Abstract
BACKGROUND:
One fifth of US counties are designated persistent child poverty counties (≥20% of children in poverty since 1980). The association between a persistent child poverty environment and mortality in children with cancer is unknown.
METHODS:
Our cohort includes 2,089 children with cancer (2000–2016) in Alabama. We used multivariable cox proportional hazards modeling (adjusted for sociodemographics/clinical characteristics) to assess mortality by persistent child poverty designation at 1, 5 and 10 years from diagnosis. Distance to treatment was subsequently explored.
RESULTS:
Forty-two percent of the cohort lived in a persistent child poverty county; they were more likely to be African American (P<0.0001), have public/no insurance (P=0.0009), and live >100 miles to treatment (P<0.0001). Children in persistent child poverty counties were 30% more likely to die by 5 years (95%CI=1.06–1.59, P=0.012). Distance (per 20-mile increase) to treatment was associated with a 9% increased mortality risk (P<0.0001). Children with both exposures (distance >100 miles and persistent child poverty) faced the highest mortality risk at 5 years (HR=1.80, 95%CI=1.39–2.33, P<0.0001). In sub-analysis, children exposed to persistent child poverty were at higher risk for cancer-related mortality. However, the risk of health-related mortality did not differ.
CONCLUSION:
Among children with cancer from the Deep South, persistent child poverty was a prevalent exposure associated with inferior overall survival. Distance to treatment was independently associated with inferior survival. Children with both exposures had the highest risk of mortality.
IMPACT:
Persistent child poverty is associated with inferior survival among children with cancer; mechanisms underlying this disparity warrant investigation.
Keywords: Poverty, child, adolescent, cancer, social determinants of health
INTRODUCTION
Survival rates for children with cancer have improved dramatically over the last 4 decades; currently 84% of children diagnosed with cancer will survive 5 years and beyond.1 These improvements are due to risk-based treatment in the setting of clinical trials, accompanied by effective supportive care.2,3 However, these improvements have not been shared equitably among all; social determinants of health (including individual-level poverty) contribute to the outcome disparities observed by children with cancer.4,5
Both individual-level and area-level poverty are associated with adverse outcomes among adults with cancer.6,7 One consideration with regards to area-level poverty is its persistence over time.8 Counties with persistent poverty face unique risk factors when compared with counties without persistent poverty, such as greater unemployment, lower educational attainment, and a much younger population.9 In the US, counties with persistent poverty have significantly higher adult cancer mortality rates when compared with counties without poverty, or with current (but not persistent) poverty.6 Observed disparities associated with persistent poverty are likely multifactorial including structural, institutional and individual level risk factors.6
The United States Department of Agriculture (USDA) has an area-level persistent poverty measure specific to children, termed “persistent child poverty” which is defined as counties in which ≥20% of children in the county were poor since 1980.10 This designation applies to 708 counties in the US (one fifth of all US counties, but 49% of counties in the state of Alabama).10 Although this exposure is prevalent, the impact of a persistent child poverty environment on the outcomes experienced by children with cancer is unknown. Further, possible mechanisms (e.g., access to care) through which persistent child poverty may impact outcomes in children with cancer have not been explored. In this study, we test the a priori hypothesis that exposure to a persistent child poverty environment is associated with inferior overall survival in children diagnosed with cancer in the state of Alabama.
MATERIALS AND METHODS
Alabama is home to 3 Children’s Hospitals, all Children’s Oncology Group sites (Children’s of Alabama, Huntsville Hospital for Women & Children, University of South Alabama Children’s and Women’s Hospital), and all available sites participated in the present study. Patients ≤19 years and diagnosed with cancer between 2000 and 2016 were included. All sites obtained local Institutional Review Board approval to submit their data as maintained for the state cancer registry. Patients lacking any of the following (not mutually exclusive) were excluded: cancer diagnosis code in accordance with the International Classification of Childhood Cancer11,12 (N=18, 0.9%), those lacking a vital status (N=7, 0.3%), and those with residence outside of Alabama (N=20, 1%).
Persistent child poverty was defined as outlined by the USDA as counties where ≥20% of related children <18y were poor as measured by three consecutive decennial censuses (1980, 1990, 2000) and the American Community Survey 5-year estimates (2007–2011).10,13 Patients were classified as exposed to a persistent child poverty county based on the county of residence at cancer diagnosis using Federal Information Processing Standard (FIPS) county codes.
Sociodemographic (sex, race/ethnicity, insurance status) and clinical (date of diagnosis, age at diagnosis, cancer site, histology, grade and stage) characteristics were reported at the patient-level by participating institutions. Primary payer at diagnosis was reported by participating institutions and categorized as: No Insurance/Public (uninsured, self-pay and Medicaid), Private and Insured-type unknown (those who had insurance at diagnosis but did not report the specific payer). Cancer type was determined using the International Classification of Childhood Cancer guidelines (relying on histology, primary site and grade).11,12 Cancer types were grouped together as appropriate when the sample size per individual cancer type was inadequate (Supplementary Table 1).
