Abstract
RSK is a serine/threonine kinase containing two distinct catalytic domains. Found at the terminus of the Ras/extracellular signal-regulated kinase (ERK)–mitogen-activated protein kinase (MAPK) kinase cascade, mitogen-stimulated ribosomal S6 kinase (RSK) activity requires multiple inputs. These inputs include phosphorylation of the C-terminal kinase domain activation loop by ERK1/2 and phosphorylation of the N-terminal kinase domain activation loop by phosphoinositide-dependent protein kinase-1 (PDK1). Previous work has shown that upon mitogen stimulation, RSK accumulates in the nucleus. Here we show that prior to nuclear translocation, epidermal growth factor-stimulated RSK1 transiently associates with the plasma membrane. Myristylation of wild-type RSK1 results in an activated enzyme in the absence of added growth factors. When RSK is truncated at the C terminus, the characterized ERK docking is removed and RSK phosphotransferase activity is completely abolished. When myristylated, however, this myristylated C-terminal truncated form (myrCTT) is activated at a level equivalent to myristylated wild-type (myrWT) RSK. Both myrWT RSK and myrCTT RSK can signal to the RSK substrate c-Fos in the absence of mitogen activation. Unlike myrWT RSK, myrCTT RSK is not further activated by serum. Only the myristylated RSK proteins are basally phosphorylated on avian RSK1 serine 381, a site critical for RSK activity. The myristylated and unmyristylated RSK constructs interact with PDK1 upon mitogen stimulation, and this interaction is insensitive to the MEK inhibitor UO126. Because a kinase-inactive CTT RSK can be constitutively activated by targeting to the membrane, we propose that ERK may have a dual role in early RSK activation events: preliminary phosphorylation of RSK and escorting RSK to a membrane-associated complex, where additional MEK/ERK-independent activating inputs are encountered.
Stimulation of the Ras/extracellular signal-regulated kinase (ERK)–mitogen-activated protein kinase (MAPK) pathway can result in cell growth and proliferation, differentiation, and cell survival. Ribosomal S6 kinases (RSK) comprise a family of serine/threonine kinases that lies at the terminus of the mitogen-regulated Ras/ERK-MAPK pathway (reviewed in reference 16). Mitogen stimulation initiates a cascade of activating events in this pathway, with resultant ERK activation and phosphorylation of RSK by ERK. Upon mitogen stimulation, ERK and RSK translocate to the nucleus, where they phosphorylate nuclear substrates.
Many RSK substrates have been identified, implicating RSK in a myriad of cellular processes. RSK phosphorylates several transcription factors, among them CREB (42), Mi (41), c-Fos (8), C/EBPβ (6), and the estrogen receptor (24), as well as interacting with the transcriptional coactivator CREB-binding protein (also known as p300) (27). RSK may also have a role in chromatin remodeling through phosphorylation of histone H3 (32). RSK promotes cell survival through phosphorylation and inactivation of the proapoptotic protein BAD (5, 33, 37). The ubiquitous Na+/H+ exchanger isoform 1, important for cell proliferation, is also a RSK substrate (36). RSK also has a role in cell cycle regulation. In Xenopus laevis oocyte extracts, the phosphorylation of Myt1 by RSK downregulates Myt1 activity, leading to p34cdc2 activation, and subsequent entry into meiosis I (28). Active RSK is also the downstream mediator of Mos-induced metaphase arrest in meiosis II, preventing cell division in mature X. laevis oocytes (4, 20, 21).
Given the diversity of the RSK substrates and their cellular roles, there has been great interest in understanding the regulation of RSK activity. RSK has two distinct and active kinase domains (15). The N-terminal kinase domain, which has homology to the AGC superfamily of kinases, is responsible for phosphorylation of all known exogenous substrates. The C-terminal kinase domain is responsible for RSK autophosphorylation. Six mitogen-regulated phosphorylation sites have been identified (14) (Fig. 1). Avian RSK1 serine 239 (rat 222), in the activation loop of the N-terminal kinase domain, is phosphorylated by phosphoinositide-dependent protein kinase-1 (PDK1) (23, 31, 40). This site is conserved in the AGC kinase superfamily, and PDK1 phosphorylates the analogous site in many of these kinases (reviewed in reference 3). Threonine 377 (rat 360) and serine 381 (rat 364) are proline-directed phosphorylation sites and are believed to be phosphorylated by ERK1/2; of these two sites, only S381 is essential for kinase activity (14). Serine 398 (rat 381) is an essential site (14, 39). When phosphorylated, this site acts as a docking site for PDK1 binding to RSK2 (17). Threonine 590 (rat 574) is phosphorylated by ERK1/2 (31, 35) and is within the C-terminal kinase domain. Finally, serine 798 (rat 733) is a nonessential site that fits a RSK (K/R)XX(S/T) phosphorylation consensus sequence. It is also within the region identified as an ERK docking site at the RSK C terminus (18, 34).
FIG. 1.
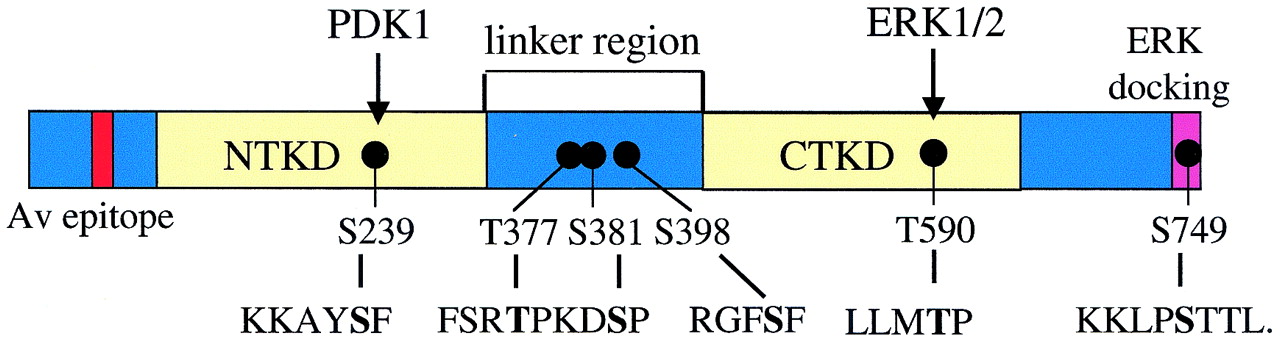
RSK structure and phosphorylation sites. Avian (Av) RSK1 contains two distinct kinase domains (yellow) separated by a linker domain. An ERK docking site is located at the extreme C terminus (purple). Six phosphorylation sites have been identified and are indicated in boldface type, using avian RSK1 numbering. An avian-specific epitope is shown in red. Two known external activating kinases are indicated: PDK1 phosphorylating S239, and ERK1/2 phosphorylating T590.
Phosphorylation is not the only important event in the activation of AGC superfamily kinases. Akt/protein kinase B (PKB) translocates to the cell membrane upon mitogen stimulation, where it can interact with similarly translocated PDK1 (reviewed in reference 7). A model suggests that once at the membrane, subsequent phosphorylation events lead to full activation of Akt/PKB kinase activity. When targeted to membranes with N-terminal myristylation, Akt/PKB becomes constitutively active, bypassing the regulated translocation event.
Like myristylated Akt/PKB, myristylated RSK has increased basal activity (33), suggesting that membrane association enables kinase activation. Like Akt/PKB, RSK interacts with PDK1 (17, 31). Unlike Akt/PKB, however, RSK does not have a pleckstrin homology domain, which would facilitate binding to membrane phosphoinositide moieties.
We have undertaken a study to determine whether membrane translocation is an important aspect of RSK activation and to elucidate the roles of ERK and PDK1 binding in this activation. We show a rapid and transient localization of RSK to the cell membrane upon mitogen stimulation. We find that ERK docking at the RSK C terminus is not required for RSK phosphotransferase activity when RSK is targeted to the membrane. While interaction of various RSK proteins with PDK1 is mitogen regulated, the elevated basal RSK activity is not due to PDK1 association, and this association is MEK independent. Membrane targeting RSK induces phosphorylation of important regulatory sites on RSK in the absence of ERK activity or C-terminal ERK docking, suggesting that these sites are not phosphorylated by ERK. These results suggest a model for RSK activation that requires ERK docking and phosphorylation, membrane association, and PDK1 inputs, as well as ERK- and PDK1-independent events.
MATERIALS AND METHODS
Materials.
Fetal calf serum was purchased from HyClone. RSK phosphospecific antibodies were purchased from Upstate Biotechnology Inc. (UBI) (Lake Placid, N.Y.) or supplied by R&D Systems (Minneapolis, Minn.). UO126 was purchased from Biomol.
