).
In breast cancer studies, Kamiya et al. found that neurotransmitters secreted from sympathetic nerves and signals of β2-AR increased cancer progression using newly generated retrograde adeno-associated virus vector-based genetic modeling (Kamiya et al., 2019). Although sympathetic nerve denervation did not change the numbers of CD4+ or CD8+ T cells, it increased the expression of programmed death ligand 1 (PD-L1) (Setordzi et al., 2021) and IFN-γ (Setordzi et al., 2021) in these cells and increased the expression of forkhead box protein 3 (Tanaka & Sakaguchi, 2017) in CD4+ T cells. These immune checkpoint molecules and cytokines play important roles in immunosuppression in the tumor microenvironment and will be influential in developing new immunotherapies to slow cancer progression. In addition to the development of new treatment methods that aim to slow growth of tumor, the prevention of breast cancer-related metastasis plays an important role in stopping the progression of cancer. Studies using beta blockers demonstrated that sympathetic nerves increase cancer cell migration into tissues by increasing tumor-associated macrophage (TAM) infiltration and receptor activator of nuclear factor kappa-B ligand expression (Campbell et al., 2012; Sloan et al., 2010). This stimulatory effect may arise directly from tumor cells or indirectly from the sites of metastases (Campbell et al., 2012).
Studies of ovarian cancers have had results similar to those of prostate, pancreatic, and breast cancers, suggesting that the sympathetic nervous system affects cancer. Thaker et al. showed a positive correlation between tumor weight and nodal metastasis number with increasing duration of exposure to chronic stress, which was also shown by using β-AR+ ovarian cancer cell lines. Researchers have not shown this correlation between tumor progression and metastasis in β-AR-null ovarian cancer cell lines. However, they did show that β-AR exerts its effects via the cAMP/PKA signaling pathway and increases tumor vascularization by increasing VEGF, MMP-2, and MMP-9 expression (Thaker et al., 2006). Furthermore, norepinephrine induces Src activation via the cAMP/PKA signaling pathway, and this activation increases tumor growth, migration, and invasion by activating Rap1 and inhibiting extracellular signal-regulated kinase (ERK) in cancer cells (Armaiz-Pena et al., 2013).
In another study of 237 ovarian cancer cases, AR expression was high in patients experiencing depression and anxiety. In the same study, more specimens with poorly differentiated histology and higher tumor stages were found in β2-AR+ tumors than in β2-AR− tumors (T. Huang et al., 2016). Also, Kang et al. showed that norepinephrine induces expression of dual-specificity phosphatase 1 (DUSP1), which inhibits the c-Jun/c-Jun N-terminal kinase (JNK) pathway, which is important for cancer cell apoptosis (Kang et al., 2016).
β3-AR, which is involved in tumor progression and metastasis, activates glycolytic enzymes by inducing uncoupling protein 2 expression, which mediates Warburg metabolism (Vander Heiden et al., 2009) in melanoma cells (Calvani et al., 2018). Whereas low blood or oxygen supply conditions activate β3-AR diminished levels of β3-AR contribute to apoptosis of melanoma cells by downregulating nitric oxide synthesis (Dal Monte et al., 2014). Moreover, this reduction in β3-AR levels was correlated with increased numbers of NK and CD8+ cells as well as reduced numbers of regulatory T cells and myeloid-derived suppressor cells (MDSCs) in melanoma cases (Calvani et al., 2019).
Isoproterenol causes an increase in the number of glioblastoma cells through the ERK1/2 pathway. β-AR activation has enhanced invasion and metastasis by increasing the expression of metalloproteinases (MMP-2 and MMP-9) in glioblastomas, which is consistent with the effect of this activation on other cancer types (He et al., 2017). Venkatesh et al. showed that neuroligin-3, a synaptic protein that acts as a mitogen, stimulates the phosphatidylinositol-3-kinase (PI3K)/mammalian target of rapamycin pathway in high-grade glioma cells and that neuroligin-3 expression correlates with poor prognosis (Venkatesh et al., 2015). In addition, blocking the release of neuroligin-3 inhibits high-grade glioma growth (Venkatesh et al., 2017).
We showed that molecules that spread from cancer cells regulate adrenergic differentiation in nerves and that the resulting newly formed adrenergic nerves increase the progression of oral squamous cell cancer. We also found a positive correlation between nerve density and aggressiveness of oral cavity cancers by examining specimens from patients with oral squamous cell cancer (Amit et al., 2020).
In another study, solute carrier family 6 member 2, which encodes norepinephrine transport protein, played a role in β-adrenergic signaling in head and neck squamous cell carcinoma cases (Lopes-Santos et al., 2021). β-adrenergic signals markedly increase the expression of metalloproteinases and laminins and reduce the levels of adhesion molecules such as epithelial cell adhesion molecule, vascular cell adhesion molecule 1, and intercellular adhesion molecule 4. Investigators showed that β2-AR increases the expression of proinflammatory genes and decreases the expression of genes related to cancer cell stemness (Lopes-Santos et al., 2021). Also, norepinephrine increased the release of interleukin(IL)-6 in oral squamous cell cancer cells, which indirectly increased the growth of cancer cells (Bernabe et al., 2011). Contrary to this finding, researchers showed that the increased effect of β2-AR in oral squamous cell cancers conversely affects the migration of tumor cells (Bravo-Calderon et al., 2020; Yamanaka et al., 2002). In view of these findings, we can say that the adrenergic system can even differentially regulate the same cancer types. However, confirming this requires further study.
Contrary to that in patients with other cancer types, researchers detected sympathetic nerves in patients with colon cancers in the early stages of the disease and found that it was associated with a good prognosis and reduced lymph node metastasis (Zhou et al., 2018). Hicks et al. reported that postdiagnostic beta blocker use had no effect on mortality in their case-control study of 4794 colorectal cancer patients (Hicks et al., 2013). In contrast, in a study of 48 patients, Ciurea et al. showed a direct correlation of expression of β-AR with tumor progression, invasion, and lymph node metastasis (Ciurea et al., 2017). Similarly, Chin et al. in their study of the mechanism of the pro-tumorigenic effect of β-AR, noted that β2-AR blockade suppressed the development of colorectal cancer cells (Chin et al., 2016). Inhibition of β2-AR signaling increased the expression of G1-checkpoint cyclin-dependent kinase (CDK) inhibitors such as p21 and p27 and decreased the formation of the cyclin D1/cyclin-dependent kinase 44 complex. This prevented tumor cells from transitioning to the G2/M phase by reducing histone H3 phosphorylation and increasing histone H2A formation. In addition, β2-AR inhibition increased caspase-3, caspase-8, caspase-9, and PARP cleavage; downregulated Bcl-2, BcL-xL, and Mcl-l; and upregulated Bcl-2 Associated X-Protein (Bax), Bcl-2 homologous antagonist/killer (Bak), p53 upregulated modulator of apoptosis (Puma), and Bcl-2 like protein 11 (Bim). Through these mechanisms, β2-AR inhibition increased apoptosis of colorectal cancer cells (Chin et al., 2016) (Fig. 1).
Fig. 1.
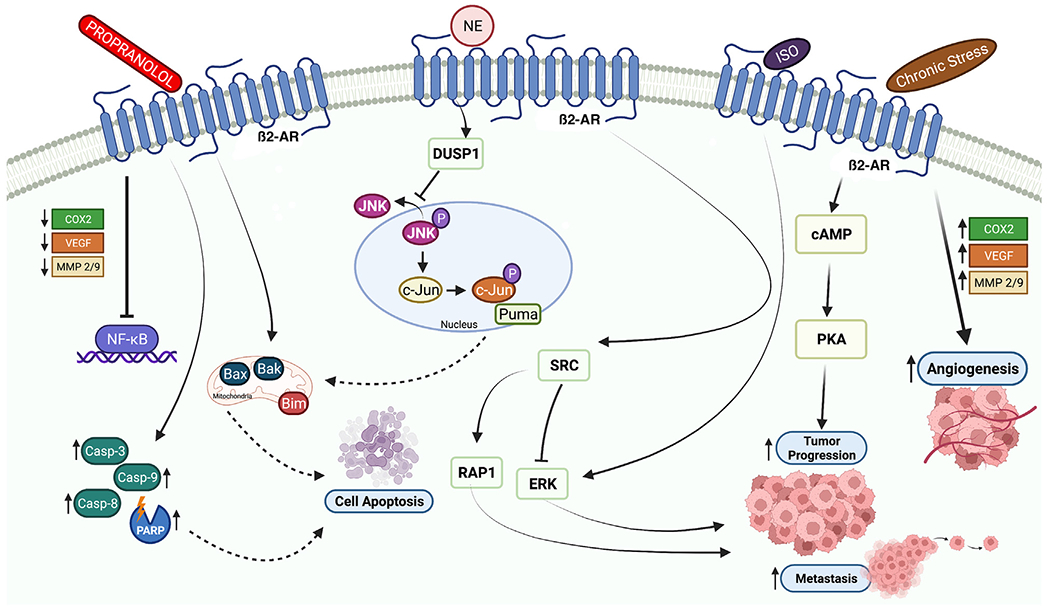
Schematic showing how inducers or blockers of the sympathetic nervous system affect tumor progression, metastasis, and apoptosis. Propranolol causes cell apoptosis through caspases or the mitochondrial cell death pathway. Norepinephrine induces expression of the DUSP1, which inhibits cell apoptosis through regulation of the JNK pathway in the nucleus and through the mitochondrial cell death pathway. As shown, isoprenaline and chronic stress promote tumor progression, angiogenesis, and metastasis. The figure was created with BioRender.com.