Because most persistent child poverty counties are in the southern half of Alabama (distant from the largest children’s hospital in Jefferson County, Supplementary Figure 1), distance to treating center (as a measure of healthcare access) was evaluated. Distance to treating center was calculated as a sphere distance (closest distance between two points on earth) measured between the center of the patient’s county of residence and the center of the treating hospital’s county. Distance was treated as a continuous variable and a categorical variable in quintiles (distance quintile in our cohort was ~20 miles). Those living >100 miles had an increased risk of mortality in our preliminary analyses so we further dichotomized distance as >100 vs. ≤100 miles.
Cause of death was provided by 1 participating center and categorized as follows: death from primary cancer, health-related cause of death (secondary malignant neoplasm, infection, cardiovascular, pulmonary, endocrine, neurological, gastrointestinal and liver causes), other/unknown, and external causes of death (accidents, homicides, suicides, etc.).
Statistical Analysis
Program and Coding:
For our statistical analysis we used SAS Version 9.4 (SAS Institute Inc., Cary, NC).
Statistical Methods:
Our primary aim was to analyze the difference in all-cause mortality among children with cancer exposed to persistent child poverty environments vs. those residing in counties not experiencing persistent child poverty (mutually exclusive groups) in the state of Alabama. Descriptive statistics were examined overall and by persistent child poverty county status. Overall survival by persistent child poverty status was estimated using the Kaplan Meier method. Univariable Cox Proportional Hazard modeling was used to evaluate the association between all-cause mortality and patient characteristics (age, sex, race/ethnicity, insurance, diagnosis year, cancer type and stage at diagnosis) including our exposure of interest (persistent child poverty county). Multivariable analysis was conducted by censoring the cohort at 3 different survival times (survival times of 1, 5 and 10 years from cancer diagnosis). When examining the association between persistent child poverty and hazard of mortality, Model 1 included sex, race/ethnicity, diagnosis year, stage, regardless of their significance level on univariate analysis. In addition, Model 1 retained any of the following variables with P<0.001 on univariate analysis: age at cancer diagnosis, cancer type and insurance. Model 2 added distance in miles to treating center (per 20-mile increase) to Model 1. Model 3 added a 3-level combined variable of persistent child poverty and prolonged distance to care (>100 miles) with patients being exposed to both, either, or neither risk factor.
In sub analysis, cause-specific death was modeled using fine-gray subdistribution hazard models adjusted for race, sex, age, diagnosis year, insurance status, cancer type and stage. We analyzed cause-specific mortality for “death from primary cancer” and “death from health-related causes.”
Data availability statement
Data collected for this work is not public due to inclusion of protected health information. Data requests for additional evaluation of childhood cancer outcomes can be addressed to the corresponding author. A data use agreement would be required due to protected health information.
RESULTS
Our cohort included 2,089 children with cancer. Based on aggregate data from the Alabama Statewide Cancer Registry over the same time period, our cohort captured 70% of all children (N=2,984) diagnosed with cancer in the state of Alabama. Data are reported as an aggregate (of the 3 sites) with Children’s of Alabama contributing 86% of the patients; Huntsville Hospital for Women & Children and University of South Alabama Children’s and Women’s Hospital contributed 9% and 5%, respectively. Clinical and sociodemographic characteristics are described in Table 1. The median age at cancer diagnosis was 7 years (range 0 to 20 years). The cohort was comprised of 54% males, and 68% non-Hispanic whites, 27% African Americans, 4% Hispanics and 2% from other racial/ethnic backgrounds. The most common insurance type was private (49%), followed by Public/No insurance (43%) and insured type unknown (8.5%). The cancer diagnoses included central nervous system (CNS) tumors (23%), precursor cell leukemias (23%), bone tumors/sarcomas (12%) and other diagnoses (42%). Approximately one-third (32%) of the sample had regional or metastatic disease at diagnosis. With a median follow up was 6 years (range 0 to 21 years), we observed 493 deaths; 183 in the first year, 423 by 5 years, 469 by 10 years from diagnosis and 24 beyond 10 years. The median distance to treating center was 60 miles (range 0–260); 23% of the cohort lived >100 miles to the respective treating center.
