Summary
Background
Repurposed drugs for treatment of new onset disease may be an effective therapeutic shortcut. We aimed to evaluate the efficacy of repurposed antivirals compared to placebo in lowering SARS-CoV2 viral load of COVID-19 patients.
Methods
REVOLUTIOn is a randomised, parallel, blinded, multistage, superiority and placebo controlled randomised trial conducted in 35 centres in Brazil. We include patients aged 18 years or older admitted to hospital with laboratory-confirmed SARS-CoV-2 infection, symptoms onset 9 days or less and SpO2 94% or lower at room air were eligible. All participants were randomly allocated to receive either atazanavir, daclatasvir or sofosbuvir/daclatasvir or placebo for 10 days. The primary outcome was the decay rate (slope) of the SARS-CoV-2 viral load logarithm assessed in the modified intention to-treat population. This trial was registered with ClinicalTrials.gov, number NCT04468087.
Findings
Between February 09, 2021, and August 04, 2021, 255 participants were enrolled and randomly assigned to atazanavir (n = 64), daclatasvir (n = 66), sofosbuvir/daclatasvir (n = 67) or placebo (n = 58). Compared to placebo group, the change from baseline to day 10 in log viral load was not significantly different for any of the treatment groups (0.05 [95% CI, −0.03 to 0.12], −0.02 [95% CI, −0.09 to 0.06], and −0.03 [95% CI, −0.11 to 0.04] for atazanavir, daclatasvir and sofosbuvir/daclatasvir groups respectively). There was no significant difference in the occurrence of serious adverse events between treatment groups.
Interpretation
No significant reduction in viral load was observed from the use of atazanavir, daclatasvir or sofosbuvir/daclatasvir compared to placebo in hospitalised COVID-19 patients who need oxygen support with symptoms onset 9 days or less.
Funding
Ministério da Ciência, Tecnologia e Inovação (MCTI) - Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ); Cia Latino-Americana de Medicamentos (Clamed); Cia Industrial H. Carlos Schneider (Ciser); Hospital Research Foundation Incorporation, Australia, HCor São Paulo; Blanver Farmoquímica; Instituto de Tecnologia em Fármacos (Farmanguinhos) da Fundação Oswaldo Cruz (Fiocruz); Coordenação Geral de Planejamento Estratégico (Cogeplan)/Fiocruz; and Fundação de apoio a Fiocruz (Fiotec, VPGDI-054-FIO-20-2-13).
Keywords: Antiviral, COVID-19, SARS-CoV2, Atazanavir, Daclatasvir, Sofosbuvir, Randomised controlled trial, Viral load, Acute respiratory failure, Hypoxemia
Research in context.
Evidence before this study
Our research group conducted pre-clinical studies from our group of investigators in repurposed drugs evaluating the effectiveness of Sofosbuvir, Daclatasvir and Atazanavir showing inhibition of SARS-CoV2 RNA replication with different mechanisms. We also searched Medline using not controlled terms on August, 2020, for pre-clinical, observational studies and randomised controlled trials with the terms (“atazanavir” OR “daclatasvir” OR “sofosbuvir”) AND (“SARS-CoV-2” OR “COVID-19”) evaluating the effectiveness of any of these drugs in patients hospitalised with COVID-19, with language restriction to English, Portuguese or Spanish. No studies were retrieved regarding viral load decrease as well as clinical outcomes. These drugs were already available for clinical use for Hepatitis-C and HIV-infected patients.
Added value of this study
REVOLUTIOn trial is a multicentre, placebo controlled, randomised trial evaluating the safety and efficacy of these repurposed drugs on viral load and clinical status of adult patients admitted to hospital with COVID-19. We found no significant difference in viral load kinetics and clinical status at days 7 and 15, time to hospital discharge, 28-day mortality, days out of the hospital and mechanical ventilation free days in 28 days in participants receiving placebo compared with those receiving active drug of atazanavir, daclatasvir or combination sofosbuvir/daclatasvir. No significant difference in the occurrence of serious adverse events was observed between groups.
Implications of all the available evidence
The inhibition of SARS-CoV2 previously reported and probable decrease in viral load was not observed in the REVOLUTIOn trial compared to placebo. Together with previous evidence, results from the REVOLUTIOn trial do not support the use of atazanavir, daclatasvir and sofosbuvir/daclatasvir in hospitalised patients with COVID-19 in a population with symptoms onset lesser than 9 days and requiring oxygen support regarding viral load kinetics, using standard approved dose of these drugs.
Introduction
Global pandemics such as coronavirus disease 2019 (COVID-19), the viral disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) impose therapeutic challenges to find new potential treatments in a very short time. Considering the time and cost required for new therapies, repurposing of available drugs for management of other diseases can be an effective therapeutic shortcut.1,2 Remdesivir, lopinavir/ritonavir and hydroxichloroquine are recent examples.3 However, only remdesivir showed evidence of clinical benefits for COVID-19 treatment4 such as shortening the time to recovery in hospitalised patients.
Other repurposed antivirals have been proposed based on the results of in vitro and small clinical studies. Sofosbuvir and daclatasvir are well tolerated and effective direct-acting antivirals (DAAs) against HCV5 which inhibit viral RNA replication via NS5A and NS5B, respectively. SARS-CoV-2 was also susceptible in vitro and in vivo to these drugs, and when combined sofosbuvir and daclatasvir displayed better efficacy to inhibit the pandemic virus replication.6, 7, 8, 9, 10 Under regular anti-HCV regimen, daclatasvir's Cmax and Cmin inhibits 90 and 50% SARS-CoV-2 replication, respectively.9 The largest study published with sofosbuvir/daclatasvir compared to standard of care for hospitalised hypoxemic COVID-19 patients failed to demonstrate significant difference in either primary outcome of hospital discharge within 10 days or overall mortality.11 Individual patient data metanalysis showed better clinical recovery within 14 days of randomisation and overall mortality in hospitalised patients with COVID-19 treated with sofosbuvir/daclatasvir.12 However, few patients were included and the effect estimate for overall mortality was no longer significant when only randomised studies were included in the analysis.
Atazanavir, an antiretroviral targeting HIV protease, inhibits competitively SARS-CoV-2 major protease (Mpro), the enzyme responsible for cleavage of the coronavirus polyprotein, inhibiting virus replication in pre-clinical studies.13,14 Although no clinical studies have been published so far with this drug alone or in combination at the reference dose against HIV, atazanavir accumulates in the lung and protects around 30% mice infected with a lethal dose of SARS-CoV-2 gamma variant from mortality.13
Implementing studies that allow more than one new treatment to be tested simultaneously may be advantageous over classic parallel group approach. The main objectives of this type of clinical trial are to quickly reject any new therapies that do not seem to be better than control and to identify those that are significantly better in terms of clinical outcomes.15 Thus, we propose a randomised, placebo-controlled, adaptive, multi-arm, multi-stage study to evaluate multiple interventions such as atazanavir, daclatasvir and sofosbuvir plus daclatasvir simultaneously to first identify, in a phase 2 trial, if any of these drugs isolated or combined can reduce viral load when compared to placebo. If so, a phase 3 would start to investigate clinical outcomes.
Methods
REVOLUTIOn is a phase 2/3, double-blinded, adaptive, multicentre, randomised, multi arm, multi stage, controlled trial for evaluating the efficacy and safety of repurposed drugs in adults admitted to hospital for COVID-19 treatment. The trial protocol and statistical analysis plan have been published.16 The trial consisted of 3 continuous stages with stage 2 and 3 depending on results of stage 1 (ClinicalTrials.gov Identifier: NCT04468087). An independent data safety and monitoring board (DSMB) was responsible for reviewing trial data in interim analyses and after each stage of the trial. The first two stages are phase II studies, and the third stage is a phase III, as shown in Supplementary Fig. S1. The trial was accomplished across 35 sites in Brazil. It was approved by the Brazilian National Committee of Ethics in Research (Comissão Nacional de Ética em Pesquisa – CONEP nº 4.303.991 September 28, 2020) and Brazilian Health Regulatory Agency (Agência Nacional de Vigilância Sanitária -ANVISA - nº 107/2020) and sponsored by the Ministry of Science, Technology and Innovation (MCTI) and Conselho Nacional de Desenvolvimento Científico e Tecnológico – (CNPQ). The trial was done in accordance with the Declaration of Helsinki, the International Conference on Harmonization Guidelines for Good Clinical Practice, and applicable local regulations. The protocol was reviewed and approved by the ethics committees of all participating centres, and patients or legal representatives provided written informed consent before study entry. This analysis is based on protocol version 4.0 of February 12, 2021.