3. Parasympathetic nervous system
The vagus nerve plays an important role in parasympathetic innervation of the subdiaphragmatic organs. Moreover, it affects tumorigenesis and cancer stem cells directly or indirectly, which results in dissimilar consequences for diverse types of cancers. The direct effect of vagotomy in gastric cancer patients is reduction of tumorigenesis by inhibition of cholinergic receptor muscarinic M3 (CHRM3) (Wang et al., 2018) through Wnt signaling (Zhao et al., 2014). The vagotomy outcome in colorectal cancer patients is similar to that in gastric cancer patients: decreased tumor proliferation. In contrast, vagotomy promotes pancreatic tumorigenesis by increasing proliferation of tumors (Renz et al., 2018). In light of these findings, the effects of the vagus nerve on tumorigenesis are diverse depending on the type of cancer.
The vagus nerve reduces cancer proliferation by inhibiting the sympathetic nervous system in the tumor microenvironment (Clancy et al., 2014). Consistently, the vagus nerve has promoted tumor progression by inducing β-adrenergic signaling indirectly (Renz, Takahashi, et al., 2018). Studies involving denervation of the vagus nerve showed that vagotomy increased the invasiveness of tumors, increased secretion of cytokines such as tumor necrosis factor-α (TNF-α), and decreased survival rates. Antitumor effects of the vagus nerve can occur as a result of TNF-α release from TAMs (Hutchings, Phillips, & Djamgoz, 2020). Also, loss of vagal signals around the cancer cells has increased the number of TAMs, resulting in increased in TNF-α levels (Partecke et al., 2017). Renz et al. showed that after parasympathetic denervation, expression of cholinergic receptor muscarinic type 1 receptor (Chrm1) in epithelial cells in pancreatic tumors increased cancer development by suppressing the epidermal growth factor receptor/mitogen-activated protein kinase (EGFR/MAPK) and PI3K/AKT cascades, whereas treatment with bethanechol after vagotomy reduced tumorigenesis and CD44 expression. They also showed that inhibition of CD11b+ myeloid cells suppressed pancreatic carcinogenesis (Renz, Tanaka, et al., 2018). Hence, vagotomy has substantial effects on pancreatic cancer growth and spread, altering tumor morphology and shortening survival (Partecke et al., 2017).
In an in vivo study, Magnon et al. showed that prostate cancer metastasis to lymph nodes increased via Chrm1 by using carbachol, a non-selective acetylcholine (ACh) receptor agonist. They showed that when Chrm1 was absent from the tumor microenvironment, no effects of carbachol on metastasis were observed, and carbachol had no effect on tumor growth. Similarly, giving pirenzepine (a Chrm1-specific antagonist) reduced metastasis of prostate cancer cells (Magnon et al., 2013). In contrast, Coarfa et al. by using chemical and physical denervation, found that bilateral botulinum toxin (Botox) application and spinal cord injury had the same effect on prostate tumor progression. They also found that the incidence of prostate tumors and overall prostate weights were reduced. Furthermore, they showed that the rate of apoptosis of tumor cells was higher in prostate cancer specimens taken from patients injected with Botox than in those from control patients (Coarfa et al., 2018).
In studies conducted with genetic manipulation in breast cancer cases, activation of the parasympathetic nervous system reduced tumor growth and distant metastasis by decreasing the expression of programmed cell death protein 1 (PD-1) and PD-L1 (Kamiya et al., 2019). Whereas the parasympathetic nervous system, like the sympathetic nervous system, did not cause any change in the number of CD4+ or CD8+ tumor-infiltrating lymphocytes, it decreased the expression of PD-1 in CD4+ T cells and the expression of PD-1 ligands in tumor tissue. It increased the release of IFN-γ from CD4+ and CD8+ tumor-infiltrating lymphocytes (Kamiya et al., 2019). Erin et al. showed an increase in the incidence of distant metastasis of breast cancers to the adrenal glands after vagotomy (Erin, Barkan, & Clawson, 2013).
The effects of vagotomy on gastric cancers were believed to be cancer-inducing in the 1980s. Researchers showed that vagotomy increased gastric pH levels, increased the atypical glandular hyperplasia level, and increased the incidence of adenocarcinomas (Tatsuta et al., 1985; Tatsuta, Iishi, Yamamura, Baba, & Taniguchi, 1988). In a rat study, the effect on tumors of vagotomy performed before carcinogen administration was unclear, but performing vagotomy after carcinogen administration did cause a significant increase in the number of gastric tumors (Tatsuta et al., 1985; Tatsuta et al., 1988). However, as in recent studies, vagotomy or treatment with Botox increased the effectiveness of chemotherapy while prolonging survival (Zhao et al., 2014).
Authors reported that vagal nerve denervation reduced the expression of Wnt signaling, a regulator of tumorigenesis (Zhao et al., 2014) (Fig. 2). They also showed that gastric tumorigenesis was suppressed by pharmacological inhibition and genetic knockout of the M3 receptor. In addition, Hayakawa et al. found that tuft cells secreted ACh, which is involved in early tumor growth and neuron formation around cancer cells. They showed that the positive-feedback loop of ACh and neurotrophic growth factor was responsible for innervation in the tumor microenvironment. The researchers further showed that tyrosine kinase is a primary receptor in this loop. In addition, they reported that muscarinic ACh receptor M3 (M3R) activates Wnt with Yes-associated protein (YAP), which is a transcriptional coactivator for Wnt/β-catenin signaling (Hayakawa et al., 2017). Another study showed that ACh, through the M3 receptor, increased gastric cancer cell proliferation and induced the phosphorylated ERK1/2 and AKT pathways. Moreover, the M3-selective antagonist 1,1-dimethyl-4-diphenylacetoxypiperidinium iodide (4-DAMP) reduced the phosphorylation of ERK and AKT. ACh also activated the EGFR signal via M3R while promoting cell proliferation, whereas blocking the EGFR signal reduced cell proliferation (Yu et al., 2017).
Fig. 2.
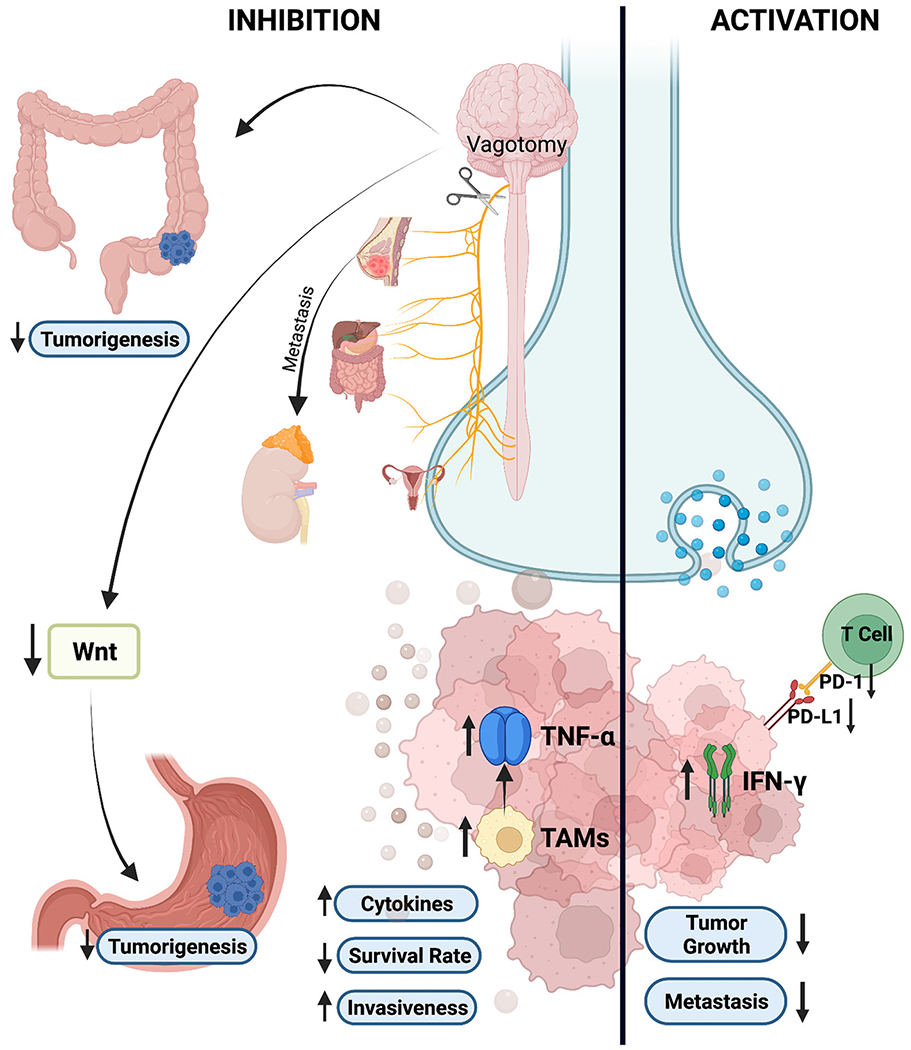
Schematic showing how parasympathetic nervous system activation reduces tumor growth and metastasis via decreased expression of PD-1 and PD-L1 while increasing release of IFN-γ from CD4+ and CD8+ tumor-infiltrating lymphocytes. As shown, denervation of the parasympathetic system or vagotomy decreases gastric tumorigenesis through Wnt signaling and causes a similar outcome in colorectal cancer cases. Increased cytokines, TNF-α, and TAMs; high invasiveness; and decreased survival rates are the results of vagotomy in patients with other cancers such as prostate, pancreatic, and breast cancer. The figure was created with BioRender.com.
Similar studies have shown that the mechanisms found in gastric cancers are also found in gastrointestinal system cancers (Belo et al., 2011; Cheng et al., 2008). Cheng et al. reported that ACh-dependent cell proliferation in colon cancers is mediated by M3R and includes an autocrine mechanism (Cheng et al., 2008). Binding of ACh to CHRM3 activates MMP-7 and increases cell proliferation, cell survival, and migration. This occurs via MAPK/ERK1/2 and PI3K/AKT pathways, which are regulated by epidermal growth factor receptor (Belo et al., 2011). Likewise, the use of scopolamine butylbromide, a muscarinic receptor antagonist, has reduced the numbers and sizes of tumors in small intestinal neoplasms (Raufman et al., 2011) (Fig. 3).
