Abstract
The role of obesity in the pathogenesis of heart failure (HF), and in particular HF with preserved ejection fraction (HFpEF), has drawn significant attention in recent years. The prevalence of both obesity and HFpEF has increased worldwide over the past decades and when present concomitantly suggests an obese-HFpEF phenotype. Anthropometrics, including body mass index, waist circumference, and waist-to-hip ratio, are associated with incident HFpEF. However, the cardiovascular effects of obesity may actually be driven by the distribution of fat, which can accumulate in the epicardial, visceral, and subcutaneous compartments. Regional fat can be quantified using non-invasive imaging techniques, including computed tomography, magnetic resonance imaging, and dual-energy X-ray absorptiometry. Regional variations in fat accumulation are associated with different HFpEF risk profiles, whereby higher epicardial and visceral fat have a much stronger association with HFpEF risk compared with elevated subcutaneous fat. Thus, regional adiposity may serve a pivotal role in the pathophysiology of HFpEF contributing to decreased cardiopulmonary fitness, impaired left ventricular compliance, upregulation of local and systemic inflammation, promotion of neurohormonal dysregulation, and increased intra-abdominal pressure and vascular congestion. Strategies to reduce total and regional adiposity have shown promise, including intensive exercise, dieting, and bariatric surgery programmes, but few studies have focused on HFpEF-related outcomes among obese. Further understanding the role these variable fat depots play in the progression of HFpEF and HFpEF-related hospitalizations may provide therapeutic targets in treating the obese-HFpEF phenotype.
Keywords: Obesity, Regional adiposity, Heart failure, Heart failure with preserved ejection fraction
Introduction
Heart failure (HF) with preserved ejection fraction (HFpEF) is a heterogeneous syndrome with multiple proposed mechanisms and is characterized by evidence of abnormal left ventricular (LV) relaxation and filling and an ejection fraction ≥50%.1 Identifying effective therapies for HFpEF has been difficult due to its multiple distinct ‘phenotypes’, marked by diastolic dysfunction, impaired exercise reserve, abnormal ventricular–arterial coupling, inflammation and endothelial dysfunction, chronotropic incompetence, altered myocardial energetics and peripheral skeletal muscle metabolism, and renal insufficiency.2,3 Obesity is a modifiable risk factor of HFpEF and represents excess total body adipose tissue.2,4 The prevalence of both obesity and HFpEF has increased worldwide over the past decades.5,6 Obesity is most commonly defined by anthropometrics, including body mass index (BMI), which is more representative of total adipose accumulation, and waist circumference (WC) and waist-to-hip ratio (WHR), which are more representative of central distribution of adiposity. While total body fat accumulation was once thought to contribute to cardiovascular risk, the deleterious cardiovascular effects of obesity may actually result from the distribution of adipose tissue within the body itself.7 Obesity is a stronger risk factor for HF than other types of cardiovascular disease,8 and among those who develop HF, higher BMI predicts HFpEF but not HF with reduced ejection fraction (HFrEF).9,10 Recent trials describe that the obesity and HFpEF conditions present comorbidly,11–17 suggesting the emergence of a distinct ‘obese-HFpEF phenotype’.18,19 Obesity is thus emerging as potentially the most important risk factor for HFpEF. While obesity contributes to the development of HFpEF, the role of regional adipose tissue distribution and its effect on HFpEF are not well understood but may play an important role in its pathophysiology. Additionally, it is not well known how regional adiposity may function differently within HFpEF groups with normal, overweight, and high BMI. In this review, we discuss the role regional adiposity has on the development and progression of HFpEF.
Obesity and risk of heart failure with preserved ejection fraction
Obesity affects approximately 40% of the United States population and exhibits a higher prevalence in people above the age of 40 years.20 As one ages, there is an increase in total body fat mass alongside a decrease in lean mass.21 Obesity serves as an important risk factor for incident HF across community cohorts,8,22 and the risk obesity confers on HF is not explained by obesity-related cardiometabolic risk factors alone.8 Longitudinal studies have demonstrated that higher BMI confers greater risk of developing HFpEF but not HFrEF.4,9,10,23 The association of obesity and HFpEF also appears to be stronger among older African American women than compared to White women.24 Additionally, higher body weights in young adulthood predicts incident HF independent of BMI later in life,25 suggesting that cumulative lifetime BMI-years drives development of HF. Average BMI has increased across HFpEF trial cohorts, as shown in Figure 1.11–17 [Of note, PARAGON-HF excluded morbid obesity (i.e. BMI >40 kg/m2) and those with low plasma brain natriuretic peptide (BNP) at the time of enrolment, which likely reflects why the mean BMI is lower compared to other recent HFpEF trials]16.
Figure 1.
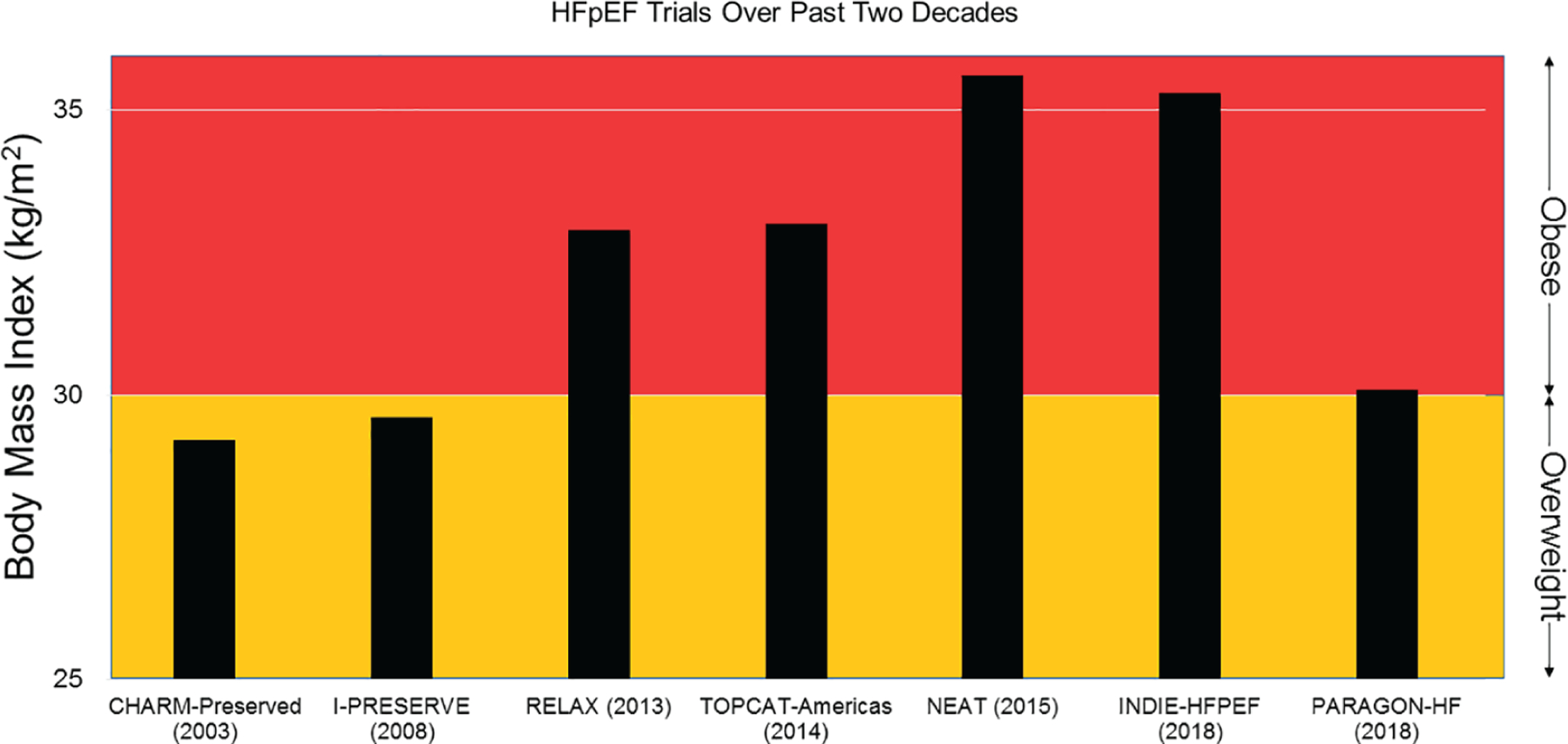
Baseline body mass index across heart failure with preserved ejection fraction (HFpEF) cohorts over the past two decades. These examples of HFpEF trials over the past two decades demonstrate an increase in average body mass index (BMI) across HFpEF cohorts.11–17 The lower mean BMI in PARAGON-HF was likely due to exclusion of morbid obesity (i.e. BMI >40 kg/m2) and those with low plasma brain natriuretic peptide at the time of enrolment.16
Obesity is associated with diabetes, hypertension, LV hypertrophy, increased LV stiffness, and reduced diastolic relaxation among individuals in community populations, factors that may promote the progression of HFpEF.26,27 Diabetes is also associated with LV remodelling, ventricular hypertrophy, and cardiac microvascular disease, likely contributing to the development of HFpEF among patients with diabetes.28 Clinical outcomes are also worse among patients with diabetes treated with insulin in HFpEF.29 The link between obesity and diabetes are likely driven by inhibited glucose transport and uptake into muscle cells caused by insulin resistance and reduced insulin sensitivity in the setting of glucotoxicity.30 Central adiposity (prevalence of adipose tissue within the abdominal compartment), and particularly visceral adiposity, is a strong predictor of insulin resistance in obesity compared to normal weight individuals.31 Visceral fat is strongly associated with increased hepatic glucose production and reduced glucose disposal,31 and among those without diabetes, visceral but not subcutaneous fat is associated with insulin resistance.32 Insulin resistance and its metabolic complications may contribute to HFpEF risk in obesity, although prospective studies are needed to characterize such mechanisms.