Participants
Patients aged 18 years or older who were admitted to hospital with laboratory-confirmed SARS-CoV-2 infection by reverse transcription-polymerase chain reaction (RT-PCR) test or rapid antigen test could be enrolled if they presented all of the following: time between symptom onset and inclusion for 9 days or less; SpO2 94% or lower at room air or need for supplemental oxygen to maintain SpO2 94% or higher. Participants of childbearing potential agreed to use two primary forms of contraception, including barrier method for 100 days. Participants were excluded if they had liver enzymes (alanine aminotransferase or aspartate aminotransferase) more than five times the upper limit of normal, a stage 4 severe chronic kidney disease or requiring dialysis (estimated glomerular filtration rate less than 30 mL/min) and pre-defined renal failure stage 3 according to Acute Kidney Injury Network17 classification with serum creatinine >4 mg/dL, total bilirubin >2 mg/dL; platelets count <50,000 cell/L; total neutrophil count <750 cell/L; liver disease with Child Pugh B and C classification,18 decompensated Congestive Heart Failure,19 pregnant or breastfeeding, known allergy or hypersensitivity to any study drug, carrier of Hepatitis C, Active Hepatitis B or HIV, currently use of nucleoside or nucleotide analog drugs for any purpose; corrected QT interval >480 ms on the electrocardiogram; heart rate <55 bpm or use of amiodarone <90 days (Supplementary Table S1).
Randomisation and masking
Participants were randomly assigned 3:3:3:1:1:1 to atazanavir, daclatasvir, sofosbuvir/daclatasvir, placebo of atazanavir, placebo of daclatasvir, placebo of sofosbuvir/daclatasvir when 6 groups were initially implemented in stage 1. They were then assigned 3:1 to receive either treatment (atazanavir, daclatasvir or sofosbuvir/daclatasvir) or placebo. Stages 2 and 3 would happen according to pre-defined rules (Supplementary Material S2 and Supplementary Fig. S2). Randomisation was performed in the electronic case report form to ensure appropriate allocation concealment through a centralised, automated, Internet-based randomisation system and used computer-generated blocks of 12 positions, with each treatment group being represented by 3 different positions and each placebo by a single position. It was stratified by centre. The global double-masking between all groups were not possible as we had 3 drugs with different physical characteristics. However, participants and investigators were masked within the allocated group, since active drug and placebo coated tablets or capsules were identical (that is, patients assigned to atazanavir or placebo were not aware if they were allocated to atazanavir or placebo of atazanavir, although they were aware they were not allocated to daclatasvir, placebo of daclatasvir, sofosbuvir/daclatasvir, or placebo of sofosbuvir/daclatasvir).
Procedures
Daclatasvir and its placebo tablet were administered orally at a loading dose of 2 tablets once daily at day 1 followed by 1 tablet once daily for a total duration of 10 days. Sofosbuvir and placebo were administered orally at a loading dose of 1 tablet twice daily at day 1 followed by 1 tablet once daily for a total duration of 10 days. Atazanavir and its placebo capsules were administered orally at a loading dose of 2 capsules twice daily at day 1 followed by 1 capsule twice daily for a total duration of 10 days (Supplementary Table S2). Drug was dispensed for home use for those discharged before the end of treatment. They were followed by telephone contact and medication diary checked in the return pre-specified visits (days 3, 6 or 10). Participant were instructed to bring all unused study drugs and any empty bottles for drug accountability.
Any experimental treatment or off-label therapy administered before enrollment was discontinued on enrollment. Concomitant therapy was recorded daily until day 10. They were properly substituted or adjusted according to possible drugs interactions (Supplementary Table S3). Other experimental treatments, besides study drugs, for the treatment of COVID-19 were not allowed. Corticosteroids were recommended for all participants as dexamethasone 6 mg once daily for 10 days or until discharge20,21 if not intubated, or 20 mg once daily for 5 days, followed by 10 mg once daily for 5 more days for critically ill with acute respiratory distress syndrome. Immunomodulatory agent Tocilizumab was left to the investigator's discretion. Anticoagulation was administered according to local protocols for venous thromboembolism prophylaxis or therapy.
Participants were assessed daily while hospitalised. Nonetheless, assessment visits at days 3, 6, 10 (plus or minus 2) were booked if already discharged. Telephone interview at day 15 and 28 (plus or minus 1) were done as well. Clinical data, concomitant medications, adverse events, blood cell counts, renal function and electrocardiogram were collected at baseline. Safety checks with liver enzymes and INR were collected at days 3, 6 and 10 (plus or minus 2) or any time according to physician discretion.
Determination of the viral load blinded to treatment group was done on nasopharyngeal swab specimens at baseline, days 3, 6, 10 (plus or minus 2). Swab specimens were stored at local sites at −20° C and transferred fortnightly to a central laboratory for analysis (Supplementary Material S3). The transportation was done in thermal boxes validated for biological material with dry ice at temperatures from −81.7 to −62.1. The SARS-CoV-2 load was measured by Quantitative Real-Time PCR via Reverse Transcriptase for quantification of target copy number (E gene) of clinical sample compared to standard curve of inactivated SARS-CoV-2 virus solution dilutions. Quantitative detection of SARS-CoV-2 nucleic acid was done using the E gene as a target, Human RNASE P endogenous control and the standard curve calibrators. The instruments include the Abbott M24sp extractor (Abbott mSample Preparation System - Promega Extraction Kit Ref.04J70-24) to perform sample extraction and purification, nucleic acid amplification and quantification using the RT (reverse transcriptase) assay on the m2000rt instrument. Preparation of biological materials and Master Mix for reaction using the Charité Protocol primers and probes and human RNASE P endogenous control (Supplementary Material S4). The device releases the results in copies/mL and logs. Detectable but not quantifiable sample has detectable target RNA (Gene E) at a level below the assay's limit of quantification. Invalid sample does not detect target RNA (Gene E) or endogenous control gene (RNAse P) and indicates the need for a new collection. The detection and quantification limits are 0.5 log copies of the target RNA. The upper limit of quantification is 6.5 log copies of the target RNA. Virus isolation culture and genetic sequencing were accomplished as well (Supplementary Material S4).
Outcomes
The primary outcome measure was the decay rate (slope) of the SARS-CoV-2 viral load logarithm in nasopharyngeal swab samples evaluated at D0, D3, D6 and D10 after randomization (Supplementary Table S4).
Secondary efficacy outcomes measures were: the clinical status at day 15 as measured on the seven-point ordinal scale of the WHO Master Protocol (version 3.0, March 3, 2020): (1) not hospitalised, no limitation on activities; (2) not hospitalised, limitation on activities; (3) hospitalised, not requiring supplemental oxygen; (4) hospitalised, requiring supplemental oxygen; (5) hospitalised, on non-invasive ventilation or high flow oxygen devices; (6) hospitalised, on invasive mechanical ventilation or ECMO; and (7) dead; the clinical status at day 7 as measured on the six-point ordinal scale: (1) not hospitalised; (2) hospitalised, not requiring supplemental oxygen; (3) hospitalised, requiring supplemental oxygen; (4) hospitalised, on non-invasive ventilation or high flow oxygen devices; (5) hospitalised, on invasive mechanical ventilation or ECMO; and (6) dead; 28 day mortality; days free from mechanical ventilation at day 28; days out of hospital in 28 days; time to discharge and days free days of respiratory support in 15 days. The secondary outcomes were assessed in all patients who were still in the hospital on day 15 exactly and in outpatients (by means of telephone interview) as close to day 15 as possible.
Safety outcomes were the cumulative incidence of any grade 3 or 4 adverse events or of any serious adverse event graded according to the Division of AIDS (DAIDS) table for grading the severity of adult and paediatric adverse events, version 2.1, July 2017.