Plasmid construction.
RSK1 (avian) was cloned into pRK7 and pKH3; pKH3 is pRK7 with a triple hemagglutinin (HA) tag at the amino terminus (26). Avian RSK also contains an avian-specific epitope between amino acids (aa) 37 and 54. C-terminally truncated RSK was generated by PCR, introducing a stop codon at aa 742, removing the last 11 aa (RRVKKLPSTTL), which encode an ERK docking site (18, 34). Point mutations in RSK were introduced using Stratagene's QuikChange kit. Myristylated RSK in pRK7 was derived from wild-type (WT) RSK in pMT2 (33). DNA encoding the Src myristylation sequence (MGSSKSKPK) was ligated to the N terminus of avian RSK1; this sequence lacks the polybasic domain necessary for predominant membrane localization. Myristylated C-terminally truncated (CTT) RSK in pRK7 was generated by cutting CTT RSK in pKH3 with BglII and EcoRI and subcloning into myristylated WT RSK in pRK7 that had also been cut with BglII and EcoRI. Flag–c-fos in pCDNA3 was generated by PCR amplification of myc–c-fos (11), replacing the N-terminal myc epitope with the Flag epitope. ERK1 in pGEX2T was a gift from Melanie Cobb, and myc-PDK1 in pCDNA3 was previously described (13). Flag–c-fos in pCDNA3 was generated by PCR amplification of myc–c-fos (11) and replacing the N-terminal myc epitope with the Flag epitope.
Cell culture, transfection, and lysis.
HEK293E cells were grown at 37°C, 5% CO2 in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum (DMEM-FCS). Sixteen hours before transfection, cells were plated onto 60- or 100-mm-diameter dishes. Using calcium phosphate, cells were transfected with the indicated DNA, using a total of 5 μg (60 mm) or 10 μg (100 mm) of DNA per dish. After 5 h, transfected cells were rinsed twice before starving for 16 to 18 h in DMEM. Cells were stimulated with 10% calf serum for 10 min or epidermal growth factor (EGF) (50 ng/ml) for 15 min at 37°C. Cells were rinsed twice on ice with phosphate-buffered saline (PBS) prior to lysis in CLB + 5 (10 mM KPO4, 1 mM EDTA, 5 mM EGTA, 10 mM MgCl2, 50 mM β-glycerophosphate, 0.5% NP-40, 0.1% Brij 35, 0.1% deoxycholic acid, 1 mM sodium orthovanadate, 2 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, peptstatin [5 μg/ml], and leupeptin [10 μg/ml]). Lysates were centrifuged at 14,000 × g for 10 min at 4°C. Supernatants were aliquoted and stored at −80°C.
Kinase assays.
For immunocomplex kinase assays, antibody to an avian-specific sequence or the HA epitope was added to cell lysates and incubated with rocking for 2 h at 4°C, with subsequent addition of inactivated Staphylococcus aureus or protein A-Sepharose for 30 min. Kinase assays were performed with glutathione-S-transferase (GST)-S6 substrate as described previously (9). Reactions were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and phosphorylation was quantitated on a Bio-Rad PhosphorImager. For in vitro kinase assays, GST-RSK D1 (K112R) (aa 1 to 385), D1′ (K112R) (aa 1 to 441), and D2 (K464R) (aa 386 to 752) and GST-PDK1 were purified from bacteria with glutathione-Sepharose beads. Activated GST-ERK1 was purified as described (15). Kinase assays were performed as described above.
Western blotting.
Lysates were subjected to SDS-PAGE on 7.5 or 10% acrylamide gels and transferred to nitrocellulose. Membrane was blocked with 3% milk–Tris-buffered saline–0.05% Tween 20 for 1 h. Primary antibody was incubated for 1 h at room temperature for polyclonal anti-avian epitope (1:5,000), polyclonal anti-MAPK (1:2,000), monoclonal anti-myc (1:2,000), and monoclonal anti-HA (1:60,000), or overnight at 4°C for polyclonal anti-c-Fos at 1:3,000 (UBI). Phosphospecific RSK antibodies were used according to the instructions of the manufacturer (UBI). Blots were washed with Tris-buffered saline–0.05% Tween 20 prior to incubation with horseradish peroxidase-conjugated anti-mouse (1:10,000), anti-rabbit (1:5,000), or anti-sheep (1:3,000) antibody. Blots were visualized by enhanced chemiluminescence.
Immunoprecipitations.
Lysates were incubated with the indicated antibody and either protein A-Sepharose (anti-HA, antiavian) or protein G-Sepharose (anti-myc) for 2 h at 4°C. Beads were washed twice with PBS–1% NP-40 + 4 (1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, pepstatin [5 μg/ml], and leupeptin [10 μg/ml]), and once in 10 mM Tris, 100 mM NaCl, 1 mM EDTA, pH 7.4.
GST pulldowns.
Cell lysates were incubated with 5 μg of GST-ERK on glutathione-S-Sepharose beads for 2 h at 4°C. Beads were washed twice with CLB + 5, and sample buffer was added. Pulldowns were subjected to SDS-PAGE and immunoblotting as described.
Confocal immunofluorescence.
HEK293E cells were transfected with WT RSK in pRK7 using Lipofectamine reagent (Life Technologies, Inc.). Four hours after transfection, cells were starved in serum-free medium for 16 h. Cells were washed twice in PBS, fixed with 4% p-formaldehyde in PBS for 20 min, permeabilized with 0.2% Triton X-100–5% goat serum–PBS for 5 min, and blocked in 10% goat serum in PBS for 1 h. The cells were then incubated with a 1:50 dilution of an affinity-purified anti-avian RSK antibody for 45 min and washed five times in PBS with 0.1% goat serum. Cells were subsequently incubated for 45 min with a 1:100 dilution of fluorescein-conjugated AffiniPure goat anti-rabbit immunoglobulin G (H+L) antibody (Jackson ImmunoResearch Laboratories, Inc.) and a 1:2,500 dilution of DAPI (1 mg/ml; Sigma Chemical Co.) and washed five times in PBS with 0.1% goat serum. Coverslips were mounted in Citifluor Mountant Media (Ted Pella, Inc.) to protect against photobleaching and viewed using a Zeiss LSM410 microscope with an epifluorescence detector.
RESULTS
It has been previously demonstrated that RSK requires at least two input signals to generate a fully active kinase: docking of ERK to the RSK C terminus and subsequent phosphorylation (18, 34), and phosphorylation of the N-terminal activation loop by PDK1 (23, 31) (Fig. 1). Recently, we generated an activated allele of avian RSK1 by encoding a myristylation sequence at the RSK N terminus (33). To investigate whether WT RSK does localize to the cell membrane, cells were transiently transfected with WT RSK and, after being quiesced by serum deprivation, stimulated with EGF. As shown in Fig. 2, RSK undergoes rapid and transient membrane localization prior to translocation into the nucleus. Maximal membrane localization is observed at 2 min, well before the maximal kinase activity seen between 10 and 15 min after mitogen stimulation (data not shown) (10), suggesting that this transient association occurs prior to full RSK activation. These data suggest that RSK association with the plasma membrane may be an important component of the activation process in order to generate a fully active RSK enzyme.
FIG. 2.