Table 1:
Descriptive statistics for children with cancer by county poverty status
| Characteristic | Overall (N=2089) | Persistent child poverty county (N=887) | Non-poverty county (N=1202) | P-value^ |
|---|---|---|---|---|
| Race/Ethnicity (n, %) * | ||||
| African American | 553 (26.6%) | 374 (42.2%) | 179 (15.0%) | <.0001 |
| Hispanic | 85 (4.1%) | 24 (2.7%) | 61 (5.1%) | |
| Non-Hispanic white | 1406 (67.6%) | 477 (53.8%) | 929 (77.9%) | |
| Other | 36 (1.7%) | 12 (1.4%) | 24 (2.0%) | |
| Sex (n, %) * | ||||
| Male | 1120 (53.7%) | 469 (52.9%) | 651 (54.3%) | 0.5334 |
| Diagnosis year (n, %) | ||||
| ≤ 2008 | 1028 (49.2%) | 443 (49.9%) | 585 (48.7%) | 0.5646 |
| >2008 | 1061 (50.8%) | 444 (50.1%) | 617 (51.3%) | |
| Insurance * | ||||
| No Insurance/Public | 892 (42.8%) | 426 (48.2%) | 466 (38.9%) | 0.0009 |
| Private | 1013 (48.6%) | 393 (44.5%) | 620 (51.7%) | |
| Insured, type unknown | 178 (8.5%) | 65 (7.4%) | 113 (9.4%) | |
| Cancer Type | ||||
| Precursor cell leukemia | 472 (22.6%) | 184 (20.7%) | 288 (24.0%) | 0.2744 |
| Acute myeloid leukemia | 122 (5.8%) | 55 (6.2%) | 67 (5.6%) | |
| Hodgkin Lymphoma | 120 (5.7%) | 48 (5.4%) | 72 (6.0%) | |
| Non-Hodgkin Lymphoma | 150 (7.2%) | 61 (6.9%) | 89 (7.4%) | |
| Brain and CNS | 471 (22.5%) | 209 (23.6%) | 262 (21.8%) | |
| Bone tumors and sarcomas | 247 (11.8%) | 111 (12.5%) | 136 (11.3%) | |
| Neuroblastoma | 163 (7.8%) | 71 (8.0%) | 92 (7.7%) | |
| Renal tumors | 113 (5.4%) | 54 (6.1%) | 59 (4.9%) | |
| Other solid tumors+ | 132 (6.3%) | 62 (7.0%) | 70 (5.8%) | |
| Other leukemia | 45 (2.2%) | 17 (1.9%) | 28 (2.3%) | |
| Other rare | 54 (2.6%) | 15 (1.7%) | 39 (3.2%) | |
| Stage | ||||
| In situ/localized | 631 (30.2%) | 340 (28.3%) | 291 (32.8%) | 0.0134 |
| Regional/metastatic | 658 (31.5%) | 371 (30.9%) | 287 (32.4%) | |
| Not applicable | 800 (38.3%) | 491 (40.8%) | 309 (34.8%) | |
| County distance to treating center in miles | ||||
| Median (range) | 60.1 (0 – 260.4) | 43.4 (0 – 236.8) | 60.1(0 – 260.4) | 0.1046 |
| County distance to treating center > 100 miles | ||||
| Residence >100 miles to treatment | 482 (23.1%) | 335 (37.8%) | 147 (12.2%) | <.0001 |
| Rural-Urban Continuum county designations | ||||
| Nonmetro Counties | 519 (24.8%) | 239 (26.9%) | 280 (23.3%) | 0.0563 |
| Completely Rural Counties | 64 (3.1%) | 39 (4.4%) | 25 (2.1%) | 0.0024 |
Missing values: sex: 2, Race: 9, Insurance 6
P-value comparing persistent child poverty counties vs. non-poverty counties
(Germ cell tumors/trophoblastic tumors/tumors of gonads (68), hepatic tumors (32), retinoblastoma (32)
Forty-two percent of children were exposed to a persistent child poverty environment at cancer diagnosis. As shown in Table 1, a higher proportion of African Americans (42% vs 15%, P<0.0001) and lower proportion of Hispanic (2.7% vs 5.1%) and Non-Hispanic White patients (54% vs 78%) lived in persistent child poverty counties. There was no difference in the proportion of non-metro counties by poverty designation (P=0.06), though persistent child poverty counties were more likely to be designated as a completely rural county (4.4% vs. 2.1% P=0.002). Those in persistent child poverty counties were more likely to have Public/no insurance (48% vs. 39%, P=0.0009). Further, a higher proportion of children in persistent child poverty counties lived >100 miles to treating center (38% vs. 12%, P <0.0001). There was no difference in sex, age at cancer diagnosis, year of diagnosis, or cancer type by county poverty status.
The overall survival was significantly greater among children living in counties without a persistent child poverty designation (Figure 1). Table 2 reports the univariable and multivariable associations between covariates and all-cause mortality. Missing data values (listed below Table 1) were considered as a separate level for that variable in the models. There was no significant difference in survival by persistent child poverty designation at a survival time of 1 year (HR=1.05, 95%CI=0.77–1.43, P= 0.78). Children exposed to a persistent child poverty environment were 30% more likely to die by 5 years (HR=1.30, 95% CI=1.06–1.59, P=0.012) after adjusting for race/ethnicity, sex and age at cancer diagnosis, diagnosis year, insurance, cancer type and stage (Model 1). The risk of mortality at 10 years was similar to the risk seen at the 5 years (HR=1.33, 95%CI=1.10–1.62, P=0.003).