Statistical analysis
The sample size was calculated assuming an average linear decay rate for patients with SARS-CoV-2 in nasopharyngeal swab samples assessed from the fourth to the fourteenth day of symptoms of 1.0 log10 (viral load) every 3 days.22 Assuming this decay rate for the placebo group, under the scenario that all treatments selected in Stage 1 have a decay rate of 1.20 log10 (viral load), the study had about 90% power to indicate that at least one of the treatments was superior to placebo, with a global significance level of 0.20 for this stage, considering a significance level of 0.067 (Bonferroni correction) for each of the 3 treatment comparisons in relation to the placebo group. The aforementioned power calculations were performed considering a 20% loss of patients from the ITT population. Simulations considering peculiarities of the design and sample size in other Stages with details on the distributions considered for the outcomes and consequent determination of the study's power are found in the Statistical Analysis Plan previous published.16
The intention-to-treat population included all randomly assigned participants with a positive or suspected SARS-CoV-2 infection, for whom a valid consent form was obtained, independently of having received any investigational treatment in the past 28 days. The modified intention-to-treat population included only those randomly assigned participants with a positive SARS-CoV-2 PCR result obtained at baseline independently of having received any investigational treatment in the past 28 days as well. Per protocol population is defined by all allocated patients with positive RT-PCR for SARS-CoV-2 at baseline, with medication adherence to the allocated treatment of at least 80%. The safety population included participants from the intention-to-treat population who received at least one dose of the treatment allocated by random assignment.
In all analyses, the three placebo groups (placebo of atazanavir, placebo of daclatasvir, and placebo of sofosbuvir/daclatasvir) were considered as a single group. Main efficacy analyses were done in the modified intention-to-treat population. Exploratory efficacy analysis also conducted in per protocol population. Safety analyses were done in the safety population. The significance level for each of the three comparisons of the primary outcome analysis was 0.067, to maintain a global significance level of 0.20 (Bonferroni adjustment) in the first stage. All other analyses were not adjusted for multiple comparisons; therefore p-values and confidence intervals should not be used to infer definitive treatment effects. The evolution of the viral load since randomisation was analysed using a mixed-effects linear model with a test of treatment effect on the slope shown in Section S6 of the Supplementary Material. For the analysis of viral load by mixed models, undetectable viral load values (<0.5 log10 copies per 10,000 cells) were imputed to the limit of detection (0.5 log10).
The analyses were performed with R software (R Core Team, 2020) and the main packages used were nlme, gamlss and mice.
Interim analysis
One interim analysis was planned during stage 1 with the purpose of assessing safety only. No adjustments on the alpha were considered for interim looks at Stage 1.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
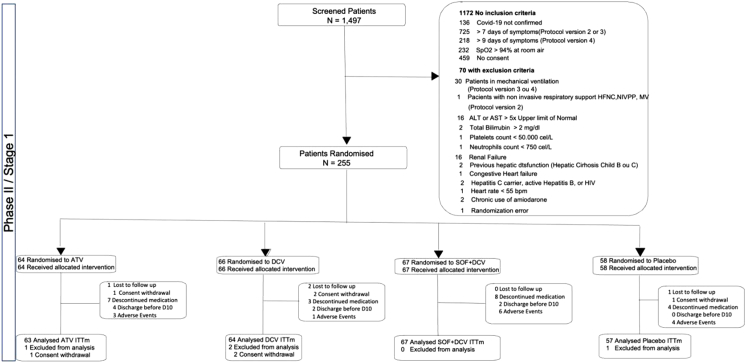
Between February 9, 2021, and August 4, 2021, 1497 participants were screened and 255 were enrolled and randomly assigned to atazanavir (n = 64), daclatasvir (n = 66), sofosbuvir/daclatasvir (n = 67) or placebo (n = 58) in 38 centres in Brazil. A total of 63 participants in the atazanavir group, 64 in daclatasvir group, 67 in sofosbuvir/daclatasvir group and 56 in the placebo group had COVID-19 confirmed by RT-PCR and were included in the modified intention-to-treat analysis with a total of 250 participants (Fig. 1). From those, only 2 participants in ATV group did not receive at least one dose of the study medication. The median duration of treatment in the atazanavir group was 9 days (interquartile range [IQR], 8–10), 10 days (IQR, 10–10) in the daclatasvir group, 10 days (IQR, 9–10) in the sofosbuvir/daclatasvir group, and 10 days (IQR, 6–10) in the placebo group.
Fig. 1.
Trial profile.
Two interim analyses (one pre-specified and another not prespecified) were conducted. In addition, the DSMB met twice after stage 1 was completed to decide whether to recommend stopping or continuing the trial to further stages based on the pre-specified rule. The DSMB reviewed data and recommended stopping the trial because none of the tested antivirals showed evidence of effect on the viral load or on clinical outcomes. The recommendation was endorsed by the REVOLUTIOn steering committee.
Participants’ baseline characteristics are shown in Table 1. Baseline characteristics were well-balanced between groups. All patients underwent randomisation within 9 days after symptom onset with a median of 7 days. The mean age of the patients was 54.2 (standard deviation [SD], 14 years), and 169 (68%) of all the included patients were men. A total of 198 (79%) of the patients were receiving supplemental oxygen at baseline. Over 90% of the cases at baseline were associated with SARS-CoV-2 variant of concern Gamma, which dominated the epidemiological weeks during the course of recruitment (Supplementary Fig. S5).
Table 1.
Baseline characteristics of the intention to treat population.
| ATV (n = 63) | DCV (n = 64) | SOF + DCV (n = 67) | Placebo (n = 56) | Total (n = 250) | |
|---|---|---|---|---|---|
| Age, mean [SD] | 54.5 [14.3] | 52.3 [14.4] | 55.9 [14.1] | 53.7 [13.4] | 54.2 [14.0] |
| Sex (male), n (%) | 44 (70%) | 41 (64%) | 44 (66%) | 40 (71%) | 169 (68%) |
| Positive COVID-19 test | |||||
| SARS-Cov 2 Antigen Test | 16 (25%) | 14 (22%) | 15 (22%) | 14 (25%) | 59 (24%) |
| POCT-PCR | 1 (2%) | 0 (0%) | 1 (1.5%) | 0 (0%) | 2 (1%) |
| RT-PCR | 46 (73%) | 50 (78%) | 51 (76%) | 42 (75%) | 189 (76%) |
| SARS-CoV-2 sequenced genomes | 46 (73%) | 44 (69%) | 47 (70%) | 43 (77%) | 180 (72%) |
| SARS-CoV-2 variants | |||||
| Alpha | 0 (0%) | 1 (2.0%) | 0 (0%) | 0 (0%) | 1 (0.6%) |
| Gamma | 42 (91%) | 40 (91%) | 43 (91.5%) | 42 (98%) | 167 (93%) |
| Delta | 1 (2%) | 0 (0%) | 1 (2%) | 0 (0%) | 2 (1%) |