Fig. 3.
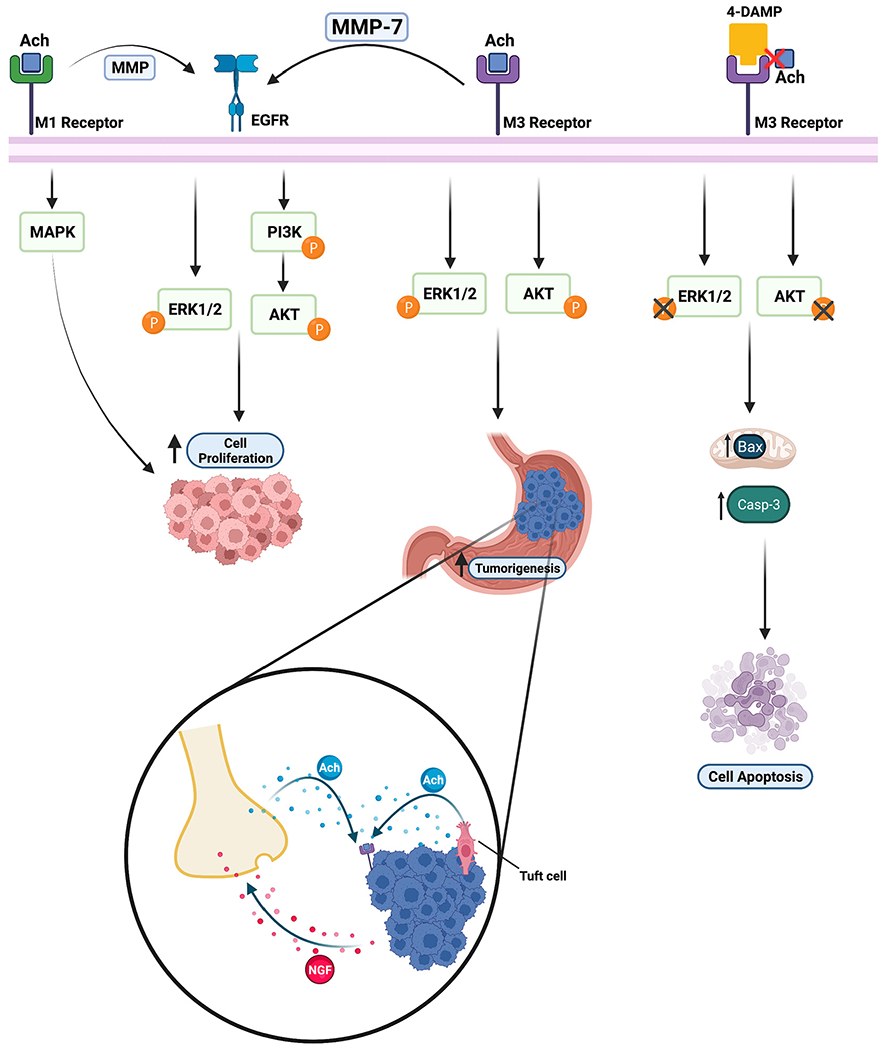
Schematic showing how acetylcholine and its antagonist affect cell apoptosis, cell proliferation, and tumorigenesis. Ach binding to muscarinic acetylcholine receptor M1 (M1R) induces EGFR-mediated tumorigenesis via MMPs and enhances cell proliferation. Muscarinic acetylcholine receptor M3 (M3R) promotes tumorigenesis through the ERK1/2 and AKT pathways. M3R increases cell proliferation by activating MMP7, which induces the ERK1/2 and PI3K/AKT pathways regulated by the EGFR signal. 4-DAMP, an M3R antagonist, promotes cell apoptosis via caspase-3 and Bax in mitochondria by inhibiting the ERK and AKT pathways. Tuft cells and nerves release ACh, upregulating NGF expression, which promotes cancer cell proliferation in gastric epithelium. The figure was created with BioRender.com.
Furthermore, investigators demonstrated that parasympathetic nerve fiber density increased the lymph node metastasis of colon cancers (Zhou et al., 2018). They showed that patients with parasympathetic nerve inhibition had a better prognosis. Also, the expression of α9 nicotinic ACh receptor (nAChR) was directly proportional to the number of parasympathetic nerves, with higher numbers found in those with lymph node metastases, and tumors with α9 nAChR were more advanced (Zhou et al., 2018). The presence of α9 nAChR has also been associated with increased metastasis with activation of vimentin and fibronectin in breast cancers cases (Lee et al., 2010). In addition, α7 nAChR has contributed to pancreatic and lung tumor development via the PI3K/AKT, nuclear factor-κB, and signal transducer and activator of transcription (STAT) pathways (Zhou et al., 2018).
4. Neurotransmitters
Neurotransmitters secreted from neurons and other cells in the tumor microenvironment link intratumoral nerves and cancer cells, thus affecting cancer invasion, metastasis, and progression. One of these neurotransmitters, glutamate, which plays a critical role in nutrition, metabolism, and signaling, is one of the most abundant amino acids (Brosnan & Brosnan, 2013). Within the central nervous system, glutamate is the major excitatory neurotransmitter, and its product, gamma-aminobutyric acid (GABA), is the major inhibitory neurotransmitter (Brosnan & Brosnan, 2013). Within cancer cells, glutamate is a potential growth factor and energy source for tumor progression (Reitzer, Wice, & Kennell, 1979; Stepulak, Rola, Polberg, & Ikonomidou, 2014; Yu, Wall, Wangari-Talbot, & Chen, 2017). Glutamate plays a role in the Krebs cycle and lipogenesis pathway, which are important sources of energy for cancer cells (Budczies et al., 2015; Koochekpour, 2013). Researchers have thought that glutamate can be used as a diagnostic biomarker because of its higher levels in cancer cells comparing with normal tissues (Budczies et al., 2015; Koochekpour, 2013). In a study of primary prostate cancer, serum glutamate levels have correlated directly with Gleason scores and tumor aggressiveness (Koochekpour, 2013). Decreased glutamate levels decrease prostate tumor growth, and invasion; and stimulate apoptosis of prostate cancer cells (Koochekpour, 2013; Koochekpour et al., 2012). Similarly, authors reported that glutamate levels were much higher in breast tumors than in normal breast tissue, whereas glutamine levels did not differ notably (Budczies et al., 2015). Based on these findings, a high glutamate/glutamine ratio, which was thought to be a positive prognostic factor, was associated with prolonged survival of breast cancer (Mohammadpour et al., 2019).
Glutamate receptors consist of two main groups: G-protein-coupled metabotropic glutamate receptors (mGluRs) and ionotropic glutamate receptors (iGluRs) (Stepulak et al., 2014). Recently, researchers found both receptors in peripheral tissues, such as the lung, breast, pancreas, bone, and skin, in addition to the central nervous system (Du, Li, & Li, 2016; Yi, Talmon, & Wang, 2019). Investigators detected increased or abnormal expression of mGluRs, which causes tumor growth via autocrine or paracrine signaling, in several cancers. Metabotropic glutamate receptor 1 can be oncogenic in epithelial cells by activating multiple cancer-signaling pathways, such as the MAPK and AKT pathways (Martino et al., 2013).
In a study of non-small cell carcinomas, Tamura et al. found aberrant expression of methylated N-methyl-d-aspartate receptor type 2B (NMDAR2B) to be an important prognostic factor. The presence of NMDAR2B has been shown associated with a better prognosis, especially for squamous cell carcinoma (Tamura et al., 2011). In a genetic study of esophageal squamous cell carcinoma, NMDAR2B was more methylated in tumors than in normal esophageal mucosa. Ifenprodil, a specific NMDAR2B inhibitor, blocks NMDAR-mediated apoptosis (Kim et al., 2006). In addition, GluN2B-mediated NMDAR signaling has been involved in metastasis of breast tumors to the brain and has been associated with poor prognosis (Zeng et al., 2019). Several studies showed that human T-cell leukemia and human T-cell lymphoma cell lines express AMPA GluR3, mGluR5, and mGluR1 (Ganor & Levite, 2014). Pharmacological inhibition of AMPA receptors in pancreatic adenocarcinoma cases reduces the invasion and migration of cancer cells and inhibits pancreatic tumor growth (Herner et al., 2011). In addition, activation of the AMPA receptor induces K-ras activity and the MAPK pathway (Herner et al., 2011).
GABA, which is primarily synthesized from glutamate, is a main inhibitory neurotransmitter in the central nervous system (Boonstra et al., 2015; Brzozowska, Burdan, Duma, Solski, & Mazurkiewicz, 2017). Similar to glutamate receptors, GABA exerts its effects on ionotropic (GABA-A or GABA-C) and metabotropic (GABA-B) GABA receptors (Brzozowska et al., 2017). GABA increases intracellular calcium levels and activates the MAPK/Erk cascade (Takehara et al., 2007). Induction of GABA-B receptor expression increases the migration of cancer cells by increasing the phosphorylation of ERK1/2 and expression of MMP-2 (Zhang et al., 2014). In a study of breast cancers, a high GABA level was directly proportional to a prolonged median overall survival duration(Brzozowska et al., 2017). GABA secreted from cancer cells increases beta-catenin signaling, which causes proliferation of tumor cells and suppression of intratumoral infiltration of CD8+T cells (Huang et al., 2022). Additionally, secretion of GABA vesicles from neuroendocrine prostate cancer cells was mediated by GABA type B receptor subunit 1 which was implicated to have a role in promotion of metastasis (Solorzano et al., 2018).