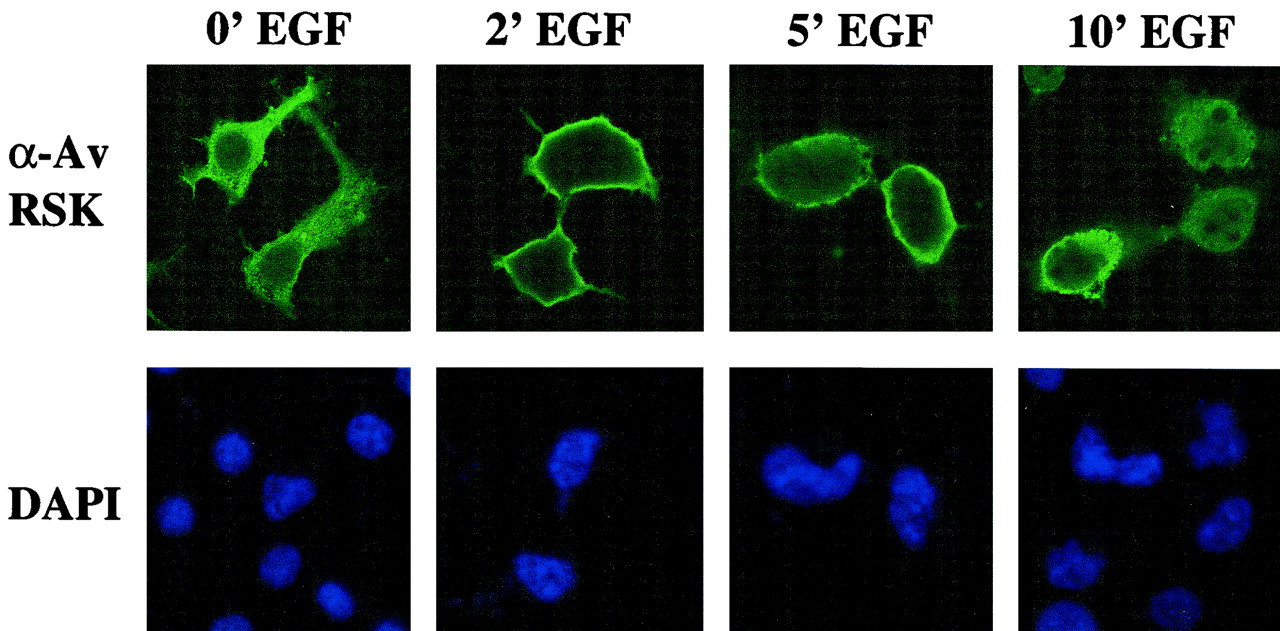
Effects of EGF stimulation on WT RSK localization in HEK293E cells. Cells were transfected with WT RSK in pRK7, starved for 16 h, and stimulated with EGF (50 ng/ml) for 0, 2, 5, and 10 min (from left to right, respectively) prior to washing and fixation. Cells were costained with DAPI and anti-Av RSK antibodies (top and bottom rows, respectively) and analyzed by immunofluorescence as described in Materials and Methods. Representative fields from confocal microscopy are shown.
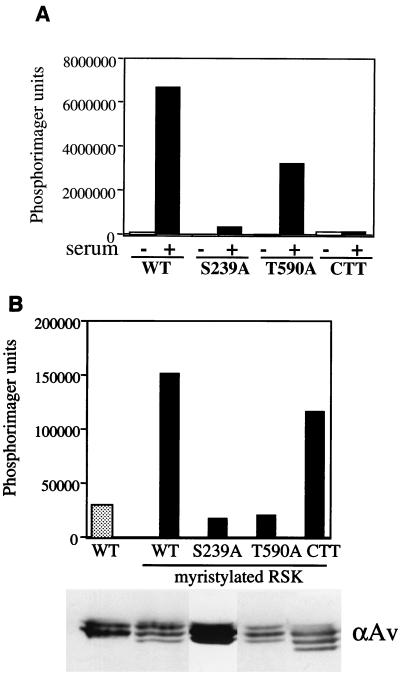
In light of this observation, we wished to address the implications for characterized events regulating RSK activation: RSK phosphorylation by ERK and PDK1, and ERK docking to the RSK C terminus. To mimic membrane localization, we encoded myristylation sequences at the N terminus of several RSK mutants and compared their basal activities with their unmyristylated counterparts (Fig. 3). Consistent with previous reports (14), mutating S239 to alanine (rat S222) abolished phosphotransferase activity of the RSK N-terminal kinase domain, as did truncating the C terminus (Fig. 3A). Mutating T590 to alanine (rat T574) resulted in a less-active RSK molecule, but it still retained some phosphotransferase activity toward GST-S6 substrate. When myristylated, S239A and T590A basal activities were unaffected compared to quiescent WT RSK (Fig. 3B). Surprisingly, the CTT RSK became active when myristylated, and the activation level was nearly equivalent to that of the myristylated WT RSK. These data confirm that the phosphorylation sites in RSK are important and that they may control macromolecule structure and/or protein-protein interactions. Once at the membrane, however, RSK does not require further ERK docking events for activation.
FIG. 3.
Myristylation can activate WT and a CTT RSK. (A) Kinase activity of HA-WT RSK and HA-RSK mutants in pKH3. HA-WT RSK and indicated mutants were expressed in HEK293E cells prior to stimulation (+) with 10% calf serum for 10 min. Cells were lysed and immunoprecipitated with anti-HA for kinase assays with GST-S6 substrate. PhosphorImager analysis was performed, and typical results are shown (n = 4). (B) Basal kinase activity of myristylated RSK proteins. HEK293E cells were transfected with indicated RSK plasmids in pRK7 and serum starved for 18 h prior to lysis. Immunoprecipitations were performed with anti-avian (αAv) antibody for kinase assays with GST-S6 substrate. Kinase activity was quantitated by PhosphorImager analysis. Typical results are shown (n = 2).
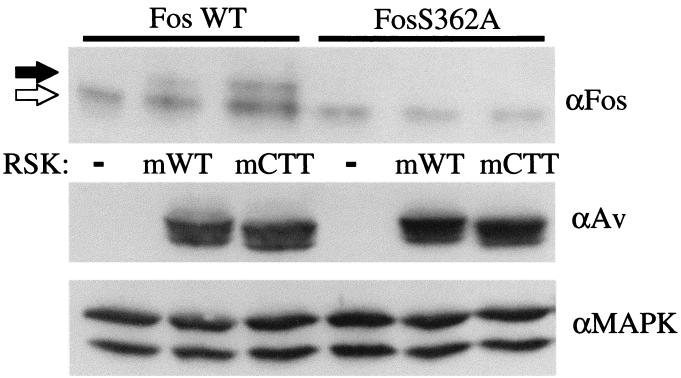
The myristylated WT (myrWT) RSK has been shown to promote cell survival in interleukin-3-dependent cells by phosphorylating the proapoptotic protein BAD, mimicking the MEK/ERK-MAPK survival signal (33). This result suggests that the myrWT RSK signals in vivo. It was not known, however, whether the myristylated CTT (myrCTT) RSK could signal in vivo; it is possible that ERK binding is necessary for downstream signaling. We therefore examined the ability of the myrWT and myrCTT RSK proteins to phosphorylate a known RSK substrate, c-Fos. RSK phosphorylates the transcription factor c-Fos on S362 (8), and phosphorylation of c-Fos protein at this site results in a mobility shift on SDS-PAGE. We coexpressed c-Fos with myrWT or myrCTT RSK or vector in the absence of serum stimulation (Fig. 4). Quiescence was indicated by immunoblotting for ERK-MAPK. With WT c-Fos, a mobility shift to the slower-migrating form was seen with both the myrWT and myrCTT RSK. That this shift was solely due to RSK phosphorylation was shown by the absence of c-Fos shifting in a c-Fos S362A mutant. Expression of transfected RSK proteins was shown by immunoblotting with the avian-specific antibody. These results led us to conclude that the myrWT RSK and the myrCTT RSK are capable of signaling to physiological targets. Therefore, ERK docking is not required for an activated RSK to phosphorylate this substrate in vivo.
FIG. 4.
myrWT RSK (mWT) and myrCTT RSK (mCTT) can phosphorylate RSK substrate in vivo. HEK293E cells were transfected with indicated plasmids and serum starved for 16 to 18 h. Cell lysates were subjected to Western blotting with anti-c-Fos (αFos) and anti-avian (αAv) antibodies. Unphosphorylated c-Fos is indicated with white arrow, and hyperphosphorylated c-Fos is indicated with black arrow (upper panel). Blotting with anti-MAPK (αMAPK) indicates that the cells are quiescent (n = 3).
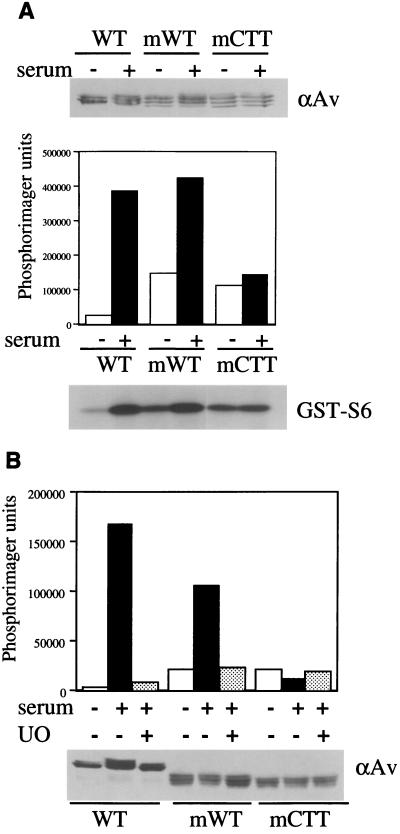
We then examined whether the myrCTT RSK could be further activated by mitogen stimulation. Unmyristylated and myrWT and myrCTT RSK were transiently expressed in HEK293E cells and subjected to serum stimulation. The WT and myrWT RSK activities are further activated by serum, but the myrCTT RSK was not affected (Fig. 5A). When S239A or T590A mutations were incorporated into myrCTT RSK, phosphotransferase activity was eliminated, reinforcing the importance of these sites in RSK activation (data not shown). The further activation of the myrWT RSK by serum was sensitive to UO126 pretreatment, returning the mitogen-stimulated activity to its elevated basal level (Fig. 5B). This result indicates that the additional activation is mediated by a UO126-sensitive target such as MEK1/2 and ERK1/2, and mitogen stimulation could be activating a cytoplasmic pool of the myrWT RSK; this myristylation sequence lacks a Src polybasic domain that anchors myristylated proteins at the membrane (30). Meanwhile, the myrCTT RSK activity was unaffected by the UO126 and mitogen treatment, consistent with the hypothesis that myrCTT activation is ERK-independent.