| Non-VoC | 3 (6.5%) | 3 (7%) | 3 (6%) | 1 (2%) | 10 (6%) |
| Comorbities | |||||
| Hypertension | 30 (48%) | 25 (39%) | 30 (45%) | 19 (34%) | 104 (41.6%) |
| Diabetes | 16 (25%) | 12 (19%) | 19 (28%) | 10 (18%) | 57 (23%) |
| Current smoker | 1 (2%) | 0 (0%) | 1 (1.5%) | 0 (0%) | 2/ (1%) |
| Former smoker | 6 (9.5%) | 5 (8%) | 2 (3%) | 3 (5%) | 16 (6%) |
| Obesity | 14 (22%) | 16 (25%) | 17 (25%) | 12 (21%) | 59 (24%) |
| Cancer | 2 (3%) | 1 (2%) | 0 (0%) | 3 (5%) | 6 (2%) |
| Heart failure | 2 (3%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (1%) |
| COPD | 2 (3%) | 0 (0%) | 1 (1.5%) | 2 (4%) | 5 (2%) |
| Chronic renal disease | 2 (3%) | 1 (2%) | 0 (0%) | 0 (0%) | 3 (1%) |
| Neuromuscular disease | 1 (2%) | 4 (6%) | 1 (1.5%) | 1 (2%) | 7 (3%) |
| Other | 1 (2%) | 1 (2%) | 1 (1.5%) | 2 (4%) | 5 (2%) |
| Baseline medications | |||||
| Corticosteroids | 55 (87%) | 53 (83%) | 54 (81%) | 45 (80%) | 207 (83%) |
| Statins | 4 (6%) | 3 (5%) | 5 (7.5%) | 2 (4%) | 14 (6%) |
| Antiarrhythmic drugs | 0 (0%) | 0 (0%) | 0/ (0%) | 1 (2%) | 1 (0.4%) |
| Anticonvulsants | 0 (0%) | 1 (2%) | 0 (0%) | 2 (4%) | 3 (1%) |
| NOAC | 2 (33%) | 1 (2%) | 0 (0%) | 1 (2%) | 4 (2%) |
| Therapeutic Dose Heparin | 17 (27%) | 15 (23%) | 22 (33%) | 21 (35.5%) | 75 (30%) |
| Tocilizumab | 0 (0%) | 0 (0%) | 1 (1.5%) | 1 (2%) | 2 (1%) |
| Antibiotics | |||||
| Ceftriaxone | 26 (41.3%) | 32 (50.0%) | 32 (47.8%) | 25 (45%) | 115 (46.0%) |
| Ceftaroline | 0 (0.0%) | 0 (0.0%) | 2 (3%) | 1 (2%) | 3 (1%) |
| Piperaciline/Tazobactan | 1 (2%) | 0 (0.0%) | 1 (1.5%) | 1 (3%) | 3 (1%) |
| Quinolone | 1 (2%) | 1 (2%) | 1 (1.5%) | 0 (0.0%) | 3 (1%) |
| Clinical data | |||||
| Systolic Blood Pressure, mmHg, mean ± [SD] | 125.8 [15.0] | 125.7 [18.1] | 124.1 [14.9] | 122.2 [14.3] | 124.5 [15.6] |
| Diastolic Blood Pressure, mmHg, mean ± [SD] | 77.8 [10.0] | 76.7 [11.3] | 75.2 [8.6] | 74.7 [8.9] | 76.1 [9.8] |
| Heart Rate, bpm, mean [SD] | 84.9 [13.7] | 85.4 [12.8] | 84.0 [15.7] | 82.2 [18.3] | 84.2 [15.1] |
| Respiratory rate, bpm, mean [SD] | 22.3 [5.9] | 20.4 [3.7] | 21.6 [4.1] | 20.9 [4.3] (n = 56) | 21.3 [4.6] |
| Peripheral saturation O2, %, mean [SD] | 93.6 [2.3] | 93.9 [2.3] | 93.6 [2.7] | 93.3 [3.7] (n = 56) | 93.6 [2.8] |
| Supplemental oxygen at randomization | |||||
| Supplemental oxygen at day 1 | 56 (90%) | 52 (81%) | 49 (73%) | 41 (73%) | 198 (79%) |
| None | 6 (9.5%) | 6 (9%) | 15 (22%) | 9 (16%) | 36 (14%) |
| Oxygen catheter | 34 (54%) | 44 (69%) | 24 (36%) | 29 (52%) | 131 (52%) |
| Venturi mask | 9 (14%) | 7/(11%) | 14 (21%) | 5 (9%) | 35 (14%) |
| NIV | 10 (16%) | 5/(8%) | 6 (9%) | 6 (11%) | 27 (11%) |
| HFNC | 4 (6%) | 2 (3%) | 8 (12%) | 6 (11%) | 20 (8%) |
| Mechanical ventilation | 0 (0%) | 0 (0%) | 0 (0%) | 1 (2%) | 1 (0.4%) |
| Laboratorial | |||||
| Creatinine, mg/dL, median [quartiles] | 0.9 [0.7; 1.1] (n = 58)a | 0.9 [0.8; 1.2] (n = 57)a | 1.0 [0.7; 1.1] (n = 62)a | 0.9 [0.8; 1.1] (n = 53)a | 0.9 [0.8; 1.1] (n = 230)a |
| Bilirrubin, mg/dL, median [quartiles] | 0.4 [0.3; 0.6] | 0.4 [0.3; 0.6] | 0.4 [0.3; 0.5] | 0.4 [0.3; 0.6] | 0.4 [0.3; 0.6] |
| Urea, mg/dL, median [quartiles] | 33 [25; 49] (n = 55)a | 35 [28; 49] (n = 55)a | 35 [29; 46] (n = 60)a | 33 [26; 45] (n = 50)a | 34 [27; 46] (n = 220)a |
| ALT, IU/L, median [quartiles] | 46 [32; 59] (n = 62)a | 44 [27; 65] | 38 [30; 51] (n = 66)a | 38 [30; 63] | 42 [30; 59] (n = 248)a |
| AST, IU/L, median [quartiles] | 36 [25; 58] (n = 62)a | 40 [23; 62] | 32 [21; 54] (n = 66) | 33 [25; 55] | 35 [23; 57] (n = 248)a |
| Hemoglobin, g/dL, mean [SD] | 14 [13; 15] (n = 61)a | 14 [12; 14] (n = 63)a | 14 [12; 14] (n = 66)a | 14 [13; 15] (n = 55)a | 14 [13; 15] (n = 245)a |
| Leukocyte, 103/mL, median [quartiles] | 7.5 [5.7; 10.6] (n = 61)a | 6.9 [5.2; 10.3] (n = 63)a | 7.4 [5.9; 9.5] (n = 66)a | 7.0 [5.2; 9.7] (n = 55)a | 7.1 [5.5; 10.0] (n = 245)a |
| Platelets, 10³/mm³, median [quartiles] | 190 [153; 234] (n = 61)a | 190 [158; 223] (n = 63)a | 184 [143; 229] (n = 66)a | 192 [153; 233] (n = 55)a | 190 [151; 231] (n = 245)a |
| Lymphocyte, 10³/mL, median [IQR] | 0.8 [0.6; 1.1] (n = 61)a | 0.9 [0.7; 1.2] (n = 63)a | 0.8 [0.5; 1.2] (n = 66)a | 0.9 [0.7; 1.2] (n = 55)a | 0.9 [0.6; 1.2] (n = 245)a |
| Days from symptoms onset to randomization, median [quartiles] | 7.0 [6.0; 8.0] | 7.0 [6.0; 8.0] | 7.0 [6.0; 8.0] | 7.0 [6.0; 8.0] | 7.0 [6.0; 8.0] |
| Days from admission to randomization, median [quartiles] | 1.0 [0.0; 1.0] | 1.0 [0.0; 2.0] | 1.0 [0.0; 2.0] | 1.0 [0.0; 1.0] | 1.0 [0.0; 2.0] |
Data are n (%), mean [SD], median [IQR], n/N (%).
Percentages may not total 100 because of rounding.
AIDS = Acquired Immuno Deficiency Syndrome, ALT = Alanine Transaminase, AST = Aspartate Transaminase, ATV = Atazanavir, COPD = Chronic Obstructive Pulmonary Disease, DCV = Daclatasvir, HFNC = High Flow Nasal Cannula, NIV = Non Invasive Ventilation, NOAC = New Oral Anticoagulants, POCT-PCR = Point of Care Test Polymerase Chain Reaction, RT-PCR = Real Time Polymerase Chain Reaction, SOF + DCV = Sofosbuvir + Daclatasvir.
Data were complete except where identified in the table.
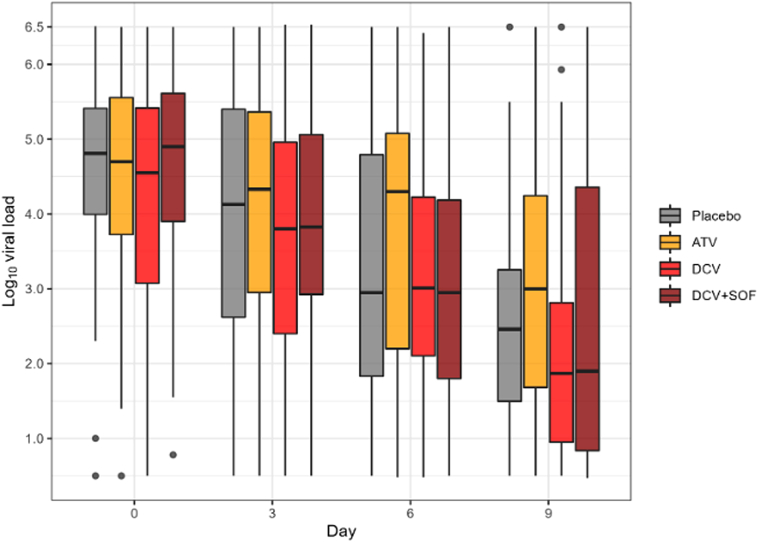
A total of 835 nasopharyngeal swabs were analysed from 250 participants. The median viral load in the treatment groups and placebo group at baseline are shown in Supplementary Tables S5 and S6. There was no significant difference between the groups in the proportion of participants with detectable viral loads at each sampling time. The median decrease in viral loads between baseline and day 10 was similar in the treatment groups and placebo group. There was no significant effect of any treatment on the viral kinetics (Fig. 2). The change in log viral load from baseline to day 10 was −0.19 (95% confidence interval [CI], −0.25 to −0.12) for the atazanavir group, −0.23 (95% CI −0.30 to −0.17) for the daclatasvir group, −0.22 (95% CI −0.30 to −0.16) for the sofosbuvir/daclatasvir group and −0.26 (95% CI −0.33 to −0.19) for the placebo group. Compared with the placebo group, the change from baseline to day 10 in log viral load was not significantly different for any of the treatment groups (0.08 (95% CI, −0.01 to 0.16), p = 0.11 for the atazanavir group; 0.03 (95% CI, −0.0606 to 0.12), p = 0.80 for the daclatasvir group; and 0.04 (95% CI, −0.0505 to 0.12), p = 0.63 for the sofosbuvir/daclatasvir group) (Table 2).