Central obesity, which is represented by the anthropometrics WC and WHR, is also associated with increased arterial stiffness, hypertension,33,34 and HFpEF risk.4,23 Weight loss and subsequent reduction in central adiposity has shown to reduce the degree of arterial stiffness.35 Bariatric surgery also contributes to reduction in hypertension.36 While regional fat is associated with arterial stiffening, observational data describe an association between obese HFpEF and lower arterial stiffness than compared to non-obese HFpEF.37 The role of arterial vascular compliance in the development and pathophysiology of obese HFpEF is unclear and warrants further investigation. Among obese individuals, regional fat distribution may serve a pivotal role in the development of HFpEF10 and provide insight into an obese HFpEF phenotype that may be more responsive to therapies such as weight loss (Figure 2).
Figure 2.
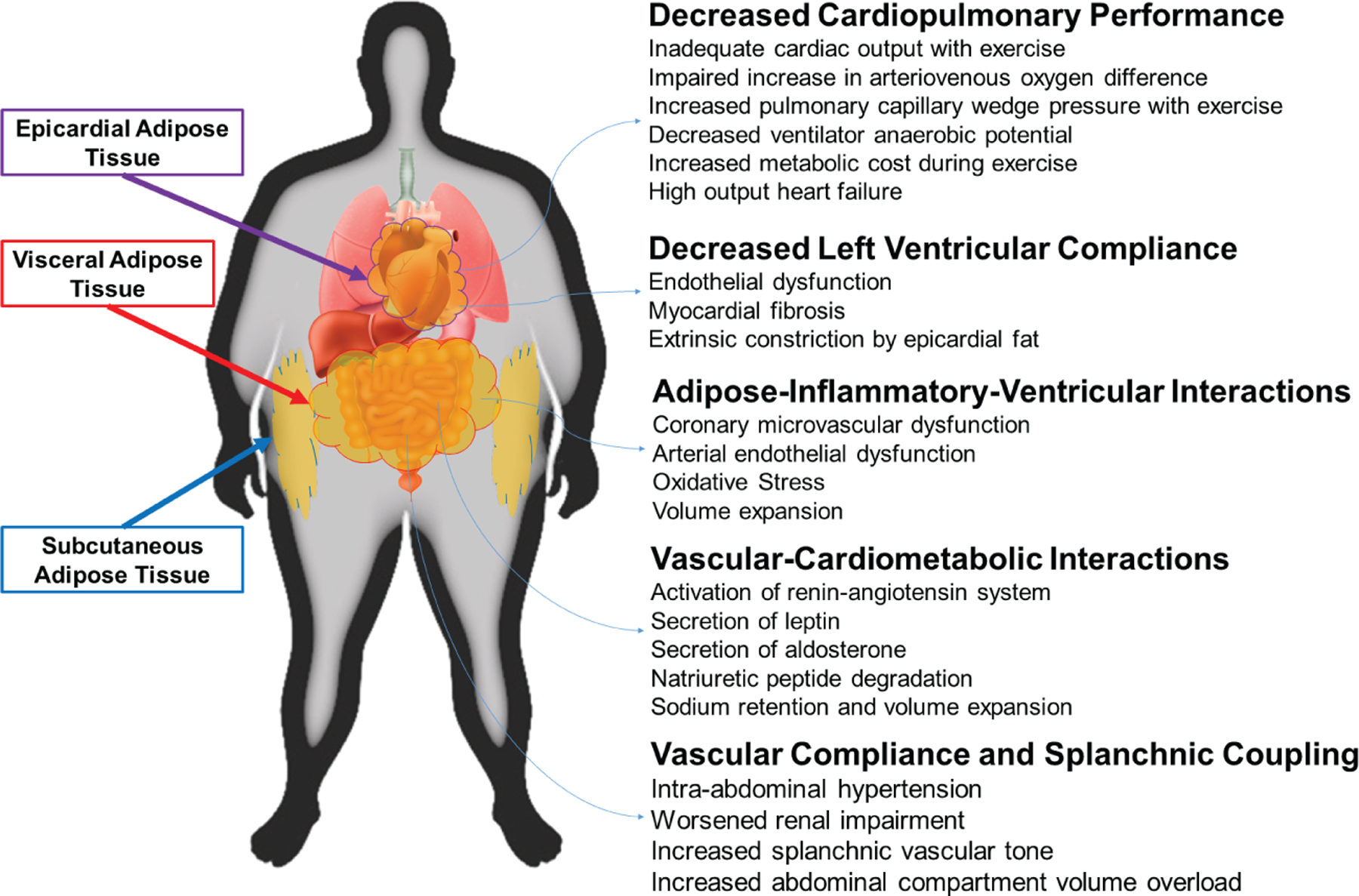
Proposed model by which regional adiposity affects heart failure with preserved ejection fraction physiology.
Regional distribution of adiposity and risk of heart failure with preserved ejection fraction
Fat accumulates within various body compartments, including subcutaneous, visceral, and epicardial compartments. While obesity, by measure of anthropometrics alone, contributes to risk of developing HFpEF, patterns of regional accumulation of adipose tissue within the body play a role in the development of HFpEF.10 Although a person may be overweight or obese by anthropometrics (i.e. BMI ≥25 kg/m2), regional fat accumulates disproportionately and does not equally contribute to the risk of HFpEF hospitalizations.10
Visceral adipose tissue
Visceral adipose tissue (VAT) refers to the intra-abdominal adipose accumulation of omental and mesenteric adipose tissue, excluding subcutaneous and intramuscular fat (Figure 3).38 Visceral adipose tissue can be quantified by averaging the fat area at single or multiple levels within the abdominal cavity. These measurements can be performed at the level of the umbilicus, L2–L3, or L4–L5 using computed tomography (CT),39 magnetic resonance imaging (MRI),40 or dual-energy X-ray absorptiometry (DXA).41 Various studies have looked at quantifying VAT between these imaging modalities and have found them to be comparable.42,43
Figure 3.
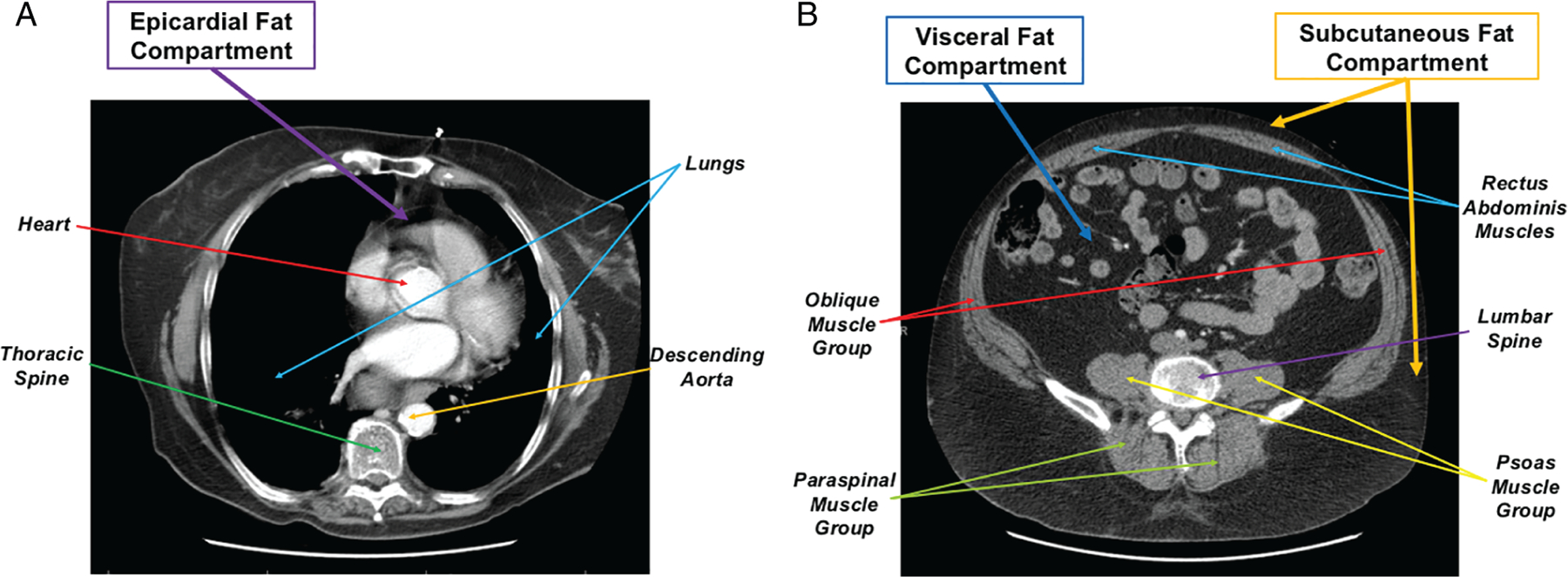
Thoracic and abdominal computed tomography slices and regional distribution of adiposity. Thoracic and abdominal computed tomography slice at level of T5 thoracic spine (A) and L2–L3 lumbar spine (B) demonstrating distribution of epicardial, visceral, and subcutaneous abdominal fat.