Dopamine is a monoamine catecholamine neurotransmitter that regulates tumor angiogenesis and vasculogenesis. In addition, it increases the effectiveness of anti-cancer drugs (Lan et al., 2017). Dopamine exerts its effects by using G-protein-coupled dopamine receptors (Lan et al., 2017). Dopamine inhibits tumor growth (Senogles, 2007), reduces vascular permeability and microvessel density (by decreasing VEGF receptor-2, focal adhesion kinase, and MAPK phosphorylation) (Sarkar et al., 2004; Sarkar, Chakroborty, Chowdhury, Dasgupta, & Basu, 2008), and reduces hypoxia-inducible factor-1α expression in cancer cases (Qin et al., 2015). When used with anticancer drugs, dopamine has prolonged life and reduced cancer progression (Sarkar et al., 2008). Dopamine inhibits insulin-like growth factor-1-induced gastric cancer cell proliferation via D2 receptors (Ganguly et al., 2010). In another study, dopamine inhibited tumor growth and neovascularization via D2 receptors by suppressing VEGFA-induced ERK1/ERK2 phosphorylation and MMP-9 synthesis (Chakroborty et al., 2008) (Table 1).
Table 1.
Neurotransmitters that promote tumor growth and dissemination.
| Neurotransmitter | Receptors | Cancer Cell Types | References |
|---|---|---|---|
| Glutamate | G-protein coupled metabotropic glutamate receptors (mGluRs) | Melanoma | (Martino et al., 2013) |
| Ionotropic glutamate receptors (iGluRs) | Non-small cell lung carcinoma Esophageal squamous cell carcinoma Breast cancer cell-brain metastasis Human T-leukemia and T-lymphoma Pancreatic adenocarcinoma |
(Tamura et al., 2011) (Kim et al., 2006) (Zeng et al., 2019) (Ganor & Levite, 2014) (Herner et al., 2011) |
|
| GABA | Metabotropic receptor (GABA-B) | Breast cancer Colorectal cancer Prostate cancer |
(Zhang et al., 2014) (Huang et al., 2022) (Solorzano et al., 2018) |
| Ionotropic receptors (GABA-A or GABA-C) | Pancreatic ductal adenocarcinoma | (Takehara et al., 2007) | |
| Dopamine | G-protein-coupled dopamine receptors (DRs) | Malignant glioma Small cell lung cancer Breast and colon cancer Glioma Gastric cancer Sarcoma |
(Lan et al., 2017) (Senogles, 2007) (Sarkar et al., 2004) (Qin et al., 2015) (Ganguly et al., 2010) (Chakroborty et al., 2008) |
5. Cancer-derived mediators
Besides cancer cells being affected by neural regulation in the tumor microenvironment, mediators released from cancer cells, for instance, extracellular vesicles (EVs), play a critical role in tumor progression and metastasis (Becker et al., 2016). These vesicles have unique compositions and carry distinct molecules, including proteins like transcription factors, enzymes, and receptors; nucleic acids like RNA, DNA, and microRNA (miRNA); and lipids (Raposo & Stoorvogel, 2013; Valadi et al., 2007). Authors reported that mRNA and tetraspanin Tspan8 proteins in tumor-derived exosomes increased angiogenesis in pancreatic tumors (Nazarenko et al., 2010). Hypoxic exosomes that carry highly expressed miR-301a-3p, which activates the PTEN/PI3K pathway and induces M2 polarization of macrophages, have been associated with poor prognosis for pancreatic cancer (Wang et al., 2018). In breast cancer studies, tumor-derived exosomes caused increased cancer cell migration and metastasis (Harris et al., 2015). In addition, an abundance of miRNAs was associated with increased tumorigenesis, invasiveness, and enhanced therapy resistance, and thus was a negative prognostic marker in breast cancer cases (Jia et al., 2017).
In addition to these findings, tumor-derived EVs, particularly exosomes, induced neurite outgrowth in a study using PC12 cells (rat pheochromocytoma cell line) and exosomes derived from head and neck cancer patients. In that study, tumor-derived exosomes with high levels of EphrinB1, an axon-guidance molecule, promoted neuritogenic effects and tumor innervation (Madeo et al., 2018). Consistently, stimulated neuritogenesis also occurred in PC12 cells treated with cervical cancer-derived exosomes (Lucido et al., 2019). Moreover, we showed that miRNAs in EVs regulate neuritogenesis, which was triggered by loss of the miR-34a or the presence of the miR-21 and miR-324 (Amit et al., 2020). These findings show that tumor-derived EVs regulate tumor innervation and progression for different cancer types.
Another group of mediators released from cancer cells, neurotrophins, also contribute to neurogenesis and tumor innervation (Wang et al., 2014). In pancreatic cancer, increased neurturin (NRTN) was positively correlated with an aggressive phenotype and invasiveness, and induced neuroplasticity. In addition, tumor-derived neurotrophic growth factor (NGF) and brain-derived neurotrophic factor have promoted tumor progression by regulating PI3K/AKT and Ras/ERK signaling pathways and axonogenesis via Trk receptors, which drive cancer modulation (Cervantes-Villagrana, Albores-Garcia, Cervantes-Villagrana, & Garcia-Acevez, 2020). Taken together, these results demonstrate that cancer-released mediators like neurotrophins and EVs are promising factors for therapeutic approaches for cancer and should be investigated further.
6. Neuroimmune interactions
Sympathetic nervous system activation enhances metastasis of cancer cells by stimulating macrophage infiltration, inflammation, angiogenesis, epithelial-mesenchymal transition, and tumor invasion and by inhibiting the immune response and apoptosis (Cole et al., 2015).
IL-6, a pro-angiogenic cytokine, plays a role in inflammation, chronic stress, and cancer development. It prevents cancer cell apoptosis via many cellular mechanisms, such as the ERK/MAPK, PI3-K, and JAK/STAT pathways (Nguyen, Li, & Tewari, 2014; Nilsson et al., 2007). Researchers showed that sympathetic nerves induce IL-6 gene expression with β-adrenergic activation or inhibition of GATA1 by using norepinephrine agonists and antagonists, respectively (Cole et al., 2010). Also, β-adrenergic stimulation and norepinephrine increased IL-6 expression via Src, a proto-oncogene product, and the tyrosine kinase signaling pathway in ovarian cancer cells (Nilsson et al., 2007). Furthermore, stimulation of norepinephrine in gastric cancers caused an increase in IL-6 expression via the cAMP/PKA signaling pathway (Yang, Lin, Gao, & Zhang, 2014).
Investigators showed that stress increased metastasis of breast cancer in both in vivo and ex vivo studies (Sloan et al., 2010). Administration of β-AR antagonists had no effect on primary tumor growth or metastatic burden in the control mouse group (non-stressed) but blocked metastasis in the chronically stressed mouse group. Also, stress increased the number of macrophages in the tumor microenvironment and the expression of the Tgfb, Arg1, Cox2, Mmp9, Vegf, and Vcam1 genes while decreasing expression of the Ifnb gene (Sloan et al., 2010). Consistent with these findings, β-adrenergic signals enhanced infiltration of CD14+ CD68+ cells and macrophages in the tumor microenvironment by increasing production of macrophages and monocyte chemoattractant protein 1 (MCP1) (Armaiz-Pena et al., 2015).
β-adrenergic signaling inhibits DNA damage repair mechanisms and prevents p53-associated apoptosis. In a study using a neuroblastoma line, propranolol upregulated p53 and caused apoptosis; in addition, it made cancer cells sensitive to the topoisomerase inhibitor SN-38 (Wolter et al., 2014). Inhibition of cytotoxic T cells and NK cells is believed to play a role in postoperative metastasis of cancer (Goldfarb et al., 2011; Inbar et al., 2011) and has been associated with increased dissemination of these tumor cells (Goldfarb et al., 2011). β-AR activation has suppressed natural killer activity and cytotoxic T cells in vivo (Inbar et al., 2011). In addition, chronic stress enhanced cancer growth via MDSCs and increased the accumulation of MDSCs in the tumor microenvironment. Furthermore, β2-AR stimulation caused phosphorylation of STAT3, which is involved in the immunosuppressive function of MDSCs, leading to increased numbers of MDSCs in tumors (Mohammadpour et al., 2019) (Fig. 4).
Fig. 4.
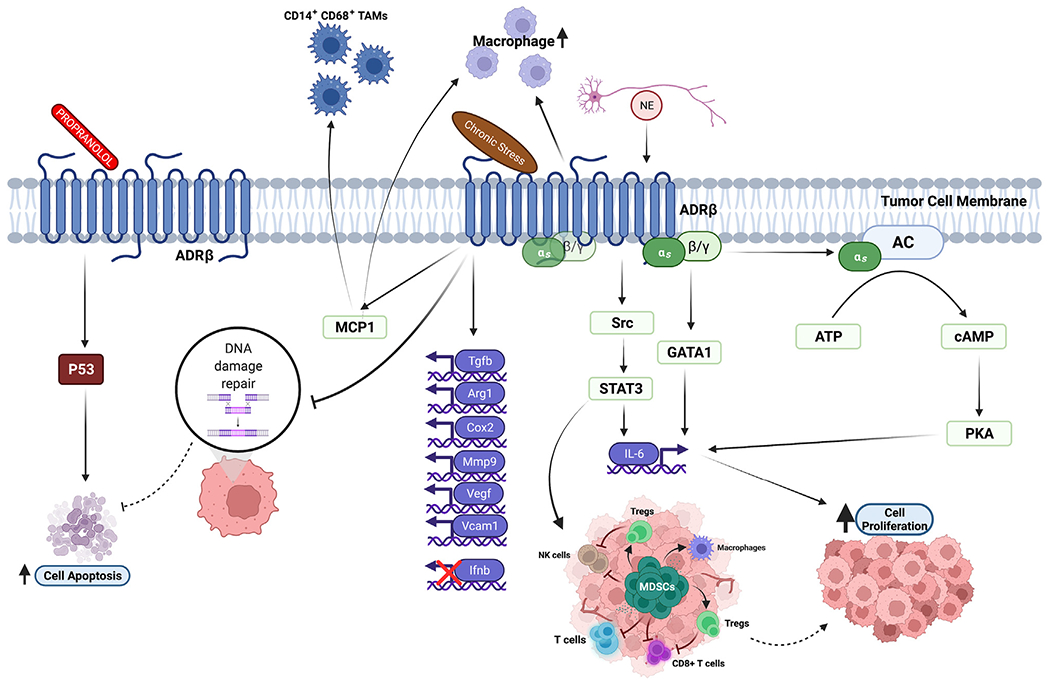
Schematic showing how activators and blockers of the sympathetic nervous system affect tumor and immune mediators. Propranolol promotes cell apoptosis by upregulating p53. Norepinephrine increases tumor cell proliferation by inducing expression of IL-6, which is regulated by the cAMP/PKA signaling pathway. Chronic stress promotes tumor growth via phosphorylated STAT3, which induces the numbers of MDSCs. β-adrenergic signals enhance the numbers of CD14+ CD68+ TAMs and macrophages via increased production of MCP1. Chronic stress and norepinephrine inhibit DNA damage repair mechanisms and prevent p53-associated cell apoptosis. The figure was created with BioRender.com.