FIG. 5.
Myristylated CTT RSK is mitogen insensitive. (A) Mitogen sensitivity of WT and myristylated RSK. WT, myrWT, and myrCTT RSK in pRK7 were expressed in HEK293E cells and serum starved for 18 h prior to serum stimulation where indicated (+). Lysates were blotted with anti-avian (αAv) antibody to detect expression levels (upper panel). Immunoprecipitations were performed with αAv antibody for in vitro kinase assays using GST-S6 substrate. PhosphorImager analysis was performed, and typical results are shown (n = 3). (B) UO126 sensitivity of WT and myristylated RSK. Cells transfected with HA-WT RSK, myrWT RSK, or myrCTT RSK in pRK7 were serum starved for 18 h prior to 30 min of pretreatment with 5 μM UO126. Where indicated, cells were serum stimulated for 10 min prior to lysis. Immunoblotting was performed with αAv antibody (lower panel), as was immunoprecipitation for in vitro kinase assays (upper panel). PhosphorImager analysis was used to quantitate RSK activity toward GST-S6 (n = 3).
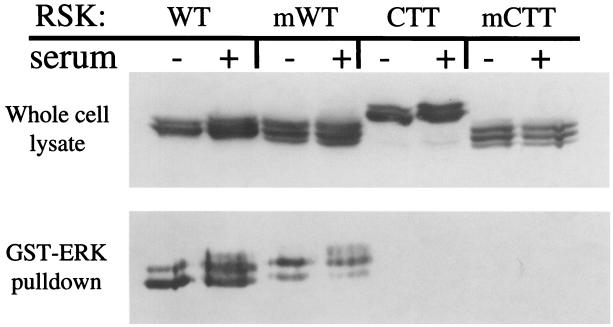
One explanation for the activation of this previously inactive CTT RSK is that by targeting it to the membrane, the myrCTT RSK undergoes a conformational change that would allow interaction with ERK, initiating activation. To test this possibility, we examined the interaction of these RSK constructs with ERK by GST-ERK binding. Cell lysates were prepared from cells expressing WT, myrWT, CTT, or myrCTT RSK, which had been starved or stimulated with serum as indicated. GST-ERK on glutathione-S-Sepharose beads was added to these lysates, and the subsequent bound proteins were examined by immunoblotting for RSK. As seen in Fig. 6, GST-ERK bound both the WT and myrWT constructs in the absence and presence of mitogen stimulation, but the CTT constructs did not interact with GST-ERK.
FIG. 6.
ERK does not bind CTT RSK. HEK293E cells were transfected with WT, myrWT (mWT), HA-CTT, or myrCTT (mCTT) RSK. Cells were serum-starved 16 to 18 h prior to serum stimulation where indicated (+). Whole-cell lysate was subjected to Western blotting with anti-avian (αAv) antibody (upper panel). Cell lysate was incubated with GST-ERK on glutathione Sepharose beads and washed as described in Materials and Methods. RSK association with GST-ERK was analyzed by Western blotting with αAv antibody (lower panel) (n = 3).
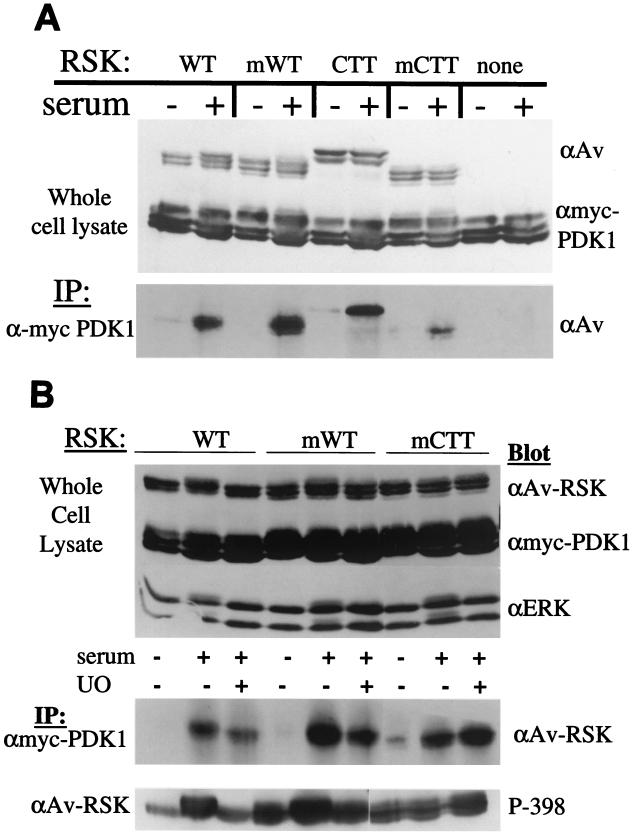
PDK1 activates several members of the AGC kinase family, including RSK (3). PDK1 interacts with RSK1 (31), and binds to RSK2 at a site analogous to the avian RSK S398 (17). Furthermore, this RSK-PDK1 interaction is reportedly dependent on the phosphorylation of this serine. Could the myrWT and myrCTT RSK proteins interact with PDK1 in the absence of mitogen stimulation and result in an activated RSK? To test this possibility we cotransfected PDK1 with WT, myrWT, CTT, and myrCTT RSK and analyzed RSK/PDK1 interaction in quiescent and serum-stimulated cells (Fig. 7A).
FIG. 7.
PDK1 association with WT, myrWT (mWT), CTT, and myrCTT (mCTT) RSK is mitogen regulated and MEK independent. (A) HEK293E cells were transfected with myc-PDK1 and WT, myrWT, HA-CTT, or myrCTT RSK where indicated. Cells were starved and stimulated where indicated (+). Cell lysates were subjected to Western blotting with anti-avian (αAv) antibody (upper panel) and anti-myc (αmyc) to detect myc-PDK1 expression (middle panel). Anti-myc PDK1 immunoprecipitations (IP) were blotted for RSK association with anti-avian antibody (lower panel) (n = 3). (B) RSK and PDK1 interactions do not require RSK activation. HEK239E cells were transfected as in panel A. Where indicated, cells were pretreated with 5 μM UO126 for 30 min prior to serum stimulation (UO). Cell lysates were immunoblotted as in panel A, with the addition of anti-ERK antibody blotting to measure MAPK-ERK pathway activation (upper panel). Anti-myc PDK1 immunoprecipitations were blotted for RSK association (middle panel) (n = 4), and anti-Av RSK immunoprecipitations were blotted with a phosphospecific antibody toward S398 (lower panel) (n = 2).
PDK1 interaction with WT, myrWT, CTT, and myrCTT RSK increased upon serum stimulation. Therefore, the lack of kinase activity in the CTT RSK is not due to an inability to bind PDK1, but is more likely a result of its inability to bind ERK. While there was a certain amount of experimental variation in the amount of interaction, basal interaction was similar for WT, myrWT, CTT, and myrCTT RSK, suggesting that the elevated kinase activities seen in the myrWT and myrCTT RSK were not due to basally increased PDK1 association as detected in this assay.
To further examine the RSK-PDK1 interaction, cells expressing different RSK proteins and PDK1 were subjected to UO126 pretreatment prior to serum stimulation. By preventing mitogen activation of MEK and ERK, WT RSK is not activated. Western blotting of whole-cell lysates indicates that UO126 treatment was effective, as seen by the lack of phosphorylation of ERK and WT RSK (Fig. 7B, top panel). Immunoprecipitation of myc-PDK1 and subsequent immunoblotting for RSK shows that RSK activation is not necessary for interaction with PDK1; PDK1 interacts with UO126-treated WT, myrWT, and myrCTT RSK (Fig. 7B, middle panel). Phosphorylation levels of S398 were monitored on WT, myrWT, and myrCTT RSK in cells coexpressing PDK1 (Fig. 7B, lower panel); phosphorylation of the analogous site in RSK2 has been reported to generate a PDK1 docking site (17). Although S398 phosphorylation levels fell for WT RSK upon UO126 pretreatment, PDK1 interaction levels appeared similar. On myrWT RSK, high levels of S398 phosphorylation were not sufficient to induce interaction in unstimulated cells. Finally, on myrCTT RSK, S398 phosphorylation levels remained similar but PDK1 interaction increased in serum-stimulated samples. These data suggest that although RSK S398 phosphorylation is necessary for interaction with PDK1 (17), it is not sufficient. A UO126-insensitive, mitogen-regulated event contributes to inducing interaction between PDK1 and RSK1.