Figure 1:
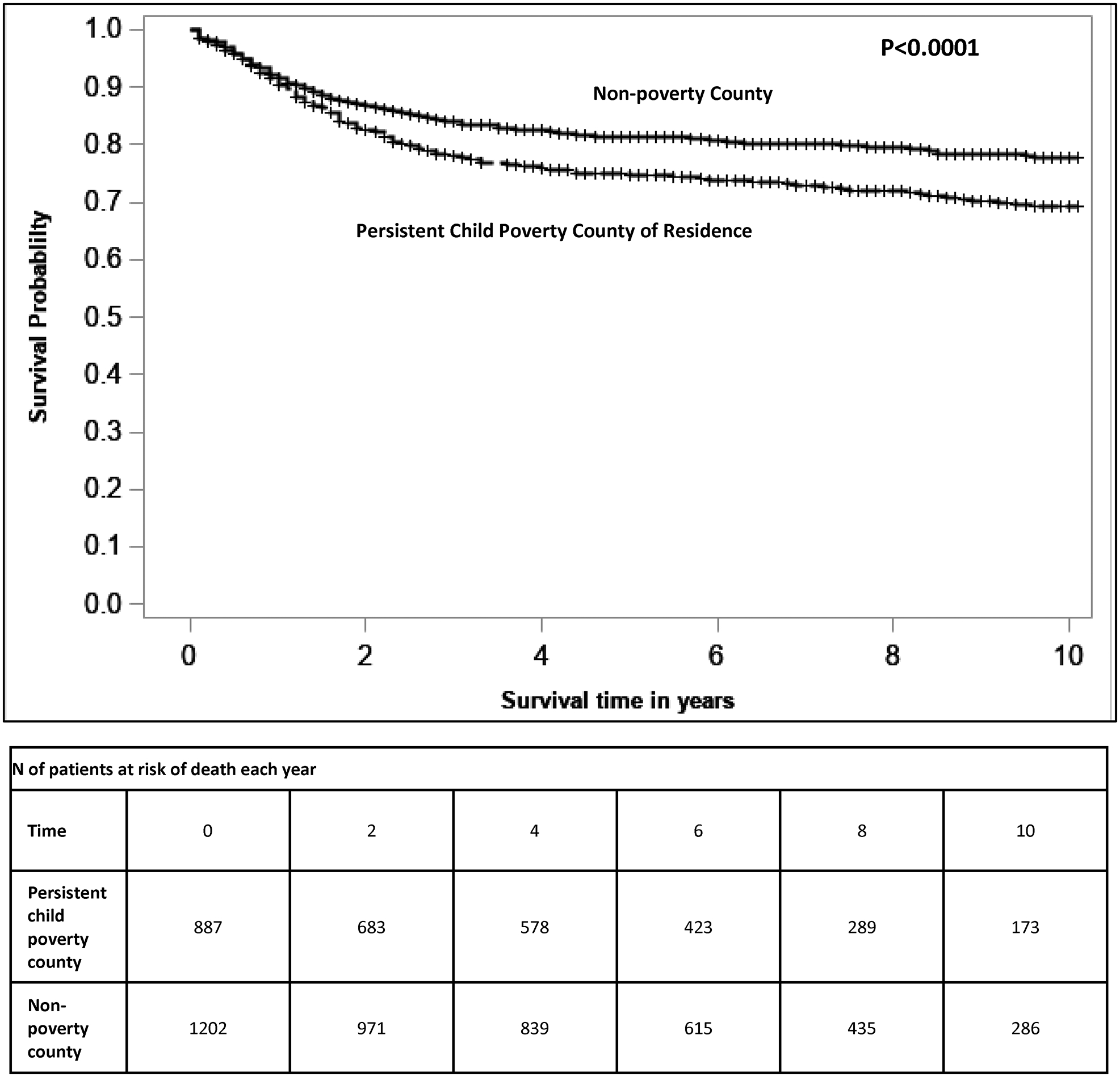
Overall survival for children with cancer in persistent child poverty counties versus non-persistent poverty counties. Based on county of residence at childhood cancer diagnosis, children in persistent child poverty counties have inferior overall survival.
Table 2:
Persistent Child Poverty County of Residence and Mortality in Unadjusted and Adjusted Models
| Characteristics | Univariable Model | Multivariable MODEL 1 | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | ||||
| Overall | 1y survival | 5y survival | 10y survival | |
| Persistent Child Poverty County (reference: no) | ||||
| Yes | 1.40 (1.17 – 1.68)^ | 1.05 (0.77 – 1.43) | 1.30 (1.06 – 1.59)* | 1.33 (1.10 – 1.62)* |
| Race/Ethnicity (reference: non-Hispanic white) | ||||
| African American | 1.13 (0.93 – 1.36) | 1.17 (0.82 – 1.66) | 1.11 (0.89 – 1.40) | 1.09 (0.88 – 1.36) |
| Hispanic | 0.61 (0.34 – 1.09) | 1.42 (0.73 – 2.77) | 0.92 (0.52 – 1.62) | 0.83 (0.47 – 1.46) |
| Other | 0.74 (0.37 – 1.49) | 1.66 (0.66 – 4.18) | 0.90 (0.40 – 2.02) | 0.94 (0.44 – 1.99) |
| Sex (reference: female) | ||||
| Male vs. female | 1.15 (0.96 – 1.38) | 1.16 (0.86 – 1.56) | 1.10 (0.91 – 1.34) | 1.15 (0.95 – 1.38) |
| Age at diagnosis | ||||
| per y increase in age | 1.03 (1.02 – 1.05)^ | 1.01 (0.99 – 1.04) | 1.04 (1.02 – 1.06)^ | 1.05 (1.03 – 1.07)^ |
| Diagnosis year (reference: ≤ 2008) | ||||
| >2008 | 0.98 (0.81 – 1.18) | 0.78 (0.58 – 1.05) | 0.82 (0.67 – 0.99)* | 0.86 (0.72 – 1.04) |
| Insurance (reference: Private) | ||||
| No Insurance/Public | 0.65 (0.54 – 0.78)^ | 1.42 (1.03 – 1.97)* | 1.19 (0.96 – 1.47) | 1.23 (1.01 – 1.51)* |
| Insured, type unknown | 0.60 (0.43 – 0.83)* | 0.79 (0.43 – 1.48) | 0.96 (0.66 – 1.39) | 1.02 (0.72 – 1.44) |
| Cancer Type (reference: lymphoma) | ||||
| Precursor cell leukemia | 1.55 (0.95 – 2.53) | 3.17 (1.11 – 9.09)* | 4.52 (2.14 – 9.58)^ | 4.24 (2.13 – 8.44)^ |
| Acute myeloid leukemia | 5.57 (3.34 – 9.29)^ | 16.23 (5.78 – 45.61)^ | 14.96 (6.99 – 32.04)^ | 13.54 (6.72 – 27.25)^ |
| Brain and CNS | 4.75 (3.04 – 7.42)^ | 10.62 (4.88 – 23.09)^ | 11.01 (6.38 – 18.99)^ | 10.83 (6.46 – 18.14)^ |
| Solid tumors/rare | 3.42 (2.20 – 5.33)^ | 2.96 (1.37 – 6.38)* | 6.01 (3.56 – 10.14)^ | 6.22 (3.80 – 10.19)^ |
| Other | 2.31 (1.31 – 4.07)* | 7.76 (2.55 – 23.64)* | 10.43 (4.72 – 23.04)^ | 8.24 (3.92 – 17.31)^ |
| Stage (reference: metastatic) | ||||
| Localized | 0.95 (0.77 – 1.16) | 0.30 (0.20 – 0.47)^ | 0.42 (0.33 – 0.55)^ | 0.43 (0.34 – 0.55)^ |
| Not applicable | 0.72 (0.50 – 1.03) | 0.49 (0.25 – 0.99) | 0.40 (0.24 – 0.67)^ | 0.46 (0.29 – 0.72)^ |
P <0.05.
P <0.001.