Fig. 2.
SARS-CoV2 viral loads in nasopharyngeal swabs in the modified intention to treat population at each time point and as change from baseline according to treatment group. ATV = Atazanavir, DCV = Daclatasvir, SOF + DCV = Sofosbuvir + Daclatasvir. Results of the median decrease in viral loads in each of the groups at baseline, days 3, 6, 10 (plus or minus 2). The depicted boxplots showed that there is no significant variation in viral load among groups on the same day of collection. The median decrease in viral loads between baseline and day 10 was similar in the treatment groups and placebo group.
Table 2.
Primary and secondary outcomes in modified intention to treat population according to treatment group.
| ATV |
DCV |
SOF + DCV |
Placebo |
Effect size |
p-value |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| ATV vs. Placebo |
DCV vs. Placebo |
SOF + DCV vs. Placebo |
ATV vs. Placebo | DCV vs. Placebo | SOF + DCV vs. Placebo | |||||
| n = 63 | n = 64 | n = 67 | n = 56 | (IC 95%) | (IC 95%) | (IC 95%) | ||||
| Primary outcome | ||||||||||
| 1. Decay rate (slope) of the. SARS-CoV-2 viral load logarithm from baseline to day 10; mean (95% CI) | −0.19 (−0.25 to −0.12) | −0.23 (−0.30 to −0.17) | −0.22 (−0.29 to −0.16) | −0.26 (−0.33 to −0.19) | 0.08 (−0.01 to 0.16) | 0.03 (−0.06 to 0.12) | 0.04 (−0.05 to 0.12) | 0.11 | 0.80 | 0.63 |
| Secondary outcomes | ||||||||||
| 1. Respiratory support free days in 15 days, mean (95% CI) | 6.19 (4.98–7.40) | 6.97 (5.68–8.27) | 6.79 (5.75–7.84) | 6.48 (5.21–7.68) | −0.28 (−1.96 to 1.49) | 0.50 (−1.24 to 2.21) | 0.32 (−1.25 to 1.94) | 0.75 | 0.58 | 0.69 |
| 2. 7-Stage ordinal scale for clinical outcomes on day 15, median [IQR] | 2.0 [1.0; 6.0] | 2.0 [1.0; 4.0] | 2.0 [1.5; 4.5] | 2.0 [1.0; 6.0] | 0.96 (0.46–1.74) | 0.95 (0.5–1.84) | 1.04 (0.5–1.8) | |||
| 3. 6-Stage ordinal scale for clinical outcomes on day 7, median [IQR] | 3.0 [1.0; 5.0] | 2.0 [1.0; 4.0] | 3.0 [1.0; 4.0] | 3.5 [2.0; 5.0] | 0.44 (0.35–1.27) | 0.59 (0.23–0.85) | 1.03 (0.31–1.1) | |||
| 4. 28-day mortality, n (%) | 8 (13%) | 10 (16%) | 9 (13%) | 8 (14%) | 0.77 (0.25–2.42) | 1.19 (0.40–3.62) | 0.77 (0.25–2.34) | |||
| 5. Days free from mechanical ventilation within 28 days, mean [SD] | 20.52 [11.82] | 22.09 [10.61] | 20.72 [11.99] | 20.36 [11.19] | 0.62 (−3.23 to 4.53) | 1.63 (−2.08 to 5.41) | 1.28 (−2.80 to 4.87) | |||
| 6. Days out of hospital in 28 days, mean [SD] | 13.44 [9.69] | 15.10 [9.25] | 13.29 [9.52] | 13.31 [9.10] | 0.61 (−3.03 to 3.95) | 1.30 (−1.80 to 4.48) | 0.61 (−2.91 to 3.76) | |||
| 7. Time to discharge, days, mean [SD] | 13.89 [8.01] | 11.75 [7.31] | 12.70 [8.15] | 12.98 [7.58] | 1.29 (−1.57 to 4.03) | −1.12 (−3.85 to 1.53) | −0.31 (−2.83 to 2.34) | |||
Data are n (%), mean [SD] median (IQR), n/N (%), mean (95% Confidence Interval).
Analysis were stratified by centre at random assignment and adjusted effect measure are reported. For primary outcome analysis, the missing data is imputed. It doesn't happen for secondary outcome analysis.
Percentages may not total 100 because of rounding.
ATV = Atazanavir, DCV = Daclatasvir, SOF + DCV = Sofosbuvir + Daclatasvir.
A total of 219 participants were included in the per protocol analysis (atazanavir, n = 51; daclatasvir, n = 61; sofosbuvir/daclatasvir, n = 62; placebo, n = 45). Compared with the placebo group, the change from baseline to day 10 in log viral load in this population was not significantly different for any of the treatment groups: 0.04 (95% CI, −0.04 to 0.13) for the atazanavir group; −0.01 (95% CI, −0.09 to 0.08) for the daclatasvir group; and −0.03 (95% CI, −0.11 to 0.05) for the sofosbuvir/daclatasvir group (Supplementary Table S7).
Clinical status of patients in the treatment groups and the placebo group according to the WHO 7-point ordinal scale at day 15 are shown in Table 2 and Supplementary Table S8. Ordinal scale data were missing from 0 (0%) participants in the atazanavir group, 2 (2%) participants in the daclatasvir group, 0 (0%) participants in the daclatasvir/sofosbuvir group and 0 (0%) in the placebo group at day 15. There were no significant differences in the distribution of the seven-point ordinal scale at day 15 between the treatment groups and placebo group (Supplementary Fig. S3 and Supplementary Table S8). There were no significant differences between treatment groups and placebo in the proportional odds of having a higher (worse) score on the seven-point ordinal scale at 15 days as follows: ATV vs. placebo: odds ratio, 0.96 (0.46–1.74); DCV vs. placebo: odds ratio, 0.95 (0.5–1.84) and SOF/DCV vs. placebo: odds ratio, 1.04 (0.50–1.80). Clinical status of patients in the treatment groups and the placebo group according to 6-point ordinal scale at day 7 are shown in Table 2 and Supplementary Table S8. There were no significant differences between the atazanavir, daclatasvir, sofosbuvir/daclatasvir and placebo groups in the distribution of the six-point ordinal scale at day 7 (Supplementary Fig. S4 and Supplementary Table S8); The proportion of deaths at day 28 was not significantly different between the treatment groups and placebo group as well. No significant difference between the treatment groups and placebo group was observed for any other secondary outcomes (Table 2).
Two sensitivity analyses were included: primary model taking the study centre effect into account as a random intercept in the primary outcome of decay rate of SARS-CoV2 viral load logarithm from baseline to day 10 and the area under the viral load which found no difference between intervention drugs ATV, DCV or SOF/DCV compared to placebo in both analysis, as shown in Supplementary Table S9.