Neurons can exert their effects on cancer through the inflammatory reflex. Specifically, they can regulate the level of IL-1β with the receptors in the afferent fibers of the vagus nerve. In response, the vagus nerve releases ACh on macrophages via α7 nAChR, thus inhibiting the release of proinflammatory cytokines and inhibiting high mobility group box protein 1 (HMGB1) secretion (Cortese, Rigamonti, Mantovani, & Marchesi, 2020).
Mediators released from tumors, EVs, not only regulate neurogenesis and cancer progression but also modulate immune cells in the tumor microenvironment. Tumor-infiltrating immune cells, regulatory B cells (Ye et al., 2018), regulatory T (Treg) cells (Wieckowski et al., 2009), TAMs (Chen et al., 2018), neutrophils, and MDSCs (Valenti et al., 2006) were modulated by tumor-derived EVs. Investigators showed that microvesicles isolated from melanoma patients caused immunosuppression by inhibiting monocyte differentiation and by generating MDSCs with tumor growth factory-β-mediated suppressive activity on T-cells (Valenti et al., 2006). Also, EVs released from melanoma cells have induced CD8+ T cells apoptosis and promoted Treg cell expansion thus contributing to immune suppression (Wieckowski et al., 2009). In one important study in the field, exosomal PD-L1 suppressed immunity and facilitated immune evasion in patients with metastatic melanoma (Chen et al., 2018). Additionally, exosomal HMGB1 expression led to immune escape via regulatory B cells expansion in hepatocellular carcinoma (Ye et al., 2018). In view of the interaction between cancer cells and immune cells through EVs within the tumor microenvironment, future studies will reveal unexplored areas of cancer.
7. Conclusions
The mechanisms of neural activity that have facilitative effects on cancer metastasis and cause poor patient outcomes are not completely understood. With an emphasis on developments in neuroimmunology, recent studies of tumor proliferation and dissemination have focused on nerves, immune cells, and interaction with cancer. Those studying the tumor microenvironment have an ongoing interest in understanding how tumors interact with other mediators such as nerves, chemokines, immune cells, and signaling molecules. Although these novel approaches highlight many undiscovered mechanisms, neuroimmune interplay in tumor dissemination is a substantial research interest in the field. In this review, we discuss sympathetic and parasympathetic regulation of different cancers, neurotransmitters, tumor-derived mediators, and pathways in the tumor. The effects of the peripheral nervous system on cancer cells and mediators released from tumor cells, such as EVs and neurotrophins, are innovative therapeutic targets for cancer. A comprehensive understanding of cancer and neuroimmune crosstalk will reveal promising treatment opportunities such as overcoming immunotherapy resistance in cancer patients.
Acknowledgments
This work was supported by The University of Texas MD Anderson Cancer Center Moon Shots Program and by the NIH/NCI under award number R37 CA242006-01A1. The authors would like to thank Tamara K. Locke from The University of Texas MD Anderson Cancer Center’s Research Medical Library for editorial assistance in preparing this manuscript.
Abbreviations:
- ACh
acetylcholine
- AR
adrenergic receptors
- β2-AR
beta 2 adrenergic receptors
- β3-AR
beta 3 adrenergic receptors
- cAMP
cyclic adenosine monophosphate
- Chrm1
cholinergic receptor muscarinic type 1 receptor
- COX
cyclooxygenase
- DUSP1
dual specificity phosphatase 1
- EGFR
epidermal growth factor receptor
- ERK
extracellular signal-regulated kinase
- EVs
extracellular vesicles
- GABA
Gamma aminobutyric acid
- HMGB1
high mobility group box protein 1
- iGluR
ionotrophic glutamate receptors
- JNK
c-Jun N-terminal kinase
- MAPK
mitogen-activated protein kinase
- MCP1
monocyte chemoattractant protein 1
- MDSCs
myeloid-derived suppressor cells
- mGluR
metabotropic glutamate receptor
- miRNA
microRNA
- MMP
matrix metalloproteinase
- nAChR
nicotinic acetylcholine receptor
- NGF
neurotrophic growth factor
- NK
natural killer
- NMDAR2B
methylated N-methyl-d-aspartate receptor type 2B
- PD-L1
programmed death ligand 1
- PI3K
phosphatidylinositol-3-kinase
- PKA
protein kinase A
- STAT
signal transducer and activator of transcription
- TAM
tumor-associated macrophage
- TNF-α
tumor necrosis factor α
- Treg
T regulatory cell
- VEGF
vascular endothelial growth factor
- 4-DAMP
1,1-dimethyl-4-diphenylacetoxypiperidinium iodide
Footnotes
Declaration of Competing Interest
The authors have no competing interest to declare.
References
- Amit M, Na’ara S, & Gil Z (2016). Mechanisms of cancer dissemination along nerves. Nature Reviews. Cancer 16(6), 399–408. 10.1038/nrc.2016.38. [DOI] [PubMed] [Google Scholar]
- Amit M, Takahashi H, Dragomir MP, Lindemann A, Gleber-Netto FO, Pickering CR, … Myers JN (2020). Loss of p53 drives neuron reprogramming in head and neck cancer. Nature 578(7795), 449–454. 10.1038/s41586-020-1996-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armaiz-Pena GN, Allen JK, Cruz A, Stone RL, Nick AM, Lin YG, … Sood AK (2013). Src activation by beta-adrenoreceptors is a key switch for tumour metastasis. Nature Communications 4, 1403. 10.1038/ncomms2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armaiz-Pena GN, Gonzalez-Villasana V, Nagaraja AS, Rodriguez-Aguayo C, Sadaoui NC, Stone RL, … Lopez-Berestein G (2015). Adrenergic regulation of monocyte chemotactic protein 1 leads to enhanced macrophage recruitment and ovarian carcinoma growth. Oncotarget 6(6), 4266–4273. 10.18632/oncotarget.2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H, & Lyden D (2016). Extracellular vesicles in cancer: Cell-to-cell mediators of metastasis. Cancer Cell 30(6), 836–848. 10.1016/j.ccell.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belo A, Cheng K, Chahdi A, Shant J, Xie G, Khurana S, & Raufman JP (2011). Muscarinic receptor agonists stimulate human colon cancer cell migration and invasion. American Journal of Physiology. Gastrointestinal and Liver Physiology 300(5), G749–G760. 10.1152/ajpgi.00306.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernabe DG, Tamae AC, Biasoli ER, & Oliveira SH (2011). Stress hormones increase cell proliferation and regulates interleukin-6 secretion in human oral squamous cell carcinoma cells. Brain, Behavior, and Immunity 25(3), 574–583. 10.1016/j.bbi.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Boonstra E, de Kleijn R, Colzato LS, Alkemade A, Forstmann BU, & Nieuwenhuis S (2015). Neurotransmitters as food supplements: The effects of GABA on brain and behavior. Frontiers in Psychology 6, 1520. 10.3389/fpsyg.2015.01520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo-Calderon DM, Assao A, Garcia NG, Coutinho-Camillo CM, Roffe M, Germano JN, & Oliveira DT (2020). Beta adrenergic receptor activation inhibits oral cancer migration and invasiveness. Archives of Oral Biology 118, Article 104865. 10.1016/j.archoralbio.2020.104865. [DOI] [PubMed] [Google Scholar]
- Brosnan JT, & Brosnan ME (2013). Glutamate: a truly functional amino acid. Amino Acids 45(3), 413–418. 10.1007/s00726-012-1280-4. [DOI] [PubMed] [Google Scholar]
- Brzozowska A, Burdan F, Duma D, Solski J, & Mazurkiewicz M (2017). Gamma-amino butyric acid (GABA) level as an overall survival risk factor in breast cancer. Annals of Agricultural and Environmental Medicine 24(3), 435–439. 10.26444/aaem/75891. [DOI] [PubMed] [Google Scholar]
- Budczies J, Pfitzner BM, Gyorffy B, Winzer KJ, Radke C, Dietel M, … Denkert C (2015). Glutamate enrichment as new diagnostic opportunity in breast cancer. International Journal of Cancer 136(7), 1619–1628. 10.1002/ijc.29152. [DOI] [PubMed] [Google Scholar]
- Calvani M, Bruno G, Dal Monte M, Nassini R, Fontani F, Casini A, … Filippi L (2019). beta3-adrenoceptor as a potential immuno-suppressor agent in melanoma. British Journal of Pharmacology 176(14), 2509–2524. 10.1111/bph.14660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvani M, Cavallini L, Tondo A, Spinelli V, Ricci L, Pasha A, … Filippi L (2018). beta3-Adrenoreceptors control mitochondrial dormancy in melanoma and embryonic stem cells. Oxidative Medicine and Cellular Longevity 2018, 6816508. 10.1155/2018/6816508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JP, Karolak MR, Ma Y, Perrien DS, Masood-Campbell SK, Penner NL, … Elefteriou F (2012). Stimulation of host bone marrow stromal cells by sympathetic nerves promotes breast cancer bone metastasis in mice. PLoS Biology 10(7), Article e1001363. 10.1371/journal.pbio.1001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes-Villagrana RD, Albores-Garcia D, Cervantes-Villagrana AR, & Garcia-Acevez SJ (2020). Tumor-induced neurogenesis and immune evasion as targets of innovative anti-cancer therapies. Signal Transduction and Targeted Therapy 5(1), 99. 10.1038/s41392-020-0205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceyhan GO, Bergmann F, Kadihasanoglu M, Altintas B, Demir IE, Hinz U, … Friess H (2009). Pancreatic neuropathy and neuropathic pain–a comprehensive pathomorphological study of 546 cases. Gastroenterology 136(1). 10.1053/j.gastro.2008.09.029 177–186 e171. [DOI] [PubMed] [Google Scholar]