Another possible explanation for the constitutive RSK activation in the absence of ERK binding is that the membrane localization allows phosphorylation of sites in the RSK linker region, a space between the two kinase domains containing three identified phosphorylation sites (Fig. 1). When either S381 (rat S363) or S398 (rat S380) is mutated to alanine, RSK phosphotransferase activity is abolished (14). Serine 381 is a proline-directed site and is postulated to be phosphorylated by ERK (14); serine 398 is believed to be an RSK autophosphorylation site (39). Using phosphospecific antibodies to these sites, we examined the phosphorylation state of WT, myrWT, CTT, and myrCTT RSK with and without serum stimulation.
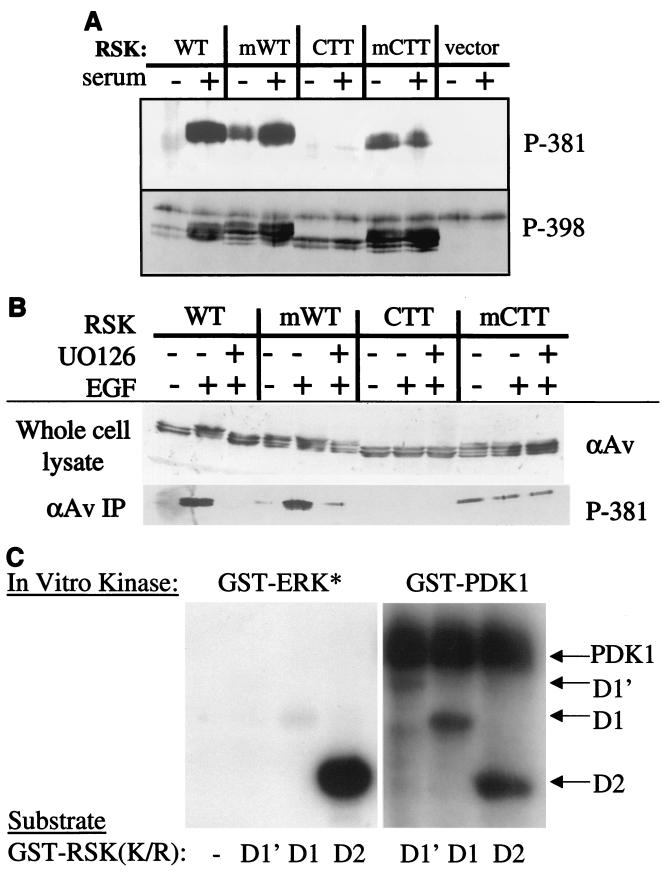
When these RSK proteins were analyzed for phosphorylation on S398 (Fig. 8A, lower panel), this important site exhibited basal phosphorylation on WT RSK in quiescent cells, with greatly increased phosphorylation upon serum stimulation. The myrWT had a high level of basal S398 phosphorylation, which increased somewhat upon exposure to serum, as was the case for S381. CTT RSK also exhibited some phosphorylation on S398. Unlike the WT RSK proteins, however, S398 phosphorylation on CTT RSK was not increased with serum stimulation. Finally, the myrCTT RSK was phosphorylated on S398 in both quiescent and serum-stimulated cells, although the phosphorylation level did not change significantly with serum. Interestingly, phosphorylation of this site in the CTT RSK proteins was in the absence of ERK docking to the C terminus, suggesting that phosphorylation of this important regulatory site does not require ERK docking. Furthermore, the lack of phosphotransferase activity in the CTT RSK even with S398 phosphorylation implies that phosphorylation of this site is not sufficient for activation.
FIG. 8.
(A) myrWT (mWT) and myrCTT (mCTT) RSK proteins have increased S381 and S398 basal phosphorylation. HEK293E cells were transfected with indicated plasmids in pRK7 and serum starved for 16 to 18 h before serum stimulation where indicated (+). Cell lysates were immunoprecipitated with anti-avian (αAv) antibody and subjected to Western blotting with indicated RSK phosphospecific antibodies toward avian RSK1 serine 381 (rat 222) and serine 398 (rat 381). The uppermost band in the lower, P-398 blot is nonspecific (n = 3). (B) Serine 381 phosphorylation is insensitive to UO126 in myristylated RSK proteins. HEK293E cells were transfected and starved as in panel A prior to pretreatment with UO126 and EGF (50 ng/ml). Cell lysates were treated as in panel A (n = 3). (C) ERK1 does not phosphorylate the RSK N terminus. Indicated GST-RSK constructs (1 μg) were incubated with either activated GST-ERK or GST-PDK1 in an in vitro kinase assay for 15 min prior to reaction termination. 32P[ATP] incorporation was visualized by autoradiography (n = 2).
The phosphorylation state of S381 was also analyzed. As seen in Fig. 8A (upper panel), S381 was phosphorylated in WT RSK upon serum stimulation. This residue was also phosphorylated on unstimulated myrWT RSK in the absence of ERK activation, and the level of phosphorylation increased upon exposure to mitogen. Phosphorylation was reduced to basal levels in cells pretreated with the MEK inhibitor UO126 (Fig. 8B). The CTT RSK was not phosphorylated on S381 under these conditions. However, the myrCTT RSK was phosphorylated at equivalent levels in both unstimulated and serum-stimulated cells (Fig. 8A). UO126 had no effect on the S381 phosphorylation of the myrCTT protein (Fig. 8B). The elevated basal S381 phosphorylation on the myristylated WT and myristylated CTT RSK suggests that the membrane targeting brings RSK into proximity with a S381 kinase, abrogating the mitogen requirement for S381 phosphorylation. The increased S381 phosphorylation on serum-stimulated myrWT RSK could be a result of additional ERK docking and phosphorylation of the myrWT RSK, or it could be due to recruitment of cytoplasmic myrWT RSK to the membrane. However, this presumably is not the case with myrCTT RSK, as it lacks the identified ERK docking site and does not bind GST-ERK (Fig. 6). The phosphorylation of S381 in myrCTT RSK is independent of ERK docking to the C-terminal ERK docking site but is dependent on membrane targeting, for the unmyristylated CTT RSK is not phosphorylated.
The serine 381 site (rat S364) is a mitogen-regulated proline-directed site that is sensitive to the MEK inhibitor PD098059, and it has been suggested that this site is phosphorylated by ERK1/2 (14). To examine whether ERK1 directly phosphorylates any residues in the RSK N terminus, in vitro kinase assays were performed using GST-RSK fusion proteins as substrates for active GST-ERK1 (Fig. 8C); these proteins contain K-to-R mutations in the ATP-binding site of the kinase domain to eliminate autophosphorylation. RSK D1 encodes aa 1 to 385 and contains two putative ERK phosphorylation sites: T377 (rat T360) and S381 (rat S364). D1′ (aa 1 to 441) extends to the beginning of the C-terminal kinase domain, and includes the S398 (rat S381) autophosphorylation site. D2 encodes the C-terminal half of RSK (aa 386 to 752), and contains S398 of the linker region, but not T377 or S381. It does contain the characterized ERK phosphorylation site, T590 (rat T574) and is used as a positive control for GST-ERK activity. Activated GST-ERK1 phosphorylates GST-RSK D2, but not D1 or D1′ (left panel). GST-RSK D1 and D1′ are capable of being phosphorylated in vitro, as demonstrated by their phosphorylation by GST-PDK1 (right panel). Serine 239 (rat S222) is phosphorylated by PDK1 in these constructs (31). GST-RSK D2 is also phosphorylated by PDK1 in vitro on S398 (rat S381) (right panel; data not shown). This result suggests that S381 and T377 are not direct ERK1 phosphorylation sites in vitro or in vivo.
DISCUSSION
RSK has been implicated in multiple cellular processes, including cell cycle regulation, transcriptional regulation, and cell survival. Understanding the regulation of this multifaceted kinase will provide insight into how it executes in vivo effects. It has been previously demonstrated that phosphorylation at multiple residues is an important component of this regulation, particularly autophosphorylation and phosphorylation by PDK1 and ERK.