A total of 248 participants were included in the safety analysis of patients who received at least one dose of the assigned treatment (atazanavir, n = 61; daclatasvir, n = 64; sofosbuvir/daclatasvir, n = 67; placebo, n = 56). Safety outcomes are shown in Table 3 and Supplementary Table S9. Among the 738 reported adverse events, 118 (35 in the atazanavir group, 35 in the daclatasvir group, 24 in the sofosbuvir/daclatasvir group and 24 in the placebo group) were graded 3 or 4 adverse events, affecting 23 (38%) of 61 participants in the atazanavir group, 15 (23%) of 64 participants in the daclatasvir group, 15 (22%) of 67 participants in the sofosbuvir/daclatatasvir group and 12 (21%) of 56 in the placebo group (Table 3). Compared to placebo there was no significant difference with treatment groups related to number grade 3 and 4 adverse events. Serious adverse events were reported in 17 (28%) participants in the atazanavir group, 12 (19%) participants in the daclatasvir group, 11 (16%) participants in the sofosbuvir/daclatasvir group and 10 (18%) participants in the placebo group with no significant difference between treatment and placebo groups (Table 3). No deaths were considered related to treatment by the investigators. The most frequently reported serious adverse events in all groups were acute renal failure, acute respiratory failure and sepsis.
Table 3.
Summary of adverse events in the safety population according to treatment group.
| ATV (n = 61) | DCV (n = 64) | SOF + DCV (n = 67) | Placebo (n = 56) | ATV vs. Placebo or (95% CI) | DCV vs. Placebo or (95% CI) | SOF + DCV vs. Placebo or (95% CI) | |
|---|---|---|---|---|---|---|---|
| Any adverse event n (%) | 32 (52.5%) | 25 (39%) | 29 (43%) | 24 (43%) | 1.47 (0.71; 3.07) p = 0.30 | 0.85 (0.41; 1.78) p = 0.67 | 1.02 (0.50; 2.09) p = 0.96 |
| Serious adverse event n (%) | 17 (28%) | 12 (19%) | 11 (16%) | 10 (18%) | 1.78 (0.74; 4.43) p = 0.20 | 1.06 (0.42; 2.73) p = 0.90 | 0.90 (0.35; 2.35) p = 0.83 |
| Drug discontinuation because of adverse event n (%) | 3 (5%) | 1 (2%) | 6 (9%) | 4 (7%) | 0.67 (0.13; 3.19) p = 0.61 | 0.21 (0.01; 1.45) p = 0.16 | 1.28 (0.35; 5.23) p = 0.71 |
| Grade 3 or 4 Adverse Event or Laboratorial Abnormality | 23 (38%) | 15 (23%) | 15 (22%) | 12 (2%) | 2.22 (0.99; 5.17) p = 0.057 | 1.12 (0.48; 2.70) p = 0.79 | 1.06 (0.45; 2.53) p = 0.90 |
| INR >2x ULN | 0 (0%) | 0 (0%) | 0 (0%) | 1 (2%) | |||
| ALT >5.1x ULN | 4 (7%) | 3 (5%) | 4 (6%) | 2 (4%) | 1.89 (0.35; 14.07) p = 0.47 | 1.33 (0.21; 10.36) p = 0.76 | 1.71 (0.32; 12.71) p = 0.54 |
| Bilirrubin >2.6x ULN | 6 (10%) | 0 (0%) | 0 (0%) | 1 (2%) | 6.00 (0.98; 115.3) p = 0.10 | a | a |
| Most Frequent Adverse Events | |||||||
| Renal failure | 7 (17%) | 7 (22%) | 2 (5%) | 5 (14%) | 1.32 (0.40; 4.72) p = 0.65 | 1.25 (0.38; 4.46) p = 0.71 | 0.31 (0.04; 1.52) p = 0.18 |
| Respiratory failure | 4 (9.5%) | 5 (15%) | 5 (13.5%) | 5 (14%) | 0.72 (0.17; 2.85) p = 0.63 | 0.86 (0.23; 3.27) p = 0.82 | 0.82 (0.22; 3.11) p = 0.77 |
| Sepsis | 5 (12%) | 1 (3%) | 4 (11%) | 5 (14%) | 0.91 (0.24; 3.45) p = 0.89 | 0.16 (0.01; 1.05) p = 0.10 | 0.65 (0.15; 2.57) p = 0.53 |
| Cardiocirculatory Arrest | 5 (12%) | 3 (9%) | 3 (8%) | 2 (6%) | 2.41 (0.50; 17.33) p = 0.30 | 1.33 (0.21; 10.36) p = 0.76 | 1.27 (0.20; 9.87) p = 0.80 |
| Septic shock | 2 (5%) | 6 (18%) | 2 (5%) | 2 (6%) | 0.92 (0.11; 7.84) p = 0.93 | 2.79 (0.61; 19.63) p = 0.22 | 0.83 (0.10; 7.11) p = 0.85 |
| Severe Hypoxemia | 2 (5%) | 2 (6%) | 4 (11%) | 2 (6%) | 0.92 (0.11; 7.84) p = 0.93 | 0.87 (0.10; 7.46) p = 0.90 | 1.71 (0.32; 12.71) p = 0.54 |
| Venous Thromboembolism | 2 (5%) | 0 (0%) | 1 (3%) | 4 (11%) | 0.44 (0.06; 2.35) p = 0.35 | a | 0.20 (0.01; 1.38) p = 0.15 |
| Pneumothorax | 2 (5%) | 0 (0%) | 3 (8%) | 0 (0%) | a | a | a |
| Respiratory infection | 0 (0%) | 0 (0%) | 2 (5%) | 2 (6%) | a | a | 0.83 (0.10; 7.11) p = 0.85 |
| Pulmonary Embolism | 0 (0%) | 0 (0%) | 2 (5%) | 1 (3%) | a | a | 1.69 (0.16; 36.99) p = 0.67 |
| Ventilator-associated pneumonia | 1 (2%) | 2 (6%) | 0 (0%) | 0 (0%) | a | a | a |
| Abdominal pain | 1 (2%) | 0 (0%) | 1 (3%) | 0 (0%) | a | a | a |
| Blood stream infection | 0 (0%) | 1 (3%) | 0 (0%) | 1 (3%) | a | 0.87 (0.03; 22.43) p = 0.92 | a |
| Bradycardia and death | 0 (0%) | 0 (0%) | 1 (3%) | 1 (3%) | a | a | 0.83 (0.03; 21.40) p = 0.90 |
| Fungal infection | 2 (5%) | 0 (0%) | 0 (0%) | 0 (0%) | a | a | a |
Data are n (%), n/N (%).
Some patients had more than a single adverse event. Analyses were done in the safety population.
Percentages may not total 100 because of rounding.
Bold indicates the level of significance is p < 0.05.
ALT = Alanine Transaminase, INR = International Normalised Ratio, OR = Odds Ratio, p = p value, ULN = Upper Limit of Normal.
Comparison not done because of lack of events (zero events).
Discussion
This report shows the result of the REVOLUTIOn trial which compared repurposed drugs atazanavir, daclatasvir or sofosbuvir/daclatasvir to placebo in hospitalised hypoxemic COVID-19 patients. These antiviral drugs were well tolerated, however none of the antiviral reduced SARS-CoV2 viral load in comparison to placebo under their reference dose for against HIV and HCV. A subsequent seamless phase III trial to assess efficacy of the antivirals in terms of clinical outcomes planned in case of efficacy of any of the antiviral to decrease viral load, was not conducted.
SARS-CoV2 virological clearance in COVID-19 patients treated with SOF/DCV was studied in few randomised controlled trials as a primary endpoint. El-Bendary et al.23 observed greater virological clearance at day 14 measured only by RT-PCR in those treated with SOF/DCV and Hydroxycloroquine for 14 days compared to Hydroxycloroquine alone. Abass et al.24 observed no difference in the percentage of patients with undetectable SARS-CoV2 RNA on 2 consecutive nasopharyngeal swabs at day 10 in those moderate to severe disease treated with SOF/DCV compared to Standard of Care. Nekoukar et al.25 is the only randomised controlled trial that studied ATV associated with ritonavir plus HCQ compared to HCQ plus Lopinavir/Ritonavir in moderate to severe COVID-19 patients and found no difference in nasopharyngeal SARS-CoV2 PCR negativity at day 14 between groups.
Clinical efficacy outcomes in COVID-19 patients treated with SOF/DCV compared to standard of care were evaluated in five previous randomised controlled trials.8,23,24,26,27 One of these studies evaluated outpatients only27 and the other four studied hospitalised patients similar to our population. All of these studies added other medications to SOF/DCV as hydroxychloroquine, ribavirin or lopinavir/ritonavir differently from our treatment groups which used isolated SOF/DCV. Clinical recovery was improved in three studies,8,24,26 shorter hospital stay in two studies23,26 and a trend to lower mortality in one study.23 However, inconsistency and imprecision of treatment effect are present. Our study didn't find any beneficial effect in clinical recovery, length of hospital stay, or 28 day mortality as well. However, it was not powered for these outcomes which were exploratory.