Visceral adipose tissue is a pro-inflammatory tissue that increases cardiovascular risk by promoting diseases such as diabetes, dyslipidaemia, and hypertension.44 While anthropometrics such as WC and WHR are intended to indirectly represent the degree of visceral adiposity by quantifying abdominal girth relative to the rest of the body, they in fact do not accurately quantify visceral fat.38 Beyond increasing the risk of cardiometabolic disease, VAT (but not BMI) is directly associated with mortality, especially among obese people with coronary artery disease.45 Even among those who are normal weight or overweight (i.e. BMI <30 kg/m2), elevated VAT appears to be associated with metabolic derangements and prevalence of cardiometabolic diseases.46
Visceral adiposity also appears to serve an active role in the development of HF. Among ambulatory individuals, visceral adiposity is associated with increased levels of N-terminal pro-BNP, a subclinical HF-related measure of vascular congestion.47 This correlation also appears among hospitalized individuals with HF. In a longitudinal, multi-ethnic cohort study, CT-measured VAT predicted incident hospitalized HFpEF but not HFrEF after adjusting for cardiovascular risk factors.10 VAT also provided additional risk prediction of HFpEF among people who were overweight or obese (i.e. BMI ≥25 kg/m2).10 Additionally, among people with normal BMI (i.e. BMI <25 kg/m2), increased VAT predicted a trend towards increased incidence of hospitalized HFpEF. Excess VAT may confer risk of developing HFpEF even among people who are identified as not overweight or obese, shedding light on a limitation of anthropometrics in qualifying risk in developing HF within a ‘silent obesity’ phenotype. The longitudinal effect on changes in VAT and subsequent changes in diastolic dysfunction or prevention of incident HFpEF or HFpEF-related hospitalizations have not been studied.
Epicardial adipose tissue
Epicardial adipose tissue (EAT) refers to regional fat surrounding the myocardium, within the pericardium. EAT is a complex, metabolically active adipose tissue that serves paracrine and exocrine functions, stores triglycerides to provide energy to the myocardium, produces pro-inflammatory adipokines and pro-oxidative substances, and may have a direct impact on cardiovascular health.48,49 EAT can be measured by quantifying epicardial fat thickness using non-invasive imaging modalities which include two-dimensional transthoracic echocardiography,50 CT,51 and MRI.52 Among these methods, CT and MRI provide the ability to quantify epicardial fat by volume and fat thickness, and MRI provides the greatest reproducibility in measurements.53
Epicardial fat appears to exert two key detrimental functions on the heart: (i) pro-inflammatory effects and driver of comorbid disease; (ii) cardio-mechanical interaction advancing diastolic dysfunction. EAT is associated with accelerated coronary atherosclerosis, insulin resistance, and hypertension both in obese and non-obese individuals.54–57 Among HF patients, EAT is associated with atrial fibrillation, diabetes, and biomarkers of myocardial injury.58 Further, EAT thickness in itself, which trends with higher BMI, correlates with the prevalence of hypertension.59 EAT displays different effects by sex and tends to increase disproportionately in older women.59 The detrimental mechanical properties of EAT become apparent through a correlation with increased ventricular wall thickness, worsened LV relaxation, and diastolic dysfunction.59–61 While epicardial fat is associated with diastolic dysfunction, no studies have investigated the role epicardial fat plays in incidence of HFpEF or whether changes in degree of EAT improves diastolic function among patients with HFpEF.
Subcutaneous adipose tissue
Subcutaneous adipose tissue (SAT) refers to the accumulation of adipose tissue outside of the abdominal cavity. The quantification of SAT is similar to visceral adiposity, and can be measured non-invasively using CT,39 MRI,42 and DXA.41 SAT appears to have different effects on cardiometabolic risk than adipose tissue in other body compartments, such as VAT. Subcutaneous adiposity is less likely associated with subclinical and clinical cardiovascular disease.62,63 Additionally, cosmetic therapies that target and remove SAT have no effect on cardiovascular risk.64 Among individuals without known cardiovascular disease, SAT served no role in predicting future HF, including HFpEF and HFrEF.10 The role of altering burden of subcutaneous fat in modulating cardiovascular health remains unclear.
Cardio-mechanical–adipose interactions
Decreased cardiopulmonary performance
Obesity is associated with impaired cardiac energy delivery with exercise among those without HF.65 Higher BMI also correlates with worse exercise performance among those with HF.66 The association between obesity and poorer cardiopulmonary performance may vary by BMI as well as the distribution of fat, suggesting a distinct obese HFpEF phenotype.19 People with obese HFpEF are different from non-obese HFpEF—they tend to have higher LV mass and develop a high output failure physiology with impaired augmentation of cardiac output during exercise despite maintaining preserved ejection fraction.67 Peak exercise oxygen consumption (VO2) drops with higher BMI among obese HFpEF.19,68 Additionally, obese HFpEF patients have higher cardiac filling pressures with exercise,19 worse HF symptoms, and higher metabolic cost of exertion than non-obese HFpEF.37
Along with excess adiposity, regional adiposity may serve a role in decreased exercise performance.69 Higher visceral fat predicts worse peak VO2, ventilatory anaerobic threshold, and 6-min walk test outcomes among obese HFpEF compared to healthy controls, and intra-abdominal adiposity predicts poorer cardiopulmonary performance than epicardial fat.70 Visceral adiposity is also associated with reduced myocardial glucose uptake,71 impaired efficiency of fatty acid metabolism,72 impaired myocardial energetics, myocardial steatosis, and concentric LV hypertrophy that mediates LV diastolic dysfunction.73 Changes in cardiac metabolism may play a significant role in reduced cardiopulmonary performance in obese HFpEF; however, longitudinal studies are needed to characterize their mechanisms.
Decreased left ventricular compliance
In obese HFpEF patients, one of the key underlying disorders in cardiac dysfunction is mediated by decreased LV distensibility.19 Changes in BMI from childhood and mid-adulthood contribute to the development of LV hypertrophy and diastolic dysfunction later in life, suggesting a harmful toll that lifetime BMI-years has on cardiac function.74,75 While the changes in weight and LV distensibility may occur concomitantly, the two are likely linked in the distinct obese HFpEF phenotype.19 VAT is associated with increased concentric LV remodelling and impaired cardiorespiratory fitness independent of BMI, whereas SAT is associated with eccentric LV remodelling and high cardiac output.76 Reduced LV compliance causes increased ventricular filling pressures, and higher BMI correlates with increased pulmonary and pulmonary capillary wedge pressures among individuals with HFpEF compared to those without HFpEF.19 A proposed mechanism by which adiposity drives the dysregulation of cardiac function and LV distensibility may be through external mechanical constraint. Obese HFpEF patients have higher EAT thickness than non-obese HFpEF and healthy individuals.19 The elevated epicardial fat develops abnormal ventricular–adipose interactions resulting in pericardial restraint: when compared to non-obese HFpEF and healthy controls, obese HFpEF demonstrates septal wall flattening by transthoracic echocardiography and higher right-sided to left-sided filling pressures along with elevated pulmonary capillary wedge pressures by invasive haemodynamics at rest and with exercise.19 These measured parameters support pressure equalization across ventricles in different physiologic states—a constrictive pattern that correlates with increased epicardial fat within a fixed pericardial space. Epicardial fat may serve a key mechanistic role within obese HF through decreased LV compliance.
Vascular–adipose interactions
Inflammatory processes
Longitudinal studies are needed to identify the role of systemic inflammation as a mediator between excess regional adiposity and HFpEF. Some investigators have proposed mechanistic relationships between increased total and regional adiposity and the upregulation of local and systemic inflammation that may contribute to oxidative stress, microvascular injury, myocardial fibrosis, and cardiac dysregulation among obese HFpEF.56,77,78 Pro-inflammatory biomarkers, interleukin-6 and tumour necrosis factor-𝛼, have been shown to predict HFpEF but not HFrEF in a community population.79 Additionally, high sensitivity C-reactive protein is elevated in obese compared to non-obese HFpEF patients.37 Thus, conceivably, obesity and excess regional adiposity may be key to producing pro-inflammatory cytokines that cause metabolic cascades leading to cardiomyocyte hypertrophy, LV remodelling, and subsequent LV diastolic dysfunction.80 The regional variability of adipose tissue among obese individuals may play a critical role in the local and systemic cardiac and vascular function, partially mediated by inflammation.44 Some investigators have proposed that higher VAT secretes pro-inflammatory cytokines that may contribute to microvascular endothelial dysfunction and reduced vascular compliance among obese HFpEF; however, prospective studies confirming this relationship are lacking.68,70,81 EAT is also a pro-inflammatory tissue that may have local paracrine effects via persistent inflammation causing coronary microvascular dysfunction and ventricular stiffness.56,82 SAT, on the other hand, does not demonstrate these metabolically active phenomena within the abdomen.44 Plasma volume expansion occurs more significantly in obese HFpEF compared to those who are not obese.83 When decompensated, these obese patients tolerate decongestion less well with a greater deterioration of renal function, a process that is key to the obese HFpEF state.84 The upregulation of systemic inflammation may result in volume expansion and adversely impair decongestive therapies in obese HFpEF. Thus, future studies investigating whether reduction in excess regional adiposity can improve systemic inflammatory dysregulation may provide potential inflammatory therapeutic targets in obese HFpEF.
Vascular–cardiometabolic factors
Vascular–adrenergic–adipokine interactions have been proposed as pathophysiologic abnormalities seen in obese HFpEF; however, prospective studies are lacking to confirm these relationships.85–87 Obese HFpEF individuals have higher plasma volume expansion compared to non-obese HFpEF.83 Obesity-related insulin resistance and compensatory hyperinsulinaemia may lead to upregulation of the sympathoadrenal system and can increase blood pressure and arterial stiffness.86 Further, dysfunctional adipose tissue triggers secretion of leptin, a hormone produced by adipocytes that regulates energy balance and modulates hunger. Leptin stimulates secretion of aldosterone,88,89 and dysfunctional adipocytes are also stimulated by leptin-regulated increases in angiotensin II.88,90 Obesity also is associated with increased activity of neprilysin, which rapidly degrades natriuretic peptide that otherwise would have an anti-aldosterone regulatory effect.87 The result of increased renin–angiotensin system activation and aldosterone synthesis is volume expansion and sodium retention, a state that in the context of impaired ventricular distensibility may further lead to decompensation in obese HFpEF.19 Prospective studies are needed to further define this relationship.