In this manuscript, we suggest that RSK activation is dependent on a combination of phosphorylation and localization events. We demonstrate that when stimulated with mitogen, RSK undergoes relocalization from the cytoplasm to the cell membrane, prior to translocation into the nucleus. Consistent with a role for membrane localization in the RSK activation process, we have found that myristylation results in constitutive RSK activation. Using membrane-targeted myristylated RSK proteins, we have endeavored to identify ERK-mediated events for RSK activation. Activation of RSK by targeting it to the membrane is independent of ERK activity and ERK docking, based on the phosphotransferase activity of a myristylated RSK that has the ERK docking site deleted (myrCTT). This constitutive RSK activation is not due to increased basal interaction with PDK1, an essential activating kinase for RSK. The RSK-PDK1 interaction does not require RSK activation by ERK, as the interaction is insensitive to the MEK inhibitor UO126. Interaction between RSK1 and PDK1 is therefore necessary, but not sufficient, for RSK1 activation, and this interaction does not require active ERK. Unmyristylated CTT RSK is not phosphorylated at a key residue, S381 (rat S364), but myristylated RSK proteins are basally phosphorylated on S381 in quiescent cells. Therefore, the S381 kinase may not be ERK1/2, as has been proposed (14). We also show that the constitutively active myrWT and myrCTT RSKs can induce phosphorylation of c-Fos, suggesting in vivo activity that is independent of ERK activity or binding. We propose, however, that ERK does play an important role in early RSK mitogen-activated events.
There is precedent for membrane localization playing a role in RSK regulation; endogenous RSK associates with the membrane fraction of HeLa cells, and an increase in the amount at the membrane was observed upon serum stimulation (12). This translocation to the membrane from the cytoplasm could be an intermediary activation step on RSK's road to the nucleus. Our observation that a kinase-inactive RSK mutant can be constitutively activated when targeted to the membrane, and that this constitutively active mutant can signal similarly to the myristylated WT RSK protein, supports this supposition. Furthermore, membrane-targeted activation can bypass the requirement for ERK docking.
How might RSK translocate to the membrane? We believe that the constitutive activation of the myrCTT RSK gives a hint. Neither the CTT nor the myrCTT RSK binds ERK, yet the CTT RSK is kinase inactive, and in quiescent cells myrCTT RSK possesses basal activity at a level nearly equivalent to that of WT RSK protein. We postulate that ERK could play a role in escorting RSK to the membrane. Indeed, ERK1 has been found to translocate to the membrane in serum-stimulated cells (19). Since the CTT RSK cannot bind ERK, it is not escorted to the proper membrane complex and is not activated. When myristylated, however, the myrCTT RSK is brought into proximity with the proper complex at the membrane; the requirement for ERK docking is abrogated. This function for ERK could be separate from its role as an T590 kinase, as the myristylation of the T590A RSK mutant does not result in constitutive RSK activity. This was true for all RSK phosphorylation site point mutants examined (Fig. 3B; data not shown), underscoring the already established importance of the phosphorylation sites. They may have important structural roles and may also determine important protein/protein interactions.
Serine 381 (rat 364) is essential for RSK activation, as mutation to an alanine residue abolishes RSK phosphotransferase activity (14). This is a proline-directed site shown to be sensitive to treatment with the MEK inhibitor PD98059, and has generally been thought to be phosphorylated by ERK1/2 (14). S381 phosphorylation increases on WT RSK when cells are mitogen stimulated, but S381 is not phosphorylated at all in CTT RSK. Therefore, S381 phosphorylation on WT RSK requires the C-terminal ERK docking site. We have also found that S381 is phosphorylated in both myrWT and myrCTT RSK in quiescent cells. Therefore, S381 phosphorylation does not require ERK1/2 activation. We cannot rule out the possibility that the in vivo serine 381 kinase may be found at the plasma membrane, and it may be a proline-directed kinase other than ERK1/2. The S381 kinase could be sensitive to PD98059; the sensitivity of ERK5/BMK1 to PD98059 and UO126 indicates that these agents are not MEK1/2 specific (25). Alternatively, the sensitivity of S381 to PD98059 may be the result of an inability of ERK to promote a competent RSK signaling complex at the membrane. Myristylation could be bringing RSK into proximity with this kinase, inducing RSK serine 381 phosphorylation and RSK activation.
Our finding that S398 is phosphorylated in each RSK construct in this study has interesting implications. Like serine 381, this site is also critical for activation of the RSK N-terminal kinase domain and phosphorylation of exogenous substrates (14). When the ERK site in the RSK C-terminal kinase domain activation loop is mutated to alanine (avian RSK1 T590), the hydrophobic serine in the linker region (analogous to avian RSK1 S398) is poorly phosphorylated upon cell stimulation, consistent with a hierarchical phosphorylation sequence of events in vivo (14, 23). Serine 398 is believed to be an autophosphorylation site, with phosphorylation by an active RSK C-terminal kinase domain (39). PDK1 can also phosphorylate RSK S398 in vitro (data not shown), and can induce S398 phosphorylation in RSK2 when PDK1 and RSK2 are coexpressed in cells (23). This site also fits the consensus for the described PDK2 activity (1). Serine 398 also sits within the identified consensus sequence for phosphorylation by the RSK N-terminal kinase domain, allowing the possibility that this is an autophosphorylation site for that domain rather than for the C-terminal kinase domain. In Akt/PKB and the conventional protein kinase C, also members of the AGC family of kinases, this motif is an autophosphorylation site (2, 38). Therefore, the identity of the in vivo kinase for S398 in RSK remains unclear.
Given the increased S398 phosphorylation, particularly in the myristylated RSK proteins in quiescent cells, we considered whether PDK1 might be responsible for the constitutive activation of membrane-associated RSK. This was not the case. Despite the increased basal S398 phosphorylation, the interaction between RSK and PDK1 was mitogen regulated and was similar for WT and CTT RSK constructs. Therefore, PDK1 interaction with RSK does not require ERK docking to RSK. The RSK-PDK1 interaction was also insensitive to treatment with the MEK inhibitor UO126, consistent with the finding that RSK activation is not necessary to support this interaction. Furthermore, RSK S398 phosphorylation is not sufficient for PDK1 interaction with RSK1 (Fig. 7B), suggesting that additional inputs are required. Phosphorylated S398 has been described as a PDK1 docking site on RSK2, and RSK2-PDK1 interaction is observed after a brief starvation period (17). The differences observed here may be attributed to potential differences in RSK1 and RSK2 regulation. PDK1 alone can induce a substantial activation of RSK2 (23), but this is not the case for RSK1, which requires cooperation with the MEK/ERK-MAPK pathway (23, 31). Furthermore, mutation of avian RSK1 tyrosine 719 to alanine has no effect on basal RSK1 activity (data not shown); in RSK2, the analogous Y707A mutation induces increased basal RSK2 activity (29). Differences in the regulation of the different RSK isoforms may reflect differences in their cellular functions.
Based on these findings, we propose a model for RSK trafficking and activation (Fig. 9). ERK is associated with RSK through the docking site at the RSK C terminus (18, 22, 34). Mitogen stimulation induces two related ERK-dependent events. First, ERK phosphorylates RSK at T590, relieving the negative regulation by the RSK C terminus. This allows phosphorylation of S398. Second, the RSK-ERK complex moves to the plasma membrane. There, ERK would associate with an as yet uncharacterized complex, possibly including Ras, MEK, Raf, and other RSK proteins. At the membrane, serine 381 would become phosphorylated. RSK would also associate with PDK1, which would phosphorylate S239 in the RSK N-terminal kinase domain activation loop. Active RSK would dissociate from the membrane complex, travel through the cytoplasm and accumulate in the nucleus, where it would phosphorylate nuclear substrates such as c-Fos, CREB, and Mi (8, 41, 42). According to this model, if ERK is unable to bind to RSK, translocation and activation would never occur. Furthermore, if RSK were targeted to the membrane, the initial ERK-dependent translocation steps would be bypassed.
FIG. 9.