The present clinical trial tested atazanavir, daclatasvir and sofosbuvir/daclatasvir at their standard doses for treatment against HIV and HCV. Nevertheless, the pre-clinical data6, 7, 8,10,13,14 suggest that higher doses could enhance the chance to achieve plasma exposure to these drugs above the threshold to inhibit more than 90% virus replication during the entire course of treatment. Although an adaptative trial proposal with different regimens was presented to the Brazilian regulatory agency, they felt the necessity to perform new phase I clinical trials to reassure the safety and tolerability of these clinically approved drugs before proceeding.
Although the study targeted a population of hospitalised patients, subjects were tested to reassure they presented detectable viral loads. The interpretation of viral load-associated endpoint seems to be challenging even for studied with early use of antivirals.28, 29, 30 Whereas early use of remdesivir decreased viral loads by 2-log10 levels, the effects of Paxlovid ranged from half-log to 1 log10 inhibition and molnupiravir's effect on RNA levels were virtually undetectable.28, 29, 30 Nevertheless, even with modest to none effect of Paxlovid and molnupiravir on viral loads, these drugs presented clinical benefit for the patients.28,29 There are some thoughtful considerations from the Paxlovid study.29 Paxlovid reduced viral RNA levels more effectively in those patients with higher (>107 copies/mL) viral loads, suggesting that high amplitude is for antiviral sensitivity. In our investigation, the average viral load was 107 copies/mL; at similar level, sensitivity to detect Paxlovid anti-SARS-CoV-2 effect decreased half-log.29
REVOLUTIOn trial, to the best of our knowledge, is the first trial that measured quantitative SARS-CoV2 viral load course in nasopharyngeal swabs during the 10 days treatment with these repurposed drugs. Furthermore, its multiple arms and stages design could accelerate the answer about efficacy of these drugs in such a pandemic scenario where time was precious for saving lives. Although drugs such as remdesivir, IL-6 receptors antagonists and Janus Kinase (JAK) inhibitors have already been proven efficacious for hospitalised COVID-19 patients,4,31,32 they are costly and may need to be administered intravenously, which might limit access to patients from low- and middle-income countries. Therefore, repurposed drugs are an important alternative to be tested in clinical studies and the REVOLUTIOn trial was designed precisely with this alternative in mind, using cheap, easily accessible drugs with easy distribution logistics and adequate supply.
Although predictive relationship of viral load reductions and clinical benefit was not well established by the time the protocol was written, the Steering Committee decided to adopt a virological measure as a primary endpoint to support progression to Phase III clinical efficacy trial, following FDA recommendations for Phase II treatment trials for COVID-19.33 The decision included to stop the study as a whole if no evidence of efficacy in decreasing viral load could be found in the first stage, and this decision was essential to stay in line with the published protocol.16 Early interruption of the study was a relevant trial limitation, because discordant findings between clinical effectiveness and viral load clearance has been published.30 Although there are other endpoints that could be implemented in the future to be complementary to viral load-based measurements, the logistic challenges are substantial. On molnupiravir clinical trials for example, drugs' effect is more pronounced on virus infectivity than on viral loads. However, the implementation of routine testing of the infectivity from patient's nasopharyngeal swabs requires high biosafety levels and strict control in the cold chain, limiting its general applicability in many clinical settings.
In conclusion, in this randomised controlled trial, the use of repurposed drugs Atazanavir, Daclatasvir, Sofosbuvir/Daclatasvir for 10 days treatment of hospitalised, hypoxemic, not intubated COVID-19 patients were not able to decrease viral load of SARS-CoV2 in nasopharyngeal swabs when compared to placebo, using standard dose regimens.
Contributors
ISM, ABC and TM were responsible for the conceptualization of the study; ISM, AM, JOG, RHNS and LPD were responsible for data curation; JOG, RHNS, LPD and TMS had access to the full data set; TMS and LPD made all formal analysis; ISM, ABC, TM and GW were responsible for funding acquisition; ISM and AM were responsible for the investigation; ISM, ABC, LPD and FGZ were responsible for the methodology of the study; ISM and AM were responsible for the administration of the study; SPCG, KLN, MBSC and LNL were responsible for resources; RHNS, JOG, LPD and FGZ were responsible for all software; ISM, AM, ABC and TM were responsible for the supervision of the study; ISM, AM, ABC, VV and TM wrote the original draft; all other authors were responsible for reviewing and editing the draft. All authors approved the final version of the manuscript submitted.
Data sharing statement
With publication, deidentified, individual participant data that underlie this Article, along with a data dictionary describing variables in the dataset, will be made available to researchers whose proposed purpose of use is approved by the REVOLUTIOn Steering Committee. To request the dataset, please address directly to the corresponding author (ismaia@ext.hcor.com.br) to obtain a data access form. All requests will be evaluated by the Trial Management Team and the REVOLUTIOn Steering Committee. For accepted requests, data will be shared after signing a data transfer agreement with the study sponsor. Data will be shared directly. Related documents, such as the study protocol, statistical analysis plan, and informed consent form, will be made available (with publication) on request to the corresponding author or to the sponsor's representative. The data will be open access for the informed consent form, protocol, and statistical analysis plan.
Declaration of interests
ISM reports devices supply from Fisher & Paykel outside the submitted work; ISM and ABC reports funding paid to HCor by Ministerio da Ciência, Tecnologia e Inovação (MCTIC)/Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ), Cia Latino Americana de Medicamentos (Clamed); Cia Industrial H. Carlos Schneider (Ciser); Hospital Research Foundation Incorporation, Australia, HCor São Paulo; Blanver Farmoquímica; Instituto de Tecnologia em Fármacos (Farmanguinhos) da Fundação Oswaldo Cruz (Fiocruz); Coordenação Geral de Planejamento Estratégico (Cogeplan)/Fiocruz; and Fundação de apoio a Fiocruz; LPD reports personal statistical Consulting fees from Servier Laboratories, Aché Laboratory and Astra Zeneca; FGZ reports grants for investigator initiated trials paid to his institution from Bactiguard, Ionis Pharmaceuticals and statistical Consulting from Bactiguard; RGR reported research grants from Pfizer and Brazilian Ministry of Health-PROADI-SUS; LCPA reported participation on advisory board of COVID-19 drugs for MSD; OB reported grants or contracts from: AstraZeneca, Amgen, Bayer, Pfizer, BMS Servier, Novartis Boehringer-Ingelheim, RDL reports grants or contracts from Bristol-Myers Squibb, Glaxo Smith Kline, Medtronic, Pfizer, Sanofi with payments to his institution, payment or honoraria for lectures, presentations, speakers, manuscript writing from Pfizer, Participation on a Data Safety Monitoring Board or Advisory Board of Glaxo Smith Kline and Consulting fees from Bayer, Boehringer Ingleheim, Bristol-Myers Squibb, Daiichi Sankyo, Glaxo Smith Kline, Medtronic, Merck, Pfizer, Portola and Sanofi; AA reports grants form Population Health Research Institute, EMS and Bayer as funding to his institution, payment for lectures for EMS and Bayer. All other authors declare no competing interests.
Acknowledgments
This work received funding from several sources: Ministério da Ciência, Tecnologia e Inovação (MCTI) e Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ), Clamed Farmácias, Cia Latino-Americana de Medicamentos, Ciser Fixadores e GP2U TeleHealth Australia, HCor São Paulo, Blanver, Farmoquímica, Instituto de Tecnologia em Fármacos (Farmanguinhos) da Fundação Oswaldo Cruz (Fiocruz), Coordenação Geral de Planejamento Estratégico (Cogeplan)/Fiocruz e Fundação de apoio a Fiocruz (Fiotec, VPGDI-054-FIO-20-2-13). We thank Prof James Wason and Otavio Ranzani for the important contribution of peer reviewing the protocol; Prof Haiyan Zheng, Otavio Ranzani and Morten Hylander for the excelent job in REVOLUTIOn Data Safety and Monitoring Board and all participants who consented to participate in the trial, as well as all study and site staff whose indispensable assistance made the conduct of the trial possible.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lana.2023.100466.