- Chakroborty D, Chowdhury UR, Sarkar C, Baral R, Dasgupta PS, & Basu S (2008). Dopamine regulates endothelial progenitor cell mobilization from mouse bone marrow in tumor vascularization. The Journal of Clinical Investigation 118(4), 1380–1389. 10.1172/JCI33125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, … Guo W (2018). Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 560(7718), 382–386. 10.1038/s41586-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Zhou J, Li X, Wang X, Lin Y, & Wang X (2018). Exosomes derived from hypoxic epithelial ovarian cancer cells deliver microRNAs to macrophages and elicit a tumor-promoted phenotype. Cancer Letters 435, 80–91. 10.1016/j.canlet.2018.08.001. [DOI] [PubMed] [Google Scholar]
- Cheng K, Samimi R, Xie G, Shant J, Drachenberg C, Wade M, & Raufman JP (2008). Acetylcholine release by human colon cancer cells mediates autocrine stimulation of cell proliferation. American Journal of Physiology. Gastrointestinal and Liver Physiology 295(3), G591–G597. 10.1152/ajpgi.00055.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin CC, Li JM, Lee KF, Huang YC, Wang KC, Lai HC, … Shi CS (2016). Selective beta2-AR blockage suppresses colorectal cancer growth through regulation of EGFR-Akt/ERK1/2 signaling, G1-phase arrest, and apoptosis. Journal of Cellular Physiology 231(2), 459–472. 10.1002/jcp.25092. [DOI] [PubMed] [Google Scholar]
- Ciurea RN, Rogoveanu I, Pirici D, Tartea GC, Streba CT, Florescu C, … Vere CC (2017). B2 adrenergic receptors and morphological changes of the enteric nervous system in colorectal adenocarcinoma. World Journal of Gastroenterology 23(7), 1250–1261. 10.3748/wjg.v23.i7.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy JA, Mary DA, Witte KK, Greenwood JP, Deuchars SA, & Deuchars J (2014). Non-invasive vagus nerve stimulation in healthy humans reduces sympathetic nerve activity. Brain Stimulation 7(6), 871–877. 10.1016/j.brs.2014.07.031. [DOI] [PubMed] [Google Scholar]
- Coarfa C, Florentin D, Putluri N, Ding Y, Au J, He D, … Ayala G (2018). Influence of the neural microenvironment on prostate cancer. Prostate 78(2), 128–139. 10.1002/pros.23454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Arevalo JM, Takahashi R, Sloan EK, Lutgendorf SK, Sood AK, … Seeman TE (2010). Computational identification of gene-social environment interaction at the human IL6 locus. Proceedings of the National Academy of Sciences of the United States of America 107(12), 5681–5686. 10.1073/pnas.0911515107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Nagaraja AS, Lutgendorf SK, Green PA, & Sood AK (2015). Sympathetic nervous system regulation of the tumour microenvironment. Nature Reviews. Cancer 15(9), 563–572. 10.1038/nrc3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese N, Rigamonti A, Mantovani A, & Marchesi F (2020). The neuro-immune axis in cancer: Relevance of the peripheral nervous system to the disease. Immunology Letters 227, 60–65. 10.1016/j.imlet.2020.07.010. [DOI] [PubMed] [Google Scholar]
- Dal Monte M, Fornaciari I, Nicchia GP, Svelto M, Casini G, & Bagnoli P (2014). beta3-adrenergic receptor activity modulates melanoma cell proliferation and survival through nitric oxide signaling. Naunyn-Schmiedeberg’s Archives of Pharmacology 387(6), 533–543. 10.1007/s00210-014-0969-1. [DOI] [PubMed] [Google Scholar]
- Du J, Li XH, & Li YJ (2016). Glutamate in peripheral organs: Biology and pharmacology. European Journal of Pharmacology 784, 42–48. 10.1016/j.ejphar.2016.05.009. [DOI] [PubMed] [Google Scholar]
- Erin N, Barkan GA, & Clawson GA (2013). Vagus nerve regulates breast cancer metastasis to the adrenal gland. Anticancer Research 33(9), 3675–3682. [PubMed] [Google Scholar]
- Ganguly S, Basu B, Shome S, Jadhav T, Roy S, Majumdar J, … Basu S (2010). Dopamine, by acting through its D2 receptor, inhibits insulin-like growth factor-I (IGF-I)-induced gastric cancer cell proliferation via up-regulation of Kruppel-like factor 4 through down-regulation of IGF-IR and AKT phosphorylation. The American Journal of Pathology 177(6), 2701–2707. 10.2353/ajpath.2010.100617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganor Y, & Levite M (2014). The neurotransmitter glutamate and human T cells: Glutamate receptors and glutamate-induced direct and potent effects on normal human T cells, cancerous human leukemia and lymphoma T cells, and autoimmune human T cells. Journal of Neural Transmission (Vienna) 121(8), 983–1006. 10.1007/s00702-014-1167-5. [DOI] [PubMed] [Google Scholar]
- Goldfarb Y, Sorski L, Benish M, Levi B, Melamed R, & Ben-Eliyahu S (2011). Improving postoperative immune status and resistance to cancer metastasis: A combined perioperative approach of immunostimulation and prevention of excessive surgical stress responses. Annals of Surgery 253(4), 798–810. 10.1097/SLA.0b013e318211d7b5. [DOI] [PubMed] [Google Scholar]
- Harris DA, Patel SH, Gucek M, Hendrix A, Westbroek W, & Taraska JW (2015). Exosomes released from breast cancer carcinomas stimulate cell movement. PLoS One 10(3), Article e0117495. 10.1371/journal.pone.0117495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa Y, Sakitani K, Konishi M, Asfaha S, Niikura R, Tomita H, … Wang TC (2017). Nerve growth factor promotes gastric tumorigenesis through aberrant cholinergic signaling. Cancer Cell 31(1), 21–34. 10.1016/j.ccell.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He JJ, Zhang WH, Liu SL, Chen YF, Liao CX, Shen QQ, & Hu P (2017). Activation of beta-adrenergic receptor promotes cellular proliferation in human glioblastoma. Oncology Letters 14(3), 3846–3852. 10.3892/ol.2017.6653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herner A, Sauliunaite D, Michalski CW, Erkan M, De Oliveira T, Abiatari I, … Kleeff J (2011). Glutamate increases pancreatic cancer cell invasion and migration via AMPA receptor activation and Kras-MAPK signaling. International Journal of Cancer 129(10), 2349–2359. 10.1002/ijc.25898. [DOI] [PubMed] [Google Scholar]
- Hicks BM, Murray LJ, Powe DG, Hughes CM, & Cardwell CR (2013). beta-blocker usage and colorectal cancer mortality: A nested case-control study in the UK clinical practice research datalink cohort. Annals of Oncology 24(12), 3100–3106. 10.1093/annonc/mdt381. [DOI] [PubMed] [Google Scholar]
- Huang T, Tworoger SS, Hecht JL, Rice MS, Sood AK, Kubzansky LD, & Poole EM (2016). Association of ovarian tumor beta2-adrenergic receptor status with ovarian cancer risk factors and survival. Cancer Epidemiology, Biomarkers & Prevention 25 (12), 1587–1594. 10.1158/1055-9965.EPI-16-0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Wang Y, Thompson JW, Yin T, Alexander PB, Qin D, … Wang XF (2022). Cancer-cell-derived GABA promotes beta-catenin-mediated tumour growth and immunosuppression. Nature Cell Biology 24(2), 230–241. 10.1038/s41556-021-00820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt PJ, Andujar FN, Silverman DA, & Amit M (2021). Mini-review: Trophic interactions between cancer cells and primary afferent neurons. Neuroscience Letters 746, Article 135658. 10.1016/j.neulet.2021.135658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchings C, Phillips JA, & Djamgoz MBA (2020). Nerve input to tumours: Pathophysiological consequences of a dynamic relationship. Biochimica Et Biophysica Acta. Reviews on Cancer 1874(2), Article 188411. 10.1016/j.bbcan.2020.188411. [DOI] [PubMed] [Google Scholar]
- Inbar S, Neeman E, Avraham R, Benish M, Rosenne E, & Ben-Eliyahu S (2011). Do stress responses promote leukemia progression? An animal study suggesting a role for epinephrine and prostaglandin-E2 through reduced NK activity. PLoS One 6(4), Article e19246. 10.1371/journal.pone.0019246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Chen Y, Wang Q, Jayasinghe U, Luo X, Wei Q, … Zhou J (2017). Exosome: Emerging biomarker in breast cancer. Oncotarget 8(25), 41717–41733. 10.18632/oncotarget.16684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobling P, Pundavela J, Oliveira SM, Roselli S, Walker MM, & Hondermarck H (2015). Nerve-cancer cell cross-talk: A novel promoter of tumor progression. Cancer Research 75(9), 1777–1781. 10.1158/0008-5472.CAN-14-3180. [DOI] [PubMed] [Google Scholar]
- Kamiya A, Hayama Y, Kato S, Shimomura A, Shimomura T, Irie K, … Ochiya T (2019). Genetic manipulation of autonomic nerve fiber innervation and activity and its effect on breast cancer progression. Nature Neuroscience 22(8), 1289–1305. 10.1038/s41593-019-0430-3. [DOI] [PubMed] [Google Scholar]