There is an inverse relationship between BMI and circulating natriuretic peptide hormone levels in obesity, although this mechanism is not well understood. Impaired cardiovascular fluid homeostasis in obese HF may be through BNP deficiency, and a correlation between higher visceral adiposity and lower BNP is possibly mediated by increased secretion of the natriuretic peptide clearance receptor (NPR-C) on adipocytes, expressing increased BNP degradation.91 A low ratio between the BNP and the clearance receptor in obese patients may reflect a circulation dominated by BNP clearance, thus prompting this deficiency.92 Further, adipocyte tissue renin–angiotensin–aldosterone system may attenuate the paracrine-metabolic system through additional secretion of angiotensinogen, ultimately increasing aldosterone secretion in plasma circulation.93 Among obese compared to non-obese HFpEF patients, there is a marked reduction in transmural LV distensibility that correlates with low circulating BNP.19 These findings lend to BNP deficiency as a marker for obese HFpEF outcomes, warranting additional studies including obese HFpEF to clarify the relationship between regional adiposity, BNP deficiency, and decompensated HFpEF.
Vascular compliance and splanchnic coupling
Heart failure with preserved ejection fraction is characterized by decreased vascular compliance and particularly so in patients with concomitant obesity.94,95 Decreased arterial, and equally if not more importantly venous compliance, are conceptually driven by (i) obesity-mediated neurohormonal and inflammatory activation, and (ii) possibly by pure mechanical properties of the adipose tissue.96 The splanchnic vascular compartment is the largest reservoir of intravascular blood volume, with the majority of the blood located in the venous system. It has been proposed that inter-compartmental volume redistribution from the abdominal/splanchnic compartment into the central compartment (chest and central vasculature) could be a key determinant of cardiovascular congestion and cause of cardiac decompensation.96,97 Since obesity (especially visceral) is associated with a higher intra-abdominal pressure (IAP),98,99 the external vascular compression could lead to a restrictive vascular physiology that is similar to the observed epicardial fat–LV distensibility relationship. Related to this, increased IAP ≥12 mmHg has been linked to organ dysfunction in general and renal impairment specifically.100,101 A reduction in IAP (contributed by ascites, gut oedema, and abdominal fat) via diuresis or paracentesis has been shown to improve renal function.101,102 Finally, as recently demonstrated, a direct reduction in splanchnic vascular tone or in other words selective increase in splanchnic vascular compliance could lead to central vascular decongestion with potentially beneficial effects on cardiopulmonary performance.103,104 The role for targeting the abdominal fat burden, elevated IAP, and modulation of vascular–splanchnic coupling in obese HFpEF remains to be studied.
Clinical implications
The role of obesity in pathogenesis of HF, and particularly HFpEF, has drawn greater attention.10,19,23,105 While anthropometrics are commonly used to categorize obesity at present, they are not always representative of excess regional adiposity.38 Focus on quantifying regional distribution of adiposity may provide more insight into incidence and disease progression of an obese HFpEF phenotype.10 The proposed mechanisms are summarized in Table 1.19,37,67–70,74,79,82,88–90,98–104,106–109 While most people do not undergo CT, MRI, or DXA imaging for purposes of screening for HF, interpreting regional fat distribution when imaging is obtained for other purposes could shed light on subpopulations who are at increased risk of developing HFpEF. Additionally, fat distribution has important implications. A patient who has an elevated BMI, WHR, and/or WC, along with elevated visceral adiposity, may have different risk of developing HFpEF or response to therapies than an individual who has similar anthropometric measures, but lower visceral fat and higher subcutaneous fat. Additionally, higher epicardial and visceral fat among people with normal anthropometric screening may be missed and have increased HFpEF risk or HFpEF-related prognosis.
Table 1.
Summary of previous studies on excess adiposity and its effects on heart failure with preserved ejection fraction
| Findings | Mechanisms | Reference |
|---|---|---|
| Decreased cardiopulmonary performance | Inadequate cardiac output with exercise Impaired increase in AVO2 difference Increased PCWP with exercise Decreased ventilator anaerobic potential Increased metabolic cost during exercise High output heart failure |
Obokata et al.19 Reddy et al.37 Mohammed et al.67 Kitzman et al.68 Li et al.69 Haykowsky et al.70 Haykowsky et al.106 Reddy et al.107 Abudiab et al.108 |
| Decreased left ventricular compliance | Endothelial dysfunction Myocardial fibrosis Extrinsic constriction by epicardial fat |
Obokata et al.19 Khan et al.74 |
| Adipose–inflammatory–ventricular interactions | Coronary microvascular dysfunction Arterial endothelial dysfunction Oxidative stress Volume expansion |
Reddy et al.37 Kalogeropoulos et al.79 Nerlekar et al.82 |
| Vascular–cardiometabolic interactions | Activation of renin–angiotensin system Secretion of leptin Secretion of aldosterone Natriuretic peptide degradation Sodium retention and volume expansion |
Xue et al.88 Huby et al.89 Briones et al.90 Clerico et al.109 |
| Vascular compliance and splanchnic coupling | Intra-abdominal hypertension Worsened renal impairment Increased splanchnic vascular tone Increased abdominal compartment volume overload |
Varela et al.98 Lambert et al.99 Malbrain et al.100 Mullens et al.101 Mullens et al.102 Fudim et al.103 Fudim et al.104 |
AVO2, arteriovenous oxygen difference; PCWP, pulmonary capillary wedge pressure.
At present, the mainstay therapeutic interventions involve healthy dieting and physical activity. Reducing caloric intake and exercise improve cardiac performance among obese HFpEF.68 However, targeting harmful fat accumulation is imperative when treating patients with obese HFpEF. Vigorous exercise reduces VAT without significant change in body weight.110,111 Invasive procedures also exist to reduce excess adiposity. Liposuction, which removes SAT, provides no significant cardiovascular protection or change in obesity-related metabolic abnormalities.112,113 Alternatively, bariatric surgeries, including Roux-en-Y gastric bypass and sleeve gastrectomy, reduce VAT and provide a remarkable improvement in 10-year cardiovascular risk and remission rates for diabetes and hypertension among morbidly obese.36 However, VAT that is surgically removed via omentectomy at time of bariatric surgery provides no significant changes in metabolic outcomes and minimal improvement in BMI compared to bariatric surgery alone.114 Bariatric surgery has in fact demonstrated a reduction in HF incidence, with the greatest improvement in HF risk among those with the greatest weight reduction.115 Further, there is sustained reduction in LV mass despite a plateau in BMI reduction over 2 years following bariatric surgery.116 While weight loss initiatives such as dieting and exercise may be effective for some, surgical treatment to reduce the burden of deleterious total and regional adiposity may prove as a more successful tool in the treatment of obese HFpEF. Comparison of these strategies among obese HFpEF in the improvement of overall cardiopulmonary functional status and HFpEF-related hospitalizations and mortality is lacking.
There is certainly a role for understanding how changes in regional fat distribution affect clinical outcomes among patients with HF, including HFpEF and HFrEF. Anthropometrics predict HFpEF but not HFrEF,10 yet there is a paradoxical relationship between BMI and HF, known as the ‘obesity paradox’.66 When looking at HFpEF subtype only, mortality appears to increase among obese individuals with HFpEF.117 Understanding whether regional elevations in adiposity contribute to these mortality trends may provide a better understanding of the long-term clinical outcomes following diagnosis and characterization of HF and its subtypes. Additionally, with regional distribution of adipose tissue drawing more attention, future investigations may shed additional light on new therapeutic targets.
Future directions
The increasing burden of obesity and HFpEF poses an imperative need to understand the pathogenesis of adiposity on cardiac function and to develop effective therapies. Regional distribution of fat serves as a vast frontier for behavioural, pharmacologic, and invasive treatment options to address this need. Ongoing clinical trials are investigating therapeutic strategies for obesity and HF through behavioural modification, including dietary adherence and physical activity, and surgical weight reduction strategies. These trials’ outcomes include weight reduction, HF-related hospitalizations, LV function, and exercise capacity (online supplementary Table S1).
Weight loss interventions may advance our understanding of the development and progression of obese HFpEF and may serve as a therapeutic target. A systematic review and meta-analysis of dietary and surgical weight loss studies revealed that both interventions improve resting systemic and cardiac filling pressures among obese people without HF.118 A prospective controlled Swedish surgical intervention study showed a marked reduction in the incidence of all HF following bariatric surgery, with the most notable HF risk reduction among those with the greatest weight loss.115 Medical drug therapies also show promise in potentially addressing the obese HFpEF phenotype: sodium–glucose co-transporter 2 inhibitors, which improve glucose control and cardiovascular outcomes in patients with diabetes, reduce adipose tissue mass with minimal impacts on muscle or lean mass.119,120 Drugs affecting lipid metabolism, such as lactoferrin, reduce visceral adiposity among overweight and obese Asians without HF.121 An ongoing prospective study aims to determine the efficacy of bariatric surgery on body weight and LV mass reduction (NCT00178633). Such surgical weight loss therapies include gastric bypass, sleeve gastrectomy, adjustable gastric banding, and biliopancreatic diversion with duodenal switch. Clinical trials of these pharmacological and surgical therapies among obese individuals are needed to help fill the gap in understanding of the change in regional adiposity and incidence of HFpEF, and among those with obese HFpEF, changes in progression of disease. Future studies can aim to answer these questions through serial imaging before and after surgical intervention, as well as serial haemodynamic assessments and serum adiposity and inflammatory biomarkers.
Among the proposed mechanisms of obese HFpEF is external pericardial constraint imposed by epicardial fat accumulation.19 In non-obese canine and swine models, percutaneous pericardial resection was tested as a novel strategy to relieve the effect of pericardial restraint in HFpEF with reduction in LV end-diastolic pressures and increase in LV end-diastolic volumes.122 Among individuals with excess epicardial fat thickness, novel pericardial interventions including pericardial resection and pericardiotomy could further our understanding on the direct mechanical compressive effect of regional adiposity among HFpEF, as well as determine the safety and comparative efficacy of these strategies on the outcomes of symptoms, haemodynamics, HFpEF-related hospitalizations, and adverse events. Through a separate mechanism, the REDUCE LAP-HF I trial demonstrated that a percutaneous interatrial shunt device creating left-to-right shunt flow safely reduced exercise pulmonary capillary wedge pressure in HF,123 and the larger REDUCE LAP-HF II trial (NCT03088033) aims to provide its efficacy in HFpEF symptoms and hospitalizations.