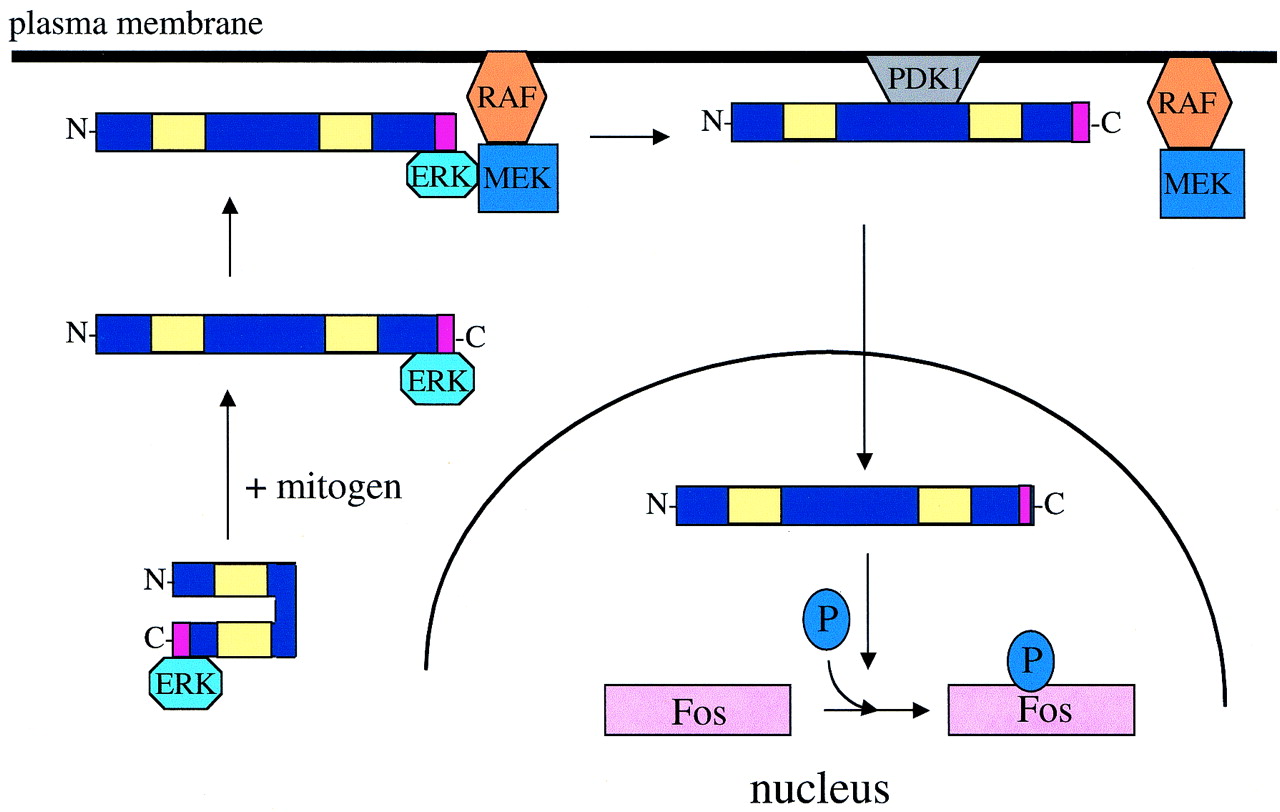
Model of RSK activation. Quiescent cytoplasmic RSK moves to the plasma membrane upon mitogen stimulation. Proper localization and association are determined by ERK, which docks at the RSK C terminus. Once at the membrane, PDK1 docks at phosphoserine 398 and induces phosphorylation of the RSK N terminus at serine 239. RSK then translocates through the cytoplasm, across the nuclear membrane, and phosphorylates nuclear substrates.
These findings suggest that regulation of RSK phosphotransferase activity is more complex than previously thought. The movement of RSK from the cytoplasm to the membrane to the nucleus adds to the complexity of this regulation, but could also better safeguard the cell from inappropriate RSK activation by increasing possible regulatory checkpoints. Given the number and diversity of identified RSK substrates, this complex activation process may lend specificity to different mitogens, substrate phosphorylation, and cellular responses. The exact mechanism that RSK employs to travel throughout the cell and the nature of the protein complexes with which RSK associates will be important to characterize. Clearly, future work dissecting RSK's regulation and cellular functions is necessary and will be exciting.
ACKNOWLEDGMENTS
We are deeply grateful to all members of the Blenis laboratory for helpful and insightful discussions, and particularly to Sue Ann Woo for the GST-ERK.
This work was supported by the Leukemia and Lymphoma Society of America (S.A.R.), the American Cancer Society (L.O.M.), and NIH grant RO1 CA46595 (J.B.).
REFERENCES
- 1.Balendran A, Casamayor A, Deak M, Paterson A, Gaffney P, Currie R, Downes C P, Alessi D R. PDK1 acquires PDK2 activity in the presence of a synthetic peptide derived from the carboxyl terminus of PRK2. Curr Biol. 1999;9:393–404. doi: 10.1016/s0960-9822(99)80186-9. [DOI] [PubMed] [Google Scholar]
- 2.Behn-Krappa A, Newton A C. The hydrophobic phosphorylation motif of conventional protein kinase C is regulated by autophosphorylation. Curr Biol. 1999;9:728–737. doi: 10.1016/s0960-9822(99)80332-7. [DOI] [PubMed] [Google Scholar]
- 3.Belham C, Wu S, Avruch J. Intracellular signalling: PDK1—a kinase at the hub of things. Curr Biol. 1999;9:R93–R96. doi: 10.1016/s0960-9822(99)80058-x. [DOI] [PubMed] [Google Scholar]
- 4.Bhatt R R, Ferrell J E., Jr The protein kinase p90 rsk as an essential mediator of cytostatic factor activity. Science. 1999;286:1362–1365. doi: 10.1126/science.286.5443.1362. [DOI] [PubMed] [Google Scholar]
- 5.Bonni A, Brunet A, West A E, Datta S R, Takasu M A, Greenberg M E. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999;286:1358–1362. doi: 10.1126/science.286.5443.1358. [DOI] [PubMed] [Google Scholar]
- 6.Buck M, Poli V, van der Geer P, Chojkier M, Hunter T. Phosphorylation of rat serine 105 or mouse threonine 217 in C/EBP beta is required for hepatocyte proliferation induced by TGF alpha. Mol Cell. 1999;4:1087–1092. doi: 10.1016/s1097-2765(00)80237-3. [DOI] [PubMed] [Google Scholar]
- 7.Chan T O, Rittenhouse S E, Tsichlis P N. AKT/PKB and other D3 phosphoinositide-regulated kinases: kinase activation by phosphoinositide-dependent phosphorylation. Annu Rev Biochem. 1999;68:965–1014. doi: 10.1146/annurev.biochem.68.1.965. [DOI] [PubMed] [Google Scholar]
- 8.Chen R-H, Abate C, Blenis J. Phosphorylation of the c-Fos transrepression domain by mitogen-activated protein kinase and 90-kDa ribosomal S6 kinase. Proc Natl Acad Sci USA. 1993;90:10952–10956. doi: 10.1073/pnas.90.23.10952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen R-H, Blenis J. Identification of Xenopus S6 protein kinase homologs (pp90rsk) in somatic cells: phosphorylation and activation during initiation of cell proliferation. Mol Cell Biol. 1990;10:3204–3215. doi: 10.1128/mcb.10.6.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen R-H, Chung J, Blenis J. Regulation of pp90rsk phosphorylation and S6 phosphotransferase activity in Swiss 3T3 cells by growth factor-, phorbol ester-, and cyclic AMP-mediated signal transduction. Mol Cell Biol. 1991;11:1861–1867. doi: 10.1128/mcb.11.4.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen R H, Juo P C, Curran T, Blenis J. Phosphorylation of c-Fos at the C-terminus enhances its transforming activity. Oncogene. 1996;12:1493–1502. [PubMed] [Google Scholar]
- 12.Chen R H, Sarnecki C, Blenis J. Nuclear localization and regulation of erk- and rsk-encoded protein kinases. Mol Cell Biol. 1992;12:915–927. doi: 10.1128/mcb.12.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chou M M, Hou W, Johnson J, Graham L K, Lee M H, Chen C S, Newton A C, Schaffhausen B S, Toker A. Regulation of protein kinase C zeta by PI 3-kinase and PDK-1. Curr Biol. 1998;8:1069–1077. doi: 10.1016/s0960-9822(98)70444-0. [DOI] [PubMed] [Google Scholar]
- 14.Dalby K N, Morrice N, Caudwell F B, Avruch J, Cohen P. Identification of regulatory phosphorylation sites in mitogen-activated protein kinase (MAPK)-activated protein kinase-1a/p90rsk that are inducible by MAPK. J Biol Chem. 1998;273:1496–1505. doi: 10.1074/jbc.273.3.1496. [DOI] [PubMed] [Google Scholar]
- 15.Fisher T L, Blenis J. Evidence for two catalytically active kinase domains in pp90rsk. Mol Cell Biol. 1996;16:1212–1219. doi: 10.1128/mcb.16.3.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frodin M, Gammeltoft S. Role and regulation of 90 kDa ribosomal S6 kinase (RSK) in signal transduction. Mol Cell Endocrinol. 1999;151:65–77. doi: 10.1016/s0303-7207(99)00061-1. [DOI] [PubMed] [Google Scholar]
- 17.Frodin M, Jensen C J, Merienne K, Gammeltoft S. A phosphoserine-regulated docking site in the protein kinase RSK2 that recruits and activates PDK1. EMBO J. 2000;19:2924–2934. doi: 10.1093/emboj/19.12.2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gavin A C, Nebreda A R. A MAP kinase docking site is required for phosphorylation and activation of p90(rsk)/MAPKAP kinase-1. Curr Biol. 1999;9:281–284. doi: 10.1016/s0960-9822(99)80120-1. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez F A, Seth A, Raden D L, Bowman D S, Fay F S, Davis R J. Serum-induced translocation of mitogen-activated protein kinase to the cell surface ruffling membrane and the nucleus. J Cell Biol. 1993;122:1089–1101. doi: 10.1083/jcb.122.5.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gross S D, Schwab M S, Lewellyn A L, Maller J L. Induction of metaphase arrest in cleaving Xenopus embryos by the protein kinase p90Rsk. Science. 1999;286:1365–1367. doi: 10.1126/science.286.5443.1365. [DOI] [PubMed] [Google Scholar]