- Kamiya A, Hiyama T, Fujimura A, & Yoshikawa S (2021). Sympathetic and parasympathetic innervation in cancer: Therapeutic implications. Clinical Autonomic Research 31 (2), 165–178. 10.1007/s10286-020-00724-y. [DOI] [PubMed] [Google Scholar]
- Kang Y, Nagaraja AS, Armaiz-Pena GN, Dorniak PL, Hu W, Rupaimoole R, … Sood AK (2016). Adrenergic stimulation of DUSP1 impairs chemotherapy response in ovarian cancer. Clinical Cancer Research 22(7), 1713–1724. 10.1158/1078-0432.CCR-15-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MS, Yamashita K, Baek JH, Park HL, Carvalho AL, Osada M, … Sidransky D (2006). N-methyl-D-aspartate receptor type 2B is epigenetically inactivated and exhibits tumor-suppressive activity in human esophageal cancer. Cancer Research 66 (7), 3409–3418. 10.1158/0008-5472.CAN-05-1608. [DOI] [PubMed] [Google Scholar]
- Kim-Fuchs C, Le CP, Pimentel MA, Shackleford D, Ferrari D, Angst E, … Sloan EK (2014). Chronic stress accelerates pancreatic cancer growth and invasion: A critical role for beta-adrenergic signaling in the pancreatic microenvironment. Brain, Behavior, and Immunity 40, 40–47. 10.1016/j.bbi.2014.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koochekpour S (2013). Glutamate, a metabolic biomarker of aggressiveness and a potential therapeutic target for prostate cancer. Asian Journal of Andrology 15(2), 212–213. 10.1038/aja.2012.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koochekpour S, Majumdar S, Azabdaftari G, Attwood K, Scioneaux R, Subramani D, … Vessella RL (2012). Serum glutamate levels correlate with Gleason score and glutamate blockade decreases proliferation, migration, and invasion and induces apoptosis in prostate cancer cells. Clinical Cancer Research 18(21), 5888–5901. 10.1158/1078-0432.CCR-12-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan YL, Wang X, Xing JS, Lou JC, Ma XC, & Zhang B (2017). The potential roles of dopamine in malignant glioma. Acta Neurologica Belgica 117(3), 613–621. 10.1007/s13760-016-0730-2. [DOI] [PubMed] [Google Scholar]
- Lee CH, Huang CS, Chen CS, Tu SH, Wang YJ, Chang YJ, … Ho YS (2010). Overexpression and activation of the alpha9-nicotinic receptor during tumorigenesis in human breast epithelial cells. Journal of the National Cancer Institute 102(17), 1322–1335. 10.1093/jnci/djq300. [DOI] [PubMed] [Google Scholar]
- Leone RD, & Powell JD (2020). Metabolism of immune cells in cancer. Nature Reviews. Cancer 20(9), 516–531. 10.1038/s41568-020-0273-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao X, Che X, Zhao W, Zhang D, Bi T, & Wang G (2010). The beta-adrenoceptor antagonist, propranolol, induces human gastric cancer cell apoptosis and cell cycle arrest via inhibiting nuclear factor kappaB signaling. Oncology Reports 24(6), 1669–1676. 10.3892/or_00001032. [DOI] [PubMed] [Google Scholar]
- Liebig C, Ayala G, Wilks JA, Berger DH, & Albo D (2009). Perineural invasion in cancer: A review of the literature. Cancer 115(15), 3379–3391. 10.1002/cncr.24396. [DOI] [PubMed] [Google Scholar]
- Lopes-Santos G, Bernabe DG, Miyahara GI, & Tjioe KC (2021). Beta-adrenergic pathway activation enhances aggressiveness and inhibits stemness in head and neck cancer. Translational Oncology 14(8), Article 101117. 10.1016/j.tranon.2021.101117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Xu Q, Zuo Y, Liu L, Liu S, Chen L, … Li Y (2017). Isoprenaline/beta2-AR activates Plexin-A1/VEGFR2 signals via VEGF secretion in gastric cancer cells to promote tumor angiogenesis. BMC Cancer 17(1), 875. 10.1186/s12885-017-3894-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucido CT, Wynja E, Madeo M, Williamson CS, Schwartz LE, Imblum BA, … Vermeer PD (2019). Innervation of cervical carcinoma is mediated by cancer-derived exosomes. Gynecologic Oncology 154(1), 228–235. 10.1016/j.ygyno.2019.04.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeo M, Colbert PL, Vermeer DW, Lucido CT, Cain JT, Vichaya EG, … Vermeer PD (2018). Cancer exosomes induce tumor innervation. Nature Communications 9 , 4284. 10.1038/s41467-018-06640-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnon C, Hall SJ, Lin J, Xue X, Gerber L, Freedland SJ, & Frenette PS (2013). Autonomic nerve development contributes to prostate cancer progression. Science 341 (6142), 1236361. 10.1126/science.1236361. [DOI] [PubMed] [Google Scholar]
- Martino JJ, Wall BA, Mastrantoni E, Wilimczyk BJ, La Cava SN, Degenhardt K, … Chen S (2013). Metabotropic glutamate receptor 1 (Grm1) is an oncogene in epithelial cells. Oncogene 32(37), 4366–4376. 10.1038/onc.2012.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadpour H, MacDonald CR, Qiao G, Chen M, Dong B, Hylander BL, … Repasky EA (2019). beta2 adrenergic receptor-mediated signaling regulates the immunosuppressive potential of myeloid-derived suppressor cells. The Journal of Clinical Investigation 129(12), 5537–5552. 10.1172/JCI129502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazarenko I, Rana S, Baumann A, McAlear J, Hellwig A, Trendelenburg M, … Zoller M (2010). Cell surface tetraspanin Tspan8 contributes to molecular pathways of exosome-induced endothelial cell activation. Cancer Research 70(4), 1668–1678. 10.1158/0008-5472.CAN-09-2470. [DOI] [PubMed] [Google Scholar]
- Nguyen DP, Li J, & Tewari AK (2014). Inflammation and prostate cancer: The role of interleukin 6 (IL-6). BJU International 113(6), 986–992. 10.1111/bju.12452. [DOI] [PubMed] [Google Scholar]
- Nilsson MB, Armaiz-Pena G, Takahashi R, Lin YG, Trevino J, Li Y, … Sood AK (2007). Stress hormones regulate interleukin-6 expression by human ovarian carcinoma cells through a Src-dependent mechanism. The Journal of Biological Chemistry 282(41), 29919–29926. 10.1074/jbc.M611539200. [DOI] [PubMed] [Google Scholar]
- O’Donnell M, Chance RK, & Bashaw GJ (2009). Axon growth and guidance: Receptor regulation and signal transduction. Annual Review of Neuroscience 32, 383–412. 10.1146/annurev.neuro.051508.135614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm D, & Entschladen F (2007). Neoneurogenesis and the neuro-neoplastic synapse. Progress in Experimental Tumor Research 39, 91–98. 10.1159/000100049. [DOI] [PubMed] [Google Scholar]
- Partecke LI, Kading A, Trung DN, Diedrich S, Sendler M, Weiss F, … Kessler W (2017). Subdiaphragmatic vagotomy promotes tumor growth and reduces survival via TNFalpha in a murine pancreatic cancer model. Oncotarget 8(14), 22501–22512. 10.18632/oncotarget.15019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin T, Wang C, Chen X, Duan C, Zhang X, Zhang J, … Yang J (2015). Dopamine induces growth inhibition and vascular normalization through reprogramming M2-polarized macrophages in rat C6 glioma. Toxicology and Applied Pharmacology 286, 112–123. 10.1016/j.taap.2015.03.021. [DOI] [PubMed] [Google Scholar]
- Raposo G, & Stoorvogel W (2013). Extracellular vesicles: Exosomes, microvesicles, and friends. The Journal of Cell Biology 200(4), 373–383. 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raufman JP, Shant J, Xie G, Cheng K, Gao XM, Shiu B, … Khurana S (2011). Muscarinic receptor subtype-3 gene ablation and scopolamine butylbromide treatment attenuate small intestinal neoplasia in Apcmin/+ mice. Carcinogenesis 32(9), 1396–1402. 10.1093/carcin/bgr118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitzer LJ, Wice BM, & Kennell D (1979). Evidence that glutamine, not sugar, is the major energy source for cultured HeLa cells. The Journal of Biological Chemistry 254 (8), 2669–2676. [PubMed] [Google Scholar]
- Renz BW, Takahashi R, Tanaka T, Macchini M, Hayakawa Y, Dantes Z, … Wang TC (2018). beta2 adrenergic-neurotrophin feedforward loop promotes pancreatic cancer. Cancer Cell 33(1), 75–90 e77. 10.1016/j.ccell.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renz BW, Tanaka T, Sunagawa M, Takahashi R, Jiang Z, Macchini M, … Wang TC (2018). Cholinergic signaling via muscarinic receptors directly and indirectly suppresses pancreatic tumorigenesis and cancer stemness. Cancer Discovery 8(11), 1458–1473. 10.1158/2159-8290.CD-18-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar C, Chakroborty D, Chowdhury UR, Dasgupta PS, & Basu S (2008). Dopamine increases the efficacy of anticancer drugs in breast and colon cancer preclinical models. Clinical Cancer Research 14(8), 2502–2510. 10.1158/1078-0432.CCR-07-1778. [DOI] [PubMed] [Google Scholar]
- Sarkar C, Chakroborty D, Mitra RB, Banerjee S, Dasgupta PS, & Basu S (2004). Dopamine in vivo inhibits VEGF-induced phosphorylation of VEGFR-2, MAPK, and focal adhesion kinase in endothelial cells. American Journal of Physiology. Heart and Circulatory Physiology 287(4), H1554–H1560. 10.1152/ajpheart.00272.2004. [DOI] [PubMed] [Google Scholar]
- Scheff NN, & Saloman JL (2021). Neuroimmunology of cancer and associated symptomology. Immunology and Cell Biology 99(9), 949–961. 10.1111/imcb.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senogles SE (2007). D2 dopamine receptor-mediated antiproliferation in a small cell lung cancer cell line, NCI-H69. Anti-Cancer Drugs 18(7), 801–807. 10.1097/CAD.0b013e3280b10d36. [DOI] [PubMed] [Google Scholar]