Model 2 (Table 3) added distance (per 20-mile increase) to Model 1. There was no interaction between distance and persistent child poverty county status (P=0.93) and patients were evenly distributed over the distance quintiles (Supplementary Figure 2). At all survival times, longer distance to treatment was associated with an increased risk of mortality (5 years: HR=1.09, 95%CI=1.05–1.14, P<0.0001). There was no difference in the risk of mortality by distance to treating center at the 10-year survival time as compared to the 5-year survival time. Adding distance to care into the model (per 20-mile increase to cancer treatment) reduced the HR of living in a persistent child poverty county from 1.30 to 1.27 (95%CI=1.04–1.56, P= 0.02).
Table 3:
Association of Persistent Child Poverty County of Residence and Distance to Treating Center with Mortality
| Characteristic | Multivariable model (Hazard Ratio, 95% CI)~ | ||
|---|---|---|---|
| 1y survival | 5y survival | 10y survival | |
| MODEL 1: Persistent Child Poverty County | |||
| Persistent Child Poverty County (ref: No) | 1.05 (0.77 – 1.43) | 1.30 (1.06 – 1.59)* | 1.33 (1.10 – 1.62)* |
| MODEL 2: Persistent Child Poverty County and Distance to care+ as separate variables | |||
| Persistent Child Poverty County (ref: No) | 1.03 (0.75 – 1.40) | 1.27 (1.04 – 1.56)* | 1.31 (1.08 – 1.59)* |
| Miles to treating center+ | 1.09 (1.03 – 1.16)* | 1.09 (1.05 – 1.14)^ | 1.08 (1.04 – 1.13)^ |
| MODEL 3: Persistent Child Poverty County and Prolonged Distance to Care as combined variable × | |||
| Persistent child poverty + distance >100 miles (ref: neither) | 1.42 (0.95 – 2.13) | 1.80 (1.39 – 2.33)^ | 1.79 (1.40 – 2.29)^ |
| Persistent child poverty or distance >100 miles (ref: neither) | 1.21 (0.86 – 1.71) | 1.30 (1.04 – 1.63)* | 1.33 (1.07 – 1.65)* |
all models adjusted for race, sex, age, diagnosis year, insurance status, cancer type and stage
P <0.05.
P <0.001.
per 20-mile increase
>100 miles to cancer treatment center
As shown in Model 3 (Table 3), patients who had the dual exposure of living in a persistent child poverty environment and distance to treating center >100 miles had the highest risk of mortality at 5 years (HR=1.80, 95%CI=1.39–2.33, P<0.0001) compared to patients with neither exposure. Patients with either exposure (distance or persistent child poverty) had a 30% increased risk of mortality at 5 years (HR=1.30, 95%CI=1.04–1.63, P= 0.021) compared to patients with neither exposure. At 1 year, there was not a significantly increased risk of mortality for children with either or both exposure (as compared to neither), while at 10 years there was a statistically significant increased risk of mortality for both groups, similar in magnitude as compared to the 5-year survival time.