- 21.Gross S D, Schwab M S, Taieb F E, Lewellyn A L, Qian Y W, Maller J L. The critical role of the MAP kinase pathway in meiosis II in Xenopus oocytes is mediated by p90(Rsk) Curr Biol. 2000;10:430–438. doi: 10.1016/s0960-9822(00)00425-5. [DOI] [PubMed] [Google Scholar]
- 22.Hsiao K M, Chou S Y, Shih S J, Ferrell J E., Jr Evidence that inactive p42 mitogen-activated protein kinase and inactive Rsk exist as a heterodimer in vivo. Proc Natl Acad Sci USA. 1994;91:5480–5484. doi: 10.1073/pnas.91.12.5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jensen C J, Buch M B, Krag T O, Hemmings B A, Gammeltoft S, Frodin M. 90-kDa ribosomal S6 kinase is phosphorylated and activated by 3-phosphoinositide-dependent protein kinase-1. J Biol Chem. 1999;274:27168–27176. doi: 10.1074/jbc.274.38.27168. [DOI] [PubMed] [Google Scholar]
- 24.Joel P B, Smith J, Sturgill T W, Fisher T L, Blenis J, Lannigan D A. pp90rsk1 regulates estrogen receptor-mediated transcription through phosphorylation of Ser-167. Mol Cell Biol. 1998;18:1978–1984. doi: 10.1128/mcb.18.4.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamakura S, Moriguchi T, Nishida E. Activation of the protein kinase ERK5/BMK1 by receptor tyrosine kinases. Identification and characterization of a signaling pathway to the nucleus. J Biol Chem. 1999;274:26563–26571. doi: 10.1074/jbc.274.37.26563. [DOI] [PubMed] [Google Scholar]
- 26.Mattingly R R, Sorisky A, Brann M R, Macara I G. Muscarinic receptors transform NIH 3T3 cells through a Ras-dependent signalling pathway inhibited by the Ras-GTPase-activating protein SH3 domain. Mol Cell Biol. 1994;14:7943–7952. doi: 10.1128/mcb.14.12.7943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakajima T, Fukamizu A, Takahashi J, Gage F H, Fisher T, Blenis J, Montminy M R. The signal-dependent coactivator CBP is a nuclear target for pp90Rsk. Cell. 1996;86:465–474. doi: 10.1016/s0092-8674(00)80119-1. [DOI] [PubMed] [Google Scholar]
- 28.Palmer A, Gavin A C, Nebreda A R. A link between MAP kinase and p34(cdc2)/cyclin B during oocyte maturation: p90(rsk) phosphorylates and inactivates the p34(cdc2) inhibitory kinase Myt1. EMBO J. 1998;17:5037–5047. doi: 10.1093/emboj/17.17.5037. . (Erratum, 18:1092, 1999.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poteet-Smith C E, Smith J A, Lannigan D A, Freed T A, Sturgill T W. Generation of constitutively active p90 ribosomal S6 kinase in vivo. Implications for the mitogen-activated protein kinase-activated protein kinase family. J Biol Chem. 1999;274:22135–22138. doi: 10.1074/jbc.274.32.22135. [DOI] [PubMed] [Google Scholar]
- 30.Resh M D. Fatty acylation of proteins: new insights into membrane targeting of myristoylated and palmitoylated proteins. Biochim Biophys Acta. 1999;1451:1–16. doi: 10.1016/s0167-4889(99)00075-0. [DOI] [PubMed] [Google Scholar]
- 31.Richards S A, Fu J, Romanelli A, Shimamura A, Blenis J. Ribosomal S6 kinase 1 (RSK1) activation requires signals dependent on and independent of the MAP kinase ERK. Curr Biol. 1999;9:810–820. doi: 10.1016/s0960-9822(99)80364-9. [DOI] [PubMed] [Google Scholar]
- 32.Sassone-Corsi P, Mizzen C A, Cheung P, Crosio C, Monaco L, Jacquot S, Hanauer A, Allis C D. Requirement of Rsk-2 for epidermal growth factor-activated phosphorylation of histone H3. Science. 1999;285:886–891. doi: 10.1126/science.285.5429.886. [DOI] [PubMed] [Google Scholar]
- 33.Shimamura A, Ballif B A, Richards S A, Blenis J. Rsk1 mediates a MEK-MAP kinase cell survival signal. Curr Biol. 2000;10:127–135. doi: 10.1016/s0960-9822(00)00310-9. [DOI] [PubMed] [Google Scholar]
- 34.Smith J A, Poteet-Smith C E, Malarkey K, Sturgill T W. Identification of an extracellular signal-regulated kinase (ERK) docking site in ribosomal S6 kinase, a sequence critical for activation by ERK in vivo. J Biol Chem. 1999;274:2893–2898. doi: 10.1074/jbc.274.5.2893. [DOI] [PubMed] [Google Scholar]
- 35.Sutherland C, Campbell D G, Cohen P. Identification of insulin-stimulated protein kinase-1 as the rabbit equivalent of rskmo-2: identification of two threonines phosphorylated during activation by mitogen-activated protein kinase. Eur J Biochem. 1993;212:581–588. doi: 10.1111/j.1432-1033.1993.tb17696.x. [DOI] [PubMed] [Google Scholar]
- 36.Takahashi E, Abe J, Gallis B, Aebersold R, Spring D J, Krebs E G, Berk B C. p90(RSK) is a serum-stimulated Na+/H+ exchanger isoform-1 kinase. Regulatory phosphorylation of serine 703 of Na+/H+ exchanger isoform-1. J Biol Chem. 1999;274:20206–20214. doi: 10.1074/jbc.274.29.20206. [DOI] [PubMed] [Google Scholar]
- 37.Tan Y, Ruan H, Demeter M R, Comb M J. p90(RSK) blocks bad-mediated cell death via a protein kinase C-dependent pathway. J Biol Chem. 1999;274:34859–34867. doi: 10.1074/jbc.274.49.34859. [DOI] [PubMed] [Google Scholar]
- 38.Toker A, Newton A C. Akt/protein kinase B is regulated by autophosphorylation at the hypothetical PDK-2 site. J Biol Chem. 2000;275:8271–8274. doi: 10.1074/jbc.275.12.8271. [DOI] [PubMed] [Google Scholar]
- 39.Vik T A, Ryder J W. Identification of serine 380 as the major site of autophosphorylation of Xenopus pp90rsk. Biochem Biophys Res Commun. 1997;235:398–402. doi: 10.1006/bbrc.1997.6794. [DOI] [PubMed] [Google Scholar]
- 40.Williams M R, Arthur J S, Balendran A, van der Kaay J, Poli V, Cohen P, Alessi D R. The role of 3-phosphoinositide-dependent protein kinase 1 in activating AGC kinases defined in embryonic stem cells. Curr Biol. 2000;10:439–448. doi: 10.1016/s0960-9822(00)00441-3. [DOI] [PubMed] [Google Scholar]
- 41.Wu M, Hemesath T J, Takemoto C M, Horstmann M A, Wells A G, Price E R, Fisher D Z, Fisher D E. c-Kit triggers dual phosphorylations, which couple activation and degradation of the essential melanocyte factor Mi. Genes Dev. 2000;14:301–312. [PMC free article] [PubMed] [Google Scholar]
- 42.Xing J, Ginty D D, Greenberg M E. Coupling of the RAS-MAPK pathway to gene activation by RSK2, a growth factor-regulated CREB kinase. Science. 1996;273:959–963. doi: 10.1126/science.273.5277.959. [DOI] [PubMed] [Google Scholar]