Neuromodulation may also serve as a potential target for obese HFpEF through mechanisms such as volume redistribution. As previously mentioned, a direct reduction in splanchnic vascular tone may reduce central vascular congestion and improve cardiopulmonary performance.103,104 The effect of neuromodulation on cardiopulmonary performance in obese HFpEF, which may theoretically have reduced vascular compliance within the abdominal compartment, increased IAP, and greater regional adiposity, has not been studied. However, future investigations are needed to understand whether neuromodulation may serve as a primary and secondary preventive strategy among obese HFpEF at risk for incident and repeat HF-related hospitalizations.
Conclusions
With the higher prevalence trends of obesity and HF over the past several decades, there is increasing recognition of the interaction between adiposity and cardiac function in the pathophysiology of an obese HFpEF phenotype. Regional adiposity, measured by non-invasive imaging, may serve as an important driver in the incidence and pathogenesis of HFpEF among overweight and obese individuals through multiple mechanisms. Behavioural and invasive weight reduction exhibit the potential to mitigate the growing burden of obese HFpEF and its disease progression. Future studies will further our understanding of the interactions between regional adiposity and HF, and randomized clinical trials will be essential in determining the safety and efficacy of promising preventive and therapeutic interventions for obese HFpEF.
Supplementary Material
Table S1. Current ongoing clinical trials on therapeutic interventions for heart failure and obesity.
Footnotes
Supplementary Information
Additional supporting information may be found online in the Supporting Information section at the end of the article.
Conflict of interest: M.F. receives research support by an American Heart Association Grant (17MCPRP33460225) and National Institute of Health T32 (5T32HL007101). He consults for Galvani and Axon Therapies. R.J.M. receives research support from the National Institutes of Health (U01HL125511–01A1, U10HL110312 and R01AG045551–01A1), Akros, Amgen, AstraZeneca, Bayer, GlaxoSmithKline, Gilead, InnoLife, Luitpold/American Regent, Medtronic, Merck, Novartis, Otsuka, and ResMed; honoraria from Abbott, Amgen, AstraZeneca, Bayer, Janssen, Luitpold Pharmaceuticals, Medtronic, Merck, Novartis, and ResMed; and has served on an advisory board for Amgen, AstraZeneca, Luitpold, Merck, Novartis and Boehringer Ingelheim. G.M.F. is supported by research grants from NHLBI, American Heart Association, Amgen, Merck, Cytokinetics, and Roche Diagnostics; he has acted as a consultant to Novartis, Amgen, BMS, Medtronic, Cardionomic, Relypsa, V-Wave, Myokardia, Innolife, EBR Systems, Arena, Abbott, Sphingotec, Roche Diagnostics, Alnylam, LivaNova, and SC Pharma. All other authors have nothing to disclose.
References
- 1.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Køber L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A; ESC Committee for Practice Guidelines. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2012;14: 803–869. [DOI] [PubMed] [Google Scholar]
- 2.Sharma K, Kass DA. Heart failure with preserved ejection fraction: mechanisms, clinical features, and therapies. Circ Res 2014;115:79–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gladden JD, Linke WA, Redfield MM. Heart failure with preserved ejection fraction. Pflugers Arch 2014;466:1037–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ho JE, Lyass A, Lee DS, Vasan RS, Kannel WB, Larson MG, Levy D. Predictors of new-onset heart failure: differences in preserved versus reduced ejection fraction. Circ Heart Fail 2013;6:279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinberg BA, Zhao X, Heidenreich PA, Peterson ED, Bhatt DL, Cannon CP, Hernandez AF, Fonarow GC; Get With the Guidelines Scientific Advisory Committee and Investigators. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation 2012;126:65–75. [DOI] [PubMed] [Google Scholar]
- 6.Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. N Engl J Med 2002;347:305–313. [DOI] [PubMed] [Google Scholar]
- 7.Bays HE, Gonzalez-Campoy JM, Bray GA, Kitabchi AE, Bergman DA, Schorr AB, Rodbard HW, Henry RR. Pathogenic potential of adipose tissue and metabolic consequences of adipocyte hypertrophy and increased visceral adiposity. Expert Rev Cardiovasc Ther 2008;6:343–368. [DOI] [PubMed] [Google Scholar]
- 8.Ndumele CE, Matsushita K, Lazo M, Bello N, Blumenthal RS, Gerstenblith G, Nambi V, Ballantyne CM, Solomon SD, Selvin E, Folsom AR, Coresh J. Obesity and subtypes of incident cardiovascular disease. J Am Heart Assoc 2016;5:e1 –e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Savji N, Meijers WC, Bartz TM, Bhambhani V, Cushman M, Nayor M, Kizer JR, Sarma A, Blaha MJ, Gansevoort RT, Gardin JM, Hillege HL, Ji F, Kop WJ, Lau ES, Lee DS, Sadreyev R, van Gilst WH, Wang TJ, Zanni MV, Vasan RS, Allen NB, Psaty BM, van der Harst P, Levy D, Larson M, Shah SJ, de Boer RA, Gottdiener JS, Ho JE. The association of obesity and cardiometabolic traits with incident HFpEF and HFrEF. JACC Heart Fail 2018;6:701–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rao VN, Zhao D, Allison MA, Guallar E, Sharma K, Criqui MH, Cushman M, Blumenthal RS, Michos ED. Adiposity and incident heart failure and its subtypes: MESA (Multi-Ethnic Study of Atherosclerosis). JACC Heart Fail 2018;6:999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borlaug BA, Anstrom KJ, Lewis GD, Shah SJ, Levine JA, Koepp GA, Givertz MM, Felker GM, LeWinter MM, Mann DL, Margulies KB, Smith AL, Tang WH, Whellan DJ, Chen HH, Davila-Roman VG, McNulty S, Desvigne-Nickens P, Hernandez AF, Braunwald E, Redfield MM; National Heart, Lung, and Blood Institute Heart Failure Clinical Research Network. Effect of inorganic nitrite vs placebo on exercise capacity among patients with heart failure with preserved ejection fraction: the INDIE-HFpEF randomized clinical trial. JAMA 2018;320:1764–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O’Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM; TOPCAT Investigators. Spironolactone for heart failure with preserved ejection fraction. N EnglJ Med 2014;370:1383–1392. [DOI] [PubMed] [Google Scholar]
- 13.Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O’Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E; RELAX Trial. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 2013;309:1268–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Redfield MM, Anstrom KJ, Levine JA, Koepp GA, Borlaug BA, Chen HH, LeWinter MM, Joseph SM, Shah SJ, Semigran MJ, Felker GM, Cole RT, Reeves GR, Tedford RJ, Tang WH, McNulty SE, Velazquez EJ, Shah MR, Braunwald E; NHLBI Heart Failure Clinical Research Network. Isosorbide mononitrate in heart failure with preserved ejection fraction. N EnglJ Med 2015;373:2314–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A; I-PRESERVE Investigators. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 2008;359:2456–2467. [DOI] [PubMed] [Google Scholar]
- 16.Solomon SD, Rizkala AR, Lefkowitz MP, Shi VC, Gong J, Anavekar N, Anker SD, Arango JL, Arenas JL, Atar D, Ben-Gal T, Boytsov SA, Chen CH, Chopra VK, Cleland J, Comin-Colet J, Duengen HD, Echeverría Correa LE, Filippatos G, Flammer AJ, Galinier M, Godoy A, Goncalvesova E, Janssens S, Katova T, Køber L, Lelonek M, Linssen G, Lund LH, O’Meara E, Merkely B, Milicic D, Oh BH, Perrone SV, Ranjith N, Saito Y, Saraiva JF, Shah S, Seferovic PM, Senni M, Sibulo AS, Sim D, Sweitzer NK, Taurio J, Vinereanu D, Vrtovec B, Widimský J, Yilmaz MB, Zhou J, Zweiker R, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, Van Veldhuisen DJ, Zannad F, Zile MR, McMurray JJ. Baseline characteristics of patients with heart failure and preserved ejection fraction in the PARAGON-HF trial. Circ Heart Fail 2018;11:e004962. [DOI] [PubMed] [Google Scholar]
- 17.Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved trial. Lancet 2003;362:777–781. [DOI] [PubMed] [Google Scholar]
- 18.Kitzman DW, Shah SJ. The HFpEF obesity phenotype: the elephant in the room. J Am Coll Cardiol 2016;68:200–203. [DOI] [PubMed] [Google Scholar]
- 19.Obokata M, Reddy YN, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation 2017;136:6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity among adults and youth: United States, 2015–2016. NCHS Data Brief 2017;288:1 –8. [PubMed] [Google Scholar]