Appendix A. Supplementary data
References
- 1.Scherman D., Fetro C. Drug repositioning for rare diseases: knowledge-based success stories. Therapie. 2020;75(2):161–167. doi: 10.1016/j.therap.2020.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Dyall J., Coleman C.M., Hart B.J., et al. Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection. Antimicrob Agents Chemother. 2014;58(8):4885–4893. doi: 10.1128/AAC.03036-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arabi Y.M., Gordon A.C., Derde L.P.G., et al. Lopinavir-ritonavir and hydroxychloroquine for critically ill patients with COVID-19: REMAP-CAP randomized controlled trial. Intensive Care Med. 2021;47(8):867–886. doi: 10.1007/s00134-021-06448-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beigel J.H., Tomashek K.M., Dodd L.E., et al. Remdesivir for the treatment of COVID-19 - final report. N Engl J Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merat S., Sharifi A.H., Poustchi H., et al. SD1000: high sustained viral response rate in 1361 patients with hepatitis C genotypes 1, 2, 3, and 4 using a low-cost, fixed-dose combination tablet of generic sofosbuvir and daclatasvir: a multicenter, phase III clinical trial. Clin Infect Dis. 2020;70(10):2206–2212. doi: 10.1093/cid/ciz628. [DOI] [PubMed] [Google Scholar]
- 6.Elfiky A.A. Ribavirin, remdesivir, sofosbuvir, galidesivir, and tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): a molecular docking study. Life Sci. 2020;253:117592. doi: 10.1016/j.lfs.2020.117592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chien M., Anderson T.K., Jockusch S., et al. Nucleotide analogues as inhibitors of SARS-CoV-2 polymerase, a key drug target for COVID-19. J Proteome Res. 2020;19(11):4690–4697. doi: 10.1021/acs.jproteome.0c00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kasgari H.A., Moradi S., Shabani A.M., et al. Evaluation of the efficacy of sofosbuvir plus daclatasvir in combination with ribavirin for hospitalized COVID-19 patients with moderate disease compared with standard care: a single-centre, randomized controlled trial. J Antimicrob Chemother. 2020;75(11):3373–3378. doi: 10.1093/jac/dkaa332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sacramento C.Q., Fintelman-Rodrigues N., Temerozo J.R., et al. In vitro antiviral activity of the anti-HCV drugs daclatasvir and sofosbuvir against SARS-CoV-2, the aetiological agent of COVID-19. J Antimicrob Chemother. 2021;76(7):1874–1885. doi: 10.1093/jac/dkab072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X., Sacramento C.Q., Jockusch S., et al. Combination of antiviral drugs inhibits SARS-CoV-2 polymerase and exonuclease and demonstrates COVID-19 therapeutic potential in viral cell culture. Commun Biol. 2022;5(1):1–14. doi: 10.1038/s42003-022-03101-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mobarak S., Salasi M., Hormati A., et al. Evaluation of the effect of sofosbuvir and daclatasvir in hospitalized COVID-19 patients: a randomized double-blind clinical trial (DISCOVER) J Antimicrob Chemother. 2021;77(3):758–766. doi: 10.1093/jac/dkab433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simmons B., Wentzel H., Mobarak S., et al. Sofosbuvir/daclatasvir regimens for the treatment of COVID-19: an individual patient data meta-analysis. J Antimicrob Chemother. 2021;76(2):286–291. doi: 10.1093/jac/dkaa418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaves O.A., Sacramento C.Q., Ferreira A.C., et al. Atazanavir is a competitive inhibitor of SARS-CoV-2 M pro, impairing variants replication in vitro and in vivo. Pharmaceuticals (Basel) 2021;15(1):21. doi: 10.3390/ph15010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fintelman-Rodrigues N., Sacramento C.Q., Lima C.R., et al. Atazanavir, alone or in combination with ritonavir, inhibits SARS-CoV-2 replication and proinflammatory cytokine production. Antimicrob Agents Chemother. 2020;64(10):e00825. doi: 10.1128/AAC.00825-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Royston P., Parmar M.K.B., Qian W. Novel designs for multi-arm clinical trials with survival outcomes with an application in ovarian cancer. Stat Med. 2003;22(14):2239–2256. doi: 10.1002/sim.1430. [DOI] [PubMed] [Google Scholar]
- 16.Maia I.S., Marcadenti A., Zampieri F.G., et al. Antivirals for adult patients hospitalized with SARS-CoV-2 infection: a randomized, phase II/III, multicenter, placebo-controlled, adaptive study, with multiple arms and stages. COALITION COVID-19 BRAZIL IX – REVOLUTIOn: protocol and statistical analysis plan. Rev Bras Med Intensiva. 2022;34(1):44–55. doi: 10.5935/0103-507X.20220002-en. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehta R.L., Kellum J.A., Shah S.V., et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pugh R.N.H., Murray-Lyon I.M., Dawson J.L., Pietroni M.C., Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60(8):646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 19.Velazquez E.J., Morrow D.A., DeVore A.D., et al. Angiotensin–neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2019;380(6):539–548. doi: 10.1056/NEJMoa1812851. [DOI] [PubMed] [Google Scholar]
- 20.Horby P., Lim W.S., Emberson J.R., et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomazini B.M., Maia I.S., Cavalcanti A.B., et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA. 2020;324(13):1307–1316. doi: 10.1001/jama.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wölfel R., Corman V.M., Guggemos W., et al. Virological assessment of hospitalized patients with COVID-19 -2019. Nature. 2020;581(7809):465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 23.El-Bendary M., Abd-Elsalam S., Elbaz T., et al. Efficacy of combined Sofosbuvir and Daclatasvir in the treatment of COVID-19 patients with pneumonia: a multicenter Egyptian study. Expert Rev Anti Infect Ther. 2022;20(2):291–295. doi: 10.1080/14787210.2021.1950532. [DOI] [PubMed] [Google Scholar]
- 24.Abbass S., Kamal E., Salama M., et al. Efficacy and safety of sofosbuvir plus daclatasvir or ravidasvir in patients with COVID-19: a randomized controlled trial. J Med Virol. 2021;93(12):6750–6759. doi: 10.1002/jmv.27264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nekoukar Z., Ala S., Moradi S., et al. Comparison of the efficacy and safety of atazanavir/ritonavir plus hydroxychloroquine with lopinavir/ritonavir plus hydroxychloroquine in patients with moderate COVID-19, a randomized, double-blind clinical trial. Iran J Pharm Res. 2021;20(4):278–288. doi: 10.22037/ijpr.2021.115157.15243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sadeghi A., Asgari A.A., Norouzi A., et al. Sofosbuvir and daclatasvir compared with standard of care in the treatment of patients admitted to hospital with moderate or severe coronavirus infection (COVID-19): a randomized controlled trial. J Antimicrob Chemother. 2020;75(11):3379–3385. doi: 10.1093/jac/dkaa334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roozbeh F., Saeedi M., Alizadeh-Navaei R., et al. Sofosbuvir and daclatasvir for the treatment of COVID-19 outpatients: a double-blind, randomized controlled trial. J Antimicrob Chemother. 2021;76(3):753–757. doi: 10.1093/jac/dkaa501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jayk Bernal A., Gomes da Silva M.M., Musungaie D.B., et al. Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients. N Engl J Med. 2022;386(6):509–520. doi: 10.1056/NEJMoa2116044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hammond J., Leister-Tebbe H., Gardner A., et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19. N Engl J Med. 2022;386(15):1397–1408. doi: 10.1056/NEJMoa2118542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gottlieb R.L., Vaca C.E., Paredes R., et al. Early remdesivir to prevent progression to severe COVID-19 in outpatients. N Engl J Med. 2022;386(4):305–315. doi: 10.1056/NEJMoa2116846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalil A.C., Patterson T.F., Mehta A.K., et al. Baricitinib plus remdesivir for hospitalized adults with COVID-19. N Engl J Med. 2021;384(9):795–807. doi: 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gordon A.C., Mouncey P.R., Al-Beidh F. Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N Engl J Med. 2021;384(16):1491–1502. doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Services USD of H and H, Administration F and D, (CDER) C for DE and R, (CBER) C for BE and R . 2021. COVID-19: developing drugs and biological products for treatment or prevention guidance for industry; pp. 1–21. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.