With regards to cause-specific mortality, our largest center contributed 1,361 patients and 438 deaths (317 from primary cancer, 53 from health related causes, 58 from other/unknown, and 10 from external causes). Those in persistent child poverty counties were more likely to die from their primary cancer at 5 years (HR=1.33, 95%CI=1.05–1.70, P=0.02) and 10 years (HR=1.37, 95%CI 1.09–1.73, P=0.008). Those in persistent child poverty counties were as likely to die of health-related causes as those in non-poverty counties (HR=1.07, 95%CI=0.59–1.94, P=0.83).
DISCUSSION
Unlike exposure to poverty at the individual level, persistent poverty examines poverty in the context of the community in which the individual lives. Persistent poverty is a longitudinal marker that reflects enduring community level poverty for over 30 years. The mechanisms underlying observed cancer disparities in persistent poverty communities are not well understood and likely reflect multi-level risk factors at the structural, institutional and individual levels.
In our cohort of children with cancer from the state of Alabama, exposure to a persistent child poverty environment was prevalent (>40% of our sample), and children in these areas were more likely to be African American, have public or no health insurance, and lived >100 miles to their cancer treatment center. We found that children in persistent child poverty counties were nearly 30% more likely to die by 5 years from their cancer diagnosis as compared to children from non-poverty counties. In sub-analysis, of cause-specific mortality, children from persistent child poverty counties were more likely to die from their primary cancer but as likely to die from health-related causes as those not living in persistent poverty counties. Children with the dual exposure of distance >100 miles and persistent child poverty environment were nearly 80% more likely to die by 5 years from their cancer diagnosis than children with neither exposure.
Exposure to a persistent poverty environment has recently been shown to be associated with higher cancer mortality among adults.6 Possible mechanisms for this finding among adults are likely multifactorial and may include risk factors at the structural level (reduced access to care including lower rates of cancer screening), institutional level and the individual level (higher rates of cancer risk behaviors including smoking, obesity.14–16 Compared to persistent poverty, persistent child poverty is a much more prevalent exposure affecting 23% of all counties in the United States, mostly in the South.10 However, the impact of persistent child poverty on children with cancer has not been examined.
Persistence is an important dimension because it describes a community level transgenerational exposure of poverty that has endured over time.8 In contrast to communities that may go into and out of poverty overtime, these areas have had a high proportion of residents that remain in poverty over the last 30 years. Persistent poverty counties (adult measure) have unique characteristics as compared to non-poverty counties including having greater unemployment, lower educational attainment, and a higher proportion of the population <18 years of age.9 Though persistent poverty counties have a higher adult cancer mortality than non-poverty counties,6 the mechanisms behind this finding remain poorly understood.
Even less is known about persistent child poverty counties,10 though the characteristics of these communities warrant further exploration, particularly given that one fifth of all US counties are classified as having persistent child poverty. Our analyses show that in Alabama, children with cancer in persistent child poverty counties are more likely to have public/no insurance, and live distant to their cancer treatment center. As such, children living in persistent child poverty counties may be more likely to have multiple simultaneous risk factors for poor cancer outcomes.
Children from persistent child poverty counties were also more likely to be African American compared to children in non-poverty counties. There are several publications showing that among African American adults with cancer, living areas with high residential segregation (a marker for structural racism), is associated with inferior outcomes.17–19 Based on our findings, specific structural barriers and challenges faced by African American children in persistent child poverty counties warrant further exploration.
By censoring our cohort at different survival times (1, 5, and 10 years from cancer diagnosis) we are able to generate hypotheses for possible mechanisms underlying the observed disparity in overall survival. At 1 year from cancer diagnosis, there was no difference in survival between those in persistent child poverty counties vs. non-poverty counties, suggesting that acute treatment-related toxicities are not the mechanism for the association. After adjusting for other prognosticators, children in our cohort were nearly 30% more likely to die by 5 years after their cancer diagnosis if they resided in a persistent child poverty county. In sub-analysis of one center, children from persistent child poverty counties were more likely to die from their primary cancer. One hypothesis for these findings is reduced access to cancer care among children in persistent child poverty environments, which we explored by examining distance to cancer treatment center. Prolonged distances to cancer treatment centers may place a repetitive burden on families and compromise treatment, follow up, and appropriate cancer surveillance over time.
Most persistent child poverty counties in Alabama are located in the southern part of the state, distant from the largest children’s hospital in Jefferson County. Though there was no interaction between persistent child poverty and distance to care, a higher proportion of children in persistent child poverty counties lived >100 miles to their cancer treatment center. Distances to treatment center of greater than 50 miles have been previously described as a risk factor for mortality among children and young adults with acute lymphoblastic leukemia,20 though the impact of distance to care on other cancer types and the impact of multiple overlapping exposures (i.e. distance and poverty) among children with cancer are not well understood. In our cohort, distance (per 20-mile increase to treatment center) was independently associated with an increased risk of mortality, and those with the dual exposure of a persistent child poverty environment and distance >100 miles to treatment were at highest risk of death.