- 21.Zong G, Zhang Z, Yang Q, Wu H, Hu FB, Sun Q. Total and regional adiposity measured by dual-energy X-ray absorptiometry and mortality in NHANES 1999–2006. Obesity (Silver Spring; ) 2016;24:2414–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ndumele CE, Coresh J, Lazo M, Hoogeveen RC, Blumenthal RS, Folsom AR, Selvin E, Ballantyne CM, Nambi V. Obesity, subclinical myocardial injury, and incident heart failure. JACC Heart Fail 2014;2:600–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pandey A, LaMonte M, Klein L, Ayers C, Psaty BM, Eaton CB, Allen NB, de Lemos JA, Carnethon M, Greenland P, Berry JD. Relationship between physical activity, body mass index, and risk of heart failure. J Am Coll Cardiol 2017;69:1129–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eaton CB, Pettinger M, Rossouw J, Martin LW, Foraker R, Quddus A, Liu S, Wampler NS, Hank Wu WC, Manson JE, Margolis K, Johnson KC, Allison M, Corbie-Smith G, Rosamond W, Breathett K, Klein L. Risk factors for incident hospitalized heart failure with preserved versus reduced ejection fraction in a multiracial cohort of postmenopausal women. Circ Heart Fail 2016;9:e002883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fliotsos M, Zhao D, Rao VN, Ndumele CE, Guallar E, Burke GL, Vaidya D, Delaney JC, Michos ED. Body mass index from early-, mid-, and older-adulthood and risk of heart failure and atherosclerotic cardiovascular disease: MESA. J Am Heart Assoc 2018;7:e009599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bello NA, Cheng S, Claggett B, Shah AM, Ndumele CE, Roca GQ, Santos AB, Gupta D, Vardeny O, Aguilar D, Folsom AR, Butler KR, Kitzman DW, Coresh J, Solomon SD. Association of weight and body composition on cardiac structure and function in the ARIC study (Atherosclerosis Risk in Communities). Circ Heart Fail 2016;9:e002978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wohlfahrt P, Redfield MM, Lopez-Jimenez F, Melenovsky V, Kane GC, Rodeheffer RJ, Borlaug BA. Impact of general and central adiposity on ventricular-arterial aging in women and men. JACC Heart Fail 2014;2:489–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tromp J, Lim SL, Tay WT, Teng TK, Chandramouli C, Ouwerkerk W, Wander GS, Sawhney JP, Yap J, MacDonald MR, Ling LH, Sattar N, McMurray JJ, Richards AM, Anand I, Lam CS; ASIAN-HF Investigators. Microvascular disease in patients with diabetes with heart failure and reduced ejection versus preserved ejection fraction. Diabetes Care 2019;42:1792–1799. [DOI] [PubMed] [Google Scholar]
- 29.Shen L, Rørth R, Cosmi D, Kristensen SL, Petrie MC, Cosmi F, Latini R, Køber L, Anand IS, Carson PE, Granger CB, Komajda M, McKelvie RS, Solomon SD, Staszewsky L, Swedberg K, Huynh T, Zile MR, Jhund PS, McMurray JJ. Insulin treatment and clinical outcomes in patients with diabetes and heart failure with preserved ejection fraction. Eur J Heart Fail 2019;21:974–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verma S, Hussain ME. Obesity and diabetes: an update. Diabetes Metab Syndr 2017;11:73–79. [DOI] [PubMed] [Google Scholar]
- 31.Bonora E Relationship between regional fat distribution and insulin resistance. Int J Obes Relat Metab Disord 2000;24 Suppl 2:S32–S35. [DOI] [PubMed] [Google Scholar]
- 32.Hsieh CJ, Wang PW, Chen TY. The relationship between regional abdominal fat distribution and both insulin resistance and subclinical chronic inflammation in non-diabetic adults. Diabetol Metab Syndr 2014;6:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zebekakis PE, Nawrot T, Thijs L, Balkestein EJ, van der Heijden-Spek J, Van Bortel LM, Struijker-Boudier HA, Safar ME, Staessen JA. Obesity is associated with increased arterial stiffness from adolescence until old age. J Hypertens 2005;23:1839–1846. [DOI] [PubMed] [Google Scholar]
- 34.Wildman RP, Mackey RH, Bostom A, Thompson T, Sutton-Tyrrell K. Measures of obesity are associated with vascular stiffness in young and older adults. Hypertension 2003;42:468–473. [DOI] [PubMed] [Google Scholar]
- 35.Dengo AL, Dennis EA, Orr JS, Marinik EL, Ehrlich E, Davy BM, Davy KP. Arterial destiffening with weight loss in overweight and obese middle-aged and older adults. Hypertension 2010;55:855–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Samson R, Milligan G, Lewine E, Sindi F, Garagliano J, Fernandez C, Moore R, DuCoin C, Oparil S, Le Jemtel TH. Effect of sleeve gastrectomy on hypertension. J Am Soc Hypertens 2018;12:e19–25. [DOI] [PubMed] [Google Scholar]
- 37.Reddy YN, Lewis GD, Shah SJ, Obokata M, Abou-Ezzedine OF, Fudim M, Sun JL, Chakraborty H, McNulty S, LeWinter MM, Mann DL, Stevenson LW, Redfield MM, Borlaug BA. Characterization of the obese phenotype of heart failure with preserved ejection fraction: a RELAX trial ancillary study. Mayo Clin Proc 2019;94:1199–1209. [DOI] [PubMed] [Google Scholar]
- 38.Tchernof A, Després JP. Pathophysiology of human visceral obesity: an update. Physiol Rev 2013;93:359–404. [DOI] [PubMed] [Google Scholar]
- 39.Ryckman EM, Summers RM, Liu J, Munoz del Rio A, Pickhardt PJ. Visceral fat quantification in asymptomatic adults using abdominal CT: is it predictive of future cardiac events? Abdom Imaging 2015;40:222–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Demerath EW, Shen W, Lee M, Choh AC, Czerwinski SA, Siervogel RM, Towne B. Approximation of total visceral adipose tissue with a single magnetic resonance image. Am J Clin Nutr 2007;85:362–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaul S, Rothney MP, Peters DM, Wacker WK, Davis CE, Shapiro MD, Ergun DL. Dual-energy X-ray absorptiometry for quantification of visceral fat. Obesity (Silver Spring; ) 2012;20:1313–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klopfenstein BJ, Kim MS, Krisky CM, Szumowski J, Rooney WD, Purnell JQ. Comparison of 3 T MRI and CT for the measurement of visceral and subcutaneous adipose tissue in humans. Br J Radiol 2012;85:e826–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reinhardt M, Piaggi P, DeMers B, Trinidad C, Krakoff J. Cross calibration of two dual-energy X-ray densitometers and comparison of visceral adipose tissue measurements by iDXA and MRI. Obesity (Silver Spring; ) 2017;25:332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bays HE. Adiposopathy is "sick fat" a cardiovascular disease? J Am Coll Cardiol 2011;57:2461–2473. [DOI] [PubMed] [Google Scholar]
- 45.Coutinho T, Goel K, Corrêa de Sá D, Kragelund C, Kanaya AM, Zeller M, Park JS, Kober L, Torp-Pedersen C, Cottin Y, Lorgis L, Lee SH, Kim YJ, Thomas R, Roger VL, Somers VK, Lopez-Jimenez F. Central obesity and survival in subjects with coronary artery disease: a systematic review of the literature and collaborative analysis with individual subject data. J Am Coll Cardiol 2011;57:1877–1886. [DOI] [PubMed] [Google Scholar]
- 46.Nazare JA, Smith JD, Borel AL, Haffner SM, Balkau B, Ross R, Massien C, Alméras N, Després JP. Ethnic influences on the relations between abdominal subcutaneous and visceral adiposity, liver fat, and cardiometabolic risk profile: the International Study of Prediction of Intra-Abdominal Adiposity and Its Relationship With Cardiometabolic Risk/Intra-Abdominal Adiposity. Am J Clin Nutr 2012;96:714–726. [DOI] [PubMed] [Google Scholar]
- 47.Cheng S, Fox CS, Larson MG, Massaro JM, McCabe EL, Khan AM, Levy D, Hoffmann U, O’Donnell CJ, Miller KK, Newton-Cheh C, Coviello AD, Bhasin S, Vasan RS, Wang TJ. Relation of visceral adiposity to circulating natriuretic peptides in ambulatory individuals. Am J Cardiol 2011;108:979–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berg G, Miksztowicz V, Morales C, Barchuk M. Epicardial adipose tissue in cardiovascular disease. Adv Exp Med Biol 2019;1127:131–143. [DOI] [PubMed] [Google Scholar]
- 49.Fitzgibbons TP, Czech MP. Epicardial and perivascular adipose tissues and their influence on cardiovascular disease: basic mechanisms and clinical associations. J Am Heart Assoc 2014;3:e000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iacobellis G, Willens HJ. Echocardiographic epicardial fat: a review of research and clinical applications. J Am Soc Echocardiogr 2009;22:1311–1319. [DOI] [PubMed] [Google Scholar]
- 51.Nakazato R, Shmilovich H, Tamarappoo BK, Cheng VY, Slomka PJ, Berman DS, Dey D. Interscan reproducibility of computer-aided epicardial and thoracic fat measurement from noncontrast cardiac CT. J Cardiovasc Comput Tomogr 2011;5:172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Doesch C, Streitner F, Bellm S, Suselbeck T, Haghi D, Heggemann F, Schoenberg SO, Michaely H, Borggrefe M, Papavassiliu T. Epicardial adipose tissue assessed by cardiac magnetic resonance imaging in patients with heart failure due to dilated cardiomyopathy. Obesity (Silver Spring; ) 2013;21:E253–E261. [DOI] [PubMed] [Google Scholar]
- 53.Talman AH, Psaltis PJ, Cameron JD, Meredith IT, Seneviratne SK, Wong DT. Epicardial adipose tissue: far more than a fat depot. Cardiovasc Diagn Ther 2014;4:416–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yerramasu A, Dey D, Venuraju S, Anand DV, Atwal S, Corder R, Berman DS, Lahiri A. Increased volume of epicardial fat is an independent risk factor for accelerated progression of sub-clinical coronary atherosclerosis. Atherosclerosis 2012;220:223–230. [DOI] [PubMed] [Google Scholar]
- 55.Okada K, Ohshima S, Isobe S, Harada K, Hirashiki A, Funahashi H, Arai K, Hayashi D, Hayashi M, Ishii H, Murohara T. Epicardial fat volume correlates with severity of coronary artery disease in nonobese patients. J Cardiovasc Med (Hagerstown: ) 2014;15:384–390. [DOI] [PubMed] [Google Scholar]