- Setordzi P, Chang X, Liu Z, Wu Y, & Zuo D (2021). The recent advances of PD-1 and PD-L1 checkpoint signaling inhibition for breast cancer immunotherapy. European Journal of Pharmacology 895, Article 173867. 10.1016/j.ejphar.2021.173867. [DOI] [PubMed] [Google Scholar]
- Sloan EK, Priceman SJ, Cox BF, Yu S, Pimentel MA, Tangkanangnukul V, … Cole SW (2010). The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Research 70(18), 7042–7052. 10.1158/0008-5472.CAN-10-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solorzano SR, Imaz-Rosshandler I, Camacho-Arroyo I, Garcia-Tobilla P, Morales-Montor G, Salazar P, … Rodriguez-Dorantes M (2018). GABA promotes gastrinreleasing peptide secretion in NE/NE-like cells: Contribution to prostate cancer progression. Scientific Reports 8(1), 10272. 10.1038/s41598-018-28538-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepulak A, Rola R, Polberg K, & Ikonomidou C (2014). Glutamate and its receptors in cancer. Journal of Neural Transmission (Vienna) 121(8), 933–944. 10.1007/s00702-014-1182-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, & Bray F (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a Cancer Journal for Clinicians 71(3), 209–249. 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Takehara A, Hosokawa M, Eguchi H, Ohigashi H, Ishikawa O, Nakamura Y, & Nakagawa H (2007). Gamma-aminobutyric acid (GABA) stimulates pancreatic cancer growth through overexpressing GABAA receptor pi subunit. Cancer Research 67 (20), 9704–9712. 10.1158/0008-5472.CAN-07-2099. [DOI] [PubMed] [Google Scholar]
- Tamura H, Suzuki M, Moriya Y, Hoshino H, Okamoto T, Yoshida S, & Yoshino I (2011).Aberrant methylation of N-methyl-D-aspartate receptor type 2B (NMDAR2B) in non-small cell carcinoma. BMC Cancer 11, 220. 10.1186/1471-2407-11-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A, & Sakaguchi S (2017). Regulatory T cells in cancer immunotherapy. Cell Research 27(1), 109–118. 10.1038/cr.2016.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsuta M, Iishi H, Yamamura H, Baba M, & Taniguchi H (1988). Effects of bilateral and unilateral vagotomy on gastric carcinogenesis induced by N-methyl-N’-nitro-N-nitrosoguanidine in Wistar rats. International Journal of Cancer 42(3), 414–418. 10.1002/ijc.2910420318. [DOI] [PubMed] [Google Scholar]
- Tatsuta M, Yamamura H, Iishi H, Ichii M, Noguchi S, Baba M, & Taniguchi H (1985). Promotion by vagotomy of gastric carcinogenesis induced by N-methyl-N’-nitro-N-nitrosoguanidine in Wistar rats. Cancer Research 45(1), 194–197. [PubMed] [Google Scholar]
- Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, … Sood AK (2006). Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nature Medicine 12(8), 939–944. 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, & Lotvall JO (2007). Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature Cell Biology 9(6), 654–659. 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- Valenti R, Huber V, Filipazzi P, Pilla L, Sovena G, Villa A, … Rivoltini L (2006). Human tumor-released microvesicles promote the differentiation of myeloid cells with transforming growth factor-beta-mediated suppressive activity on T lymphocytes. Cancer Research 66(18), 9290–9298. 10.1158/0008-5472.CAN-06-1819. [DOI] [PubMed] [Google Scholar]
- Vander Heiden MG, Cantley LC, & Thompson CB (2009). Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 324(5930), 1029–1033. 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh HS, Johung TB, Caretti V, Noll A, Tang Y, Nagaraja S, … Monje M (2015). Neuronal activity promotes glioma growth through neuroligin-3 secretion. Cell 161(4), 803–816. 10.1016/j.cell.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh HS, Tam LT, Woo PJ, Lennon J, Nagaraja S, Gillespie SM, … Monje M (2017). Targeting neuronal activity-regulated neuroligin-3 dependency in high-grade glioma. Nature 549(7673), 533–537. 10.1038/nature24014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Demir IE, D’Haese JG, Tieftrunk E, Kujundzic K, Schorn S, … Ceyhan GO (2014). The neurotrophic factor neurturin contributes toward an aggressive cancer cell phenotype, neuropathic pain and neuronal plasticity in pancreatic cancer. Carcinogenesis 35(1), 103–113. 10.1093/carcin/bgt312. [DOI] [PubMed] [Google Scholar]
- Wang L, Xu J, Xia Y, Yin K, Li Z, Li B, … Xu Z (2018). Muscarinic acetylcholine receptor 3 mediates vagus nerve-induced gastric cancer. Oncogenesis 7(11), 88. 10.1038/s41389-018-0099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Luo G, Zhang K, Cao J, Huang C, Jiang T, … Qiu Z (2018). Hypoxic tumor-derived exosomal miR-301a mediates M2 macrophage polarization via PTEN/PI3Kgamma to promote pancreatic Cancer metastasis. Cancer Research 78(16), 4586–4598. 10.1158/0008-5472.CAN-17-3841. [DOI] [PubMed] [Google Scholar]
- Wieckowski EU, Visus C, Szajnik M, Szczepanski MJ, Storkus WJ, & Whiteside TL (2009). Tumor-derived microvesicles promote regulatory T cell expansion and induce apoptosis in tumor-reactive activated CD8+ T lymphocytes. Journal of Immunology 183(6), 3720–3730. 10.4049/jimmunol.0900970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolter JK, Wolter NE, Blanch A, Partridge T, Cheng L, Morgenstern DA, … Irwin MS (2014). Anti-tumor activity of the beta-adrenergic receptor antagonist propranolol in neuroblastoma. Oncotarget 5(1), 161–172. 10.18632/oncotarget.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka Y, Mammoto T, Kirita T, Mukai M, Mashimo T, Sugimura M, … Nakamura H (2002). Epinephrine inhibits invasion of oral squamous carcinoma cells by modulating intracellular cAMP. Cancer Letters 176(2), 143–148. 10.1016/s0304-3835(01)00764-9. [DOI] [PubMed] [Google Scholar]
- Yang R, Lin Q, Gao HB, & Zhang P (2014). Stress-related hormone norepinephrine induces interleukin-6 expression in GES-1 cells. Brazilian Journal of Medical and Biological Research 47(2), 101–109. 10.1590/1414-431X20133346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, Zhang Q, Cheng Y, Chen X, Wang G, Shi M, … Chen G (2018). Tumor-derived exosomal HMGB1 fosters hepatocellular carcinoma immune evasion by promoting TIM-1(+) regulatory B cell expansion. Journal for Immunotherapy of Cancer 6(1), 145. 10.1186/s40425-018-0451-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi H, Talmon G, & Wang J (2019). Glutamate in cancers: From metabolism to signaling. Journal of Biomedical Research 34(4), 260–270. 10.7555/JBR.34.20190037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Xia H, Tang Q, Xu H, Wei G, Chen Y, … Bi F (2017). Acetylcholine acts through M3 muscarinic receptor to activate the EGFR signaling and promotes gastric cancer cell proliferation. Scientific Reports 7, 40802. 10.1038/srep40802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu LJ, Wall BA, Wangari-Talbot J, & Chen S (2017). Metabotropic glutamate receptors in cancer. Neuropharmacology 115, 193–202. 10.1016/j.neuropharm.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Peng Z, Qin M, Liu Y, Wang J, Zhang C, … Sun S (2021). Interferon-gamma induces tumor resistance to anti-PD-1 immunotherapy by promoting YAP phase separation. Molecular Cell 81 (6), 1216–1230 e1219. 10.1016/j.molcel.2021.01.010. [DOI] [PubMed] [Google Scholar]
- Zahalka AH, Arnal-Estape A, Maryanovich M, Nakahara F, Cruz CD, Finley LWS, & Frenette PS (2017). Adrenergic nerves activate an angio-metabolic switch in prostate cancer. Science 358(6361), 321–326. 10.1126/science.aah5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Q, Michael IP, Zhang P, Saghafinia S, Knott G, Jiao W, … Hanahan D (2019). Synaptic proximity enables NMDAR signalling to promote brain metastasis. Nature 573(7775), 526–531. 10.1038/s41586-019-1576-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Li X, Yao Z, Wei C, Ning N, & Li J (2014). GABAergic signaling facilitates breast cancer metastasis by promoting ERK1/2-dependent phosphorylation. Cancer Letters 348(1–2), 100–108. 10.1016/j.canlet.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Huang H, Coleman M, Ziemys A, Gopal P, Kazmi SM, & Brekken RA (2021). VEGFR2 activity on myeloid cells mediates immune suppression in the tumor microenvironment. JCI. Insight 6(23). 10.1172/jci.insight.150735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao CM, Hayakawa Y, Kodama Y, Muthupalani S, Westphalen CB, Andersen GT, … Chen D (2014). Denervation suppresses gastric tumorigenesis. Science Translational Medicine 6(250), 250ra115. 10.1126/scitranslmed.3009569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Shi B, Jia Y, Qiu G, Yang W, Li J, … Li Z (2018). Expression and significance of autonomic nerves and alpha9 nicotinic acetylcholine receptor in colorectal cancer. Molecular Medicine Reports 17(6), 8423–8431. 10.3892/mmr.2018.8883. [DOI] [PubMed] [Google Scholar]