Other pathways through which a persistent child poverty environment may negatively impact children at the individual level warrant further exploration. Numerous studies have described the negative physiologic impact of living in a low socioeconomic status (SES) environment on child health. Lower levels of SES are associated with higher levels of obesity, sedentary lifestyle, and increased levels of chronic stress among children.21,22 Among children with cancer, there may be physiologic differences secondary to the stress of living in a high poverty environment that could impact their response and resiliency to cancer treatment.
It is also important to consider the compound effect of individual (household) risk factors combined with a persistent poverty environment in future studies. For example, housing insecurity, food insecurity and financial toxicity are commonly described among families undergoing childhood cancer treatment and in survivorship.23,24 Persistent poverty communities, may lack the infrastructure and support systems to assist patients and families who face these individual issues while undergoing cancer treatment. Individual household poverty may pose a particular risk to those in persistent poverty communities. Parents and caregivers with household poverty may lack the resources needed to adhere to cancer treatment over time,25 and the burden of seeking cancer treatment (i.e., lost work, lost wages – particularly among those traveling long distances) may be insurmountable without additional community safety nets in place.23,24
Not all participating centers reported cause of death, which is a limitation of the current study. By censoring the cohort at various survival times we can generate hypotheses regarding mechanisms for our findings, and we were able to complete a cause-specific death analysis for the largest contributing center. Though most children with cancer are treated on clinical trials,3 specific treatment information was not available for analyses, nor were patient-level comorbidities or functional status. Though stage at diagnosis was included as a prognosticator in our analysis, prognostic markers specific for childhood leukemia (such as the National Cancer Institute risk group) were not able to be calculated with the available data thus was not included. We did not examine cancer-specific models due to limited sample size of the individual cancer types. However, an examination by cancer type is warranted in future studies to understand how the exposure of persistent child poverty and distance to care may differentially impact different cancer types. Without residential address, more specific measures of healthcare access, including patient commute time, were not able to be analyzed. Because household level poverty is not routinely collected for cancer registry reporting, it was not available in the current study, though we were able to include Medicaid status (as a proxy measure of income) in our analysis. Given the smaller sample sizes from two contributing institutions, outcomes were not analyzed by individual treatment center. Lastly, our sample mirrors the racial/ethnic make-up of the population in Alabama,26 however compared to the US as a whole, our sample has relatively fewer Hispanic and Asian patients, and a greater proportion of African Americans.
The present study has numerous strengths including a cohort that is large and racially diverse, with data from all children’s hospitals in the state representing most children diagnosed with cancer in Alabama over the observation period. Our analyses report findings regarding an under-represented group of children in the literature, children with cancer from the Deep South, and include a high proportion of children exposed to persistent child poverty environments and living physically distant from the treating center.
In conclusion, children with cancer living in persistent child poverty counties in Alabama face an increased risk of mortality at 5 years and 10 years as compared to children in non-poverty counties. This risk was present regardless of their distance to treatment (both children near to and far from treatment centers faced an increased risk of mortality if they lived in a persistent child poverty county). However, distance to care was independently associated with inferior survival, and children with the dual exposure of prolonged distance to care and a persistent child poverty environment faced the highest risk of mortality. In sub-analysis, children in persistent child poverty counties were more likely to die from their primary cancer and at equal risk for mortality from health-related causes. Future research with longer follow up (beyond 10 years) is warranted to investigate health-related mortality in this vulnerable group of children given the long latency of health-related complications following childhood cancer diagnosis.
In contrast to the concept of poverty, persistent poverty spans generations, and reflects enduring deprivation in a community for over 30 years. To mitigate disparities, further etiologic research is urgently needed to understand the multilevel causes of cancer disparities faced by children in persistent child poverty communities. Understanding structural issues, as well as institutional policies and practices, and individual risk factors that contribute to ongoing disparities in these communities will be critical to inform future interventions and policy changes. Improved access to care is of paramount importance in improving cancer related outcomes for this vulnerable population in Alabama; strategies including expansion of telehealth and satellite clinic systems are under exploration.
Supplementary Material
Financial Support:
This work was supported by the National Institutes of Health, National Cancer Institute (CA 047888 to A.H.); and by a Mentored Research Scholar Grants in Applied and Clinical Research from the American Cancer Society (MRSG-18-020-01- CPPB to K.K.).