- 56.Packer M Epicardial adipose tissue may mediate deleterious effects of obesity and inflammation on the myocardium. J Am Coll Cardiol 2018;71:2360–2372. [DOI] [PubMed] [Google Scholar]
- 57.Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev 2010;11:11–18. [DOI] [PubMed] [Google Scholar]
- 58.van Woerden G, Gorter TM, Westenbrink BD, Willems TP, van Veldhuisen DJ, Rienstra M. Epicardial fat in heart failure patients with mid-range and preserved ejection fraction. Eur J Heart Fail 2018;20:1559–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim SA, Kim MN, Shim WJ, Park SM. Epicardial adipose tissue is related to cardiac function in elderly women, but not in men. Nutr Metab Cardiovasc Dis 2017;27:41 –47. [DOI] [PubMed] [Google Scholar]
- 60.Chang HX, Zhao XJ, Zhu QL, Hou Q, Li Y. Removal of epicardial adipose tissue after myocardial infarction improves cardiac function. Herz 2018;43:258–264. [DOI] [PubMed] [Google Scholar]
- 61.Smail H, Baciu A, Dacher JN, Litzler PY. Surgical resection of circumferential epicardial adipose tissue hypertrophy: case report and systematic review of the literature. J Thorac Cardiovasc Surg 2016;151:e27–30. [DOI] [PubMed] [Google Scholar]
- 62.Narumi H, Yoshida K, Hashimoto N, Umehara I, Funabashi N, Yoshida S, Komuro I. Increased subcutaneous fat accumulation has a protective role against subclinical atherosclerosis in asymptomatic subjects undergoing general health screening. Int J Cardiol 2009;135:150–155. [DOI] [PubMed] [Google Scholar]
- 63.Neeland IJ, Ayers CR, Rohatgi AK, Turer AT, Berry JD, Das SR, Vega GL, Khera A, McGuire DK, Grundy SM, de Lemos JA. Associations of visceral and abdominal subcutaneous adipose tissue with markers of cardiac and metabolic risk in obese adults. Obesity (Silver Spring; ) 2013;21:E439–E447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goossens GH. The metabolic phenotype in obesity: fat mass, body fat distribution, and adipose tissue function. Obes Facts 2017;10:207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rayner JJ, Peterzan MA, Watson WD, Clarke WT, Neubauer S, Rodgers CT, Rider OJ. Myocardial energetics in obesity: enhanced ATP delivery through creatine kinase with blunted stress response. Circulation 2020;141:1152–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sharma A, Lavie CJ, Borer JS, Vallakati A, Goel S, Lopez-Jimenez F, Arbab-Zadeh A, Mukherjee D, Lazar JM. Meta-analysis of the relation of body mass index to all-cause and cardiovascular mortality and hospitalization in patients with chronic heart failure. Am J Cardiol 2015;115:1428–1434. [DOI] [PubMed] [Google Scholar]
- 67.Mohammed SF, Borlaug BA, Roger VL, Mirzoyev SA, Rodeheffer RJ, Chirinos JA, Redfield MM. Comorbidity and ventricular and vascular structure and function in heart failure with preserved ejection fraction: a community-based study. Circ Heart Fail 2012;5:710–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kitzman DW, Brubaker P, Morgan T, Haykowsky M, Hundley G, Kraus WE, Eggebeen J, Nicklas BJ. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 2016;315:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li J, Li S, Feuers RJ, Buffington CK, Cowan GS. Influence of body fat distribution on oxygen uptake and pulmonary performance in morbidly obese females during exercise. Respirology 2001;6:9–13. [DOI] [PubMed] [Google Scholar]
- 70.Haykowsky MJ, Nicklas BJ, Brubaker PH, Hundley WG, Brinkley TE, Upadhya B, Becton JT, Nelson MD, Chen H, Kitzman DW. Regional adipose distribution and its relationship to exercise intolerance in older obese patients who have heart failure with preserved ejection fraction. JACC Heart Fail 2018;6:640–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim G, Jo K, Kim KJ, Lee YH, Han E, Yoon HJ, Wang HJ, Kang ES, Yun M. Visceral adiposity is associated with altered myocardial glucose uptake measured by 18FDG-PET in 346 subjects with normal glucose tolerance, prediabetes, and type 2 diabetes. Cardiovasc Diabetol 2015;14:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peterson LR, Herrero P, Schechtman KB, Racette SB, Waggoner AD, Kisrieva-Ware Z, Dence C, Klein S, Marsala J, Meyer T, Gropler RJ. Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation 2004;109:2191 –2196. [DOI] [PubMed] [Google Scholar]
- 73.Rayner JJ, Banerjee R, Holloway CJ, Lewis AJ, Peterzan MA, Francis JM, Neubauer S, Rider OJ. The relative contribution of metabolic and structural abnormalities to diastolic dysfunction in obesity. Int J Obes (Lond) 2018;42:441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Khan SS, Shah SJ, Colangelo LA, Panjwani A, Liu K, Lewis CE, Shay CM, Goff DC Jr, Reis J, Vasconcellos HD, Lima JAC, Lloyd-Jones D, Allen NB. Association of patterns of change in adiposity with diastolic function and systolic myocardial mechanics from early adulthood to middle age: the coronary artery risk development in young adults study. J Am Soc Echocardiogr 2018;31:1261–1269.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang H, Zhang T, Li S, Guo Y, Shen W, Fernandez C, Harville E, Bazzano LA, Urbina EM, He J, Chen W. Long-term excessive body weight and adult left ventricular hypertrophy are linked through later-life body size and blood pressure: the Bogalusa Heart Study. Circ Res 2017;120:1614–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Neeland IJ, Gupta S, Ayers CR, Turer AT, Rame JE, Das SR, Berry JD, Khera A, McGuire DK, Vega GL, Grundy SM, de Lemos JA, Drazner MH. Relation of regional fat distribution to left ventricular structure and function. Circ Cardiovasc Imaging 2013;6:800–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gomberg-Maitland M, Shah SJ, Guazzi M. Inflammation in heart failure with preserved ejection fraction: time to put out the fire. JACC Heart Fail 2016;4:325–328. [DOI] [PubMed] [Google Scholar]
- 78.Kitzman DW, Nicklas BJ. Pivotal role of excess intra-abdominal adipose in the pathogenesis of metabolic/obese HFpEF. JACC Heart Fail 2018;6:1008–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kalogeropoulos A, Georgiopoulou V, Psaty BM, Rodondi N, Smith AL, Harrison DG, Liu Y, Hoffmann U, Bauer DC, Newman AB, Kritchevsky SB, Harris TB, Butler J; Health ABC Study Investigators. Inflammatory markers and incident heart failure risk in older adults: the Health ABC (Health, Aging, and Body Composition) study. J Am Coll Cardiol 2010;55:2129–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013;62:263–271. [DOI] [PubMed] [Google Scholar]
- 81.Kitzman DW, Lam CS. Obese heart failure with preserved ejection fraction phenotype: from pariah to central player. Circulation 2017;136:20–23. [DOI] [PubMed] [Google Scholar]
- 82.Nerlekar N, Muthalaly RG, Wong N, Thakur U, Wong DTL, Brown AJ, Marwick TH. Association of volumetric epicardial adipose tissue quantification and cardiac structure and function. J Am Heart Assoc 2018;7:e009975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Miller WL, Borlaug BA. Impact of obesity on volume status in patients with ambulatory chronic heart failure. J Card Fail 2020;26:112–117. [DOI] [PubMed] [Google Scholar]
- 84.Reddy YN, Obokata M, Testani JM, Felker GM, Tang WH, Abou-Ezzeddine OF, Sun JL, Chakrabothy H, McNulty S, Shah SJ, Lewis GD, Stevenson LW, Redfield MM, Borlaug BA. Adverse renal response to decongestion in the obese phenotype of heart failure with preserved ejection fraction. J Card Fail 2020;26:101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Packer M Derangements in adrenergic-adipokine signalling establish a neurohormonal basis for obesity-related heart failure with a preserved ejection fraction. Eur J Heart Fail 2018;20:873–878. [DOI] [PubMed] [Google Scholar]
- 86.Reaven GM, Lithell H, Landsberg L. Hypertension and associated metabolic abnormalities–the role of insulin resistance and the sympathoadrenal system. N EnglJ Med 1996;334:374–381. [DOI] [PubMed] [Google Scholar]
- 87.Packer M Leptin-aldosterone-neprilysin axis: identification of its distinctive role in the pathogenesis of the three phenotypes of heart failure in people with obesity. Circulation 2018;137:1614–1631. [DOI] [PubMed] [Google Scholar]
- 88.Xue B, Yu Y, Zhang Z, Guo F, Beltz TG, Thunhorst RL, Felder RB, Johnson AK. Leptin mediates high-fat diet sensitization of angiotensin II-elicited hypertension by upregulating the brain renin-angiotensin system and inflammation. Hypertension 2016;67:970–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huby AC, Antonova G, Groenendyk J, Gomez-Sanchez CE, Bollag WB, Filosa JA, Belin de Chantemèle EJ. Adipocyte-derived hormone leptin is a direct regulator of aldosterone secretion, which promotes endothelial dysfunction and cardiac fibrosis. Circulation 2015;132:2134–2145. [DOI] [PubMed] [Google Scholar]
- 90.Briones AM, Nguyen Dinh Cat A, Callera GE, Yogi A, Burger D, He Y, Corrêa JW, Gagnon AM, Gomez-Sanchez CE, Gomez-Sanchez EP, Sorisky A, Ooi TC, Ruzicka M, Burns KD, Touyz RM. Adipocytes produce aldosterone through calcineurin-dependent signaling pathways: implications in diabetes mellitus-associated obesity and vascular dysfunction. Hypertension 2012;59:1069–1078. [DOI] [PubMed] [Google Scholar]
- 91.Gruden G, Landi A, Bruno G. Natriuretic peptides, heart, and adipose tissue: new findings and future developments for diabetes research. Diabetes Care 2014;37:2899–2908. [DOI] [PubMed] [Google Scholar]
- 92.Kovacova Z, Tharp WG, Liu D, Wei W, Xie H, Collins S, Pratley RE. Adipose tissue natriuretic peptide receptor expression is related to insulin sensitivity in obesity and diabetes. Obesity (Silver Spring; ) 2016;24:820–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schütten MT, Houben AJ, de Leeuw PW, Stehouwer CD. The link between adipose tissue renin-angiotensin-aldosterone system signaling and obesity-associated hypertension. Physiology (Bethesda; ) 2017;32:197–209. [DOI] [PubMed] [Google Scholar]
- 94.Reddy YN, Andersen MJ, Obokata M, Koepp KE, Kane GC, Melenovsky V, Olson TP, Borlaug BA. Arterial stiffening with exercise in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol 2017;70:136–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brunner EJ, Shipley MJ, Ahmadi-Abhari S, Tabak AG, McEniery CM, Wilkinson IB, Marmot MG, Singh-Manoux A, Kivimaki M. Adiposity, obesity, and arterial aging: longitudinal study of aortic stiffness in the Whitehall II cohort. Hypertension 2015;66:294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fudim M, Hernandez AF, Felker GM. Role of volume redistribution in the congestion of heart failure. J Am Heart Assoc 2017;6:e1–e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fudim M, Yalamuri S, Herbert JT, Liu PR, Patel MR, Sandler A. Raising the pressure: hemodynamic effects of splanchnic nerve stimulation. J Appl Physiol (1985) 2017;123:126–127. [DOI] [PubMed] [Google Scholar]
- 98.Varela JE, Hinojosa M, Nguyen N. Correlations between intra-abdominal pressure and obesity-related co-morbidities. Surg Obes Relat Dis 2009;5: 524–528. [DOI] [PubMed] [Google Scholar]
- 99.Lambert DM, Marceau S, Forse RA. Intra-abdominal pressure in the morbidly obese. Obes Surg 2005;15:1225–1232. [DOI] [PubMed] [Google Scholar]
- 100.Malbrain ML, Chiumello D, Pelosi P, Wilmer A, Brienza N, Malcangi V, Bihari D, Innes R, Cohen J, Singer P, Japiassu A, Kurtop E, De Keulenaer BL, Daelemans R, Del Turco M, Cosimini P, Ranieri M, Jacquet L, Laterre PF, Gattinoni L. Prevalence of intra-abdominal hypertension in critically ill patients: a multicentre epidemiological study. Intensive Care Med 2004;30:822–829. [DOI] [PubMed] [Google Scholar]
- 101.Mullens W, Abrahams Z, Skouri HN, Francis GS, Taylor DO, Starling RC, Paganini E, Tang WH. Elevated intra-abdominal pressure in acute decompensated heart failure: a potential contributor to worsening renal function? J Am Coll Cardiol 2008;51:300–306. [DOI] [PubMed] [Google Scholar]
- 102.Mullens W, Abrahams Z, Francis GS, Taylor DO, Starling RC, Tang WH. Prompt reduction in intra-abdominal pressure following large-volume mechanical fluid removal improves renal insufficiency in refractory decompensated heart failure. J Card Fail 2008;14:508–514. [DOI] [PubMed] [Google Scholar]
- 103.Fudim M, Ganesh A, Green C, Jones WS, Blazing MA, DeVore AD, Felker GM, Kiefer TL, Kong DF, Boortz-Marx RL, Hernandez AF, Patel MR. Splanchnic nerve block for decompensated chronic heart failure: splanchnic-HF. Eur Heart J 2018;39:4255–4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fudim M, Jones WS, Boortz-Marx RL, Ganesh A, Green CL, Hernandez AF, Patel MR. Splanchnic nerve block for acute heart failure. Circulation 2018;138:951–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pandey A, Kondamudi N, Patel KV, Ayers C, Simek S, Hall ME, Musani SK, Blackshear C, Mentz RJ, Khan H, Terry JG, Correa A, Butler J, Neeland IJ, Berry JD. Association between regional adipose tissue distribution and risk of heart failure among blacks. Circ Heart Fail 2018;11:e005629. [DOI] [PubMed] [Google Scholar]
- 106.Haykowsky MJ, Brubaker PH, John JM, Stewart KP, Morgan TM, Kitzman DW. Determinants of exercise intolerance in elderly heart failure patients with preserved ejection fraction. J Am Coll Cardiol 2011;58:265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Reddy YN, Olson TP, Obokata M, Melenovsky V, Borlaug BA. Hemodynamic correlates and diagnostic role of cardiopulmonary exercise testing in heart failure with preserved ejection fraction. JACC Heart Fail 2018;6:665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Abudiab MM, Redfield MM, Melenovsky V, Olson TP, Kass DA, Johnson BD, Borlaug BA. Cardiac output response to exercise in relation to metabolic demand in heart failure with preserved ejection fraction. Eur J Heart Fail 2013;15:776–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Clerico A, Passino C, Emdin M. The paradox of low B-type natriuretic peptide levels in obesity revisited: does sex matter? Eur J Heart Fail 2018;20: 1215–1216. [DOI] [PubMed] [Google Scholar]
- 110.Ismail I, Keating SE, Baker MK, Johnson NA. A systematic review and meta-analysis of the effect of aerobic vs. resistance exercise training on visceral fat. Obes Rev 2012;13:68–91. [DOI] [PubMed] [Google Scholar]
- 111.Slentz CA, Aiken LB, Houmard JA, Bales CW, Johnson JL, Tanner CJ, Duscha BD, Kraus WE. Inactivity, exercise, and visceral fat. STRRIDE: a randomized, controlled study of exercise intensity and amount. J Appl Physiol (1985) 2005;99:1613–1618. [DOI] [PubMed] [Google Scholar]
- 112.Mohammed BS, Cohen S, Reeds D, Young VL, Klein S. Long-term effects of large-volume liposuction on metabolic risk factors for coronary heart disease. Obesity (Silver Spring; ) 2008;16:2648–2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Klein S, Fontana L, Young VL, Coggan AR, Kilo C, Patterson BW, Mohammed BS. Absence of an effect of liposuction on insulin action and risk factors for coronary heart disease. N EnglJ Med 2004;350:2549–2557. [DOI] [PubMed] [Google Scholar]
- 114.Lee Y, Pedziwiatr M, Major P, Brar K, Doumouras AG, Hong D. The effect of omentectomy added to bariatric surgery on metabolic outcomes: a systematic review and meta-analysis of randomized controlled trials. Surg Obes Relat Dis 2018;14:1766–1782. [DOI] [PubMed] [Google Scholar]
- 115.Jamaly S, Carlsson L, Peltonen M, Jacobson P, Karason K. Surgical obesity treatment and the risk of heart failure. Eur Heart J 2019;40:2131 –2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Algahim MF, Lux TR, Leichman JG, Boyer AF, Miller CC, Laing ST, Wilson EB, Scarborough T, Yu S, Snyder B, Wolin-Riklin C, Kyle UG, Taegtmeyer H. Progressive regression of left ventricular hypertrophy two years after bariatric surgery. Am J Med 2010;123:549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tsujimoto T, Kajio H. Abdominal obesity is associated with an increased risk of all-cause mortality in patients with HFpEF. J Am Coll Cardiol 2017;70:2739–2749. [DOI] [PubMed] [Google Scholar]
- 118.Reddy YN, Anantha-Narayanan M, Obokata M, Koepp KE, Erwin P, Carter RE, Borlaug BA. Hemodynamic effects of weight loss in obesity: a systematic review and meta-analysis. JACC Heart Fail 2019;7:678–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sugiyama S, Jinnouchi H, Kurinami N, Hieshima K, Yoshida A, Jinnouchi K, Nishimura H, Suzuki T, Miyamoto F, Kajiwara K, Jinnouchi T. Dapagliflozin reduces fat mass without affecting muscle mass in type 2 diabetes. J Atheroscler Thromb 2018;25:467–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nagai Y, Fukuda H, Kawanabe S, Nakagawa T, Ohta A, Tanaka Y. Differing effect of the sodium-glucose cotransporter 2 inhibitor ipragliflozin on the decrease of fat mass vs. lean mass in patients with or without metformin therapy. J Clin Med Res 2019;11:297–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ono T, Murakoshi M, Suzuki N, Iida N, Ohdera M, Iigo M, Yoshida T, Sugiyama K, Nishino H. Potent anti-obesity effect of enteric-coated lactoferrin: decrease in visceral fat accumulation in Japanese men and women with abdominal obesity after 8-week administration of enteric-coated lactoferrin tablets. Br J Nutr 2010;104:1688–1695. [DOI] [PubMed] [Google Scholar]
- 122.Borlaug BA, Carter RE, Melenovsky V, DeSimone CV, Gaba P, Killu A, Naksuk N, Lerman L, Asirvatham SJ. Percutaneous pericardial resection: a novel potential treatment for heart failure with preserved ejection fraction. Circ Heart Fail 2017;10:e003612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shah SJ, Feldman T, Ricciardi MJ, Kahwash R, Lilly S, Litwin S, Nielsen CD, van der Harst P, Hoendermis E, Penicka M, Bartunek J, Fail PS, Kaye DM, Walton A, Petrie MC, Walker N, Basuray A, Yakubov S, Hummel SL, Chetcuti S, Forde-McLean R, Herrmann HC, Burkhoff D, Massaro JM, Cleland JGF, Mauri L. One-year safety and clinical outcomes of a transcatheter interatrial shunt device for the treatment of heart failure with preserved ejection fraction in the Reduce Elevated Left Atrial Pressure in Patients With Heart Failure (REDUCE LAP-HF I) trial: a randomized clinical trial. JAMA Cardiol 2018;3:968–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Current ongoing clinical trials on therapeutic interventions for heart failure and obesity.