Footnotes
Disclosure Statement: The authors declare no potential conflicts of interest.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 2.Hudson MM, Meyer WH, Pui CH. Progress Born From a Legacy of Collaboration. J Clin Oncol. 2015;33(27):2935–7. [DOI] [PubMed] [Google Scholar]
- 3.Gelijns AC, Gabriel SE. Looking beyond translation--integrating clinical research with medical practice. N Engl J Med. 2012;366(18):1659–61. [DOI] [PubMed] [Google Scholar]
- 4.Bona K, Li Y, Winestone LE, Getz KD, Huang Y, Fisher BT, et al. Poverty and Targeted Immunotherapy: Survival in Children’s Oncology Group Clinical Trials for High-Risk Neuroblastoma. J Natl Cancer Inst. 2021;113(3):282–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bona K, Brazauskas R, He N, Lehmann L, Abdel-Azim H, Ahmed AI, et al. Neighborhood poverty and pediatric allogeneic hematopoietic cell transplantation outcomes: a CIBMTR analysis. Blood. 2021;137(4):556–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moss JL, Pinto CN, Srinivasan S, Cronin KA, Croyle RT. Persistent Poverty and Cancer Mortality Rates: An Analysis of County-Level Poverty Designations. Cancer Epidemiol Biomarkers Prev. 2020;29(10):1949–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Unger JM, Moseley AB, Cheung CK, Osarogiagbon RU, Symington B, Ramsey SD, et al. Persistent Disparity: Socioeconomic Deprivation and Cancer Outcomes in Patients Treated in Clinical Trials. J Clin Oncol. 2021;39(12):1339–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Descriptions and Maps: County Economic Types, 2015. Edition. Available from: https://www.ers.usda.gov/data-products/county-typology-codes/descriptions-and-maps/#ppov.
- 9.Miller K, Weber BA. Persistent poverty across the rural-urban continuum RPRC Working Paper No 03–01. Rural Poverty Research Center; 2003. [Google Scholar]
- 10.Descriptions and Maps: County Economic Types, 2015. Edition. Available from: https://www.ers.usda.gov/data-products/county-typology-codes/descriptions-and-maps/#pcpov.
- 11.International Classification of Childhood Cancer (ICCC). Available at: https://seer.cancer.gov/iccc/.
- 12.Swerdlow SH, Campo; Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, International agency for research on cancer, 2008. [Google Scholar]
- 13.American Community Survey (ACS). Available from: https://www.census.gov/programs-surveys/acs/.
- 14.Bennett KJ, Probst JC, Pumkam C. Obesity among working age adults: the role of county-level persistent poverty in rural disparities. Health Place. 2011;17(5):1174–81. [DOI] [PubMed] [Google Scholar]
- 15.Moss JL, Liu B, Feuer EJ. Urban/Rural Differences in Breast and Cervical Cancer Incidence: The Mediating Roles of Socioeconomic Status and Provider Density. Womens Health Issues. 2017;27(6):683–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bennett KJ, Pumkam C, Bellinger JD, Probst JC. Cancer screening delivery in persistent poverty rural counties. J Prim Care Community Health. 2011;2(4):240–9. [DOI] [PubMed] [Google Scholar]
- 17.Poulson M, Cornell E, Madiedo A, Kenzik K, Allee L, Dechert T, et al. The Impact of Racial Residential Segregation on Colorectal Cancer Outcomes and Treatment. Ann Surg. 2021;273(6):1023–1030. [DOI] [PubMed] [Google Scholar]
- 18.Poulson MR, Beaulieu-Jones BR, Kenzik KM, Dechert T, Ko NY, Sachs T, et al. Residential Racial Segregation and Disparities in Breast Cancer Presentation, Treatment, and Survival. Ann Surg. 2021;273(1):3–9. [DOI] [PubMed] [Google Scholar]
- 19.Poulson MR, Helrich SA, Kenzik KM, Dechert TA, Sachs TE, Katz MH. The impact of racial residential segregation on prostate cancer diagnosis and treatment. BJU Int. 2021;127(6)636–644. [DOI] [PubMed] [Google Scholar]
- 20.Rotz SJ, Wei W, Thomas SM, Hanna R. Distance to treatment center is associated with survival in children and young adults with acute lymphoblastic leukemia. Cancer. 2020;126(24):5319–5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newacheck PW, Hung YY, Park MJ, Brindis CD, Irwin CE Jr. Disparities in adolescent health and health care: does socioeconomic status matter? Health Serv Res. 2003;38(5):1235–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen E, Paterson LQ. Neighborhood, family, and subjective socioeconomic status: How do they relate to adolescent health? Health Psychol. 2006;25(6):704–14. [DOI] [PubMed] [Google Scholar]
- 23.Bona K, London WB, Guo D, Frank DA, Wolfe J. Trajectory of Material Hardship and Income Poverty in Families of Children Undergoing Chemotherapy: A Prospective Cohort Study. Pediatr Blood Cancer. 2016;63(1):105–11. [DOI] [PubMed] [Google Scholar]
- 24.Bilodeau M, Ma C, Al-Sayegh H, Wolfe J, Bona K. Household material hardship in families of children post-chemotherapy. Pediatr Blood Cancer. 2018;65(1): 10.1002/pbc.26743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhatia S, Landier W, Hageman L, Heeyoung K, Chen Y, Crews K, et al. 6MP adherence in a multiracial cohort of children with acute lymphoblastic leukemia: a Children’s Oncology Group study. Blood. 2014;124(15):2345–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.United States Census Bureau. available at: https://data.census.gov/cedsci/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data collected for this work is not public due to inclusion of protected health information. Data requests for additional evaluation of childhood cancer outcomes can be addressed to the corresponding author. A data use agreement would be required due to protected health information.


